Abstract
Background and Aims Plant–pollinator interactions shape the evolution of flowers. Floral attraction and reward traits have often been shown to affect pollinator behaviour, but the possible effect of efficiency traits on visitation behaviour has rarely been addressed. Anther position, usually considered a trait that influences efficiency of pollen deposition on pollinators, was tested here for its effect on pollinator visitation rates and visit duration in flowers of wild radish, Raphanus raphanistrum.
Methods Artificial selection lines from two experiments that expanded the naturally occurring phenotypic variation in anther position were used. In one experiment, plant lines were selected either to increase or to decrease anther exsertion. The other experiment decreased anther dimorphism, which resulted in increased short stamen exsertion. The hypothesis was that increased exsertion would increase visitation of pollen foragers due to increased visual attraction. Another hypothesis was that exsertion of anthers above the corolla would interfere with nectar foragers and increase the duration of visit per flower.
Key Results In the exsertion selection experiment, increased exsertion of both short and long stamens resulted in an increased number of fly visits per plant, and in the dimorphism experiment bee visits increased with increased short stamen exsertion. The duration of visits of nectar feeders declined significantly with increasing long stamen exsertion, which was opposite to the hypothesis.
Conclusions Until now, anther position was considered to be an efficiency trait to enhance pollen uptake and deposition. Anther position in wild radish is shown here also to have an ecological significance in attracting pollen foragers. This study suggests an additional adaptive role for anther position beyond efficiency, and highlights the multiple ecological functions of floral traits in plant–pollinator interactions.
Keywords: Anther position, artificial selection, Brassicaceae, pollen-foraging insects, pollinator-mediated selection, Raphanus raphanistrum, visitation rate
INTRODUCTION
Plant–pollinator interactions play a major role in plant speciation (Grant, 1949; van der Pijl, 1961; Levin and Kerster, 1967; Grant, 1994) and are a classic example of evolution mediated through biotic interactions (Grant, 1949; van der Pijl, 1961; Fægri and van der Piji, 1979; Proctor et al., 1996). A useful way to understand the role of floral traits in pollination is to group traits by their specific functions. First, advertisement traits attract the attention of animal pollinators and provide cues for associative learning (e.g. Laverty, 1994; Fenster et al., 2006; Higginson et al., 2006; Ushimaru et al., 2007). Secondly, reward traits are the ultimate reason for pollinator visits (e.g. Fægri and van der Pijl, 1979; Burd, 1995; Fenster et al., 2006). Last are floral traits that influence pollination efficiency via the fit of flower and pollinator, and include flower and flower-part size, shape and position (e.g. Mitchell and Shaw, 1993; Campbell et al., 1994; Hansen et al., 2003; Gómez et al., 2009). For example, the position of the anthers relative to the corolla tube opening (anther exsertion) affects pollen removal in wild radish (Conner et al., 2003).
Floral attraction and reward traits have been shown to affect visitation behaviour of pollinators in numerous studies (e.g. Conner and Rush, 1996; Melendez-Ackerman et al., 1997; Jones and Reithel, 2001; Kunze and Gumbert, 2001; Ne'eman and Kevan, 2001; Biernaskie and Cartar, 2004; Harder et al., 2004; Armbruster et al., 2005; Huber et al., 2005; Irwin and Strauss, 2005; Wolfe et al., 2005; Buide, 2006; Fenster et al., 2006; Hoballah et al., 2007). Less obvious and less well studied are the effects of efficiency traits on visitation behaviour. We propose two possible mechanisms for how anther position might affect pollinator visitation. First, prominent anthers can be part of the visual display of the flower (Nakanishi, 1982; Langanger et al., 2000; Lunau, 2000; Andersson and Jorgensen, 2005), providing a direct signal of the reward for pollen foragers. On the other hand, prominent or exserted anthers may interfere with visitors foraging for nectar and may result in longer duration visits, which in turn can increase pollen removal or deposition (Young and Stanton, 1990; Conner et al., 1995; Kudo, 2003). These hypotheses are not mutually exclusive, but both predict that anther position in the flower has a role beyond efficiency of pollen deposition.
Dimorphic or polymorphic positions of anthers, where anthers are at different heights or in whorls, is common in some angiosperm groups, such as in Narcissus, Linum and most Polemoniaceae and Brassicaceae species. Flowers of most of the approx. 4000 species in the mustard family (Brassicaceae) exhibit tetradynamy, in which there are four long and two short stamens in each flower. This trait is used as a diagnostic trait for the family (Zomlefer, 1994), but the reasons for the evolutionary stasis of this trait are unclear. There is significant genetic variation for the difference in anther heights in Brassica rapa and Raphanus raphanistrum (Karoly and Conner, 2000), and in the latter species tetradynamy is under stabilizing selection and affects pollinator efficiency (Conner et al., 2003).
Experimental studies of anther position effects on pollinator behaviour are usually categorical, i.e. they use experimental manipulation of the numbers of anthers at different heights to assess the effect of anther position on the duration of visits and pollen removal per visit (e.g. Golding et al., 1999; Kudo, 2003; Ornelas et al., 2004; Syafaruddin et al., 2006). While these studies provide insight to the function of anthers in whorls, they do not address the effects of continuous natural variation in anther positions. Using continuous variation in anther position is a complementary approach that more closely mimics natural variation for most traits (Stanton et al., 1986; Campbell et al., 1991; Conner and Rush, 1996; Conner et al., 2003). The weakness of this approach is that traits that are well adapted will show reduced variation, as the unfit variants have been removed by selection in the past. Therefore, it is difficult to determine the effect of variation in these traits on fitness. Artificial selection is a solution to this problem of a lack of variation in continuous traits (Conner, 2003, 2006; Fuller et al., 2005; Lehtila and Brann, 2007). Artificial selection is the source of most domesticated species (Meyer et al., 2012), and can be used in quantitative genetic studies for evolutionary inferences (Callahan, 2005; Fuller et al., 2005). However, it has been rarely, if ever, applied to pollination studies.
