Abstract
Injection drug use is increasingly an important route of HIV transmission in Romania (from 1.5% of the newly diagnosed cases prior to 2010 to 31% in 2013). In this study we investigated the viral characteristics and relationships in newly HIV infected persons who inject drugs in Bucharest, Romania.
Results
HIV-1 pol sequencing, followed by phylogenetic and clustering analysis was performed on blood from 37 injecting drug users (IDUs) newly diagnosed with HIV infection. While HIV subtype F1, the dominant strain in Romania since 1990, remains prevalent, new subtypes were found including G, B, B/G and B/F recombinants. Overall, 27 of the available sequences (72.9%) clustered with at least one other. Network and phylogenetic analysis revealed tight monophyletic clusters for both subtypes F and G, with short genetic distances between sequences, suggesting recent numerous acute to acute transmissions or single burst-type episodes. No transmitted drug-resistance mutations were identified. Greater immunosuppression was present in subjects forming the subtype G cluster, possibly indicating a faster rate of progression associated with this subtype.
Conclusions
The recent increasing numbers of IDU related HIV transmissions in Bucharest, has resulted in closely-knit transmission networks that maychange the genetic profile of the local HIV epidemic.
Keywords: HIV, transmission networks, drug use, subtype G, subtype F
1. Introduction
According to the latest European Centre for Disease Prevention and Control report (ECDC [1]), 29,381 human immunodeficiency virus (HIV) diagnoses were reported in 2012 by European countries (a rate of 5.8 per 100 000 population, with a 3.2 male to female ratio). Male-to-male sexual contact was the predominant mode of transmission (40% of the cases), followed by heterosexual contact (34% of infections). Across Europe, a significant decline in the number of HIV infections related to injected drug use (IDU) has been reported during the last decade, following a peak in 2001–2002, which coincided with epidemics in the Baltic States (UUSKULA & al.[2]; BALODE & al. [3]). This downward trend was interrupted in 2011–2012, when Romania and Greece experienced large HIV outbreaks in people who inject drugs (PWID) (PHARRIS & al. [4]); consequently, the two countries now account for 23% of the total European new HIV cases attributed to injected drug-use (ECDC [1]).
In Romania, most of the 12,273 diagnosed people living with HIV (PLHIV) were infected during the nosocomial pediatric epidemic (1988–1991) and now are between 22 and 26 years of age (CNLAS [5]). Heterosexually transmitted cases increased slowly after 2000, but injection drug use accounted for less than 1.5% of the newly diagnosed HIV cases prior to 2010. However, a striking increase in injected drug-related HIV cases has been observed during the last 3 years (18.2% of total cases in 2011 and 31% in 2012 and 2013) (CNLAS [5]). Moreover, outbreaks of multiple blood-borne viral infections were reported in PWID requiring hospitalization for severe systemic infections (OPREA & al. [6]). HIV-1 subtype F1 has predominated in HIV-infected patients from Romania since 1990, while introduction of other strains remained limited, with subtypes B and C identified in less than 10% of the newly diagnosed cases before 2010 (MANOLESCU & al. [7]). This study aimed to assess if the recent shift toward IDU-related transmission of HIV infection was associated with changes in circulating viral subtypes and with the presence of transmission clusters.
2. Materials and Methods
Study patients
This analysis was a retrospective study on stored plasma samples collected from PWID admitted between 2010 and 2013 in a single tertiary facility (Victor Babes Hospital for Infectious Diseases). All patients had previously unknown HIV status, were hospitalized mainly for severe drug-related bacterial infections, and had provided informed consent. The study was approved by the Institutional Review Board of the Institute of Virology, Bucharest, Romania. Sixty-one patients were newly diagnosed with HIV infection (ELISA followed by Western Blot confirmation). Thirty-seven of these had both a viral load of >1000 copies/ml (determined with COBAS TaqMan HIV-1 Test Version 2.0, Roche Molecular Systems, Branchburg, NJ, USA; lower detection limit: 20 HIV RNA copies/mL) and a stored sample available for sequencing; all subjects were naïve to antiretroviral therapy.
HIV-1 sequencing
Pol genotyping and resistance testing were performed using the ViroSeq HIV-1 Genotyping System (Abbott Laboratories, Des Plaines, IL, USA). HIV-1 subtype was determined with the REGA HIV-1 subtyping tool (http://www.bioafrica.net/rega-genotype/html/subtypinghiv.html) and transmitted drug resistance was predicted using the 2009 WHO list (http://cpr.stanford.edu/cpr.cgi); GenBank accession numbers for the sequences are KP231374-KP231406 and JX966527-JX966530.
