Abstract
Development of metastatic castration-resistant prostate cancer is a result of the lack of an apoptotic response by the tumor cells and loss of the ability to stick to adjacent cells through epithelial–mesenchymal transition. Although there are several strongly recommended biomarkers for determining prognosis of metastatic castration-resistant prostate cancer, only few of them may help decide the selection of the optimal treatment option. The mode of treatment sequencing in metastatic castration-resistant prostate cancer will be based on the individual characteristics of the patient. In this study, we aimed to explain the correlation between the expression characteristics of periostin, integrin-α4, and fibronectin in metastatic castration-resistant prostate cancer patients and their clinico-pathological data comprising Gleason score, PSA levels, and metastatic sites in the process of epithelial–mesenchymal transition. We evaluated by using Western blotting, periostin, integrin-α4, and fibronectin expressions in peripheral blood samples of metastatic castration-resistant prostate cancer patients (n = 40), benign prostatic hyperplasia patients (n = 20), and the healthy control group (n = 20). Associations between changes in the protein expressions and clinico-pathological parameters were also analyzed in the metastatic castration-resistant prostate cancer group. When comparing BPH and healthy groups with the metastatic castration-resistant prostate cancer group, a reduced expression of integrin-α4 was found in metastatic patients, albeit being statistically insignificant (P > 0.05). Protein expressions of periostin and fibronectin in the metastatic castration-resistant prostate cancer group were higher than those in the BPH and heathy groups (P < 0.001). Increased periostin expression in metastatic patients was significantly associated with bone metastasis (P < 0.05). Elevated periostin and fibronectin levels in metastatic castration-resistant prostate cancer patients may be appropriate targets of therapeutic intervention in the future.
Impact statement
Prostate cancer is the third most common cancer in the world and the most common cancer among men. Development of metastatic castration-resistant prostate cancer (mCRPC) is a result of the lack of an apoptotic response by the tumor cells and loss of the ability to stick to adjacent cells through epithelial–mesenchymal transition (EMT). The present study analyzes for the first time the expressions of EMT marker proteins – periostin, integrin α4, fibronectin – in mCRPC and in benign prostatic hyperplasia (BPH) with the aim to determine the clinical relevance of changes in these three proteins vis-a-vis the PCa aggressive phenotype. In doing so, it sheds light on the molecular mechanism underlying the disease. We concluded that elevated periostin and fibronectin levels in mCRPC patients may be appropriate targets of therapeutic intervention in the future; hence, adopting methods that target these proteins may help treat prostate cancer effectively.
Keywords: Epithelial–mesenchymal transition, fibronectin, gene expression, integrin α4, metastatic castration-resistant prostate cancer, periostin
Introduction
The recurrence and metastasis of prostate cancer (PCa) point to the acquisition of epithelial to mesenchymal transition (EMT) phenotype.1 During this transition, cancer cells become more fibroblastic and gain invasive and metastatic properties due to the loss of ability to adhere to adjacent neighboring cells and extracellular matrix (ECM) proteins.2 Bornstein and Sage3 defined a group of de-adhesive extracellular proteins as “matricellular.” These matricellular proteins do not contribute directly to the formation of structural elements but serve to modulate cell–matrix interactions and cell functions. They also characteristically contain binding sites for ECM structural proteins and cell surface receptors and may initiate, inhibit, or modulate activities of specific growth factors.4 The factors that are used to define the clinical state of PCa are the primary tumor’s status, presence or absence of noticeable metastases, prior and current treatment and serum testosterone levels (non-castrate/castrate).5 This dynamic clinical states transition model estimated that the point prevalence of PCa which was predicted as 2.2 million for 2009 would increase to 3.07 million in 2020.5 Castration-resistant prostate cancer (CRPC) presents involves patients without metastases or symptoms with rising prostate-specific antigen (PSA) levels despite androgen depletion therapy (ADT), and those with metastases. Metastatic CRPC (mCRPC) – bearing the highest mortality risk – is characterized by disease progression despite first-line chemotherapy associated with a steady increase in serum PSA levels and/or the emergence of new metastases. The majority (86%) of mCRPC incidence was observed from within the non-metastatic CRPC (nmCRPC) clinical state, with <15% from the non-castrate state.5
