Abstract
Aerobic exercise reduces blood pressure (BP) on average 5 to 7 mmHg among those with hypertension; limited evidence suggests similar or even greater BP benefits may result from isometric handgrip (IHG) resistance exercise. We conducted a randomized controlled trial investigating the antihypertensive effects of an acute bout of aerobic compared to IHG exercise in the same individuals. Middle-aged adults (n=27) with prehypertension and obesity randomly completed three experiments: aerobic [60% peak oxygen uptake, 30 minutes]; IHG [30% maximum voluntary contraction, 4x2 minutes bilateral]; and non-exercise control. Subjects were assessed for carotid-femoral pulse wave velocity (PWV) pre and post exercise, and left the laboratory wearing an ambulatory BP monitor. Systolic and diastolic BP (SBP/DBP) were lower after aerobic versus IHG (4.8±1.8/3.1±1.3mmHg, p=0.01/0.04) and control (5.6±1.8/3.6±1.3mmHg, p=0.02/0.04) over the awake hours, with no difference between IHG versus control (p=0.80/0.83). PWV changes following acute exercise did not differ by modality (aerobic increased 0.01±0.21m•s−1, IHG decreased 0.06±0.15m•s−1, control increased 0.25±0.17m•s−1, p>0.05). A subset of participants then completed either 8 weeks of aerobic or IHG training. Awake SBP was lower after versus before aerobic training (7.6±3.1mmHg, p=0.02), while sleep DBP was higher after IHG training (7.7±2.3mmHg, p=0.02). Our findings did not support IHG as antihypertensive therapy but that aerobic exercise should continue to be recommended as the primary exercise modality for its immediate and sustained BP benefits.
Keywords: Ambulatory Blood Pressure Monitoring, Acute Exercise, Arterial Stiffness, Exercise Training, Isometric Handgrip, Lifestyle, Postexercise Hypotension, Prehypertension, Resistance Exercise
INTRODUCTION
Hypertension is the most prevalent, costly, and modifiable risk factor for cardiovascular disease affecting over 1 billion adults globally [1]. Both acute [short-term or postexercise hypotension (PEH)] and chronic (long-term or training) aerobic exercise lower blood pressure (BP) 5 to 7 mmHg among adults with hypertension [2, 3]. Therefore, moderate intensity, aerobic exercise is universally recommended on most, preferably all days of the week to lower BP among those with hypertension [4, 5]. Unfortunately, the majority of people with hypertension do not adhere to these recommendations to achieve these BP benefits [6, 7].
Seven randomized controlled trials have reported BP reductions from acute [8, 9] and chronic [10–14] IHG resistance exercise of 3 to 15 mmHg among young [12, 14] and older [8, 10, 11, 13] adults with normal [8, 10, 14] and high BP [11–13]. Notably these BP reductions following acute and chronic IHG resistance exercise rival the magnitude observed following acute and chronic aerobic exercise among adults with high BP [15], while the volume of IHG resistance exercise was considerably less [8–14]. On the other hand, three other trials observed no antihypertensive benefit from a similar volume of IHG resistance exercise [16–18]. As a result, the American Heart Association (AHA) cautioned that more data are needed to establish the efficacy and safety of IHG resistance exercise as antihypertensive therapy [19].
Accordingly, we conducted a randomized, crossover trial to assess the magnitude and duration of PEH after acute IHG resistance compared to acute aerobic exercise in the same group of adults with high BP. In addition, we assessed the BP response in a smaller subset of these adults after completing an IHG resistance exercise training program compared to aerobic exercise training program. We hypothesized acute and chronic IHG resistance exercise would elicit equal or even greater antihypertensive benefits than acute and chronic aerobic exercise.
METHODS
Overview
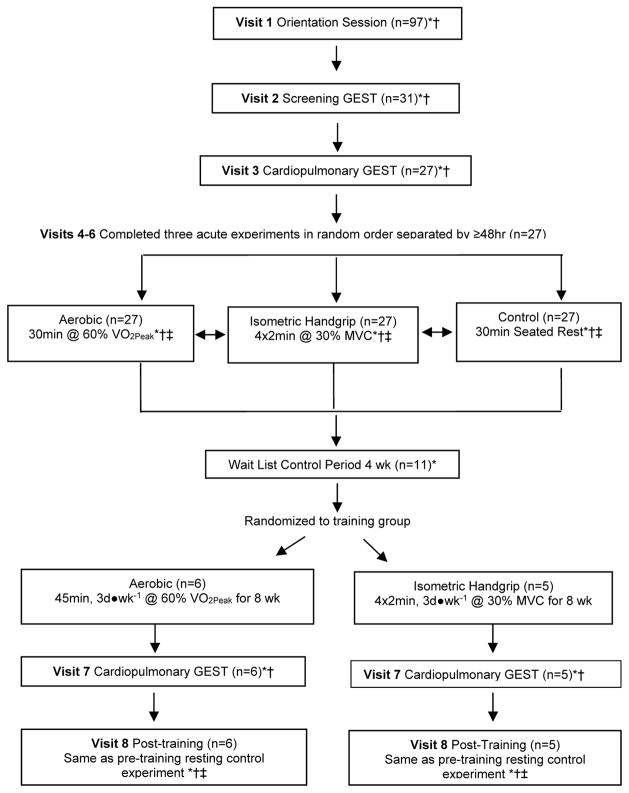
We employed a randomized controlled crossover design based upon our previous PEH studies (Figure 1) [20–22]. Subjects completed an orientation, two stress tests, and provided a fasting blood sample for an assessment of cardiometabolic profile (Visits 1–3). They next completed three randomly assigned acute experiments at the same time of the morning separated by at least 48 hours: 30 minutes of aerobic cycling at 60% peak oxygen consumption (VO2peak), 4x2 minutes bilateral IHG contractions at 30% maximum voluntary contraction (MVC), and a 30 minute control session of seated rest. We measured BP and pulse wave velocity (PWV), an index of arterial stiffness, in the laboratory before and for 60 minutes after each experiment. Subjects then left the laboratory wearing an ambulatory BP monitor over the next 19 hours.
