Abstract
Background:
Prior data on iron deficiency anemia’s (IDA) prevalence and associated risk factors among female university students are scarce in the Saudi Arabian context. This study therefore recruited a sample of female students at the University of Tabuk, Saudi Arabia, to investigate IDA prevalence and risk factors and fill the identified research gap.
Methods:
A cross-sectional study of 200 apparently healthy female students aged between 19 and 25 years was performed between February and June 2016. Data on the participants’ sociodemographics, diet, health, anthropometry, and hematological and biochemical iron status indices were gathered. A logistic regression analysis then revealed the IDA risk factors.
Results:
The IDA prevalence was 12.5%. The factors associated via logistic regression with an elevated anemia risk were inadequate iron and vitamin C intakes, infrequent (≤2 times per week) consumption of red meat, frequent (≥2 times per week) tea consumption, and a past personal history of IDA.
Conclusions:
The findings suggest that focused education and awareness strategies are needed to improve nutritional habits by encouraging the consumption of rich dietary iron sources and by raising awareness of the food and drinks which facilitate or hinder the bioavailability of iron.
Keywords: Iron deficiency anemia, prevalence, risk factors, female university students, Saudi Arabia
Introduction
Anemia is a worldwide public health issue affecting approximately 1.62 billion sufferers or approximately a quarter of the world’s population in both developed and developing states.1,2 Many different forms of anemia exist, and among these, iron deficiency anemia (IDA) is the most widespread.3,4 Indeed, IDA has reached epidemic levels in numerous developing countries5 and is currently the most prevalent micronutrient deficiency in the world.6,7 Iron deficiency anemia has extremely negative implications for affected individuals, who are at risk of impaired growth and cognitive development, lower mental and motor function, poorer work capacity, and a generally lower quality of life.8
Iron deficiency is caused by a prolonged negative imbalance between a person’s dietary intake of iron and their body’s physiological demand.8,9 Various nonmodifiable and modifiable factors exert an influence on an individual’s iron balance, either in combination or alone,10 ranging from sociodemographic characteristics (including the individual’s age, sex, marital status, level of education, income, and ethnicity) to the amount and quality of the food and beverages they consume, their mental and physical health, the medication they take, any abnormalities they have, and their genetic makeup.10–12
It is particularly true for women that if IDA is not promptly identified and treated, it has lifelong effects, including negative maternal and neonatal outcomes, and an elevated risk of disability in later life.13 Information on IDA rates and the risk factors associated with the condition is scarce for female Saudi Arabian university students. In the only relevant previous study which could be located, Al Hassan14 reported an estimated prevalence of IDA of 64% for a sample of female university students of Saudi nationality, but their research omitted any consideration of the potential risk factors connected with IDA. Because of this gap in the prior research, this work aimed to recruit and evaluate a sample of female university students in Tabuk, Saudi Arabia, to establish their prevalence of IDA and the risk factors associated with the condition.
Methods
Subjects
This cross-sectional observational study was performed between February and June 2016 with a sample of 200 healthy women aged between 19 and 25 years, who were studying at the medicine, science, and education colleges of the University of Tabuk, Saudi Arabia. The study sample excluded students who reported having been diagnosed with an eating disorder, those who were currently pregnant or breastfeeding, those who were taking medication or nutritional supplements, and those not enrolled to study at the university at the time of recruitment. All the participants provided written informed consent acknowledging the investigation’s purpose and were assured of the confidentiality of the results. Ethical approval for the research was sought and obtained from the University of Tabuk’s Committee of Research Ethics.
Data collection
The present research was conducted on the campus of the University of Tabuk, Saudi Arabia. The data were collected at various locations which had been specifically chosen for their convenience to students across all the university’s colleges. A team comprising a medical doctor, a dietician, 2 nurses, and 2 laboratory technicians performed the dietary and anthropometric data collection and blood sample extraction.