Ding et al.6 and Gao et al.7 demonstrated that PCa cells acquired mobility and invasive potential throughout EMT. EMT of cancer cells could be characterized by the expression of hallmark proteins such as periostin and fibronectin. Periostin, also called osteoblast-specific factor 2 (OSF-2), is a multifunctional glycoprotein that belongs in the group of “matricellular” proteins. Periostin can interact with other ECM proteins such as fibronectin – one of the ECM proteins, and integrins – a cell-surface receptor.8 It has been reported that the overexpression of periostin was correlated with the development of various tumors, such as colon,9 breast,10 lung,11 ovarian cancer,12 and PCa.13,14 These reports have suggested that a number of periostin-associated signaling pathways (e.g. PI3-K/Akt) promote numerous processes, such as cell growth and survival, resistance to hypoxia-induced cell death, epithelial–mesenchymal transition, invasion, tumor angiogenesis, and metastasis. Hu et al.15 showed that overexpression of periostin in PC3 and DU145 prostate cell lines increased the expression of EMT-associated factors. Few studies have investigated periostin expression in PCa patients. Periostin, as a potential biomarker, may predict the pathological grade and prognosis of PCa.16,17 Periostin expression was found to be closely correlated with aggressive phenotype in radical prostatectomy cases.18
Integrins – heterodimers consisting of α and β subunits – are one of the major families of cell surface receptors that bind to ECM proteins. Integrin signaling has deregulated in several types of cancers, including PCa.19 Integrin α4 is a “matricellular” protein receptor that forms a complex with integrin β1 or β7, which then adheres to fibronectin.20 Several reports on integrin expression in PCa showed that some α subunits including α4 were downregulated.19,21 In the occurrence and development of PCa, a reduction in the expression of α5-integrin and α7-integrin was related to Gleason score, pathological stage, lymph node metastasis, and PSA level.22 On the other hand, upregulation of β1 integrin promotes the growth and invasion of PCa cells.23
Fibronectin is an essential ECM glycoprotein involved in both physiological and pathological processes. Fibronectin pre-mRNA undergoes alternative splicing to generate over 20 splicing variants, all of which contain integrin binding motifs.24 Within tumors, changes may occur in deposition of certain matrix proteins including fibronectin. The EMT process is accelerated by increased deposition of fibronectin into the matrix during EMT via continual stimulation of integrin signaling.25 An increased expression of fibronectin was observed during androgen deprivation in PCa patients.26 In human prostate cell lines, fibronectin can upregulate the expression of matrix metalloproteinases (MMPs) which are directly involved in PCa progression.27 Lee et al.28 found that fibronectin matrix induced mesenchymal phenotypes in human PCa cells with zero or low expression levels of CD82 – a transmembrane protein which was identified as a metastasis suppressor of PCa. They showed that repression of adhesion signaling mediated by CD82 interacting with fibronectin-receptor integrins resulted in the blocking of fibronectin-induced EMT by high CD82 expression levels. This both suggests a mechanism of EMT inhibition by CD82 and supports the importance of integrin signaling in EMT.28
Although EMT in PCa has been mostly studied in cell line models, the gene expression levels and the mechanism underlying the regulation have not yet been characterized in mCRPC patients. This is the first study to analyze the expressions of EMT marker proteins – periostin, integrin α4, fibronectin – in mCRPC and in BPH with the aim to determine the clinical relevance of changes in these three proteins vis-a-vis the PCa aggressive phenotype.
Materials and methods
Patient selection and sample preparation
All 40 mCRPC (median 68; range for the patient ages 40–85) and 20 BPH (median 57; range for the patient ages 39–83) patients admitted to the Urology Clinic, Hacettepe University Hospital (Ankara, TR) were recruited into this prospective single-center study from May 2015 through June 2016. Control group (n = 20) was composed of healthy volunteer men at the urology clinic within the same age range. An a priori power analysis (R 3.0.1. open source software) indicated that 20 individuals per group would be required to detect correlations between clinico-pathological variables and expression levels (α = 5% probability type 1 error, significance level; >90% power) of the selected three EMT genes. The Ethics Committee of Gazi University Faculty of Medicine approved the study and informed consents were obtained from all subjects. All clinical information concerning mCRPC patients, including Gleason score, serum PSA values and TNM classification, were gathered from surgical records of the Urology Department. Thirty-five patients have been recently diagnosed with metastatic PCa; 5 out of 40 patients who had been pre-screened earlier were metastatic despite having been castrated during the follow-up, and PSA levels of these patients were less than 10 ng/ml and they had low tumor load. These five patients had received luteinizing hormone-releasing hormone (LHRH) analogue and metastases were observed approximately three to five years after treatment. In all mCRPC patients (stage T3–T4), the cancer had spread to lymph nodes and/or distant sites. The metastasis sites of the patients were also recorded. Peripheral blood from each patient was collected before commence of subsequent therapy.