Here we used artificially selected lines of wild radish (Raphanus raphanistrum) with expanded variation in anther position to test the hypothesis that anther position, usually considered an efficiency trait, can affect pollinator visitation behaviour. Specifically, we tested the effect of long and short stamen exsertion (relative to the corolla opening) on number of visits to the plant, number of flowers probed in each such visit and the duration of these visits. We tested two predictions. (1) More exserted anthers may act as a visual attractant and increase the accessibility of reward for pollen foragers. Therefore, we predict that increased short and long stamen exsertion will increase the number of visits by pollen foragers and the time spent foraging per flower. (2) More exserted anthers may interfere with visitors foraging for nectar and may thus cause increased time spent per flower.
MATERIALS AND METHODS
Study organism
Wild radish (Raphanus raphanistrum) is an annual or short-lived perennial native to the Mediterranean region, growing mainly in disturbed areas, and was introduced to North America in the middle of the 19th century (based on herbarium specimens; Panetsos and Baker, 1967; J. Conner, unpubl. data). Flowers of wild radish exhibit tetradynamy, in which there are four long and two short stamens in each flower. Wild radish is self-incompatible and thus its reproduction is entirely dependent on insect pollination; therefore, floral adaptations for successful pollination are crucial for fitness. The most common pollinators of wild radish in Europe and North America are honey-bees, small bees, bumble-bees, syrphid flies and butterflies, particularly Pieris rapae (Conner et al., 2009); 14 of the 15 genera of these taxa tested were effective pollinators of wild radish (Sahli and Conner, 2007).
Artificial selection
Artificial selection was carried out in separate experiments for stamen dimorphism and for anther exsertion of the long stamen, with two replicates of all selected and control lines. Anther exsertion is defined as filament length minus the length of the corolla tube (Conner, 1997). Before selection, mean exsertion of the long stamen was near zero, meaning that the entire anther is exserted, whereas mean short stamen exsertion is about –2 mm (Conner and Via, 1993), which means the top of the anther is at the corolla tube opening (mean short anther length is about 2 mm; J. Conner, unpubl. res.). The initial base population for the exsertion lines was derived from a single natural population (Conner and Via, 1993) that had been maintained in large random-mating greenhouse populations (Ne ≈ 600) for seven generations (Conner, 2002); the dimorphism lines were established directly from the seeds collected from the same natural population. Filament and corolla tube lengths were measured on one early flower on each plant, usually the third (the first two often develop abnormally; J. Conner, pers. obs.). For both traits, within-family selection was used, in which the most extreme individual within each full-sib family was chosen as a parent for the next generation; completely randomized crosses were performed, with each selected plant serving as a male and a female, with no reciprocal crosses. This technique maximizes Ne and thus minimizes genetic drift (Falconer, 1989). One member of each family was randomly chosen for crosses in the control lines.
The anther exsertion experiment involved 12 families in each of six lines: two selected for increased exsertion, two for decreased exsertion and two randomly selected controls. After three generations of selection performed at Kellogg Biological Station (MI, USA; hereafter KBS), replicates of each line were split between KBS and Reed College (OR, USA; hereafter RC) for two (RC) or three (KBS) more generations of selection. For more details of this artificial selection experiment, see Conner et al. (2011).
Stamen dimorphism has a lower heritability than anther exsertion in R. raphanistrum (0·25 vs. 0·47, respectively; Karoly and Conner, 2000); thus, 20 families were used in each of four lines for the stamen dimorphism experiment. Two lines were selected for decreased dimorphism, and the other two were randomly mated controls. Each pair of lines was initiated from a base population consisting of one plant from each of 200 field-collected sibships grown in either the KBS or RC greenhouse, and selection continued for five generations, one pair of selection and control lines at KBS and the other at RC. On average, 7·4 offspring were grown and measured from each of the 20 full-sib families in each generation. We selected for an increased ratio of short to long stamen lengths, ensuring that selection is not biased toward plants with smaller flowers.
There was no evidence for significant genetic correlations between short and long stamen exsertion and reward traits: pollen production, nectar volume and nectar concentration did not differ between the selection and control lines. This is not surprising, because since exsertion is the difference between two linear dimensions, it is not correlated with overall flower size. Phenotypic correlations between short and long stamen exsertion and the attraction traits of floral display (number of flowers open when the plant was placed in the field) and petal area were all <0·3 and with one exception not significant (Supplementary Data Table S1). Still, these two advertisement traits were included in the visitation rate analyses to correct for these low correlations (see below).
Field experimental arrays
After the fifth generation of selection (sixth in the case of KBS exsertion lines), four plants from each family (anther exsertion, n = 288; stamen dimorphism, n = 320) were grown in a stratified random design in the KBS greenhouse and exsertion or dimorphism was measured on each; this was done in spring 2001 for the exsertion selection lines and spring 2002 for the dimorphism lines. One plant from each family was selected to produce the most uniform distribution of exsertion and dimorphism possible across all lines, resulting in a group of 72 plants for the exsertion lines and 80 for the dimorphism lines. For the pollinator observations, the exsertion selection plants were divided into three arrays of 24 plants each in the summer of 2001, and the dimorphism selection plants into four arrays of 20 plants each in the summer of 2002. Field work was done at KBS, which consists of a mixture of old fields, woodlands and agricultural fields. The visitors to wild radish at this site are primarily small native bees and syrphid flies (Sahli and Conner, 2007).
