Short abstract
The main mediator of the canonical Wnt pathway, β-catenin, is a major effector of embryonic development, postnatal tissue homeostasis, and adult tissue regeneration. The requirement for β-catenin in cardiogenesis and embryogenesis has been well established. However, many questions regarding the molecular mechanisms by which β-catenin and canonical Wnt signaling regulate these developmental processes remain unanswered. An interesting question that emerged from our studies concerns how β-catenin signaling is modulated through interaction with other factors. Recent experimental data implicate new players in canonical Wnt signaling, particularly those which modulate β-catenin function in many its biological processes, including cardiogenesis. One of the interesting candidates is plakoglobin, a little-studied member of the catenin family which shares several mechanistic and functional features with its close relative, β-catenin. Here we have focused on the function of β-catenin in cardiogenesis. We also summarize findings on plakoglobin signaling function and discuss possible interplays between β-catenin and plakoglobin in the regulation of embryonic heart development.
Impact statement
Heart development, function, and remodeling are complex processes orchestrated by multiple signaling networks. This review examines our current knowledge of the role of canonical Wnt signaling in cardiogenesis and heart remodeling, focusing primarily on the mechanistic action of its effector β-catenin. We summarize the generally accepted understanding of the field based on experimental in vitro and in vivo data, and address unresolved questions in the field, specifically relating to the role of canonical Wnt signaling in heart maturation and regeneration. What are the modulators of canonical Wnt, and particularly what are the potential roles of plakoglobin, a close relative of β-catenin, in regulating Wnt signaling?Answers to these questions will enhance our understanding of the mechanism by which the canonical Wnt signaling regulates development of the heart and its regeneration after damage.
Keywords: β-catenin, Wnt signaling, cardiogenesis, plakoglobin, development, heart
Introduction
The heart is the first organ to form and function in the embryo. All subsequent events during an organism’s lifetime depend on the heart’s ability to match its output with physiological demands for oxygen and nutrient requirements. The heart is formed through multiple developmental steps that are driven and controlled by a plethora of signaling pathways. These include bone morphogenic proteins (BMPs), transforming growth factor-beta (TGF-β) family members, fibroblast growth factors (FGFs) and, more recently proteins of the Wnt family.1,2 Mutations within the canonical Wnt pathway or perturbation of components of this pathway can induce developmental abnormalities and various types of diseases including cancer.3 In addition, Wnt signaling plays a critical role in the maintenance and expansion of cardiac progenitor cell (CPC) lineages at the initial stage of cardiac progenitor proliferation. At later stages of cardiogenesis, Wnt signaling is suppressed to allow specification and differentiation of CPCs into cardiomyocytes. Wnt signaling is also involved in cardiac remodelling due, in part, to its key role in regulating the intersection between cell proliferation and differentiation. Several studies have implicated Wnt signaling in the development of hypertrophic response to insult or injury, which often leads to pathological hypertrophy, heart fibrosis and, ultimately, disruption of the contractile function of the heart. Full function of Wnt signaling ultimately results in the transcriptional regulation of its target genes. Two of its known transcriptional effectors are β-catenin and plakoglobin (γ-catenin) which are suggested to play distinct roles in cardiogenesis, presumably through the activation of a separate set of target genes.