Interview questionnaire
Each participant was interviewed to complete the structured questionnaire which had been developed by the researchers based on the large body of relevant prior literature to meet the objectives of the research.10–12,15–17 The questionnaire covered 5 main topics: the participants’ personal sociodemographic data (eg, their age, marital status, and monthly household income); dietary information relating to their intakes of iron-rich foods and of iron absorption–inhibiting or enhancing foods, and whether or not they followed particular dietary regimens; their obstetric and gynecological history (ie, their menstrual history, including its frequency and duration; their usual menstrual flow during their cycle; whether or not they encountered blood clotting; their use of oral and other contraceptives, and if so, how long they had done so; and their number of children and the birth intervals between them); any current or past diagnoses of medical conditions (eg, chronic diseases, blood disorders, or blood transfusions); their history of smoking (if any); and, finally, their personal and family histories of IDA.
Before the participant interviews were conducted to complete the questionnaires, a pilot study was performed with a sample of 20 students to test the design of the questionnaire and to gather feedback which could be used to make any alterations which might be needed. This pilot study also established the time required for questionnaire completion, which was measured at an average of 15 minutes.
Dietary intake data collection
A trained dietician administered a 24-hour dietary recall to estimate each participant’s nutrients intake by asking them to report all the foods, drinks, condiments, and sauces they had consumed during the day prior to the interview. In each interview, different serving plates, cups, glasses, and spoons were shown to the participant to assist them in estimating the portion sizes of the foods and drinks they had consumed. Before completing the 24-hour dietary recall, the dietician checked whether the participant regarded the previous 24 hours as having been typical in terms of their diet. If they did not (due, eg, to having attended a party or family gathering or to having dined out at a restaurant rather than at home), then they were instead asked to report on their diet 2 days prior to the interview date to capture data for a typical day. Tinuviel, WISP version 4.0 nutritional analysis software (Warrington, UK) was then used to analyze the dietary data captured by the questionnaires. In instances where nutritional information was unavailable in the software, other sources (eg, manufacturer product labels or information supplied by fast-food companies) were consulted. The adequacy or otherwise of each participant’s food consumption was then determined using dietary reference intake.18
Anthropometric data collection
Each participant’s weight was measured to the nearest 100 g using medical weighing scales (Seca Ltd, Hamburg, Germany) while they were barefoot and wearing minimal outer clothing. To ensure accuracy of measurement, the scales were calibrated at regular intervals as per the manufacturer’s guidelines and checked for zero readings prior to each measurement. Every participant’s height was also measured to the nearest 0.5 cm using a portable height measure (Seca Ltd), and their body mass index was worked out via the formula: body mass (kg)/height (m2) in line with the World Health Organization’s (WHO) criteria for the classification of overweight and obesity.19
Blood sample collection
On the same day when the dietary and anthropometric data were collected, a nurse took a 10-mL sample of venous blood from each participant for hematological and biochemical screening tests. The laboratory technicians in the research team used 2 types of vacutainers to perform venipunctures; one contained EDTA for hematological tests, including hemoglobin (Hgb), hematocrit, mean corpuscular hemoglobin, mean corpuscular volume, and mean corpuscular hemoglobin concentration. These were performed using a Beckman coulter LH750 machine (Beckman Coulter Inc., Miami, FL, USA) which was regularly checked and calibrated during the study according to the standard quality assurance protocols set at the beginning of the research. In the case of the biochemical screening, the participants’ blood was collected using vacutainers without any additional anticoagulant, so each blood sample was allowed to clot as it was assigned to serum separation. Serum iron, ferritin, and total iron capacity were each measured using a modular machine (Hitachi, Finchampstead, UK), which, again, was regularly checked and calibrated during the study as per the standard quality assurance protocols set at the beginning of the research.
Data analysis
The collected data were analyzed using the SPSS for Windows program (version 23.0, SPSS, Chicago, IL, USA). The level of statistical significance was set at P < .05. A univariate logistic regression analysis was chosen as the screening method to assess the relationships between the respective putative predictors and the outcome variable (anemia), which were categorized into either anemic or nonanemic in line with the WHO’s criteria, as ferritin <15 ng/mL and hemoglobin <12 g/dL.20 The predictor variables identified as significant by the univariate logistic analysis were then entered into a multiple logistic regression model to establish which of them could most accurately predict occurrences of IDA among the study’s sample of participants. The odds ratios (ORs) and their 95% confidence intervals (CIs) for each of the variables were then generated to clarify the respective association of each of the risk factors with the participants’ anemia status.
