Abstract
A number of signaling pathways underlying pathological cardiac hypertrophy have been identified. However, few studies have probed the functional significance of these signaling pathways in the context of exercise or physiological pathways. Exercise studies were performed on females from six different genetic mouse models that have been shown to exhibit alterations in pathological cardiac adaptation and hypertrophy. These include mice expressing constitutively active glycogen synthase kinase-3β (GSK-3βS9A), an inhibitor of CaMK II (AC3-I), both GSK-3βS9A and AC3-I (GSK-3βS9A/AC3-I), constitutively active Akt (myrAkt), mice deficient in MAPK/ERK kinase kinase-1 (MEKK1−/−), and mice deficient in cyclin D2 (cyclin D2−/−). Voluntary wheel running performance was similar to NTG littermates for five of the mouse lines. Exercise induced significant cardiac growth in all mouse models except the cyclin D2−/− mice. Cardiac function was not impacted in the cyclin D2−/− mice and studies using a phospho-antibody array identified six proteins with increased phosphorylation (greater than 150%) and nine proteins with decreased phosphorylation (greater than 33% decrease) in the hearts of exercised cyclin D2−/− mice compared to exercised NTG littermate controls. Our results demonstrate that unlike the other hypertrophic signaling molecules tested here, cyclin D2 is an important regulator of both pathologic and physiological hypertrophy.
Impact statement
This research is relevant as the hypertrophic signaling pathways tested here have only been characterized for their role in pathological hypertrophy, and not in the context of exercise or physiological hypertrophy. By using the same transgenic mouse lines utilized in previous studies, our findings provide a novel and important understanding for the role of these signaling pathways in physiological hypertrophy. We found that alterations in the signaling pathways tested here had no impact on exercise performance. Exercise induced cardiac growth in all of the transgenic mice except for the mice deficient in cyclin D2. In the cyclin D2 null mice, cardiac function was not impacted even though the hypertrophic response was blunted and a number of signaling pathways are differentially regulated by exercise. These data provide the field with an understanding that cyclin D2 is a key mediator of physiological hypertrophy.
Keywords: Cardiac hypertrophy, physiological hypertrophy, exercise, signaling molecules, voluntary wheel running
Introduction
Cardiac hypertrophy is a natural response to mechanical and neurohormonal stimuli and acts to reduce ventricular wall stress and maintain or increase cardiac function.1–3 An increase in cardiac mass is primarily the result of increases in cardiomyocyte size.4,5 Depending on the nature of the stimulus, cardiac hypertrophy can be classified as either physiological or pathological.3 Pathological hypertrophy is a maladaptive response associated with increased morbidity and mortality resulting from diseases such as hypertension, aortic stenosis, myocardial infarction, and genetic mutations.6,7 In contrast, physiological stimuli such as chronic exercise training elicit an adaptive response that is fundamentally different from pathological hypertrophy and is not associated with cardiac dysfunction or the development of heart failure.8,9 In fact, activation of physiological hypertrophy may even prevent or reverse cardiac disease.3,10
A growing body of literature has elucidated a number of functional, molecular and biochemical changes that take place during physiological cardiac growth. For example, exercise-mediated cardiac hypertrophy is associated with normal cardiac structure, normal or enhanced cardiac function,11,12 and is reversible upon cessation of exercise.13,14 There is a notable absence of the induction of the fetal gene program, fibrosis, and apoptosis in physiological hypertrophy.15,16 Physiological hypertrophy induces both fatty acid and glucose oxidation.17 Finally, angiogenesis takes place at a rate that matches cardiac growth so that capillary density remains normal or even enhanced during physiological growth.18
The hypertrophic signaling pathways that coordinate physiological hypertrophy are distinct from pathological hypertrophy (see Maillet et al.9 for recent review). Briefly, exercise training stimulates the release of growth hormones and factors and mechanical forces that facilitate the growth response of the heart. These processes activate a number of cellular receptors and intracellular signaling pathways coordinating the myocardial changes uniquely associated with physiological cardiac hypertrophy including the IGF1-PI3K-Akt signaling pathway.3,9,19 Interestingly, many of the molecular pathways that regulate pathological cardiac hypertrophy have not been evaluated for their role in the development of physiological hypertrophy. For example, although glycogen synthase kinase-3β (GSK-3β) negatively regulates pathological hypertrophy,20–22 an increase in its phosphorylation state in response to voluntary wheel running suggests that GSK-3β signaling may be involved in physiological hypertrophy as well.22 CaMK signaling has also been implicated in the development of pathological hypertrophy and inhibition of CaMKII signaling has been shown to be protective in the context of cardiac disease.22–25 Akt plays an important role in mediating cardiomyocyte size, is required for physiological hypertrophy, and overexpression of Akt leads to massive cardiac hypertrophy.26–29 MAPK/ERK kinase (MEK) kinase-1 (MEKK1) signaling is also important for pathological hypertrophy and can play opposite roles in the development of pathological hypertrophy depending on the context.30–32 Lastly, increases in the cell cycle regulators, including cyclin D2, are associated with pathological cardiac hypertrophy33 and mice lacking cyclin D2 have attenuated pathological hypertrophy.34,35
In order to determine whether these hypertrophic signaling molecules would impact the exercise response, we used a number of genetic mouse models to assess exercise performance and the hypertrophic response to exercise training. The mice used here include the following: overexpression of a cardiac-specific constitutively active GSK-3β (GSK-3βS9A), overexpression of a cardiac-specific constitutively active (myristoylated) Akt 1 (myrAkt), overexpression of a cardiac-specific CaMKII inhibitor (AC3-I), and mice deficient in either MEKK1 (MEKK1−/−) or cyclin D2 (cyclin D2−/−). We and others have previously shown that, on a voluntary cage wheel, female mice run longer distances and achieve more cardiac hypertrophy per kilometer run than male mice.22,36 Because of these findings, we used female mice in this study and exercised the mice for 21 days on a voluntary cage wheel. Our findings are that all genetic mouse models had similar exercise performance compared to littermate controls. Perhaps more important is that physiological hypertrophy occurred in all genetic mouse models except cyclin D2−/− mice. Cardiac function was not impacted concomitant with attenuated cardiac growth in response to exercise in cyclin D2−/− mice and we identified a number of proteins with increased or decreased phosphorylation in exercised cyclin D2−/− mouse hearts compared to exercised NTG mice. Thus, these data establish cyclin D2 as an important regulator of physiological hypertrophy.
