Abstract
microRNAs are small single-stranded non-coding RNA molecules which modify gene expression by silencing potential target genes. The aberrant expression of RhoA, a small GTPase protein of Rho family, is involved in gastric cancer tumorigenesis. Since miR-31 is a pleomorphic molecule, we evaluated the miR-31/RhoA axis in inducing the malignant phenotype of gastric cancer cells MKN-45. Also, the clinicopathological significance of RhoA was investigated in a well-defined collection of gastric carcinomas which were embedded in tissue microarray blocks. Induction of miR-31 in MKN-45 followed by suppression of RhoA expression resulted in increased sensitivity to 5-fluorouracil, inhibition of cell proliferation, and invasion compared to the control groups. Immunohistochemical analysis in gastric adenocarcinoma patients’ samples showed significantly higher expression of RhoA in diffuse versus intestinal subtype tumors (P = 0.009), poorly differentiated versus well and moderately differentiated tumors (P = 0.03) and the presence of vascular invasion versus the absence of vascular invasion (P = 0.04). Our findings suggest a critical role for miR-31, as a tumor suppressor gene, in gastric cancer tumorigenesis by targeting the RhoA.
Impact statement
Gastric cancer ranks as the third leading cause of cancer-associated deaths worldwide. The RhoA gene encodes a small GTPase protein of Rho family (RhoA) that its dysregulation is associated with cell motility and invasion. A strong line of evidence supports the regulation of RhoA by a number of miRs, including miR-31 in tumors. Our findings revealed that miR-31 is involved in gastric cancer tumorigenesis as a tumor suppressor gene. Through down-regulation of RhoA, miR-31 decreased cell proliferation, migration, and invasion in gastric cancer cells. In addition, induction of miR-31 increased sensitivity to 5-FU; thus, increasing its tissue concentrations could be a potential target for treatment of gastric cancer in the future.
Keywords: RhoA, gastric cancer, miR-31, invasion
Introduction
Gastric cancer is the fifth most common cancer and third fatal malignant tumor worldwide.1,2 The incidence of gastric cancer has been declining in many countries, but gastric cancer-related mortality does not follow as a favorable trend.3 At the present time, the only curative therapy for gastric cancer is complete resection of the whole tumor in the absence of distant or locoregional metastasis.4 Perioperative chemotherapy or adjuvant chemotherapy improves the survival of gastric patients, which, however, remain dismal.5,6 Cumulative evidence suggests dysregulation of RhoA is linked to the cell survival, apoptosis, metastasis, and tumorigenesis in gastric cancer.7,8 Ras homolog gene family, member A (RhoA) is a small GTPase protein of Rho family that mediates cytoskeletal remodeling and facilitates cell migration and invasion.9 Knock-down of RhoA using siRNA in gastric cancer cells causes inhibition of cancer cell proliferation and tumorigenicity and increased the sensitivity of gastric tumor cells to Adriamycin and 5-fluorouracil (5-FU).10 MicroRNAs (miRNAs) are small non-coding RNA that are involved in RNA silencing and post-transcriptional regulation of gene expression.11 MicroRNAs have received increasing attention because of their role in tumorigenesis, drug resistance, metastasis, and relapse.11 miR-31 may act as oncomiRs by targeting tumor suppressor genes or as tumor suppressive miR by targeting oncogenes in a tissue-dependent manner.12 MicroRNAs which are down-regulated in cancer tissues, called tumor suppressor genes, while miRNAs which are up-regulated in cancer tissues, are called oncomiRs.13 In the previous clinical studies, down-regulation of miR-31 in gastric cancer tissues was shown to be strongly associated with undifferentiated tumors, advanced stage, lymph node metastasis, and shorter survival.14,15 It has been shown that induction of miR-31 in gastric tumor cells can decrease the viability of gastric tumor cells, enhance apoptosis, reduce tumor cell invasion, and inhibit the in vivo tumorigenesis.15–17 Compelling evidence suggests the regulation of RhoA by a number of miRs, including miR-31 in tumors (Figure 1).18–20 The current study was designed to analyze for the first time the role of miR-31/RhoA axis in mediating cellular proliferation, chemotherapy drug resistance, cell mobility, and invasion in an in vitro model of gastric cancer.