For phylogenetic analysis, maximum likelihood trees, using the Tamura-Nei 93 (TN93) evolutionary model and 1000 bootstraps, were generated for each subtype, using PHYML implemented in Geneious® (Biomatters, Auckland, USA), after filtering sequences for APOBEC hypermutations. For network clustering analysis, a pairwise distance comparison of all sequences was performed. Putative transmission links were inferred for sequences with a TN93 genetic distance of ≥1.5% between two sequences, as described previously (WERTHEIM & al. [8]). Transmission clusters were assembled by connecting all patients sharing putative transmission links. The time of most recent common ancestor (tMRCA) for the two largest clusters was estimated with a Bayesian Markov chain Monte Carlo framework implemented in BEAST v1.8. Analyses were run under a strict clock, TN93 substitution model, and a constant population size for 10,000,000 generations, discarding the first 10% as burn-in. The data did not support more complicated evolutionary models (e.g., uncorrelated log normal relaxed molecular clock, GTR+4, or an exponential growth rate). Mixing and convergence were evaluated with Tracer v1.5, and an estimated sample size > 200 was reached for all parameters.
Statistical Analyses
were performed using IBM SPSS Statistics, Version 21.0. (Armonk, NY: IBM Corp). Categorical variables were compared using χ2 or Fisher’s exact test and non-categorical variables were evaluated using the independent samples t-test; p<0.05 was considered significant.
3. Results and discussion
Patients characteristics
Most of the 61 newly diagnosed HIV infected IDUs were young urban males (85.2%), unemployed (78.7%), and without health insurance (75.4%). Only 8.8% had started drug use within the last year and 78.7% were currently using new psychoactive substances (NPS). All reported frequent needle sharing, but only 7 (11.4%) had been imprisoned. HIV infection appeared to be recently acquired in most of the cohort, since only 16.4% were late-presenters (defined as having <350 CD4 cells/ml). There were no significant differences related to gender, age, drug use or clinical parameters between the 37 subjects with available samples for genotyping compared to all newly diagnosed IDUs (Table 1).
Table 1.
Comparison between IDUs with and without available samples for genotyping
| Patient characteristics | Total HIV positives n=61 |
Total sequenced n=37 |
p |
|---|---|---|---|
| Malesa | 52 (85.2) | 33 (89.1) | 0.5 |
| Median age, years (range) | 26 (15– 45) | 27 (15– 45) | 0.44 |
| Median age at initiation of drug use, years (range) | 17 (16– 39) | 17 (16–38) | 0.12 |
| Median time on drugs, months (range) | 66 (4–180) | 78 (6–180) | 0.3 |
| Heroin onlya | 13 (21.3) | 8 (21.6) | 0.47 |
| NPS onlyab | 8 (13.1) | 6 (16.2) | 0.8 |
| NPSb with/after heroina | 40 (65.6) | 23 (62.1) | 0.8 |
| Median CD4 count, cells/ml (range) | 586 (29– 1988) | 507 (29–1988) | 0.8 |
| CD4 < 200 cells/mla | 8 (13.1) | 7 (18.9) | 0.8 |
| Median HIV RNA, log10 copies/ml (range) | 4.9 (2.4– 6.7) | 4.9 (3.7–6.7) | 0.9 |
Legend of table:
All values are patients numbers, (%), unless otherwise indicated.
NPS- new psychoactive substances.
p<0.05 was considered statistically significant.
Prevalence of HIV-1 subtypes and drug resistance mutations
The most common HIV-1 subtype was F1 (25/37, 67.5%), followed by subtypes G (8/37; 21.6%), and B (2/37; 5.4%); B/G and B/F recombinants were in single subjects (1/37, 2.7% in both cases). Patients infected with non-F1 HIV1 strains presented with significantly lower CD4+ cell counts (Table 2). No other differences related to demographic or risk factors were found. Mutations associated with resistance to antiretrovirals were not detected.
Table 2.
