Abstract
Objective:
Depression screening among children and adolescents is controversial. In 2009, the United States Preventive Services Task Force first recommended routine depression screening for adolescents, and this recommendation was reiterated in 2016. However, no randomized controlled trials (RCTs) of screening were identified in the original 2009 systematic review or in an updated review through February 2015. The objective of this systematic review was to provide a current evaluation to determine whether there is evidence from RCTs that depression screening in childhood and adolescence improves depression outcomes.
Method:
Data sources included the MEDLINE, MEDLINE In-Process, EMBASE, PsycINFO, Cochrane CENTRAL and LILACS databases searched February 2, 2017. Eligible studies had to be RCTs that compared depression outcomes between children or adolescents aged 6 to 18 years who underwent depression screening and those who did not.
Results:
Of 552 unique title/abstracts, none received full-text review. No RCTs that investigated the effects of screening on depression outcomes in children or adolescents were identified.
Conclusions:
There is no direct RCT evidence that supports depression screening among children and adolescents. Groups that consider recommending screening should carefully consider potential harms, as well as the use of scarce health resources, that would occur with the implementation of screening programs.
Keywords: depressive disorders, screening, child and adolescent psychiatry
Abstract
Objectif:
Le dépistage de la dépression chez les enfants et les adolescents est controversé. En 2009, le groupe de travail des services préventifs des États-Unis a été le premier à recommander le dépistage systématique de la dépression pour les adolescents, recommandation qui a été réitérée en 2016. Cependant, aucun essai randomisé contrôlé (ERC) de dépistage n’a été identifié dans la revue systématique originale de 2009 ou dans une revue mise à jour jusqu’en février 2015. L’objectif de cette revue systématique était de fournir une évaluation actuelle afin de déterminer si oui ou non les ECT offrent des données probantes à l’effet que le dépistage de la dépression chez les enfants et les adolescents améliore les résultats de la dépression.
Méthode:
Les sources des données recherchées le 2 février 2017 étaient entre autres les bases de données MEDLINE, MEDLINE In-Process, EMBASE, PsycINFO, Cochrane CENTRAL et LILACS. Les études admissibles devaient être des ERC qui comparaient les résultats de la dépression entre des enfants ou adolescents de 6 à 18 ans qui ont subi un dépistage de la dépression et ceux qui n’en ont pas subi.
Résults:
Sur les 552 titres/résumés isolés, aucun n’a fait l’objet d’une révision du texte intégral. Aucun ERC qui recherchait les effets du dépistage sur les résultats de la dépression chez les enfants et les adolescents n’a été identifié.
Conclusions:
Il n’y a pas de données probantes directes des ERC qui soutiennent le dépistage de la dépression chez les enfants et les adolescents. Les groupes qui envisagent de recommander le dépistage devraient considérer attentivement les effets nuisibles potentiels ainsi que l’utilisation des rares ressources de santé qu’entraînerait la mise en oeuvre des programmes de dépistage.
Depression in children and adolescents is a disabling condition associated with long-term mental and physical health problems.1 Screening is one possible solution to improve depression management. Depression screening involves the use of a self-report depression symptom questionnaire with a pre-specified cutoff score to identify patients who may have depression but who have not sought treatment and have not otherwise been recognized as depressed.2,3 Screening differs from routine assessment, in which a health care provider employs careful observation and thoughtful, appropriate questions to evaluate a patient’s health and determine if more focused examination is needed. It is also different from case-finding, in which questionnaires may be used to evaluate symptoms in people suspected of having depression.4
Depression screening is controversial.5–11 The United States Preventive Services Task Force (USPSTF) recently reiterated their recommendation for screening adults for depression in primary care.12 This recommendation has been criticized, however, for relying on indirect evidence from studies of screening tool accuracy and treatment effectiveness, rather than evidence from randomized controlled trials (RCTs) that show that patients who are screened have better mental health outcomes than patients who are not screened. Trials cited as evidence by the USPSTF have evaluated whether collaborative care for patients already identified as depressed is more effective than usual care or that have otherwise conflated treatment and screening.5–7,10,11,13 Concern has also been raised that the USPSTF recommendation fails to adequately consider possible harms from screening, such as overdiagnosis and overtreatment, as well as the consumption of scarce healthcare resources in a context where people with known mental health problems struggle to obtain adequate care.5–7,10,11 In the UK, neither the National Institute for Health and Care Excellence (NICE)4 nor the National Screening Committee14 recommend depression screening. Similarly, the Canadian Task Force on Preventive Health Care (CTFPHC) recommends against screening adults for depression.13,15,16
Depression screening guidelines for children and adolescents are similarly inconsistent. In 2016, the USPSTF reiterated its 2009 recommendation17 that adolescents, but not younger children, should be routinely screened for depression in primary care settings when depression care systems are in place to ensure accurate diagnosis, treatment and follow-up.18 This recommendation was made even though neither the 2009 systematic review nor an updated review through February 2015 identified any RCTs that compared depression outcomes between screened and unscreened adolescents.1,19 In Canada, the CTFPHC determined in 2005 that there was insufficient evidence to recommend screening children and adolescents for depression, and this recommendation has not been updated.20 On the other hand, the Canadian Paediatric Society endorses the use of a clinical tool for periodic health visits that recommends depression screening for adolescents per USPSTF guidelines.21 In the UK, NICE has emphasized that primary care practitioners should be alert to symptoms of depression in children and adolescents, but does not recommend screening.22,23
Experts have argued that guideline makers should refrain from recommending new screening services based on only low-quality indirect evidence.7 Thus, recommendations for screening should ideally be based on the demonstration of benefits in excess of potential harms in well-conducted RCTs. These RCTs should clearly separate any effects of being screened from the effects of different depression management options that may be available for those identified as depressed through screening or without screening.5–7,24
The objective of the present systematic review was to evaluate whether there is direct RCT evidence that depression screening improves depression outcomes in children and adolescents.