Peripheral blood samples in EDTA-containing tubes (Monovette, Sarstedt, Numbrecht, Germany) were immediately centrifuged at 2000g for 30 min at 4℃. The separated plasma was quickly frozen in aliquots at −80℃ for later analysis. The frozen plasma was then diluted in proper amount and protein concentrations were determined in all samples using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL 61105, USA). For this method, we used 25 µl from each sample and also added working reagents to these samples. After incubation at 37℃ for 30 min, absorbance was measured at a wavelength of 562 nm using ELISA reader (Spectramax® M3; Molecular Devices LLC, Sunnyvale, CA, USA).
Western blotting
Lysates containing 25 ug of protein were mixed with a loading buffer with 5% β-mercaptoethanol and heated for 5 min at 95℃. Equal quantities of protein were loaded and separated by 12% SDS-PAGE, then transferred to a polyvinylidene difluoride membrane (Thermo Fisher Scientific, Inc., Rockford, lL 61105, USA). Following blocking with 5% w/v non-fat milk or 5% w/v bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 (TBST-T), the membrane was incubated overnight at 4℃ with rabbit anti-human Periostin antibody (Abcam, Cambridge, MA), Integrin-α4 antibody (Elabscience Biotechnology Co., Ltd, Bethesda, MD), and Fibronectin antibody (Elabscience Biotechnology Co., Ltd, Bethesda, MD), as well as rabbit anti-human β-actin monoclonal antibody (Cell Signaling Technology, Inc., Danvers, MA) as the loading control. All primary antibodies were diluted in 1: 500–1000. After three washes in TBS/0.1% Tween 20, the membranes underwent hybridization with a horseradish peroxidase-conjugated secondary rabbit IgG antibody (1:5000 dilution; Thermo Fisher Scientific, Inc., Rockford, lL 61105, USA) for 2 h at room temperature. After washing extensively in TBS/0.1% Tween 20, proteins were visualized using a Kodak Gel Logic 2200 imaging system (Kodak, Rochester, NY, USA) with Luminata™ Crescendo Western HRP substrate (EMD Millipore, Billerica, MA, USA).
Statistical analysis
Densitometry of the Western Blot protein bands was analyzed using Image J program and normalized against β-actin. Variables were processed and analyzed by using SPSS Statistics 21.0. Descriptive statistics were presented as mean ± standard deviation (SD) and median (min-max). Non-parametric tests were used for inferential statistics. Mann–Whitney-U and Kruskal–Wallis tests were performed to compare the three groups and clinico-pathological parameters (Gleason Score, PSA levels, metastatic sites). P < 0.05 was considered statistically significant.
Results
Differential expressions of periostin, integrin-α4 and fibronectin proteins
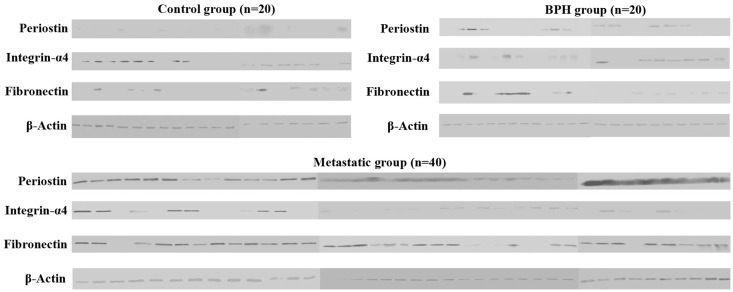
We firstly investigated expression levels of these three EMT members at protein level in control, BPH, and metastatic plasma samples. This comparison was carried out by Western Blotting with isolated total proteins from control, BPH, and metastatic blood plasma samples. Western Blot results revealed that expression levels of these genes showed variations within all groups, indicating heterogeneity of gene expression in each sample (Figure 1). Periostin and fibronectin expression levels increased in BPH group when compared to controls. Also, the greatest increase in expression levels was observed in metastatic group in comparison to the control and BPH groups. Conversely, integrin-α4 expression decreased in the BPH and metastatic groups.