A brief overview of canonical Wnt signaling
The discovery of Wnt signaling derives in part from the identification in 1982 in the mouse of a gene and its oncogenic product designated Int1 (integration 1).4 Subsequent realization in 1987 that the previously characterized Drosophila melanogaster Wingless (Wg) gene was the same as the mouse Int gene5,6 resulted in the contraction of the Wg and Int to form to the Wnt designation. The Wnt family of genes encodes a large evolutionarily conserved class of secreted molecules that initiate signaling cascades in recipient cells that express appropriate receptors. The Wnt proteins comprise a diverse family of secreted lipid-modified signaling glycoproteins of ∼40 kDa that harbor many conserved cysteines and which has a highly unusual two-domain structure with amino-terminal and carboxy-terminal domains (NTD and CTD) characteristic of the Wnt protein family.5 Several cell types with signaling function secrete Wnt into the extracellular milieu where responding cells recognise Wnt via a heterodimeric receptor complex, consisting of a Frizzled (Fz) and an LRP5/6 protein. Following Wnt binding, the receptors form larger multiprotein complexes which can be detected as distinct punctate structures in the cytoplasm. These protein complexes, designated signalosomes, are the sites at which cascades of protein phosphorylation occur which ultimately result in either transcriptional activation of downstream target genes, rearrangement of cytoskeletal rearrangements, or cell movement.6
There are 19 mammalian Wnt proteins including 10 frizzled (Fz) receptors and two LRP5 coreceptors, indicative of the enormous complexity required to confer Wnt signaling specificity.6,7 Conventionally Wnt proteins have been classified into two groupings depending on their ability to induce secondary axis formation in frog embryos and to transform mammary epithelial cells. Those that have such capabilities, such as Wnt1, Wnt3, and Wnt8,6 are known as the canonical Wnts; other Wnt proteins, such as Wnt5a and Wnt11 act through the non-canonical pathway. However, it is now apparent that individual Wnt proteins can activate either the canonical or the non-canonical Wnt signaling pathways, depending on cellular context.8,9
The canonical and non-canonical Wnt pathways are differentiated by their downstream effectors. Signaling through canonical Wnt usually involves β-catenin as the primary effector. In contrast, non-canonical Wnt signaling is more diverse in its roles which include Wnt-mediated polarity, Wnt-Ca2+, and Wnt-a typical protein kinase C pathways. These pathways contribute to developmental processes such as cell polarity in Drosophila, convergent extension movements during gastrulation, and cell migration. Non-canonical Wnt signaling has been reviewed more extensively elsewhere.6,7 In this review we focus on the function of canonical Wnt and β-catenin signaling.
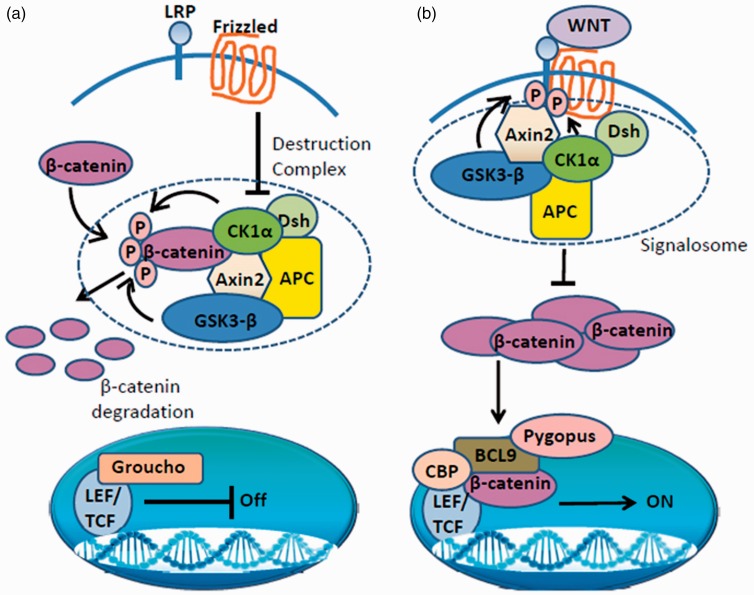
The intensity of Wnt/β-catenin signaling appears to be determined by the nuclear localization of β-catenin and its overall stability. In the absence of Wnt ligands, cytoplasmic β-catenin is maintained at a low level and is sequestered into a multiprotein destruction complex.10 At the core of the degradation complexes are axin and adenomatous polyposis coli (APC), two scaffold proteins which facilitate the phosphorylation of β-catenin by casein kinase 1α (CK1α) and glycogen synthase kinase 3β (GSK3β). When β-catenin is phosphorylated, it undergoes ubiquitination and proteasome-mediated degradation. Under this condition, canonical Wnt signaling and β-catenin are not active (Figure 1[a]) and the T-cell factor/lymphoid enhancer factor (LEF/TCF) transcription factor associates with the Groucho co-repressor to repress target gene expression.11,12 Binding of Wnt to the Frizzled (Fzd) receptor and its co-receptor Ryk leads to signalosome formation which prevents β-catenin proteasomal degradation.13,14 Consequently, β-catenin is stabilized, accumulates in the cytoplasm, and then is translocated to the nucleus. In the nucleus, β-catenin displaces Groucho by binding to LEF/TCF. It interacts with the BCL9 protein, the nuclear Pygopus protein, and the cAMP-response element binding (CREB) protein (CBP) as well as tissue-specific transcriptional activators. These interactions act as switches to convert LEF/TCF from a transcriptional repressor to an activator that turns on gene expression in a cell-type specific manner15,16 (Figure 1[b]). Canonical Wnt signaling is involved in the control of cell proliferation, particularly through target genes such as c-myc and the CCND1 gene encoding cyclin D1. A subset of cellular β-catenin can be translocated into the nucleus involving a process that requires activated Ras signaling.17,18
Figure 1.