Comparison between IDUs infected with HIV subtype F vs. non-F
| F subtype n=25 |
non-F subtypes n=12 |
P | |
|---|---|---|---|
| Malesa | 22 (88) | 11 (91.6) | 0.9 |
| Median age, years (range) | 25 (15–45) | 27 (15–42) | 0.62 |
| Median age at initiation of drug use, years (range) | 16.5 (11–34) | 17 (14–30) | 0.56 |
| Median time on drugs, months (range) | 78 (6–180) | 82 (12–180) | 0.64 |
| Patients on heroin onlya | 5 (20) | 3 (25) | 0.7 |
| Patients on NPS onlyab | 4 (16) | 2 (16.6) | 0.9 |
| Patients on NPSb with/after heroina | 15 (60) | 8 (66.6) | 0.6 |
| Median CD4 count, cells/ml (range) | 697 (39–1988) | 226 (25–530) | 0.001* |
| Patients with CD4 < 200 cells/mla | 2 (8) | 5 (41.6) | 0.02* |
| Median HIV RNA, log10 copies/ml (range) | 4.8 (3.7–6.2) | 5.5 (3.9–6.7) | 0.07 |
Legend of table:
All values are patients numbers, (%), unless otherwise indicated.
NPS- new psychoactive substances.
p<0.05 was considered statistically significant.
Phylogenetic and Network analyses
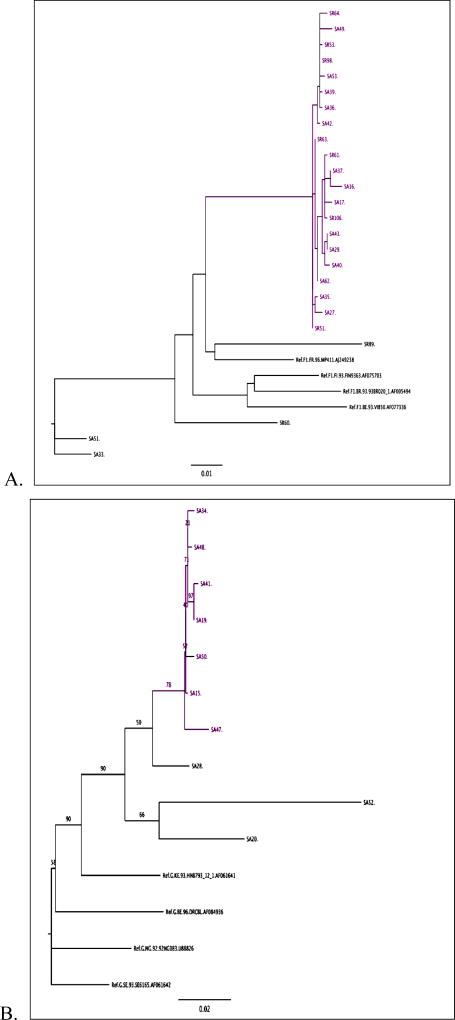
Overall, 27 out of the 37 available sequences (72.9%) clustered with at least one other sequence, forming two large clusters of subtype F1 (Fig. 1.A) and G (Fig. 1.B) plus a pair of B sequences. When compared with other sequences from the Los Alamos database, all F1 sequences were most closely related to a Romanian strain (isolated from an adult sample in 1997), whereas the G sequences show high similarities with three G strains isolated from Portugal and one from Romania. Dating analysis using BEAST suggested that the most recent common ancestor of the subtype F cluster was present approximately 10 years prior to 2013 (95% HPD: 5–17 years). Unfortunately the subtype G cluster was too small and lacked enough temporal information to obtain accurate dating. Nevertheless, the branch lengths separating these isolates are very short, suggesting a recent introduction into the PWID population.
Figure 1. Phylogenetic trees for (A) subtype F and (B) subtype G.
(A) A maximum likelihood phylogenetic tree demonstrated a large HIV transmission cluster within the sequences subtyped as F1. (B) A maximum likelihood phylogenetic tree demonstrated grouping of all the 10 sequences subtyped as G, with an HIV transmission cluster of 7 samples.
