Abstract
Mast cells are multifunctional immune cells that participate in many important processes such as defense against pathogens, allergic reactions, and tissue repair. These cells perform their functions through the release of a wide variety of mediators. This release occurs mainly through cross-linking IgE (immunoglobulin E) bound to high affinity IgE receptors by multivalent antigens. The abundance of mast cells in connective tissue, surrounding blood vessels, and their involvement in the early stages of bone repair support the possibility of physiological and pathological interactions between mast cells and osteoblasts. However, the participation of mast cell mediators in osteogenesis is not fully understood. Therefore, the objective of this work was to investigate the role of mast cell mediators in the acquisition of the osteogenic phenotype in vitro. The results show that pooled mast cell mediators can affect proliferation, morphology, and cytoskeleton of osteoblastic cells, and impair the activity and expression of alkaline phosphatase as well as the expression of bone sialoprotein. Also, mast cell mediators inhibit the expression of mRNA for those proteins and inhibit the formation and maturation of calcium nodules and consequently inhibit mineralization. Therefore, mast cell mediators can modulate osteogenesis and are potential therapeutic targets for treatments of bone disorders.
Keywords: Alizarin red, bone remodeling, cell shape, co-culture, energy dispersive x-ray spectrometry, fluorescence microscopy, rat
Introduction
Mast cells are multifunctional immune cells with important roles in innate and acquired immunity. These cells are found mainly in perivascular connective tissue and at the interface between the host and the external environment.1–5 Mast cell activation can be dependent or independent of the high affinity receptor for IgE (immunoglobulin E; FcεRI), located on the surface of mast cells.1–12 In FcεRI-dependent activation, the most well-studied form of activation, the interaction of multivalent antigens (allergens) with IgE bound to FcεRI cross-links the IgE-receptor complex, which results in aggregation of FcεRI. This aggregation initiates several biochemical events that culminate in the release of three classes of biologically active substances: preformed mediators, newly formed mediators, and newly synthesized mediators.4,8,10–14 Preformed mediators, such as histamine, heparin, β-hexosaminidase, TNF-α (tumor necrosis factor alpha), and SCF (stem cell factor), are stored in cytoplasmic granules and are released immediately after stimulation. Newly formed mediators such as PGD2 and PGE2 (prostaglandins D2 and E2), LTB4 and LTC4 (leukotrienes B4 and C4), PAF (platelet activing factor), and thromboxanes are derived from the metabolism of arachidonic acid through the activation of phospholipase A2 and are released at the plasma membrane immediately after activation. Newly synthesized mediators such as interleukins IL-3, IL-4, IL-5, and IL-6, and TGF-β (transforming growth factor-beta) are synthesized following transcription factor activation and released 8 to 12 hr after stimulation. Through the release of these mediators, mast cells participate in many biological events, such as host defense against pathogens, allergic reactions, and tissue repair.4,8,11–20
The exact interaction between mast cells and bone tissue cells is not well understood. Urist and McLean21 first noted the relationship between bone and mast cells in 1957 and since that time, there have been various studies trying to resolve the role mast cells play in bone remodeling.22–25 Because of the wide variety of substances secreted by mast cells,1–14 it is now recognized that they can exert an effect on non-immunological functions such as bone remodeling.23–26 Previous investigations have largely examined the effect of a single mast cell mediator on bone metabolism.4,27–33 In vivo, stimulated mast cells do not release just a single mediator, but rather they release a wide variety of mediators. Because some mast cell mediators stimulate osteoblast differentiation and mineralization and others inhibit this,30,31,34–36 the present in vitro study was undertaken to investigate and characterize the influence of a pool of mast cell mediators on osteoblastic cells and on the capacity of these cells to mineralize the extracellular matrix. The results of this investigation demonstrate that pooled mast cell mediators inhibit osteoblastic differentiation and extracellular matrix mineralization.
Materials and Methods
Cells
The rat mast cell line, RBL-2H3,37 and the rat osteoblastic cell line, UMR-106,38 were grown attached in tissue culture flasks (Greiner Bio-One North America, Inc.; Monroe, NC) in DMEM supplemented with 15% (RBL-2H3) or 10% (UMR-106) heat-inactivated fetal bovine serum (Sigma-Aldrich; St. Louis, MO), an antibiotic-antimycotic mixture containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 µg/mL amphotericin B (Gibco, Thermo Fisher; Waltham, MA), as previously described,39–41 and maintained at 37C in a humidified atmosphere containing 5% CO2 in air. For experiments, mast cells and osteoblastic cells were cultured in osteogenic medium, α-MEM (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich), 50 µg/mL gentamycin (Gibco), 5 µg/mL ascorbic acid (Gibco), and 7 mM β-glycerophosphate (Sigma-Aldrich).
Osteogenic Medium Containing Mediators Released by RBL-2H3 Mast Cells
To obtain mast cell mediators in osteogenic medium, the RBL-2H3 cells were adapted to osteogenic medium. RBL-2H3 cells were grown in 75% DMEM and 25% osteogenic medium for 3 days. Then, the cells were grown in 50% DMEM and 50% osteogenic medium for another 3 days, and the medium was then changed to 25% DMEM and 75% osteogenic medium, and, finally, after another 3 days, the RBL-2H3 cells were cultured only in osteogenic medium. Adapted RBL-2H3 cells were then cultured in osteogenic medium (1 × 106 cells/75 cm2 flask) and subsequently stimulated via FcεRI. Cells were sensitized with IgE anti-TNP (Trinitrophenol; courtesy of Dr. Reuben Siraganian, National Institute of Dental and Cranial Research, National Institutes of Health; Bethesda, MD) at a concentration of 1:5000 for 16 hr and stimulated with DNP48-HSA (Sigma-Aldrich) at a concentration of 50 ng/mL. After 24 hr, the culture supernatant was collected, centrifuged for 5 min at 129 × g and frozen at −20C. Preformed, newly formed, and newly synthesized mediators are all released after 24 hr.4,8 The released mediators were characterized using the Proteome Profiler Rat Cytokine Array Kit, Panel A (R&D Systems, Inc.; Minneapolis, MN), as previously described (Supplemental Fig. 1).42 Before use, the concentration of mediators in the osteogenic medium was normalized to the activity of released β-hexosaminidase per mL of osteogenic medium. To evaluate the influence of pooled mast cell mediators on the physiology of osteoblastic cells, the UMR-106 cells were cultured in DMEM with 10% fetal bovine serum, osteogenic medium, or in osteogenic medium containing mast cell mediators.
Assay for β-Hexosaminidase Activity
To confirm activation of RBL-2H3 cells cultured in osteogenic medium and also to standardize the concentration of mast cell mediators per mL of osteogenic medium, culture supernatants from stimulated RBL-2H3 cells were assayed for β-hexosaminidase activity. RBL-2H3 cells were stimulated for 24 hr, and 25 μL aliquots of osteogenic medium containing mast cell mediators were transferred to a 96-well plate. The adherent cells were solubilized in 1% Triton X-100 diluted in osteogenic medium, and 25 μL aliquots of the solubilized cells were also transferred to a 96-well plate. Then, 50 µL of 8 mM NAG (p-Nitrophenyl-N-acetyl-β-D-Glucosaminide; Sigma-Aldrich), in citrate buffer (0.1 M citric acid/sodium citrate), pH 4.5, was added to each well. The reaction was stopped by adding 25 µL of glycine buffer (0.4 M glycine, 0.4 M NaCl, pH 10). The β-hexosaminidase activity was determined by measuring the reaction product at 405 nm using a PowerWave X Plate Reader (Bio-Tek Instruments; Winooski, VT). The total amount of β-hexosaminidase activity (100%) was determined by the sum of the values of the supernatant and the solubilized cells from each well. The percentage of released β-hexosaminidase activity was then calculated from the reading of the supernatant in relation to the total value.
Co-cultures
Initially, to verify the influence of mast cells in osteogenesis, three proportions of UMR-106 cells and RBL-2H3 cells were co-cultured in DMEM or osteogenic medium: 20% mast cells (104 UMR-106 cells: 2 × 103 RBL-2H3 cells), 10% mast cells (104 UMR-106 cells: 103 RBL-2H3 cells), and 5% mast cells (104 UMR-106: 500 RBL-2H3 cells), for 4 and 7 days. RBL-2H3 cells were sensitized via FcεRI and stimulated with DNP48-HSA at days 0 and 3 of cultivation. UMR-106 cells alone were used as controls for the co-cultures. After 4 days, cells were analyzed by phase contrast microscopy, and after 7 days, cells were stained with Alizarin red, for detection of bone-like nodule formation (methods described below).