Pollinator observations
On 20 d from 18 June to 30 August 2001 and 28 d from 13 June to 21 August 2002, one group of plants was transported to the field site; thus, each array was in the field seven times (six times for one array in 2001), spaced across the normal core flowering season of wild radish. On each day of observation, floral traits were measured from one recently opened flower on each plant, the height of the plant was measured with a metre stick from the base of the plant to the top of the central inflorescence, and the number of open flowers on the plant was counted. The plants were placed randomly in a rectangular grid with 1 m spacing between plants (4 × 6 in 2001, 4 × 5 in 2002). Each plant was observed for 10 min, and all pollinators foraging on flowers were recorded. Total observation times were 76 h and 40 min in 2001 and 84 h and 10 min in 2002. For a sub-set of focal visitors (see the Results), we recorded the number of flowers probed by that visitor, the total duration of its visit and whether it was foraging for pollen or nectar. Flies and most bees feed only on pollen because their proboscides are too short to reach the nectar at the bottom of the 12 mm corolla tubes. The mouthparts of butterflies only allow them to feed on nectar. Honey-bees and long-tongued bees (families Anthophroridae and Megachilidae) forage for both pollen and nectar; nectar feeding is obvious because the tongues are <12 mm, so the bees struggle to insert their heads into the corolla tube. Mean time per flower was calculated as the total time a visitor spent on a plant divided by the number of flowers probed.
Statistical analysis
Individual plants were the unit of analysis; thus, for all analyses, we averaged the floral and pollinator visitation measurements across each of the six or seven field days for each plant. Exsertion of long and short filaments was calculated as the length of the filament minus the length of the corolla tube. Analysis of variance was performed to test for the effect of the artificial selection on mean exsertion compared with the control lines.
Insect visitors were lumped into three groups (bees, flies and butterflies) to ensure adequate sample sizes for analysis; other visitors (wasps, beetles and ants) totalled <3 % of visitors and were excluded from the analyses. Two response variables were used to assess the effect of floral morphology on pollinator behaviour: the average number of pollinators visiting the plant per 10 min observation period and the average time spent foraging per flower probed. The latter was obtained from the observations on the sub-sample of focal visitors (see above). The residuals were checked for normality using the Box–Cox transformation family (MASS package for R; Venables and Ripley, 2002); based on this test, time per flower was log-transformed for the significance testing.
To analyse the effect of anther position on attraction to pollinators, we used analysis of co-variance (ANCOVA) of the number of visits and time per flower against both long and short filament exsertion separately for the 2001 and 2002 experiments. In order to control for the effect of advertisement other than anthers, we added both petal area and number of open flowers as predictor variables. Poisson link-function was implemented in the model to account for the nearly Poisson distribution of the number of visits. For all analyses, we used generalized linear models (GLMs) performed in R version 3.1.2 (R Development Core Team, 2014) using RStudio. All data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1gc03.
RESULTS
Effects of artificial selection on anther exsertion
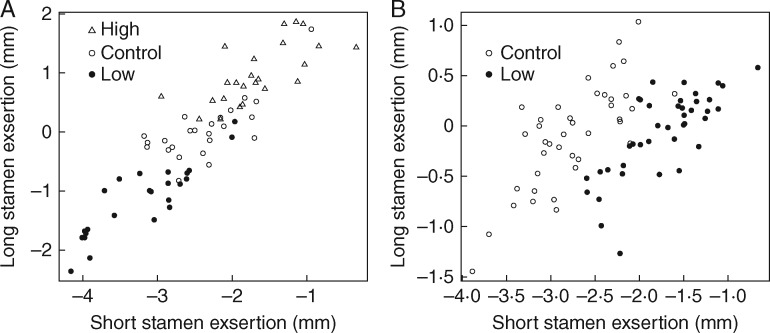
Selection on long stamen anther exsertion resulted in significant differences among treatment groups for this trait and for short anther exsertion through a correlated response (Fig. 1A;Table 1; see also Conner et al., 2011). In the dimorphism selection experiment, where the target of selection was the relative lengths of the two filaments, no difference was found in mean long stamen exsertion, but short stamen exsertion was significantly less in the selection lines compared with the control (Fig. 1B;Table 2). These mean differences among lines caused increased variance in the traits when lines were combined for the field studies, increasing our power to test the effects of exsertion on pollinators. Thus, variance in both short and long stamen exsertion was increased relative to controls alone (representing the natural population) in the 2001 study, and only variance in short stamen exsertion was increased in the 2002 experiment (Fig. 1; Table 2B).
Fig. 1.
Values of long and short stamen exsertion in experimental populations of wild radish. Points represent mean values across flowers for each plant used in the field pollination experiment. Exsertion was measured as the difference between filament length and tube length, hence, negative values indicate insertion of the bottom of the anther into the corolla tube. Because the anthers are 2 mm long on average, an exsertion of –2 means that the top of the anther is even with the tube opening, and values above this mean at least part of the anther is exserted. (A) Exsertion selection experiment (n = 72); plants were selected either to increase exsertion (‘High’) or to decrease exsertion (‘Low’). (B) Dimorphism selection experiment (n = 80); plants were selected for decreased dimorphism (‘Low’).
