Abstract
Yorkie (Yki), the transcriptional co-activator of the Hippo signaling pathway, has well-characterized roles in balancing apoptosis and cell division during organ growth control. Yki is also required in diverse tissue regenerative contexts. In most cases this requirement reflects its well-characterized roles in balancing apoptosis and cell division. Whether Yki has repair functions outside of the control of cell proliferation, death, and growth is not clear. Here we show that Yki and Scalloped (Sd) are required for epidermal wound closure in the Drosophila larval epidermis. Using a GFP-tagged Yki transgene we show that Yki transiently translocates to some epidermal nuclei upon wounding. Genetic analysis strongly suggests that Yki interacts with the known wound healing pathway, Jun N-terminal kinase (JNK), but not with Platelet Derived Growth Factor/Vascular-Endothelial Growth Factor receptor (Pvr). Yki likely acts downstream of or parallel to JNK signaling and does not appear to regulate either proliferation or apoptosis in the larval epidermis during wound repair. Analysis of actin structures after wounding suggests that Yki and Sd promote wound closure through actin regulation. In sum, we found that Yki regulates an epithelial tissue repair process independently of its previously documented roles in balancing proliferation and apoptosis.
Keywords: Drosophila, Wound healing, Yorkie, JNK, Actin, Tissue repair, Scalloped
1. Introduction
To cope with inevitable injury, organisms possess efficient wound healing mechanisms that maintain tissue integrity and guard against infection. However, the detailed genetic basis of wound healing is still poorly defined. Epidermal wound healing in Drosophila larvae serves as a powerful system to uncover the genes that are required for wound closure (Galko and Krasnow, 2004; Kwon et al., 2010; Lesch et al., 2010; Stevens and Page-McCaw, 2012). In this model, wounds made in the monolayer barrier epidermal sheet close through directed cell migration (Galko and Krasnow, 2004; Lesch et al., 2010). The receptor tyrosine kinase (RTK) Pvr, which shares homology to vertebrate platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) receptors (Cho et al., 2002; Duchek et al., 2001; Heino et al., 2001) and its ligand, Pvf1, are required for larval wound closure (Wu et al., 2009), as are other genes (Stevens and Page-McCaw, 2012; Kwon et al., 2010). Pvr regulates cellular protrusions at the wound edge (Wu et al., 2009) and also controls the directed migration of border cells (Duchek et al., 2001; Prasad and Montell, 2007) and embryonic hemocytes (Wood et al., 2006).
In addition to the Pvr pathway, the JNK pathway is also essential for larval epidermal wound healing (Galko and Krasnow, 2004; Lesch et al., 2010). Loss of function of JNK upstream kinases, Slpr and Hep, or downstream transcriptional factors, D-fos and D-jun, all cause wound closure defects in Drosophila (Lesch et al., 2010). Moreover, the JNK pathway regulates an actin regulator, Profilin, at the transcriptional level during healing (Brock et al., 2012). Pvr and JNK act in parallel during epidermal wound healing (Wu et al., 2009). However, whether the Pvr or JNK pathways interact with other signaling pathways during wound healing is still unclear.
Yorkie (Yki) is a transcriptional co-activator that functions as a downstream effector of the Hippo pathway (Halder et al., 2012; Huang et al., 2005; Oh and Irvine, 2010; Pan, 2007; Yu and Guan, 2013). The core of the Hippo pathway is a kinase cascade of the Hippo (MST2 in vertebrates) and Warts kinases (LATS1/2 in vertebrates) that phosphorylate Yki thereby inhibiting its nuclear localization and ability to interact with the TEAD family of transcription factors (Halder and Johnson, 2011; Pan, 2010; Yu and Guan, 2013). During normal development, the Hippo pathway regulates organ size by controlling cell proliferation (Lin and Pearson, 2014) and apoptosis (Huang et al., 2005; Udan et al., 2003). The Hippo pathway also regulates Drosophila intestinal and imaginal disc regeneration by promoting proliferation (Grusche et al., 2011; Karpowicz et al., 2010; Ren et al., 2010; Shaw et al., 2010; Staley and Irvine, 2010; Sun and Irvine, 2011) and adult skin regeneration by inhibiting cell-cell fusion and promoting ploidization (Losick et al., 2013). However, the signals that regulate the activity of Yki during normal development and regeneration are not well understood (Staley and Irvine, 2012).
In this study, we found that Yki and its TEAD binding partner Scalloped (Sd) are required for wound closure (WC) in the larval epidermis. We also observed that a GFP-tagged Yki fusion protein translocates to the nucleus in some wound edge epidermal cells upon wounding. Yki's requirement for wound closure is intriguing since the larval epidermis is an endoreduplicated tissue that is likely incapable of cell division. We found that neither blocking nor ectopically promoting cell division in the epidermis could rescue UAS-ykiRNAi-induced wound closure. Likewise, we found no role for apoptosis in wound healing, and loss of Yki did not have apparent effects on apoptosis in this context. Thus, unlike other regenerative contexts, Yki does not control the balance of mitosis or apoptosis in the healing larval epidermis, but rather seems to regulate actin polymerization in the migrating wound-edge epidermal cells.
2. Materials and methods
2.1. Drosophila stocks and genetics
Pvrc02859 is a hypomorphic allele (Cho et al., 2002; Wu et al., 2009). Pvrc02195 (Cho et al., 2002) is referred to as Pvrnull#1. PvrMI04181 (Venken et al., 2011), referred to as Pvrnull#2, is a null allele based on its known molecular lesion and WC phenotype. Pvf1EP1624, here referred to as Pvf1null, is a null allele (Cho et al., 2002; Wu et al., 2009). ykiB5, here referred to as ykinull, is a null allele (Huang et al., 2005). Hepr75, here referred to as Hepnull, is a null allele (Glise et al., 1995).
The GAL4/UAS system was used to drive tissue-specific gene expression of transgenes under UAS control (Brand and Perrimon, 1993). For the embryonic and larval epidermis, e22c-Gal4 was used (Lawrence et al., 1995); for the larval epidermis, A58-Gal4 was used (Galko and Krasnow, 2004). For live imaging of larvae, we used e22c-Gal4, UAS-src-GFP, UAS-DsRed2-Nuc or A58-Gal4, UAS-src-GFP, UAS-DsRed2Nuc (Lesch et al., 2010). e22c-Gal4, UAS-src-GFP, UAS-DsRed2Nuc; tubP-gal80ts was used where temporal control of the Gal4/UAS system was needed (McGuire et al., 2003). UAS-RNAi lines employed were: UAS-ykiRNAi (N-terminal+ C-terminal, N+C) (yki#3), UAS-ykiRNAi (N-terminal) (yki#4), UAS-ykiRNAi (C-terminal) (yki#5), and P{Diap1-GFP.HREx8} (Zhang et al., 2008), which were gifts from Dr. Jin Jiang. AjubaRNAi#4 was a gift from Dr. Gregory D. Longmore (Das Thakur et al., 2010). P{UAS-Mer+.myc} (LaJeunesse et al., 1998). P{UAS-ex. B} (Boedigheimer et al., 1997). Bantam sensor, P{Tub-EGFP.ban} (Brennecke et al., 2003).
UAS-RNAi lines from Vienna Drosophila Research Center (VDRC) were: GD7185 (#38442/AjubaRNAi#2 and #38443/AjubaRNAi#3), KK109756 (ykiRNAi#1), GD11187 (ykiRNAi#2), GD1570 (hpoRNAi#1), KK101055 (wtsRNAi#1), GD1563 (wtsRNAi#3), KK100140 (matsRNAi), GD16019 (savRNAi#1), KK107562 (savRNAi#3), KK107857 (hipkRNAi), GD14350 (dsRNAi#1), GD2646 (dsRNAi#2), GD8808 (α-catRNAi), GD4047 (l(2)glRNAi), GD14463 (crbRNAi#1), GD14463 (crbRNAi#2), KK101128 (scribRNAi#1), GD11663 (scribRNAi#2), KK111409 (kibraRNAi), GD12284 DriceRNAi#1, KK108877 (sdRNAi#2), GD10696 (ZyxinRNAi#2), and GD14749 (cycERNAi).