Cell Proliferation
UMR-106 cells were cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators at a concentration of 2 × 104 cells/well in 24-well plates (Corning Life Sciences; Tewksbury, MA). Cell proliferation was assessed after 1, 4, and 7 days in culture. The cells were washed twice with PBS, fixed with methanol (Dinâmica Química Contemporânea Ltda; Diadema, SP, Brazil) for 10 min, washed twice with PBS, and stained with 0.2% crystal violet (Grübler & Co.; Berlin, Germany) in 2% ethanol (Synth; Diadema, SP, Brazil) for 15 min. Then, the wells were washed 10 times with PBS, and the solution of 0.1 M sodium citrate in 50% ethanol was added. The plates were agitated for 30 min, and then 100 μL of supernatant from each well was transferred to another 96-well plate. The absorbance of the samples was measured by ELISA PowerWave X Plate Reader (Bio-Tek Instruments) at 550 nm.
Phase Contrast Microscopy
For co-cultures, the cells were plated in DMEM or osteogenic medium. For other experiments, UMR-106 cells (2 × 104 cells/well in 24-well plates) were plated in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators. Accordingly, unfixed co-cultures were observed after 4 days, and unfixed UMR-106 cells were observed after 1, 4, and 7 days in culture by phase contrast microscopy using a Nikon Eclipse TS100 inverted microscope (Nikon USA; Melville, NY) equipped with a Nikon DXM 1200 digital camera.
F-actin Staining
UMR-106 cells (2 × 104 cells/well in 24-well plates) were plated in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators, on glass coverslips placed in the wells of 24-well plates. After 1, 4, and 7 days, cells were fixed with 2% formaldehyde (Electron Microscopy Sciences; Hatfield, PA) in PBS for 15 min at room temperature, washed twice with PBS, and permeabilized with 0.3% Triton X-100 diluted in PBS for 10 min at room temperature. Subsequently, the cells were washed twice with PBS, once with PBS containing 0.1 M glycine (Sigma-Aldrich) for 5 min at room temperature, and again washed twice with PBS. Then, the cells were incubated for 50 min with phalloidin conjugated to Alexa Fluor 488 (Molecular Probes, Thermo Fisher) at a final concentration of 2.6 U/mL together with the nuclear marker DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride; Molecular Probes) at a final concentration of 0.2 μg/mL. Next, the cells were washed 5 times in PBS, rinsed quickly in Milli-Q water, and coverslips were mounted onto glass slides with Fluoromount G (Electron Microscopy Sciences). The samples were analyzed using an Olympus BX50F4 (Olympus Corporation of America; Waltham, MA) fluorescence microscope. The images were acquired with a SPOT RT3 digital camera (Diagnostic Instruments, Inc.; Sterling Heights, MI).
Alizarin Red Staining of Calcium Nodules
Co-cultures were plated in DMEM or osteogenic medium, and UMR-106 cells (2 × 104 cells/well in 24-well plates) were plated in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators, and allowed to grow for 7 days. Cells were washed in Hank’s Balanced Salt Solution (Sigma-Aldrich) and fixed with 70% ethanol in PBS for 1 hr at 4C. Then, the cells were washed in PBS followed by Milli-Q water and stained with 2% Alizarin red in ethanol for 15 min at room temperature. Cells were washed with Milli-Q water and dried at room temperature. The samples were analyzed using an Olympus BX50F4 (Olympus) fluorescence microscope. Images were acquired with a SPOT RT3 digital camera (Diagnostic Instruments, Inc.). For co-cultures, the Alizarin red extraction was performed as previously described.43
Scanning Electron Microscopy (SEM)
UMR-106 cells (2 × 104 cells/well) were plated in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators, on glass coverslips placed in the wells of 24-well plates. After 7 days, osteoblastic cells were washed in warm (37C) PBS, fixed in 2% glutaraldehyde (Electron Microscopy Sciences) in warm PBS (containing 0.90 mM Ca2+ and 0.50 mM Mg2+) for 2 hr at room temperature. After fixation, cells were washed twice with 0.1 M cacodylate buffer, pH 7.4, and post-fixed in 1% OsO4 (Electron Microscopy Sciences) in Milli-Q water for 2 hr at room temperature. Subsequently, cells were washed in Milli-Q water and incubated in a saturated solution of thiocarbohydrazide (Electron Microscopy Sciences) for 10 min at room temperature. After this, the samples were washed 5 times with Milli-Q water and then incubated in 1% OsO4 in Milli-Q water. The cells were then dehydrated in increasing ethanol solutions (30, 50, 70, 90, and 100%) and critically point dried using CO2 (BAL-TEC-DPC-030 Critical Point Dryer; Balzers, Germany). Each coverslip was then attached with silver paint (Electron Microscopy Sciences) to an aluminum stub and covered with gold in a sputter coater (BAL-TEC-CPD-050 Sputter Coater; Balzers, Germany). The samples were then observed in a JEOL JSM-6610 LV (Tokyo, Japan) scanning electron microscope.
Energy Dispersive X-Ray Spectrometry (EDS)
UMR-106 cells (2 × 104 cells/well) were cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators, on glass coverslips placed in the wells of 24-well plates. After 7 days, cells were rinsed in PBS and fixed for 60 min in 4% formaldehyde in PBS, pH 7.2, at room temperature. Subsequently, the cells were washed in PBS followed by Milli-Q water.44 After this process, the samples were analyzed by EDS (Oxford Instruments; Abingdon, Oxfordshire, UK) coupled to a JEOL JSM-6610LV scanning electron microscope equipped with a backscatter detector. Data were analyzed using Aztec 1.0 software (Oxford).
Immunoblotting
Lysate Preparation
UMR-106 cells were lysed in 0.5 mL lysis buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 0.1% SDS, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 15 µL/mL Protease Inhibitor Cocktail (Sigma-Aldrich)) at 4C for 20 min, followed by centrifugation (16,000 × g) for 15 min at 4C. The protein concentration of the lysates was determined by the Bradford method.45
SDS-PAGE and Western Blot
Cell lysates were added to the sample buffer (1:1) containing 5% β-mercaptoethanol and boiled for 5 min. 10 µg of protein was applied per lane to 7.5% polyacrylamide gels46 and electrophoresed under reducing and dissociating conditions using a miniVE Vertical Electrophoresis System (GE Healthcare; Piscataway, NJ), at 30 mA. After separation, the proteins were electrotransferred to nitrocellulose membranes by the method of Towbin et al.47 After transfer, the nitrocellulose membranes were stained with Ponceau Red (Sigma-Aldrich). The membranes were then washed in TBS/Tween (100 mM NaCl; 10 mM Tris-HCl, pH 8.0; and 0.05% Tween-20, v/v) and incubated for 1 hr with blocking buffer (TBS/Tween plus 4% BSA, w/v; Sigma-Aldrich). Then, the membranes were incubated for 1 hr with anti-alkaline phosphatase (ALP) antibody (sc-137213, Santa Cruz Biotechnology, Inc.; Dallas, TX) at 1:200 dilution in TBS/Tween containing 1% BSA (w/v) and subsequently with goat anti-mouse IgG-HRP (Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA) at 1:5000 dilution in TBS/Tween for 1 hr at room temperature. After incubation, the membranes were washed 10 times with TBS/Tween. Proteins were detected by chemoluminescence using the ECL kit (GE Healthcare). Controls included omission of the primary antibody and substitution of the primary antibody with normal mouse IgG. All controls were negative. The blots were scanned, and the optical densities of the bands were calculated using Adobe Photoshop (Adobe Systems, San Jose, CA).
Total Protein Measurement
UMR-106 cells (2 × 104 cells/well) were cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators in 24-well plates. After 1, 4, and 7 days, the total protein concentration was determined using the method of Lowry et al.48 Wells were washed with warm PBS (37C) and then with 2 mL of deionized water. The samples were then subjected to 5 cycles of thermal shock (20 min at −20C and for 15 min at 37C). At the end of the cycles, 1 mL from each well of the cell lysate was transferred to test tubes containing 1 mL of Lowry solution (Sigma-Aldrich) and allowed to stand at room temperature for 20 min. After this period, 0.5 mL of Folin and Ciocalteu’s phenol solution (Sigma-Aldrich) was added to each tube, and the tubes were allowed to stand for 30 min at room temperature. The absorbance of each tube was measured in a spectrophotometer (CE3021, Cecil, Cambridge, UK) at 680 nm, and the total protein concentration (μg/mL) for each well was calculated from a standard curve of bovine serum albumin (Sigma-Aldrich).