Table 1.
Mean short and long stamen anther exsertion in the exsertion selection experiment (2001) and dimorphism selection experiment (2002)
| Stamen | 2001 | Control | Low | 2002 | Low |
|---|---|---|---|---|---|
| High | Control | ||||
| Short filament exsertion | –1·67 ± 0·07 | –2·37 ± 0·06 | –3·21 ± 0·08 | –2·75 ± 0·06 | –1·73 ± 0·05 |
| Long filament exsertion | 0·98 ± 0·07 | 0·03 ± 0·06 | –1·14 ± 0·08 | –0·09 ± 0·06 | –0·07 ± 0·05 |
Values are the mean ± s.e. of exsertion of the anthers, expressed as the difference between filament length and tube length (in mm).
In 2001 all means were significantly different among treatments for both short and long stamens (ANOVA: F2,69 = 40·57, P < 0·001 and F2,69 = 94·17, P < 0·001, respectively; Tukey post-hoc: P < 0·001 for all comparisons).
In 2002 mean length of the long filament was not different between treatments (Student’s t-test: t74·6 = 0·17, P = 0·866), but short filament length was different between treatments (t77·6 = 9·29, P < 0·001).
Table 2.
Number of visitors recorded in wild radish plants in each year, including the sub-set that were tracked and their behaviour recorded in detail (see the Materials and Methods)
| 2001 |
2002 |
|||
|---|---|---|---|---|
| Recorded | Tracked | Recorded | Tracked | |
| Flies | 1249 | 488 | 811 | 317 |
| Bees | 437 | 269 | 3825 | 1229 |
| Butterflies | 137 | 84 | 13 | 5 |
| Other taxa | 21 | 5 | 18 | 5 |
| Total | 1844 | 846 | 4667 | 1556 |
2001: exsertion selection experiment; total observation time 76 h and 40 min.
2002: dimorphism selection experiment; total observation time 84 h and 10 min.
In order to test for an increase in variance, we performed an F-test of equality of variances (Snedecor and Cochran, 1989). In the 2001 exsertion selection experiment, the variance in exsertion of short filaments in the full array, including both control and selection lines, was 0·74, more than 2·5 times higher than the variance measured in control lines (0·296; F47,23 = 3·287, P = 0·003); the latter represent the natural variation. Variance in long filament exsertion was 1·447 in selection lines, about six times larger than in control lines (0·242, F47,23 = 5·977, P < 0·001; Fig. 1A). Nonetheless, the overall variance in short filaments across all the plants in this experiment was expanded, compared with variance in the control plants alone. Variance was 0·497 for the full array of all the plants, 2·25 times higher than control lines alone (F79,39 = 2·249, P = 0·006). Variance of long filaments was not significantly increased by the selection treatment, where all the plants combined exhibited variance of 0·216, only 1·25 times larger than control lines (F79,39 = 1·256, P = 0·436; Fig. 1B).
Composition of pollinators
The composition of pollinators differed between years (Table 2): in the 2001 study on the exsertion selection lines, flies were the majority of visitors, while in the 2002 study on the dimorphism lines bees were dominant. Bees in both years foraged mostly for pollen (69 and 88 % of bee visits in 2001 and 2002, respectively).
Effect of anther position on pollinator visitation rates
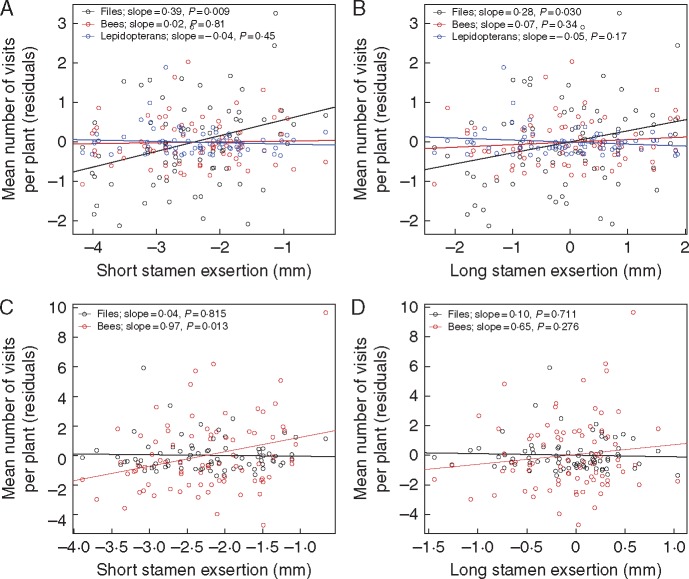
The results from both years supported the hypothesis that more exserted anthers increase attractiveness to pollen foragers, with the effect on the dominant pollinator and the traits with expanded variation in each year being statistically significant. In 2001, increased exsertion of both short and long stamen anthers resulted in an increased number of fly visits per plant (Fig. 2A, B). In 2002, number of visits of bees per plant increased with increased short stamen exsertion (Fig. 2C), but long stamen exsertion did not have a significant effect (Fig. 2D); recall that variance in long filament exsertion was not increased significantly in this experiment.
Fig. 2.
Effect of short and long anther exsertion on mean number of visits to plants by bees and flies. Data are residuals after controlling for petal area and number of open flowers per plant. In 2002, butterflies were not included because of the low number of visits (see Table 2). P-values for the significance of the difference from zero of the regression slopes are denoted for each visitor type in the key. (A and B) Effects of short and long stamen exsertion in the 2001 exsertion selection experiment. (C and D) Effects of short and long stamen exsertion in the 2002 dimorphism selection experiment.