UAS-RNAi lines from the TRiP Bloomington collection were: JF02470 (hpoRNAi#2), JF02471 (wtsRNAi#2), JF02840 (savRNAi#2), JF03120 (exRNAi), JF02841 (merRNAi), JF03270 (zyxRNAi#1), JF02744 (hthRNAi), JF02856 (tshRNAi), HMS00563 (wbp2RNAi), GLV21013 (madRNAi#1), JF01263 (madRNAi#2), JF01264 (madRNAi#3), JF02514 (sdRNAi#3), JF02843 (fjRNAi), GL00199 (aurBRNAi#1), JF03107 (aurBRNAi#2), GL00262 (cdc2RNAi#1), HMS00752 (Diap1RNAi), HMS02335 (AjubaRNAi#1), JF01355 (LuciferaseRNAi), and JF03004 (cdc2RNAi#2).
UAS-RNAi lines from NIG-Fly (http://www.shigen.nig.ac.jp/fly/nigfly/index.jsp) were: 8544R-3 and 8544R-2 (sdRNAi#1 and #4, respectively), 5680R-1 and 5680R-2 (JNKRNAi), 31196R-4 (14-3-3εRNAi), 7788R-2 (DriceRNAi#2), 8624R-1 (meltedRNAi), 8091R-1 (DroncRNAi), 9553R-3 and 9553R-4 (profilinRNAi). Other transgenic lines from Bloomington Stock Center: #11173, puc-lacZ. #9674, w1118; P{UAS-Myc. Z}132. #64196, w*; P{UAS-Ras85D.V12}2. #44248, P{lacW}ex697. #28819, w*; P{UAS-yki. V5.O}attP2. #44258, w*; P{UAS-wts. MYC}3/TM6B, Tb1. #44254, y1 w*; P{UAS-dMST.FLAG}3/TM2. #5072, w*; P{UAS-p35. H}BH1. #44253, y1 w*; P{lacW}fjp1. #12093, y1 w*; P{lacW}Diap1j5C8/TM3, Sb1. #28815, y1 w*; P{UAS-yki. GFP}4–12-1 (Oh and Irvine, 2008); #35545, y1 w*; P{UAS-Lifeact-Ruby}VIE-19A.
2.2. Wounding assays
Pinch and puncture wounding of the larvae was carried out according to our detailed protocol (Burra et al., 2013). In cases where early expression of a UAS transgene was lethal (UAS-Myc and UAS-rasV12), larvae bearing tub-gal80ts, the Gal4 driver and toxic UAS transgene were raised for six days at 18 °C to begin development, shifted to 30 °C for two days to reach mid-third-instar, and then allowed to recover at 25 °C following pinch wounding. Pinch wounds were scored as “open” if the initial wound gap remained after 24 h, and as “closed” if a continuous epidermal sheet was observed at the wound site. To calculate the percentage of larvae with open wounds, approximately 30 larvae per genotype were pinched and scored for open wounds under a stereo microscope (Leica MZ16FA).
2.3. Whole mount immunostaining and cell labeling
The third instar larval epidermis was dissected and processed as detailed previously (Burra et al., 2013). To highlight wound morphology, a mouse monoclonal antibody against Fasciclin III was used (1:50; Developmental Studies Hybridoma Bank). Rat anti-Sd was a gift from Kirsten Guss (Guss et al., 2013) and was used at 1:20. Mouse anti-GFP monoclonal antibody (Life Technologies) was used at 1: 500. Mouse anti-β-galactosidase monoclonal antibody (Promega #Z3781) was used at 1:1000. Anti-activated Caspase 3 (Cell Signaling) was used at 1:150. Mouse anti-Drosophila Profilin (Developmental Studies Hybridoma Bank) was used undiluted. Rabbit anti-DsRed (Clontech) was used at 1:1000. Rabbit anti-Lethal (2) giant larvae (l(2)gl) was used at 1:50 (Santa Cruz, sc-98260). Alexa 546 conjugated Phalloidin (Invitrogen) was used at 1:200. A TUNEL staining kit (Roche Diagnostics) was also used to detect DNA double-stranded breaks.
2.4. Imaging and analysis
An Olympus FV1000 Confocal microscope and Fluoview software were used to obtain images of the dissected epidermal whole mounts. Leica MZ16FA stereomicroscope with Planapo 1.6× objective and appropriate filters was used for live imaging of epidermal wounds. ImageJ software was used for image processing. For statistics, Student's T-test was used.
2.5. Quantitation of Yki nuclear translocation
Epidermal cells in unwounded or in wounded larvae at different times post-wounding were counted as containing nuclear Yki if the nuclear Yki-GFP signal was obviously greater than the cytoplasmic signal in the same cell. n =4 for each genotype.
2.6. Quantitation of wound-edge F-actin intensity
Wound-edge F-actin was labeled with UAS-Lifeact-Ruby. The fluorescent signal around wounds of different genotypes six hours following wounding was measured using the following protocol: In ImageJ, the wound edge was first selected based on the F-actin signals using the “wand”. Next the wound edge area was designated from the wound edge using the “make band” tool (width =6 µm) and the average fluorescent intensity was measured within the selection. The wound edge intensities were first normalized to the adjacent unwounded segments. The normalized intensities of the UAS-ykiRNAi#2 and UAS-sdRNAi#3 groups were then normalized to the UAS-LucRNAi control. n ≥7 for each genotype.
2.7. Quantitation of F-actin mean intensity and Profilin protein levels in the unwounded larval epidermis
For F-actin, whole-mount epidermis expressing different transgenes was stained with Alexa546-condugated phalloidin. Mean phalloidin signal of one middle segment (usually abdominal segment 2 or 3) of larvae expressing UAS-ykiRNAi, UAS-sdRNAi and UAS-ProfilinRNAi were normalized to UAS-LucRNAi control. n≥4 for each genotype. For measuring the mean Profilin protein level, whole-mount epidermis expressing different transgenes was stained with anti-Drosophila Profilin. Mean Profilin levels were measured as for F-actin mean intensity above. n≥4 for each genotype.
3. Results
3.1. Yorkie and Scalloped are required for epidermal wound closure
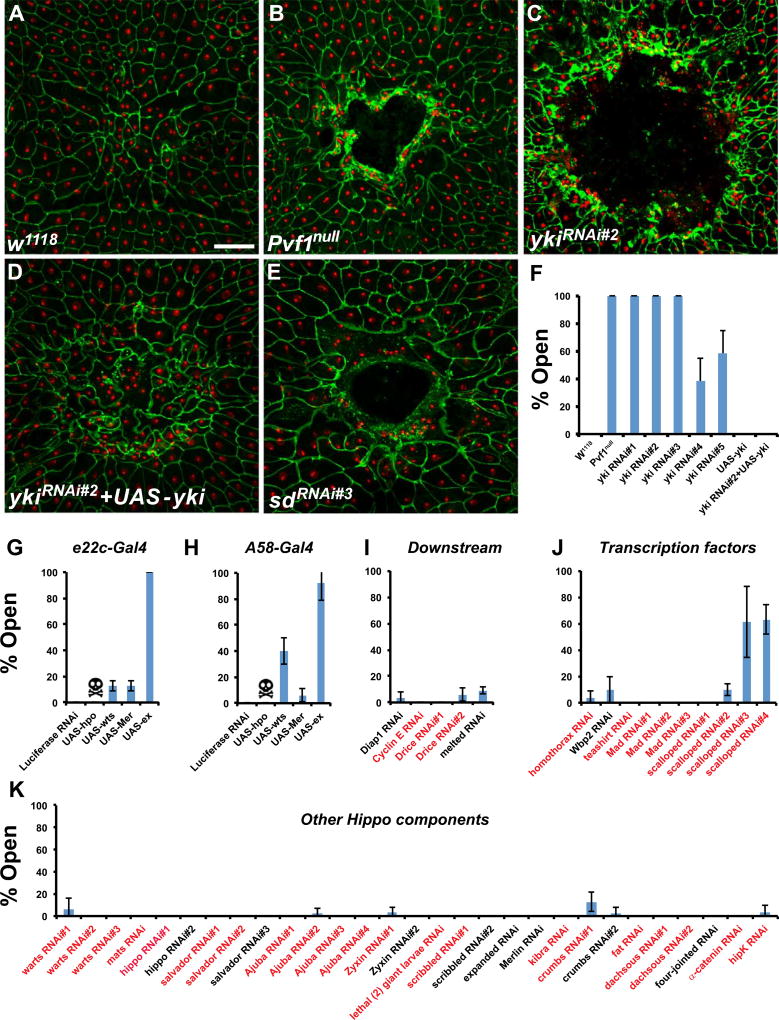
We have been performing a tissue-specific in vivo RNAi screen for genes required in the larval epidermis for WC (Lesch et al., 2010). While surveying the function of Drosophila transcriptional regulators in this context we used an established pinch wounding assay (Galko and Krasnow, 2004; Wu et al., 2009) to test whether expression of RNAi transgenes targeting yki in the larval epidermis affects WC. Wounds in control larvae were invariably closed 24 h after wounding (Fig. 1A,F). In contrast, wounds made in Pvf1null mutant larvae remained open after 24 h (Fig. 1B,F and Wu et al., 2009). Similarly, expression of multiple UAS-ykiRNAi transgenes resulted in fully or partially penetrant WC defects (Fig. 1C,F). Open wounds were observed with two different UAS-ykiRNAi transgenes that target non-overlapping regions of the yki gene (Fig. 1F and Fig. S1A). Overexpression of Yki in the larval epidermis via UAS-Yki completely rescued the UAS-ykiRNAi-induced WC defects, indicating that these effects are due to loss of Yki function in the larval epidermis and not to off-target effects (Fig. 1D,F). Expression of UAS-YkiRNAi or Yki overexpression did not affect normal epidermal architecture. Other UAS transgenes co-expressed with UAS-YkiRNAi (see Fig. 4E,H) did not rescue, indicating the rescue observed here is not solely a function of titration of the Gal4/UAS system by the presence of multiple UAS transgenes.