Measurement of ALP Activity
UMR-106 cells (2 × 104 cells/well) were cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators in 24-well plates. After 1, 4, and 7 days of culture, the ALP activity was assessed by measuring the release of thymolphthalein by the hydrolysis of the substrate thymolphthalein monophosphate, using the Alkaline Phosphatase Kit (Labtest Diagnostica SA; Belo Horizonte, MG, Brazil), according to manufacturer’s instructions. The activity of ALP in µmol thymolphthalein/h/mg protein was calculated by measuring a standard tube and normalized to total protein concentration.
Immunolocalization of Bone Sialoprotein (BSP)
UMR-106 cells (2 × 104 cells/well) were cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators, on glass coverslips placed in the wells of 24-well plates, for 7 days. The cells were then fixed in 2% formaldehyde (Electron Microscopy Sciences) in PBS for 15 min and then washed 3 times with PBS. Cells were permeabilized with 0.1% triton X-100 (Sigma-Aldrich) in PBS for 10 min at room temperature. Then, cells were blocked with PBS plus 1% BSA containing 5 μg/mL goat IgG (Jackson ImmunoResearch Laboratories, Inc.) for 15 min. After blocking, the cells were washed 3 times with PBS and incubated with rabbit anti-BSP antibody (Abcam; Cambridge, MA), at a final concentration of 2 μg/mL for 1 hr. After incubation, cells were washed 5 times with PBS and incubated with goat anti-rabbit IgG conjugated to Alexa 594 (Molecular Probes) at a final concentration of 2 μg/mL, for 30 min. Next, cells were washed 10 times in PBS, quickly rinsed in Milli-Q water, and coverslips were mounted with Fluoromount G (Electron Microscopy Sciences). The samples were analyzed with BX50F4 Olympus fluorescence microscope (Olympus). The images were acquired with a SPOT RT3 digital camera (Diagnostic Instruments, Inc.).
BSP Expression Analysis
The fluorescence intensity of the osteoblastic cells immunostained for BSP was quantified using Image-Pro Plus version 4.5.1 software (Media Cybernetics; Silver Spring, MD). For each experimental group, 10 fields were randomly selected using a 40× objective. The fields were thresholded, and the intensity of each cell was automatically measured, and the mean of fluorescence intensity was calculated by the program. The data are expressed as the average of 3 independent experiments.
Analysis of Gene Expression by Real-Time Polymerase Chain Reaction (PCR)
Quantitative real-time PCR was carried out to evaluate the gene expression of ALP, BSP, OPN (osteopontin), OC (osteocalcin), Runx2 (runt-related transcription factor 2), and OSX (osterix), in osteoblastic cells. UMR-106 cells (2 × 104 cells/well) were cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators in 24-well plates for 7 days. Total RNA was extracted with TRIzol (Invitrogen, Thermo Fisher Scientific), and the concentration was determined by reading the optical density at 230, 260, 280, and 320 nm (GE Healthcare; Milwaukee, WI). Complementary DNA (cDNA) was synthesized using 1 µg of RNA through a reverse transcription reaction (Applied Biosystems, Thermo Fisher Scientific) according to the manufacturer’s instructions. Real-time PCR was performed in a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories; Philadelphia, PA) using TaqMan probes (Applied Biosystems) for the target genes (Table 1). The standard PCR conditions were 50C (2 min), 95C (10 min), 40 cycles of 95C (15 sec), and 60C (1 min). The relative gene expression was calculated in reference to both β-actin and HPRT1 (hypoxanthine phosphoribosyltransferase 1) expression and its respective control using the cycle threshold (Ct) method.49
Table 1.
Probes for TaqMan Gene Expression Assay.
| Gene | Probe | Bp |
|---|---|---|
| Alpl | Rn01516028_m1 | 68 |
| Ibsp | Rn00561414_m1 | 138 |
| Spp1 | Rn01449972_m1 | 124 |
| Bglap | Rn00566386_g1 | 104 |
| Runx2 | Rn01512298_m1 | 86 |
| Sp7 | Rn02769744_s1 | 76 |
| ACTB | 4352931 | 91 |
| Hprt1 | Rn01527840_m1 | 64 |
Abbreviations: Alpl, alkaline phosphatase gene; Ibsp, bone sialoprotein gene; Spp1, osteopontin gene; Bglap, osteocalcin gene; Runx2, runt-related transcription factor 2 gene; Sp7, osterix gene; ACTB, β-actin gene; Hprt1, hypoxanthine phosphoribosyltransferase 1 gene.
Statistical Analysis
GraphPad Prism 5.0 (GraphPad Software; San Diego, CA) was used to compare experimental groups by one- or two-way ANOVA. Data were expressed as mean ± standard deviation of at least three independent experiments. p Values below 0.05 were considered significant.
Results
High Concentrations of Mast Cells Inhibit Mineralization
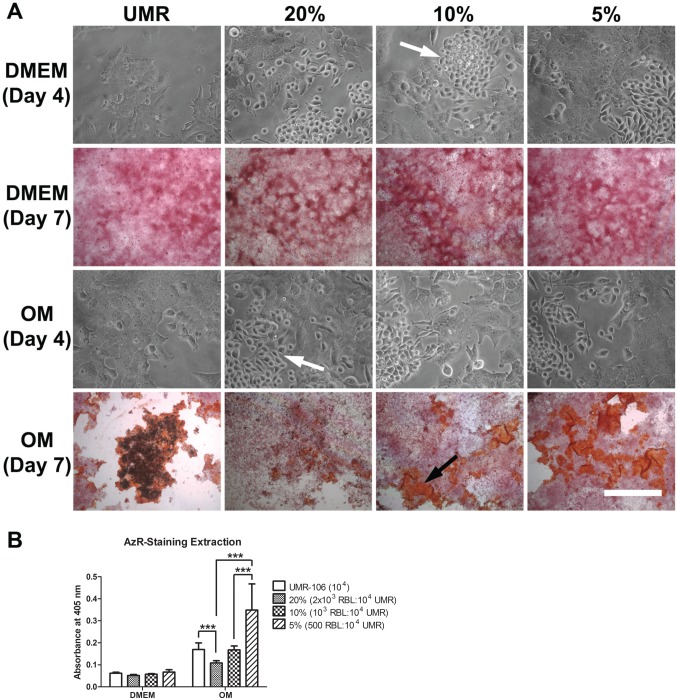
To assess the influence of mast cells on osteoblast mineralization, osteoblastic cells were co-cultured with various concentrations of RBL-2H3 mast cells (Fig. 1) in either DMEM or osteogenic medium. By phase contrast microscopy, it was possible to see that in either medium, the mast cells and the osteoblastic cells tended to grow in clusters independent of each other. In DMEM, mineralization was not detected either with or without the presence of mast cells. In contrast, in osteogenic medium in the presence of mast cells, the pattern of mineralization was altered. When the osteoblastic cells were cultured without mast cells, the structure of the mineralized nodules was dense and compact. In the presence of mast cells, the nodules were more dispersed, less compact, and there were fewer dense nodules. Furthermore, there was a significant inhibition of mineralization when the mast cell concentration reached 20% in the co-cultures. There was also an inverse ratio between the percent of mast cells in the co-cultures and the area occupied by mineralized nodules. With 5% mast cells in the co-cultures, the mineralized area was most extensive as reflected by the Alizarin red staining. The amount of mineralization seen with the osteogenic medium alone, as analyzed by Alizarin red extraction, may not reflect the true degree of mineralization due to the difficulty in extracting the Alizarin red from the mature nodules (Fig. 1B). Thus, the presence of mast cells, especially at high concentrations, does affect the process of mineralization.
Figure 1.
Co-culture of UMR-106 osteoblastic cells with RBL-2H3 mast cells inhibits mineralization. (A) Alizarin red staining and phase contrast microscopy of co-cultures of UMR-106 osteoblastic cells and RBL-2H3 mast cells. The mast cells (white arrows) tend to grow as clusters separate from the osteoblastic cells. No bone-like nodule formation was observed when co-cultures were grown in DMEM. When grown in OM, the co-cultures have progressively more bone-like formation (black arrow) as the proportion of RBL-2H3 mast cells decreases. Bar = 50 µm for phase contrast microscopy images and 500 µm for Alizarin red-stained images. (B) Quantification of Alizarin red-staining extraction. In co-cultures with 20% RBL-2H3 mast cells, significantly less Alizarin red-stained areas were observed when compared with UMR-106 alone and co-cultures with 5% RBL-2H3 mast cells. Co-cultures with 10% RBL-2H3 mast cells have significantly less Alizarin red-stained areas than co-cultures with 5% RBL-2H3 mast cells. Data are representative of three independent experiments. Abbreviations: UMR-106, rat osteoblastic cell line; RBL-2H3, rat mast cell line; OM, osteogenic medium. ***p<0.001.