Results
In all, 200 female students of Saudi nationality enrolled at the University of Tabuk consented to participate in this research. Their ages ranged between 19 and 25 years, with a mean average age of 21.8 years. The study population’s overall prevalence of IDA was found to be 12.5% (Figure 1).
Figure 1.
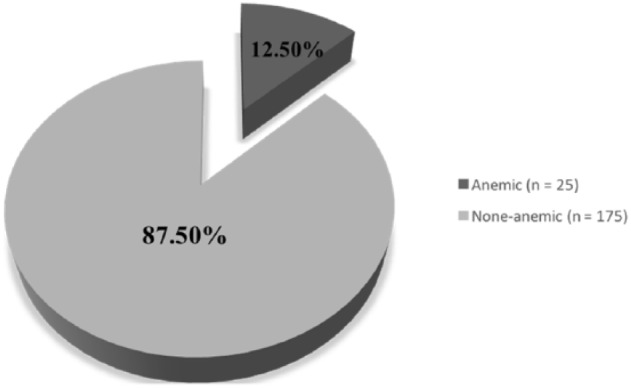
Prevalence of iron deficiency anemia (IDA) among the study sample of female students (n = 200).
Table 1 shows the unadjusted ORs for the factors associated with IDA among the research sample of Saudi Arabian female students. The univariate analysis found that insufficient iron (OR = 6.08; 95% CI: 2.40-15.44) and vitamin C intakes (OR = 4.44; 95% CI: 1.87-10.55), frequent (≥2 times per week) tea consumption (OR = 0.10; 95% CI: 0.04-0.27), infrequent (≤2 times per week) red meat consumption (OR = 7.70; 95% CI: 2.91-20.39), reports of blood clots with periods (OR = 4.75; 95% CI: 1.99-11.36), personal histories of IDA (OR = 7.37; 95% CI: 3.00-18.11), and family histories of IDA (OR = 3.27; 95% CI: 1.39-7.70) were each significantly associated with an increased risk of IDA for the participating students.
Table 1.
Univariate logistic regression analysis of the factors associated with iron deficiency anemia among the study sample of female students (n = 200).
| Variables | Anemic (n = 25, 12.5%) |
Nonanemic (n = 175, 87.5%) |
Unadjusted |
|---|---|---|---|
| No. (%) | No. (%) | OR (95% CI) | |
| Sociodemographic information | |||
| Age, y | |||
| 19-22 | 16 (14.3) | 96 (85.7) | 1.46 (0.61-3.49) |
| >22-≥25 | 9 (10.2) | 79 (89.8) | 1 (Ref.) |
| Marital status | |||
| Single | 14 (16.1) | 73 (83.9) | 1.78 (0.76-4.14) |
| Married | 11 (9.7) | 102 (90.3) | 1 (Ref.) |
| Monthly household income, SR | |||
| <5000 | 5 (12.8) | 34 (87.2) | 0.96 (0.23-3.92) |
| 5000-15 000 | 16 (12.2) | 115 (87.8) | 0.90 (0.28-2.93) |
| >15 000 | 4 (13.3) | 26 (86.7) | 1 (Ref.) |
| Body mass index, kg/m2 | |||
| <18 | 5 (17.2) | 24 (82.8) | 1.48 (0.45-4.87) |
| ≥25 | 11 (11.2) | 87 (88.8) | 0.90 (0.35-2.30) |
| 18-24.9 | 9 (12.3) | 64 (87.7) | 1 (Ref.) |
| Participants’ dietary data | |||
| Intake of iron | |||
| < Recommended intake | 18 (25.7) | 52 (74.3) | 6.08 (2.40-15.44) |
| ≥ Recommended intake | 7 (5.4) | 123 (94.6) | 1 (Ref.) |
| Intake of vitamin C | |||
| < Recommended intake | 14 (26.4) | 39 (73.6) | 4.44 (1.87-10.55) |
| ≥ Recommended intake | 11 (7.5) | 136 (92.5) | 1 (Ref.) |