Classical scheme of canonical Wnt signaling. (a) When Wnt ligands are absent, β-catenin in the cytoplasm is constantly targeted for proteasome-mediated degradation by a multiprotein destruction complex. The nuclear transcription factor lymphoid enhancer-binding factor/T cell-specific factor (LEF/TCF) associates with Groucho and represses target gene expression. (b) Upon binding of a Wnt ligand to its receptor, the Wnt-associated Frizzled binds aco-receptor which together form a large multiprotein complex or signalosome. This complex initiates a cascade of phosphorylation events which prevents the degradation of β-catenin. As a result, β-catenin accumulates in the cytoplasm and is translocated into the nucleus, where it displaces Groucho and forms a complex with BCL9, Pygopus, histone modifier CBP and converts LEF/TCF from a transcriptional repressor to an activator which activates the expression downstream target genes. These include CK1a—case in kinase 1α; Dsh—dishevelled; Axin2—scaffold protein; APC—adenomatous polyposis coli; GSK3-β—glycogen synthase kinase 3β; LRP—lipoprotein receptor-related protein 5/6; BCL9—B-cell lymphoma 9 protein; histone modifier CBP— cAMP response element-binding (CREB) protein. (A color version of this figure is available in the online journal.)
Two faces of β-catenin
Although β-catenin is a key regulator of canonical Wnt signaling, it is located at the cytoplasmic side of the cell membrane and also serves as a member of the cadherin–catenin intercellular connections even in the absence of a Wnt stimulus. The adherens junctions, for example, contain cadherin–catenin complexes and contribute to the formation of polarized epithelial tissues and are necessary for organismal integrity.19,20 The β-catenin in these complexes interacts with cadherins to link them to the actin cytoskeleton via binding with α-catenin. Such interactions that can result in signaling activity are highly dependent on phosphorylation. For instance, phosphorylation of β-catenin by Src kinase at Tyr654 and by protein kinase (PK) D1 at Thr112 or Thr120 promotes its binding to E-cadherin.21 Similarly, phosphorylation of E-cadherin at Ser834, Ser836, and Ser842 by CK2 and GSK3β enhances its interaction with β-catenin. Conversely, interaction of E-cadherin and β-catenin is inhibited when Tyr831 and Tyr860 are phosphorylated by Src and Ser846 by CK1.22 Thus the involvement of β-catenin in Wnt signaling or intercellular interactions is subtely regulated and highly orchestrated by its phosphorylation at multiple sites.23,24 Genetic knockouts and overexpression of β-catenin in embryos, as well as cultured cells, have shown that canonical Wnt signaling and regulation of cadherin–catenin complexes in cell-to-cell connections both depend on the same pool of β-catenin.25,26 Therefore, cells modulate the level of Wnt signaling and regulate cell–cell adhesion or proliferation via competition for cytoplasmic β-catenin.
Studies using animal models show that epithelial-mesenchymal (EMT) or mesenchymal-epithelial transitions (MET) are exquisitely regulated by cadherin-mediated cell adhesion. How the signaling function of β-catenin is linked to cell adhesion has previously been discussed.27 In short, the absence of E-cadherin/catenin-based cell adhesion promotes β-catenin stabilization and signaling, which in turn leads to EMT during embryogenesis as well as invasion and metastasis in cancer cells. Alternatively, the absence of E-cadherin can promote EMT even without activation of β-catenin signaling.28,29 Interestingly, nuclear β-catenin down-regulates E-cadherin expression and activates the proteases and other EMT regulating factors. Thus, the loss of cadherins leads to the degradation of β-catenin complexes which appears to be a prerequisite for canonical Wnt signaling. Taken together, β-catenin has several functional cellular niches, and mediates the interaction between different biological processes with particular influence on cell adhesion and signaling.
