Abstract
Personalized features in the treatment of knee injuries and articular replacement therapies play an important role in modern life with increasing demand. Therefore, cell-based therapeutic approaches for the regeneration of traumatic defects of cartilage tissue were developed. However, great variations in the quality of repair tissue or therapeutic outcome were observed. The aim of the study was to capture and visualize individual differentiation capacities of chondrocytes derived from different donors with regard to a possible personal regeneration capacity using a cell-based therapy. The redifferentiation potential of monolayer cultured cells was analyzed in a scaffold-free three-dimensional tissue model. Furthermore, stimulating options using cartilage maturation factors such as L-ascorbic acid and transforming growth factor beta 2 (TGF-β2) on this process were of special interest. Cells and tissues were analyzed via histological and immunohistochemical methods. Gene expression was measured by quantitative real-time polymerase chain reaction. In monolayer culture, cells from all donors showed an almost identical differentiation profile. In contrast, the differentiation state of cartilage-like three-dimensional microtissues revealed clear differences with respect to individual donors. Analyses at the protein and mRNA levels showed high variations regarding cartilage-typical matrix components (e.g. proteoglycans, collagen type II) and intracellular proteins (e.g. S100). Interestingly, only donor chondrocytes with a basic tendency to re-differentiate in a three-dimensional environment were able to increase this tissue-specific maturation when exposed to L-ascorbic acid and/or TGF-β2. Our approach revealed clear-cut possibilities for classification of individual donors into responders or non-responders. On the basis of these results an in vitro platform could be designed to discriminate responders from non-responders. This in vitro three-dimensional test system may be a suitable basis to establish a “personalized diagnostic tool” with the opportunity to assess the capacity of expanded chondrocytes to respond to an autologous cell-based therapy.
Impact statement
A challenge in cell-based cartilage regeneration therapies is the identification of a “personalized diagnostic tool” to predict the chondrogenic potency of cells from patients who are going to be treated with autologous cells.
Comparing the phenotype of isolated chondrocytes from different donors in vitro revealed an individual cartilage-specific differentiation capacity. These personalized features are not detectable in vitro until the monolayer cells have the possibility to rearrange in 3D tissues.
Cells from articular cartilage in monolayer culture may not be a suitable basis to discriminate responders from non-responders with respect to a personalized cell-based therapy to treat cartilage defects. A more physiological 3D (micro-)environment enable the cells to present their individual differentiation capacity.
The here described microtissue model might be the basis for an in vitro platform to predict the therapeutic outcome of autologous cell-based cartilage repair and/or a suitable tool to identify early biomarkers to classify the patients.
Keywords: Articular cartilage, 3D culture, microtissue, scaffold-free, personalized differentiation, responder/non-responder, personalized diagnostic platform
Introduction
Modern society is influenced by an increasing life expectancy and a growing number of people participating in all kinds of sports with high risk for injuries. Therefore, the frequency of traumatic injuries of the knee joint resulting in articular cartilage lesions is growing. Despite the remarkable durability of cartilage tissue, its capacity for self-repair is very limited. Furthermore, lesions in adult cartilage tissue can induce degenerative processes that result in osteoarthritis.1 To reverse this loss of functional hyaline cartilage, several repair strategies have emerged, including replacement of lost cartilage using autologous tissues like osteochondral grafts2 or the regeneration potential of bone marrow.3 To improve the treatment of chondral defects of the knee joint, cell-based strategies like autologous chondrocyte transplantation (ACT) were established.4,5 To overcome the difficulties associated with the first generation of cell-based repair strategies, such as a graft failure or delamination,6 further improvements were introduced, like seeding cells on a collagen membrane or embedding chondrocytes in three-dimensional (3D) matrices.7–9 Several partly randomized controlled studies were published comparing the outcomes of different repair strategies with respect to defect sizes and locations as well as short-term and long-term follow-up. Taking study types, patient groups, and readout parameters into account, a great variation in the quality of repair tissues was obtained.10 This may imply the necessity to include further validation parameters that have been underestimated to date.
Furthermore, the influences of scaffold materials on chondrocyte behavior with respect to cell signaling, cell–matrix interactions, and composition of the extracellular matrix (ECM) produced by embedded cells have not been fully elucidated.10 To promote self-organization of chondrocytes into a 3D tissue architecture avoiding unwanted scaffold effects, a scaffold-free approach is recommended.11
It is already known that isolation of chondrocytes from their natural environment and subsequent monolayer expansion leads to a loss of cell–matrix interactions and a shift from type II collagen to type I collagen expression.12,13 This phenotypic shift is also visible by a loss of S100 in monolayer culture. 12,13 Culturing these cells in a 3D environment is able to reverse these effects. Furthermore, various growth factors such as transforming growth factor beta (TGF-β) family members, e.g. TGF-β2,14 or bone morphogenetic protein family members, e.g. BMP-2, BMP-4, BMP-6, and BMP-7,15 are suitable to support redifferentiation.
Besides these variations in culture and differentiation conditions, the individual genetic imprinting of chondrocytes might interfere with cell-based therapies. The aim of this study was to investigate the influence of donor characteristics on the redifferentiation potential of isolated chondrocytes after propagation in monolayer cultures. Chondrocytes isolated from human condyles where initially cultured as monolayers and subsequently transferred to a scaffold-free 3D culture system.11,16 Taking previous findings into account, TGF-β2 was utilized to support the redifferentiation process.17
Materials and methods
Tissue preparation and cell culture
Human articular cartilage samples were obtained from femoral condyles of patients undergoing knee surgery (knee joint endoprosthesis). An informed, written consent was obtained from all patients. Data were generated by performing independent experiments on cartilage samples from six different patients. Age was in between 64 years and 80 years, five male and one female donor and the histological grading of ostheoarthritis was in between 1 and 2 (Table 1). Isolation of chondrocytes was performed as previously described.11 Cartilage tissue was peeled from the condyles with a scalpel. Chondrocytes were isolated by mechanical mincing of the tissue with a scalpel followed by enzymatic treatment (collagenase, 350 U/mL in MEM alpha medium and HAM’s F12 (1:2), Biochrom, Berlin, Germany). The closed tube was placed on a shaker (Thermomixer comfort, Eppendorf, Hamburg, Germany) at 300 r/min interval mixing and incubated at 37℃ for 20 h. The isolated chondrocytes were centrifuged at 300 × g for 5 min. The supernatant was removed and the cell pellet was resuspended with 10 ml of MEM alpha medium plus HAM’s F12 enriched with 1% L-glutamine (Biochrom), 10% human serum (serum pool from voluntary donors), further designated as basal medium. The chondrocytes were plated and expanded as monolayers at 37℃ and 5% CO2. Cells were removed for subcultures using 0.05% trypsin and 0.02% EDTA (Biochrom), and plated at a defined ratio (1:3). Second passage (P2) cells were transferred to a 3D-promoting environment as described below (Figure 1). During the expansion stage, chondrocytes were cultured in basal medium without the addition of growth factors.