| Intake of protein | |||
| < Recommended intake | 8 (20.0) | 32 (80.0) | 2.10 (0.84-5.30) |
| ≥ Recommended intake | 17 (10.6) | 143 (89.4) | 1 (Ref.) |
| Intake of energy | |||
| < Estimated average requirement | 6 (20.7) | 23 (79.3) | 2.09 (0.76-5.77) |
| ≥ Estimated average requirement | 19 (11.1) | 152 (88.9) | 1 (Ref.) |
| Frequency of tea consumption | |||
| ≤2 times a week | 5 (3.8) | 126 (96.2) | 0.10 (0.04-0.27) |
| ≥3 times a week | 20 (29.0) | 49 (71.0) | 1 (Ref.) |
| Frequency of citrus fruit consumption | |||
| ≤2 times a week | 7 (14.6) | 41 (85.4) | 1.27 (0.50-3.26) |
| ≥3 times a week | 18 (11.8) | 134 (88.2) | 1 (Ref.) |
| Frequency of red meat consumption | |||
| ≤2 times a week | 19 (27.1) | 51 (72.9) | 7.70 (2.91-20.39) |
| ≥3 times a week | 6 (4.6) | 124 (95.4) | 1 (Ref.) |
| Frequency of poultry consumption | |||
| ≤2 times a week | 13 (18.8) | 56 (81.2) | 2.30 (0.99-5.37) |
| ≥3 times a week | 12 (9.2) | 119 (90.8) | 1 (Ref.) |
| Frequency of fish consumption | |||
| ≤2 times a week | 21 (14.4) | 125 (85.6) | 2.10 (0.69-6.43) |
| ≥3 times a week | 4 (7.4) | 50 (92.6) | 1 (Ref.) |
| Participants following a dietary regime | |||
| Yes | 4 (10.3) | 35 (89.7) | 0.76 (0.25-2.36) |
| No | 21 (13.0) | 140 (87.0) | 1 (Ref.) |
| Smoking | |||
| Yes | 1 (14.3) | 6 (85.7) | 1.17 (0.14-10.17) |
| No | 24 (12.4) | 169 (87.6) | 1 (Ref.) |
| Participants’ menstrual history | |||
| Menstruation frequency | |||
| Once per month | 9 (17.3) | 43 (82.7) | 1.73 (0.71-4.19) |
| Twice or more per month | 16 (10.8) | 132 (89.2) | 1 (Ref.) |
| Menstruation duration, d | |||
| ≤7 | 11 (10.2) | 97 (89.8) | 0.63 (0.27-1.47) |
| ≥8 | 14 (15.2) | 78 (84.8) | 1 (Ref.) |
| Blood clotting during menstruation | |||
| Yes | 15 (26.3) | 42 (73.7) | 4.75 (1.99-11.36) |
| No | 10 (7.0) | 133 (93.0) | 1 (Ref.) |
| Heavy flow of menstrual blood | |||
| Yes | 12 (19.4) | 50 (80.6) | 2.31 (0.99-5.40) |
| No | 13 (9.4) | 125 (90.6) | 1 (Ref.) |
| Participants’ obstetric history | |||
| No. of children | |||
| <5 | 20 (11.6) | 153 (88.4) | 0.58 (0.20-1.69) |
| ≥5 | 5 (18.5) | 22 (81.5) | 1 (Ref.) |
| Birth interval, y | |||
| ≤1 | 6 (17.6) | 28 (82.4) | 1.66 (0.61-4.52) |
| >1 | 19 (11.4) | 147 (88.6) | 1 (Ref.) |
| Use of oral contraceptive pills | |||
| Yes | 5 (11.6) | 38 (88.4) | 0.90 (0.32-2.56) |
| No | 20 (12.7) | 137 (87.3) | 1 (Ref.) |
| Duration of use of oral contraceptives, y | |||
| <2 | 4 (13.3) | 26 (86.7) | 1.09 (0.35-3.44) |