Canonical Wnt signaling is one of the main driving forces in cardiogenesis
Heart development is a complex and highly dynamic process. The heart is the first organ to be formed in the embryo around mouse embryonic day E 6.5, during gastrulation when primitive CPCs migrate and undergo lineage specification. Cells migrate from the primitive streak and align themselves in an anterior-lateral position on either side of the midline.30 Later, at stage E7.5, one can detect these CPCs as a “cardiac crescent” in the mesoderm underlying the head folds. At stage E8, the progenitors fuse to form the first primitive cardiac structure, the cardiac tube, which then loops to form an S-shaped structure. Finally, by E14.5 the clearly formed septated heart chambers are discernible as the result of a finely orchestrated series of rearrangements and cell expansions.31,32
The region in which the heart forms is divided into two distinctive cell populations: the first heart field (FHF) and the second heart field (SHF) field, respectively.2 Others have also considered a third heart field (THF),33 referring to an additional population of mesodermal cells which give rise to a pro-epicardium and sinus node. The FHF constitutes the cardiac crescent and forms the primitive heart tube, giving rise to the left ventricle (LV) and atrium. The expansion of the primitive heart tube depends on the SHF, which provides a cell reservoir for subsequent heart growth. The SHF forms the outflow tract (OFT), atria and the right ventricular region. Specific genetic markers for both heart fields have also been defined. For example, Tbx5 is specific for the FHF, while Isl1, Fgf10, Tbx1, and Fgf8 delineate the SHF.31,32
Vertebrate heart formation and development are strongly regulated by a balanced interaction between the Wnt/β-catenin, nodal/activin, FGF, and BMP signaling pathways.31,34,35 Although Wnt/β-catenin signaling is known to be important for vertebrate cardiogenesis, data regarding its mechanism of action of this biological process remains scarce and even contradictory. Experiments with Drosophila and Xenopus embryos, and mice and mammalian stem cells have contributed to our appreciation of the diverse roles played by Wnt/β-catenin signaling leading to the observation that Wnt signaling can have different effects depending on the spatiotemporal context.
During embryogenesis, induction of precardiac mesoderm and subsequent FHF formation requires inhibition of Wnt/β-catenin signaling by adjacent ectoderm. In mice lacking β-catenin, respecification of endoderm into precardiac mesoderm is disrupted which leads to the formation of multiple heart primordia.36 Depletion of β-catenin also produces higher level of Bmp2 ectopic expression following canonical Wnt signaling downregulation.37 This finding suggests that canonical Wnt inhibition and BMP upregulation are necessary for proper cardiac mesoderm specification. Interaction between canonical Wnt and other regulatory pathways contributing to initiation and development in the FHF was shown with a Notch-inducible mouse embryonic stem (ES) cell line. Under the conditions used, Notch signaling in these ES cells was able to redirect hemangioblasts into cardiac mesoderm by downregulation of Wnt/β-catenin signaling and upregulation of the BMP pathway.37 Similarly, heart formation requires high BMP2 and low Wnt signaling activities in zebrafish, Xenopus, and chicken.38 In chicken, heart progenitors migrate anteriorly into a region in which expression of Dkk1 and Crescent is detected, resulting in inhibition of canonical Wnt.39 It is now known that Dkk1 and Crescent down-regulate canonical Wnt signaling, which is necessary for initiation of the cardiac gene program by cooperative BMP and FGF signaling.40,41 Among the most critical cardiac transcription factors regulated in this manner are Nkx2.5 and cardiogenic Gata4 and Gata6. In Xenopus, canonical Wnt signaling functionally cooperates with the Gata4, Gata6, and Nkx2.5 transcription factors. This interaction occurs at two different stages of heart development and is necessary for cardiac specification. Thus, at stages 8 and 18, Wnt/β-catenin down-regulates expression of Gata4 and Gata6 in the precardiac mesoderm. This event precedes the migration of cardiac progenitors to the ventral midline of the embryo.42
