Short abstract
Reductions in hydrogen sulfide (H2S) production have been implicated in the pathogenesis of hypertension; however, no studies have examined the functional role of hydrogen sulfide in hypertensive heart disease. We hypothesized that the endogenous production of hydrogen sulfide would be reduced and exogenous hydrogen sulfide would ameliorate cardiac dysfunction in Nω-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rats. Therefore, this study investigated the cardioprotective effects of hydrogen sulfide on L-NAME-induced hypertensive heart disease and explored potential mechanisms. The rats were randomly divided into five groups: Control, Control + sodium hydrosulfide (NaHS), L-NAME, L-NAME + NaHS, and L-NAME + NaHS + glibenclamide (Gli) groups. Systolic blood pressure was monitored each week. In Langendorff-isolated rat heart, cardiac function represented by ±LV dP/dtmax and left ventricular developing pressure was recorded after five weeks of treatment. Hematoxylin and Eosin and Masson’s trichrome staining and myocardium ultrastructure under transmission electron microscopy were used to evaluate cardiac remodeling. The plasma nitric oxide and hydrogen sulfide concentrations, as well as nitric oxide synthases and cystathionine-γ-lyase activity in left ventricle tissue were determined. The protein expression of p-Akt, Akt, p-eNOS, and eNOS in left ventricle tissue was analyzed using Western blot. After five weeks of L-NAME treatment, there was a time-dependent hypertension, cardiac remodeling, and dysfunction accompanied by a decrease in eNOS phosphorylation, nitric oxide synthase activity, and nitric oxide concentration. Meanwhile, cystathionine-γ-lyase activity and hydrogen sulfide concentration were also decreased. NaHS treatment significantly increased plasma hydrogen sulfide concentration and subsequently promoted the Akt/eNOS/NO pathway which inhibited the development of hypertension and attenuated cardiac remodeling and dysfunction. The cardioprotective effects of NaHS were counteracted by Gli which inhibited the Akt/eNOS/NO pathway. This suggests that the effects of hydrogen sulfide were mediated by the activation of the KATP channels. In conclusion, hydrogen sulfide ameliorated L-NAME-induced hypertensive heart disease via the activation of the Akt/eNOS/NO pathway, which was mediated by KATP channels.
Impact statement
1. We found that H2S ameliorated L-NAME-induced cardiac remodeling and dysfunction, and played a protective role in L-NAME-induced hypertensive heart disease, which the existing studies have not reported.
2. H2S activated the Akt/eNOS/NO pathway, thereby playing a cardioprotective role in L-NAME-induced hypertensive heart disease.
3. The cardioprotective effect of H2S was mediated by ATP-sensitive potassium channels.
Keywords: Hydrogen sulfide, hypertensive heart disease, cardiac function, nitric oxide, cardiac remodeling, Nω-nitro-L-arginine methyl ester
Introduction
Hypertension is a major contributory factor in cardiovascular disease and increases the mechanical load of the heart. If not controlled, it can lead to pathological cardiac remodeling and dysfunction, resulting in hypertensive heart disease (HHD), which eventually leads to heart failure.1 Several different mechanisms are considered to underlie the functional and histological cardiac damage in this process, including defects in the endogenous synthesis of nitric oxide (NO) and loss of NO bioavailability.2 NO is endogenously produced from L-arginine by a family of nitric oxide synthase (NOS) including three isoforms, namely neuronal, endothelial, and inducible NOS (nNOS, eNOS, and iNOS, respectively) and plays various important physiological and pathophysiological roles in the cardiovascular system. A decrease in NO production and loss of its bioactivity induces endothelial dysfunction, which occurs during the early stage of hypertension.3 Nω-nitro-L-arginine methyl ester (L-NAME), a non-specific NOS inhibitor, significantly decreases serum NO levels and induces elevation of blood pressure; over time, this leads to cardiac remodeling and dysfunction.4 It is well known that the serine/threonine protein kinase Akt (protein kinase B) can activate eNOS by direct phosphorylation of serine 1177, thereby leading to NO production. Furthermore, an altered Akt/eNOS signaling plays a contributing role in hypertension. In addition, congestive heart failure induced by coronary artery ligation in rats is associated with endothelial dysfunction and decreased activation of Akt/eNOS signaling.
Hydrogen sulfide (H2S), the most recently identified endogenously produced gasotransmitter, has been found to play a pivotal role in the regulation of cardiovascular function, and a decrease in the endogenous production of H2S has been associated with several pathological conditions in the cardiovascular system such as hypertension, heart failure, atherosclerosis, and diabetes.5–8 H2S is endogenously synthesized by three enzymes: cystathionine β-synthase, cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase. Similar to NO, H2S is also generated by the endothelium and affects vascular tone. Studies have shown that mice with a deletion of the key enzymes in NO and H2S production, eNOS and CSE, display hypertension.9,10 In addition, there is growing evidence that a crosstalk may exist between H2S and NO in the endothelium. Various studies have demonstrated that biological effects of H2S are regulated by NO.11,12 However, recent studies suggest that H2S influences the bioavailability of NO and activities of its synthesis enzymes. H2S increases eNOS activity by inducing the S-sulfhydration of eNOS, promoting its phosphorylation, inhibiting its S-nitrosylation, and increasing eNOS dimerization.13,14 In contrast, other studies have reported that H2S inhibits eNOS activity in endothelial cells.15 However, it is not yet clear whether H2S modulates the biological effects of NO in HHD.
We hypothesized that endogenous production of H2S would be reduced and exogenous H2S would ameliorate cardiac dysfunction in L-NAME-induced hypertensive rats. Therefore, the present study was designed to investigate the cardioprotective effects of H2S on L-NAME-induced HHD, to clarify the presence of a cross-talk between NO and H2S, and to explore the potential mechanisms.
Materials and methods
Drugs and chemicals
Sodium hydrosulfide (NaHS), L-NAME, glibenclamide (Gli), L-cysteine and pyridoxal-5’-phosphate were purchased from Sigma-Aldrich Ltd (St. Louis, MO, USA). NaHS, a donor of H2S, was freshly prepared before use. Detection kits for NO and NOS activities were purchased from Jiancheng Biomedical Engineering (Nanjing, Jiangsu, China). Bicinchoninic acid (BCA) reagent was purchased from Generay Biotechnology (Shanghai, China). Other chemicals and reagents were of analytical grade.