Mast Cell Mediators Inhibit Osteoblastic Cell Proliferation
Because the mast cells and osteoblastic cells tended to grow independent of each other, it was of interest to determine if the direct interaction of mast cells with osteoblastic cells was necessary to alter the process of mineralization as seen in the co-cultures or if the released mediators alone were sufficient to affect mineralization. Therefore, subsequent experiments were focused on evaluating the effect of released mast cell mediators on UMR-106 cells. Initially, the influence of the mast cell mediators on osteoblastic proliferation was evaluated using UMR-106 cells cultured with DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators (Fig. 2). No differences in osteoblastic proliferation were observed after 1 day in culture with any of the media. After 4 days, no differences in proliferation were seen when osteoblastic cells were cultured in DMEM or in osteogenic medium. In contrast, a reduction in osteoblastic proliferation was found when the cells were cultured in osteogenic medium containing mast cell mediators. After 7 days in culture with DMEM, a significant increase in osteoblastic proliferation was observed when compared with the other media. However, there was no difference in proliferation between osteoblastic cells cultured for 7 days in osteogenic medium or in osteogenic medium containing mast cell mediators.
Figure 2.
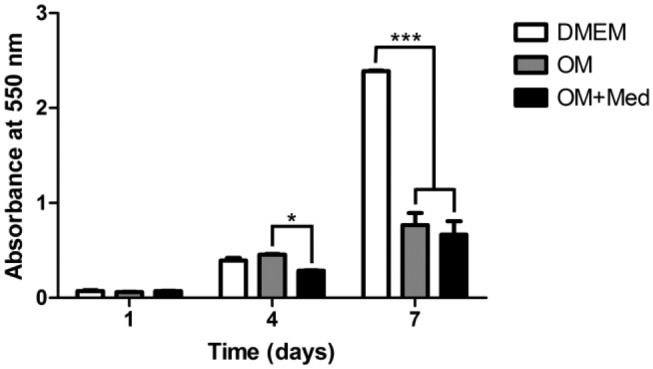
Mast cell mediators affect osteoblastic proliferation. Osteoblastic cells were cultured for 1, 4, and 7 days in DMEM, OM, or OM+Med. The cells were stained with crystal violet. Data are representative of five independent experiments. Abbreviations: OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators. *p<0.05, ***p<0.001.
Mast Cell Mediators Induce Morphological Changes in Osteoblastic Cells
The effect of mast cell mediators on osteoblastic morphology was then evaluated (Fig. 3). Osteoblastic cells cultured for 1 and 4 days in DMEM or in osteogenic medium were polygonal, resembling epithelial cells, while osteoblastic cells cultured in osteogenic medium containing mast cell mediators were fusiform. After 7 days, the osteoblastic cells cultured in both osteogenic medium and osteogenic medium containing mast cell mediators showed areas consistent with mineralization. These mineralized areas were most evident in the cultures with only osteogenic medium.
Figure 3.
Mast cell mediators affect osteoblastic morphology. Osteoblastic cells were cultured for 1, 4, and 7 days in DMEM, OM, or OM+Med. Cell morphology was assessed by phase contrast microscopy. Mineralized areas (arrows). Bar = 50 μm. Abbreviations: OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators.
Mast Cell Mediators Stimulate Rearrangement of the Actin Cytoskeleton
Because the actin cytoskeleton reflects the cell morphology, it was of interest to investigate if changes in the actin cytoskeleton occurred when osteoblastic cells were cultured in the different media (Fig. 4). In osteoblastic cells cultured in DMEM, osteogenic medium, or in osteogenic media containing mast cell mediators, actin filaments were seen at the inner surface of the plasma membrane, forming a framework for the cell. In osteoblastic cells cultured with osteogenic medium containing mast cell mediators, the actin filaments were organized into parallel bundles along the major axis of the cells. In addition, after 7 days in osteogenic medium, the osteoblastic cells had many pyknotic nuclei, indicating cell death (Fig. 4).
Figure 4.
Mast cell mediators promote reorganization of the actin cytoskeleton. Osteoblastic cells were cultured for 1, 4, and 7 days in DMEM, OM, or OM+Med. The cytoskeleton was analyzed by fluorescence microscopy. Actin filaments are organized into parallel bundles under the plasma membrane (small arrows) and in the cells grown in OM+Med, the actin filaments were found along the major axis of the cells (inset, arrow). Pyknotic nuclei (inset, arrowhead). Green (Alexa 488) = F-actin, Blue (DAPI) = Nuclei. Bar = 50 μm. Abbreviations: OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
Mast Cell Mediators Inhibit Mineralization
After 7 days in culture with osteogenic medium with or without mast cell mediators, the cultures had areas that appeared mineralized. Thus, the influence of the mast cell mediators on the formation of calcium nodules was evaluated (Fig. 5). After 7 days in culture in osteogenic medium with or without mast cell mediators, the osteoblastic cultures had areas with calcium nodules as assessed by Alizarin red staining. In contrast, no mineralized areas were observed when osteoblastic cells were cultured in DMEM. Calcification could also be seen by SEM. The areas with calcium nodules were more extensive and the surface of nodules was more complex and rough in osteoblastic cells cultured in osteogenic medium, when compared with osteoblastic cells cultured in osteogenic medium containing mast cell mediators. Furthermore, when the osteoblastic cells were cultured in osteogenic medium, these calcium nodules could be detected by intense red fluorescence using a 590 nm emission filter. No fluorescent nodules were seen in osteoblastic cells cultured in the other 2 media (Fig. 5).
Figure 5.
Mast cell mediators inhibit mineralization. Osteoblastic cells were cultured for 7 days in DMEM, OM, or OM+Med. The cells were analyzed by AzR, Fluo, and SEM. Mineralization areas were detected by Alizarin red staining and SEM (arrows). Surfaces of nodules (insets). Mineralized areas with red Ca++ autofluorescence (arrowheads). Bars = 50 μm. Abbreviations: OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators; AzR, Alizarin red; Fluo, fluorescence microscopy; SEM, scanning electron microscopy; Red, autofluorescence of calcium; Blue (DAPI), nuclei; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
Mast Cell Mediators Reduce Calcium Deposition During Mineralization
Because mast cell mediators inhibited the formation of calcified nodules and no calcified nodules were seen with DMEM, the composition of the hydroxyapatite crystals was investigated. The osteoblastic cultures were analyzed by EDS (Fig. 6, Supplemental Fig. 2). Analysis of the mineralized matrix with EDS showed higher phosphorus content in osteoblastic cultures in osteogenic medium, as compared with osteoblastic cells cultured in DMEM. Calcium was detected in the matrix when osteoblastic cells were cultured in osteogenic medium and osteogenic medium containing mast cell mediators, but not in cultures with DMEM. The content of calcium was significantly higher in cultures grown in osteogenic medium without mediators, when compared with cultures grown in osteogenic medium containing mast cell mediators.
Figure 6.

Mast cell mediators affect the composition of calcified nodules. Osteoblastic cells were cultured for 7 days in DMEM, OM, or OM+Med. Phosphorus and calcium levels were determined by EDS. % Molecular Weight = Percentage of the sample molecular weight calculated by the software Aztec. P = phosphorus; Ca = calcium. Data are representative of three independent experiments. Abbreviations: OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators; EDS, energy dispersive x-ray spectrometry. *p<0.05, ***p<0.001.
Mast Cell Mediators Inhibit ALP Expression
After 7 days in culture in osteogenic medium with mast cell mediators, there was less calcium present than in osteogenic medium. Because ALP acts in the formation of hydroxyapatite crystals, the expression of ALP in osteoblastic cells cultured in DMEM, osteogenic medium, or osteogenic medium containing mast cell mediators was evaluated. At 1 and 4 days in culture, ALP expression was lower in osteoblastic cells cultured in osteogenic medium containing mast cell mediators, compared with ALP expression in osteoblastic cells cultured with osteogenic medium alone or DMEM. In addition, at 4 days, ALP expression in osteoblastic cells cultured in osteogenic medium was lower compared with osteoblastic cells cultured in DMEM. In contrast, after 7 days, ALP expression in osteoblastic cells cultured in DMEM was higher compared with ALP expression in osteoblastic cells cultured with osteogenic media, with or without mast cell mediators. In addition, after 7 days, there was no significant difference between the ALP expression in osteoblastic cells cultured in osteogenic medium and osteogenic medium containing mast cell mediators (Fig. 7A and B).
Figure 7.