Table 1.
Characterization and staging of donor samples
| Donor | Gender | Age | Macroscopic appearance | Hist. gradinga | Weight of tissue sampleb | Cell yieldc | Cell viability (%)d |
|---|---|---|---|---|---|---|---|
| 1 | Male | 77 | Surface discontinuity, superficial fibrillation | 2 | 3.1 g | 8.8ċ106 | 96.3 |
| 2 | Male | 80 | Smooth, intact surface | 1 | 1.7 g | 5.8ċ106 | 94.5 |
| 3 | Female | 79 | Smooth, intact surface | 1 | 2.4 g | 7.3ċ106 | 91.8 |
| 4 | Male | 64 | Uneven, intact surface | 1.5 | 4.0 g | 10.5ċ106 | 91.4 |
| 5 | Male | 72 | Smooth, intact surface | 1 | 3.3 g | 9.2ċ106 | 93.3 |
| 6 | Male | 79 | Smooth, intact surface | 1 | 2.8 g | 7.8ċ106 | 97.1 |
According to Pritzker et al.18
Used for cell isolation.
Total number of cells isolated from healthy (unaffected) parts of cartilage.
Cell viability measured directly after cell isolation.
Figure 1.
Schematic overview showing the experimental approach used in the presented study. The different steps during the generation of scaffold free 3D cartilage like microtissues are displayed. The experimental in vitro study comprised six human cartilage condyles. They were used for (1) characterizing the initial tissue material and (2) isolating cells for monolayer culture. After expansion of cells in monolayer (characterization via IH, in part via qRT-PCR), the cells were transferred to agar overlay culture to allow microtissues formation and differentiation. (A color version of this figure is available in the online journal)
Generation of microtissues and chondrogenic redifferentiation
To induce microtissue formation, P2 chondrocytes were suspended in basal medium and seeded in agarose-coated 96-well plates at 3 × 105 cells/well. A schematic overview of the isolation and differentiation process is visualized in Figure 1. 3D chondrocyte constructs were further cultured using this agar overlay technique.11 To investigate the influence of differentiation-promoting factors, the following media were applied: basal medium, basal medium supplemented with 50 µg/ml L-ascorbic acid (Biochrom), basal medium plus 5 ng/ml TGF-β2 (Miltenyi Biotec, Bergisch Gladbach, Germany), or basal medium supplemented with 50 µg/ml L-ascorbic acid and 5 ng/ml TGF-β2.
Histological and immunohistochemical analysis
After four weeks of in vitro tissue development, constructs were harvested, embedded in Neg-50 frozen section medium (Richard Alan scientific, Kalmazoo, USA) and sectioned using a cryomicrotom (Microm GmbH, Walldorf, Germany). Cryosections on glass slides were fixed in a two-step process. A formalin fixation (4% at 4℃ for 10 min, AppliChem, Darmstadt, Germany) was followed by incubation in a mixture of methanol/acetone (1:1 at −20℃ for 10 min, Roth, Karlsruhe, Germany).17 Histological staining was performed with hematoxylin and eosin (H&E) (AppliChem) for morphological analysis and Safranin O-Fast Green (SO) (AppliChem) to visualize glycosaminoglycans. Immunohistochemical analyses were carried out to detect human collagen type I, collagen type II, and S100 in fixed cryosections or monolayer-cultured cells.13 Sections were rinsed with phosphate-buffered saline (PBS) and incubated for 20 min at room temperature (RT) with normal goat serum (Dianova, Hamburg, Germany) diluted 1:50 in PBS/0.1% BSA (Roth). Primary antibodies were diluted in PBS/0.1% bovine serum albumin (BSA) as follows: anti-collagen type I and anti-collagen type II (1:1000, MP Biomedicals, Ohio, USA), and anti-S100 (1:400, DakoCytomation, Glostrup, Denmark). The cryosections were incubated with primary antibodies in a humified chamber overnight at 4℃. After washing three times with PBS, the slides were incubated for 1 h at RT with Cy3-conjugated goat anti-mouse (Type I and II Collagen) and goat anti-rabbit (S100) antibody (Dianova, Hamburg) diluted 1:600 in PBS/0.1% BSA including DAPI (1 µg/ml; Fluka, Seelze, Germany) to stain cell nuclei. The preparations were mounted in fluorescent mounting medium (DakoCytomation) and analyzed by fluorescence microscopy. Cryosections of native human articular cartilage were used as positive control for collagen type II and S100 and as negative control for collagen type I. In order to test for unspecific binding of the secondary antibody, staining without primary antibody was included in all experiments.
Microscopy of living cells and microtissues
Microscopic imaging of histological preparations was carried out using a BX41 microscope (Olympus, Hamburg, Germany) equipped with a Color View I camera (Olympus) and CellD-Imaging software (Soft Imaging Systems, Muenster, Germany). Fluorescence imaging was performed using a fluorescence microscope system (IX81, Olympus) with a xenon burner (MT20, Olympus). Image documentation and evaluation were performed using a digital camera (F-View II, Olympus) and CellR-Imaging Software for Life Science Microscopy (Soft Imaging Systems). Immunohistochemical images were taken with a black-and-white camera and subsequently colored using the CellD-Imaging Software for Life Science Microscopy (Soft Imaging Systems). Living cells and microtissues were visualized by phase contrast microscopy (CKX 41, Olympus), reflected light microscopy (SZX10 stereo microscope, Olympus), and evaluated using a DP 71 camera (Olympus) and CellD-Imaging software.