Animal model of hypertension and experimental protocol
Male normotensive Sprague Dawley rats (3 weeks old) from the Experimental Animal Center of Hebei Medical University were housed in plastic cages in a room with a controlled humidity of 60%, at a temperature of 22°C–24°C and on a regular 12-h light and dark cycle. They were fed on standard rat chow and tap water ad libitum. The rats were allowed to acclimate for one week before the beginning of the experiment and the daily mean value of water consumption was estimated. During the experiment, drinking fluid consumption was controlled and adjusted, when necessary. All animal experimental procedures were approved by the Ethics Committee for Laboratory Animals Care and Use of Hebei Medical University and in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health.
The four-week-old rats with an initial body weight of 100 g were randomly divided into five groups: Control, Control + NaHS group, L-NAME, L-NAME + NaHS, and L-NAME + NaHS + Gli groups. All rats received 50 mg/100 mL L-NAME, which was dissolved in their drinking water for five weeks, except those in the Control and Control + NaHS groups which received tap water for the same period. In the L-NAME + NaHS + Gli group, Gli (5 mg/100 mL) was added to the drinking water. Rats in the NaHS treatment group were intraperitoneally injected with 56 μmol/kg/d of NaHS in the same volume of 0.2 mL at the same time each day for five weeks and stopped two days before measurement of cardiac function by Langendorff perfusion for the study of the long-term effects of drugs without the involvement of the acute effects of drugs. Rats in the other groups were injected with the same volume of sterile saline.
Measurement of blood pressure
To assess the development of hypertension, blood pressure was serially determined in conscious and quiet rats using a non-invasive tail-cuff plethysmograph (Chengdu, Sichuan, China). Before the onset of the experiment, rats were acclimated to the tail-cuff plethysmography procedure for one week, and their blood pressure was measured before the treatments started and each week thereafter. The value was taken as the average of at least five measurements after removing the outliers and any associated with excess noise or animal movement on each occasion.
Measurement of cardiac function by Langendorff perfusion
After five weeks, the rats were anesthetized by intraperitoneal injection of 25% urethane (1.25 g/kg) and then anticoagulated with heparin (500 U/kg). A polyethylene catheter was introduced into the right femoral artery to collect blood. The plasma was prepared by centrifuging the blood samples at 4000 rpm for 10 min and frozen at −80°C for subsequent experimental analysis. Thereafter, the chest was opened, and the heart was rapidly excised and placed in cold (4°C) modified Krebs–Henseleit (K–H) solution with the following composition (in mmol/L): NaCl 118.0, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.0, and glucose 11.0 (pH 7.4). The heart, mounted on the Langendorff apparatus, was perfused retrogradely through the aorta with K–H solution, which was held at 37°C and aerated with 95% O2 and 5% CO2. A saline-filled latex balloon was inserted into the left ventricle (LV) through the left atrium and connected via a rigid fluid-filled catheter to a pressure transducer for the measurement of left ventricular pressure. After 20 min of stabilization, parameters of left ventricular function including maximal positive and negative velocity of left ventricular pressure (±LV dP/dtmax) and left ventricular developing pressure (LVDP) were measured using an RM-6240BD (Chengdu, Sichuan, China) with left ventricular end diastolic pressure prestabilized at 4–10 mmHg.
Evaluation of LV hypertrophy
At the end of the experiment, the heart was promptly removed from the Langendorff apparatus. The heart weight and LV weight were determined, and the heart/body weight index (HWI) and left ventricular/body weight index (LVWI) were calculated (by dividing heart weight and left ventricular weight by body weight) as an indicator of left ventricular hypertrophy. Thereafter, the LV tissues were immediately frozen in liquid nitrogen and kept at −80°C for protein extraction.
Histological analysis
Heart tissues were fixed in 4% paraformaldehyde for 48 h, embedded in paraffin and cut into 4 μm sections. Hematoxylin and Eosin (H&E) and Masson’s trichrome staining of left ventricular heart sections were visualized using a light microscope (Olympus BX40, Tokyo, Japan).
Observation of the heart ultrastructure
The hearts were dissected, and small pieces were fixed with 2.5% glutaraldehyde and postfixed in 1% osmium tetroxide. The sections were stained with uranyl acetate and lead citrate and examined using a transmission electron microscopy (Model H-7500; Hitachi, Japan).
Measurement of NO concentration in plasma and NOS activity in LV tissue
The NO concentration in plasma was determined by evaluation of its oxidation products (nitrate and nitrite) using the nitrate reductase method. The nitrate was reduced to nitrite by nitrate reductase, and the nitrite was measured by the Griess reaction. The plasma was mixed with the reagents supplied in an NO assay kit (Jiancheng Biomedical Engineering, Nanjing, Jiangsu, China) and incubated at 37°C for 60 min. The absorbance was spectrophotometrically measured at 550 nm. All operations were according to the manufacturer’s instructions.
The NOS activity in LV tissues was also analyzed using the Griess reaction principle. In brief, LV tissues were homogenized and centrifuged at 12,000 g for 20 min at 4°C. The supernatant were mixed with the reagents supplied in an NOS assay kit (Jiancheng Biomedical Engineering, Nanjing, Jiangsu, China). The NO level in the supernatant was calculated according to the results of a standard curve to reflect the total biological activity of NOS.
Measurement of plasma H2S concentration
The plasma H2S was measured according to previously described methods.16 Thirty microliters of plasma were mixed with 80 μL monobromobimane (MBB; Sigma Aldrich) and 10 μL 0.1% ammonia by shaking for 1 h at room temperature for derivatization of sulfide. MBB reacts with sulfide to produce sulfide-dibimane (SDB). SDB is more hydrophobic than most physiological thiols and can be separated by gradient elution and analyzed by liquid chromatography–tandem mass spectrometry. The reaction was then terminated with 10 μL 20% formic acid and centrifuged at 15,000 g for 10 min. The supernatants were stored at −80°C until H2S measurements were done. H2S concentrations were determined using a curve generated with sodium sulfide (0–40 μmol/L) standards.
Measurement of CSE activity
CSE activity in heart tissues was measured according to previously described methods with some methods modified.17 Briefly, heart tissues were homogenized in ice-cold PBS and centrifuged at 12,000 g for 20 min at 4°C. The supernatant was immediately used to measure the activity of CSE, and proteins in the supernatant were quantified using BCA reagent. To measure the CSE activity, the enzyme substrate L-cysteine (10 mmol/L) and the cofactor pyridoxal-5′-phosphate (2 mmol/L) were added to the supernatant for an incubation of 0.5 h at 37°C. The H2S concentrations in the reaction system were then measured and the amount of H2S produced per microgram protein per hour was calculated as the activity of CSE.