Fig. 1.
Yki and Sd are required for larval epidermal wound closure. (A–E) Dissected epidermal whole mounts of pinch wounded larvae expressing UAS-DsRed2Nuc via the e22c-Gal4 driver and the indicated mutations or transgenes. Epidermal nuclei (red); cell boundaries (immunostained with anti-Fasciclin III, green). (A) Control (B) Pvf1Null (C) UAS-ykiRNAi#2 (D) UAS-ykiRNAi#2 and UAS-yki (E) UAS-SdRNAi#3. Scale bar: 100 µm. (F–K) Percentage of open wounds in larvae expressing the indicated transgenes via the e22c-Gal4 driver (G, I–K) or A58-Gal4 driver (H). (F) UAS-ykiRNAi transgenes, Yki overexpression, and rescue. (G, H) Overexpression of yki negative regulators. (I) UAS-RNAi transgenes targeting yki downstream genes (genes that also act upstream of yki are included in Fig. 1K). (J) UAS-RNAi transgenes targeting yki transcriptional partners. (K) UAS-RNAi transgenes targeting Hippo pathway components upstream of yki. 3 sets of n≥8 for each genotype. Error bars, standard deviation. Previously published UAS-RNAi transgenes are labeled in red.
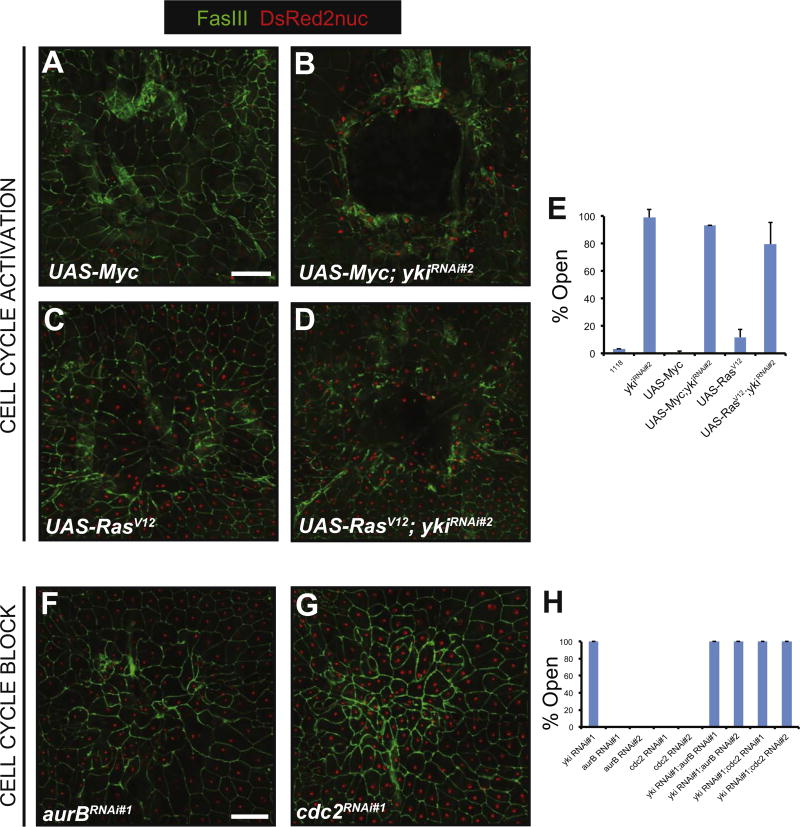
Fig. 4.
Yki does not regulate cell proliferation in epidermal wound closure. (A–D) Dissected epidermal whole mounts of pinch wounded larvae expressing the indicated transgenes via the e22c-Gal4 driver. Cell membranes (immunostained with anti-Fasciclin III), green; nuclei labeled with UAS-DsRed2Nuc; Scale bar: 100 µm. (A) UAS-Myc (B) UAS-Myc + UAS-ykiRNAi#2 (C) UAS-RasV12 (D) UAS-RasV12 + UAS-ykiRNAi#2. (E) Percentage of open wounds on expression of the indicated UAS-RNAi lines or transgenes. (F, G) Dissected epidermal whole mounts of pinch wounded larvae expressing the indicated transgenes via the e22c-GAL4 driver. Cell membranes (immunostained with anti-Fasciclin III), green; nuclei labeled with UAS-DsRed2Nuc; Scale bar: 100 µm. (F) UAS-aurBRNAi#1 (G) UAS-cdc2RNAi#1 (H) Percentage of open wounds on expression of indicated UAS-RNAi transgenes.
Next, we tested whether other Hippo pathway components regulate larval epidermal wound closure. Yki is commonly negatively regulated by the upstream Hippo kinase cascade (Huang et al., 2005). If Hippo/Warts regulates Yki during WC, then overexpression of these genes should phenocopy the WC defect of UAS-ykiRNAi. Overexpression of Hippo (dMst) via both the e22c-Gal4 and A58-Gal4 epidermal drivers resulted in lethality (Fig. 1G,H). By contrast, overexpression of Warts via the A58-Gal4 driver was viable and caused 40% open wounds (Fig. 1H). The other upstream pathway component whose epidermal overexpression resulted in open wounds was Expanded (Fig. 1G,H). Expanded can inhibit Yki through direct binding to Yki as well as through activation of Warts (Badouel et al., 2009). Expression of UAS-RNAi transgenes targeting several known Yki downstream genes, such as Diap1 and Cyclin E, did not affect WC (Fig. 1I). We also expressed UAS-RNAi transgenes targeting the factors that commonly act upstream of Yki to test if they are also required for WC. Since overexpression of Yki did not block WC (Fig. 1F), and many of the upstream components of the pathway negatively regulate Yki, expression of UAS-RNAi transgenes targeting these factors was not necessarily expected to affect WC. Indeed, expression of UAS-RNAi transgenes targeting warts, hippo, mats, salvador, merlin, expanded, and other negative regulators of Yki did not affect WC (Fig. 1K). Taken together, our results suggest that Yki is required in the larval epidermis for WC and is likely at least partially regulated by the Warts kinase in the larval epidermis.
Yki cannot bind DNA by itself (Oh and Irvine, 2010). Instead, Yki complexes with a variety of different transcription factors to regulate gene expression (Goulev et al., 2008; Oh and Irvine, 2011; Peng et al., 2009; Wu et al., 2008; Zhang et al., 2008, 2011; Zhao et al., 2008). We surveyed many of these factors for their potential roles in WC, and found only one binding partner, Scalloped (Sd), whose knockdown resulted in WC defects. Epidermal expression of two non-overlapping UAS-sdRNAi transgenes (Fig. S1B, UAS-sdRNAi#3 and UAS-sdRNAi#4) resulted in WC defects (Fig. 1J). Expression of either of these UAS-sdRNAi transgenes that blocked WC showed a significant reduction of Sd protein (compare Fig. S1E,F to Fig. S1C). A third UAS-sdRNAi transgene that failed to block WC (UAS-sdRNAi#1, Fig. 1J) showed little or no knockdown of Sd (Fig. S1D). These results suggest that Yki may preferentially partner with Sd during larval WC.