Figure 1.
The communication between RhoA transcripts with miR-31 recognition site(s)
Materials and methods
Cell line and culture conditions
Human gastric adenocarcinoma MKN-45 cell line (NCBI Code: C615) and human embryonic kidney (HEK) 293T cells (NCBI Code: C497) were purchased from Pasteur Institute of Iran, Tehran, Iran. These cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco, Invitrogen, USA) in humidified air at 37℃ with 5% CO2.
Retroviral transduction and GFP expression assay
HEK 293 T cells were transduced with psPAX2, pMD2G, and pLEX-miR-31 or pLEX-control; all of them were purchased from Bon Yakhteh Cell Bank, Tehran, Iran. Then lenti-miR-31 and lentiviruses containing control vector were purified using nanofilters and used for transduction of MKN-45 cells. Selection of lenti-miR-31 or bare lentivirus transducted cells was made with puromycin. After incubation for 24 h, transduction efficiency was investigated by detecting GFP expression under a fluorescence microscope.
mRNA and miRNA extraction, cDNA synthesis and quantitative RT-PCR
Total RNA from MKN-45 transduced with lentivirus containing miR-31 (lenti-miR-31) or lentivirus alone and parental MKN-45 was extracted using RNX-plus solution (cat number: RN7713C, CinnaGen Inc., Tehran, Iran) as described previously.21 After the assessment of quality and quantity of RNA, reverse transcription was completed using a cDNA synthesis kit for mRNA (cat number: K1641, Fermentas Life Sciences, Germany) and Expand™ Reverse Transcriptase (cat number: 11785826001, Sigma-Aldrich, USA) for miRNA. Quantitative RT-PCR (qPCR) for RhoA and miR-31 was conducted using a Rotor-Gene 6000 system (Corbett, Concorde, NSW, Australia). Based on the miRBase database (http://www.mirbase.org/), the 5′ arm is main product of mature form of miR-31 and thus, in the current survey, specific primers were designed for analysis of this region.22 Triplicate reactions of RhoA and miR-31 were normalized with the housekeeping genes β-actin and SNORD 47 and analyzed using the relative expression software tool (REST®).23
Viability and proliferation of MKN-45 miR-31-expressing cells
The effects of 5-FU on the viability and proliferation of MKN-45 cells transduced with lenti-miR-31 and two control cell lines were investigated using the MTT assay. MKN-45 cells transduced with lenti-miR-31 and the two control cell lines were plated at a density of 1 × 104 in 96-well plates and incubated with different concentrations of 5-FU (0-10 nanomolar) for 48 h.
Cell cycle analysis using flow cytometry
The MKN-45 cells expressing miR-31, MKN-45-control vector, and parental MKN-45 were harvested and washed with PBS. Single cells were fixed in 70% ethanol and stained using propidium iodide (PI) staining solution containing PI (50 mg/L), RNase A (1 g/L), and 0.1% Triton X-100. Samples were analyzed using a fluorescence-activated cell sorting (FACS) flow cytometer (Partec, Germany) and data were analyzed using FlowJo software (Tree Star, Ashland, OR).24
Cell migration and invasion assay
Evaluation of cell migration was performed using transwell insert with a pore size of 8 µm from SPL (cat number: CBA- 100, Life Bioscience, Korea). The stably transduced MKN-45 cells by lenti-miR-31 or control lenti vector and parental MKN-45 cells were seeded at a density of 3 × 105 in the upper chamber. After 24 h, media in the lower chambers was collected, and cells grown on the chambers were trypsinized, neutralized with FBS, and counted.