Gene expression analysis
In addition to the expression check for chondrogenic markers on protein level, the gene expression on RNA level was analyzed for two samples (one from each donor group). These two tissue samples were big enough to end up with enough cells in P2 to perform also the RT-PCR analysis. For RNA isolation, monolayer cells were centrifuged for 5 min at 300 × g. The pellet was washed with PBS and subsequently resuspended in TriPure extraction buffer (Roche, Mannheim, Germany) using 1 × 106 cells/ml. Total RNA was extracted according to the manufacturers’ protocol. For RNA preparation from spheroids, the microtissues were washed in PBS followed by homogenization in extraction buffer (TriPure) using a pestle. The isolated total RNA was quantified by using NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) at 260/280 nm. cDNA synthesis was performed with 1.0 µg of RNA using a Transcriptor High Fidelity cDNA Kit (Roche) according to the manufacturer’s guidelines. Gene expression was quantified by quantitative real-time polymerase chain reaction (qRT-PCR) using a LightCycler 2.0 and LightCycler® FastStart DNA SYBR Green I MasterPlus-Mix (Roche) according to the manufacturers’ guidelines. Amplifications were carried out with primers for collagen type IAII (COL1A2), collagen type IIA1 (COL2A1), proteoglycan 4 (PRG4), s100B (S100B), and cartilage oligomeric matrix protein (COMP). The levels of expression were analyzed in triplicate and normalized by the expression of beta-actin (ACTB). The primer sequences used for the analysis are listed in Table 2. Relative gene expression was calculated using the 2−ΔCT method. In case of the comparison of monolayer cells and microtissues, all data were normalized to the reference gene ACTB by Delta Delta CT method. Values were displayed as x-fold change of controls.
Table 2.
Primers sequences used for quantitative qRT-PCR
| Primer Name | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|
| COL1A2 Forward COL1A2 Reverse | GGACTATGAAGTTGATGCTACT GTCACTCCTTCAACATTATATTC | 345 |
| COL2A1 Forward COL2A1 Reverse | CAACACTGCCAACGTCCAGAT CTGCTTCGTCCAGATAGGCAAT | 107 |
| PRG4 Forward PRG4 Reverse | GTGGAGAGGACTTCCAAATG GGATAAGGTCTGCCCAGAAC | 166 |
| COMP Forward COMP Reverse | TGGGTTGGAAGGACAAGAAG TTGGCCCAGATGATGTTCTC | 103 |
| S100B Forward S100B Reverse | GGGAGGGAGACAAGCACAAG CGTGGCAGGCAGTAGTAACC | 198 |
| ACTB Forward ACTB Reverse | TGATCCACATCTGCTGGAAGGT GACAGGATGCAGAAGGAGATTACT | 142 |
qRT-PCR: quantitative real-time polymerase chain reaction.
Results
Characterization of donor tissues and cells
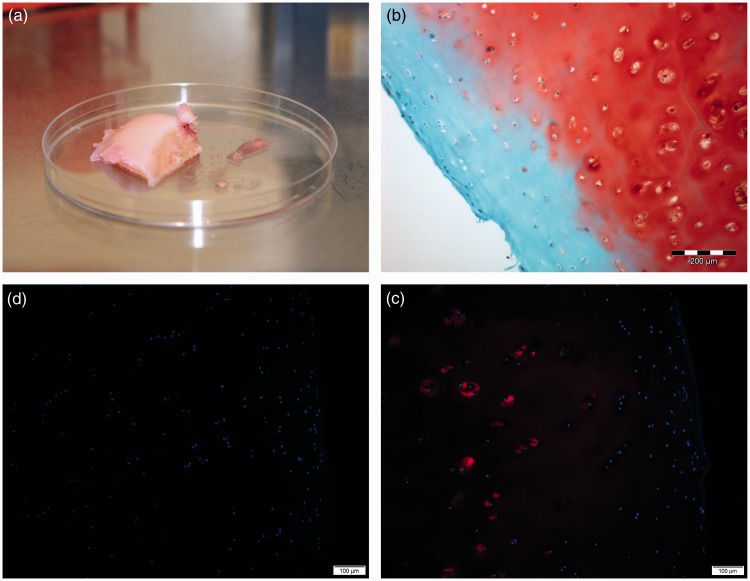
The histological gradings of the used tissues are summarized in Table 1. Exemplarily, the macroscopic appearance of a condyle of donor 2 is shown in Figure 2(a). Cartilage pieces cut out of femoral condyles were analyzed histologically to assess the overall quality of the original cartilage tissues as starting materials. H&E staining visualized the typical tissue architecture of hyaline cartilage (data not shown). Cartilage-typical proteoglycans (PGs) were detected within the ECM visualized with Safranin O staining in red, omitting only a small region linked to the superficial zone of articular cartilage (Figure 2(b), red), which reflects histopathological grade 1 of osteoarthritic cartilage in the specimens (cartilage histopathology grade assessment-grading methodology18). In general, no significant differences between the individual specimens from the donors were observed. Based on this histological grading system of osteoarthritic cartilage, four samples were assessed grade 1, one sample grade 1.5, and one sample grade 2 (Table 1). The original tissue samples expressed cartilage typical collagen type II. Highest expression is seen in the pericellular regions, reduced positivity was detected in middle and deep zones of the specimens (Figure 2(c), red). The outer zone was negative for collagen type II. This region was also negative for PGs. All donor cartilage tissues were negative for collagen type I (Figure 2(c), red).
Figure 2.