Figure 1.
Western Blot results of three EMT members from 20 controls, 20 BPH, and 40 metastatic samples. Periostin and Fibronectin showing quite high expression levels in metastatic group compared to control and BPH groups. In the majority of metastatic samples, integrin-α4 showed very low or no expression in comparison to the control and BPH groups (C: control; BPH: benign prostatic hyperplasia; MET: metastatic)
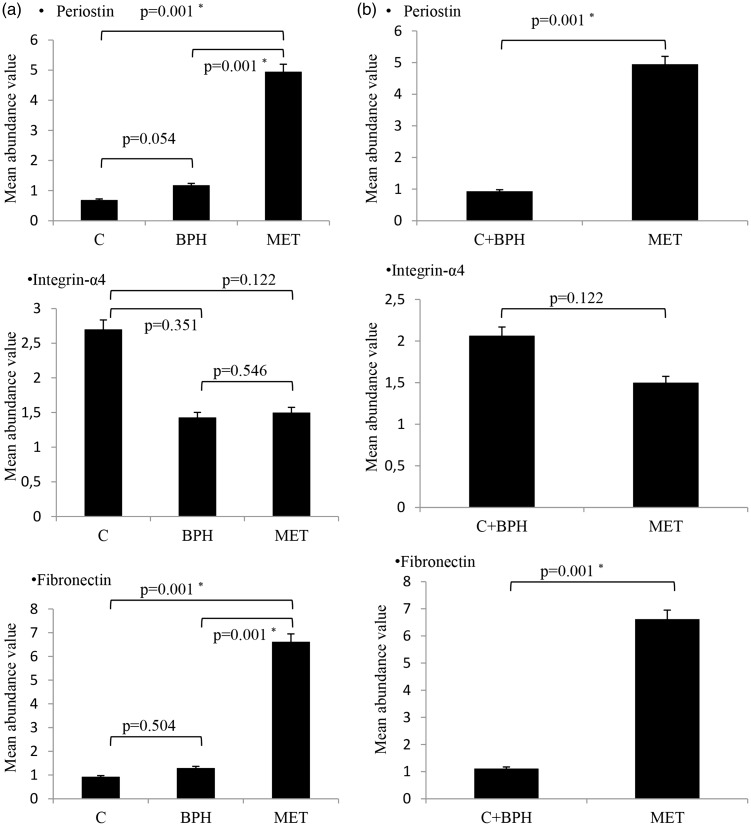
Secondly, we transformed expression levels of these proteins obtained from Western Blot bands into numerical data by using Image J software and calculated mean abundance values. As shown in Figure 2(a), Mann–Whitney U test revealed that expression levels of integrin-α4 decreased, whereas those of periostin and fibronectin increased in metastatic samples. Periostin protein expression levels significantly increased in the metastatic group in comparison to the BPH and control groups (P = 0.001). Between BPH and control groups, there were no statistical differences in periostin expression (P = 0.054). Expression levels of integrin-α4 protein decreased in metastatic and BPH groups; however, no statistically significant changes were found between control-BPH (P = 0.351), control-metastatic (P = 0.122), and BPH-metastatic (P = 0.546) groups. Among the investigated proteins, the greatest expression change was seen in fibronectin protein. About 7-fold increase was observed in the expression levels of fibronectin in the metastatic group (P = 0.001) when compared to BPH and control groups. There was no statistically significant difference in terms of fibronectin expression between control and BPH groups (P = 0.504).
Figure 2.