Cell invasion was also investigated using transwell inserts coated with Extracellular Matrigel Matrix (ECM, cat number: ECM550, Sigma-Aldrich, USA). For this purpose, the stably transduced MKN-45 cells by lenti-miR-31 or control vector, and parental MKN-45 cells were plated at 3 × 105 cells/well. After 24 h, the invaded cells at the bottom of the filters and chambers were counted.
Western blotting
For evaluation of the effect of miR-31 overexpression on RhoA, the protein from stably transduced MKN-45 cells by lenti-miR-31 or control vector and parental MKN-45 cell lysates were extracted using RIPA buffer containing a protease inhibitor. An equal protein amount from three groups was resolved by SDS-PAGE, transferred to nitrocellulose membrane, and incubated with primary antibodies against RhoA (ab152151, Abcam, Cambridge, UK) and β-actin. The specific bands were detected using an anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase and visualized using the ECL Western blot detection kit (Amersham, Life Science, USA).
Immunohistochemical analysis of RhoA expression in intestinal subtype of gastric adenocarcinoma clinical specimens
The expression level of RhoA was evaluated in the gastric adenocarcinoma samples embedded in a tissue microarray (TMA). Immunohistochemistry staining was performed using mouse monoclonal anti-RhoA antibody (ab54835, Abcam, Cambridge, UK), as described previously.25–27 The immunostained TMA slides were examined and scored by two trained pathologists (A. K. and Z. M.) in a blinded fashion. Intensity of staining was scored as 1 (weak), 2 (moderate), and 3 (strong), while percentage of positive cells was scored as >25%, 25%–50%, 50%–75%, and 75% <. Histochemical score (H-score) was obtained by multiplying the staining intensity by the percentage of positive tumor cells. Median of H-score for RhoA expression was 200 and considered as a cut-off point to categorize samples as low (H-score ≤ 200) or high (200 < H-score) expression. In addition, the correlation between the level of RhoA expression and clinicopathological parameters was explored.
Statistical analysis
Data were analyzed using the SPSS software version 20 using one-way ANOVA method (SPSS, Chicago, IL, USA). All the results were expressed as a mean ± standard error. The relationship of RhoA status with clinicopathological parameters was explored using Pearson’s Chi-square or Fisher’s exact test. A P-value of ≤ 0.05 was considered statistically significant.
Results
Confirmation of stable miR-31 expression
Considering the presence of GFP in lentiviral vector, transduction of MKN-45 was considered successful when green color staining of more than 85% of cells was confirmed under a fluorescence microscope. Stable transduction of MKN-45 cells was validated using a qRT-PCR assay for miR-31. Our analysis showed a significant up-regulation in miR-31 expression in MKN-45-miR-31 compared to MKN-45-control vector (P = 0.01) and parental MKN-45 (P < 0.001). In the current study, all comparisons were made among the MKN-45-miR-31 (termed test), MKN-45-control vector (termed control), and parental MKN-45 (termed control).