Western blot analysis
Frozen LV tissues were homogenized with ice-cold RIPA lysis buffer. The proteins were extracted and quantified by the BCA method. Equal amounts of protein samples (80 μg/lane) were separated on 10% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% non-fat milk for 1 h and incubated with primary antibodies that recognized p-eNOS (Ser1177, 1:1000; Abcam, USA), eNOS (1:1000, Abcam, USA), p-Akt (Ser473, 1:1000, Signalway, USA), Akt (1:1000, Signalway, USA), and GAPDH (1:1000; Proteintech, USA) at 4°C overnight. The membranes were then incubated with the appropriate secondary antibodies (1:2000; Gaithersburg, USA) for 1 h after washing with Tris-buffered saline containing Tween-20 (TBST). Specific bands were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo, Scientific-Pierce, Waltham, MA, USA). The band intensity was quantified by Image J software.
Statistical analysis
Results were presented as mean ± SEM. Statistical analysis was performed using the SPSS software package, version 13.0 (SPSS, Inc., Chicago, IL, USA). The results for three or more groups were compared using one-way ANOVA followed by Student–Newman–Keuls test. Comparisons between two groups were made using Student’s t-test. P < 0.05 was considered statistically significant.
Results
Exogenous H2S ameliorated cardiac dysfunction in L-NAME-induced hypertensive rats
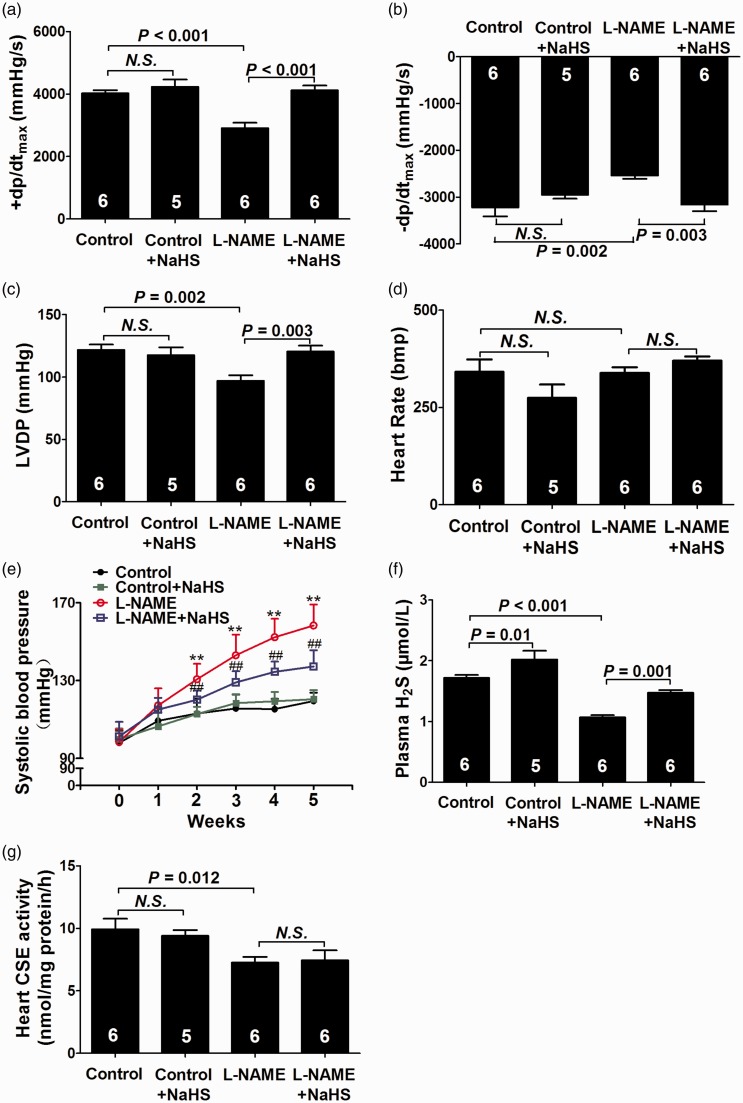
After five weeks of treatment, ±LV dP/dtmax (Figure 1(a) and (b)) and LVDP (Figure 1(c)) were significantly decreased (both P < 0.01) accompanied by a significant and time-dependent increase in blood pressure (Figure 1(e)) in the L-NAME group as compared with the control group (P < 0.01). Plasma H2S levels (Figure 1(f)) and myocardial CSE activity (Figure 1(g)) were significantly reduced in the L-NAME group compared to those in the control group (P < 0.01 and P < 0.05, respectively).
Figure 1.
Exogenous H2S ameliorated cardiac dysfunction in L-NAME-induced hypertensive rats. (a–d) Hemodynamic parameter analysis. (e) Changes of systolic blood pressure at the beginning and after five weeks of the experimental period. (f) H2S levels in plasma. (g) CSE activity in heart tissues. Results are means ± SEM. **P < 0.01 vs. Control group; ##P < 0.01 vs. L-NAME group. A P of <0.05 was considered significant. CSE: cystathionine-γ-lyase; LVDP: left ventricular developing pressure; HR: heart rate; ±LV dP/dtmax: maximal positive and negative velocity of left ventricular pressure. (A color version of this figure is available in the online journal.)
Five-week NaHS treatment significantly increased plasma H2S levels in the L-NAME group (P < 0.01), but did not change the myocardial CSE activity. NaHS treatment also increased ±LV dP/dtmax and LVDP (both P < 0.01), while decrease blood pressure in L-NAME-induced hypertensive rats (P < 0.01).