Characterization of the original cartilage tissues. (a) Macrosopic appearance of a donor condyle on a 10 cm petri dish; (b) Histological analyses of a representative native human articular cartilage sample from donor condyles using cryosections. Detection of sulfated PGs (red) by Safranin O (SO) staining. Scale bar = 200 µm. (c and d) Immunohistochemical analyses using cryosections of articular cartilage samples. Indirect immunofluorescence for collagen type II (c, red) and for collagen type I (d), blue: cell nuclei stained with DAPI. Scale bars = 100 µm. The outer surface of the articular cartilage is on the right hand side, respectively
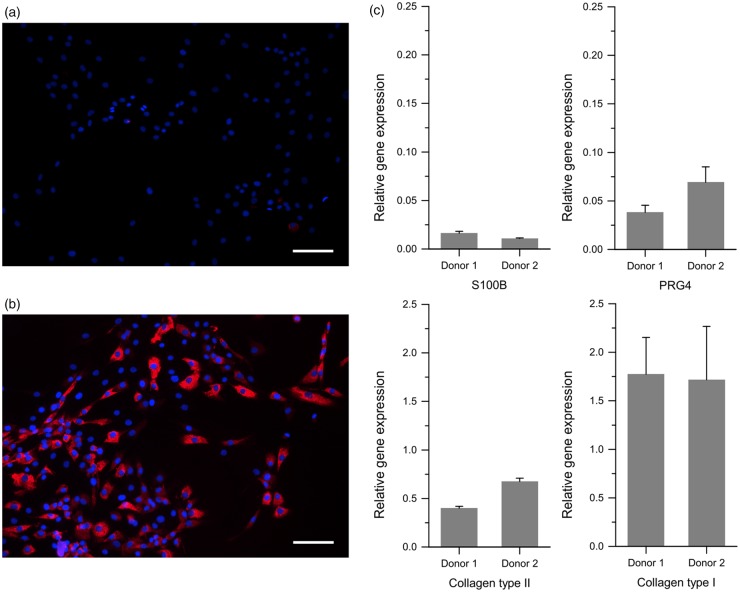
Chondrocytes expanded in monolayer cultures started to dedifferentiate, indicated by a shift from a rounded cell shape to a more fibroblastic morphology accompanied by down-regulation of collagen type II and up-regulation of collagen type I in P2 cells (Figure 3(a) and (b)). The immunohistological data were similar for all individual cell cultures established from different donor tissues. The supplementary data with respect to the expression analyses on mRNA level of cartilage-specific genes in P2 monolayer cells verified these results. The relative gene expression levels for collagen type II, s100b, and prg4 were quite low, whereas that for collagen type I was three times higher than the expression level for collagen type II. The relative gene expression values were similar among the individual donors, (Figure 3(c)).
Figure 3.
Characterization of isolated cells from cartilage tissues. Analysis of human chondrocytes in monolayer cultures at P2 (after ∼4 population doublings). Indirect immunofluorescence for collagen type II (a) and collagen type I (b) are shown. Red: antigens stained by Cy3 labeling; blue: cell nuclei stained with DAPI. Scale bars = 200 µm. (c) Relative gene expression levels of cartilage-specific markers (collagen type II, PRG4, S100B) and collagen type I normalized by β-actin expression. Here, three independent experiments were performed
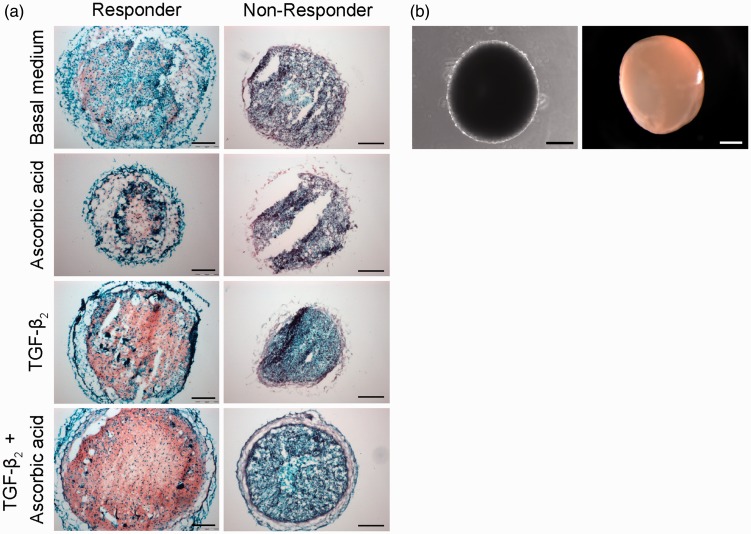
To investigate the donor-related redifferentiation capacity of the propagated chondrocytes, microtissues were generated. Individual cells were still visible on the surface of the spheroids after one week in culture (Figure 4(b) left), whereas a smooth glossy hyaline-like surface was strikingly indicative for the synthesis of ECM embedding the cells after four weeks in culture (Figure 4(b), right).
Figure 4.
Histological and morphological analyses of cartilage-like microtissues. (a) Safranin O-staining of cryosections (red: PGs; dark blue: cell nuclei). Comparison of different donors and culture medium conditions are shown. Scale bars = 200 µm. (b) Microscopic and macroscopic appearances of microtissues generated using the agar overlay technique. (Left) Phase-contrast microscopic image after one week in culture. Scale bar = 500 µm. (Right) Stereo-microscopic image after four weeks in culture, allowing visualization of the hyaline-like surface via the reflected light technique. Scale bar = 1000 µm
Microtissue morphology and PG synthesis
Safranin O-Fast Green staining revealed remarkable differences between the analyzed personalized cells and the used supplements. One group of donor cells displayed deposition of PGs for all medium compositions used in variable amounts (Figure 4(a), left column). Even microtissues cultured in basal medium only displayed Safranin O-positive regions (Figure 4, basal). Addition of TGF-β2 caused marked induction of PG synthesis, while the combination of ascorbic acid and TGF-β2 was superior and resulted in very high PG contents in the whole microtissues. Therefore, the cells in this group were designated “responders.” In contrast, no PGs were visible in the other donor-related microtissues (Figure 4, right column). Even TGF-β2 and ascorbic acid supplementation did not result in any detectable PG deposition. Therefore, the cells in this group were classified as “non-responders.”