| ≥2 | 21 (12.4) | 149 (87.6) | 1 (Ref.) |
| Use of intrauterine contraceptive device | |||
| Yes | 3 (13.6) | 19 (86.4) | 1.12 (0.31-4.10) |
| No | 22 (12.4) | 156 (87.6) | 1 (Ref.) |
| Participants’ medical history | |||
| Chronic disease | |||
| Yes | 2 (10.5) | 17 (89.5) | 0.81 (0.18-3.73) |
| No | 23 (12.7) | 158 (87.3) | 1 (Ref.) |
| Blood disorders | |||
| Yes | 1 (14.3) | 6 (85.7) | 1.17 (0.14-10.17) |
| No | 24 (12.4) | 169 (87.6) | 1 (Ref.) |
| Blood transfusion | |||
| Yes | 3 (17.6) | 14 (82.4) | 1.57 (0.42-5.90) |
| No | 22 (12.0) | 161 (88.0) | 1 (Ref.) |
| Past personal history of iron deficiency anemia | |||
| Yes | 16 (32.0) | 34 (68.0) | 7.37 (3.00-18.11) |
| No | 9 (6.0) | 141 (94.0) | 1 (Ref.) |
| Past family history of iron deficiency anemia | |||
| Yes | 14 (22.2) | 49 (77.8) | 3.27 (1.39-7.70) |
| No | 1 (8.0) | 126 (92.0) | 1 (Ref.) |
Abbreviations: CI, confidence interval; OR, odds ratio; Ref., reference; SR, Saudi riyal.
The only factors which emerged as statistically significant from the adjusted logistic regression analysis model (Table 2) were insufficient intakes of iron (OR = 7.39; 95% CI: 1.45-37.57) and vitamin C (OR = 6.14; 95% CI: 1.34-28.27), frequent (≥2 times per week) tea consumption (OR = 0.01; 95% CI: 0.01-0.08), infrequent (≤2 times per week) red meat consumption (OR = 3.71; 95% CI: 1.01-13.61), and the possession of a personal history of IDA (OR = 6.00; 95% CI: 1.45-24.76).
Table 2.
Multivariate logistic regression analysis of the factors associated with iron deficiency anemia among the study sample of female students (n = 200).
| Variables | Adjusted |
|---|---|
| OR (95% CI) | |
| Intake of iron | |
| < Recommended intake | 7.39 (1.45-37.57) |
| ≥ Recommended intake | 1 (Ref.) |
| Intake of vitamin C | |
| < Recommended intake | 6.14 (1.34-28.27) |
| ≥ Recommended intake | 1 (Ref.) |
| Frequency of tea consumption | |
| ≤2 times a week | 0.01 (0.01-0.08) |
| ≥3 times a week | 1 (Ref.) |
| Frequency of red meat consumption | |
| ≤2 times a week | 3.71 (1.01-13.61) |
| ≥3 times a week | 1 (Ref.) |
| Blood clotting during menstruation | |
| Yes | 1.66 (0.42-6.47) |
| No | 1 (Ref.) |
| Past personal history of iron deficiency anemia | |
| Yes | 6.00 (1.45-24.76) |
| No | 1 (Ref.) |
| Past family history of iron deficiency anemia | |
| Yes | 1.04 (0.26-4.17) |
| No | 1 (Ref.) |
Abbreviations: CI, confidence interval; OR, odds ratio; Ref., reference.