Materials and Methods
Experimental animals
The transgenic mouse model overexpressing a cardiac-specific, constitutively active form of GSK-3β (GSK-3βS9A)20 or cardiac-specific constitutively active form of Akt (myrAkt)29 were detailed previously. Transgenic mice with CaMKII inhibition (AC3-I) and transgenic control mice (AC3-C) are described elsewhere.24 AC3-I transgenic mice were bred with GSK-3βS9A generating the GSK-3βS9A × AC3-I double transgenic mouse. Transgenic mice deficient in MEKK1 (MEKK1−/−)37 and cyclin D2 (cyclin D2−/−)38 were previously described. Each transgenic mouse model was bred and offspring were genotyped by PCR for the presence of the specific transgene. For all transgenic mouse models except the MEKK1−/− mice, littermate female mice lacking the respective transgene (NTG) were used for controls. There are no littermate controls as the MEKK1−/− mice were bred to each other for this study. Female CB6F1 hybrid mice (The Jackson Laboratory) were also used. All research involving the use of mice was performed in strict accordance to approved protocols by the Institutional Animal Use and Care Committee at the University of Colorado and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Voluntary wheel running and exercise performance
Female mice 12–16 weeks of age were randomly placed into the sedentary or exercise group. Exercised mice were individually placed in a cage with access to a free wheel for 7 or 21 days as previously described.39 Briefly, cages were fitted with an 11.5 cm-diameter cage wheel with a 5.0 cm-wide running surface (model 6208, PetsMart; Phoenix, AZ) equipped with a digital magnetic counter (model BC 600, Sigma Sport; Olney, IL). Exercise values of distance and time were recorded daily and speed was calculated for each exercised animal. Sedentary mice were placed into the same cage without a cage wheel.
Morphometric analysis
Within 8 h of completing the exercise protocol, mice were euthanized by cervical dislocation. Body mass was measured and hearts were harvested and washed in ice-cold PBS. The hearts were weighed and immediately either fixed in 10% phosphate-buffered formalin or snap-frozen in liquid nitrogen and stored at −80˚C. The ratio of heart weight-to-body weight (HW/BW) was calculated.
Histological analysis
Whole hearts were fixed and processed as previously described.21 Briefly, fixed hearts were processed by Premier Histology (Boulder, CO), embedded in paraffin, sectioned, and stained with hematoxylin-eosin (H&E) or Masson’s trichrome stain. In H&E slides, left ventricular sections with suitable cross-sections, defined as having circular-to-oval myocytes sections, were analyzed by light microscopy and photographed. The outline of individual myocytes was traced and the mean area for each cell was quantified using NIH Image J software. The mean data reflect results from three to four hearts in each group (approximately 15–20 cells per heart).
Quantitative RT-PCR
Total RNA was extracted from frozen left ventricular tissue using the RNeasy kit (Qiagen) as per the manufacturer’s instructions. RNA was quantified and its integrity was assessed using the Agilent 2100 Bioanalyzer. Using equal concentrations of total RNA, cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad) following manufacturer’s instructions. Using the synthesized cDNA for the PCR amplifications, gene expression levels of cell cycle genes were measured by quantitative RT-PCR using iTaq Universal SYBR Green (Bio-Rad) on the CFX96 Touch Real-Time PCR Detections System (Bio-Rad). Predesigned primer pairs for 18S, cyclin D1, cyclin D2, cyclin E1, and cyclin-dependent kinase (CDK) 4 were purchased from Bio-Rad (PrimePCR Primers). We used a standard curve to estimate the relative levels of cDNA in each sample and results were plotted as fold change relative to NTG hearts after normalization to 18S rRNA values.
Echocardiography
Left ventricular function was evaluated by transthoracic echocardiography on sedentary and mice exercised for 21 days as previously described.40 M mode measurements determined the left ventricular internal dimension in diastole (LVIDd), left ventricular internal dimension in systole (LVIDs), left ventricular posterior wall thickness in diastole (LVPWd), left ventricular posterior wall thickness in systole (LVPWs), left ventricular anterior wall thickness in diastole (LVAWd), left ventricular anterior wall thickness in systole (LVAWs), left ventricular fractional shortening (FS), and left ventricular ejection fraction (EF).
Phospho-kinase array
The phosphorylation status of 46 different kinases was evaluated using the human phospho-kinase antibody arrays according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN) as detailed previously.41,42 Mice were exercised for seven days, the hearts were harvested, and left ventricular lysates were pooled from three mice. Equal amounts of protein were incubated with the arrays.
Data and statistical analysis
All data are reported as mean ± SEM. The percent increase in HW/BW ratio was determined by dividing the HW/BW ratio of each mouse subjected to exercise to the mean of the HW/BW ratio of the sedentary group. Statistical significance of difference between respective genetic mouse model and littermate controls, and sedentary and exercised groups was determined using unpaired, two-tailed Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results
Exercise performance and cardiac adaptation in GSK-3βS9A, AC3-I, and GSK-3βS9A × AC3-I mice
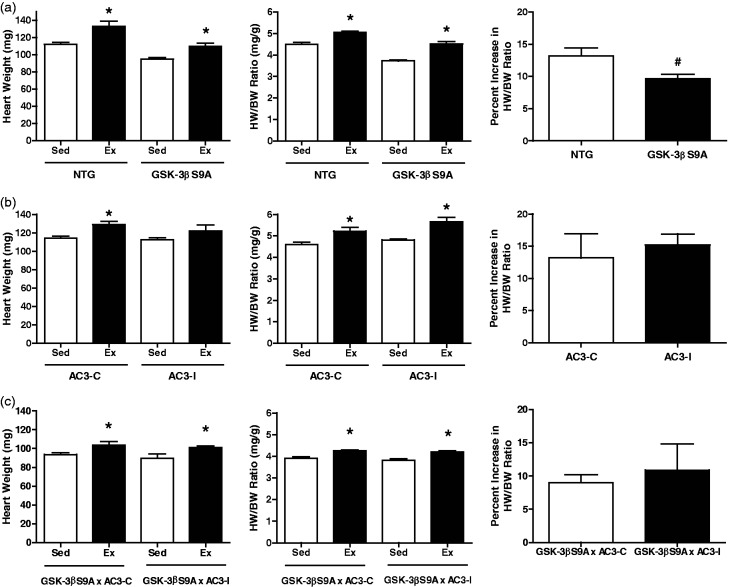
Because constitutive activation of GSK-3β and inhibition of CaMKII can attenuate hypertrophy associated with pathological stimuli,20,24 we examined whether these genetic mouse models would also be anti-hypertrophic in the context of exercise-mediated cardiac growth. Exercise training was conducted for 21 days using a cage free-wheel and exercise performance was measured by distance, duration, and average speed as previously described.22,39 At the end of the exercise program, mice were euthanized and the hearts were harvested and analyzed. Consistent with previous studies,22 exercise in NTG female mice led to a significant increase in heart weight and heart weight-to-body weight (HW/BW) ratio (Figure 1). GSK-3βS9A female mice exercised similarly compared to NTG littermate control female mice (9.9 ± 0.7 km/d versus 8.5 ± 0.7 km/d) (Table 1).43 Following exercise training, both average heart weights and HW/BW ratios were significantly increased in GSK-3βS9A mice compared to sedentary controls (HW/BW ratio, 4.51 ± 0.11 mg/g vs. 3.73 ± 0.04 mg/g). This indicates that while the hearts of this transgenic model have a blunted hypertrophic response to a pathological stimulus,20,21 their physiological hypertrophic response remains intact (Figure 1(a)), even though the percent increase in HW/BW ratio was slightly less than NTG littermate controls (9.6% ± 0.7% vs. 13.2% ± 1.2%; Figure 1(a)). Female mice with CaMKII inhibition (AC3-I) also had similar exercise performance values compared to female littermate controls (AC3-C) (Table 1). Exercise also resulted in increased heart weights and HW/BW ratio in AC3-I mice (Figure 1(b)). Compared to female controls, AC3-I mice had a similar percent increase in HW/BW ratio following exercise (13.2% ± 3.7% vs. 15.2% ± 1.7%) (Figure 1(b)). Since neither constitutive activation of GSK-3β nor inhibition of CaMKII blocked the hypertrophic response of the heart to exercise, a doubly transgenic mouse was created expressing both constitutively active GSK-3β and the CaMKII inhibitory peptide in the heart (GSK-3βS9A x AC3-I). The GSK-3βS9A × AC3-I female mice had a normal exercise capacity and achieved a 10.9% ± 4.0% increase in HW/BW ratio (Figure 1(c)). Thus, expression of constitutive activation of GSK-3β or CaMKII inhibition alone or even the simultaneous expression of both transgenes in the heart does not affect exercise performance and, more importantly, physiological hypertrophy is achievable in these mice.