Expression of cartilage-specific proteins
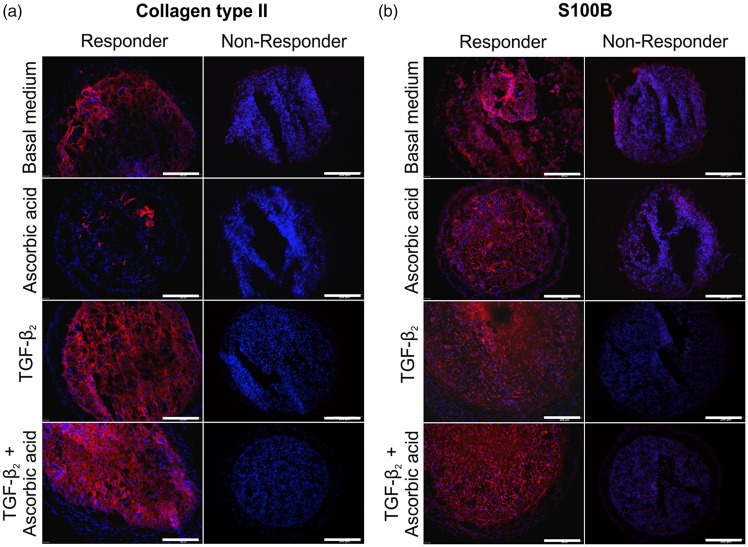
To investigate the distribution of hyaline cartilage-specific proteins within the in vitro microtissues, collagen type II and S100 were analyzed. In microtissues from responder cells (Figure 5(a), left column), variations in collagen type II expression were seen with respect to the medium composition (Figure 5(a)). Microtissues cultured in basal medium showed moderate collagen type II expression (Figure 5(a), control). Addition of TGF-β2 or TGF-β2 plus ascorbic acid resulted in broad collagen type II deposition throughout the microtissues. Addition of L-ascorbic acid only did not lead to elevated collagen type II expression. Microtissues generated from non-responder cells, identified by Safranin O analysis, did not express collagen type II at all (Figure 5(a), right column). Even supplementation with TGF-β2 or TGF-β2 plus ascorbic acid was unable to induce collagen type II expression in these cells.
Figure 5.
Immunohistochemical analyses of cartilage-specific proteins in cartilage-like microtissues. (a and b) Indirect immunofluorescence for collagen type II (a) and S100B (b) with respect to different culture medium conditions (red: collagen type II or S100B; blue: cell nuclei stained with DAPI). Scale bars = 200 µm
At first sight, the expression of the intracellular protein S100 revealed comparable results to the collagen type II findings with respect to the individual donor cells. One group of cells was able to express S100 (Figure 5(b), responders), while the other group of cells was not (Figure 5(b), non-responders). Similar to the collagen type II situation, supplementation with TGF-β2 or TGF-β2 plus ascorbic acid augmented the expression of S100 in responder cells. In contrast to the collagen type II situation, almost all cells in microtissues cultured in basal medium or supplemented with ascorbic acid only expressed intracellular S100 (Figure 5(b)), whereas collagen type II was only marginally expressed (Figure 5(a)). According to the findings of the histochemical and immunohistochemical analysis, four out of six donors were classified as responders and two as non-responders. Collagen type I was also expressed in the microtissues of the responder group, whereas microtissues of non-responders did not express collagen type I (supplementary Figure 1). For comparison, monolayer cells of all donors expressed collagen type I and did not express collagen type II (see Figure 3). Representative controls for IH are shown in Figure 7. The primary antibody was omitted and secondary antibodies detecting mouse antibodies (Figure 7(a)) or rabbit antibodies (Figure 7(b)) were applied on microtissue cryosections.
Figure 7.
Representative controls for immunohistochemistry. The primary antibody was omitted and secondary antibodies detecting mouse primary antibodies (a) or rabbit primary antibodies (b) were applied on microtissue cryosections. Scale bars = 50 µm. (A color version of this figure is available in the online journal)
Quantitative real-time PCR analyses of cartilage- specific gene expression
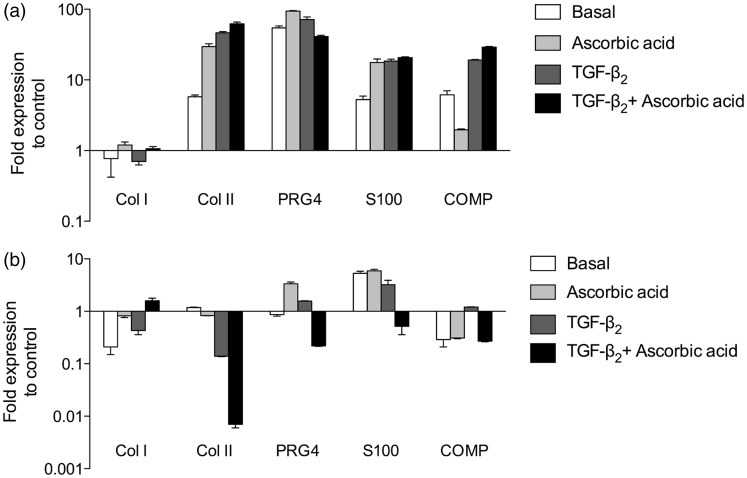
In order to investigate this variable behavior of the donor cells – responder or non-responder – during the process of redifferentiation, a quantitative real-time PCR analysis was done. This complementary analysis accompanying the analysis on protein level was done on the basis of two samples (one of each donor group). This restriction was necessary because only a limited number of cells in P2 were available for monolayer and 3D microtissue experiments. Gene expression analyses for cartilage-related genes comparing monolayer-cultured cells and their corresponding cartilage microtissues further supported the results of the immunofluorescence analyses. As shown in Figure 6, clear differences in the gene expression levels were detected between the cells from different donors. In the case of the responding donor cell group col2a1, prg4, and s100b displayed remarkable increases in gene expression in microtissues (Figure 6(a)). Furthermore, the amount of col2a1 mRNA increased stepwise by the addition of ascorbic acid via TGF-β2 towards TGF-β2 plus ascorbic acid. In contrast, the formation of 3-D microtissues was not sufficient to induce cartilage-specific gene expressions in the non-responding cell group (Figure 6(b)). Besides a slight up-regulation of s100b, no increases in gene expressions were observed. In case of the hyaline cartilage-specific gene col2a1, even a clear down regulation was observed. These quantitative PCR data as well as the immunohistochemical analyses demonstrate divergence in the redifferentiation potential of cells isolated from individual donors, thereby confirming the grouping of these cells into responders and non-responders.
Figure 6.