Discussion
The present research focused on establishing the prevalence of IDA among female university students of Saudi nationality and identifying the risk factors associated with that condition. This demographic group (ie, women of childbearing age) is at a heightened risk of deficiency in comparison with the general population due to their greater nutritional needs for the maintenance of their metabolic stores and because of their potentially high nutritional demands due to menstrual blood loss, pregnancy, and/or lactation.21–23
The study observed an overall IDA prevalence of 12.5%, a significantly lower rate than that found by a prior study among a sample of female Saudi university students, which reported a prevalence of IDA of 64% (defined as hemoglobin <12 g/dL).14 In developing countries, the overall prevalence of anemia has been estimated at 43%, but in highly developed countries, it has been reported at a far lower level of 9%.24 To take an example, research in an Indian setting reported a prevalence of 44.0% among female university students,25 and widespread IDA has also been found among female students in other developing countries such as Bangladesh, where 63.3% of a female student sample was found to have IDA.22 In contrast, in Australia, a developed country, only a 3% prevalence of IDA was found by a study using a sample of female university students.21
This research has found that most of the female students in the study sample who were anemic reported inadequate intakes of iron, along with a lower level of consumption (≤2 times per week) of red meat. These 2 factors were found to be associated with a statistically significant increased risk of IDA. In the human diet, iron exists either in the form of heme or nonheme iron; the former is mostly consumed via meat, with a rate of absorption of up to 50%, whereas the latter is mainly found in dairy products, fruit, and vegetables, and its variable level of absorption depends on the enhancers and inhibitors present.26,27 Red meat is a key source of bioavailable heme iron in human diets,28 and various prior studies have identified a negative association between low levels of red meat consumption and a heightened risk of IDA.10,11,16 For example, a study by AlQuaiz et al10 of the risk factors among women of childbearing age in Riyadh, Saudi Arabia, in relation to anemia reported that infrequent (≤2 times per week) meat consumption (OR = 1.54, 95% CI: 1.15-2.05) was associated with an increased anemia risk. The relatively high cost of meat can prevent poor families living in developing countries from regularly buying and consuming it.29 For instance, a study in the context of Turkey reported that participants from poorer households had lower consumption rates of red meat and fish and therefore also lower intakes of easily bioavailable sources of iron.30 Its authors proposed that advice targeting the poorest members of Turkey’s society on how to reduce their risk of insufficient iron intake should encourage them to consume rich dietary iron sources such as red meat, poultry, and fish. For this to have a positive impact, practical low-cost campaigns are likely to be required to increase poor families’ access to highly bioavailable iron as part of their diets.
Because any examination of the dietary contribution to the cause of IDA must evaluate the possible dietary factors which inhibit or enhance iron absorption,30 the present research also explored the dietary factors influencing the absorption and subsequent bioavailability of iron. In doing so, it found that most of the anemic group in the study had inadequate intakes of vitamin C, a factor inversely associated with anemia (OR = 6.14; 95% CI: 1.34-28.27). Vitamin C (ascorbic acid) has previously been identified as a strong iron absorption enhancer for nonheme foods,31 and citrus fruit has a high vitamin C content.32 Previous research has associated low citrus fruit consumption with an elevated risk of anemia.11,12 In terms of the iron absorption–inhibiting dietary factors, frequent (≥2times per week) tea consumption (OR = 0.01; 95% CI: 0.01-0.08) was found to be associated with a heightened risk of anemia. This finding is in line with previous studies which have found that anemic groups reported higher levels of tea consumption,11,12,16,33 and tea is a beverage which harmfully affects an individual’s iron levels due to its high polyphenol content, which inhibits nonheme iron absorption.3 Furthermore, tea drinking is a popular custom across Muslim countries, including Saudi Arabia, and it is usually consumed both before and after all meals.30,34,35 Educational campaigns aiming to raise general awareness of the food and drinks that can facilitate or restrict iron absorption would therefore help to lower the prevalence of IDA.
The present research also found that a personal history of IDA was a statistically significant determinant of anemia. However, it should be explained in this respect that having examined various nondietary factors (ie, personal sociodemographic variables, individual obstetric and gynecological history variables, and medical history variables), the personal history of IDA was the sole statistically significant nondietary factor found by the present research. Although some prior studies in the Saudi Arabian context have reported a significant association between the risk of IDA and a past personal history of IDA,11,16 2 research papers in the same context did not identify that association.10,11 Instead, the latter 2 studies identified that an individual with a past family history of IDA was at a significantly heightened risk of IDA themselves. It is therefore strongly recommended that screening for IDA is provided to those with either prior personal or family histories of IDA.