Mast cell mediators decrease ALP expression. Osteoblastic cells were cultured for 1, 4, and 7 days in DMEM, OM, or OM+Med. (A) Western blot. (B) Quantification of the Western blot bands was done using Image-Pro Plus. Data are representative of three independent experiments. Abbreviations: ALP, alkaline phosphatase; OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators. ***p<0.001.
Mast Cell Mediators Inhibit ALP Activity
Because the formation of calcified nodules and calcium content are decreased in osteoblastic cells cultured in osteogenic medium containing mast cell mediators, ALP activity was evaluated. ALP activity was normalized to the total protein content of the osteoblastic cells grown in different media. At 1 day of culture, there was no difference in protein content among the culture media analyzed. After 4 days, it was possible to observe a decrease in the protein content in osteoblastic cells cultured in osteogenic medium containing mast cell mediators compared with osteoblastic cells cultured in osteogenic medium alone. There was also an increased protein content in osteoblastic cells cultured in osteogenic media, compared with osteoblastic cells cultured in DMEM. After 7 days of culture, the protein content was higher in osteoblastic cells cultured in DMEM, compared with those cultured in osteogenic medium or osteogenic medium containing mast cell mediators (Fig. 8A).
Figure 8.
Mast cell mediators affect total protein content and ALP activity. Osteoblastic cells were cultured for 1, 4, and 7 days in DMEM, OM, or OM+Med. Total protein content was determined using the method of Lowry. ALP activity was assessed by the release of thymolphthalein by the hydrolysis of the substrate thymolphthalein monophosphate. Data are representative of three independent experiments. Abbreviations: ALP, alkaline phosphatase; OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators. *p<0.05, ***p<0.001.
At 1 and 4 days of culture, the ALP activity levels were low, and there was no significant difference between the culture media tested. After 7 days, the ALP activity was significantly higher for osteoblastic cells cultured in osteogenic media compared to osteoblastic cells cultivated in DMEM. In addition, ALP activity levels in osteoblastic cells cultured in osteogenic medium containing mast cell mediators was significantly higher than that seen in cells cultured in DMEM, but lower when compared with levels of ALP activity in osteoblastic cells cultured in osteogenic medium (Fig. 8B).
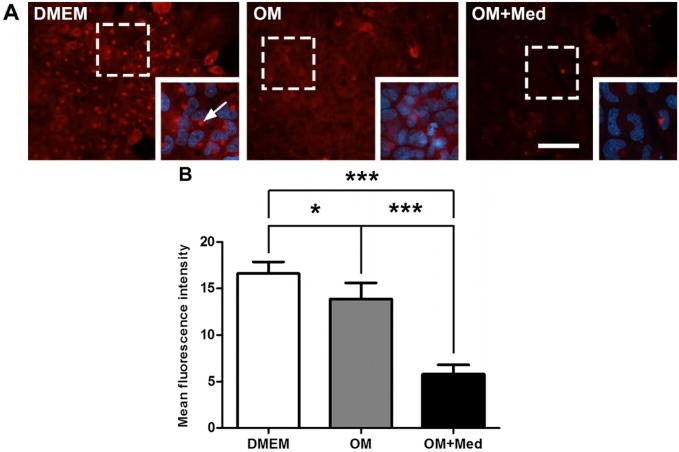
Mast Cell Mediators Alter the Localization and Expression of BSP
BSP is also related to the development of the osteoblastic phenotype and participates in mineralization of the extracellular matrix. Therefore, the influence of mast cell mediators in the localization and expression of BSP was evaluated. At 7 days, BSP was localized in the cytoplasm of osteoblastic cells under all culture conditions. However, in osteoblastic cells cultured in DMEM, there was an accumulation of BSP in the juxtanuclear region, consistent with its localization in the Golgi apparatus (Fig. 9). The expression of BSP was significantly lower when osteoblastic cells were cultured in osteogenic medium containing mast cell mediators, compared with osteoblastic cells cultured in osteogenic medium or DMEM. In addition, the expression of BSP was less in osteoblastic cells cultured in osteogenic medium, when compared with osteoblastic cells cultured in DMEM (Fig. 9).
Figure 9.
Mast cell mediators modify the localization and expression of BSP. Osteoblastic cells were cultured for 7 days in DMEM, OM, or OM+Med. (A) The cells were immunostained with the rabbit polyclonal antibody anti-BSP followed by goat anti-rabbit IgG conjugated to Alexa 594. Juxtanuclear region (arrow). Red = BSP; Blue = nuclei (DAPI). Bar = 50 μm. The images are representative of three independent experiments. (B) Fluorescence intensity was quantified using Image-Pro Plus. Data are representative of three independent experiments. Abbreviations: BSP, bone sialoprotein; OM = osteogenic medium; OM+Med = osteogenic medium containing mast cell mediators; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride. *p<0.05, ***p<0.001.
Mast Cell Mediators Modulate the Expression of mRNA of Proteins Involved in Osteoblastic Differentiation
Mast cell mediators influenced the expression and activity of ALP and BSP, proteins important in the mineralization process. Therefore, mRNA expression of ALP and BSP as well as other proteins involved in osteoblastic differentiation and mineralization were evaluated by Real-Time PCR. ALP and BSP mRNA levels in osteoblastic cells cultured in osteogenic medium containing mast cell mediators were significantly lower than those levels found when osteoblastic cells were cultured in DMEM or osteogenic medium. Furthermore, mRNA expression levels for ALP were less for osteoblastic cells cultured in osteogenic medium, compared with osteoblastic cells cultured in DMEM (Fig. 10).
Figure 10.
Mast cell mediators modulate the expression of mRNA of osteoblastic differentiation proteins. Osteoblastic cells were cultured for 7 days in DMEM, OM, or OM+Med. The expression of mRNA of osteoblastic differentiation proteins was evaluated by Real-Time PCR using TaqMan PCR Master Mix. The graphs represent the relative expression of mRNA of osteoblastic differentiation proteins. Data are representative of 3 independent experiments. Abbreviations: OM, osteogenic medium; OM+Med, osteogenic medium containing mast cell mediators; ALP, alkaline phosphatase; BSP, bone sialoprotein; OPN, osteopontin; OC, osteocalcin; PCR, polymerase chain reaction; Runx2, runt-related transcription factor 2; OSX, osterix. *p<0.05, **p<0.01, ***p<0.001.
In addition, there were no differences in the levels of mRNA for OPN (osteopontin) among the 3 tested culture media, and mRNA expression levels for OC (osteocalcin) were significantly lower in osteoblastic cells cultured with osteogenic medium, when compared with DMEM (Fig. 10). The mRNA levels for Runx2 (runt-related transcription factor 2) and OSX (osterix) were significantly lower when osteoblastic cells were cultured in osteogenic medium or in osteogenic medium containing mast cell mediators, when compared with DMEM. However, no significant difference was observed in the levels of mRNA for Runx2 and OSX between osteoblastic cells cultured with osteogenic medium or osteogenic medium containing mast cell mediators (Fig. 10).
Discussion
Although several previous studies have evaluated the effects of a single mast cell mediator on bone cell behavior, in vivo stimulated mast cells release a wide variety of mediators. Thus, to simulate in vivo conditions, the present study was carried out with pooled mast cell mediators, and the data demonstrate, for the first time, that this combination of mast cell mediators influences the proliferation and morphology of osteoblastic cells and modulates the location, expression, and activity of proteins involved in osteoblastic differentiation. Although specific mast cell mediators can either stimulate or inhibit bone repair and remodeling,21–28 in the present study, the combined effects of the mediators was to inhibit osteoblastic proliferation and mineralization of the extracellular matrix. In the presence of mast cell mediators, the mRNA expression of key osteoblastic markers was inhibited as well as the formation and maturation of calcium nodules. This consequently resulted in the inhibition of mineralization of the extracellular matrix.