Expression levels of mRNAs in human in vitro cartilage-like microtissues cultured under different medium conditions. (a) Gene expression profile of cells in microtissues responding to the cartilage redifferentiation promoting treatment. (b) Expression profile of cartilage-specific genes in non-responding donor cells. (a, b) Relative gene expression levels of the cartilage-specific markers collagen type II, PRG4, S100B, and COMP were analyzed and supplemented by the expression level of the dedifferentiation marker collagen type I. The expression levels were measured by quantitative real-time RT-PCR and normalized by the b-actin expression levels. Monolayer-cultured cells from the same donor at P2 (start of 3D culture) served as controls and are depicted as baseline (number “1” at the y-axis). Data are expressed as means ± SD of three independent triplicate experiments
Discussion
ACT or autologous chondrocyte implantation (ACI) is a routine technique for the regeneration of focal cartilage lesions. Next-generation techniques were developed with the common aim for transplanting 3D tissue-like structures. Clinical studies revealed great variation in the examined characteristics such as study types, patient groups, and readout parameters.10 Identification of patients as responders is an important aim for successful application of cell-based regenerative treatments in cartilage repair. Dell’Accio et al.19 used a nude mouse assay to identify molecular markers in expanded chondrocytes for stable cartilage formation in vivo. Saris et al.20 used their results to introduce a specific gene marker cut-off score as a criterion for implantation in a clinical trial. To our knowledge, it is not currently known whether low gene scores are indeed associated with less successful outcomes, and whether high gene scores are potentially predictive of better clinical outcomes. Moreover, Stenberg et al.,21 did not observe any significant difference in the expression of the predefined gene marker set of ACI chondrocytes between successful and failed implants after three years follow up. The expression analysis was done using surplus chondrocytes in passage 2 in monolayer culture of five patients with graft failures and five patients with clinical improvement. In the present study, an in vitro 3D model was used to identify differences in the cellular capacity for redifferentiation.16 Despite almost identical RNA profiles of chondrocytes propagated in monolayer cultures from different donors (see Figure 3), we could identify two distinct groups of cell populations. The cells in one group showed a clear redifferentiation potential (designated responders), while the cells in the other group did not respond to the redifferentiation treatment (designated non-responders).
In general, chondrocytes transferred from their natural environment to an artificial monolayer culture start to express collagen type I instead of the hyaline cartilage-specific collagen type II.19,22 This phenotypic shift was also observed in all cells examined in the present study. One of the basic problems in cell-based cartilage repair strategies is to circumvent this dedifferentiation process and enable chondrocytes to maintain their redifferentiation potential.10
To avoid undesired influences from scaffold materials in the tissue engineering process and provide a system quite close to the natural process of chondrogenesis, a scaffold-free culture system was selected.11,13,16 The medium used for chondrogenic differentiation varies widely throughout the literature including serum-free, serum-containing, and a high variety of specific additives with the aim to achieve the highest differentiation of chondrocytes in vitro.12,23–25 Our study has the basic aim to capture and visualize individual differentiation capacities of chondrocytes derived from different donors with regard to a possible personal regeneration capacity using a cell-based therapy. Therefore, we want to use a differentiation strategy, which does not change the personalized intrinsic potential of cells isolated from an individual-related cartilage tissue. We want to avoid culture conditions, which force cells in a special direction. That is why we try to mimic the natural situations as close as possible. Tissue regeneration is thought to recapitulate the first steps of chondrogenesis in embryonic development of vertebrates,26 e.g. cell condensation. Therefore, we selected the scaffold-free agar overlay technique to allow an aggregation process and a self-organization of the cells as a tissue using intrinsic capacities like cell–cell contacts and paracrine communication. On the other hand, we used only human serum as medium additive for cell nutrition and assistance to differentiate.16 To get any information about a possible enhancement of this basic redifferentiation process of individual chondrocytes, we selectively added well-known specific differentiation factors (TGF-β2, L-ascorbic acid).17,27 Also, other studies use 5% or 10% serum in the differentiation medium with/without the addition of a specific growth factor28,29 or even increase the serum concentration in the differentiation medium from 5% to 20% compared to the medium for monolayer culture.27 Since Tallheden et al.23 2005 were able to show comparable differentiation capacities for chondrocytes originating from OA patients or young individuals, we decided to use one of these cell sources, OA cartilage.
Several studies have demonstrated positive effects of TGF-β on collagen type II expression in cartilage and chondrocyte maturation,24,30,31 which were confirmed in the present study within the group of responders. Addition of TGF-β2 had a promoting effect on the redifferentiation of the generated microtissues, visualized by increased PG and collagen type II synthesis and increased expression of hyaline cartilage-related genes. Besides TGF-β2, ascorbic acid was used as an inducing factor for chondrogenesis in several studies, resulting in conflicting outcomes.32,33 In our study, ascorbic acid did not have any influence on the PG, collagen type II, or S100 contents in microtissues.
Collagen type II, a hallmark of articular cartilage, was down regulated within one or two passages after chondrocyte isolation, whereas S100 was detected even after several population doublings in monolayer cultures.13,25 The first description of S100 in human chondrocytes was published 30 years ago.34 Nevertheless, S100 is still not widely accepted as a marker for the chondrogenic phenotype.16,25,35 To further support the concept of S100 as an early indicator of chondrocyte differentiation, collagen type II and S100 expressions during self-aggregation and microtissue maturation of the expanded chondrocytes were compared. For the group of responders, clear differences in the expressions of S100 and collagen type II with respect to the medium conditions used were obvious. A comparison of Figure 3(a) with Figure 3(b) supports the conceptual idea that S100 is expressed at an earlier time point during the process of redifferentiation than collagen type II. S100 was ubiquitously distributed throughout the cryosections of microtissues of responders cultured without any special additives. In contrast, the group of non-responders did not show any expression of S100 irrespective of the added redifferentiation stimuli. In addition, the early detection of S100 compared with collagen type II is another hint for the classification of S100 as an early chondrospecific marker.