Method
Detailed methods were registered in the PROSPERO prospective register of systematic reviews (CRD42012003194), and a review protocol was published.25
Search Strategy
The MEDLINE, MEDLINE In-Process, EMBASE, PsycINFO, Cochrane CENTRAL and LILACS databases were searched through to February 2, 2017 using a peer-reviewed search strategy (Supplemental File 1). Searches included articles published from January 2006 or later because an earlier Agency for Healthcare Research and Quality systematic review, which identified no trials that investigated the effects of screening on depression outcomes,1 searched through to May 2006. To identify unpublished or ongoing trials, we also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform. Search results were downloaded into the citation management database RefWorks (RefWorks-COS, Bethesda, MD, USA), and the software’s duplication check was used to identify citations retrieved from multiple sources.
Identification of Eligible Studies
Eligible articles were original studies in any language with data on children and adolescents aged 6 to 18 years, conducted in general medicine clinics, schools, and community settings. Studies of college and university populations were excluded, as college and university students have a different pathway to mental health treatment than adolescents under their parents’ care. Studies with mixed population samples were eligible if data for children or adolescents aged 6 to 18 years were reported separately or if at least 80% of the sample were aged 18 years or younger.
Eligible studies had to be RCTs that compared depression outcomes between children or adolescents who underwent depression screening and those who did not. For a trial to be considered a depression screening trial, the trial must have (1) determined patient eligibility and randomized patients before screening; (2) excluded patients already diagnosed with a recent episode of depression or being treated for depression at the time of trial enrollment; and (3) provided similar depression management options to patients found to have depression in the screening arm of the trial (via screening or otherwise) and patients in the non-screening arm identified as depressed (via other mechanisms, such as patient report or unaided physician diagnosis).6 Per the UK National Screening Committee’s definition of screening,2 eligible trials had to include a case identification strategy based on an a priori-defined cutoff score on a depression screening tool to make decisions regarding further assessment or treatment.
Two investigators independently reviewed titles/abstracts for eligibility. As there were no titles/abstracts identified as potentially eligible by either investigator, no articles underwent full-text review.
Evaluation of Eligible Studies
We did not extract data or conduct a risk of bias assessment because no trials met inclusion criteria.
Results
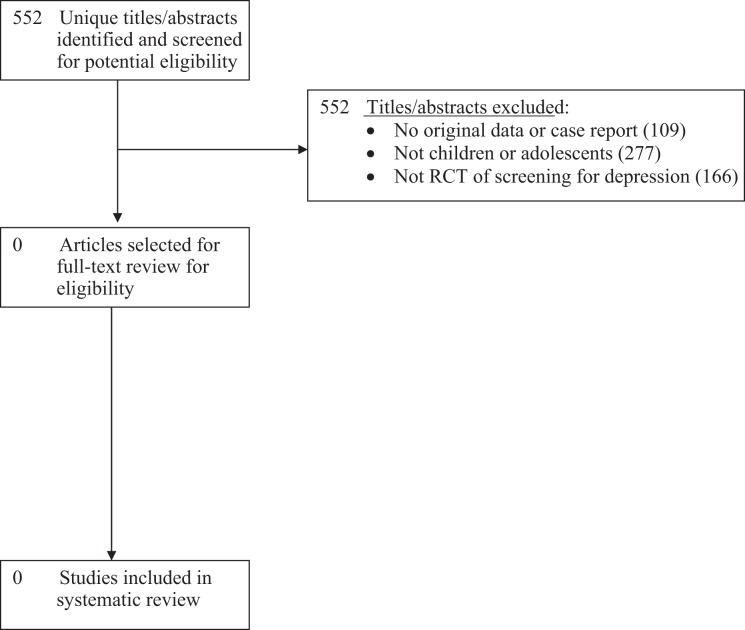
Of 552 unique titles/abstracts identified from the database search, all were excluded after title/abstract review (Figure 1). No eligible RCTs of depression screening were identified. No pre-post or other non-randomized controlled trials were identified and excluded.