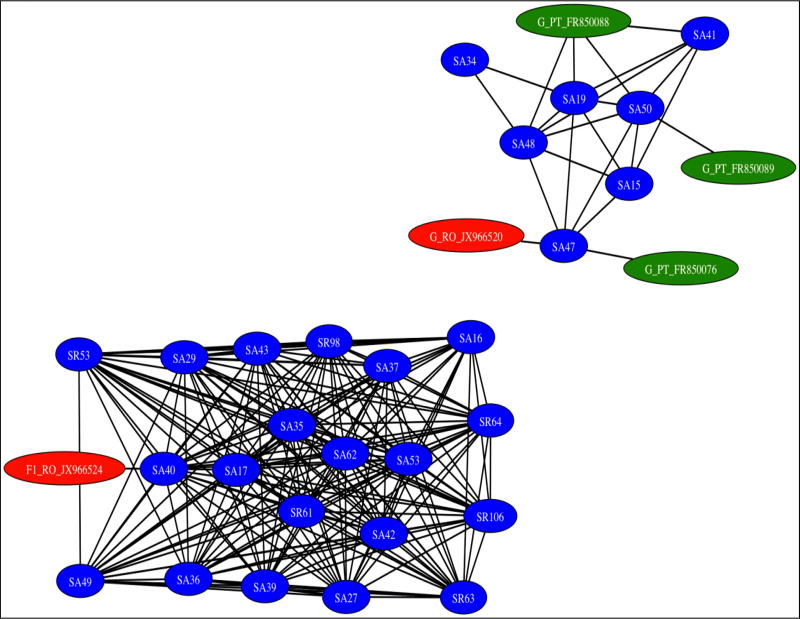
The low genetic distance between sequences and the monophyletic nature of the clusters suggest a recent emergence of local HIV transmission networks. Further network analysis revealed highly interconnected sequences, with pronounced genetic similarity, indicating transmission clusters of both subtypes F and G (Fig. 2). Interestingly, 89.7% of IDUs using NPS clustered, compared with only 50% of those using heroin exclusively (p=0.04).
Figure 2. Inferred transmission clusters of subtypes F and G by genetic distance analysis.
Linkages are represented by network edges, and individuals by network nodes. A cluster includes all connected individuals, and all are less than 1.5% divergent from at least one other, but not necessarily less than 1.5% divergent between all within the cluster.
The subtype F1 cluster, which included 20 subjects (9 diagnosed in 2010 and 11 in 2012–2013) were all highly interconnected (Fig. 2). The genetic similarity of these viruses suggests that transmission occurred rapidly from one individual to another, indicating high-risk behavior in this group, presumably by sharing needles. The mean age of the subjects was 26.2 years, with a significant increase with later year of diagnosis (21.8 years for 2010–11 vs. 30.7 years for 2012–13, p=0.04). More than a third of patients in this cluster (38%) started injecting drugs at a young age (11–15 years), and 85.7% of them were currently using NPS.
Most of the patients within this cluster (18/20, 90%) had CD4 cell counts >500 cells/ml and high viral loads (mean: 4.89 log 10 HIV RNA copies/ml) at diagnosis, unrelated to the year of diagnosis, but suggesting recent infection.
The other cluster of subtype G included 7 individuals (3 diagnosed in 2011 and 4 in 2012–2013), with a mean age of 23 years. Interestingly, 71.4% (5/7) of the patients from this cluster presented with severe immunosuppression (<200 CD4 cells/ml), whereas only one had >500 CD4 cells/ml. Two other male patients, infected with subtype B, diagnosed in 2012 and 2013 respectively, clustered together and were late presenters having less than 350 CD4 cells/ml.
We report interconnected clusters of HIV transmission among newly infected IDUs, suggesting numerous transmissions early in infection and/or a single burst-type episode (e.g. a contaminated syringe shared between several individuals). The low genetic distance between sequences and the monophyletic nature of the clusters suggest the existence of recent local networks of HIV transmission in this risk group. This pattern can be explained by high-risk behavior driven by firstly the use of NPS, which were legal and affordable until 2011 and require a higher frequency of daily injections than heroin, and secondly a dramatic decrease in harm reduction programs, due to economic limitations (1.7 million sterile syringes were distributed in 2009 vs. less than 900,000 in 2011; 76% of IDUs reported visiting exchange programs in 2009 vs. 49% in 2011) (NAAFR & al. [9]). These changes combined to increase sharing and reuse of needles and syringes. A similar tendency towards HIV clustering in PWID during the financial crisis has recently been described in Greece (PARASKEVIS & al. [10]), where increased rates of unemployment and homelessness have correlated with higher prevalence rates of both HIV and HCV.