(a) Comparative mean abundance values of Western Blot bands of periostin, integrin-α4 and fibronectin proteins in all groups. Statistical analyses showed decreased expression levels of integrin-α4, increased expression levels of periostin and fibronectin in metastatic samples compared to other two groups. (b) We merged the control and BPH groups and compared with the metastatic group. Results showed that expression levels of periostin and fibronectin increased in metastatic samples in comparison to control + BPH. P < 0.05 was considered statistically significant (*P < 0.05) (C: control; BPH: benign prostatic hyperplasia; MET: metastatic)
Control and BPH groups were combined and then compared with the metastatic group in terms of gene expression. Our results showed that expression levels of periostin and fibronectin proteins significantly increased in the metastatic group when compared to the combined control + BPH group (P = 0.001). There was no statistically significant difference in terms of integrin-α4 expression between the two groups (P = 0.122) (Figure 2(b)).
Associations among periostin, integrin-α4, fibronectin protein levels, and Gleason score and PSA levels
We analyzed the association between the two conventional clinico-pathological parameters – Gleason score and serum PSA level – and expression levels of three EMT proteins in metastatic samples (Table 1). Patients were classified according to Gleason scores (from 8 to 10) obtained after the prostate biopsy. Then, plasma expression levels of periostin, integrin-α4, and fibronectin were calculated and matched with the Gleason scores. Kruskal–Wallis test revealed that there were no statistically significant differences among plasma periostin (P = 0.329), integrin-α4 (P = 0.795), and fibronectin (P = 0.321) levels of patients classified according to Gleason scores.
Table 1.
Association among Gleason scores, PSA levels, periostin, integrin-α4, fibronectin expressions in the mCRPC patient group
| Periostin |
Integrin-α4 |
Fibronectin |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | P | Mean | Median | P | Mean | Median | P | |
| Gleason score | ||||||||||
| 8 | 12 | 5.8 ± 2.75 | 5.4 (2–9.9) | 0.329 | 1.6 ± 2.81 | 0.15 (0.03–7.6) | 0.795 | 8.1 ± 3.28 | 8.5 (0.01–12.5) | 0.321 |
| 9 | 16 | 4.3 ± 2.57 | 3.6 (0.9–9.1) | 1.4 ± 1.43 | 0.6 (0.02–4.1) | 5.5 ± 4.19 | 5.2 (0.09–14.1) | |||
| 10 | 12 | 4.9 ± 2.58 | 5 (2–10.8) | 1.5 ± 1.97 | 0.3 (0.02–5.5) | 6.6 ± 3.82 | 7.4 (0.04–11.4) | |||
| PSA (ng/ml) | ||||||||||
| <20 | 9 | 4.2 ± 2.00 | 3.8 (0.9–6.5) | 0.486 | 1.3 ± 2.37 | 0.1 (0.03–7.4) | 0.446 | 5.7 ± 3.76 | 5.8 (0.8–10.2) | 0.549 |
| ≥20 | 31 | 5.2 ± 2.78 | 4.5 (1.6–10.9) | 1.5 ± 1.96 | 0.5 (0.01–7.6) | 6.9 ± 3.93 | 7.3 (0.01–14.1) | |||
PSA: prostate-specific antigen; mCRPC: metastatic castration-resistant prostate cancer.
We did not find a significant association between serum PSA and plasma periostin (P = 0.486), integrin-α4 (P = 0.446), and fibronectin (P = 0.549) levels. On the other hand, samples with PSA levels higher than or equal to 20 ng/ml showed higher periostin and fibronectin expression values than samples with PSA levels lower than 20 ng/ml.
Associations among periostin, integrin-α4, fibronectin protein levels, and metastasis sites
We analyzed expression levels of the three proteins in connection to the metastasis sites of the patients. We divided patients into three groups of metastasis sites for this purpose. The first group is bone (B), second is bone + lymph (B + L) and third is bone + lymph + organ (B + L + O) (Table 2). Our results showed that periostin had a significantly higher expression in patients with bone metastasis (Group 1), when compared to Group 2 (P = 0.004). Moreover, periostin showed an increased expression in Group 1 when compared to combined Group 2 + Group 3 (P = 0.039). There were no significant changes in terms of periostin expression between groups other than those mentioned above (P > 0.05). As opposed to periostin, we did not detect any statistically significant changes in terms of integrin-α4 and fibronectin expression levels between any groups (P > 0.05). Finally, when we compared Group 1 + 2 with Group 3, we did not observe any statistically significant changes in any of these proteins (P > 0.05).