Figure 1.
Morphometric data and cardiac adaptation in response to 21 days of exercise for GSK-3βS9A and AC3-I female mice. (a) GSK-3βS9A and NTG littermate controls: Left: heart weight. Middle: heart weight normalized for body weight (HW/BW) ratio. Right: percent increase in HW/BW ratio. (b) AC3-I and AC3-C controls: Left: heart weight. Middle: heart weight normalized for body weight (HW/BW) ratio. Right: percent increase in HW/BW ratio. (c) GSK-3βS9A × AC3-I and GSK-3βS9A × AC3-C female mice: Left: heart weight. Middle: heart weight normalized for body weight (HW/BW) ratio. Right: percent increase in HW/BW ratio. *, P < 0.05 relative to sedentary counterparts; #, P < 0.05 relative to NTG exercised mice. n = 5–14 animals per group. Data are reported as mean ± SE
Table 1.
Voluntary wheel running performance of female transgenic mice
| Distance (km/day) | Time (h/day) | Speed (km/h) | |
|---|---|---|---|
| Constitutively active GSK-3β | |||
| NTG (n = 9) | 8.49 ± 0.70 | 6.98 ± 0.55 | 1.21 ± 0.04 |
| GSK-3βS9A (n = 14) | 9.92 ± 0.66 | 8.11 ± 0.64 | 1.25 ± 0.03 |
| CaMKII Inhibition | |||
| AC3-C (n = 5) | 4.48 ± 1.28 | 3.22 ± 0.89 | 1.33 ± 0.13 |
| AC3-I (n = 5) | 4.32 ± 0.90 | 3.11 ± 0.66 | 1.29 ± 0.10 |
| Constitutively active GSK-3β and CaMKII Inhibition | |||
| GSK-3βS9A × AC3-C (n = 7) | 7.97 ± 0.54 | 6.74 ± 0.46 | 1.20 ± 0.04 |
| GSK-3βS9A × AC3-I (n = 7) | 7.66 ± 0.77 | 6.23 ± 0.53 | 1.24 ± 0.05 |
| Constitutively active Akt | |||
| NTG (n = 10) | 8.04 ± 0.70 | 5.75 ± 0.52 | 1.39 ± 0.07 |
| myrAkt (n = 9) | 7.12 ± 0.41 | 5.14 ± 0.27 | 1.40 ± 0.06 |
| MEKK1 null | |||
| MEKK1−/− (n = 4) | 4.88 ± 0.82 | 3.81 ± 0.50 | 1.28 ± 0.09 |
| Cyclin D2 null | |||
| NTG (n = 6) | 6.91 ± 0.85 | 5.49 ± 0.60 | 1.25 ± 0.04 |
| Cyclin D2−/− (n = 14) | 5.77 ± 0.81 | 4.63 ± 0.49 | 1.26 ± 0.09 |
Values are mean ± SEM.
Exercise performance and cardiac adaptation in myrAkt mice
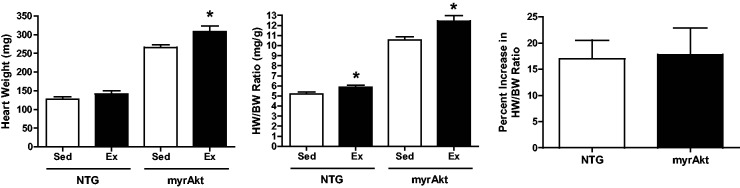
A number of studies have implicated Akt in physiologic cardiac growth.19,27,29 In the current study, we used transgenic mice that express a constitutively active form of Akt through myristoylation (myrAkt) that leads to massive hypertrophy in males and females.44 Consistent with these prior findings, we observed a similar significant increase in heart size and HW/BW ratio in sedentary myrAkt female mice (Figure 2). Because of the already large hearts, it was unclear whether these massive hearts could undergo further hypertrophy. We found that expression of myrAkt did not have a significant impact on exercise performance as myrAkt mice ran on average 7.1 ± 0.4 km/d compared to NTG controls which ran 8.0 ± 0.7 km/d (Table 1). Additionally, both heart size and HW/BW ratio increased as a result of exercise training in myrAkt female mice. In fact, no difference in percent increase in HW/BW ratio was observed in response to exercise between female myrAkt and female littermate control mice (17% ± 3.6 vs. 17.8% ± 5.1). These data show that physiological hypertrophy can still occur in the context of constitutive activation of Akt.
Figure 2.
Morphometric data and cardiac adaptation in response to 21 days of exercise for myrAkt and NTG female mice. (a) myrAkt and NTG littermate controls: Left: heart weight. Middle: heart weight normalized for body weight (HW/BW) ratio. Right: percent increase in HW/BW ratio. *, P < 0.05 relative to sedentary counterparts; n = 9–10 animals per group. Data are reported as mean ± SE
Exercise performance and cardiac adaptation in MEKK1−/− mice
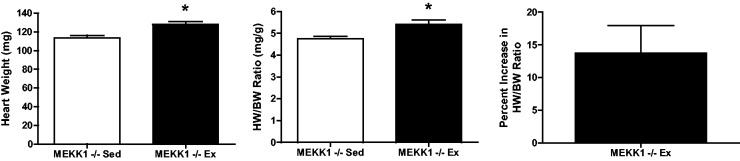
MEKK1 signaling has been demonstrated to both mediate Gαq-induced pathologic hypertrophy32 and confer a protective role in cardiac disease.30,31 Because of the opposing roles of MEKK1 in hypertrophy, we examined whether exercise performance and cardiac adaptation to exercise were affected in MEKK1−/− female mice. Since MEKK1−/− mice were bred to each other, there were no littermate controls. MEKK1−/− mice ran an average of 4.9 ± 0.8 km during each 24-h period (Table 1). Cardiac growth in MEKK1−/− mice was observed as a result of voluntary wheel running as indicated by an increase in cardiac mass and HW/BW ratio compared to sedentary MEKK1−/− mice resulting in a 13.8% increase in HW/BW ratio (Figure 3), similar to the increase observed in other mice.