Fig. 1.
Study Design. *Blood pressure measured throughout (see text for details). †Ambulatory blood pressure monitor worn afterwards until waking the next morning. ‡Pulse wave velocity measured throughout (see text for details). GEST, graded exercise stress test. MVC, maximum voluntary contraction. VO2peak, peak oxygen consumption
Subjects
Sedentary subjects aged 18 to 55 years with pre- to stage 1 hypertension and a body mass index (BMI) ≥25 to <40 kg•m−2 were enrolled. Any medications that could potentially influence BP including inhaled or oral steroids, nonsteroidal anti-inflammatory agents, aspirin, antihypertensive and hyperlipidemic medications, nutritional supplements besides one-a-day vitamin, cold medications, hormone-altering contraception, or herbal supplements were stopped at least four weeks before any testing. Subjects with osteoarthritis and orthopedic problems were not recruited if these conditions compromised their ability to complete the acute exercise experiments. Four participants with mild hypertension discontinued their antihypertensive medications ≥6 weeks prior to study participation with physician permission. Women were pre-menopausal and regularly menstruating. Subject remained weight stable throughout study participation, defined as gaining or losing <2.25 kg of orientation body weight. Informed consent was obtained from all individual participants included in the study. The procedures were approved by the institutional review boards of the University of Connecticut and Hartford Hospital.
Body Composition
BMI (kg•m−2) was calculated from body weight and height using a calibrated balance beam scale. Waist circumference was measured at the narrowest part of the torso using a non-distensible Guilick tape measure [23].
Blood Pressure
A trained investigator (GIA) measured BP with an automated BPTRU BPM-100 monitor (Coquitlam, Canada) according to AHA standards [24] at the orientation session to determine BP status. This investigator also measured BP before the acute experiments every 2 minutes for 20 minutes in the non-dominant arm with the automated BPTRU monitor that was averaged as baseline BP. After each study visit using our established protocols [20–22, 24–26], subjects were attached to an Oscar2 ambulatory BP monitor (SunTech Medical, Raleigh, NC). A calibration check was done with a mercury sphygmomanometer using a t-tubule upon attachment of the ambulatory BP monitor to the subject.
The ambulatory BP monitor was programmed to record BP at regular intervals three times per waking hour and two times per sleeping hour. The monitor obtained a second reading if consecutive readings differ by >50 mmHg for SBP, >40 mmHg for DBP, or >50 mmHg for pulse pressure. Subjects were instructed to leave the laboratory and proceed with normal activities and diet, avoid unusual activities including napping, not to exercise, and when each ambulatory BP measurement was being taken to keep their arm still and extended at their side. Subjects carried a standard journal, recording activities performed during each measurement, any unusual physical or emotional events, and sleep and wake times. The next morning they detached the monitor and physically returned it that day along with the journal to the investigator. The investigator inspected the journal and reports after each visit, and reminded the subjects of all protocol instructions.
Computerized ambulatory BP reports were acceptable if at least 80% of the potential BP readings were obtained. We omitted ambulatory BP readings of SBP >220 or <80 mmHg, or DBP >130 or <40 mmHg according to the manufacturer’s exclusion criteria. Ambulatory BP studies following the screening visits (Visits 1–3) were used to familiarize subjects with the technology [25], confirm their ability to follow all protocols, and lastly that they met the inclusion criteria of having prehypertension to stage 1 hypertension [26].
Stress Tests
The first peak graded exercise stress test (Visit 2) was performed on a treadmill following the Bruce Protocol with continuous 12-lead electrocardiograph recording (Cardiosoft, GE Healthcare, Port Washington, NY) to exclude atherosclerotic ischemic heart disease. The second (Visit 3) was performed on a Monark 893E Digital Cycle Ergometer (Stockholm, Sweden) to determine VO2peak. Subjects cycled continuously at a constant cadence of 60 revolutions per minute with resistance increased by 0.5 kiloponds every 2 minutes until volitional exhaustion [23]. VO2peak was determined using breath-by-breath analysis of expired gases (ParvoMedics TrueOne 2400 Metabolic Measurement System, Sandy, UT).
Cardiometabolic Profile
Subjects provided a fasting blood sample (Visit 3) for baseline values of serum lipids, lipoproteins, glucose, and insulin (see Supplemental Digital Content 1-A, which describes blood sampling and analysis).
Acute Experiments
To measure PEH, subjects completed three experiments in random order at the same time of the morning at least 48 hours apart (Visits 4 through 6). Subjects were instructed to consume a standard breakfast two to three hours before all experiments consisting of 250 mL orange juice, 125 mL skim or 1% milk, and either 125 mL of plain cereal such as cornflakes, two slices white toast, one English muffin, or one bagel 9 cm in diameter [20–22]. They were also instructed to refrain from caffeinated beverages for 6 hours before all experiments. Each experiment began with 10 minutes of supine rest followed by a baseline measurement of pulse wave velocity (PWV), an index of arterial stiffness (see Supplemental Digital Content 1-B, which describes arterial stiffness assessment). Volunteers then sat for 20 minutes with BP recordings taken every 2 minutes from the non-dominant arm, which were averaged to determine baseline.