Figure 1.
PRISMA Flow Diagram of Study Selection Process.
Discussion
The main finding of this systematic review was that no RCTs have evaluated whether depression screening improves depression outcomes among children and adolescents. A potential limitation might be that we did not include non-randomized studies on the effects of depression screening. The systematic review done for the recent 2016 USPSTF guideline did include them, however, and none were identified. Furthermore, we did not identify and exclude any pre-post or other non-randomized trials.
The 2016 USPSTF recommendation to screen adolescents for depression in medical settings where depression management programs are available was based on the existence of screening tools and depression treatment options, but not on data from trials comparing mental health outcomes between screened and unscreened adolescents.18,19 A recent systematic review, however, found that there is no single screening tool and cutoff that can consistently identify children or adolescents with depression and rule out those without depression. Furthermore, the small number of studies that have been conducted on screening tools in children and adolescents suggest that many children and adolescents would be falsely identified as likely depressed by these tools, in some cases due to normal variations in mood.26
As with children and adolescents, among adults, no well-conducted clinical trials that have randomized untreated patients prior to screening for depression and then provided the same depression treatment to patients identified as depressed in both unscreened and screened trial arms have found that screening improves mental health outcomes.6,27 To address the question of whether depression screening improves outcomes in childhood and adolescence, welldesigned RCTs are needed that determine eligibility and randomize patients before screening; exclude patients already known to have depression or already receiving treatment for depression; and provide similar depression care to patients in either trial group who are identified as depressed via screening or via other methods.5
Despite the lack of direct evidence in support of depression screening among children and adolescents and disagreement between current guidelines, screening programs have already been implemented in many schools and medical contexts. Before its unexplained closure in 2012, the TeenScreen program, based at Columbia University, aggressively promoted routine depression screening in adolescents and was reportedly active at numerous sites across the United States and internationally.28 In Canada, several provincial governments have called for depression screening in schools and medical settings.29–31
Depression screening may be useful only to the extent that it improves patient outcomes beyond those of standard care. In the absence of evidence that depression screening benefits children and adolescents, the USPSTF and Canadian organizations that consider recommending screening should carefully consider potential harms, such as false-positive screens and overdiagnosis,26 as well as the use of costly health care resources, which would occur with routine depression screening.
Conclusions
In conclusion, recommendations for depression screening in children and adolescents are not supported by evidence from any RCTs that screening programs would improve depression outcomes. Implementation of screening programs would result in harm to some children and adolescents who are screened. They would also consume scarce healthcare resources that would thus not be available to treat youth who have mental health problems but do not obtain adequate treatment. There is particular concern about the use of resources and unintended harm that may result from the implementation of depression screening programs for children and adolescents, because major guideline organizations, including the CTFPHC, USPSTF, and NICE, have not yet identified any well-conducted RCTs that have shown health benefits from depression screening, even among adults.5–11,13,15,16 Further increasing uncertainty, none of these guideline organizations have identified any trials that have shown that screening with questionnaires for any other presently experienced but unreported problems, including suicidal ideation, intimate partner violence, alcohol or drug abuse, and developmental delays, improves outcomes.32 Screening is not the only way to improve depression identification and care. Without evidence of benefit from screening, a better option would be to direct resources toward improving access to mental health services and ensuring that healthcare professionals are adequately trained to recognize, assess, and treat depression. Well-designed and conducted RCTs that test the possible benefits and potential harms of depression screening in children and adolescents are needed.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that they have no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the Canadian Institutes for Health Research (KA1 – 119795). MR was supported by a Murray R. Stalker Primary Care Research Bursary and a Mach-Gaensslen Foundation of Canada Student Grant as part of the McGill University Faculty of Medicine Research Bursary Program. BDT was supported by an Investigator Award from the Arthritis Society. No funding body had any involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716–e735. [DOI] [PubMed] [Google Scholar]
- 2. UK National Screening Committee. Second report of the UK National Screening Committee. London (GB): Departments of Health for England, Scotland, Northern Ireland and Wales; 2000. [Google Scholar]
- 3. Raffle A, Gray M. Screening: evidence and practice London (GB): Oxford University Press; 2007. [Google Scholar]