Figure 3.
Morphometric data and cardiac adaptation in response to 21 days of exercise for MEKK1−/− female mice. Left: heart weight. Middle: heart weight normalized for body weight (HW/BW) ratio. Right: percent increase in HW/BW ratio. *, P < 0.05 relative to sedentary counterparts; n = 4–6 animals per group. Data are reported as mean ± SE
Expression levels of cell cycle genes in NTG mice, and exercise performance, cardiac adaptation, and histology in cyclin D2−/− mice
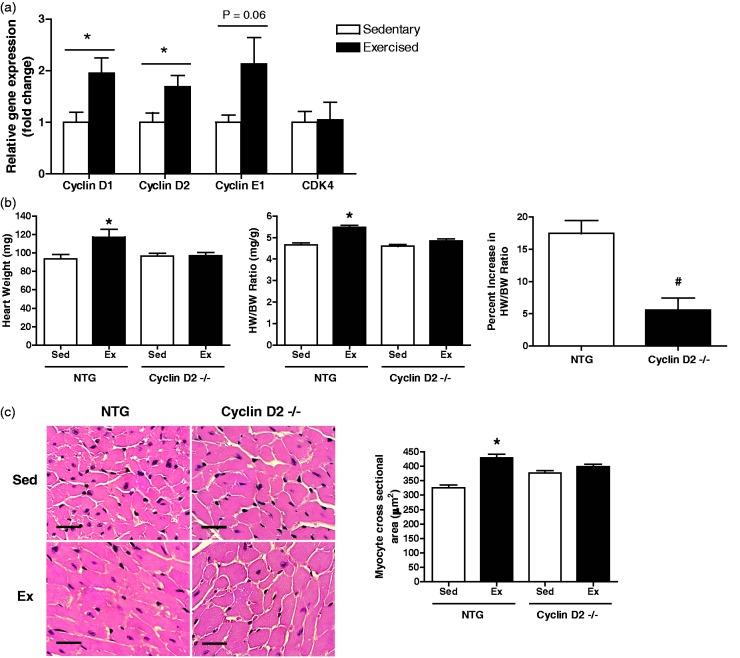
Although there is evidence that cyclin D2 signaling mediates both cardiac hypertrophy and cardiomyocyte proliferation during cardiac disease,34,35 the role of cyclin D2 in physiological hypertrophy is not clear. Therefore, we evaluated expression levels of cell cycle genes including cyclin D1, cyclin D2, cyclin E1, and cyclin-dependent kinase (CDK) 4 in sedentary and exercise CB6F1 NTG female mice. In the exercised hearts, cyclin D1 and cyclin D2 mRNA levels were significantly increased compared to the sedentary group (Figure 4(a)) while there are no statistically significant differences in gene expression of CDK4 and cyclin E1 (P value = 0.06). These data suggest that increases in gene expression of a subset of cell cycle proteins, including cyclin D2, are associated with physiological hypertrophy. Next, we subjected female mice deficient in cyclin D2 to 21 days of exercise training and determined that cyclin D2−/− female mice exercised to a similar extent compared to their littermate controls (Table 1). In contrast to the other transgenic mouse lines, female cyclin D2−/− mice had limited hypertrophic growth when subjected to exercise. Both heart weight and HW/BW ratio of exercised cyclin D2−/− female mice were not significantly different from sedentary mice (HW/BW ratio: 4.6 ± 0.08 mg/g vs. 4.9 ± 0.08 mg/g) (Figure 4(b)). Consistent with heart weights and HW/BW ratios, myocyte cross-sectional area in NTG hearts was increased after 21 days of exercise training, whereas exercise did not affect myocyte cross-sectional area in cyclin D2−/− mice (Figure 4(c)). The percent increase in HW/BW ratio in response to exercise was significantly reduced in cyclin D2−/− mice compared to NTG littermates (Figure 4(b)). Finally, we did not observe differences in the degree of interstitial fibrosis in the hearts of exercised and sedentary cyclin D2−/− mice (data not shown). Taken together, loss of cyclin D2 does not impact exercise performance but exercise-induced hypertrophy is attenuated in these mice. Interestingly, we also observed a similar reduction in exercise-mediated cardiac growth in male cyclin D2−/− mice suggesting that this effect is not sex-specific (data not shown).
Figure 4.
Expression analysis of a subset of cell cycle genes in sedentary and exercised NTG female mice, and morphometric data, cardiac adaptation, and histological analysis in response to 21 days of exercise for cyclin D2−/− and NTG female mice. (a) Expression of cell cycle genes including cyclin D1, cyclin D2, cyclin E1, and CDK4 in sedentary and exercised (21 days) NTG mice (CB6F1 hybrid line). (b) Morphometric data and cardiac adaptation in cyclin D2−/− and NTG littermate controls: Left: heart weight. Middle: heart weight normalized for body weight (HW/BW) ratio. Right: percent increase in HW/BW ratio. (c) Cardiomyocyte cell size analysis: Left: left ventricular cross-sections stained with hematoxylin and eosin (scale bar = 25 µm). Right: quantification of myocyte cross sectional area (n = 3–4 per group, 3–4 regions per heart, 5–10 cells per region). *, P < 0.05 relative to sedentary counterparts. n = 5–14 animals per group. Data are reported as mean ± SE. (A color version of this figure is available in the online journal.)
Cardiac contractile function in sedentary and exercised NTG and cyclin D2−/− mice
To determine the impact of reduced hypertrophic growth observed in the cyclin D2−/− exercised female mice, left ventricular dimensions and function were measured by echocardiography. After 21 days of voluntary wheel running, NTG mice had markedly increased parameters of hypertrophy including left ventricular posterior wall end-systolic (LVPWs) thickness (0.99 ± 0.01 mm vs. 1.15 ± 0.05 mm) and left ventricular anterior wall end-systolic (LVAWs) thickness (1.02 ± 0.03 mm vs. 1.30 ± 0.06 mm) (Table 2). In contrast, assessment of left ventricular dimensions in cyclin D2−/− female mice did not show any differences between sedentary and exercised mice (LVPWs, 0.91 ± 0.06 mm vs. 0.96 ± 0.01 mm) (Table 2). Fractional shortening and ejection fraction were also not different between sedentary and exercised NTG or cyclin D2−/− female mice (Table 2). Taken together, these data support our findings indicating that cyclin D2−/− females did not increase heart size in response to exercise. Moreover, despite the reduced hypertrophic response to exercise, cardiac function was not affected in these mice.
Table 2.