This was followed by one of three experiments assigned in random blinded order by a single block design (www.randomization.com) [27]: 1) Aerobic Exercise: cycling for 20 minutes at 60% VO2peak with a 5 minute warm up and cool down. Intensity was monitored using a Polar Heart Rate Monitor (Lake Success, NY) based upon a linear regression plot of work and heart rate versus VO2 achieved on the cardiopulmonary GEST; 2) IHG Resistance Exercise: a digital handgrip device (Zonaplus, Boise, ID) was held while sitting upright in a chair with feet flat on the floor and a single maximal contraction of the hand flexor muscles with each hand was completed to determine MVC. Subjects then performed four, 2 minute alternating bilateral contractions of the hand flexor muscles at 30% MVC with 1 minute rest between contractions. We selected this protocol because it has been shown in previous research to lower BP [8–14], and thus, has been adopted for use in clinical settings as antihypertensive lifestyle therapy [19, 28]. Subjects were provided feedback and encouragement to sustain 30% MVC. The percentage of time they held this tension was registered by the digital device and averaged 85.1±1.5%; and 3) Control: sitting quietly for 30 minutes. All experiments concluded with 30 minutes of seated recovery followed by 30 minutes of supine recovery, with BP measured every 2 minutes and PWV measured 35 and 60 minutes into the recovery period. The investigator monitored the subjects closely to ensure they did not fall asleep. Subjects left the laboratory each visit wearing the same ambulatory BP monitor as they wore during the screening visits until the next morning.
Training Study
After completion of the PEH experiments, 11 of the 27 subjects volunteered to participate in an exercise training program to investigate whether chronic aerobic versus IHG resistance exercise training influenced resting BP (Figure 1). These individuals completed a four week wait list control period [29] after which systolic and diastolic BP (SBP/DBP) were similar to their before wait list value (p=0.32 / 0.45). The subjects were then randomly assigned by a single block design (www.randomization.com) [27] to complete either a supervised aerobic (n=6, 60% VO2peak, 45 minutes per day) or IHG (n=5, 30% MVC, 4x2 minutes bilateral) exercise training program three days per week for eight weeks (see Supplemental Digital Content 1-C, which describes exercise training). During the last week of training, each subject completed a stress test (Visit 7, Figure 1). Beginning 48 hours after the last training session, each subject completed post-training assessments of resting baseline BP, PWV, and ambulatory BP over 19 hours (Visit 8, Figure 1) following the identical protocol of the pre-training resting control experiment. The interval of 48 hours was long enough to avoid the confounding effects of PEH from the last session but short enough to avoid the confounding effects of detraining on BP [2].
Statistical Analysis
Postexercise Hypotension (PEH)
Data are reported as mean ± standard error. The PEH dependent variable was calculated as the BP change following control versus the BP change following exercise (aerobic and IHG) and tested for normality by the Shapiro-Wilk test. Repeated measures analysis of covariance (RMANCOVA) compared BP between experiments (control, aerobic, and IHG) over four 15 minute intervals following exercise in the laboratory and then hourly intervals under ambulatory conditions with age and BMI as covariates over awake (first 10 hours post-exercise), sleep (11 to 19 hours), and 19 hours. All dependent variables and covariates in the RMANCOVA were tested for normality by the Shapiro-Wilk test and log-transformed if needed to satisfy the underlying assumption of normality. No sex- or racial-based differences in BP response were present (p>0.05) so subjects were analyzed as a single cohort.
The Ambulatory Blood Pressure Response to Exercise Training
The resting BP response to exercise training was calculated as resting ambulatory BP over awake, sleep, and 19 hours after training versus the day of the resting control experiment before training. Mixed linear models compared BP before versus after training over four 15 minute intervals in the laboratory and then hourly intervals under ambulatory conditions over awake (first 10 hours), sleep (11 to 19 hours), and 19 hours. Analysis of arterial stiffness outcomes followed similar methods (see Supplemental Digital Content 1-D).
Statistical Power Calculations
Given expected SBP reduction of 6.9±1.1 mmHg [3] following aerobic exercise and 13.4±1.1 mmHg [30] following IHG, we needed 24 subjects to assess PEH magnitude differences by modality (β=80%, α=0.05). Thus, our sample of 27 subjects performing acute PEH experiments was adequately powered although the subpopulations completing chronic exercise training were not. However, power among the chronic training groups was increased by employing mixed linear models with hourly intervals as an alternative to RMANOVA; thus representing each subject as 19 hourly BP measurements and making the effective sample size n=5x19=95. All statistical analyses utilized SPSS 14.0 (Chicago, IL) except for mixed linear models (SAS 9.3, Cary, NC) and power calculations (SAS 9.3 and PASS 2008, NCSS, Kaysville, UT).
RESULTS
Subjects
Subjects were middle-aged and overweight to obese with prehypertension. They were below average physical fitness for their age [23], and had fasting glucose and lipids-lipoproteins within normal ranges [7] (Table 1). Over half (52%) of the participants reported a family history of hypertension among first degree relatives. Baseline subject characteristics did not differ between the exercise modality groups (p>0.05) (Table 1) and were also plotted at the subject level (Supplemental Digital Figures S2-1, S2-2). Exercise training performance and outcomes are detailed in Supplemental Digital Content 3-A.
Table 1.