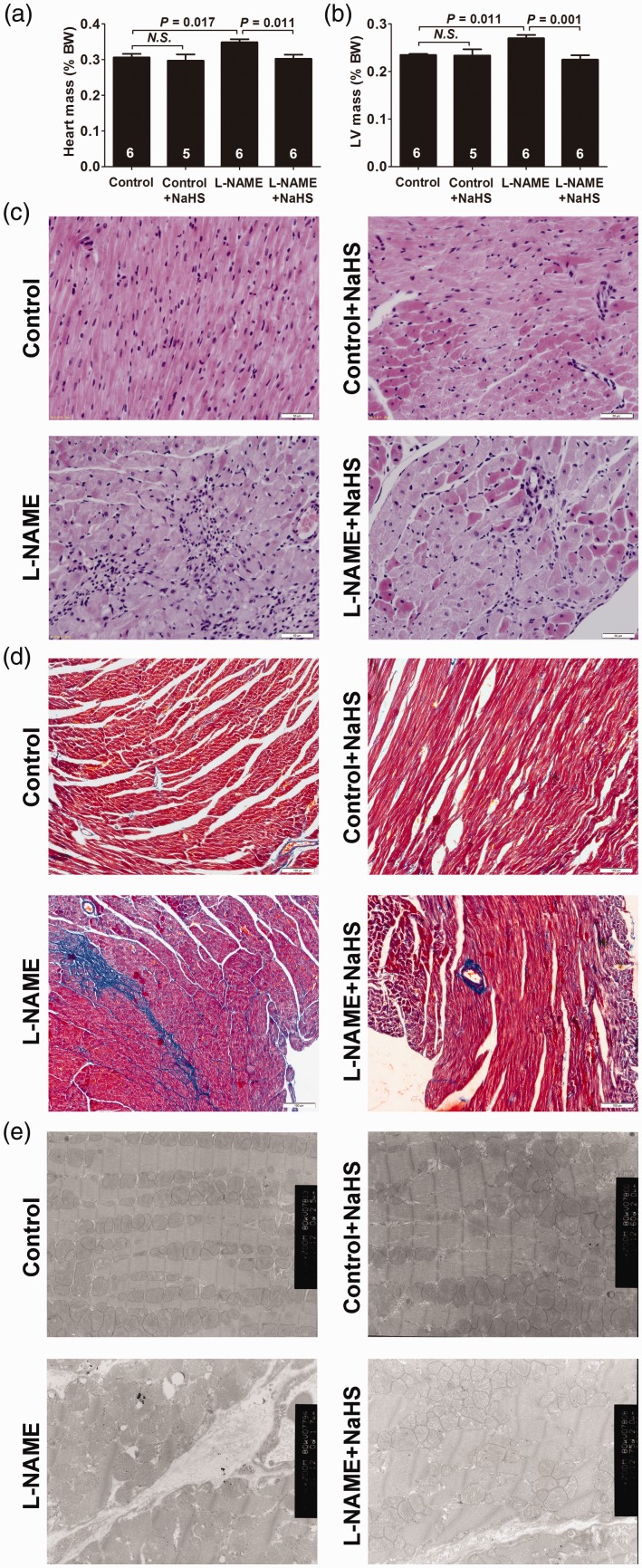
Exogenous H2S attenuated cardiac remodeling in myocardium in L-NAME-induced hypertensive rats
After five weeks of treatment, increased HWI and LVWI were found in L-NAME-induced hypertensive rats (both P < 0.05) (Figure 2(a) and (b)). H&E and Masson’s trichrome staining, respectively, showed fibroblast aggregation and increased interstitial collagen volumes in these rats (Figure 2(c) and (d)). Myocardial ultrastructure was shown in Figure 2(e). Collagen fiber hyperplasia, mitochondrial swelling and cristae vague could be observed in the L-NAME group. Treatment with NaHS decreased HWI and LVWI (both P < 0.05), reduced fibroblast aggregation and collagen hyperplasia, and ameliorated the ultrastructure of cardiomyocytes.
Figure 2.
Exogenous H2S attenuated cardiac remodeling in myocardium of L-NAME-induced hypertensive rats. (a) Heart-to-body weight ratios (HW/BW × 100%). (b) Left ventricle-to-body weight ratios (LVW/BW × 100%). (c) Representative HE-stained left ventricular sections (scale bar = 50 μm). (d) Representative Masson’s trichrome-stained left ventricular sections (scale bar = 100 μm). (e) Representative myocardium ultrastructure under transmission electron microscopy (5000×). Results are expressed as mean ± SEM. A P of <0.05 was considered significant. (A color version of this figure is available in the online journal.)
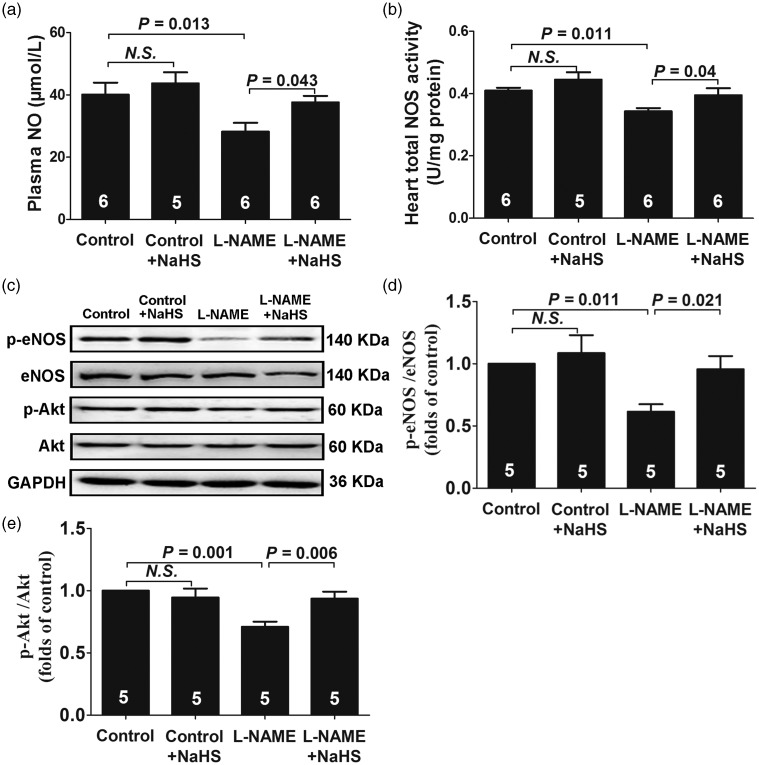
Exogenous H2S upregulated the eNOS/NO pathway in L-NAME-induced hypertensive rats
As shown in Figure 3, the NO concentration in plasma (Figure 3(a)) and NOS activity in LV tissues (Figure 3(b)) were decreased after five weeks of L-NAME treatment (both P < 0.05). L-NAME treatment also decreased the protein levels of phospho-eNOS at Ser1177 (P < 0.05) (Figure 3(d)), accompanied by a decrease in phosphorylations of Akt at Ser473 (P < 0.05) (Figure 3(e)). NaHS treatment significantly reversed the downregulation of phospho-Akt (P < 0.05) and phospho-eNOS (P < 0.05) and increased the NO concentration in plasma (P < 0.05) and NOS activity in LV tissues (P < 0.05).