Echocardiographic analysis of mice in response to voluntary wheel running
| NTG |
Cyclin D2−/− |
|||
|---|---|---|---|---|
| Sedentary (n = 4) | Exercised (n = 4) | Sedentary (n = 4) | Exercised (n = 3) | |
| Heart Rate (bpm) | 486.0 ± 12.9 | 527.2 ± 16.4 | 484 ± 10.0 | 498.1 ± 14.0 |
| LVIDd (mm) | 3.93 ± 0.12 | 4.02 ± 0.02 | 3.51 ± 0.24 | 3.85 ± 0.06 |
| LVIDs (mm) | 2.69 ± 0.22 | 2.51 ± 0.06 | 2.31 ± 0.21 | 2.44 ± 0.08 |
| LVPWd (mm) | 0.65 ± 0.03 | 0.66 ± 0.03 | 0.66 ± 0.04 | 0.58 ± 0.03 |
| LVPWs (mm) | 0.99 ± 0.01 | 1.15 ± 0.05* | 0.91 ± 0.06 | 0.96 ± 0.01 |
| LVAWd (mm) | 0.65 ± 0.05 | 0.76 ± 0.02 | 0.64 ± 0.02 | 0.63 ± 0.02 |
| LVAWs (mm) | 1.02 ± 0.3 | 1.30 ± 0.06* | 1.04 ± 0.11 | 1.06 ± 0.04 |
| FS (%) | 31.7 ± 3.9 | 37.6 ± 1.6 | 34.0 ± 4.8 | 36.6 ± 0.9 |
| EF (%) | 59.7 ± 5.6 | 68.0 ± 1.9 | 63.0 ± 6.2 | 67.0 ± 1.2 |
Note: Anesthetized mice underwent transthoracic echocardiography. Values are mean ± SEM.
LVIDd: LV internal diameter during diastole; LVIDs: LV internal diameter during systole; LVPWd: LV posterior wall thickness during diastole; LVPWs: LV posterior wall thickness during systole; LVAWd: LV anterior wall thickness during diastole; LVAWs: LV anterior wall thickness during systole; FS: fractional shortening; EF: ejection fraction.
P < 0.05 vs sedentary mice.
Activation states of cellular signaling pathways in sedentary and exercised NTG and cyclin D2−/− mice
To address the mechanisms underlying the lack of exercise-induced cardiac hypertrophy in cyclin D2−/− mice, we measured the phosphorylation states of 46 intracellular signaling molecules in NTG and cyclin D2−/− sedentary and exercised hearts. To accomplish this, we used a well-described phospho-antibody array (41, 42). We carried out this analysis after seven days of exercise, reasoning that signaling events would precede hypertrophic events. Phosphorylation of 23 proteins increased more than 1.5-fold in both NTG and cyclin D2−/− hearts as a result of exercise when compared to sedentary animals (Table 3). Remarkably, no proteins had a decrease in phosphorylation of more than 33% in either NTG or cyclin D2−/− mice when comparing exercised to sedentary mice. We also compared exercised cyclin D2−/− mice to exercised NTG mice and identified six proteins with phosphorylation levels more than 1.5-fold higher in exercised cyclin D2−/− mice including Src, Fyn, Yes, Hck, Chk2, Fgr (Table 3). By comparison, phosphorylation of nine proteins was lower by more than 33.3% (Table 3). In other words, phosphorylation of these proteins was increased in exercised NTG mice compared to cyclin D2−/− mice. These proteins include Pyk2, eNOS, AMPKα1, PLCγ1, paxillin, p27 (both T198 and T157), c-Jun, and MSK 1/2. Consistent with a previous investigation,22 we also report here increased Akt and GSK-3β phosphorylation in NTG hearts following seven days of exercise. These data suggest that signaling pathways are differentially regulated in hearts that undergo physiologic hypertrophic growth compared to hearts that cannot.
Table 3.
Phosphorylation of selected kinases in cyclin D2−/− and NTG female mice exercised for seven days
| Kinase: Phosphorylation site | NTG Ex/Sed (Fold change) | Cyclin D2−/− Ex/ Sed (Fold change) | Cyclin D2−/−/NTG (Fold change) |
|---|---|---|---|
| Src: Y419 | 1.7 | 8.2 | ↑ 4.82 |
| Fyn: Y420 | 1.7 | 4.8 | ↑ 2.82 |
| Yes: Y426 | 1.7 | 4.5 | ↑ 2.65 |
| Hck: Y411 | 0.9 | 2.2 | ↑ 2.44 |
| Chk2: T68 | 1.8 | 3.5 | ↑ 1.94 |
| Fgr; Y412 | 2.1 | 3.6 | ↑ 1.71 |
| TOR: S2448 | 1.6 | 2.2 | 1.38 |
| STAT5b: Y699 | 1.5 | 1.8 | 1.20 |
| STAT5 a/b:Y694/Y699 | 1.8 | 2.1 | 1.17 |
| HSP27: S78/S82 | 2.0 | 2.3 | 1.15 |
| β-catenin | 3.5 | 3.8 | 1.09 |
| Lyn: Y397 | 2.9 | 3.1 | 1.07 |
| STAT6: Y641 | 2.0 | 2.1 | 1.05 |
| STAT2: Y689 | 2.2 | 2.3 | 1.05 |
| FAK: Y397 | 3.7 | 3.7 | 1.00 |
| STAT3: Y705 | 4.5 | 4.5 | 1.00 |
| AMPKα2: T172 | 2.0 | 1.9 | 0.95 |
| GSK-3α/β: S21/S9 | 1.7 | 1.4 | 0.82 |
| CREB: S133 | 3.9 | 3.0 | 0.77 |
| Lck: Y394 | 3.4 | 2.5 | 0.74 |
| MEK1/2: S218/S222, S222/S226 | 2.5 | 1.8 | 0.72 |
| Akt: S473 | 2.1 | 1.5 | 0.71 |
| Pyk2: Y402 | 2.8 | 1.5 | ↓ 0.54 |
| eNOS: s1177 | 2.3 | 1.1 | ↓ 0.48 |
| AMPKα1: t174 | 2.9 | 1.4 | ↓ 0.48 |
| PLCγ-1: Y783 | 3.8 | 1.5 | ↓ 0.39 |
| Paxillin: Y118 | 3.7 | 1.4 | ↓ 0.38 |
| p27: T198 | 3.7 | 1.1 | ↓ 0.30 |
| c-Jun: S63 | 5.9 | 1.3 | ↓ 0.22 |
| p27: T157 | 5.7 | 1.0 | ↓ 0.18 |
| MSK1/2: S376/S360 | 15.5 | 1.8 | ↓ 0.12 |
Note: Bold text indicates an increase in phosphorylation greater than 1.5-fold in both NTG and cyclin D2−/− exercised mice compared to sedentary mice.
Discussion
Many signaling pathways have been identified in the context of pathological hypertrophy. Here we evaluated a number of these pathways to determine their role in physiological hypertrophy in response to an exercise stimulus. Our data demonstrate that the pathways studied do not impact exercise performance and that only cyclin D2 was found to be an important regulator of physiologic hypertrophy. We conclude that unlike many hypertrophic signaling molecules tested here, cyclin D2 appears to be required for both pathologic and physiologic cardiac hypertrophy. These data underscore and provide further evidence that the signaling pathways that mediate physiological and pathological hypertrophy are distinct.