Expression of miR-31 increased sensitivity of MKN-45 cells to 5-FU
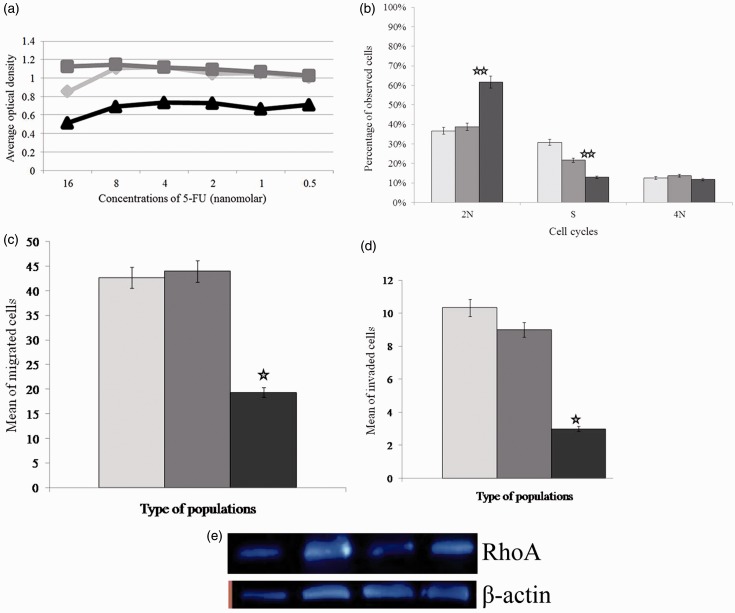
Resistance to 5-FU in MKN-45 cells stably transduced by lenti-miR-31 or control vector and parental MKN-45 was compared using the MTT assay. Stably transduced MKN-45 cells by pLEX-miR-31 were significantly more sensitive to 5-FU compared to control groups (P = 0 < 0001), whereas there was no significant difference between two control groups (P = 0.098) (Figure 2(a)).
Figure 2.
The effect of miR-31 on the sensitivity of MKN-45 to 5-FU, cell proliferation, invasion, migration, and expression of RhoA. (a) miR-31 increased the sensitivity of MKN-45 cells to 5-FU compared to control groups. (b) miR-31 decreased cell proliferation, (c) migration (d) invasion, and (e) Western blotting analysis of RhoA (from left to right: parental MKN-45, Hela cells as positive control, MKN-45-miR-31, MKN-45-control vector).
Note: In all images, parental MKN-45 cells, MKN-45-control vector, and MKN-45-miR-31 are shown with light grey, dark grey, and black colors, respectively. (A color version of this figure is available in the online journal.) *P = 0.01, and **P < 0.001
Overexpression of miR-31 decreased cell proliferation, migration, and invasion of MKN-45 cells
Analysis of cell cycle in MKN-45 cells expressing miR-31 showed a significant increase in the percentage of cells in G1/pre-G1 phase compared to the control groups (P < 0.001) and a significant decrease in the percentage of cells in S phase compared to the control groups (P < 0.001), meanwhile the percentage of cells in G2 phase was similar in all groups (P = 0.28) (Figure 2(b)). Cell migration assay revealed that MKN-45 cells expressing miR-31 had a significant reduction in the migration compared to control groups (P = 0.01), whereas the difference was not significant for control groups (P = 0.99) (Figure 2(c)). Moreover, evaluation of cell invasion showed a significant difference between the test and control groups (P = 0.01), but not between control sub-groups (P = 0.75) (Figure 2(d)).
Decreased expression of RhoA in MKN-45 cells expressing miR-31
The effect of induction of miR-31 on RhoA expression as assayed by qRT-PCR showed a significant decrease of RhoA expression in MKN-45 cells expressing miR-31 relative to MKN-45-control vector (P < 0.001) and parental MKN-45 (P < 0.001). Protein expression of RhoA using Western blotting indicated down-regulation in MKN-45 cells expressing miR-31, but not in MKN-45 cells transduced with the control vector and parental MKN-45 (Figure 2(e)).
RhoA expression in clinical gastric cancer tumors and its correlation with clinicopathological features
Immunohistochemical analysis of RhoA in gastric adenocarcinoma samples showed mainly a cytoplasmic pattern of staining. From a total of 101 gastric cancer patients’ samples, 52 (52%) cases showed low expression of RhoA, while 49 (48%) expressed high levels of RhoA expression. The expression of RhoA showed a significant difference between diffuse and intestinal subtype of gastric adenocarcinoma (P = 0.009). Higher expression of RhoA was mainly seen in poorly differentiated gastric adenocarcinoma compared to the well and moderately differentiated tumors (P = 0.03). We also found increased expression of RhoA in gastric adenocarcinoma cases with vascular invasion compared to the absence of vascular invasion (P = 0.04). No significant association was found between RhoA expression with TNM stage (P = 0.65), neural invasion (P = 0.81), and omental involvement (P = 0.87) (Table 1).