Figure 3.
Exogenous H2S upregulated eNOS/NO pathway in L-NAME-induced hypertensive rats. (a) NO levels in plasma. (b) Total NOS activity in heart tissues. (c–e) Representative Western blots and quantitative analysis for p-eNOS/eNOS and p-Akt/Akt expression. Results are means ± SEM. A P of <0.05 was considered significant.
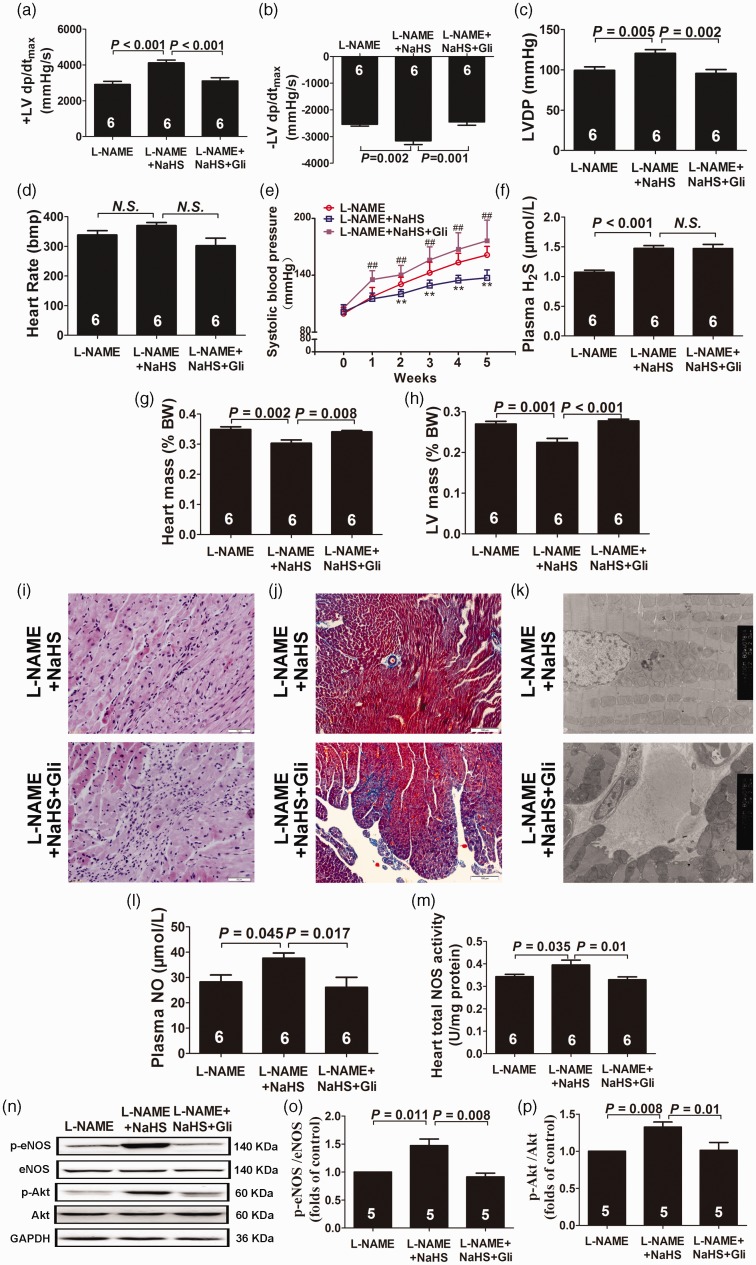
The cardioprotective effect of H2S was mediated by ATP-sensitive potassium (KATP) channels
As shown in Figure 4, the cardioprotective effects of NaHS were counteracted by Gli, a KATP channel inhibitor. After five weeks of treatment, ±LV dP/dtmax (Figure 4(a) and (b)) and LVDP (Figure 4(c)) were significantly decreased accompanied by a significant and time-dependent increase in blood pressure (Figure 4(e)) in the L-NAME + NaHS + Gli group as compared with the L-NAME + NaHS group (P < 0.01, respectively), but there was no statistical difference in plasma H2S levels between the two groups (Figure 4(f)). There was also no difference between the L-NAME and the L-NAME + NaHS + Gli groups in HWI and LVWI (Figure 4(g) and (h)). H&E and Masson’s trichrome staining, respectively, showed fibroblast aggregation and increased interstitial collagen volumes in the L-NAME + NaHS + Gli group as compared to the L-NAME + NaHS group. Meanwhile, myocardial ultrastructure showed collagen fiber hyperplasia, mitochondrial swelling, and cristae vague (Figure 4(i) to (k)). Five-week Gli treatment decreased the protein levels of phospho-eNOS (Figure 4(o)) accompanied by a decrease in phosphorylations of Akt in the L-NAME + NaHS group (both P < 0.05) (Figure 4(p)). The detailed statistics of the results have been supplied in the online supplementary material.
Figure 4.
The cardioprotective effect of H2S was mediated by KATP channels. (a–d) Hemodynamic parameter analysis. (e) Changes of systolic blood pressure at the beginning and after five weeks of the experimental period. (f) H2S levels in plasma. (g) Heart-to-body weight ratios (HW/BW × 100%). (h) Left ventricle-to-body weight ratios (LVW/BW × 100%). (i) Representative HE-stained left ventricular sections (scale bar = 50 μm). (j) Representative Masson’s trichrome-stained left ventricular sections (scale bar = 100 μm). (k) Representative myocardium ultrastructure under transmission electron microscopy (5000×). (l) NO levels in plasma. (m) Total NOS activity in heart tissues. (n–p) Representative Western blots and quantitative analysis for p-eNOS/eNOS and p-Akt/Akt expression. Results are expressed as mean ± SEM. **P < 0.01 vs. L-NAME group; ##P < 0.01 vs. L-NAME + NaHS group. A P of <0.05 was considered significant. HR, heart rate; LVDP, left ventricular developing pressure; ±LV dP/dtmax, maximal positive and negative velocity of left ventricular pressure. (A color version of this figure is available in the online journal.)