GSK-3β is a serine/threonine kinase that is ubiquitously expressed and, in contrast to most other proteins kinases, is normally active and negatively regulated by phosphorylation. GSK-3β regulates many biological functions45 and evidence has clearly shown that GSK-3β is a negative regulator of pathologic cardiac hypertrophy in vivo.20 Using the same transgenic mouse model as that used here, overexpression of constitutively active GSK-3β attenuated various forms of pathological hypertrophy including pressure overload, β-adrenergic stimulation, and hypertrophic cardiomyopathy.20,21 Researchers also studied mice with inducible overexpression of constitutively active GSK-3β and determined that active GSK-3β mediates the reversal of established pressure overload-induced hypertrophy.46 Finally, a recent investigation used a genetic homozygous GSK-3β knock-in (KI) mouse model in which serine 9 was changed to alanine, rendering the isoform constitutively active.47 These investigators found that GSK-3β KI prevented pathological hypertrophy following pressure overload.47 With respect to physiological hypertrophy, previous studies have demonstrated that voluntary wheel running and treadmill exercise stimulate GSK-3β phosphorylation.22,48 Interestingly, our laboratory previously demonstrated that active GSK-3β blunts the hypertrophic response to pregnancy using the same mice used here.49 While much evidence supports GSK-3β acting in an anti-hypertrophic manner, recent investigations have questioned this role (as reviewed in Cheng et al.50) and additional studies will be required to determine the precise role of GSK-3β activation in cardiac health and disease. Nevertheless, we found that constitutive activation of GSK-3β in the heart does not affect exercise performance nor does it inhibit physiological hypertrophy. These findings are important because if chronic activation of GSK-3β prevents pathologic hypertrophy, therapeutic strategies to maintain or increase GSK-3β activity would allow for physiological hypertrophy and its associated benefits to occur.
CaMKII is also a serine/threonine kinase activated by Ca2+/calmodulin that regulates intracellular Ca2+ handling or regulatory proteins.7 Upregulation of the delta3 isoform of CaMKII was demonstrated in human heart failure51 and subsequent animal studies have determined that CaMKII is a critical modulator of pathological hypertrophy.7 Anderson and his colleagues23,24 created a unique genetic mouse model that expresses a CaMKII inhibitory peptide, and demonstrated that CaMKII inhibition prevented various forms of cardiac disease including maladaptive remodeling and apoptosis as a result of excessive β-adrenergic receptor stimulation and myocardial infarction, and reduced cardiac dysfunction, arrhythmia susceptibility, and sudden death in calcineurin-mediated cardiomyopathy.52 Using the same genetic mouse model here, we found that cardiac-specific CaMKII inhibition does not affect the ability of a mouse to exercise nor does it prevent exercise-mediated cardiac growth. Taken together, these data indicate that myocardial inhibition of CaMKII can reduce pathological hypertrophy and, at the same time, allow the beneficial aspects of physiological hypertrophy to take place. These findings further underscore the importance of CaMK inhibition as an effective therapeutic strategy for patients with heart failure as suggested by Anderson et al.53
Akt is a serine/threonine kinase and an important part of the IGF1/phosphoinositide 3-kinase [PI3K, (p100α)]/Akt signaling cascade, the best characterized signaling cascade regulating physiological hypertrophy.3,9 We previously observed increased Akt phosphorylation during physiological hypertrophy,22 and more importantly, DeBosch et al.27 showed mice deficient in Akt1 were not capable of increasing their heart size in response to exercise. The mouse model studied in the current investigation (constitutive activation of Akt through myristoylation) has heart sizes that are ∼2–3 times larger than wild type hearts with no evidence of pathology at 16 weeks of age.29 On the other hand, long-term activation of Akt in these mice was found to be maladaptive in ischemia/reperfusion injury.54 Because of these findings, it was unclear whether these mice would have the capacity to exercise on the voluntary wheel and whether their hearts could undergo further hypertrophy. We observed normal exercise capacity and, surprisingly, their hearts were able to undergo additional growth (Figure 2). In fact, these mice had a similar percent increase in their HW/BW ratio as compared to their NTG counterparts. It is unclear whether physiological hypertrophy resulted in these mice from the activation of signaling pathways independent of Akt or whether the physiological hypertrophy was mediated solely through the increased Akt signaling. Nevertheless, these data indicate that even with cardiac-specific constitutive activation Akt, cardiac growth occurs in response to exercise.
Stimulation of G protein-coupled receptors activates the MAPK signaling cascade leading to the sequential phosphorylation and activation of the three major terminal effectors, ERKs, JNKs, and p38-MAPKs.55 Both human studies and animals models of heart failure have implicated MAPK signaling in cardiac disease.56 Investigations evaluating the role of MEKK1, a MAPKKKK that regulates JNK and ERK1/2, in cardiac disease have found opposite roles for MEKK1 in cardiac hypertrophy. One group observed that mice deficient in MEKK1 were protected against pathological cardiac hypertrophy induced by Gαq.32 In contrast, pathological cardiac hypertrophy and cardiac dysfunction were increased in these mice with pressure overload31 and familial hypertrophic cardiomyopathy.30 Here we demonstrate that expression of MEKK1 is not required for exercise capacity nor is it required for cardiac growth in response to the exercise stimulus. It appears, then, that although the role of MEKK1 in pathological hypertrophy may be stimulus-dependent, MEKK1 is not required for physiological hypertrophy.
Although D-type cyclins including cyclin D2 are important for cell cycle progression,57 an association between increased expression of cyclin D2 and the development of pathological hypertrophy was previously observed.33,58,59 More recently, studies have provided a mechanistic link between cyclin D2 signaling in myc- and pressure overload-induced hypertrophy.34,60 In these two studies, cyclin D2-deficient mice failed to achieve hypertrophy comparable to NTG mice in both models. Cardiac function was not measured in either study so the effect of blocking pathological hypertrophy on function in cyclin D2−/− mice is not known. Little is known about the role of cyclin D2 in physiological hypertrophy. To our knowledge, this is the first study to demonstrate that cyclin D2 is a critical mediator of physiologic hypertrophy. Interestingly, the lack of hypertrophic growth, at least initially, does not detrimentally impact cardiac function or increase myocardial fibrosis.