3.2. Yorkie transiently translocates to the nucleus in some wound-edge epidermal cells
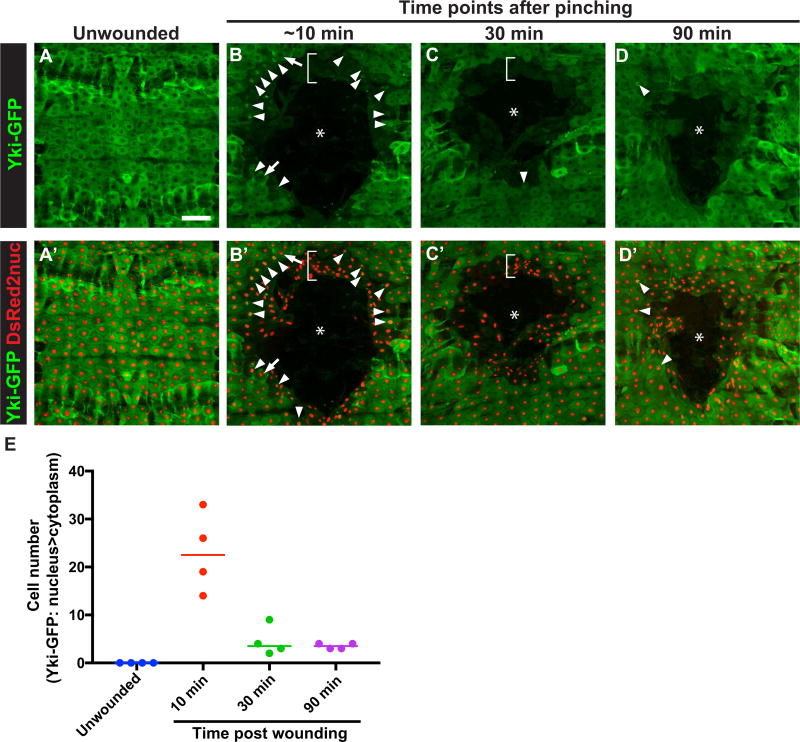
Yki translocates to the nucleus upon activation (Dong et al., 2007; Zhao et al., 2007). To examine the localization of Yki following wounding, we expressed a GFP-tagged version of YKi, UAS-yki-GFP, in the larval epidermis. Overexpression of UAS-yki-GFP did not cause a WC defect (Fig. S2B,D). The transgene was functional as it completely rescued the UAS-YkiRNAi-induced open wound phenotype (Fig. S2C,D). UAS-Yki-GFP also rescued the WC defect of UAS-wts (Fig. S2D), suggesting that the Wts WC defect is related to Yki function, as suggested by previous studies (Dong et al., 2007; Oh and Irvine, 2008; Zhao et al., 2007). Next, we examined the cellular localization of Yki-GFP in the larval epidermis. In unwounded larvae, Yki-GFP was predominately cytoplasmic (Fig. 2A). Ten minutes after wounding, Yki-GFP signal in the first few rows around the wound showed an overall reduction (brackets in Fig. 2B,B′). However, certain cells around the wound edge showed predominant nuclear localization (Fig. 2B,B′,E). The number of cells that exhibited nuclear Yki-GFP signal declined quickly thereafter (Fig. 2C–D′,E). Taken together, these results indicate that at least some wound-edge cells transiently translocate Yki-GFP to the nucleus upon wounding.
Fig. 2.
Yki-GFP rapidly translocates to the nucleus in some wound-edge epidermal cells upon wounding. (A–D) Dissected epidermal whole mounts of unwounded (A) or pinch wounded (B–D) larvae expressing the indicated transgenes via e22c-Gal4 at the indicated time points following wounding. Cell nuclei were labeled by DsRed2nuc in A′–D′. Asterisks in (B–D′) indicate the center of the wounds. Arrowhead in (B–D) show nuclei with prominent Yki-GFP localization. Arrows in (B) show examples of cells with Yki-GFP cytoplasmic localization. Scale bar in (A) indicates 100 µm is for (A–D′). (E) Quantitation of cells with predominant Yki-GFP nuclear localization at different time points after wounding. n=4 for each condition. Each dot represents one larva.
3.3. Yorkie interacts genetically with the Jun N-terminal Kinase (JNK) pathway during wound closure
Both the JNK and Pvr pathways are essential for Drosophila larval WC (Galko and Krasnow, 2004; Lesch et al., 2010; Wu et al., 2009). We asked if Yki interacts genetically with either of these pathways during WC. For genetic interactions with Pvr, we used a Pvrnull allele and a ykinull allele that did not show a WC defect as heterozygotes (Fig. S3A). All of these null alleles are lethal when homozygous. Combining heterozygosity for the Pvrnull alleles with the Ykinull allele result in normal WC (Fig. S3A) as might be expected if the pathways were independent. Likewise, overexpression of Yki did not rescue the Pvrhypo/hypo WC defect (Fig. S3B). These data indicate that the Pvr pathway and Yki do not genetically interact in the context of larval epidermal WC.
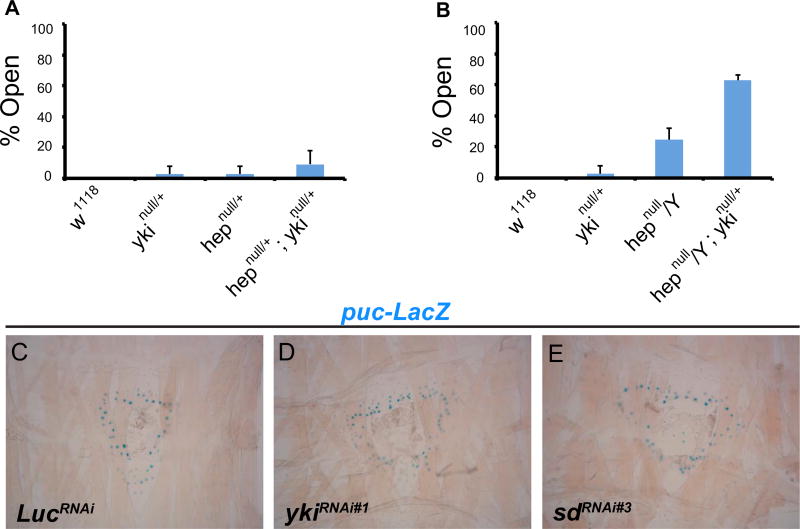
Next, we analyzed genetic interactions between Yki and the JNK pathway. We used a null allele of the JNK upstream kinase, hemipterous (hepnull) (Glise et al., 1995), which yields only 2.8% open wounds when heterozygous (Fig. 3A). Combining heterozygosity for the hepnull and ykinull alleles enhanced wound closure defects by almost three fold (9.2%), hinting at a bona fide interaction between hep and yki (Fig. 3A). We then examined hepnull hemizygous males, which yielded 24.4% open wounds. When combined with ykinull, the occurrence of open wounds was dramatically increased (to 62.7%, Fig. 3B). The percentage of open wounds in hepnull/Y; ykinull/+ animals was greater than that expected from the sum of open wounds from ykinull and hepnull/Y, strongly suggesting synergy between Yki and hep.
Fig. 3.
yki genetically interacts with the JNK pathway. (A, B) Percentage of open wounds in larvae of indicated genotypes. 3 sets of n≥8 for each genotype. Error bars, standard deviation. (C–E) X-gal staining of dissected epidermal whole mounts of larvae expressing puc-lacZ and the indicated transgenes via e22c-Gal4. All wounds are 6 h post-wounding. Blue dots; puc-lacZ positive nuclei.
Given the observed genetic interaction, Yki could act either upstream or downstream of the JNK pathway during WC. To ask if Yki acts upstream of JNK during wound closure we used puc-lacZ, an effective reporter of JNK activity during WC (Brock et al., 2012; Galko and Krasnow, 2004; Rämet et al., 2002). In control larvae, puc-lacZ is strongly induced in the cells adjacent to the wound six hours after wounding (Fig. 3C). Expression of UAS-ykiRNAi (Fig. 3D and Fig. S3C,D) or knockdown of sd (Fig. 3E and Fig. S3E) failed to alter the expression of puc-lacZ at the wound edge. The converse analysis (examining activation of a potential Yki reporter upon JNK knockdown) was not possible given that multiple such reporters (fj-lacZ, exlacZ, Diap1-lacZ, Diap1-GFP and Bantam sensor) were not activated in a wound-specific manner (Fig. S4). Our results suggest that Yki and Sd are not required for wound-induced JNK activation and thus are not likely to act upstream of JNK activation during WC.