Mean (±SEM) baseline characteristics of the subjects
| Acute Exercise Experiments (n=27) |
Aerobic Exercise Training (n=6) |
Isometric Handgrip Exercise Training (n=5) |
|
|---|---|---|---|
| Age (yr) | 40.6±2.0 | 39.7±4.9 | 43.4±5.3 |
| Race (African American / Caucasian / Other) | 15 / 10 / 2 | 1 / 4 / 1 | 3 / 2 / 0 |
| Gender (male / female) | 23 / 4 | 6 / 0 | 4 / 1 |
| Body mass index (kg·m−2) | 30.7±0.7 | 29.7±0.9 | 33.0±2.2 |
| Waist circumference (cm) | 91.6±1.8 | 92.3±2.4 | 96.2±2.6 |
| Relative peak oxygen consumption (mL·kg−1·min−1) | 27.4±1.1 | 32.7±2.2 | 24.4±3.2 |
| 19 hour systolic blood pressure (mmHg) | 138.4±1.9 | 140.7±2.3 | 134.0±2.7 |
| 19 hour diastolic blood pressure (mmHg) | 82.9±1.5 | 83.5±1.6 | 78.4±2.3 |
| Awake systolic blood pressure (mmHg) | 145.7±2.0 | 150.9±2.2 | 142.4±3.2 |
| Awake diastolic blood pressure (mmHg) | 89.4±1.5 | 92.1±1.4 | 87.2±3.3 |
| Sleep systolic blood pressure (mmHg) | 130.3±2.3 | 129.4±4.1 | 124.6±3.1 |
| Sleep diastolic blood pressure (mmHg) | 75.7±1.9 | 74.0±2.5 | 68.7±2.1 |
| Fasting glucose (mmol·L−1) | 5.39±0.11 | 5.56±0.35 | 5.60±0.37 |
| Fasting insulin (pmol·L−1) | 70.8±10.4 | 54.2±15.3 | 105.6±39.6 |
| HOMA | 2.4±0.3 | 2.0±0.7 | 3.6±1.2 |
| Fasting low-density lipoproteins (mmol·L−1) | 2.958±0.150 | 3.124±0.235 | 3.289±0.160 |
| Fasting high-density lipoproteins (mmol·L−1) | 1.269±0.070 | 1.252±0.137 | 1.167±0.184 |
| Fasting triglycerides (mmol·L−1) | 1.294±0.157 | 1.718±0.558 | 1.341±0.172 |
HOMA, Homeostatic Model Assessment of Insulin Resistance.
One subject declined to provide a blood sample so glucose, insulin, and lipids were measured for the other 26 subjects.
Postexercise Hypotension (PEH)
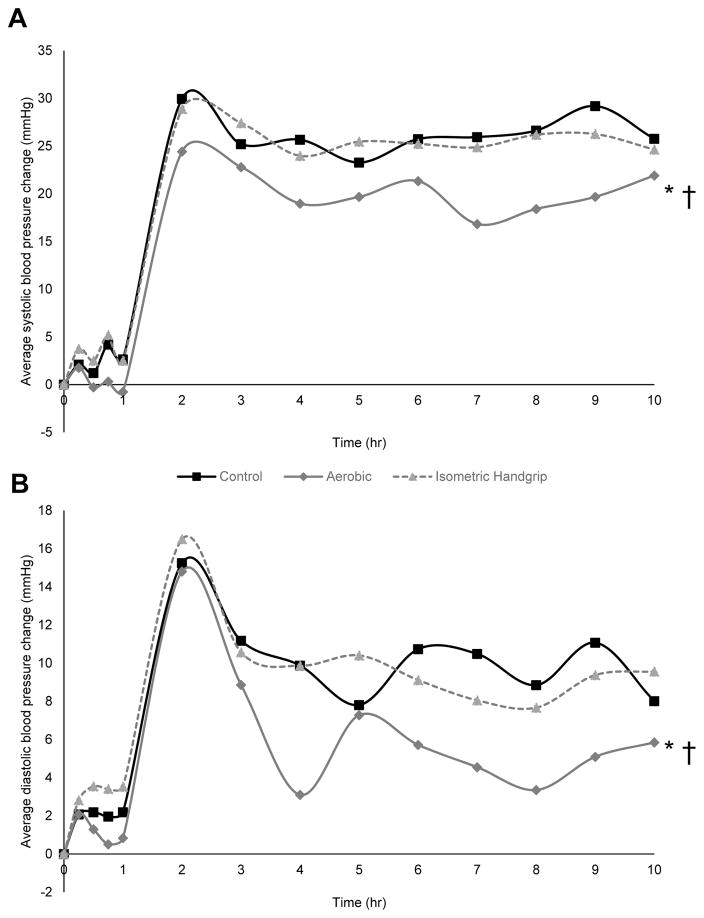
Sedentary participants (n=27) underwent each of three exercise regimens (aerobic, IHG or control) on different days and were assessed for 19 hours after each exercise regimen. After non-exercise control, SBP/DBP increased by 2.4±1.2 / 2.1±0.9 mmHg over one hour in the laboratory (p=0.05 / p=0.02) and 16.7±1.9 / 3.1±1.3 mmHg over 19 hours under ambulatory conditions (p<0.001 / p=0.03) from a baseline of 121.7±2.4 / 79.9±1.5 mmHg (Figure 2). The inclusion of a control session is crucial in the assessment of PEH to account for sources of BP variation unrelated to exercise and the normal circadian variation in BP [31]. The BP increase during the first hour in the laboratory also seen in our previous work [21, 22] and others [31] reflects the typical morning circadian increase in BP [20–22, 24–26, 32]. The BP increase during subsequent hours under ambulatory compared to laboratory conditions has also been observed in our previous work [21, 25, 33, 34] and that of others [35–37]. Higher ambulatory than laboratory BP has been noted especially among male subjects with high BMI but without diagnosed hypertension [36, 38, 39] as characterized our sample (Table 1). Aerobic exercise attenuated SBP and DBP elevations during the awake hours by 5.6±1.9 / 3.6±1.3 mmHg (p=0.02 / 0.04). In contrast, following IHG there were no significant differences in BP versus control during the awake hours (p=0.80 / 0.83) (Figure 2). In direct comparison, aerobic exercise attenuated SBP and DBP elevations versus IHG over the awake hours by 4.8±1.6 / 3.1±1.3 mmHg (p=0.01 / 0.04) (Figure 2). Over sleep hours there were no significant differences between conditions (aerobic vs control p=0.85 / 0.91, IHG vs control p=0.82 / 0.79, aerobic vs IHG p=0.67 / 0.81). The PWV response to acute exercise did not differ by modality (see Supplemental Digital Content 3-B which reports the arterial stiffness response to acute exercise).