It is well established that during cell cycle progression, cyclin D2 binds to CDK4 and CDK661 and the cyclin D2-CDK4/6 complex phosphorylates a number of cellular targets including the pocket protein, Rb.62 The role of the cyclin D2-CDK4/6 complex has only recently been explored in cardiac hypertrophy. As previously mentioned, mice deficient in cyclin D2 have attenuated hypertrophy in response to pressure overload and this inhibition of cardiac growth is mediated through Rb.34 Additional studies implicating this pathway in cardiac growth include a study that observed partial inhibition of pathological hypertrophy when the CDK4/6 inhibitor, p16 was overexpressed.63 Furthermore, mice lacking p27, a CDK4/6 inhibitor, have baseline cardiac hypertrophy and exaggerated pathological hypertrophy following pressure overload.64 Interestingly, our study observed increased p27 phosphorylation in NTG exercised mice but not in cyclin D2−/− mice (Table 3). Since phosphorylation of p27 reduces the ability of p27 to inhibit CDK4/6 activity,65 we hypothesize that p27 is also involved in physiological hypertrophy. Although the levels of mRNAs encoding cell cycle proteins including cyclin D1, cyclin E1, and CDK4 were not measured in the cyclin D2−/− mice, these data and our findings clearly demonstrate that the cyclin D2-CDK4/6 pathway is regulated in cardiac hypertrophy. Future investigations aimed at the elucidation of the mechanism preventing physiological hypertrophy in the cyclin D2−/− mice will help further our understanding of exercise-mediated hypertrophy.
In addition to the changes in the phosphorylation state of p27, there was increased phosphorylation in molecules known to play roles in cardiac hypertrophy including Akt, MAPK kinase 1/2 (MEK1/2), GSK-3α/β, AMPKα2, and mTOR in NTG exercised mice.9 Interestingly, a number of Src family tyrosine kinases had increased phosphorylation levels in exercised cyclin D2−/− mice as compared to NTG exercised mice. Both Src and Fyn have been previously demonstrated to play a role in cardiac hypertrophy66,67 and evidence suggests that Fyn may be a negative regulator of hypertrophy.66 However, the role these tyrosine kinases play in regulation of cardiac hypertrophy in the cyclin D2−/− mice is unknown. Finally, phosphorylation of mitogen and stress activated kinase 1/2 (MSK1/2) is dramatically increased in the NTG exercised mice as compared to the cyclin D2−/− exercised mice (15.5-fold versus 1.8-fold, respectively). Little is known about the role of MSK 1/2 in cardiac hypertrophy except that increased phosphorylation of MSK1/2 is associated with phenylephrine-mediated hypertrophy of isolated cardiac myocytes.68 Further investigations are required to better understand the role of MSK1/2 in physiological hypertrophy.
In summary, we find that of the signaling pathways tested, only cyclin D2 is an important mediator of physiological hypertrophy. We also demonstrate that cardiac function and interstitial fibrosis is not affected by the attenuated cardiac growth in the cyclin D2−/− mice and that a number of signaling pathways in these mice are differentially regulated. A limitation of this study is that the levels of cyclin D2 were only measured in a wild type mouse line and additional research is required to determine the mechanistic role of cyclin D2 in physiological hypertrophy. Nevertheless, this is the first study to demonstrate that cyclin D2 is a critical mediator of exercise-mediated hypertrophy and these data provide novel insights into the understanding of several well-characterized hypertrophic signaling molecules and their role in physiological hypertrophy. These data further underscore the differences in signaling pathways between physiological and pathological hypertrophy.
Acknowledgements
We are grateful to Margaret Isenhart for her care of the mice and Kelly Ambler for echocardiography. We also greatly appreciate Eric Olson for the GSK-3βS9A mice, Anthony Rosenzweig for the myrAkt mice, Mark Anderson for the AC3-I and AC3-C mice, Gary Johnson for the MEKK1−/− mice, and Peter Sicinski for the cyclin D2−/− mice.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript: SWL, CDH, JPK, EDL, and AMK conducted the experiments; SWL and LAL wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by National Heart, Lung, and Blood Institute HL 50560 (to L.A. Leinwand), F32 HL 72565, National Heart, Lung, and Blood Ruth L. Kirschstein National Research Service Award (to S.W. Luckey), and F32 HL 70509, National Heart, Lung, and Blood Ruth L. Kirschstein National Research Service Award (to J.P. Konhilas).
References
- 1.Dorn GW., 2nd The fuzzy logic of physiological cardiac hypertrophy. Hypertension 2007; 49: 962–70. [DOI] [PubMed] [Google Scholar]
- 2.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975; 56: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 2010; 128: 191–227. [DOI] [PubMed] [Google Scholar]
- 4.Kellerman S, Moore JA, Zierhut W, Zimmer HG, Campbell J, Gerdes AM. Nuclear DNA content and nucleation patterns in rat cardiac myocytes from different models of cardiac hypertrophy. J Mol Cell Cardiol 1992; 24: 497–505. [DOI] [PubMed] [Google Scholar]
- 5.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis during hypertrophy in adult mice. Am J Physiol 1994; 266(4 Pt 2): H1439–45. [DOI] [PubMed] [Google Scholar]
- 6.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–6. [DOI] [PubMed] [Google Scholar]
- 7.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 2013; 123: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buttrick PM, Scheuer J. Physiologic, biochemical, and coronary adaptation to exercise conditioning. Cardiol Clin 1987; 5: 259–70. [PubMed] [Google Scholar]
- 9.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol 2013; 14: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res 2006; 98: 540–8. [DOI] [PubMed] [Google Scholar]
- 11.Fagard RH. Impact of different sports and training on cardiac structure and function. Cardiol Clin 1997; 15: 397–412. [DOI] [PubMed] [Google Scholar]
- 12.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation 2000; 101: 336–44. [DOI] [PubMed] [Google Scholar]
- 13.Maron BJ, Pelliccia A, Spataro A, Granata M. Reduction in left ventricular wall thickness after deconditioning in highly trained Olympic athletes. Br Heart J 1993; 69: 125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehsani AA, Hagberg JM, Hickson RC. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol 1978; 42: 52–6. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 2004; 94: 110–8. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Van Peborgh J, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 2000; 279: H2994–3002. [DOI] [PubMed] [Google Scholar]
- 17.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest 1988; 82: 2017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 2012; 302: H10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev 2006; 20: 3347–65. [DOI] [PubMed] [Google Scholar]
- 20.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A 2002; 99: 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luckey SW, Mansoori J, Fair K, Antos CL, Olson EN, Leinwand LA. Blocking cardiac growth in hypertrophic cardiomyopathy induces cardiac dysfunction and decreased survival only in males. Am J Physiol Heart Circ Physiol 2007; 292: H838–45. [DOI] [PubMed] [Google Scholar]