Table 1.
The correlation between RhoA expression and clinicopathological features in patients with gastric cancer (P-value; Pearson χ2)
| Clinicopathologic characteristics | Total number (%) | RhoA expression (Median H-score = 200) |
P | |
|---|---|---|---|---|
| Low | High | |||
| Median age (ranged: 30–100 years) | ||||
| ≤62 | 50 (49) | 22 (44) | 28 (56) | 0.13 |
| >62 | 51 (51) | 30 (59) | 21 (41) | |
| Gender | ||||
| Male | 83 (82) | 43 (52) | 40 (48) | 0.88 |
| Female | 18 (18) | 9 (50) | 9 (50) | |
| Depth of invasion | ||||
| pT1 | 6 (6) | 2 (33) | 4 (67) | 0.35 |
| pT2–T4 | 95 (94) | 50 (53) | 45 (47) | |
| Lymph node metastasis | ||||
| N 0 | 23 (23) | 10 (43) | 13 (57) | 0.38 |
| N 1–3 | 78 (77) | 42 (54) | 36 (46) | |
| Tumor stage (TNM) | ||||
| I | 16 (16) | 6 (37) | 10 (63) | 0.22 |
| II - IV | 85 (84) | 46 (54) | 39 (46) | |
| Histological subtypes | ||||
| Intestinal | 72 (71) | 43 (60) | 29 (40) | 0.009 |
| Diffuse | 29 (29) | 9 (31) | 20 (69) | |
| Tumor differentiation | ||||
| Well and moderately | 46 (45) | 29 (63) | 17 (37) | 0.03 |
| poorly | 55 (55) | 23 (42) | 32 (58) | |
| Tumor location | ||||
| Cardia and body | 59 (58) | 26 (44) | 33 (56) | 0.07 |
| Antrum and diffuse | 42 (42) | 26 (62) | 16 (38) | |
| Vascular invasion | ||||
| Yes | 57 (58) | 25 (44) | 32 (56) | 0.04 |
| No | 41 (42) | 26 (63) | 15 (37) | |
| Neural invasion | ||||
| Yes | 59 (62) | 31 (53) | 28 (47) | 0.81 |
| No | 36 (38) | 18 (50) | 18 (50) | |
| Omental involvement | ||||
| Yes | 4 (6) | 2 (50) | 2 (50) | 0.34 |
| No | 61 (94) | 28 (46) | 33 (54) | |
Note: The values shown in bold, are statistically significant.
Discussion
Cumulative evidence points that aberrant expression of Rho GTPases might be associated with gastric cancer tumorigenesis.28 These are key molecules in signaling pathways that regulate cell proliferation, invasion, and death.29 Based on the regulation mode, Rho GTPases are categorized into two major groups; typical and atypical. Typical Rho GTPases act as molecular switch between the active (GTP-bound form) and inactive (GTP-bound form) states.28 RhoA is the most well-known member of typical Rho GTPases family and its dysregulation has been reported in gastric cancer cell lines and tissues.7,8,30 Hence, exploration of molecular regulators of RhoA can shed light on drug resistance, migration, and invasion mechanisms in gastric cancer cells. miR-31, a small non-coding RNA molecule, can both act as tumor suppressor gene or conversely, an oncogene and is proposed as regulator for RhoA.12,31,32 In the current study, for the first time, we examined the role of miR-31 in RhoA expression, cell cycle, drug resistance, cell motility, and invasion in MKN-45 gastric cancer cells. In our experiment, overexpression of miR-31 resulted in cell cycle inhibition, and decrease in cell motility and invasion in MKN-45 cells. Furthermore, induction of miR-31 increased sensitivity to 5-FU, a major drug in a chemotherapeutic regimen of gastric cancer, and resulted in decreased expression of RhoA at the gene and protein levels. In a prior study, down-regulation of miR-31 was found in gastric cancer tissues compared with adjacent normal tissues.14 Ruoming et al.16 showed low expression of miR-31 in gastric tumor specimens from patients with stages III/IV and distant metastasis. They also showed that miR-31, by targeting Smad4 and SGPP2, diminished gastric cancer cell invasion and progression.16 A more recent report has shown down-regulation of miR-31 in gastric cancer tissues was correlated with advanced tumor stage, lymph node metastasis, and poor survival.15 The same group also showed induction of miR-31 expression in gastric cancer cells by inhibiting E2F2 suppressed the malignant phenotype of gastric cancer cells.15 In another study, Zhang et al.17 demonstrated that miR-31, by employing integrin α5, can suppress cell invasion and tumor progression.