Fig. 2.
Average awake systolic (A) and diastolic (B) blood pressure change from baseline at hourly intervals for 10 hours after control and exercise among 27 sedentary adults. *p<0.05 aerobic versus control. †p<0.05 aerobic versus isometric handgrip. Baseline values were control 121.7±2.4 / 79.7±1.5 mmHg, aerobic 121.4±2.0 / 79.7±1.4 mmHg, isometric handgrip 120.1±1.9 / 77.9±1.3 mmHg.
The Ambulatory Blood Pressure Response to Exercise Training
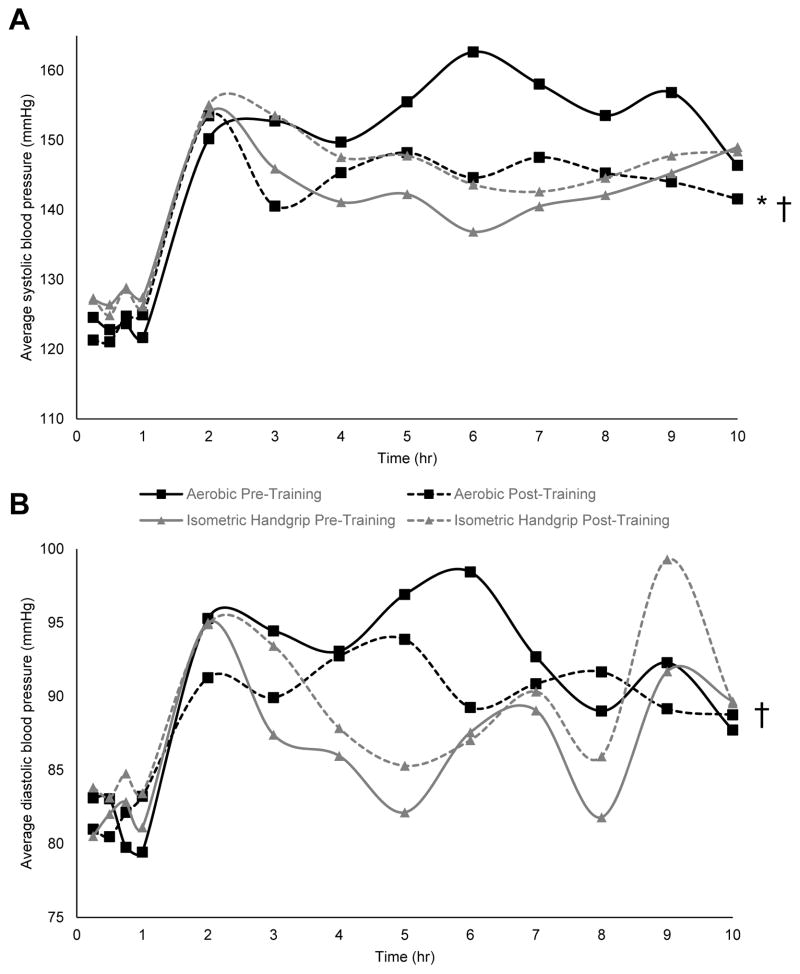
Starting at 48 hours following aerobic (n=6) or IHG exercise training (n=5), the impact of exercise training on ambulatory BP over 19 hours was assessed and compared to pre-training levels over this same time period in a smaller subset of the subjects. Aerobic exercise training reduced awake SBP (7.6±3.1 mmHg, p=0.01) but not DBP (p=0.18), while IHG resistance exercise training did not influence awake SBP or DBP (p=0.15 / 0.18) (Figure 3). In direct comparison, awake SBP and DBP decreased more after versus before aerobic exercise training than IHG resistance exercise training (10.8±3.9 / 4.9±2.2 mmHg, p=0.01 / p=0.04, Figure 3). Over sleep hours aerobic exercise training did not influence SBP or DBP (p=0.81 / 0.43), while IHG resistance exercise training did not influence sleep SBP (p=0.06) and increased sleep DBP by 7.7±2.3 mmHg (p=0.02). The PWV response to chronic exercise did not differ by modality (see Supplemental Digital Content 3-C which reports the resting arterial stiffness response to exercise training).
Fig. 3.
Average awake systolic (A) and diastolic (B) blood pressure at hourly intervals before and after exercise training among participants assigned to the aerobic (n=6) and isometric handgrip (n=5) training groups. *p<0.05 versus pre-training. †p<0.05 change following aerobic exercise training versus change following isometric handgrip exercise training
DISCUSSION
We conducted a randomized controlled crossover trial designed to test the efficacy of acute and chronic aerobic and IHG resistance exercise as antihypertensive therapy. Our major findings were that aerobic exercise induced a clinically meaningful PEH effect versus control, attenuating the rise in BP due to circadian variation by 4 to 6 mmHg over the awake hours (Figure 2). In contrast, IHG did not elicit PEH. A subset of these subjects then underwent 8 weeks of either IHG resistance or aerobic exercise training. We found that aerobic exercise training lowered resting SBP by 7 mmHg over the awake hours, while DBP was not different. In contrast, IHG resistance exercise training increased resting DBP by 5 mmHg over the awake hours and 7 mmHg over the sleep hours, while SBP was not different. In direct comparison, SBP was 11 mm Hg and DBP 5 mmHg lower over the awake hours (Figure 3) and DBP 5 mmHg lower over the sleep hours after aerobic than IHG resistance training. Collectively, our findings indicated that aerobic exercise is superior to IHG resistance exercise as antihypertensive therapy and should continue to be recommended for its immediate and sustained BP benefits.