The largest identified cluster in our study are subtype F1 which continues to dominate the Romanian HIV epidemic, suggesting that HIV spread is still driven by local transmission, as observed also in other European studies (FRENTZ & al. [11]). We found extension of a previously reported small subtype F transmission cluster related to IDU in four newly HIV diagnosed patients (TEMEREANCA & al. [12]). Multiple additional members of this cluster were identified during this study, with dating analysis suggesting initiation of the cluster within the last 10 years. Importantly, the mean age of these subjects is congruous with the present age of long term survivors of the pediatric epidemic, a fact that is particularly concerning in regard to future HIV transmission, both by sexual contact and by IDU. Long-term survivors have been treated since early childhood with multiple antiretroviral regimens, acquiring cumulative drug-related toxicities, resulting in poor adherence, overall fatigue with the healthcare system and diminished compliance with measures to prevent HIV transmission. Sustainable risk reduction interventions should rapidly be implemented to avoid an escalation of new HIV infection.
The phylogenetic and network analysis of samples from IDUs infected with non-F subtypes, show evidence of a subtype G transmission cluster, closely related with sequences from Portuguese HIV infected IDUs. Subtype G was first reported in IDUs in Italy (CICCOZZI & al. [13]), then in Portugal, where it led to a well-established epidemic with secondary spread to non-IDU heterosexuals (ABECASIS & al. [14]; ESTEVES & al. [15]) and in Spain (THOMSON & al. [16]). Although we did not gather information about travel outside the country, infection with subtype G while abroad cannot be excluded, as most of the 2.12 million Romanian migrants in the European Union have settled in Italy and Spain (IOM’s & al. [17]).
Interestingly, we found low levels of immunosuppression in the subtype F transmission cluster, whereas most of the individuals in the subtype G cluster had CD4 counts <200 cells/ml. Although the higher level of immunosuppression could suggest earlier transmission of subtype G, it might also be related to a faster rate of progression driven by this subtype. Differences in progression rates by subtype have previously been reported: subtype D is associated with a faster progression (AMORNKUL & al. [18]; SSEMWANGA & al. [19], whereas subtype A with a slower progression (SPIRA & al. [20]; PANT & al. [21]). An accelerated loss of CD4 cells has also been shown in patients infected with BF recombinants when compared to those infected with subtype B (TAROSSO & al. [22]). Further work is required to clarify the dissimilarities in pathogenicity of different subtypes.
Two other male patients (one diagnosed in 2012 at 28 years, and another diagnosed in 2013 at 30 years) were infected with subtype B and clustered together. The epidemiology of HIV infections in Europe is dominated by subtype B found in almost 70% of the cases, especially in men having sex with men (MSM). However, in Romania, this subtype is uncommon, except in MSM, although the rates are rising. The existence of highly stratified epidemics in specific European countries, with different subtypes compartmentalized in distinct transmission groups has been already suggested (ABECASIS & al. [23]; LUNAR & al. [24]).
Although the relatively small size of the patients sample is a limitation to our study, the observed emergence of new HIV subtypes in Romania is supported by a recent study that points to a decrease in the number of F1 HIV infections in IDUs and the appearance of a circulating recombinant form (CRF 14_BG), bearing similarity with sequences found in Greece (NICULESCU & al. [25]). It also remains to be seen if the existence of a conflict zone in neighbouring Ukraine, where a B variant is widespread among PWID (KAZENNOVA & al. [26]), will trigger further changes in the distribution of subtypes in Romania. Continuous monitoring of the evolution of this HIV epidemic is necessary to monitor and understand shifting trends in HIV transmission.
4. Conclusion
The recent rapid increase in numbers of injection drug related HIV transmissions in Romania has resulted in closely-knit transmission networks and diversification of the circulating subtypes, representing a novel change in the genetic profile of the Romanian HIV epidemic.
Acknowledgments
The authors thank Carmen Diaconu, PhD and Claudia Dita, MD from Stefan S Nicolau Institute of Virology, Bucharest, for technical support.
Funding: Partially supported by NIH grants AI036211-19 (subcontract PO 5600167489, through the Baylor International Pediatric AIDS Initiative), AI11018 and AI093163.
Footnotes
This work was presented in part as an oral communication at the 24th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain 10–13 May, 2014, abstract O167.