The observation that the S100B expression is closely spaced by collagen type II is in accordance with the report of Diaz-Romero et al.36 observing a simultaneous upregulation of S100B and collagen type II during redifferentiation of human articular chondrocytes. Furthermore, S100 is proposed as marker to assess chondrogenicity of human articular chondrocytes, of potential value for cell-based cartilage repair treatment.24 S100 proteins seem to be dispensable for chondrogenesis.29 S100A1 and S100B have no obvious effect on chondrogenic differentiation and cartilage matrix production.37 Consequently, inhibition of S100 would not necessarily lead to inhibition of chondrogenic differentiation. Silencing of S100A1 and S100B genes by siRNA did not affect early differentiation markers but the terminal differentiation markers were markedly enhanced. S100 proteins as targets of SOX9 and its coactivators SOX5 and SOX6 did not induce early differentiation of chondrocytes but suppressed terminal (hypertropic) differentiation.37 These transcription factors show early expression during cartilage differentiation and are essential for the underlying signaling pathway.38
In summary, the cells of all analyzed individual donors displayed quite similar passage-dependent loss of cartilage-specific proteins during monolayer culture. Transfer of these dedifferentiated cells to a scaffold-free 3D culture system led to the formation of microtissues. Regarding the redifferentiation potential of the chondrocytes, clear donor-specific behaviors were obvious, leading to a classification of responders and non-responders. In addition to the individual abilities of the cells to redifferentiate in the described 3D culture, the cells also revealed donor-dependent responses to the cartilage differentiation-promoting factors ascorbic acid and TGF-β2 or TGF-β2 alone. Chondrocytes with a capacity to redifferentiate without supplementation (responders) displayed strong responses to TGF-β2 and L-ascorbic acid with respect to the expressions of hyaline cartilage-specific markers. In contrast, non-responder cells were able to form microtissues, but lacked the molecular signs of cartilaginous redifferentiation. To visualize this donor-dependent features, we focused on the analysis of the main differentiation markers of chondrocytes in monolayer cells and cryosections of 3D spheroids on protein level. Relating to cell-based therapeutic approaches to repair/regenerate cartilage defects, the evaluation of the outcome is mainly based on protein/ECM analyses using immunohistochemistry or histology (beside the ratings via appropriate clinical scores). Here, emphasis is placed on the detection and localization of proteins on sections. This approach is also recommended by the International Cartilage Repair Society (ICRS) in the “Guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials.”39 This methodological approach was also used by Mumme et al.40 in their first-in-human trial using nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects.
In conclusion, our data indicate that individual donor prerequisites may influence the therapeutic outcomes when using propagated cells for autologous cell-based cartilage regeneration therapies. We could show that analysis of common cartilage-specific markers may not be sufficient to identify responders in monolayer cultures prior to a cell-based therapy. Therefore, the identification of other attributes may be necessary to predict the personalized potential of cells to regenerate cartilage defects.
First hints regarding new markers for quality control and evaluation of ACI were described on the basis of a global gene array comparison of grafts with successful and failed outcomes.21,41 Further investigations are necessary to analyze possible personal-based factors characterizing the biological response of individual cells in a redifferentiation process in vitro. Identification of factors that regulate cell redifferentiation allowing the identification of responders and non-responders is still elusive. Obvious factors may be components of the microenvironment of the cells (e.g. cell–cell and cell–matrix contacts, specific growth factors/signaling molecules) as well as parts of the macroenvironment of the individual articular cartilage. The list of influencing factors of the macroenvironment could include the activity of the patients, the compression load applied to the knee joint, asymmetry of the joints, influence of drugs for pain treatment, other drugs, or further diseases. Broader studies are necessary to identify the factor(s) involved in the personalized differentiation capacity of monolayer-expanded human chondrocytes. These studies should be based on a higher donor number and should include the recording of clinical data of the donors to establish a “patient profile” typical for responders or non-responders.
Above all, the described in vitro platform using the formation of scaffold-free 3D microtissues to discriminate responders from non-responders may be a suitable basis to establish a “personalized diagnostic tool.” This tool would give the opportunity to assess the capacity of expanded chondrocytes to respond to an autologous cell-based therapy. Avoiding any kind of animal experiments is a suitable basis for standardizing and validating this projected “personalized diagnostic platform.” Moreover, in vitro cartilage microtissues expressing donor-specific features may be a suitable tool to look for and identify an early biomarker which would allow the identification of responders and non-responders.
Supplementary Material
Acknowledgments
The authors would like to thank colleagues from the Translational Centre for Regenerative Medicine (TRM) at the University of Leipzig for discussing the manuscript. We thank Dr. Heiko Richter (Department of Orthopedics, Hospital Senftenberg), Dr. Dietrich Lorenz and Dr. Frank Heublein (Department of Orthopedics, Hospital Hoyerswerda) for the provided cartilage tissues (left overs) and the fruitful discussions. We are grateful to Dr. Kai-Uwe Schmidtke (Faculty of Environment and Natural Sciences, Brandenburg University of Technology Cottbus-Senftenberg) for arranging the figures. The authors acknowledge the financial support of the project from the Federal Ministry of Education and Research, Germany (Code 1787X08) and from the Ministry of Science, Research, and Culture, Brandenburg, Germany.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. FM did most of the design, the experimental work, and the writing of the manuscript. ML did parts of the experimental work. US critically revised the manuscript. UA was responsible for the whole project, supervised the study, contributed to the writing and gave final approval of the version to be published. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol 2005; 17: 195–200. [DOI] [PubMed] [Google Scholar]
- 2.Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 1998; 21: 751–6. [DOI] [PubMed] [Google Scholar]
- 3.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 2001; October(391 Suppl): 362–9. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331: 889–95. [DOI] [PubMed] [Google Scholar]
- 5.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 2002; 30: 2–12. [DOI] [PubMed] [Google Scholar]