Our findings are in agreement with three reports that acute [16, 18] and chronic [17] IHG resistance exercise did not lower BP, while they are in contrast with others that concluded acute [8, 9] and chronic IHG [10–14] resistance exercise reduced BP to the same or greater levels as acute [2, 15, 20, 21] and chronic aerobic exercise [2, 3, 15]. The relative volume of IHG resistance exercise (4x2 minutes, 30% MVC) we prescribed in this study was equivalent to these other reports [8–14]. Nonetheless, discrepancies in our results compared to others [8–14] may be partially attributed to other differences in study design. We enrolled the sample size double that of previous reports minimizing the possibility of a Type 2 statistical error [8–14]. We measured ambulatory BP over 19 hours under conditions of daily living following our established rigorous protocols known to induce PEH [4, 5, 20–22]. Similar to our finding, investigators found that IHG resistance exercise did not lower ambulatory BP over 24 hours [17, 18]. In contrast, those finding antihypertensive benefits of IHG measured BP by auscultation in the laboratory [8, 10–14]. One study found antihypertensive benefit of IHG under ambulatory conditions over 7 hours [9] but may have been limited by high subject attrition (33%) and missing ambulatory BP data (up to 30%).
Differences in the demographic characteristics among study populations may also have contributed to discrepancies among our findings and those of other reports [8–14]. Subjects from studies reporting antihypertensive benefit from IHG had either normal BP [8, 10, 14] or BP controlled to normal levels with antihypertensive medication [10, 11, 13]. These subjects were also normal weight [8–14], physically active [8, 10], of average cardiorespiratory fitness [8–10], and likely to be Caucasian based upon geographic location although race was not reported [8–14]. By contrast, subjects from our study were in the early stages of hypertension, not receiving antihypertensive medication, sedentary, obese, and of low cardiorespiratory fitness with the majority (56%) African Americans (Table 1). The AHA scientific statement [19] has cautioned that limited patient populations have been evaluated to date [8, 10–14, 16, 17] regarding the antihypertensive efficacy of IHG resistance exercise so that generalizing findings to adults populations per se may not be warranted. Our findings reinforce this caution, as it is known that the exercise response of BP and regulatory factors that influence BP differ by racial/ethnic groups [40–48].
A growing body of evidence indicates that health outcomes following exercise exhibit inter-individual variability that is belied by mean results [3, 49]. We found the standard deviation of the BP change following acute aerobic (−5.6±9.2 mmHg, Supplemental Digital Figure S4-1) and acute IHG exercise (−0.3±9.3 mmHg, Supplemental Digital Figure S4-2) exceeded the mean value of the change. Furthermore, nine of the 27 participants in our study did not achieve clinically significant antihypertensive benefit (i.e., SBP and/or DBP reduced by ≥2 mmHg [2, 22]) following acute aerobic exercise (Supplemental Digital Figure S3–S1); while 13 did achieve such benefit following IHG (see Supplemental Digital Figure S3-2). Nonetheless, ambulatory BP was on average 3 to 5 mmHg lower after acute aerobic than acute IHG resistance exercise. In addition, most of the participants (i.e., 85%) individually experienced a greater BP reduction following acute aerobic than acute IHG resistance exercise, reinforcing our major finding that acute aerobic was superior to acute IHG resistance exercise in lowering ambulatory BP.
One factor that can partially account for variability in the BP response to exercise is technical error related to obtaining repeated measurements [49]. We minimized technical error by having all BP assessments performed by a single investigator (GIA) at the same time of day using the same ambulatory BP monitor for the same subject throughout study duration. Accordingly, the coefficients of variation for baseline SBP / DBP before the acute experiments were 2.9% / 3.5%, respectively, supporting that these study design features minimized unexplained variability in repeated ambulatory BP measurements.
Strengths and Limitations
Although this study bore methodological strengths including a randomized controlled crossover design and the clinical gold standard assessment of ambulatory BP [24, 26], there are three limitations of note. First, our study was not designed to determine mechanisms so that mechanistic explanations for our finding that aerobic exercise elicited PEH while IHG did not are beyond the scope of our study. Second, our sample size of 27 adults with prehypertension and obesity limited our power to conduct sub-group analysis (eg, sex and racial differences) as well as generalizability to other populations (eg, older adults with more advanced hypertension). Third, our subpopulation undergoing chronic exercise training regimens was small, and these findings should be treated as preliminary and with caution. Nonetheless the lack of benefit from IHG resistance exercise was consistent across each individual participant and are shown in Supplemental Digital Figure S2-3.
Conclusion
We found that IHG resistance exercise did not elicit PEH and surprisingly increased resting BP following 8 weeks of exercise training. Contrary to our hypothesis, IHG does not seem to be a viable antihypertensive exercise modality alternative for aerobic exercise among adults with high BP. Our findings are consistent with the current recommendations from a variety of professional organizations [4, 5] regarding exercise as antihypertensive lifestyle therapy, that aerobic exercise should be the primary modality of choice for its immediate and sustained BP lowering effects among adults with high BP. Our findings are intriguing due to strengths of our randomized controlled crossover design, the size of our patient population versus previous investigations, and our use of ambulatory blood pressure monitoring, yet they merit confirmation.
Supplementary Material
Acknowledgments
Essential assistance with supervision of exercise testing and training, recruitment of participants, data entry, and specimen analysis was provided by Patrick Armstrong, Jeffrey Capizzi, Paul Dalton, TaShauna Goldsby, Laura Kratochvil, Spencer Lau, Harold Lee, Lindsay Lorson, Amanda Missimer, Emily Moker, Donna Chelle Morales, Purvi Parwani, William Roman, John Slim, and Hyungyu Suh.