Effect of anther position on duration of visits
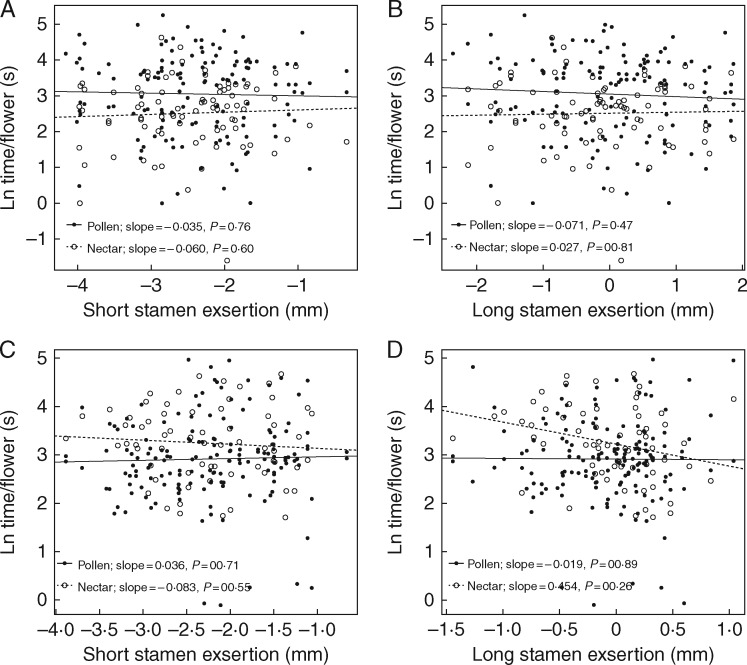
In 2001, time per flower was higher for pollen feeders (mean visit duration 35·3 s, n = 135) compared with nectar feeders (mean visit duration 18·0 s, n = 83; t = 3·68, d.f. = 193·23, P < 0·001; data were log-transformed for significance testing). Recall that individual bees were classified as either pollen or nectar feeders based on their behaviour during each visit. Visit time per flower was not significantly affected by anther exsertion and there was no significant interaction between foraging mode and exsertion of either of the stamens (ANCOVA: P > 0·3; Table 3; Fig. 3A, B). In 2002, the mean difference was opposite to 2001 – time per flower was significantly higher for nectar feeders (mean visit duration 33·8 s, n = 65) compared with pollen feeders (mean visit duration 26·1 s, n = 160; t = –2·82, d.f. = 131·56, P = 0·005). The long stamen exsertion by foraging mode interaction was marginally significant (Table 3), and the visit duration of nectar feeders declined significantly with increasing long stamen exsertion (Fig. 3D).
Table 3.
Analysis of covariance for the effect of foraging mode (pollen or nectar collection) and short and long stamen exsertion on duration per flower
| Experiment | Source | d.f. | Sum of squares | F | Significamce |
|---|---|---|---|---|---|
| 2001 | Forage | 1 | 14·7 | 12·46 | P < 0·001 |
| Long exsertion | 1 | 0·27 | 0·23 | P = 0·630 | |
| Short exsertion | 1 | 0·98 | 0·83 | P = 0·364 | |
| Forage × long exsertion | 1 | 0·51 | 0·44 | P = 0·510 | |
| Forage × short exsertion | 1 | 1·03 | 0·03 | P = 0·868 | |
| Residuals | 212 | 1302·5 | |||
| 2002 | Forage | 1 | 5·1 | 7·29 | P < 0·001 |
| Long exsertion | 1 | 1·1 | 1·50 | P = 0·222 | |
| Short exsertion | 1 | 0·5 | 0·71 | P = 0·401 | |
| Forage × long exsertion | 1 | 1·95 | 2·79 | P = 0·096 | |
| Forage × short exsertion | 1 | 0·1 | 0·17 | P = 0·680 | |
| Residuals | 219 | 2147·3 |
Duration per flower data were log-transformed for significance testing.
Fig. 3.
Mean duration of insect visits to single flowers as a function of stamen exsertion and foraging mode (pollen or nectar). (A and B) Effects of short and long stamen exsertion in the 2001 exsertion selection experiment. (C and D) Effects of short and long stamen exsertion in the 2002 dimorphism selection experiment. Values of time per flower are log-transformed.
DISCUSSION
In this study, we used artificial selection to expand the variance of anther position within a species (Fig. 1) and found that pollinator visitation was affected by this expanded variance in anther exsertion, which suggests that anther position functions in pollinator attraction as well as efficiency. The effect of floral traits on pollinator behaviour has been shown in many studies across many angiosperm families (Fægri and van der Pijl, 1979; Proctor et al., 1996; Johnson and Steiner, 2000; Willmer, 2011). These studies usually rely on natural floral variation in wild populations, and only a few studies have experimentally expanded trait variation beyond the natural range of the traits, using inter- or intra-specific hybrids (e.g. Bradshaw and Schemske, 2003; Martin et al., 2008; Sapir, 2009; Anton et al., 2013). We used artificial selection within a species to achieve a similar increase in statistical power (Conner, 2003). Previous work on wild radish, including one study using the selection lines used here, found evidence for selection on stamen exsertion (Morgan and Conner, 2001; Sahli and Conner, 2011), and here we provide evidence for one possible mechanism of this selection by examining the effects of anther exsertion on the behaviour of the main pollinators of wild radish. This enables us to test hypotheses regarding the role of anthers as advertisement, as well as their interference for nectar-foraging insects.