References
- 1.European Centre For Disease Prevention And Control/Who Regional Office For Europe. HIV/AIDS surveillance in Europe 2012. Stockholm: World Health Organization; 2013. available online at http://www.ecdc.europa.eu/en/publications/Publications/annual-epidemiological-report-2013.pdf. [Google Scholar]
- 2.Uuskula A, Kalikova A, Zilmer K, Tammai L, Dehovitz J. The role of injection drug use in the emergence of Human Immunodeficiency Virus infection in Estonia. Int. J. Infect. Dis. 2002;6(1):23–7. doi: 10.1016/s1201-9712(02)90131-1. [DOI] [PubMed] [Google Scholar]
- 3.Balode D, Ferdats A, Dievberna I, Viksna L, Rozentale B, Kolupajeva T, Konicheva V, Leitner T. Rapid epidemic spread of HIV type 1 subtype A1 among intravenous drug users in Latvia and slower spread of subtype B among other risk groups. AIDS Res. Hum. Retroviruses. 2004;20(2):245–9. doi: 10.1089/088922204773004978. [DOI] [PubMed] [Google Scholar]
- 4.Pharris A, Wiessing L, Sfetcu O, Hedrich D, Botescu A, Fotiou A, Nikolopoulos GK, Malliori M, Salminen M, Suk JE, Griffiths P, Van Delaar MJ. Human immunodeficiency virus in injecting drug users in Europe following a reported increase of cases in Greece and Romania. Euro Surveil. 2011;16(48):20032. [PubMed] [Google Scholar]
- 5.National Aids Committee, Department For Aids Monitoring From Romania. Statistic data on HIV/AIDS infection in Romania. CNLAS; Bucharest: 2013. available online at http://www.cnlas.ro/date-statistice.html. [Google Scholar]
- 6.Oprea C, Ceausu E, Ruta S. Ongoing outbreak of multiple blood-borne infections in injecting drug users in Romania. Public Health. 2013;127(11):1048–50. doi: 10.1016/j.puhe.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Manolescu LS, Temereanca A, Ruta S. HIV-1 circulating subtypes in Romania. Roum. Arch. Microbiol. Immunol. 2013;72(2):121–34. [PubMed] [Google Scholar]
- 8.Wertheim JO, Leigh Brown AJ, Hepler NL, Mehta SR, Richman DD, Smith DM, Kosakovsky Pond SL. The global transmission network of HIV-1. J. Infect. Dis. 2014;209(2):304–13. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Antidrug Agency From Romania. 2013 National report to EMCDDA by the Reitox National Focal Pointon drugs situation for Romania. Bucharest: 2013. Available at http://www.ana.gov.ro/rapoartenationale/en/NationalReportonDrugs2013.pdf. [Google Scholar]
- 10.Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, Lazanas M, Gargalianos P, Psichogiou M, Skoutelis A, Wiessing L, Friedman SR, Jarlais DC, Terzidou M, Kremastinou J, Malliori M, Hatzakis A. Economic Recession and Emergence of an HIV-1 Outbreak among Drug Injectors in Athens Metropolitan Area: A Longitudinal Study. PLoS One. 2013;8(11):e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frentz D, Wensing AM, Albert J, Paraskevis D, Abecasis AB, Hamouda O, Jorgensen LB, Kücherer C, Struck D, Schmit JC, Åsjö B, Balotta C, Beshkov D, Camacho RJ, Clotet B, Coughlan S, De Wit S, Griskevicius A, Grossman Z, Horban A, Kolupajeva T, Korn K, Kostrikis LG, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer Stöckl E, Sönnerborg A, Stanekova D, Stanojevic M, Vandamme AM, Boucher CA, Van De Vijver DA. SPREAD Programme, Limited cross-border infections in patients newly diagnosed with HIV in Europe. Retrovirology. 2013;10:36. doi: 10.1186/1742-4690-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temereanca A, Ene L, Mehta S, Manolescu L, Duiculescu D, Ruta S. Transmitted HIV drug resistance in treatment-naive Romanian patients. J. Med. Virol. 2013;85(7):1139–47. doi: 10.1002/jmv.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccozzi M, Montieri S, Salemi M, De Oliveira T, Dorrucci M, Sinicco A, De Luca A, Giuliani M, Balotta C, Rezza G. An outbreak of HIV-1 subtype G among Italian injecting drug users. AIDS. 2007;21(9):1213–25. doi: 10.1097/QAD.0b013e32813aee1a. [DOI] [PubMed] [Google Scholar]