Research reported in this publication was supported by the University of Connecticut Research Council, the Institute for Collaboration on Health, Intervention, and Policy, and the Connecticut Institute for Clinical and Translational Science (CICATS). NIH/NIDDK (T32DK097718) also supported GIA during manuscript writing, and the Brazilian Council for the Scientific and Technological Development (CNPq) supported PF.
Footnotes
This manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as abstracts at American College of Sports Medicine Annual Meetings in 2013–2015.
All authors declare that they have no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding sources.
References
- 1.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: 2011. [Google Scholar]
- 2.Pescatello LS, Kulikowich JM. The aftereffects of dynamic exercise on ambulatory blood pressure. Med Sci Sports Exerc. 2001;33:1855–1861. doi: 10.1097/00005768-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 4.Pescatello LS, MacDonald HV, Lamberti L, Johnson BT. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr Hypertens Rep. 2015;17 doi: 10.1007/s11906-015-0600-y. 87-015-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pescatello LS, MacDonald HV, Ash GI, Lamberti LM, Farquhar WB, Arena R, Johnson BT. Assessing the Existing Professional Exercise Recommendations for Hypertension: A Review and Recommendations for Future Research Priorities. Mayo Clin Proc. 2015;90:801–812. doi: 10.1016/j.mayocp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Churilla JR, Ford ES. Comparing physical activity patterns of hypertensive and nonhypertensive US adults. Am J Hypertens. 2010;23:987–993. doi: 10.1038/ajh.2010.88. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 8.Millar PJ, MacDonald MJ, McCartney N. Effects of isometric handgrip protocol on blood pressure and neurocardiac modulation. Int J Sports Med. 2011;32:174–180. doi: 10.1055/s-0030-1268473. [DOI] [PubMed] [Google Scholar]
- 9.VAN Assche T, Buys R, DE Jaeger M, Coeckelberghs E, Cornelissen V. One single bout of low intensity isometric handgrip exercise reduces blood pressure during daily activities in healthy pre- and hypertensive individuals. J Sports Med Phys Fitness. 2016 doi: 10.23736/S0022-4707.16.06239-3. [DOI] [PubMed]
- 10.Millar PJ, Bray SR, MacDonald MJ, McCartney N. The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. J Cardiopulm Rehabil Prev. 2008;28:203–207. doi: 10.1097/01.HCR.0000320073.66223.a7. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AC, McCartney N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;35:251–256. doi: 10.1249/01.MSS.0000048725.15026.B5. [DOI] [PubMed] [Google Scholar]
- 12.Wiley RL, Dunn CL, Cox RH, Hueppchen NA, Scott MS. Isometric exercise training lowers resting blood pressure. Med Sci Sports Exerc. 1992;24:749–754. [PubMed] [Google Scholar]
- 13.Badrov MB, Horton S, Millar PJ, McGowan CL. Cardiovascular stress reactivity tasks successfully predict the hypotensive response of isometric handgrip training in hypertensives. Psychophysiology. 2013;50:407–414. doi: 10.1111/psyp.12031. [DOI] [PubMed] [Google Scholar]
- 14.Badrov MB, Bartol CL, DiBartolomeo MA, Millar PJ, McNevin NH, McGowan CL. Effects of isometric handgrip training dose on resting blood pressure and resistance vessel endothelial function in normotensive women. Eur J Appl Physiol. 2013;113:2091–2100. doi: 10.1007/s00421-013-2644-5. [DOI] [PubMed] [Google Scholar]
- 15.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 16.Olher Rdos R, Bocalini DS, Bacurau RF, Rodriguez D, Figueira A, Jr, Pontes FL, Jr, et al. Isometric handgrip does not elicit cardiovascular overload or post-exercise hypotension in hypertensive older women. Clin Interv Aging. 2013;8:649–655. doi: 10.2147/CIA.S40560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiller-Moldovan C, Kenno K, McGowan CL. Effects of isometric handgrip training on blood pressure (resting and 24 h ambulatory) and heart rate variability in medicated hypertensive patients. Blood Press Monit. 2012;17:55–61. doi: 10.1097/MBP.0b013e32835136fa. [DOI] [PubMed] [Google Scholar]
- 18.Goessler K, Buys R, Cornelissen V. Low intensity isometric handgrip exercise has no transient effect on blood pressure in patients with coronary artery disease. Journal of the American Society of Hypertension. doi: 10.1016/j.jash.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013;61:1360–1383. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 20.Pescatello LS, Fargo AE, Leach CN, Jr, Scherzer HH. Short-term effect of dynamic exercise on arterial blood pressure. Circulation. 1991;83:1557–1561. doi: 10.1161/01.cir.83.5.1557. [DOI] [PubMed] [Google Scholar]
- 21.Pescatello LS, Guidry MA, Blanchard BE, Kerr A, Taylor AL, Johnson AN, et al. Exercise intensity alters postexercise hypotension. J Hypertens. 2004;22:1881–1888. doi: 10.1097/00004872-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Ash GI, Eicher JD, Pescatello LS. The Promises and Challenges of the Use of Genomics in the Prescription of Exercise for Hypertension: The 2013 Update. Curr Hypertens Rev. 2013;9:130–147. doi: 10.2174/15734021113099990010. [DOI] [PubMed] [Google Scholar]
- 23.Pescatello LS, Arena R, Riebe D, Thompson PD, editors. ACSM’s Guidelines for Exercise Testing and Prescription. Baltimore, ML: Lippincott Williams & Wilkins: American College of Sports Medicine; 2013. [DOI] [PubMed] [Google Scholar]