- 22.Konhilas JP, Maass AH, Luckey SW, Ikeda K, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in the mouse. Am J Physiol Heart Circ Physiol 2004; 287: H2768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Zhu WZ, Joiner ML, Zhang R, Oddis CV, Hou Y, Yang J, Price EE, Gleaves L, Eren M, Ni G, Vaughan DE, Xiao RP, Anderson ME. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am J Physiol Heart Circ Physiol 2006; 291: H3065–75. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr., Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 2005; 11: 409–17. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr., Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 2003; 92: 912–9. [DOI] [PubMed] [Google Scholar]
- 26.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol 2002; 22: 2799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 2006; 113: 2097–104. [DOI] [PubMed] [Google Scholar]
- 28.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 2005; 115: 2108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 2002; 277: 22896–901. [DOI] [PubMed] [Google Scholar]
- 30.Konhilas JP, Boucek DM, Horn TR, Johnson GL, Leinwand LA. The role of MEKK1 in hypertrophic cardiomyopathy. Int Heart J 2010; 51: 277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Liu J, Takagi G, Karoor V, Hong C, Johnson GL, Vatner DE, Vatner SF. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest 2002; 110: 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minamino T, Yujiri T, Terada N, Taffet GE, Michael LH, Johnson GL, Schneider MD. MEKK1 is essential for cardiac hypertrophy and dysfunction induced by Gq. Proc Natl Acad Sci U S A 2002; 99: 3866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busk PK, Bartkova J, Strom CC, Wulf-Andersen L, Hinrichsen R, Christoffersen TE, Latella L, Bartek J, Haunso S, Sheikh SP. Involvement of cyclin D activity in left ventricle hypertrophy in vivo and in vitro. Cardiovasc Res 2002; 56: 64–75. [DOI] [PubMed] [Google Scholar]
- 34.Angelis E, Garcia A, Chan SS, Schenke-Layland K, Ren S, Goodfellow SJ, Jordan MC, Roos KP, White RJ, MacLellan WR. A cyclin D2-Rb pathway regulates cardiac myocyte size and RNA polymerase III after biomechanical stress in adult myocardium. Circ Res 2008; 102: 1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 2005; 96: 110–8. [DOI] [PubMed] [Google Scholar]
- 36.Foryst-Ludwig A, Kreissl MC, Sprang C, Thalke B, Bohm C, Benz V, Gurgen D, Dragun D, Schubert C, Mai K, Stawowy P, Spranger J, Regitz-Zagrosek V, Unger T, Kintscher U. Sex differences in physiological cardiac hypertrophy are associated with exercise-mediated changes in energy substrate availability. Am J Physiol Heart Circ Physiol 2011; 301: H115–22. [DOI] [PubMed] [Google Scholar]
- 37.Yujiri T, Sather S, Fanger GR, Johnson GL. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 1998; 282: 1911–4. [DOI] [PubMed] [Google Scholar]
- 38.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384: 470–4. [DOI] [PubMed] [Google Scholar]
- 39.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 2001; 90: 1900–8. [DOI] [PubMed] [Google Scholar]
- 40.Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. Soy diet worsens heart disease in mice. J Clin Invest 2006; 116: 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haines CD, Harvey PA, Leinwand LA. Estrogens mediate cardiac hypertrophy in a stimulus-dependent manner. Endocrinology 2012; 153: 4480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen PC, Wakimoto H, Conner D, Araki T, Yuan T, Roberts A, Seidman C, Bronson R, Neel B, Seidman JG, Kucherlapati R. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome-associated Sos1 mutation. J Clin Invest 2010; 120: 4353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 2011; 90: 234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem 2002; 277: 22528–33. [DOI] [PubMed] [Google Scholar]
- 45.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3 – an overview of an over-achieving protein kinase. Curr Drug Targets 2006; 7: 1377–88. [DOI] [PubMed] [Google Scholar]
- 46.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res 2003; 92: 609–16. [DOI] [PubMed] [Google Scholar]
- 47.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci U S A 2008; 105: 20900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iemitsu M, Maeda S, Miyauchi T, Matsuda M, Tanaka H. Gene expression profiling of exercise-induced cardiac hypertrophy in rats. Acta Physiol Scand 2005; 185: 259–70. [DOI] [PubMed] [Google Scholar]
- 49.Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol 2012; 112: 1564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng H, Woodgett J, Maamari M, Force T. Targeting GSK-3 family members in the heart: a very sharp double-edged sword. J Mol Cell Cardiol 2011; 51: 607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res 1999; 84: 713–21. [DOI] [PubMed] [Google Scholar]
- 52.Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation 2006; 114: 1352–9. [DOI] [PubMed] [Google Scholar]
- 53.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 2011; 51: 468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 2005; 115: 2128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clerk A, Sugden PH. Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists. Am J Cardiol 1999; 83: 64H–9H.. [DOI] [PubMed] [Google Scholar]
- 56.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev 2010; 90: 1507–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherr CJ. D-type cyclins. Trends Biochem Sci 1995; 20: 187–90. [DOI] [PubMed] [Google Scholar]
- 58.Sadoshima J, Aoki H, Izumo S. Angiotensin II and serum differentially regulate expression of cyclins, activity of cyclin-dependent kinases, and phosphorylation of retinoblastoma gene product in neonatal cardiac myocytes. Circ Res 1997; 80: 228–41. [DOI] [PubMed] [Google Scholar]
- 59.Li JM, Poolman RA, Brooks G. Role of G1 phase cyclins and cyclin-dependent kinases during cardiomyocyte hypertrophic growth in rats. Am J Physiol 1998; 275(3 Pt 2): H814–22. [DOI] [PubMed] [Google Scholar]
- 60.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM, MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J 2006; 25: 3869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reed SI. G1/S regulatory mechanisms from yeast to man. Prog Cell Cycle Res 1996; 2: 15–27. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 1995; 81: 323–30. [DOI] [PubMed] [Google Scholar]
- 63.Nozato T, Ito H, Watanabe M, Ono Y, Adachi S, Tanaka H, Hiroe M, Sunamori M, Marum F. Overexpression of CDK Inhibitor p16INK4a by adenovirus vector inhibits cardiac hypertrophy in vitro and in vivo: a novel strategy for the gene therapy of cardiac hypertrophy. J Mol Cell Cardiol 2001; 33: 1493–504. [DOI] [PubMed] [Google Scholar]
- 64.Hauck L, Harms C, An J, Rohne J, Gertz K, Dietz R, Endres M, von Harsdorf R. Protein kinase CK2 links extracellular growth factor signaling with the control of p27(Kip1) stability in the heart. Nat Med 2008; 14: 315–24. [DOI] [PubMed] [Google Scholar]
- 65.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 2002; 8: 1153–60. [DOI] [PubMed] [Google Scholar]
- 66.Matsushima S, Kuroda J, Zhai P, Liu T, Ikeda S, Nagarajan N, Oka S, Yokota T, Kinugawa S, Hsu CP, Li H, Tsutsui H, Sadoshima J. Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. J Clin Invest 2016; 126: 3403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Gong H, Jiang G, Ye Y, Wu J, You J, Zhang G, Sun A, Komuro I, Ge J, Zou Y. Src is required for mechanical stretch-induced cardiomyocyte hypertrophy through angiotensin II type 1 receptor-dependent beta-arrestin2 pathways. PLoS One 2014; 9: e92926–e92926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Markou T, Cieslak D, Gaitanaki C, Lazou A. Differential roles of MAPKs and MSK1 signalling pathways in the regulation of c-Jun during phenylephrine-induced cardiac myocyte hypertrophy. Mol Cell Biochem 2009; 322: 103–12. [DOI] [PubMed] [Google Scholar]