In the present study, RBL-2H3 cells were activated via FcεRI and media collected 24 hr later to obtain all classes of mediators. As the newly formed mediators (bioactive lipids) are unstable in solution,50 the osteogenic medium with mast cell mediators probably contains little or no newly formed mediators, such as PGD2, PGE2, LTB4, LTC4, and PAF.4,51 However, TGF-β, a preformed and newly synthesized mast cell mediator, and PDGF, a newly synthesized mast cell mediator, among others, most likely are present in the osteogenic medium and can play a role in osteoblastic cell proliferation and differentiation.4,44,52–56 There are a lack of studies that evaluate the effects of mast cell mediators on the acquisition of the osteoblastic phenotype. However, there are similarities between the granule content of mast cells and those of platelets.57 The results of the present study are in general agreement with the results seen with platelet extracts. A mixture of growth factors and proteins simulating platelet extracts, including TGF-β1, TGF-β2, and PDGF-BB, was shown to inhibit the development of the osteogenic phenotype both in human and rat osteoblastic cell cultures, and led to an increase in cell proliferation and the inhibition of osteoblastic differentiation.44,58 However, in the present study, although a transient reduction in the cell population was detected at day 4 in cultures exposed to mast cell mediators, no differences in the amount of osteoblastic cells between cultures grown in osteogenic medium containing or not containing mast cell mediators were noticed at day 7, when the UMR-106 cells achieve their full osteoblastic phenotype.59 This reduction in cell population after 7 days was correlated with extracellular matrix mineralization. This was also reflected in the total protein content of the cultures. At least in part, the differential impact on the proliferation rate seen with osteoblasts from different origins suggests that they respond differently to growth factor exposure. Moreover, the composition and the relative proportions of the different constituents in the cellular extracts could exhibit synergistic and/or antagonistic effects.44,58,60–63
Growth factors can also affect reorganization of the cytoskeleton and might have an impact on migration and cell shape.52–54,58,64,65 The present study demonstrated that exposure to mast cell mediators induced morphological changes in the osteoblastic cells. UMR-106 cells are polygonal in shape, but in the presence of mast cell mediators, the actin cytoskeleton is reorganized and the cells become fusiform taking on the characteristic morphology of mesenchymal cells,66–68 most likely reflecting a change in function.
In addition to the effects on cell proliferation and morphology, the present study also shows that when UMR-106 cells were cultured in osteogenic medium containing mast cell mediators, there were fewer areas of bone formation, the calcium content was decreased, calcium nodules were smoother and less complex, and no fluorescent calcium-containing nodules were present. These results suggest that the formation of hydroxyapatite crystals was defective, and that the calcified nodules did not mature. Consequently, the process of mineralization was inhibited. Hydroxyapatite crystals are the main inorganic constituent of mineralized tissues and are composed of crystalline calcium phosphate (Ca10(PO4)6(OH)2).69–73 Biological mineralization is a dynamic process involving several proteins that enhance or inhibit formation and growth of hydroxyapatite crystals.74–77 ALP, a marker of osteoblastic differentiation, is an ectoenzyme that promotes the mineralization process by hydrolyzing pyrophosphate, an inhibitor of mineralization.44,58,74,78 BSP, OPN, and OC are non-collagenous extracellular matrix proteins that play a crucial role in the mineralization process. BSP is implicated in the growth and nucleation of hydroxyapatite crystals, and its expression is also an important marker of osteoblastic differentiation.79–81 OPN and OC act in the early (calcium deposition) and late (homeostasis of calcium ions) stages of the mineralization process.82–86 Moreover, Runx2 and OSX are transcription factors that are involved in osteoblastic differentiation.87–89 In the current investigation, the presence of mast cell mediators leads to a decrease in ALP expression, and a decrease in the expression of BSP. Also, the intracellular distribution of BSP was modified. Furthermore, in the presence of mast cell mediators, expression of mRNA for both ALP and BSP was reduced, although the expression of mRNA for OPN, OC, Runx2, and OSX was unaffected. OPN has been considered a potent mineralization inhibitor.90–94 Thus, although mast cell mediators did not affect the expression of mRNA for OPN in UMR-106 cells, the exogenous OPN, produced by mast cells and probably present in the osteogenic medium, could inhibit the mineralization process by maintaining high levels of pyrophosphate.34,90–94,95 In addition, the inhibition of mineralization could also be due to the presence of TNF-α, which is a pro-inflammatory cytokine found in mast cell granules that inhibits the expression and activity of osteoblast markers, such as ALP, eventually impairing the mineralization of the extracellular matrix96–98; TNF-α has also an inhibitory influence on bone formation through the induction of osteoblast apoptosis. Another important mast cell mediator is histamine, which can also affect bone metabolism.27–33 Several studies have demonstrated that histamine has a direct effect on osteoblasts, stimulating the maturation and differentiation of osteoblastic cells.30,31 In the present study, differentiation of UMR-106 was inhibited, suggesting that the stimulation of differentiation by histamine might be counterbalanced by the presence of inhibitory factors in the medium.
In conclusion, the present study has demonstrated that mast cell mediators act synergistically to reduce the osteogenic potential of the UMR-106 cells and consequently inhibit the mineralization of the extracellular matrix. Further studies are needed to elucidate the precise effects of mast cell mediators in vivo. Nevertheless, the results of the present study contribute to the understanding of the influence of mast cells and their mediators in bone repair and remodeling and support the concept that immune system cells and bone cells interact and influence bone metabolism in health and disease. Thus, mast cells are a potential therapeutic target for the treatment of bone disorders.
Acknowledgments
We would like to thank Anderson R. Souza (Ribeirão Preto Medical School, University of Sao Paulo), and Roger Rodrigo Fernandes and Fabíola Singaretti de Oliveira (Faculdade de Odontologia de Ribeirão Preto, University of Sao Paulo) for technical support. We also thank José A. Maulin, Maria Dolores S. Ferreira, and Maria Teresa P. Maglia (Laboratório Multiusuário de Microscopia Eletrônica, Ribeirão Preto Medical School, University of Sao Paulo) for support and assistance with the scanning electron microscopy.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: WMAM, CO, and MCJ made substantial contributions to the conception and design of the work. WMAM, ACS, and EZMS were responsible for the acquisition of the data. WMAM, CO, MCJ, PTO, ACS, and EZMS participated in the analysis and interpretation of the data. WMAM, CO, MCJ, and PTO drafted and critically revised the manuscript. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was provided by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, http://www.fapesp.br): Research grant 14/113396-5 to C.O., Research Grant 16/50298-4 to P.T.O., Predoctoral Fellowship 2010/51850-6 to W.M.A.M., Predoctoral Fellowship 2012/06373-0 to E.Z.M.S., and Equipment Grant 09/54013-0 to M.C.J. and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, http://www.cnpq.br): Research Grant 476209/2013-7 and Fellowship 304740/2015-2 to C.O., Fellowship 308981/2016-2 to P.T.O., and Predoctoral Fellowship 166166/2014 to A.C.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
William Marcatti Amarú Maximiano, Department of Cell and Molecular Biology and Pathogenic Bioagents, Ribeirão Preto Medical School, University of São Paulo, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Elaine Zayas Marcelino da Silva, Department of Cell and Molecular Biology and Pathogenic Bioagents, Ribeirão Preto Medical School, University of São Paulo, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Ana Carolina Santana, Department of Cell and Molecular Biology and Pathogenic Bioagents, Ribeirão Preto Medical School, University of São Paulo, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Paulo Tambasco de Oliveira, Department of Morphology, Stomatology, and Basic Pathology, School of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Maria Célia Jamur, Department of Cell and Molecular Biology and Pathogenic Bioagents, Ribeirão Preto Medical School, University of São Paulo, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Constance Oliver, Department of Cell and Molecular Biology and Pathogenic Bioagents, Ribeirão Preto Medical School, University of São Paulo, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Literature Cited