Proper tissue specification requires defined protein–protein interactions as well as temporal regulation of signaling. For example, canonical Wnt and β-catenin have a biphasic signaling role in heart development. In the FHF the Wnt/β-catenin pathway has an inhibitory effect but it also plays an inductіve role in proliferation in the SHF (Figure 2). Deactivation of β-catenin in the SHF leads to a decrease in cell proliferation and results in developmental abnormalities as well as inhibition of SHF expansion.38 Several studies support the idea that proliferation in the SHF is modulated by Wnt2 through the canonical Wnt pathway.43,44 It is noteworthy, however, that canonical Wnt signaling not only maintains the SHF cells in a proliferative state, but also maintains them in an undifferentiated state.45,46 Conditional knockout of β-catenin in SHF cells utilizing Isl1-Cre to remove β-catenin reduces the cell number within the SHF and thereby impairs the formation and development of OFT and the right ventricle (RV).45 The same activity for Wnt/β-catenin signaling has been observed in ES cells. Using conditioned media or feeder cells that secrete Wnt3a mimics the requirement of canonical Wnt for cardiac ISL1+ progenitor expansion and the formation of beating embryoid bodies. In contrast, the presence of dickkopf-1 (DKK1), an inhibitor of canonical Wnt, in the culture medium, dramatically reduces the number of ISL1+ progenitor cells and beating embryoid bodies.47 These data are also supported by results in which Notch 1 had been knocked out in embryos and ES cells. Mutant cells lacking Notch 1 displayed increased CPC proliferation mediated by Wnt/β-catenin up-regulation.48 Cells from the Notch1 null embryos are unable to populate the developing RV (which originates from ISL1+ cells). Furthermore, Notch knockout ES cells do not express genes that are associated with cardiomyocyte differentiation. These data clearly indicate that downregulation of canonical Wnt is necessary for enabling progenitor cells of the SHF to exit the proliferative state and to initiate cardiac differentiation.
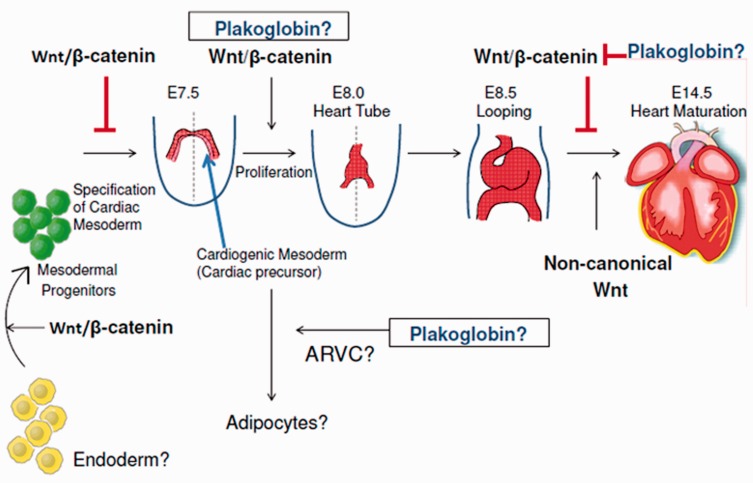
Figure 2.
Stage specific roles of Wnt/β-catenin signaling in heart development. Wnt/β-catenin is spatiotemporally activated or repressed to orchestrate normal cardiac formation. During stem cell renewal and mesendodermal specification, Wnt/β-catenin signaling is activated. Subsequently, repression of Wnt/β-catenin becomes necessary for specification of cardiogenic mesodermal progenitors. It is inhibitory in the FHF but plays an inductive proliferative role in SHF where it promotes proliferation of multipotent progenitor cells and early ventricular myocytes. Plakoglobin may contribute to this process, providing functional redundancy with β-catenin. The precise mechanisms by which Wnt/β-catenin exerts its effects on adult cardiomyocytes remain unknown. However, plakoglobin is capable of downregulating Wnt/β-catenin signaling in the adult mouse heart. Plakoglobin is also implicated in arrhythmogenic ventricular cardiomyopathy due to its ability to drive the differentiation of cardiac precursors into adipocytes. (A color version of this figure is available in the online journal.)