3.4. Yorkie does not regulate wound closure by balancing cell proliferation and apoptosis
Next, we investigated the cellular mechanism by which Yki regulates wound closure in the Drosophila epidermis. In the context of organ growth control Yki regulates the balance between cell proliferation and survival (Huang et al., 2005). The cells in the larval epidermis do not appear capable of proliferation as they are endoreduplicated (Wang et al., 2015) and there is no phosphohistone 3 staining after wounding (Lesch et al., 2010; Wang et al., 2015). However, to completely rule out the potential contribution of proliferation or programmed cell death to WC, we first sought to ectopically “force” the cell cycle in the epidermis by expressing UAS-Myc (Johnston et al., 1999) or an activated version of Ras, UAS-RasV12 (Karim and Rubin, 1998). Expression of these transgenes did not adversely affect WC (Fig. 4A,C,E). Next, we tested if UAS-Myc or UAS-RasV12 could rescue the WC defect caused by expression of UAS-ykiRNAi. Neither cell-cycle activating transgene was able to rescue UAS-ykiRNAi-induced WC defects (Fig. 4B,D,E).
We then decided to block the cell cycle, independently or together with UAS-ykiRNAi, and test for effects on WC. We previously identified UAS-RNAi transgenes targeting aurB (aurora B) and cdc2 (cyclin dependent kinase-2) that inhibit the G2 > M cell cycle transition and block potential mitotic progression in the larval epidermis without affecting endoreduplication or normal epithelial architecture (Wang et al., 2015). We expressed these transgenes in the larval epidermis and assessed WC following pinch wounding. Neither transgene perturbed epidermal WC (Fig. 4F–H). Moreover, combining these RNAi transgenes with UAS-ykiRNAi expression in the epidermis did not alter the ykiRNAi-induced WC defects (Fig. 4H). Taken together, these results suggest that regulation of mitotic progression is not involved in larval epidermal WC, either by Yki or other factors.
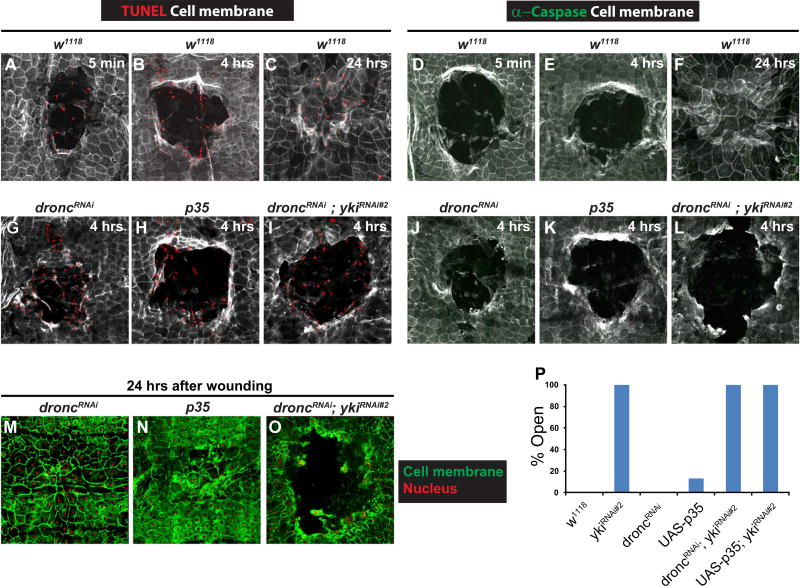
Does Yki promote WC by blocking epidermal apoptosis? Interestingly, we saw a differential induction of two common markers of apoptotic activity following wounding. TUNEL staining was observed immediately post-wounding (Fig. 5A) in leading edge cells and scattered cells in the wound gap. This staining persisted throughout (Fig. 5B) and past WC (Fig. 5C). We suspected based on its immediate appearance (Fig. 5A) that the observed TUNEL reactivity was more likely to be due to DNA fragmentation related to physical cell damage than to rapid activation of apoptosis. Consistent with this idea, the TUNEL reactivity was still observed at an early time point upon expression of UAS-p35 or UAS-DroncRNAi (Fig. 5G,H), both potent inhibitors of apoptosis that efficiently block UV-induced apoptosis (Fig S5B–D). Activated Caspase-3 staining, a direct readout of apoptotic caspase activation by contrast, was never observed following wounding (Fig. 5D–F). These results suggest that epidermal wounding does induce DNA double strand breaks but does not activate the actual machinery of apoptosis.
Fig. 5.
Yki does not regulate apoptosis during epidermal wound closure. (A–C, G–I) Dissected epidermal whole mounts of pinch wounded larvae of the indicated genotypes and at the indicated time points post wounding stained with anti-Fasciclin III antibody (white) and TUNEL (red). (A–C) w1118 control. (A) 5 mins. (B) 4 h. (C) 24 h. (G) UAS-droncRNAi, 4 h. (H) UAS-p35, 4 h. (I) UAS-droncRNAi + UAS-ykiRNAi#2, 4 h. (D–F, J–L) Dissected epidermal whole mounts of pinch wounded larvae of the indicated genotypes and at the indicated time points post wounding immunostained with anti-Fasciclin III antibody (white) and anti-cleaved-Caspase 3 antibody (green). (D–F) w1118 control. (D) 5 mins. (E) 4 h. (F) 24 h. (J) UAS-droncRNAi, 4 h. (K) UAS-p35, 4 h. (L) UAS-droncRNAi + UAS-ykiRNAi#2, 4 h. (m–O) Dissected epidermal whole mounts of larvae expressing UAS-dsRed2Nuc (red) and the indicated transgenes 24 h after pinch wounding, stained with anti-Fasciclin III antibody (green). (M) UAS-droncRNAi, 24 h. (N) UAS-p35, 24 h. (O) UAS-droncRNAi + UAS-ykiRNAi#2, 24 h. (P) Percentage of open wounds on expression of the indicated UAS-RNAi line or transgenes.
Expression of UAS-droncRNAi alone, UAS-p35 alone, or combination of either transgene with UAS-ykiRNAi did not block wound-induced TUNEL reactivity (Fig. 5G–I) or affect the absence of caspase activation (Fig. 5J–L). Expression of UAS-DroncRNAi or UAS-p35 alone also did not affect WC (Fig. 5M,N,P) or alter the UAS-ykiRNAi mediated WC defect (Fig. 5O,P). These results, in combination with the proliferation results above, suggest that Yki mediates cellular functions other than regulating cell proliferation and apoptosis during WC.
3.5. Yorkie is required for formation of an effective actin cable during wound closure
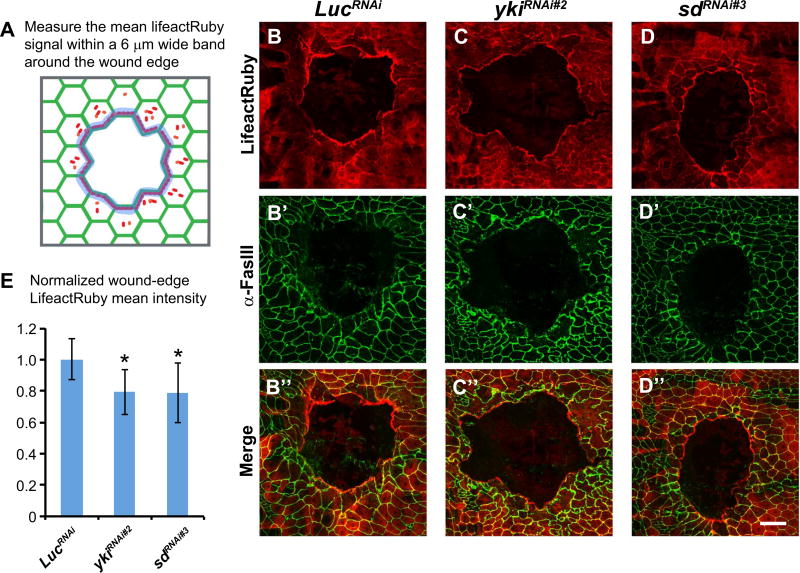
If Yki does not regulate mitosis or apoptosis, what is its function during WC? We previously showed that an actin-based cable is formed at the wound-edge (Wu et al., 2009; Brock et al., 2012). We therefore examined F-actin intensity using a UAS-lifeact-Ruby transgene co-expressed in the larval epidermis with UAS-LucRNAi (control) or UAS-ykiRNAi (Fig. 6A). In control larvae, actin formed a characteristic discontinuous cable around the wound six hours post wounding (Fig. 6B). The UAS-lifeact-Ruby label in the epidermis was reduced at the wound edge when co-expressed with UAS-ykiRNAi, (Fig. 6C,E). A similar reduction in actin cable intensity was observed upon coexpression of UAS-sdRNAi with UAS-lifeact-Ruby (Fig. 6D,E).