Our first hypothesis was that anthers are used as a direct signal of the reward to pollen foragers and thus are a visual attraction trait, as suggested by studies on the UV absorbance and reflectance by anthers (Nakanishi, 1982; Langanger et al., 2000). Support for this hypothesis in R. raphanistrum was found here: increased exsertion of short filaments in both years and of long filaments in 2001 significantly increased the number of visits by the dominant pollinator (Fig. 2A–C). Recall that the main group of insects that visited flowers each year was either exclusively or mainly foraging for pollen (flies in 2001 and bees in 2002). To our knowledge, this is the first report that anther position, largely considered as an efficiency trait (Kudo, 2003; Castellanos et al., 2004; Armbruster et al., 2009; Conner et al., 2009), is also used as an advertisement.
As with any study, it is possible that the increased attraction is not caused by anther exsertion, but rather by unmeasured traits that are genetically correlated to anther exsertion, and thus responded to our artificial selection. However, by focusing on exsertion, which is a difference between two dimensions, we remove inter-trait correlations that are caused by floral size; indeed, there were no significant responses to selection for reward and display traits (see the Materials and Methods). Further, because we included display traits in the models, we are correcting for unmeasured traits correlated with these as well. Given these facts, it is likely that the effect of anthers on pollinator behaviour is direct rather than through correlation with other traits.
Our second hypothesis was that increased anther exsertion increases the duration of nectar foraging visits through interference. This hypothesis was not supported; the only significant effect of exsertion was a decrease in time per flower of nectar feeders with increasing long stamen exsertion in 2002 (Fig. 3D). In 2001, we did not find evidence for the effect of anther exsertion on duration of visit per flower (Fig. 3A, B), while in 2002 we did observe a decrease in the duration per flower of nectar foragers with increase of exsertion of the long stamens (Fig. 3D). This evidence is opposite to our expectation and requires further detailed observation to understand this effect.
A few studies have tried to assess the adaptive role of anther position in Brassicaceae, usually proposing pollination efficiency as an adaptive explanation (Conner and Via, 1993; Conner and Sterling, 1995; Conner et al., 1995; Morgan and Conner, 2001). Here we show that anther position can act as a visual attraction, providing an alternative adaptive role for anther position, other than efficient pollen delivery. Pollen presentation as a visual attraction, as well as interference of anthers with visiting pollinators, had been proposed in the past (Lunau, 2000; Cocucci et al., 2014; Ren and Bu, 2014). However, our study is the first to test the combination of both attraction and interference and to partition it between two foraging modes, which suggest a possible mechanism of selection on anther position. Moreover, while past studies examined the natural variation of anther position, this is the first study that we are aware of that expanded the variation beyond the natural limits of the traits by using a few generations of artificial selection. These effects found here might not have been uncovered without the expanded variation in anther position traits introduced experimentally through artificial selection.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Table S1: correlations between anther position traits and advertisement traits.
Supplementary Material
ACKNOWLEDGEMENTS
We thank many undergraduates and especially C. Stewart for help with the artificial selection and field work. We thank C. Pélabon, J. Karron and an anonymous reviewer for valuable comments. This research was supported by grants from the National Science Foundation (DEB-9903880 to J.K.C. and K.K., and DEB-0919452 to J.K.C.) and the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture (agreement no. 2002* 35320-11538 to J.K.C.). This is KBS contribution no. 1996.
LITERATURE CITED
- Andersson S, Jorgensen TH.. 2005. The genetic basis of naturally occurring pollen color dimorphisms in Nigella degenii (Ranunculaceae). Journal of Heredity 96: 550–556. [DOI] [PubMed] [Google Scholar]
- Anton KA, Ward JR, Cruzan MB.. 2013. Pollinator-mediated selection on floral morphology: evidence for transgressive evolution in a derived hybrid lineage. Journal of Evolutionary Biology 26: 660–673. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Antonsen L, Pélabon C.. 2005. Phenotypic selection on Dalechampia blossoms: honest signaling affects pollination success. Ecology 86: 3323–3333. [Google Scholar]
- Armbruster WS, Pélabon C, Pérez-Barrales R, Maad J.. 2009. The adaptive accuracy of flowers: measurement and microevolutionary patterns. Annals of Botany 103: 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie JM, Cartar RV.. 2004. Variation in rate of nectar production depends on floral display size: a pollinator manipulation hypothesis. Functional Ecology 18: 125–129. [Google Scholar]
- Bradshaw HD, Schemske DW.. 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426: 176–178. [DOI] [PubMed] [Google Scholar]
- Buide ML. 2006. Pollination ecology of Silene acutifolia (Caryophyllaceae): floral traits variation and pollinator attraction. Annals of Botany 97: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd M. 1995. Pollinator behavioural response to reward size in Lobelia deckennii: no escape from pollen limitation of seed set. Journal of Ecology 83: 865–872. [Google Scholar]