Discussion
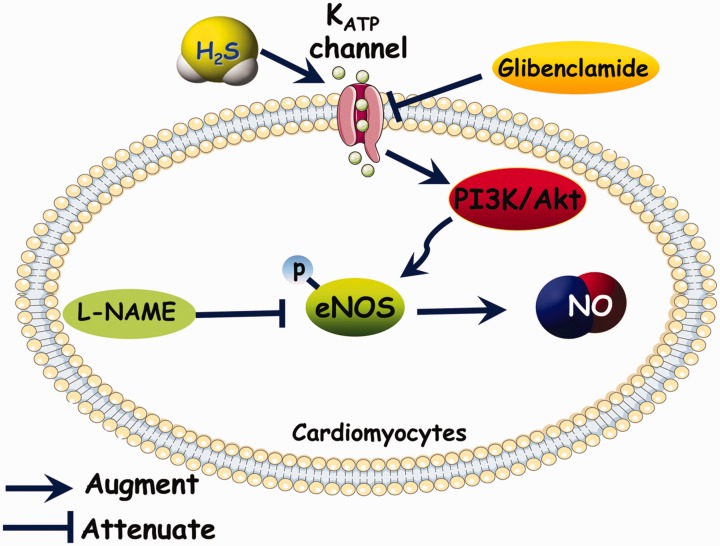
In the present study, we used the L-NAME-induced hypertensive rat to investigate the cardioprotective effects of H2S on HHD. The results showed that H2S ameliorated L-NAME-induced cardiac remodeling and dysfunction via activation of the Akt/eNOS/NO pathway, which was mediated by KATP channels (Figure 5).
Figure 5.
Schematic representation of the ameliorative effect of NaHS on cardiac dysfunction in L-NAME-induced hypertensive rats. (A color version of this figure is available in the online journal.)
Hypertension is a major global health problem which is becoming worse as the population ages. The sustained increase in blood pressure on the heart eventually leads to overt HHD, which is characterized by cardiac remodeling and dysfunction, a considerable cause of cardiovascular morbidity and mortality worldwide.1 Multiple mechanisms for the development of HHD have been proposed; the most important being defects in the endogenous synthesis of NO or loss of NO bioavailability.2 In the present study, we chronically blocked the generation of NO by the administration of L-NAME for five weeks to induce HHD in rats. This is a well-established hypertension model resembling patients with vascular endothelial dysfunction, who also develop functional and structural cardiac dysfunction caused by decreased NO bioavailability.18 As shown in our results, the NO concentration in plasma and NOS activity in LV tissues decreased after five weeks L-NAME treatment, subsequently causing a time-dependent hypertension and cardiac remodeling. To exclude interferences from neuroregulation, humoral regulation, and afterload, we used the Langendorff apparatus to observe cardiac function. In order to exclude interferences of preload, left ventricular end diastolic pressure was prestabilized at 4–10 mmHg. The ±LV dP/dtmax and LVDP were significantly decreased, which indicated the cardiac dysfunction induced by L-NAME treatment. These results are congruent with K Sonoda et al.19 who found that L-NAME treatment could lead to the development of HHD and dietary nitrite supplementation improved HHD by inhibiting AT II/AT1R -mediated cardiac remodeling.
After chronic depletion of NO by L-NAME treatment, our results showed that there was a significant decrease in plasma H2S levels and myocardial CSE activity which was also found to occur in numerous cardiovascular diseases such as atherosclerosis,7 myocardial infarction,20 and takotsubo cardiomyopathy.21 This suggests that endogenous NO may regulate the endogenous CSE/H2S pathway. As two important gasotransmitters, it has generally been considered that H2S and NO exert their biological effects via independent signaling pathways. However, recent experimental evidence suggests that there is a crosstalk between the two pathways. It has been proposed that the molecules generated by the chemical interaction of H2S and NO show a greater activity than the parental molecules.22,23 Besides a direct reaction, NO can increase H2S generation by upregulation of CSE expression24 or by increasing the activity of cGMP-dependent protein kinases, which in turn stimulate CSE.25 Some studies also report that sodium nitroprusside (a donor of NO) increases CSE activity in the aortic smooth muscle cells of spontaneously hypertensive rats.26 The mechanism by which NO regulates CSE has been well studied, while the mechanisms by which H2S regulates eNOS remain to be clarified. On the one hand, H2S increases eNOS activity by inducing S-sulfhydration, inhibiting S-nitrosylation, and promoting phosphorylation.13 On the other hand, H2S reduces eNOS activity and eNOS transcript abundance in cultured endothelial cells and isolated rat aortas27 and inhibits the activity of purified, recombinant eNOS.28 To date, the precise mechanisms by which H2S affects the NOS/NO pathways are unable to be predicted based on existing knowledge, and this needs to be studied further.