Fig. 6.
Yki regulates wound-edge F-actin levels during epidermal wound closure. (A) Illustration of wound-edge F-actin measurement. (B–D) Dissected epidermal whole mounts of wounded larvae expressing the indicated transgenes and UAS-lifeact-Ruby (F-actin, red, B–D) via the e22c-Gal4 six hours post wounding. (B′–D′) Green, anti-Fasciclin III. (B”–D”) Merged images. Scale bar: 100 µm. (E) Average F-actin intensities around the wound sites of larvae expressing the indicated transgenes. Error bars indicate standard deviation. Student's t-test was used to test for significance. *, P < 0.05.
Drosophila profilin, encoded by the chickadee (chic) gene, is required to form the actin-based cable around the wound-edge (Brock et al., 2012). Therefore, we tested if Yki and/or Sd regulate Profilin levels. Expression of UAS-ykiRNAi or UAS-sdRNAi in the epidermis did not affect Profilin levels (Fig. S6A’–C′,E), whereas UAS-profilinRNAi did, even in unwounded larvae (Fig. S6A’,D′,E). Next, we tested if Yki regulates the overall F-actin level in the unwounded larvae using an alternative F-actin marker, phalloidin. UAS-ykiRNAi- and UAS-sdRNAi-expressing larvae showed similar F-actin distributions and F-actin mean intensity as the UAS-LucRNAi control (Fig. S6A”–C”,F), suggesting that Yki and Sd do not regulate F-actin levels in the absence of wounding. In sum, these results suggest that Yki and Sd are required for formation of a fully functional actin cable at the wound edge, and likely regulate WC primarily through control of cell migration.
4. Discussion
4.1. Proliferation, apoptosis, and Yorkie/YAP function in regenerative contexts and larval wound healing
Xenopus YAP is required for limb bud regeneration where it regulates proliferation and apoptosis in the regenerative blastema (Hayashi et al., 2014). Yki also regulates cell proliferation in Drosophila imaginal discs (Grusche et al., 2011; Sun and Irvine, 2011) although assessing regulation of apoptosis in this model is challenging as apoptosis is the trigger for genetically-induced regeneration. In the Drosophila gut Yki regulates cell proliferation following damage-induced regeneration (Karpowicz et al., 2010; Ren et al., 2010; Shaw et al., 2010; Staley and Irvine, 2010). A similar role has been found in the mouse heart (Heallen et al., 2013; Xin et al., 2013) and in the Zebrafish blastema during fin regeneration (Mateus et al., 2015). In the skin, YAP is required for basal stem cell proliferation in the mouse epidermis and thus for epithelial maintenance (Lee et al., 2014; Schlegelmilch et al., 2011).
Our results indicate that cell proliferation and apoptosis do not play important roles during Drosophila larval wound healing, either by Yki or other factors. Consistent with this, well-established Yki/Sd reporters (Diap1-LacZ and ex-LacZ) that are typically activated upon suppression of apoptosis or cell-cycle activation during organ growth control are not activated or repressed upon wounding. Our results suggest that there is substantial tissue specificity to Yki functions during repair processes. They further suggest that there may be distinct target genes that are activated and/or repressed in non-proliferative tissues during repair. In adult Drosophila, Yorkie regulates wound-induced polyploidization but is not directly required for wound closure (Losick et al., 2013) again suggesting stage-specific differences in action even within lineage-related tissues. Taken together, our results suggest that Yki/Sd signaling must regulate some other aspect of epidermal cell behavior during epithelial wound healing.
4.2. Yki/YAP regulates actin polymerization
Our results show that Yki likely functions during WC by regulating actin polymerization. The actin cytoskeleton has been linked to Hippo signaling (Matsui and Lai, 2013). For example, F-actin regulates Hippo pathway and cell proliferation in the Drosophila wing imaginal disc (Fernández et al., 2011; Sansores-Garcia et al., 2011). In mammalian cell culture, YAP/TAZ activity is also regulated by tension of the actin cytoskeleton (Dupont et al., 2011). These effects may be bidirectional in some cases; the Hippo pathway also regulates the actin cytoskeleton in Drosophila imaginal discs (Fernández et al., 2011; Lin et al., 2014). Other studies suggest that overexpression of Yorkie can directly influence actin levels at wing hair initiation sites (Fang and Adler, 2010). In cultured mammalian hepatocytes, YAP regulates the intensity of the peripheral actin cytoskeleton (Bai et al., 2016). These studies suggest that a role for Yki in regulating actin during damage-induced cell motility is certainly plausible.
We found that Yki and Sd are required for larval WC and for efficient actin polymerization at the wound edge. During border cell migration, a developmentally programmed process, Yki gain-of-function led to enhanced migration (Lucas et al., 2013). In this context, the effects of Yki or Sd loss-of-function are not yet clear (Lin et al., 2014; Lucas et al., 2013). However, both of these studies argue that the upstream Hippo kinases may regulate actin independently of Yki. In the larval epidermis, overexpression of Wts caused a weaker WC defect than Yki loss-of-function, suggesting that Yki may have independent effects on actin depending on the cell type examined.
How does Yki/YAP regulate the actin cytoskeleton? In a mouse heart regeneration study, loss of salvador (sav) leads to transcriptional activation of several actin-related genes (Morikawa et al., 2015), suggesting that Yki may regulate actin regulators at the transcriptional level. Indeed, activation of migration-related genes by YAP/TEAD factors has also been observed in metastatic contexts (Liu et al., 2016). If this mechanism is conserved during larval WC, the specific Yki/Sd target genes that might be related to wound-induced migration have yet to be elucidated. These may include Yki/Sd targets that are induced in other contexts or genes whose regulation are important for larval WC (Brock et al., 2012). In breast cancer cells, lysophosphatidic acid (LPA)/GPCR/YAP signaling regulates cell migration (Yu et al., 2012). However, the identity of a putative LPA-like receptor in Drosophila is not entirely clear. The detailed mechanism(s) of how Yki regulates actin dynamics during larval WC will be an interesting topic for further investigation.
4.3. Relationship of Yki translocation to the requirement of Yki for wound closure
Yki-GFP shows predominant nuclear localization in some woundedge epidermal cells within ten minutes of wounding. Other studies have shown that Yap/Yki nuclear translocation can be regulated through physical and mechanical cues (Aragona et al., 2013; Dupont et al., 2011; Rauskolb et al., 2014). The Yki translocation we observed here may be due to the mechanical force of wounding or to alterations in epidermal tension following tissue damage, as the predominant rapid nuclear localization decreases quickly over the next hour. The functional consequence of this rapid translocation, in terms of Yki transcriptional regulation or other functions related to WC is not yet clear, especially given the rapid kinetics of translocation versus the long timescale of wound closure. One possibility here is that Yki may have non-transcriptional functions during WC as previously reported during cytokinesis (Bui et al., 2016).
4.4. The Yki/Sd signaling axis in larval wound healing
Our genetic interaction experiments suggest that Yki interacts strongly with the JNK pathway. Previous work has linked the JNK pathway to the Hippo pathway. In the wing imaginal disc, JNK phosphorylates Ajuba/LIM1D, which results in Warts inhibition, Yki activation, and cell proliferation (Sun and Irvine, 2013; Codelia et al., 2013). Two observations suggest that Ajuba may not be the link between JNK and Yki during WC. First, there is no cell proliferation in the larval epidermis. Second, epidermal expression of a phenotypic UAS-AjubaRNAi transgene (Das Thakur et al., 2010; Rauskolb et al., 2014) does not phenocopy expression of UAS-YkiRNAi.
Additional connections between JNK activation and Hippo signaling have been found during gut regeneration. In particular, Misshapen (msn), a MAP4K sterol family kinase acting upstream of JNK, phosphorylates and activates Warts leading to Yki inhibition in the intestinal stem cells (Li et al., 2014). In larval WC, expression of UAS-msnRNAi leads to a peculiar and specific phenotype of enhancing wound-induced epithelial cell-cell fusion (Lesch et al., 2010; Wang et al., 2015). However, expression of UAS-YkiRNAi or UAS-Yki did not result in this epidermal cell fusion phenotype, suggesting that Msn > Wts mediated Yki regulation may not occur in the larval epidermis.