- 24.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005;7:102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ash GI, Walker TJ, Olson KM, Stratton JH, Gomez AL, Kraemer WJ, et al. The Reproducibility in Ambulatory Blood Pressure Change from Initial Values on Two Different Days. Clinics (Sao Paulo) 2013;68(12):1509–1515. doi: 10.6061/clinics/2013(12)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 27.Dallal GE. Randomization.com. 2008. 2011. [Google Scholar]
- 28.Brook RD, Jackson EA, Giorgini P, McGowan CL. When and how to recommend ‘alternative approaches’ in the management of high blood pressure. Am J Med. 2015;128:567–570. doi: 10.1016/j.amjmed.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE) Med Sci Sports Exerc. 2001;33:1774–1784. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Kelley GA, Kelley KS. Isometric handgrip exercise and resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2010;28:411–418. doi: 10.1097/HJH.0b013e3283357d16. [DOI] [PubMed] [Google Scholar]
- 31.de Brito LC, Rezende RA, da Silva ND, Junior, Tinucci T, Casarini DE, Cipolla-Neto J, Forjaz CL. Post-Exercise Hypotension and Its Mechanisms Differ after Morning and Evening Exercise: A Randomized Crossover Study. PLoS One. 2015;10:e0132458. doi: 10.1371/journal.pone.0132458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stergiou GS, Parati G. How to best assess blood pressure? The ongoing debate on the clinical value of blood pressure average and variability. Hypertension. 2011;57:1041–1042. doi: 10.1161/HYPERTENSIONAHA.111.172924. [DOI] [PubMed] [Google Scholar]
- 33.Pescatello LS, Miller B, Danias PG, Werner M, Hess M, Baker C, Jane De Souza M. Dynamic exercise normalizes resting blood pressure in mildly hypertensive premenopausal women. Am Heart J. 1999;138:916–921. doi: 10.1016/s0002-8703(99)70017-7. [DOI] [PubMed] [Google Scholar]
- 34.Pescatello LS, Bairos L, Vanheest JL, Maresh CM, Rodriguez NR, Moyna NM, et al. Postexercise hypotension differs between white and black women. Am Heart J. 2003;145:364–370. doi: 10.1067/mhj.2003.107. [DOI] [PubMed] [Google Scholar]
- 35.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243–250. doi: 10.1016/j.amjhyper.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard JP, Fletcher B, Gill P, Martin U, Roberts N, McManus RJ. Predictors of the Home-Clinic Blood Pressure Difference: A Systematic Review and Meta-Analysis. Am J Hypertens. 2016;29:614–625. doi: 10.1093/ajh/hpv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorostidi M, Vinyoles E, Banegas JR, de la Sierra A. Prevalence of white-coat and masked hypertension in national and international registries. Hypertens Res. 2015;38:1–7. doi: 10.1038/hr.2014.149. [DOI] [PubMed] [Google Scholar]
- 38.Ogedegbe G. Causal mechanisms of masked hypertension: socio-psychological aspects. Blood Press Monit. 2010;15:90–92. doi: 10.1097/MBP.0b013e3283380df5. [DOI] [PubMed] [Google Scholar]
- 39.Wang GL, Li Y, Staessen JA, Lu L, Wang JG. Anthropometric and lifestyle factors associated with white-coat, masked and sustained hypertension in a Chinese population. J Hypertens. 2007;25:2398–2405. doi: 10.1097/HJH.0b013e3282efeee7. [DOI] [PubMed] [Google Scholar]
- 40.Bond V, Millis RM, Adams RG, Oke LM, Enweze L, Blakely R, et al. Attenuation of exaggerated exercise blood pressure response in African-American women by regular aerobic physical activity. Ethn Dis. 2005;15:S5-10-3. [PMC free article] [PubMed] [Google Scholar]
- 41.Brandon LJ, Elliott-Lloyd MB. Walking, body composition, and blood pressure dose-response in African American and white women. Ethn Dis. 2006;16:675–681. [PubMed] [Google Scholar]
- 42.Enweze L, Oke LM, Thompson T, Obisesan TO, Blakely R, Adams RG, et al. Acute exercise and postexercise blood pressure in African American women. Ethn Dis. 2007;17:664–668. [PMC free article] [PubMed] [Google Scholar]
- 43.Headley SA, Keenan TG, Manos TM, Phillips K, Lachowetz T, Keenan HA, Mahar MT. Renin and hemodynamic responses to exercise in borderline hypertensives. Ethn Dis. 1998;8:312–318. [PubMed] [Google Scholar]
- 44.Jones JM, Dowling TC, Park JJ, Phares DA, Park JY, Obisesan TO, Brown MD. Differential aerobic exercise-induced changes in plasma aldosterone between African Americans and Caucasians. Exp Physiol. 2007;92:871–879. doi: 10.1113/expphysiol.2007.037408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones JM, Park JJ, Johnson J, Vizcaino D, Hand B, Ferrell R, et al. Renin-angiotensin system genes and exercise training-induced changes in sodium excretion in African American hypertensives. Ethn Dis. 2006;16:666–674. [PMC free article] [PubMed] [Google Scholar]
- 46.Pescatello LS, Bairos L, Vanheest JL, Maresh CM, Rodriguez NR, Moyna NM, et al. Postexercise hypotension differs between white and black women. Am Heart J. 2003;145:364–370. doi: 10.1067/mhj.2003.107. [DOI] [PubMed] [Google Scholar]
- 47.Santa-Clara H, Szymanski L, Fernhall B. Effect of exercise training on blood pressure in postmenopausal Caucasian and African-American women. Am J Cardiol. 2003;91:1009–11. A8. doi: 10.1016/s0002-9149(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 48.Yan H, Behun MA, Cook MD, Ranadive SM, Lane-Cordova AD, Kappus RM, et al. Differential Post-Exercise Blood Pressure Responses between Blacks and Caucasians. PLoS One. 2016;11:e0153445. doi: 10.1371/journal.pone.0153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Hakkinen K, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7:e37887. doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.