To determine the regulatory effects of exogenous H2S on cardiac dysfunction and the production of NO in L-NAME-induced hypertensive rats, NaHS was intraperitoneally injected every day for five weeks in our study. Our data showed that five-week NaHS treatment significantly increased plasma H2S levels and inhibited the development of HHD induced by L-NAME. Although it is not possible to exclude that the cardioprotective effects of H2S in HHD result from its antihypertensive effect, G Meng et al. found that a low dose of GYY4137 (a donor of H2S) also attenuated myocardial hypertrophy without any discernible antihypertensive effect. This suggests that the inhibitory effect of H2S on myocardial hypertrophy is created, at least in part, through a blood pressure independent mechanism.29
We also found that the long-term administration of NaHS significantly promoted eNOS phosphorylation, increased NOS activity in LV tissues and the NO concentration in plasma of the L-NAME-treated rats. Therefore, H2S may augment the NOS/NO pathway in the pathogenesis of L-NAME-induced HHD. Our findings are consistent with the previous observation. R Mazza et al.30 reported that NaHS dose-dependently decreased inotropism by increasing phosphorylation of eNOS (Ser-1177) and enhancing NO production in isolated and perfused frog and rat hearts. A recent study found that H2S improved intestinal recovery following ischemia by endothelial NO-dependent mechanisms.31 Similarly, AL King et al. found that mice lacking CSE exhibit dysfunctional eNOS, diminished NO levels, and exacerbated myocardial and hepatic ischemic-reperfusion (I/R) injuries. In CSE knockout (KO) mice, H2S restored eNOS function and NO bioavailability and attenuated I/R injury; while H2S failed to protect against I/R in eNOS KO and eNOS phospho-mutant mice (S1179A).32 In addition, similar studies have been done by Ji et al.33 and Zhong et al.34 who also found the antihypertensive effects of H2S were mediated by the eNOS/NO pathway in L-NAME-induced hypertensive rats. Ji et al. focused on the protective role of H2S in the pathological injury of the liver and lipid metabolism abnormalities induced by hypertension, while our study demonstrated the cardioprotective effects of H2S in HHD which was one of the common hypertension-related complications. Zhong et al. showed for the first time that exogenous H2S prevented L-NAME induced hypertension. However, long-term administration of NaHS (20 μmolmol/kg/day) did not change the NO production. In our study, higher dose of NaHS (56 μmolmol/kg/day) was given to the mice and significantly increased the NO concentration in plasma and NOS activity in LV tissues. In pressure overload-induced heart failure, cardiac nitrite levels were also increased after oral SG-1002 (active H2S releasing compound) treatment for 12 weeks.35
It is well established that eNOS can undergo post-translational modifications, including phosphorylation or dephosphorylation, which tightly regulate NO production. Specifically, phosphorylation of the amino acid Ser1177, which increases the activity of eNOS, is facilitated by Akt activation.36 In the present study, NaHS increased Akt phosphorylation at Ser473 followed by an increase in eNOS phosphorylation at Ser1177. These results are comparable to the findings of K Kondo et al. who supported that H2S protected against pressure overload-induced heart failure via a VEGF-Akt-eNOS-NO-cGMP signaling pathway.35 NaHS-induced eNOS phosphorylation was abolished by LY 294002 (an inhibitor of the Akt pathway)37 and was also absent in Akt-heterogenic deficient mice.35 Recent studies provide strong evidence that the stimulatory effect on Akt activity is likely to be related to H2S-mediated inhibition of phosphatase and tensin homolog, and protein tyrosine phosphatase 1B (the two essential counterregulatory enzymes of the PI3K pathway that is upstream of Akt) via sulfhydration of Cys124 or Cys215, respectively.38,39
KATP channel opening is known to mediate many of the biological activities of H2S. Both vessel relaxation and the negative inotropic effects of H2S are reported to occur through sulfhydration of the Kir 6.1 subunit in the KATP channel.40 However, whether the cardioprotective effects of H2S on HHD are mediated by KATP channels is not yet fully defined. As a result, Gli, a KATP channel inhibitor, has been used for subsequent experiments. As shown in our result, the cardioprotective effects of NaHS were counteracted by Gli, suggesting that the effects of H2S were mediated by the activation of the KATP channel. We also found that five-week Gli treatment down-regulated the eNOS/NO pathway by decreasing Akt phosphorylation. These data are in agreement with the reports of J Kong et al., who found that Nicorandil (a KATP channel opener) increases the levels of phospho-Akt by PI3K activation in the heart and brain.41 The opening of KATP channels leads to an influx of Ca2+, as a result of K+ efflux, and then up-regulates the Akt/eNOS pathway to facilitate the function of human endothelial colony-forming cells.42
There are several limitations in the present study. Firstly, it is important to note that the endogenous production of H2S appears to be significantly reduced in HHD. However, whether the reduction of H2S levels is the cause or consequence of this pathological state is not well understood. Further studies should, therefore, be done to explore the roles of endogenous H2S in the basal physiological functions and pathogenesis of HHD. Additionally, we find that H2S plays a protective role in L-NAME-induced HHD via activation of the eNOS/NO pathway, but it is relatively simple and insufficient. Another area needing further clarification is the interaction between H2S and NO, and perhaps with CO. Finally, if it is applied to clinical treatment, it is important to consider fully the highly toxic actions of supraphysiological levels of H2S, and great care must be taken during the development of H2S-based therapeutic agents. This means that there is still a long way to go.
Conclusion
In conclusion, our studies revealed that H2S ameliorated L-NAME-induced cardiac remodeling and dysfunction, and played a protective role in L-NAME-induced HHD via activation of the Akt/eNOS/NO pathway, which was mediated by KATP channels.
Supplementary Material
Funding
This study was supported by the National Natural Science Foundation of China (Grant 31171098, 31671185), the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20121323110008), the Hebei Province for Innovation Talents Support Plan (Grant LJRC017), the Natural Science Foundation of Hebei Province of China (H2017206269), and the office of Education Foundation of Hebei Province of China (QN2016144).
Authors' contributions
SJ, XT, and YW designed and performed experiments, analyzed data, and contributed to writing the manuscript; LX performed molecular biology experiment; HX measured plasma H2S concentration; QG, XD, and YC performed transmission electron microscopy, H&E, and Masson’s trichrome staining experiment.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Uraizee I, Cheng S, Hung CL, Verma A, Thomas JD, Zile MR, Aurigemma GP, Solomon SD. Relation of N-terminal pro-B-type natriuretic peptide with diastolic function in hypertensive heart disease. Am J Hypertens 2013; 26:1234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L, Gao JY, Ma J, Xu X, Wang Q, Xiong L, Yang J, Ren J. Cardiac-specific overexpression of metallothionein attenuates myocardial remodeling and contractile dysfunction in L-NAME-induced experimental hypertension: role of autophagy regulation. Toxicol Lett 2015; 237:121–32. [DOI] [PubMed] [Google Scholar]