Wound-induced JNK activation was not blocked by UAS-RNAi targeting Yki or Sd. This makes it similarly unlikely that Yki acts upstream of JNK (Ma et al., 2015) during larval WC. However, the JNK downstream transcriptional factors, Activation Protein-1 family genes (AP-1), D-Jun and D-Fos, are required for larval WC (Lesch et al., 2010). Jun physically interacts with TEAD4 in colon cancer cells (Liu et al., 2016) and could potentially interact directly with Yki to regulate transcription as observed in cancer cells (Verfaillie et al., 2015; Zanconato et al., 2015). Some of the transcriptional targets that shared AP-1 and TEAD binding sites include actin regulators (Liu et al., 2016). The Drosophila ortholog of at least one of these target genes, DOCK, is a known larval WC gene (Lesch et al., 2010). Additionally, JNK may directly phosphorylate and activate Yki, as mammalian JNK has been shown to phosphorylate YAP to regulate apoptosis in keratinocytes and a squamous cell carcinoma (Tomlinson et al., 2010). Finally, JNK inhibits Yki activation and wound-induced polyploidization in the Drosophila adult epidermis (Losick et al., 2016). Since ploidization is not directly related to cell division, this observation, together with the other instances of transcriptional co-regulation, provide potentially fertile leads for future work as to how Yki and JNK cooperate to effect wound closure.
Supplementary Material
Acknowledgments
We thank members of the Galko lab for comments on the manuscript. We thank Kirsten A. Guss for anti-Sd, Jin Jiang, Benny Shilo, Gregory D. Longmore, Renate Renkawitz-Pohl, the Vienna Drosophila RNAi Center, the Bloomington Drosophila Stock Center, and the Japanese NIG-Fly stock centers for Drosophila stocks, and the Developmental Studies Hybridoma Bank for antibodies. CRT was supported by American Heart Association (AHA) predoctoral fellowship 16PRE30880004, AEA was supported by NIH training grant NIH T32 CA 9299-33 and an MD Anderson Center for Cancer Epigenetics Scholar Award, SB was supported by American Heart Association (AHA) postdoctoral fellowship 16560021, JJ was supported by NIH R01 NS069828 to MJG. MJG was supported by NIH R01 GM083031 and an MD Anderson Center for Cancer Epigenetics internal research award.
Abbreviations
- β-Gal
β-Galactosidase
- crb
crumbs
- D-fos
Drosophila fos
- D-Jun
Drosophila Jun
- ds
dachsous
- ex
expanded
- fj
four-jointed
- hep
hemipterous
- JNK
c-Jun N-terminal kinase
- l(2)gl
lethal (2) giant larvae
- Luc
luciferase
- MAP4k
mitogen-activated protein 4 kinase
- puc
puckered
- Pvr
Platelet Derived Growth Factor/Vascular-Endothelial Growth Factor receptor
- PDGF
platelet-derived growth factor
- RTK
receptor tyrosine kinase
- sd
scalloped
- sav
salvador
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP nick end labeling
- VEGF
vascular endothelial growth factor
- WC
wound closure
- yki
yorkie
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2017.05.006.
References
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. http://dx.doi.org/10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Bai H, Zhu Q, Surcel A, Luo T, Ren Y, Guan B, Liu Y, Wu N, Joseph NE, Wang T-L, Zhang N, Pan D, Alpini G, Robinson DN, Anders RA. Yes-Associated Protein impacts adherens junction assembly through regulating actin cytoskeleton organization. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;00027:2016. doi: 10.1152/ajpgi.00027.2016. http://dx.doi.org/10.1152/ajpgi.00027.2016, ajpgi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedigheimer MJ, Nguyen KP, Bryant PJ. expanded functions in the apical cell domain to regulate the growth rate of imaginal discs. Dev. Genet. 1997 doi: 10.1002/(SICI)1520-6408(1997)20:2<103::AID-DVG3>3.0.CO;2-B. http://dx.doi.org/10.1002/(SICI)1520-6408(1997)20:2 < 103::AID-DVG3 > 3.0.CO;2-B. [DOI] [PubMed]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. http://dx.doi.org/10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. http://dx.doi.org/10.1016/S0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Brock AR, Wang Y, Berger S, Renkawitz-Pohl R, Han VC, Wu Y, Galko MJ. Transcriptional regulation of Profilin during wound closure in Drosophila larvae. J. Cell Sci. 2012;125:5667–5676. doi: 10.1242/jcs.107490. http://dx.doi.org/10.1242/jcs.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui DA, Lee W, White AE, Harper JW, Schackmann RCJ, Overholtzer M, Selfors LM, Brugge JS. Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP. Sci. Signal. 2016:9. doi: 10.1126/scisignal.aaa9227. http://dx.doi.org/10.1126/scisignal.aaa9227, ra23-ra23. [DOI] [PMC free article] [PubMed]
- Burra S, Wang Y, Brock AR, Galko MJ. Using drosophila larvae to study epidermal wound closure and inflammation. Methods Mol. Biol. 2013;1037:449–461. doi: 10.1007/978-1-62703-505-7_26. http://dx.doi.org/10.1007/978-1-62703-505-7_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow M. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Codelia V, Sun G, Irvine K. Regulation of YAP by mechanical strain through Jnk and hippo signaling. Curr. Biol. 2013 doi: 10.1016/j.cub.2014.07.034. http://dx.doi.org/10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed]
- Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the hippo signaling pathway. Curr. Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. http://dx.doi.org/10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. http://dx.doi.org/10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jékely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. http://dx.doi.org/10.1016/S0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. http://dx.doi.org/10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fang X, Adler PN. Regulation of cell shape, wing hair initiation and the actin cytoskeleton by Trc/Fry and Wts/Mats complexes. Dev. Biol. 2010;341:360–374. doi: 10.1016/j.ydbio.2010.02.029. http://dx.doi.org/10.1016/j.ydbio.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. http://dx.doi.org/10.1242/jcs.092866. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004:2. doi: 10.1371/journal.pbio.0020239. http://dx.doi.org/10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed]
- Glise B, Bourbon H, Noselli S. hemipterous encodes a novel drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. http://dx.doi.org/10.1016/0092-8674(95)90123-X. [DOI] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the Hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. http://dx.doi.org/10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev. Biol. 2011;350:255–266. doi: 10.1016/j.ydbio.2010.11.020. http://dx.doi.org/10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Guss KA, Benson M, Gubitosi N, Brondell K, Broadie K, Skeath JB. Expression and function of scalloped during Drosophila development. Dev. Dyn. 2013;242:874–885. doi: 10.1002/dvdy.23942. http://dx.doi.org/10.1002/dvdy.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012 doi: 10.1038/nrm3416. http://dx.doi.org/10.1038/nrm3416. [DOI] [PubMed]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. http://dx.doi.org/10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Tamura K, Yokoyama H. Yap1, transcription regulator in the Hippo signaling pathway, is required for Xenopus limb bud regeneration. Dev. Biol. 2014;388:57–67. doi: 10.1016/j.ydbio.2014.01.018. http://dx.doi.org/10.1016/j.ydbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. http://dx.doi.org/10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino TI, Kärpänen Terhi, Wahlström Gudrun, Pulkkinen M, Eriksson U, Alitalo K, Roos C. The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech. Dev. 2001;109:69–77. doi: 10.1016/s0925-4773(01)00510-x. http://dx.doi.org/10.1016/S0925-4773(01)00510-X. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. http://dx.doi.org/10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Johnston La, Prober Da, Edgar Ba, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. (doi:S0092-8674)(00)(81512-3)(pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. http://dx.doi.org/10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YC, Baek SH, Lee H, Choe KM. Nonmuscle myosin II localization is regulated by JNK during Drosophila larval wound healing. Biochem. Biophys. Res. Commun. 2010;393:656–661. doi: 10.1016/j.bbrc.2010.02.047. http://dx.doi.org/10.1016/j.bbrc.2010.02.047. [DOI] [PubMed] [Google Scholar]
- LaJeunesse DR, McCartney BM, Fehon RG. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J. Cell Biol. 1998;141:1589–1599. doi: 10.1083/jcb.141.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Bodmer R, Vincent JP. Segmental patterning of heart precursors in Drosophila. Development. 1995;121:4303–4308. doi: 10.1242/dev.121.12.4303. [DOI] [PubMed] [Google Scholar]