- Callahan HS. 2005. Using artificial selection to understand plastic plant phenotypes. Integrative and Comparative Biology 45: 475–485. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ.. 1991. Components of phenotypic selection: pollen export and flower corolla width in Ipomopsis aggregata. Evolution 45: 1458–1467. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Price MV.. 1994. Indirect selection of stigma position in Ipomopsis aggregata via a genetically correlated trait. Evolution 48: 55–68. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD.. 2004. ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology 17: 876–885. [DOI] [PubMed] [Google Scholar]
- Cocucci AA, Marino S, Baranzelli M, Wiemer AP, Sérsic A.. 2014. The buck in the milkweed: evidence of male–male interference among pollinaria on pollinators. New Phytologist 203: 280–286. [DOI] [PubMed] [Google Scholar]
- Conner JK. 1997. Floral evolution in wild radish: the roles of pollinators, natural selection, and genetic correlations among traits. International Journal of Plant Sciences 158: S108–S120. [Google Scholar]
- Conner JK. 2002. Genetic mechanisms of floral trait correlations in a natural population. Nature 420: 407–410. [DOI] [PubMed] [Google Scholar]
- Conner JK. 2003. Artificial selection: a powerful tool for ecologists. Ecology 84: 1650–1660. [Google Scholar]
- Conner JK. 2006. Ecological genetics of floral evolution In: Harder L, Barrett S, eds. Ecology and evolution of flowers. New York: Oxford University Press. [Google Scholar]
- Conner JK, Rush S.. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105: 509–516. [DOI] [PubMed] [Google Scholar]
- Conner JK, Sterling A.. 1995. Testing hypotheses of functional relationships: a comparative survey of correlation patterns among floral traits in five insect-pollinated plants. American Journal of Botany 82: 1399–1406. [Google Scholar]
- Conner JK, Via S.. 1993. Patterns of phenotypic and genetic correlations among morphological and life-history traits in wild radish, Raphanus raphanistrum. Evolution 47: 704–711. [DOI] [PubMed] [Google Scholar]
- Conner JK, Davis R, Rush S.. 1995. The effect of wild radish floral morphology on pollination efficiency by four taxa of pollinators. Oecologia 104: 234–245. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rice AM, Stewart C, Morgan MT.. 2003. Patterns and mechanisms of selection on a family-diagnostic trait: evidence from experimental manipulation and lifetime fitness selection gradients. Evolution 57: 480–486. [DOI] [PubMed] [Google Scholar]
- Conner JK, Sahli HF, Karoly K.. 2009. Tests of adaptation: functional studies of pollen removal and estimates of natural selection on anther position in wild radish. Annals of Botany 103: 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JK, Karoly K, Stewart C, Koelling VA, Sahli HF, Shaw FH.. 2011. Rapid independent trait evolution despite a strong pleiotropic genetic correlation. American Naturalist 178: 429–441. [DOI] [PubMed] [Google Scholar]
- Fægri K, van der Pijl L.. 1979. The principles of pollination ecology. Oxford: Pergamon Press. [Google Scholar]
- Falconer DS. 1989. Introduction to quantitative genetics. New York: Academic Press. [Google Scholar]
- Fenster CB, Cheely G, Dudash MR, Reynolds RJ.. 2006. Nectar reward and advertisement in hummingbird-pollinated Silene virginica (Caryophy llaceae). American Journal of Botany 93: 1800–1807. [DOI] [PubMed] [Google Scholar]
- Fuller RC, Baer CF, Travis J.. 2005. How and when selection experiments might actually be useful. Integrative and Comparative Biology 45: 391–404. [DOI] [PubMed] [Google Scholar]
- Golding YC, Sullivan MS, Sutherland JP.. 1999. Visits to manipulated flowers by Episyrphus balteatus (Diptera: Syrphidae): partitioning the signals of petals and anthers. Journal of Insect Behavior 12: 39–45. [Google Scholar]
- Gómez JM, Abdelaziz M, Munoz-Pajares J, Perfectti F.. 2009. Heritability and genetic correlation of corolla shape and size in Erysimum mediohispanicum. Evolution 63: 1820–1831. [DOI] [PubMed] [Google Scholar]
- Grant V. 1949. Pollination systems as isolating mechanisms in angiosperms. Evolution 3: 82–97. [DOI] [PubMed] [Google Scholar]
- Grant V. 1994. Modes and origins of mechanical and ethological isolation in Angiosperms. Proceedings of the National Academy of Sciences, USA 91: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TF, Pélabon C, Armbruster WS, Carlson ML.. 2003. Evolvability and genetic constraint in Dalechampia blossoms: components of variance measures of evolvability. Journal of Evolutionary Biology 16: 754–766. [DOI] [PubMed] [Google Scholar]
- Harder LD, Jordan CY, Gross WE, Routley MB.. 2004. Beyond floricentrism: the pollination function of inflorescences. Plant Species Biology 19: 137–148. [Google Scholar]
- Higginson AD, Gilbert FS, Barnard CJ.. 2006. Morphological correlates of nectar production used by honeybees. Ecological Entomology 31: 269–276. [Google Scholar]
- Hoballah ME, Gubitz T, Stuurman J, et al. 2007. Single gene-mediated shift in pollinator attraction in Petunia. The Plant Cell 19: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP.. 2005. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 142: 564–575. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Strauss SY.. 2005. Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. American Naturalist 165: 225–237. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE.. 2000. Generalization versus specialization in plant pollination systems. Trends in Evolution and Ecology 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Jones KN, Reithel JS.. 2001. Pollinator-mediated selection on a flower color polymorphism in experimental populations of Anthirrhinum (Scrophulariaceae). American Journal of Botany 88: 447–454. [PubMed] [Google Scholar]