- 1. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2. Sur R, Cavender D, Malaviya R. Different approaches to study mast cell functions. Int Immunopharmacol. 2007;7(5):555–67. doi: 10.1016/j.intimp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 3. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62(10):698–738. doi: 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oettgen HC, Burton OT. IgE and mast cells: the endogenous adjuvant. Adv Immunol. 2015;127:203–56. doi: 10.1016/bs.ai.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 6. Beaven MA, Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993;14(5):222–6. [DOI] [PubMed] [Google Scholar]
- 7. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–79. [DOI] [PubMed] [Google Scholar]
- 8. Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124(4):639–46; quiz 47–8. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalesnikoff J, Galli SJ. Anaphylaxis: mechanisms of mast cell activation. Chem Immunol Allergy. 2010;95:45–66. doi: 10.1159/000315937. [DOI] [PubMed] [Google Scholar]
- 10. Barbosa-Lorenzi VC, Buranello PA, Roque-Barreira MC, Jamur MC, Oliver C, Pereira-da-Silva G. The lectin ArtinM binds to mast cells inducing cell activation and mediator release. Biochem Biophys Res Commun. 2011;416(3–4):318–24. doi: 10.1016/j.bbrc.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 11. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol. 2016;38(5):581–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galli SJ, Nakae S. Mast cells to the defense. Nat Immunol. 2003;4(12):1160–2. doi: 10.1038/ni1203-1160. [DOI] [PubMed] [Google Scholar]
- 14. Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117(6):1214–25; quiz 26. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15. Noli C, Miolo A. The mast cell in wound healing. Vet Dermatol. 2001;12(6):303–13. [DOI] [PubMed] [Google Scholar]
- 16. Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38(1):4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 17. Hofmann AM, Abraham SN. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr Opin Immunol. 2009;21(6):679–86. doi: 10.1016/j.coi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wulff BC, Wilgus TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol. 2013;22(8):507–10. doi: 10.1111/exd.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Succar J, Douaiher J, Lancerotto L, Li Q, Yamaguchi R, Younan G, Pejler G, Orgill DP. The role of mouse mast cell proteases in the proliferative phase of wound healing in microdeformational wound therapy. Plast Reconstr Surg. 2014;134(3):459–67. doi: 10.1097/PRS.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 20. Murakami M, Taketomi Y. Secreted phospholipase A2 and mast cells. Allergol Int. 2015;64(1):4–10. doi: 10.1016/j.alit.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 21. Urist MR, McLean FC. Mast cells in bones. Surg Forum. 1957;7:590–2. [PubMed] [Google Scholar]
- 22. McKenna MJ, Frame B. The mast cell and bone. Clin Orthop Relat Res. 1985;(200):226–33. [PubMed] [Google Scholar]
- 23. Silberstein R, Melnick M, Greenberg G, Minkin C. Bone remodeling in W/Wv mast cell deficient mice. Bone. 1991;12(4):227–36. [DOI] [PubMed] [Google Scholar]
- 24. Fouilloux I, Duplan MB, Baroukh B, Cherruau M, Saffar JL, Lesclous P. Mast cell activation and degranulation occur early during induction of periosteal bone resorption. Bone. 2006;38(1):59–66. doi: 10.1016/j.bone.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 25. Behrends DA, Cheng L, Sullivan MB, Wang MH, Roby GB, Zayed N, Gao C, Henderson JE, Martineau PA. Defective bone repair in mast cell deficient mice with c-Kit loss of function. Eur Cell Mater. 2014;28:209–21; discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 26. Crivellato E, Ribatti D, Mallardi F, Beltrami CA. The mast cell: a multifunctional effector cell. Adv Clin Path. 2003;7(1):13–26. [PubMed] [Google Scholar]
- 27. Lesclous P, Guez D, Baroukh B, Vignery A, Saffar JL. Histamine participates in the early phase of trabecular bone loss in ovariectomized rats. Bone. 2004;34(1):91–9. [DOI] [PubMed] [Google Scholar]
- 28. Lesclous P, Schramm F, Gallina S, Baroukh B, Guez D, Saffar JL. Histamine mediates osteoclastic resorption only during the acute phase of bone loss in ovariectomized rats. Exp Physiol. 2006;91(3):561–70. doi: 10.1113/expphysiol.2006.033217. [DOI] [PubMed] [Google Scholar]
- 29. Zdolsek J, Eaton JW, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;5:31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ikawa Y, Yonekawa T, Ohkuni Y, Kuribayashi M, Fukino K, Ueno K. A comparative study of histamine activities on differentiation of osteoblasts and osteoclasts. J Toxicol Sci. 2007;32(5):555–64. [DOI] [PubMed] [Google Scholar]
- 31. Meh A, Sprogar S, Vaupotic T, Cör A, Drevenšek G, Marc J, et al. Effect of cetirizine, a histamine (H(1)) receptor antagonist, on bone modeling during orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2011;139(4):e323–9. doi: 10.1016/j.ajodo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32. Ezzat BA, Abbass MM. The ability of H1 or H2 receptor antagonists or their combination in counteracting the glucocorticoid-induced alveolar bone loss in rats. J Oral Pathol Med. 2014;43(2):148–56. doi: 10.1111/jop.12104. [DOI] [PubMed] [Google Scholar]
- 33. Biosse-Duplan Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am J Path. 2009;174(4):1426–34. doi: 10.2353/ajpath.2009.080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bulfone-Paus S, Paus R. Osteopontin as a new player in mast cell biology. Eur J Immunol. 2008;38(2):338–41. doi: 10.1002/eji.200738131. [DOI] [PubMed] [Google Scholar]
- 35. Greene LW, Asadipooya K, Corradi PF, Akin C. Endocrine manifestations of systemic mastocytosis in bone. Rev Endocr Metab Disord. 2016;17(3):419–31. [DOI] [PubMed] [Google Scholar]
- 36. Chatakun P, Núñez-Toldrà R, Díaz López EJ, Gil-Recio C, Martínez-Sarrà E, Hernández-Alfaro F, Ferrés-Padró E, Giner-Tarrida L, Atari M. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: a current review of the literature. Cell Mol Life Sci. 2014;71(1):113–42. doi: 10.1007/s00018-013-1326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981;11(4):317–23. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 38. Partridge NC, Alcorn D, Michelangeli VP, Kemp BE, Ryan GB, Martin TJ. Functional properties of hormonally responsive cultured normal and malignant rat osteoblastic cells. Endocrinology. 1981;108(1):213–9. doi: 10.1210/endo-108-1-213. [DOI] [PubMed] [Google Scholar]
- 39. Morita Y, Siraganian RP. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J Immunol. 1981;127(4):1339–44. [PubMed] [Google Scholar]
- 40. Basciano LK, Berenstein EH, Kmak L, Siraganian RP. Monoclonal antibodies that inhibit IgE binding. J Biol Chem. 1986;261(25):11823–31. [PubMed] [Google Scholar]
- 41. Pierini L, Holowka D, Baird B. Fc epsilon RI-mediated association of 6-micron beads with RBL-2H3 mast cells results in exclusion of signaling proteins from the forming phagosome and abrogation of normal downstream signaling. J Cell Biol. 1996;134(6):1427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. da Silva EZM, Freitas-Filho EG, de Souza-Júnior DA, daSilva LLP, Jamur MC, Oliver C. Adaptor protein-3: a key player in RBL-2H3 mast cell mediator release. PLoS ONE. 2017;12(3):e0173462. doi: 10.1371/journal.pone.0173462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. [DOI] [PubMed] [Google Scholar]
- 44. de Oliva MA, Maximiano WM, de Castro LM, da Silva PE, Fernandes RR, Ciancaglini P, Beloti MM, Nanci A, Rosa AL, de Oliveira PT. Treatment with a growth factor-protein mixture inhibits formation of mineralized nodules in osteogenic cell cultures grown on titanium. J Histochem Cytochem. 2009;57(3):265–76. doi: 10.1369/jhc.2008.952713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 46. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. [DOI] [PubMed] [Google Scholar]
- 47. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 49. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50. Maddipati KR, Zhou SL. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat. 2011;94(1–2):59–72. doi: 10.1016/j.prostaglandins.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 51. Azouz NP, Fukuda M, Rothenberg ME, Sagi-Eisenberg R. Investigating mast cell secretory granules; from biosynthesis to exocytosis. J Vis Exp. 2015;(95):52505. doi: 10.3791/52505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lomri A, Marie PJ. Effects of transforming growth factor type beta on expression of cytoskeletal proteins in endosteal mouse osteoblastic cells. Bone. 1990;11(6):445–51. [DOI] [PubMed] [Google Scholar]
- 53. Colciago A, Celotti F, Casati L, Giancola R, Castano SM, Antonini G, et al. In vitro effects of PDGF isoforms (AA, BB, AB and CC) on migration and proliferation of SaOS-2 osteoblasts and on migration of human osteoblasts. Int J Biomed Sci. 2009;5(4):380–9. [PMC free article] [PubMed] [Google Scholar]