Table 2.
Association among metastasis sites of mCRPC patients and periostin, integrin-α4, fibronectin expressions
| Periostin |
Integrin-α4 |
Fibronectin |
|||||
|---|---|---|---|---|---|---|---|
| Metastasis sites | n | Mean | Median | Mean | Median | Mean | Median |
| Group 1:B | 15 | 6.1 ± 2.83 | 6.32 (1.6–10.8) | 0.9 ± 9.57 | 0.26 (0.01–2.7) | 6.0 ± 3.43 | 7.0 (0.04–9.7) |
| Group 2: B + L | 17 | 4.1 ± 2.25 | 3.5 (0.88–9.9) | 1.4 ± 2.21 | 0.22 (0.03–7.4) | 6.2 ± 4.5 | 5.8 (0.01–12.5) |
| Group 3: B + L + O | 8 | 4.7 ± 2.46 | 4.6 (2–9.7) | 2.7 ± 2.76 | 2.0 (0.03–7.6) | 8.8 ± 2.7 | 8.3 (5.1–14.1) |
| Statistics |
P
|
P | P | ||||
| Group1 vs. Group2 | 0.040* | 0.650 | 0.821 | ||||
| Group1 vs. Group3 | 0.208 | 0.100 | 0.093 | ||||
| Group2 vs. Group3 | 0.641 | 0.308 | 0.294 | ||||
| Group1 vs. Group(2 + 3) | 0.039* | 0.295 | 0.371 | ||||
| Group(1 + 2) vs.Group3 | 0.697 | 0.146 | 0.144 | ||||
B: bone; L: lymph; O: organ; mCRPC: metastatic castration-resistant prostate cancer.
P < 0.05.
Discussion
The search for markers of tumor metastasis depends heavily on molecular mechanisms underlying EMT process.29,30 In this study, we analyzed the expression levels of three EMT-related proteins in mCRPC and BPH patients and healthy controls. Then we investigated the possible association between expression levels of these proteins and conventional clinico-pathological prognostic factors in metastatic samples.
Tsunoda et al.16 reported significantly increased periostin expression in the early stages of PCa (Gleason score 6–7), but not in the advanced stages of PCa. Our data indicate that periostin is remarkably upregulated in metastatic castration-resistance group (Gleason score 8–10) compared to control and BPH groups (P < 0.001). This provides strong evidence pointing to the fact that increased expression of periostin can facilitate invasive and metastatic potential of tumor cells. Tischler et al.13 have reported upregulation of periostin in high grade and high stage human PCa tissues. Another work showed that periostin increased 9.12 folds in PCa when compared with BPH.14 It has also been suggested that periostin expression was correlated with cancer metastasis. Indeed, the periostin increased in prostate tumor tissues with Gleason score 6 and higher in comparison to paired non-tumorous prostate tissues.17 Our results were consistent with aforementioned studies. Although we did not find any statistically significant differences among Gleason scores and PSA levels in the expression levels of periostin in mCRPC patients, bony metastasis was found all closely correlated to the increased periostin expression (P < 0.05). The significant increase in periostin expression in only bone metastasis group may indicate that this protein is important in the adhesion of the tumor cell to the bone surface.
We showed that integrin α4 expression was downregulated in mCRPC group compared with control and BPH groups, but this downregulation was not correlated with Gleason scores, PSA levels, and metastatic sites. α4 subunits and β subunits have been shown to be downregulated in PCa.21,22,31 We found that integrin α4 expression was negatively correlated with fibronectin expression. This result may be due to degradation of integrins which were ubiquitinated in migrating cells in response to fibronectin binding and degraded in lysosomes.32 Another cause of the molecular mechanism underlying the loss of integrin α4 expression in mCRPC is the transcriptional silencing of this gene. Hypermethylation of integrin α4 was observed in 66.6% of PCa patients, whereas no hypermethylation was observed in BPH patients.33 Another study suggested that metastasis suppressor CD82 repressed adhesion signaling through lateral interactions with the associated α3β1 and α5β1 integrins, and hence inhibited fibronectin adhesion-induced EMT in PCa cells.28
The role of fibronectin as marker of EMT is less straightforward in clinical PCa than it is in vitro. Although expression of mesenchymal marker fibronectin increased in PCa patients, overexpression of fibronectin was not found statistically significant in BPH and malignant prostate epithelium.34 Kolijn et al.34 showed that fibronectin was expressed in only a small percentage of tumor cells. In our study, as compared to BPH and control groups, nearly a 7-fold increase was observed in expression level of fibronectin in mCRPC patients. According to Moroz et al.,27 expression of MMPs that are directly involved in PCa aggressiveness can be upregulated by fibronectin. As demonstrated by Han et al.,35 fibronectin may be a potential therapeutic target for PCa. There was also an increased fibronectin expression in both mRNA and protein levels in metastatic PCa cell line DU145.36 Our results are in parallel with those of Han et al.35 in clinical terms and with those of Kang et al.36 in vitro. We did not find any significant correlations between fibronectin expression and any of the clinico-pathological parameters, including Gleason scores, PSA levels, and metastatic sites, of mCRPC patients.