- 4. National Collaborating Center for Mental Health. The NICE guideline on the management and treatment of depression in adults (updated edition) London (GB): National Institute for Health and Clinical Excellence; 2010. [Google Scholar]
- 5. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253. [DOI] [PubMed] [Google Scholar]
- 6. Thombs BD, Ziegelstein RC, Roseman M, et al. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. BMC Med. 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lenzer J. Is the United States Preventive Services Task Force still a voice of caution? BMJ. 2017;356:j743. [DOI] [PubMed] [Google Scholar]
- 8. Gilbody S, Sheldon T, Wessely S. Should we screen for depression? BMJ. 2006;332:1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer SC, Coyne JC. Screening for depression in medical care: pitfalls, alternatives, and revised priorities. J Psychosom Res. 2003;54(4):279–287. [DOI] [PubMed] [Google Scholar]
- 10. Thombs BD, Coyne JC, Cuijpers P, et al. Rethinking recommendations for screening for depression in primary care. CMAJ. 2012;184(4):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thombs BD, Ziegelstein RC. Depression screening in primary care: why the Canadian Task Force on Preventive Health Care did the right thing. Can J Psychiatry. 2013;58(12):692–696. [DOI] [PubMed] [Google Scholar]
- 12. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380–387. [DOI] [PubMed] [Google Scholar]
- 13. Joffres M, Jaramillo A, Dickinson J, et al. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pittam G, Allaby M. Appraisal of screening for depression. Oxford, UK: Solutions for Public Health, 2014. [Google Scholar]
- 15. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. CMAJ Open. 2013;1(4):E159–E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bland RC, Streiner DL. Why screening for depression in primary care is impractical. CMAJ. 2013;185(9):753–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Preventive Services Task Force. Screening and treatment for major depressive disorder in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;123(4):1223–1228. [DOI] [PubMed] [Google Scholar]
- 18. Siu AL, US Preventive Services Task Force. Screening for depression in children and adolescents: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(5):360–366. [DOI] [PubMed] [Google Scholar]
- 19. Forman-Hoffman V, McClure E, McKeeman J, et al. Screening for major depressive disorder in children and adolescents: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164(5);342–349. [DOI] [PubMed] [Google Scholar]
- 20. MacMillan HL, Patterson CJ, Wathen CN, et al. Screening for depression in primary care: recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2005;172(1):33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greig AA, Constantin E, LeBlanc CM, et al. An update to the Greig Health Record: executive summary. Paediatr Child Health. 2016;21(5):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Collaborating Centre for Mental Health. Depression in children and young people: identification and management in primary, community, and secondary care London (GB): National Institute for Health and Clinical Excellence; 2005. [Google Scholar]
- 23. National Collaborating Centre for Mental Health. Depression in children and young people: identification and management in primary, community, and secondary care London (GB): National Institute for Health and Clinical Excellence; 2015. [Google Scholar]
- 24. UK National Screening Committee. Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. London (UK): Public Health England; 2015. [cited 2017 Feb 24]. Available from: https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme. [Google Scholar]
- 25. Thombs BD, Roseman M, Kloda LA. Depression screening and mental health outcomes in children and adolescents: a systematic review protocol. Syst Rev. 2012;1:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roseman M, Kloda LA, Saadat N, et al. Accuracy of depression screening tools to detect major depression in children and adolescents: a systematic review. Can J Psychiatry. 2016;61(12):746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thombs BD, Roseman M, Coyne JC, et al. Does evidence support the American Heart Association’s recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lenzer J. Controversial mental health program closes down. BMJ. 2012;345:e8100. [DOI] [PubMed] [Google Scholar]
- 29. Alberta Health and Wellness Communications. Positive futures—optimizing mental health for Alberta’s children & youth: a framework for action (2006–2016). Edmonton (Alberta): Government of Alberta; 2006. [cited 2017 Feb 24]. Available from: http://www.health.alberta.ca/documents/mental-health-framework-child-06.pdf. [Google Scholar]
- 30. British Columbia Guidelines and Protocols Advisory Committee. Anxiety and depression in children and youth—diagnosis and treatment. Victoria: B.C. Government; 2010. [cited 2017 Feb 24]. Available from: http://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/depressyouth.pdf. [Google Scholar]
- 31. Manitoba Healthy Living. Reclaiming hope: Manitoba’s youth suicide prevention strategy. Winnipeg: Manitoba Government; 2008. [cited 2017 Feb 24]. Available from: http://www.gov.mb.ca/healthyliving/mh/docs/hope.pdf. [Google Scholar]
- 32. Thombs BD, Saadat N, Riehm KE, et al. Consistency and sources of divergence in recommendations on screening with questionnaires for presently experienced health problems or symptoms: a comparison of recommendations from the Canadian Task Force on Preventive Health Care, UK National Screening Committee, and US Preventive Services Task Force. BMC Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.