- 3.Ruschitzka F, Quaschning T, Noll G, deGottardi A, Rossier MF, Enseleit F, Hürlimann D, Lüscher TF, Shaw SG. Endothelin 1 type a receptor antagonism prevents vascular dysfunction and hypertension induced by 11 beta-hydroxysteroid dehydrogenase inhibition: role of nitric oxide. Circulation 2001; 103:3129–35. [DOI] [PubMed] [Google Scholar]
- 4.Biwer LA, Broderick TL, Xu H, Carroll C, Hale TM. Protection against L-NAME-induced reduction in cardiac output persists even after cessation of angiotensin-converting enzyme inhibitor treatment. Acta Physiol (Oxf) 2013; 207:156–65. [DOI] [PubMed] [Google Scholar]
- 5.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 2017; 69:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 2013; 6:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L, Gu Y, Wen M, Zhao S, Wang W, Ma Y, Meng G, Han Y, Wang Y, Liu G, Moore PK, Wang X, Wang H, Zhang Z, Yu Y, Ferro A, Huang Z, Ji Y. Hydrogen sulfide induces keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes 2016; 65:3171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin S, Pu SX, Hou CL, Ma FF, Li N, Li XH, Tan B, Tao BB, Wang MJ, Zhu YC. Cardiac H2S generation is reduced in ageing diabetic mice. Oxid Med Cell Longev 2015; 2015:758358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol 2003; 284:R628–38. [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008; 322:587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal 2015; 8:ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesseling S, Fledderus JO, Verhaar MC, Joles JA. Beneficial effects of diminished production of hydrogen sulfide or carbon monoxide on hypertension and renal injury induced by NO withdrawal. Br J Pharmacol 2015; 172:1607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 2014; 7:ra87. [DOI] [PubMed] [Google Scholar]
- 14.Li XH, Xue WL, Wang MJ, Zhou Y, Zhang CC, Sun C, Zhu L, Liang K, Chen Y, Tao BB, Tan B, Yu B, Zhu YC. H2S regulates endothelial nitric oxide synthase protein stability by promoting microRNA-455-3p expression. Sci Rep 2017; 7:44807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloesch B, Steiner G, Mayer B, Schmidt K. Hydrogen sulfide inhibits endothelial nitric oxide formation and receptor ligand-mediated Ca2+ release in endothelial and smooth muscle cells. Pharmacol Rep 2016; 68:37–43. [DOI] [PubMed] [Google Scholar]
- 16.Tan B, Jin S, Sun J, Gu Z, Sun X, Zhu Y, Huo K, Cao Z, Yang P, Xin X, Liu X, Pan L, Qiu F, Jiang J, Jia Y, Ye F, Xie Y, Zhu YZ. New method for quantification of gasotransmitter hydrogen sulfide in biological matrices by LC-MS/MS. Sci Rep 2017; 7:46278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 2013; 19:448–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndisang JF, Chibbar R, Lane N. Heme oxygenase suppresses markers of heart failure and ameliorates cardiomyopathy in L-NAME-induced hypertension. Eur J Pharmacol 2014; 734:23–34. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda K, Ohtake K, Uchida H, Ito J, Uchida M, Natsume H, Tamada H, Kobayashi J. Dietary nitrite supplementation attenuates cardiac remodeling in L-NAME-induced hypertensive rats. Nitric Oxide 2017; 67:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Miao L, Shen X, Whiteman M, Xin H, Shen Y, Xin X, Moore PK, Zhu YZ. Hydrogen sulfide mitigates myocardial infarction via promotion of mitochondrial biogenesis-dependent M2 polarization of macrophages. Antioxid Redox Signal 2016; 25:268–81. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Jin S, Teng X, Duan X, Chen Y, Wu Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017; 67:10–25. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA, Bikiel D, Dorsch K, Leffler A, Babes A, Lampert A, Lennerz JK, Jacobi J, Martí MA, Doctorovich F, Högestätt ED, Zygmunt PM, Ivanovic-Burmazovic I, Messlinger K, Reeh P, Filipovic MR. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun 2014; 5:4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A 2015; 112:E4651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao WM, Zhang J, Lu YJ, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 2001; 20:6008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao WM, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 2003; 81:848–53. [DOI] [PubMed] [Google Scholar]
- 26.Jin HF, Du JB, Tang CS, Bu DF. Regulatory effect of nitric oxide on hydrogen sulfide/cystathionine-γ-lyase of vascular smooth muscle cells in spontaneously hypertensive rats. J Appl Clin Pediatr 2006; 21:140–1. [Google Scholar]
- 27.Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, Zhang Z, Tang C, Du J. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol 2007; 293:R1608–18. [DOI] [PubMed] [Google Scholar]
- 28.Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology 2007; 241:92–7. [DOI] [PubMed] [Google Scholar]
- 29.Meng G, Xiao Y, Ma Y, Tang X, Xie L, Liu J, Gu Y, Yu Y, Park CM, Xian M, Wang X, Ferro A, Wang R, Moore PK, Zhang Z, Wang H, Han Y, Ji Y. Hydrogen sulfide regulates Krüppel-like factor 5 transcription activity via specificity protein 1 S-sulfhydration at Cys664 to prevent myocardial hypertrophy. J Am Heart Assoc 2016; 5:e004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazza R, Pasqua T, Cerra MC, Angelone T, Gattuso A. Akt/eNOS signaling and PLN S-sulfhydration are involved in H2S-dependent cardiac effects in frog and rat. Am J Physiol Regul Integr Comp Physiol 2013; 305:R443–51. [DOI] [PubMed] [Google Scholar]
- 31.Jensen AR, Drucker NA, Khaneki S, Ferkowicz MJ, Markel TA. Hydrogen sulfide improves intestinal recovery following ischemia by endothelial nitric oxide-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol 2017; 312:G450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A 2014; 111:3182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji W, Liu S, Dai J, Yang T, Jiang X, Duan X, Wu Y. Hydrogen sulfide defends against the cardiovascular risk of Nw-nitro-L-arginine ester-induced hypertension in rats via the nitric oxide/endothelial nitric oxide synthase pathway. Clin Med J (Engl) 2014; 127:375–7. [PubMed] [Google Scholar]
- 34.Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens 2003; 12:1879–85. [DOI] [PubMed] [Google Scholar]
- 35.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr, Gojon G, Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 2013; 127:1116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anwar MA, Samaha AA, Ballan S, Saleh AI, Iratni R, Eid AH. Salvia fruticosa induces vasorelaxation in rat isolated thoracic aorta: role of the PI3K/Akt/eNOS/NO/cGMP signaling pathway. Sci Rep 2017; 7:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 2007; 76:29–40. [DOI] [PubMed] [Google Scholar]
- 38.Ohno K, Okuda K, Uehara T. Endogenous S-sulfhydration of PTEN helps protect against modification by nitric oxide. Biochem Biophys Res Commun 2015; 456:245–9. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 2011; 4:ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 2011; 109:1259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong J, Ren G, Jia N, Wang Y, Zhang H, Zhang W, Chen B, Cao Y. Effects of nicorandil in neuroprotective activation of PI3K/AKT pathways in a cellular model of Alzheimer’s disease. Eur Neurol 2013; 70:233–41. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, He MY, Ye JK, Ma SY, Huang W, Wei YY, Kong H, Wang H, Zeng XN, Xie WP. Activation of ATP-sensitive potassium channels facilitates the function of human endothelial colony-forming cells via Ca2+/Akt/eNOS pathway. J Cell Mol Med 2017; 21:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.