The small sample size in terms of patients and the small number of proteins studied are the main limiting factors in the present study. Despite this limitation, our findings presented here have highlighted the role of EMT in facilitating PCa progression and metastasis. The serum levels of these markers – periostin, integrin-α4 and fibronectin – should be explored in non-recurrent, non-metastatic PCa to determine whether they are markers of metastasis or simply present in the serum of PCa patients.
Conclusion
Western Blot results showed increased expression in fibronectin and periostin and decreased expression in integrin-α4 in the mCRPC group compared to BPH and control groups. Our findings indicate that elevated periostin might be a co-stimulator of PCa cell progression in bone. Therefore, periostin might be a potential therapeutic target for suppressing the metastatic progression of PCa.
Authors’ contributions
Study design: EK, IK, GA, CYB. Patient selection and sample preparation: ES, CYB. Performed experiments: IK. Statistical analyses: AUD. Analysed output data: EK, IK, CYB. Manuscript preparation: EK, CYB.
Funding
This study with the project code number 01/2015-40 has been supported by the Gazi University Research Fund.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One 2010; 5: e12445–e12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin SK, Kamelgarn M, Kyprianou N. Cytoskeleton targeting value in prostate cancer treatment. Am J Clin Exp Urol 2014; 2: 15–26. [PMC free article] [PubMed] [Google Scholar]
- 3.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002; 14: 608–16. [DOI] [PubMed] [Google Scholar]
- 4.Roberts DD. Emerging functions of matricellular proteins. Cell Mol Life Sci 2011; 68: 3133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, Solo K, Valant J, Todd MB, Mehra M. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One 2015; 10: e0139440–e0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding G, Fang J, Tong S, Qu L, Jiang H, Ding Q, Liu J. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 2015; 75: 957–68. [DOI] [PubMed] [Google Scholar]
- 7.Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget 2015; 6: 5947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 2009; 66: 2219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao ZM, Wang XY, Wang AM. Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem 2015; 62: 401–6. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H, Lu P. Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS One 2012; 7: e46670– e46670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morra L, Rechsteiner M, Casagrande S, von Teichman A, Schraml P, Moch H, Soltermann A. Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer 2011; 76: 183–190. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Fejzo MS, Anderson L, Dering J, Ginther C, Ramos L, Gasson JC, Karlan BY, Slamon DJ. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol 2010; 119: 337–44. [DOI] [PubMed] [Google Scholar]
- 13.Tischler V, Fritzsche FR, Wild PJ, Stephan C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J, Schraml P, Jung K, Moch H, Soltermann A, Kristiansen G. Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer 2010; 10: 273–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun C, Zhao X, Xu K, Gong J, Liu W, Ding W, Gou Y, Xia G, Ding Q. Periostin: a promising target of therapeutical intervention for prostate cancer. J Transl Med 2011; 9: 99–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Q, Tong S, Zhao X, Ding W, Gou Y, Xu K, Sun C, Xia G. Periostin mediates TGF-β-induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem 2015; 36: 799–809. [DOI] [PubMed] [Google Scholar]
- 16.Tsunoda T, Furusato B, Takashima Y, Ravulapalli S, Dobi A, Srivastava S, McLeod DG, Sesterhenn IA, Ornstein DK, Shirasawa S. The increased expression of periostin during early stages of prostate cancer and advanced stages of cancer stroma. Prostate 2009; 69: 1398–403. [DOI] [PubMed] [Google Scholar]