Our immunohistochemical analysis showed higher expression of RhoA in diffuse subtype as well as in gastric adenocarcinoma cases with poor differentiation and vascular invasion. These results reflect that overexpression of RhoA can confer aggressive behavior to gastric adenocarcinoma. In parallel with our findings, Kakiuchi et al.,8 demonstrated that RhoA expression is up-regulated in diffuse subtype of gastric cancer. Another previous study also showed up-regulation of RhoA in gastric cancer cells and that its suppression leads to inhibition of cell proliferation.30 In summary, our findings revealed that miR-31 is involved in gastric cancer tumorigenesis as a tumor suppressor gene. Through down-regulation of RhoA, miR-31 decreased cell proliferation, migration, and invasion in gastric cancer cells. In addition, induction of miR-31 increased sensitivity to 5-FU; thus, increasing its tissue concentrations could be a potential target for treatment of gastric cancer in the future.
Authors’ contributions
AK and ZM contributed to the design and interpretation of the study. AK conducted experiments. AK, RR, and ZM analyzed data. All authors reviewed the manuscript. AK, RR and AS wrote the manuscript.
Funding
This study was supported by a grant from Iran University of Medical Sciences (Grant # 94-04-126-26858).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Cancer IAfRo, Organization WH. Estimated cancer incidence, mortality, and prevalence worldwide in 2012, http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (2015, accessed 22 August 2017).
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Song H, Zhu J, Lu D. Molecular-targeted first-line therapy for advanced gastric cancer. Cochrane Database Syst Rev 2016; 7: CD011461–CD011461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725–30. [DOI] [PubMed] [Google Scholar]
- 5.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007; 357: 1810–20. [DOI] [PubMed] [Google Scholar]
- 6.Power DG, Kelsen DP, Shah MA. Advanced gastric cancer–slow but steady progress. Cancer Treat Rev 2010; 36: 384–92. [DOI] [PubMed] [Google Scholar]
- 7.Maeda M, Ushijima T. RHOA mutation may be associated with diffuse-type gastric cancer progression, but is it gain or loss? Gastric Cancer 2016; 19: 326–8. [DOI] [PubMed] [Google Scholar]
- 8.Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y, Ichimura T, Ushiku T, Funahashi S, Tateishi K, Wada I, Shimizu N, Nomura S, Koike K, Seto Y, Fukayama M, Aburatani H, Ishikawa S. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet 2014; 46: 583–7. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases 2013; 4: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N, Bi F, Pan Y, Sun L, Xue Y, Shi Y, Yao X, Zheng Y, Fan D. Reversal of the malignant phenotype of gastric cancer cells by inhibition of RhoA expression and activity. Clin Cancer Res 2004; 10: 6239–47. [DOI] [PubMed] [Google Scholar]
- 11.Hata A, Kashima R. Dysregulation of microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol 2016; 51: 121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of MicroRNAs in Cancer. Cancer Res 2016; 76: 3666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar FH, Li Y, Wang Z, Kong D, Ali S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist Updat 2010; 13: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Guo J, Li D, Xiao B, Miao Y, Jiang Z, Zhuo H. Down-regulation of miR-31 expression in gastric cancer tissues and its clinical significance. Med Oncol 2010; 27: 685–9. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Zhang X, Liu Y, Ni Z, Lin Y, Duan Z, Shi Y, Wang G, Li F. Downregulated miR-31 level associates with poor prognosis of gastric cancer and its restoration suppresses tumor cell malignant phenotypes by inhibiting E2F2. Oncotarget 2016; 7: 36577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruoming W, Zhen Y, Tengteng Z, Jisheng H. Tumor suppressor microRNA-31 inhibits gastric carcinogenesis by targeting Smad4 and SGPP2. Cancer Gene Ther 2015; 22: 564–72. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XB, Song L, Wen HJ, Bai XX, Li ZJ, Ma LJ. Upregulation of microRNA-31 targeting integrin α5 suppresses tumor cell invasion and metastasis by indirectly regulating PI3K/AKT pathway in human gastric cancer SGC7901 cells. Tumour Biol 2016; 37: 8317–25. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Bi F, Zhou X, Zheng Y. Rho GTPase regulation by miRNAs and covalent modifications. Trends Cell Biol 2012; 22: 365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang KW, Kao SY, Wu YH, Tsai MM, Tu HF, Liu CJ, Lui MT, Lin SC. Passenger strand miRNA miR-31*regulates the phenotypes of oral cancer cells by targeting RhoA. Oral Oncol 2013; 49: 27–33. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Liu S, Xia Y, Wu K. MiR-31 regulates Rho-associated kinase-myosin light chain (ROCK-MLC) pathway and inhibits gastric cancer invasion: roles of RhoA. Med Sci Monit 2016; 22: 4679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roudi R, Madjd Z, Ebrahimi M, Najafi A, Korourian A, Shariftabrizi A, Samadikuchaksaraei A. Evidence for embryonic stem-like signature and epithelial-mesenchymal transition features in the spheroid cells derived from lung adenocarcinoma. Tumour Biol 2016; 37: 11843–59. [DOI] [PubMed] [Google Scholar]
- 22.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 2014; 42: D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acid Res 2002; 30: e36–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugli E, Roederer M, Cossarizza A. Data analysis in flow cytometry: the future just started. Cytometry A 2010; 77: 705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korourian A, Roudi R, Shariftabrizi A, Kalantari E, Sotoodeh K, Madjd Z. Differential role of Wnt signaling and base excision repair pathways in gastric adenocarcinoma aggressiveness. Clin Exp Med. Epub ahead of print 1 December 2016. DOI: 10.1007/s10238-016-0443-0. [DOI] [PubMed] [Google Scholar]
- 26.Roudi R, Madjd Z, Korourian A, Mehrazma M, Molanae S, Sabet MN, Shariftabrizi A. Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer Biomark 2014; 14: 457–67. [DOI] [PubMed] [Google Scholar]
- 27.Erfani E, Roudi R, Rakhshan A, Sabet M, Shariftabrizi A, Madjd Z. Comparative expression analysis of putative cancer stem cell markers CD44 and ALDH1A1 in various skin cancer subtypes. Int J Biol Mark 2016; 31: e53–61. [DOI] [PubMed] [Google Scholar]
- 28.Haga RB, Ridley AJ. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases 2016; 7: 207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Peyrollier K, Kilic G, Brakebusch C. Rho GTPases and cancer. Biofactors 2014; 40: 226–35. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M, Liao Z, Li Z, Luo D, Shi F, Zheng Y, Bi F. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res 2009; 7: 570–80. [DOI] [PubMed] [Google Scholar]
- 31.Bhatnagar N, Li X, Padi SK, Zhang Q, Tang M, Guo B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis 2010; 1: e105–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Sempere LF, Ouyang H, Memoli VA, Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, Dragnev KH, Li H, Direnzo J, Bak M, Freemantle SJ, Kauppinen S, Dmitrovsky E. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest 2010; 120: 1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]