- 14.Abecasis AB, Martins A, Costa I, Carvalho AP, Diogo I, Gomes P, Camacho RJ. Molecular epidemiological analysis of paired pol/env sequences from Portuguese HIV type 1 patients. AIDS Res. Hum. Retroviruses. 2011;27(7):803–5. doi: 10.1089/AID.2010.0312. [DOI] [PubMed] [Google Scholar]
- 15.Esteves A, Parreira R, Piedade J, Venenno T, Franco M, Germano De Sousa J, Patricio L, Brum P, Costa A, Canas-Ferreira WF. Spreading of HIV-1 subtype G and envB/gagG recombinant strains among injecting drug users in Lisbon, Portugal. AIDS Res. Hum. Retroviruses. 2003;19(6):511–7. doi: 10.1089/088922203766774568. [DOI] [PubMed] [Google Scholar]
- 16.Thomson MM, Delgado E, Manjon N, Ocampo A, Villahermosa ML, Mariňo A. HIV-1 genetic diversity in Galicia Spain: BG intersubtype recombinant viruses circulating among injecting drug users. AIDS. 2001;15(4):509–16. doi: 10.1097/00002030-200103090-00010. [DOI] [PubMed] [Google Scholar]
- 17.International Organization For Migration. The World Migration Report 2013: Migrant Well-being and Development - the 7th report in IOM’s World Migration Report series. Geneva: 2013. available on line at www.iom.int. [Google Scholar]
- 18.Amornkul PN, Karita E, Kamali A, Rida WN, Sanders EJ, Lakhi S, Price MA, Kilembe W, Cormier E, Anzala O, Latka MH, Bekker LG, Allen SA, Gilmour J, Fast PE. Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS. 2013;27(17):2775–86. doi: 10.1097/QAD.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ssemwanga D, Nsubuga RN, Mayanja BN, Lyagoba F, Magambo B, Yirrell D, Van Der Paal L, Grosskurth H, Kaleebu P. Effect of HIV-1 subtypes on disease progression in rural Uganda: a prospective clinical cohort study. PLoS One. 2013;8(8):e71768. doi: 10.1371/journal.pone.0071768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spira S, Wainberg MA, Loemba H, Turner D, Brenner BG. Impact of clade diversity on HIV-1 virulence, antiretroviral drug sensitivity and drug resistance. J. Antimicrob. Chemother. 2003;51(2):229–40. doi: 10.1093/jac/dkg079. [DOI] [PubMed] [Google Scholar]
- 21.Pant Pai N, Shivkumar S, Cajas JM. Does genetic diversity of HIV-1 non-B subtypes differentially impact disease progression in treatment-naive HIV-1-infected individuals? A systematic review of evidence: 1996–2010. J. Acquir. Immune Defic Syndr. 2012;59(4):382–8. doi: 10.1097/QAI.0b013e31824a0628. [DOI] [PubMed] [Google Scholar]
- 22.Tarosso LF, Sanabani SS, Ribeiro SP, Sauer MM, Tomiyama HI, Sucupira MC. Short communication: HIV type 1 subtype BF leads to faster CD4+ T cell loss compared to subtype B. AIDS Res. Hum. Retroviruses. 2014;30(2):190–4. doi: 10.1089/AID.2012.0243. [DOI] [PubMed] [Google Scholar]
- 23.Abecasis AB, Wensing AM, Paraskevis D, Vercauteren J, Theys K, Van De Vijver DA, Albert J, Asjo B, Balotta C, Beshkoy D, Camacho RJ, Clotet B, De Gascun C, Griskevicius A, Grossman Z, Hamouda O, Horban A, Kolupajeva T, Kom K, Kostrikis LG, Kucherer C, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer-Stockl E, Schmit JC, Sonnerborg A, Stanekova D, Stanojevic M, Struck D, Boucher CA, Vandamme AM. HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology. 2013;10:7. doi: 10.1186/1742-4690-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunar MM, Vandamme AM, Tomažič J, Karner P, Vovko TD, Pečavar B. Bridging epidemiology with population genetics in a low incidence MSM-driven HIV-1 subtype B epidemic in Central Europe. BMC Infectious Diseases. 2015;15:65. doi: 10.1186/s12879-015-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niculescu I, Paraschiv S, Paraskevis D, Abagiu A, Batan I, Banica L, Otelea D. Recent HIV-1 outbreak among intravenous drug users in Romania: evidence for cocirculation of CRF14_BG and subtype F1 strains. AIDS Res. Hum. Retroviruses. 2014 doi: 10.1089/aid.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazennova E, Laga V, Lapovok I, Glushchenko N, Neshumaev D, Vasilyev A, Bobkova M. HIV-1 genetic variants in the Russian Far East. AIDS Res. Hum. Retroviruses. 2014;30(8):742–52. doi: 10.1089/aid.2013.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]