- 6.Hettrich CM, Crawford D, Rodeo SA. Cartilage repair: third-generation cell-based technologies–basic science, surgical techniques, clinical outcomes. Sports Med Arthrosc 2008; 16: 230–5. [DOI] [PubMed] [Google Scholar]
- 7.Zheng MH, Willers C, Kirilak L, Yates P, Xu J, Wood D, Shimmin A. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng 2007; 13: 737–46. [DOI] [PubMed] [Google Scholar]
- 8.Kon E, Di MA, Filardo G, Tetta C, Busacca M, Iacono F, Delcogliano M, Albisinni U, Marcacci M. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur J Radiol 2011; 79: 382–8. [DOI] [PubMed] [Google Scholar]
- 9.Stein S, Strauss E, Bosco J., III Advances in the surgical management of articular cartilage defects: autologous chondrocyte implantation techniques in the pipeline. Cartilage 2013; 4: 12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Osch GJ, Brittberg M, Dennis JE, Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT, Luyten FP. Cartilage repair: past and future – lessons for regenerative medicine. J Cell Mol Med 2009; 13: 792–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin F, Lehmann M, Anderer U. Generation of scaffold free 3-D cartilage-like microtissues from human chondrocytes. In: Daskalaki A. (ed). Medical advancements in aging and regenerative technologies: clinical tools and applications, Hershey, PA, USA: IGI Global, 2012, pp. 169–194. [Google Scholar]
- 12.Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol 2005; 202: 731–42. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann M, Martin F, Mannigel K, Kaltschmidt K, Sack U, Anderer U. Three-dimensional scaffold-free fusion culture: the way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur J Histochem 2013; 57: e31–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenkel SR, Saadeh PB, Mehrara BJ, Chin GS, Steinbrech DS, Brent B, Gittes GK, Longaker MT. Transforming growth factor beta superfamily members: role in cartilage modeling. Plast Reconstr Surg 2000; 105: 980–90. [DOI] [PubMed] [Google Scholar]
- 15.Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC. Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth Fact 2008; 26: 275–83. [DOI] [PubMed] [Google Scholar]
- 16.Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Miner Res 2002; 17: 1420–9. [DOI] [PubMed] [Google Scholar]
- 17.Martin F, Lehmann M, Schläger P, Sack U, Anderer U. Differentiation capacity of chondrocytes in microtissues depends on TGF-beta subtype. J Biochip Tissue Chip 2012; S2: 002.. DOI: 10.4172/2153-0777.S2-002–002. DOI: 10.4172/2153-0777.S2-002. [Google Scholar]
- 18.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoart Cartilage 2006; 14: 13–29. [DOI] [PubMed] [Google Scholar]
- 19.Dell'Accio F, De BC, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum 2001; 44: 1608–19. [DOI] [PubMed] [Google Scholar]
- 20.Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 200937 Suppl 1): 10S–19S. [DOI] [PubMed] [Google Scholar]
- 21.Stenberg J, de Windt TS, Synnergren J, Hynsjo L, van der Lee J, Saris DBF, Brittberg M, Peterson L, Lindahl A. Clinical outcome 3 years after autologous chondrocyte implantation does not correlate with the expressionof a predefined gene marker set in chondrocytes prior to implantation but is associated with critical signaling pathways. Orthopedic J Sports Med 2014; 2: 2325967114550781–2325967114550781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darling EM, Athanasiou KA. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res 2005; 322: 463–73. [DOI] [PubMed] [Google Scholar]
- 23.Tallheden T, Bengtsson C, Brantsing C, Sjogren-Jansson E, Carlsson L, Peterson L, Brittberg M, Lindahl A. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther 2005; 7: R560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res 1997; 237: 318–25. [DOI] [PubMed] [Google Scholar]
- 25.Giovannini S, Diaz-Romero J, Aigner T, Mainil-Varlet P, Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol 2010; 222: 411–20. [DOI] [PubMed] [Google Scholar]
- 26.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays 2000; 22: 138–47. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed N, Gan L, Nagy A, Zheng J, Wang C, Kandel RA. Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng 2009; 15: 665–73. [DOI] [PubMed] [Google Scholar]
- 28.Yoon DM, Fischer JP. Effects of exogenous IGF-1 delivery on the early expression of IGF-1 signaling molecules by alginate embedded chondrocytes. Tissue Eng 2008; 14: 1263–73. [DOI] [PubMed] [Google Scholar]
- 29.Mori Y, Mori D, Chung U, Tanaka S, Heierhorst J, Buchou T, Baudier J, Kawaguchi H, Saito T. S100A1 and S100B are dispensable for endochondral ossification during skeletal development. BiomedRes 2014; 35: 243–50. [DOI] [PubMed] [Google Scholar]
- 30.Nugent MA, Edelman ER. Transforming growth factor beta 1 stimulates the production of basic fibroblast growth factor binding proteoglycans in Balb/c3T3 cells. J Biol Chem 1992; 267: 21256–64. [PubMed] [Google Scholar]
- 31.Li TF, Chen D, Wu Q, Chen M, Sheu TJ, Schwarz EM, Drissi H, Zuscik M, O'Keefe RJ. Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J Biol Chem 2006; 281: 21296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aulthouse AL, Becker K, Beck M. Proteoglycan synthesis by cultured human chondrocytes. Microsc Res Tech 1994; 28: 520–6. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt TA, Schumacher BL, Klein TJ, Voegtline MS, Sah RL. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum 2004; 50: 2849–57. [DOI] [PubMed] [Google Scholar]
- 34.Stefansson K, Wollmann RL, Moore BW, Arnason BG. S-100 protein in human chondrocytes. Nature 1982; 295: 63–4. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann M, Martin F, Herzog N, Küpper JH, Linke R, Anderer U. Human articular chondrocytes with potential extended life span and cartilage specific phenotype as model system for pharmacological studies. Cartilage 2009; 1: 107–107. [Google Scholar]
- 36.Diaz-Romero J, Quintin A, Schoenholzer E, Pauli C, Despont A, Zumstein MA, Kohl S, Nesic D. S100A1 and S100B expression patterns identify differentiation status of human articular chondrocytes. J Cell Physiol 2014; 229: 1106–17. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. S100A1 and S100B, transcriptional targets of SOX trio, inhibit terminal differentiation of chondrocytes. EMBO Rep 2007; 8: 504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de CB. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 2002; 16: 2813–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoemann C, Kandel R, Roberts S, Saris DBF, Creemers L, Mainil-Varlet P, Méthot S, Hollander AP, Buschmann MD. International Cartilage Repair Society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2011; 2: 153–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, Asnaghi AM, Baumhoer D, Bieri O, Kretzschmar M, Pagenstert G, Haug M, Schaefer DJ, Martin I, Jakob M. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet 2016; 388: 1985–94. [DOI] [PubMed] [Google Scholar]
- 41.Stenberg J, De Windt T, Van Der Lee J, Brittberg M, Saris D, Lindahl A. Global gene array comparison between successful and failed outcome after autologous chondrocyte implantation. J Tissue Eng Regen Med 2012; 6: 61–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.