- 17.Tian Y, Choi CH, Li QK, Rahmatpanah FB, Chen X, Kim SR, Veltri R, Chia D, Zhang Z, Mercola D, Zhang H. Overexpression of periostin in stroma positively associated with aggressive prostate cancer. PLoS One 2015; 10: e0121502–e0121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu G, Liu Z, Epstein JI, Davis C, Christudass CS, Carter HB, Landis P, Zhang H, Chung JY, Hewitt SM, Miller MC, Veltri RW. A novel quantitative multiplex tissue immunoblotting for biomarkers predicts a prostate cancer aggressive phenotype. Cancer Epidemiol Biomarkers Prev 2015; 24: 1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel HL, Alam N, Johnson IN, Languino LR. Integrin signaling aberrations in prostate cancer. Am J Transl Res 2009; 1: 211–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL. Role of focal adhesion kinase and phosphatidylinositol 3'-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res 2006; 66: 8091–9. [DOI] [PubMed] [Google Scholar]
- 21.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer 2008; 3: 657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drivalos A, Chrisofos M, Efstathiou E, Kapranou A, Kollaitis G, Koutlis G, Antoniou N, Karanastasis D, Dimopoulos MA, Bamias A. Expression of α5-integrin, α7-integrin, Ε-cadherin, and N-cadherin in localized prostate cancer. Urol Oncol 2016; 34: 165. e11–65.e18. [DOI] [PubMed] [Google Scholar]
- 23.Ruppender N, Larson S, Lakely B, Kollath L, Brown L, Coleman I, Coleman R, Nguyen H, Nelson PS, Corey E, Snyder LA, Vessella RL, Morrissey C, Lam HM. Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS One 2015; 10: e0130565–e0130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Ni H. Fibronectin maintains the balance between hemostasis and thrombosis. Cell Mol Life Sci 2016; 73: 3265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial mesenchymal transition. Oncogene 2014; 33: 1649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, Settleman J, Johnson L. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res 2012; 72: 527–36. [DOI] [PubMed] [Google Scholar]
- 27.Moroz A, Delella FK, Lacorte LM, Deffune E, Felisbino SL. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochem Biophys Res Commun 2013; 430: 1319–21. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Byun HJ, Lee MS, Jin YJ, Jeoung D, Kim YM, Lee H. The metastasis suppressor CD82/KAI1 inhibits fibronectin adhesion-induced epithelial-to-mesenchymal transition in prostate cancer cells by repressing the associated integrin signaling. Oncotarget 2017; 8: 1641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MI, Hamid A, Adhami VM, Lall RK, Mukhtar H. Role of epithelial mesenchymal transition in prostate tumorigenesis. Curr Pharm Des 2015; 21: 1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol 2011; 8: 428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev 2007; 26: 567–78. [DOI] [PubMed] [Google Scholar]
- 32.Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerød L, Stenmark H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 2010; 19: 148–59. [DOI] [PubMed] [Google Scholar]
- 33.Mostafavi-Pour Z, Kianpour S, Dehghani M, Mokarram P, Torabinejad S, Monabati A. Methylation of integrin α4 and E-cadherin genes in human prostate cancer. Pathol Oncol Res 2015; 21: 921–7. [DOI] [PubMed] [Google Scholar]
- 34.Kolijn K, Verhoef EI, van Leenders GJ. Morphological and immunohistochemical identification of epithelial-to-mesenchymal transition in clinical prostate cancer. Oncotarget 2015; 6: 24488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Z, Zhou Z, Shi X, Wang J, Wu X, Sun D, Chen Y, Zhu H, Magi-Galluzzi C, Lu ZR. EDB fibronectin specific peptide for prostate cancer targeting. Bioconjug Chem 2015; 26: 830–8. [DOI] [PubMed] [Google Scholar]
- 36.Kang R, Zhao S, Liu L, Li F, Li E, Luo L, Xu L, Wan S, Zhao Z. Knockdown of PSCA induces EMT and decreases metastatic potentials of the human prostate cancer DU145 cells. Cancer Cell Int 2016; 16: 20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]