- 54. Casati L, Celotti F, Negri-Cesi P, Sacchi MC, Castano P, Colciago A. Platelet derived growth factor (PDGF) contained in Platelet Rich Plasma (PRP) stimulates migration of osteoblasts by reorganizing actin cytoskeleton. Cell Adh Migr. 2014;8(6):595–602. doi: 10.4161/19336918.2014.972785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suzuki E, Ochiai-Shino H, Aoki H, Onodera S, Saito A, Azuma T. Akt activation is required for TGF-β1-induced osteoblast differentiation of MC3T3-E1 pre-osteoblasts. PLoS ONE. 2014;9(12):e112566. doi: 10.1371/journal.pone.0112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang X, Wang Y, Han X, Shu R, Chen T, Zeng H, Xu X, Huang L, Ren A, Song J, Cao L, Bai D. Effects of TGF-β1 on OPG/RANKL expression of cementoblasts and osteoblasts are similar without stress but different with mechanical compressive stress. ScientificWorldJournal. 2015;2015:718180. doi: 10.1155/2015/718180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–89. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Oliveira PT, de Oliva MA, Maximiano WM, Sebastião KE, Crippa GE, Ciancaglini P, Beloti MM, Nanci A, Rosa AL. Effects of a mixture of growth factors and proteins on the development of the osteogenic phenotype in human alveolar bone cell cultures. J Histochem Cytochem. 2008;56(7):629–38. doi: 10.1369/jhc.2008.950758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van den Dolder J, Spauwen PH, Jansen JA. Evaluation of various seeding techniques for culturing osteogenic cells on titanium fiber mesh. Tissue Eng. 2003;9(2):315–25. doi: 10.1089/107632703764664783. [DOI] [PubMed] [Google Scholar]
- 60. Giannobile WV, Whitson SW, Lynch SE. Non-coordinate control of bone formation displayed by growth factor combinations with IGF-I. J Dent Res. 1997;76(9):1569–78. [DOI] [PubMed] [Google Scholar]
- 61. Schmidmaier G, Wildemann B, Lübberstedt M, Haas NP, Raschke M. IGF-I and TGF-beta 1 incorporated in a poly(D,L-lactide) implant coating stimulates osteoblast differentiation and collagen-1 production but reduces osteoblast proliferation in cell culture. J Biomed Mater Res B Appl Biomater. 2003;65(1):157–62. doi: 10.1002/jbm.b.10513. [DOI] [PubMed] [Google Scholar]
- 62. Chaudhary LR, Hofmeister AM, Hruska KA. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone. 2004;34(3):402–11. doi: 10.1016/j.bone.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 63. Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20(5):848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 64. Celotti F, Colciago A, Negri-Cesi P, Pravettoni A, Zaninetti R, Sacchi MC. Effect of platelet-rich plasma on migration and proliferation of SaOS-2 osteoblasts: role of platelet-derived growth factor and transforming growth factor-beta. Wound Repair Regen. 2006;14(2):195–202. doi: 10.1111/j.1743-6109.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 65. Kim SJ, Kim SY, Kwon CH, Kim YK. Differential effect of FGF and PDGF on cell proliferation and migration in osteoblastic cells. Growth Factors. 2007;25(2):77–86. doi: 10.1080/08977190701398977. [DOI] [PubMed] [Google Scholar]
- 66. Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–6. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 67. Li J, Yang S, Lu S, Zhao H, Feng J, Li W, Ma F, Ren Q, Liu B, Zhang L, Zheng Y, Han ZC. Differential gene expression profile associated with the abnormality of bone marrow mesenchymal stem cells in aplastic anemia. PLoS ONE. 2012;7(11):e47764. doi: 10.1371/journal.pone.0047764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Skardal A, Mack D, Atala A, Soker S. Substrate elasticity controls cell proliferation, surface marker expression and motile phenotype in amniotic fluid-derived stem cells. J Mech Behav Biomed Mater. 2013;17:307–16. doi: 10.1016/j.jmbbm.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990;10(6):1031–48. doi: 10.1148/radiographics.10.6.2175444. [DOI] [PubMed] [Google Scholar]
- 70. Midura RJ, Vasanji A, Su X, Wang A, Midura SB, Gorski JP. Calcospherulites isolated from the mineralization front of bone induce the mineralization of type I collagen. Bone. 2007;41(6):1005–16. doi: 10.1016/j.bone.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Midura RJ, Midura SB, Su X, Gorski JP. Separation of newly formed bone from older compact bone reveals clear compositional differences in bone matrix. Bone. 2011;49(6):1365–74. doi: 10.1016/j.bone.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tsui FW. Genetics and mechanisms of crystal deposition in calcium pyrophosphate deposition disease. Curr Rheumatol Rep. 2012;14(2):155–60. doi: 10.1007/s11926-011-0230-6. [DOI] [PubMed] [Google Scholar]
- 73. Lin K, Wu C, Chang J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014;10(10):4071–102. doi: 10.1016/j.actbio.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 74. Ciancaglini P, Yadav MC, Simão AM, Narisawa S, Pizauro JM, Farquharson C, Hoylaerts MF, Millán JL. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res. 2010;25(4):716–23. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ikeda K, Takeshita S. Factors and mechanisms involved in the coupling from bone resorption to formation: how osteoclasts talk to osteoblasts. J Bone Metab. 2014;21(3):163–7. doi: 10.11005/jbm.2014.21.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ. Biomimetic approaches in bone tissue engineering: integrating biological and physicomechanical strategies. Adv Drug Deliv Rev. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 77. Hlaing TT, Compston JE. Biochemical markers of bone turnover—uses and limitations. Ann Clin Biochem. 2014;51(Pt 2):189–202. doi: 10.1177/0004563213515190. [DOI] [PubMed] [Google Scholar]
- 78. Millán JL. Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2(2):335–41. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10(1):79–98. [DOI] [PubMed] [Google Scholar]
- 80. Wade-Gueye NM, Boudiffa M, Vanden-Bossche A, Laroche N, Aubin JE, Vico L, Lafage-Proust MH, Malaval L. Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: the marrow ablation model under PTH challenge. Bone. 2012;50(5):1064–73. [DOI] [PubMed] [Google Scholar]
- 81. Holm E, Aubin JE, Hunter GK, Beier F, Goldberg HA. Loss of bone sialoprotein leads to impaired endochondral bone development and mineralization. Bone. 2015;71:145–54. doi: 10.1016/j.bone.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 82. Nagao M, Feinstein TN, Ezura Y, Hayata T, Notomi T, Saita Y, Hanyu R, Hemmi H, Izu Y, Takeda S, Wang K, Rittling S, Nakamoto T, Kaneko K, Kurosawa H, Karsenty G, Denhardt DT, Vilardaga JP, Noda M. Sympathetic control of bone mass regulated by osteopontin. Proc Natl Acad Sci U S A. 2011;108(43):17767–72. doi: 10.1073/pnas.1109402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228(6):1149–53. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- 84. Subraman V, Thiyagarajan M, Malathi N, Rajan ST. OPN-revisited. J Clin Diagn Res. 2015;9(6):ZE10–3. doi: 10.7860/JCDR/2015/12872.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Oliveira PT, Zalzal SF, Irie K, Nanci A. Early expression of bone matrix proteins in osteogenic cell cultures. J Histochem Cytochem. 2003;51(5):633–41. [DOI] [PubMed] [Google Scholar]
- 86. de Oliveira PT, Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials. 2004;25(3):403–13. [DOI] [PubMed] [Google Scholar]
- 87. Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–9. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 88. Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, Wakisaka S, Kiyonari H, Shioi G, Yamaguchi A, Tsumaki N, Akiyama H, Yoneda T. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem. 2012;287(40):33179–90. doi: 10.1074/jbc.M111.337063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Choi YH, Han Y, Lee SH, Cheong H, Chun KH, Yeo CY, Lee KY. Src enhances osteogenic differentiation through phosphorylation of Osterix. Mol Cell Endocrinol. 2015;407:85–97. doi: 10.1016/j.mce.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 90. Narisawa S, Yadav MC, Millán JL. In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin. J Bone Miner Res. 2013;28(7):1587–98. doi: 10.1002/jbmr.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yadav MC, Huesa C, Narisawa S, Hoylaerts MF, Moreau A, Farquharson C, Millán JL. Ablation of osteopontin improves the skeletal phenotype of phospho1(-/-) mice. J Bone Miner Res. 2014;29(11):2369–81. doi: 10.1002/jbmr.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millán JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164(4):1199–209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M, Terkeltaub R, Millán JL. Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(-/-) mice. J Bone Miner Res. 2006;21(9):1377–86. doi: 10.1359/jbmr.060619. [DOI] [PubMed] [Google Scholar]
- 94. Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282(21):15872–83. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 95. Nagasaka A, Matsue H, Matsushima H, Aoki R, Nakamura Y, Kambe N, Kon S, Uede T, Shimada S. Osteopontin is produced by mast cells and affects IgE-mediated degranulation and migration of mast cells. Eur J Immunol. 2008;38(2):489–99. doi: 10.1002/eji.200737057. [DOI] [PubMed] [Google Scholar]
- 96. Abuna RP, de Oliveira FS, De S, Santos T, Guerra TR, Rosa AL, Beloti MM. Participation of TNF-α in inhibitory effects of adipocytes on osteoblast differentiation. J Cell Physiol. 2016;231:204–14. doi: 10.1002/jcp.25073. [DOI] [PubMed] [Google Scholar]
- 97. Fan JZ, Yang X, Bi ZG. The effects of 6-gingerol on proliferation, differentiation, and maturation of osteoblast-like MG-63 cells. Braz J Med Biol Res. 2015;48(7):637–43. doi: 10.1590/1414-431X20154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang G, Zhang X, Yu B, Ren K. Gliotoxin potentiates osteoblast differentiation by inhibiting nuclear factor-κB signaling. Mol Med Rep. 2015;12(1):877–84. doi: 10.3892/mmr.2015.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]