- Lee M-JJ, Ran Byun M, Furutani-Seiki M, Hong J-HH, Jung H-SS. YAP and TAZ regulate skin wound healing. J. Invest. Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. http://dx.doi.org/10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics. 2010;186:943–957. doi: 10.1534/genetics.110.121822. http://dx.doi.org/10.1534/genetics.110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, RothFlach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, Cotton JL, Mao J, McCollum D, Jiang J, Czech MP, Xu L, Ip YT. The conserved misshapen-warts-yorkie pathway acts in enteroblasts to regulate intestinal stem cells in drosophila. Dev. Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. http://dx.doi.org/10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AYT, Pearson BJ. Planarian yorkie/YAP functions to integrate adult stem cell proliferation, organ homeostasis and maintenance of axial patterning. Development. 2014;141:1197–1208. doi: 10.1242/dev.101915. http://dx.doi.org/10.1242/dev.101915. [DOI] [PubMed] [Google Scholar]
- Lin TH, Yeh TH, Wang TW, Yu JY. The hippo pathway controls border cell migration through distinct mechanisms in outer border cells and polar cells of the drosophila ovary. Genetics. 2014;198:1087–1099. doi: 10.1534/genetics.114.167346. http://dx.doi.org/10.1534/genetics.114.167346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, Davis RJ, Mao J. Tead and AP1 Coordinate Transcription and Motility. Cell Rep. 2016;14:1169–1180. doi: 10.1016/j.celrep.2015.12.104. http://dx.doi.org/10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr. Biol. 2013;23:2224–2232. doi: 10.1016/j.cub.2013.09.029. http://dx.doi.org/10.1016/j.cub.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Jun AS, Spradling AC. Wound-Induced Polyploidization: regulation by Hippo and JNK signaling and conservation in mammals. PLoS One. 2016;11:e0151251. doi: 10.1371/journal.pone.0151251. http://dx.doi.org/10.1371/journal.pone.0151251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EP, Khanal I, Gaspar P, Fletcher GC, Polesello C, Tapon N, Thompson BJ. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 2013;201:875–885. doi: 10.1083/jcb.201210073. http://dx.doi.org/10.1083/jcb.201210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Chen Y, Xu W, Wu N, Li M, Cao Y, Wu S, Li Q, Xue L. Impaired Hippo signaling promotes Rho1-JNK-dependent growth. Proc. Natl. Acad. Sci. USA. 2015;112:1065–1070. doi: 10.1073/pnas.1415020112. http://dx.doi.org/10.1073/pnas.1415020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus R, Lourenco R, Fang Y, Brito G, Farinho A, Valerio F, Jacinto A. Control of tissue growth by Yap relies on cell density and F-actin in zebrafish fin regeneration. Development. 2015;142:2752–2763. doi: 10.1242/dev.119701. http://dx.doi.org/10.1242/dev.119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Lai ZC. Mutual regulation between Hippo signaling and actin cytoskeleton. Protein Cell. 2013 doi: 10.1007/s13238-013-3084-z. http://dx.doi.org/10.1007/s13238-013-3084-z. [DOI] [PMC free article] [PubMed]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. http://dx.doi.org/10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Zhang M, Heallen T, Leach J, Tao G, Xiao Y, Bai Y, Li W, Willerson JT, Martin JF. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci. Signal. 2015;8:ra41. doi: 10.1126/scisignal.2005781. http://dx.doi.org/10.1126/scisignal.2005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. Cooperative regulation of growth by Yorkie and Mad through bantam. Dev. Cell. 2011;20:109–122. doi: 10.1016/j.devcel.2010.12.002. http://dx.doi.org/10.1016/j.devcel.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 2010 doi: 10.1016/j.tcb.2010.04.005. http://dx.doi.org/10.1016/j.tcb.2010.04.005. [DOI] [PMC free article] [PubMed]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. http://dx.doi.org/10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev. Cell. 2010 doi: 10.1016/j.devcel.2010.09.011. http://dx.doi.org/10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007 doi: 10.1101/gad.1536007. http://dx.doi.org/10.1101/gad.1536007. [DOI] [PubMed]
- Peng HW, Slattery M, Mann RS. Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 2009;23:2307–2319. doi: 10.1101/gad.1820009. http://dx.doi.org/10.1101/gad.1820009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Montell DJ. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev. Cell. 2007;12:997–1005. doi: 10.1016/j.devcel.2007.03.021. http://dx.doi.org/10.1016/j.devcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Rämet M, Lanot R, Zachary D, Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 2002;241:145–156. doi: 10.1006/dbio.2001.0502. http://dx.doi.org/10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. http://dx.doi.org/10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun E-Y, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. USA. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. http://dx.doi.org/10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Mol. Biol. Cell. 2012;23(6):1068–1079. doi: 10.1091/mbc.E11-09-0745. http://dx.doi.org/10.1016/S1534-5807(02)00400-8, (Epub 2012 Jan 19.PMID:22262460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K-I, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. http://dx.doi.org/10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. http://dx.doi.org/10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. http://dx.doi.org/10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Hippo signaling in Drosophila: recent advances and insights. Dev. Dyn. 2012 doi: 10.1002/dvdy.22723. http://dx.doi.org/10.1002/dvdy.22723. [DOI] [PMC free article] [PubMed]
- Staley BK, Irvine KD. Warts and yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr. Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. http://dx.doi.org/10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD. Ajuba family proteins link JNK to Hippo signaling. Sci. Signal. 2013;6:ra81. doi: 10.1126/scisignal.2004324. http://dx.doi.org/10.1126/scisignal.2004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev. Biol. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. http://dx.doi.org/10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson V, Gudmundsdottir K, Luong P, Leung K-Y, Knebel a, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. http://dx.doi.org/10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. http://dx.doi.org/10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- Venken KJT, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, Carlson JW, Levis RW, Spradling AC, Hoskins RA, Bellen HJ. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods. 2011 doi: 10.1038/nmeth.1662. http://dx.doi.org/10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed]
- Verfaillie A, Imrichova H, Atak ZK, Dewaele M, Rambow F, Hulselmans G, Christiaens V, Svetlichnyy D, Luciani F, Van den Mooter L, Claerhout S, Fiers M, Journe F, Ghanem G-E, Herrmann C, Halder G, Marine J-C, Aerts S. Decoding the regulatory landscape of melanoma reveals TEADS as regulators of the invasive cell state. Nat. Commun. 2015;6:6683. doi: 10.1038/ncomms7683. http://dx.doi.org/10.1038/ncomms7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Antunes M, Anderson AE, Kadrmas JL, Jacinto A, Galko MJ. Integrin adhesions suppress syncytium formation in the Drosophila larval epidermis. Curr. Biol. 2015;25:2215–2227. doi: 10.1016/j.cub.2015.07.031. http://dx.doi.org/10.1016/j.cub.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. http://dx.doi.org/10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. http://dx.doi.org/10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Wu Y, Brock AR, Wang Y, Fujitani K, Ueda R, Galko MJ. A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr. Biol. 2009;19:1473–1477. doi: 10.1016/j.cub.2009.07.019. http://dx.doi.org/10.1016/j.cub.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. http://dx.doi.org/10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013 doi: 10.1101/gad.210773.112. http://dx.doi.org/10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. http://dx.doi.org/10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. http://dx.doi.org/10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. http://dx.doi.org/10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Milton CC, Poon CLC, Hong W, Harvey KF. Wbp2 cooperates with Yorkie to drive tissue growth downstream of the Salvador-Warts-Hippo pathway. Cell Death Differ. 2011;18:1346–1355. doi: 10.1038/cdd.2011.6. http://dx.doi.org/10.1038/cdd.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. http://dx.doi.org/10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Zhao B, Wei X, Wei X, Li W, Li W, Udan RS, Udan RS, Yang Q, Yang Q, Kim J, Kim J, Xie J, Xie J, Ikenoue T, Ikenoue T, Yu J, Yu J, Li L, Li L, Zheng P, Zheng P, Ye K, Ye K, Chinnaiyan A, Chinnaiyan A, Halder G, Halder G, Lai Z, Lai Z, Guan K-L, Guan K-L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. http://dx.doi.org/10.1101/gad.1602907.Hpo/Sav. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.