Some limitations are inherent in the present research. First, no causal associations could be established between the influencing factors and personal IDA due to the cross-sectional nature of the study. Second, the student participants who formed the study sample were all volunteers, so they may not be taken to represent the wider female student population in Saudi Arabia. Finally, even though a 24-hour dietary recall method is suitable for gathering detailed data regarding the amounts and types of food and beverages which a person has consumed on a specific day, a single day’s data on personal dietary intake may not sufficiently closely reflect a typical day. However, this potential limitation was minimized in the study by checking with each participant that they regarded their dietary intake on the day prior to the study as typical. If they did not, then they were instead asked to report on their diet 2 days before the date of the interview.
Conclusions
This study found an overall prevalence of IDA among its sample of apparently healthy young Saudi female university students of 12.5%. The study also reported that the main risk factors in relation to contracting anemia were inadequate intakes of iron and vitamin C, frequent tea consumption, infrequent red meat consumption, and a past personal history of IDA. The findings presented here suggest a need for focused education and awareness strategies designed to improve nutritional habits by encouraging the consumption of rich sources of iron in the diet (eg, red meat), as well as by building understanding of which food and beverages can improve (eg, vitamin C–rich foods) and hinder (eg, polyphenol-rich beverages, such as tea) iron bioavailability. The current work has set a benchmark for possible future research in the form of randomized trials which would help to build greater understanding of this health issue and would also support the development of a strong and suitable public health policy which can efficiently tackle IDA.
Acknowledgments
The authors of this study would like to thank all the students who participated in the research for agreeing to do so. They are also grateful to the team who offered their assistance in collecting the data.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RAA and OA-A designed this study. RAA was responsible for the data analyses and the drafting of the manuscript, and OA-A was responsible for the study protocol and fieldwork. Both authors contributed to the interpretation of the results and approved the final manuscript.
References
- 1. World Health Organization. Worldwide Prevalence of Anemia 1993 to 2005. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 2. Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102:1585–1594. [DOI] [PubMed] [Google Scholar]
- 3. Anand T, Rahi M, Sharma P, Ingle GK. Issues in prevention of iron deficiency anemia in India. Nutrition. 2014;30:764–770. [DOI] [PubMed] [Google Scholar]
- 4. Shander A, Goodnough LT, Javidroozi M, et al. Iron deficiency anemia—bridging the knowledge and practice gap. Transfus Med Rev. 2014;28:156–166. [DOI] [PubMed] [Google Scholar]
- 5. Sirdah MM, Yaghi A, Yaghi AR. Iron deficiency anemia among kindergarten children living in the marginalized areas of Gaza Strip, Palestine. Rev Bras Hematol Hemoter. 2014;36:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandyo RK, Henjum S, Ulak M, et al. The prevalence of anemia and iron deficiency is more common in breastfed infants than their mothers in Bhaktapur, Nepal. Eur J Clin Nutr. 2016;70:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abu-Ouf NM, Jan MM. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med J. 36:2015;2:146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 9. Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S-1467S. [DOI] [PubMed] [Google Scholar]
- 10. Alquaiz AM, Gad MA, Khoja TA, et al. Prevalence of anemia and associated factors in child bearing age women in Riyadh, Saudi Arabia. J Nutr Metab. 2013;636585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Quaiz JM. Iron deficiency anemia. A study of risk factors. Saudi Med J. 2001;22:490–496. [PubMed] [Google Scholar]
- 12. Alquaiz AJ, Khoja TA, Alsharif A, et al. Prevalence and correlates of anaemia in adolescents in Riyadh city, Kingdom of Saudi Arabia. Public Health Nutr. 2015;18:3192–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev. 2013;71:35–51. [DOI] [PubMed] [Google Scholar]
- 14. Al Hassan NN. The prevalence of iron deficiency anemia in Saudi University female students. J Microsc Ultrastruct. 2015;3:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bekele A, Tilahun M, Mekuria A. Prevalence of anemia and its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch Town Gamo Gofa Zone, Ethiopia: a cross-sectional study. Anemia. 2016;2016:1073192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Sayes F, Gari M, Qusti S, Bagatian N, Abuzenadah A. Prevalence of iron deficiency and iron deficiency anemia among females at university stage. J Med Lab Diagn. 2011;2:5–11. [Google Scholar]
- 17. Abdelhafez AM, El-Soadaa SS. Prevalence and risk factors of anemia among a sample of pregnant females attending primary health care centers in Makkah, Saudi Arabia. Pak J Nutr. 2012;11:1113–1120. [Google Scholar]
- 18. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 19. World Health Organization (WHO). Global database on Body Mass Index. http://www.assessmentpsychology.com/icbmi.htm. Accessed June 18, 2016.