- Karoly K, Conner J.. 2000. Heritable variation in a family-diagnostic trait. Evolution 54: 1433–1456. [DOI] [PubMed] [Google Scholar]
- Kudo G. 2003. Anther arrangement influences pollen deposition and removal in hermaphrodite flowers. Functional Ecology 17: 349–355. [Google Scholar]
- Kunze J, Gumbert A.. 2001. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behavioral Ecology 12: 447–456. [Google Scholar]
- Langanger M, Jokl S, Musso M.. 2000. UV-reflectance in flowers of Nymphaea alba L. and Nuphar lutea (L.) Sm. (Nymphaeaceae). Aquatic Botany 67: 13–21. [Google Scholar]
- Laverty TM. 1994. Bumble bee learning and flower morphology. Animal Behaviour 47: 531–545. [Google Scholar]
- Lehtila K, Brann KH.. 2007. Correlated effects of selection for flower size in Raphanus raphanistrum. Canadian Journal of Botany 85: 160–166. [Google Scholar]
- Levin DA, Kerster HW.. 1967. Natural selection for reproductive isolation in Phlox. Evolution 21: 679–687. [DOI] [PubMed] [Google Scholar]
- Lunau K. 2000. The ecology and evolution of visual pollen signals. Plant Systematics and Evolution 222: 89–111. [Google Scholar]
- Martin NH, Sapir Y, Arnold ML.. 2008. The genetic architecture of reproductive isolation in Louisiana irises: pollination syndromes and pollinator preferences. Evolution 62: 740–752. [DOI] [PubMed] [Google Scholar]
- Melendez-Ackerman EJ, Campbell DR, Waser NM.. 1997. Hummingbird behavior and mechanisms of selection on flower color in Ipomopsis. Ecology 78: 2532–2541. [Google Scholar]
- Meyer RS, DuVal AE, Jensen HR.. 2012. Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytologist 196: 29–48. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Shaw RG.. 1993. Heritability of floral traits for the perennial wild flower Penstemon centranthifolius (Scrophulariaceae) – clones and crosses. Heredity 71: 185–192. [Google Scholar]
- Morgan MT, Conner JK.. 2001. Using genetic markers to directly estimate male selection gradients. Evolution 55: 272–281. [DOI] [PubMed] [Google Scholar]
- Nakanishi T. 1982. Morphological and ultraviolet absorption differences between fertile and sterile anthers of Japanese apricot cultivars in relation to their pollination stimuli. Scientia Horticulturae 18: 57–63. [Google Scholar]
- Ne'eman G, Kevan PG.. 2001. The effect of shape parameters on maximal detection distance of model targets by honeybee workers. Journal of Comparative Physiology A 187: 653–660. [DOI] [PubMed] [Google Scholar]
- Ornelas JF, González C, Jiménez L, Lara C, Martínez AJ.. 2004. Reproductive ecology of distylous Palicourea padifolia (Rubiaceae) in a tropical montane cloud forest. II. Attracting and rewarding mutualistic and antagonistic visitors. American Journal of Botany 91: 1061–1069. [DOI] [PubMed] [Google Scholar]
- Panetsos CA, Baker HG.. 1967. The origin of variation in ‘wild’ Raphanus sativus (Cruciferae) in California. Genetics 38: 243–274. [Google Scholar]
- van der Pijl L. 1961. Ecological aspects of flower evolution. II. Zoophilous flower classes. Evolution 15: 44–59. [Google Scholar]
- Proctor M, Yeo P, Lack A.. 1996. The natural history of pollination .London: Harper Collins. [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ren M-X, Bu Z-J.. 2014. Is there ‘anther–anther interference’ within a flower? evidences from one-by-one stamen movement in an insect-pollinated plant. PLoS One 9: e86581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli HF, Conner JK.. 2007. Visitation, effectiveness, and efficiency of 15 genera of visitors to wild radish, Raphanus raphanistrum (Brassicaceae). American Journal of Botany 94: 203–209. [DOI] [PubMed] [Google Scholar]
- Sahli HF, Conner JK.. 2011. Testing for conflicting and nonadditive selection: floral adaptation to multiple pollinators through male and female fitness. Evolution 65: 1457–1473. [DOI] [PubMed] [Google Scholar]
- Sapir Y. 2009. Effects of floral traits and plant genetic composition on pollinator behavior. Arthropod-Plant Interactions 3: 115–129. [Google Scholar]
- Snedecor GW, Cochran WG.. 1989. Statistical methods. Iowa State University Press. [Google Scholar]
- Stanton ML, Snow AA, Handel SN.. 1986. Floral evolution: attractiveness to pollinators increases male fitness. Science 232: 1625–1627. [DOI] [PubMed] [Google Scholar]
- Syafaruddin S, Horisaki A, Niikura S, Yoshioka Y, Ohsawa R.. 2006. Effect of floral morphology on pollination in Brassica rapa L. Euphytica 149: 267–272. [Google Scholar]
- Ushimaru A, Watanabe T, Nakata K.. 2007. Colored floral organs influence pollinator behavior and pollen transfer in Commelina communis (Commelinaceae). American Journal of Botany 94: 249–258. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD.. 2002. Modern applied statistics with S. Berlin: Springer. [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- Wolfe BE, Husband BC, Klironomos JN.. 2005. Effects of a belowground mutualism on an aboveground mutualism. Ecology Letters 8: 218–223. [Google Scholar]
- Young HJ, Stanton ML.. 1990. Influences of floral variation on pollen removal and seed production in wild radish. Ecology 71: 536–547. [Google Scholar]
- Zomlefer WR. 1994. Guide to flowering plant families. Chapel Hill, NC: University of North Carolina Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.