- 20. World Health Organization. Iron Deficiency Anemia Assessment, Prevention and Control. A Guide for Programme Managers (Document WHO/NHD/01.3). Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 21. Fayet-Moore F, Petocz P, Samman S. Micronutrient status in female university students: iron, zinc, copper, selenium, vitamin B12 and folate. Nutrients. 2014;6:5103–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shill KB, Karmakar P, Kibria MG, et al. Prevalence of iron-deficiency anaemia among university students in Noakhali region, Bangladesh. J Health Popul Nutr. 2014;32:103–110. [PMC free article] [PubMed] [Google Scholar]
- 23. Shams S, Asheri H, Kianmehr A, et al. The prevalence of iron deficiency anaemia in female medical students in Tehran. Singapore Med J. 2010;51:116–119. [PubMed] [Google Scholar]
- 24. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12:444–454. [DOI] [PubMed] [Google Scholar]
- 25. Bano R, Ahmad N, Sharma BC, Agarwal A. Nutritional anemia in the medical students. Indian Med Gaz. 2012;1:16–18. [Google Scholar]
- 26. McDermid JM, Lönnerdal B. Iron. Adv Nutr. 2012;3:532–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–1467S. [DOI] [PubMed] [Google Scholar]
- 28. Hooda J, Shah A, Zhang L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients. 2014;6:1080–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhargava A, Bouis H, Scrimshaw N. Dietary intakes and socioeconomic factors are associated with the hemoglobin concentration of Bangladeshi women. J Nutr. 2001;131:758–764. [DOI] [PubMed] [Google Scholar]
- 30. Keskin Y, Moschonis G, Dimitriou M, et al. Prevalence of iron deficiency among school children of different socio-economic status in urban Turkey. Eur J Clin Nutr. 2005;59:64–71. [DOI] [PubMed] [Google Scholar]
- 31. Chiplonkar SA, Agte VV, Mengale SS, Tarwadi KV. Are lifestyle factors good predictors of retinol and vitamin C deficiency in apparently healthy adults? Eur J Clin Nutr. 2002;56:96–104. [DOI] [PubMed] [Google Scholar]
- 32. Cook J, Reddy M. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am J Clin Nutr. 2001;73:93–98. [DOI] [PubMed] [Google Scholar]
- 33. Sato APS, Fujimori E, Szarfarc SC, Borges ALV, Tsunechiro MA. Food consumption and iron intake of pregnant and reproductive aged women. Revista Latino-Americana de Enfermagem. 2010;18:247–254. [DOI] [PubMed] [Google Scholar]
- 34. Al-Othaimeen A, Osman A, al Orf S. Prevalence of nutritional anaemia among primary school girls in Riyadh City, Saudi Arabia. Int J Food Sci Nutr. 1999;50:237–243. [DOI] [PubMed] [Google Scholar]
- 35. Musaiger AO. Iron deficiency anaemia among children and pregnant women in the Arab Gulf countries: the need for action. Nutr Health. 2002;16:161–171. [DOI] [PubMed] [Google Scholar]