In addition, non-canonical Wnt signaling is required for the regulation of SHF progenitor cell differentiation. It was shown, that the absence of Wnt5a and Wnt11 increases the nuclear level of β-catenin and affects SHF differentiation.49 High levels of Wnt5a and Wnt1 induce cardiogenesis and turn on cardiac-associated gene expression in differentiating ES cells. These data support the idea that formation of the FHF- and SHF-associated progenitors are instructed by non-canonical Wnt.49 Moreover, ectopic expression of Wnt2 increases cardiomyogenic differentiation of mouse ES cells.20 Taken together, these results point out that Notch and non-canonical Wnt signaling are negatively regulated by the Wnt/β-catenin pathway and that such interplay is critically necessary for retaining progenitors in the SHF in their proliferative state.
When SHF cells become part of the heart tube, the cardiac progenitors enter a region where Wnt/β-catenin dependent transcription is slowly downregulated and BMP signaling is rapidly increased. At this point, the SHF progenitors activate their cardiac gene program and initiate specification and differentiation into cardiomiocytes.50–52 The SHF determines the atrial and venous poles of the embryonic heart. For successful atrial pole specification, Wnt/β-catenin pathway is downregulated mainly via the actions of non-canonical Wnt11 and Wnt5a.49 At the same time signaling activity of β-catenin is required for inflow tract development. Wnt2 knockout in mice results in abnormalities of the heart atrial pole, affecting the atrioventricular canal as well as the atrial and pulmonary veins.44 In these mutant embryos, there is downregulation of the canonical Wnt components, Axin2 and Lef1, as well as Isl1 and SHF markers. Moreover, the number of SHF cells is highly reduced. A similar phenotype was observed in an Isl1-Cre-mediated conditional knockout of β-catenin mice.53 This finding supports the idea that canonical Wnt is critical for formation of the inflow tract in the embryonic myocardium.
Heart development is complex in nature, with its three-dimensional structure and dynamic cellular composition that undergo progressive changes throughout development. The Wnt/β-catenin signaling is an important player in the signaling network that orchestrates the process of cardiogenesis and that is activated in at least two stages of heart development. It is also worth noting that the canonical Wnt pathway is a key for normal heart development. Its collective interactions with other signaling pathways ensure proper initiation of heart progenitor specification and its role in driving subsequent heart development.
Reactivation of canonical Wnt signaling during adult heart pathology
Studies in several animal models have shown that various Wnt factors involved in hypertrophic response and wound healing following cardiac injury are induced after experimental myocardial infarction (MI).54,55 Furthermore, inhibition of the canonical Wnt pathway has a favorable effect on adult heart remodeling.56,57 For example, a secreted frizzled-related protein (SFRP), which is a known antagonist of canonical Wnt, prevents Wnt binding to the frizzled receptor, reduces infarct size, and improves heart function. Interestingly, the infarct size reduction was achieved by either inducing an MI in transgenic mice overexpressing Sfrp158 or by altering local secretion of SFRP2 or by its exogenous administration.59,60 This finding is supported by the observation that depletion of cardiospecific β-catenin significantly improved survival up to four-weeks and restored LV function post MI.
It is noteworthy that some reports indicate that inhibition of β-catenin after MI can have a positive effect61 while other studies have shown beneficial outcomes following its activation.55,62–64 A favorable response to β-catenin signaling in heart regeneration was also shown with TOPGAL reporter mice.62 In this study β-catenin signaling was observed in epicardial cells and cardiac fibroblasts after MI. Downregulation of β-catenin transcriptional activity attenuated epicardial cell expansion and even led to a deterioration of cardiac function and ventricular dilatation in the injured heart. Furthermore, abrogation of the canonical Wnt pathway in cardiac fibroblasts led to less robust wound healing and cardiac performance. These data are consistent with the idea that β-catenin activation is required for heart tissue regeneration after MI in epicardial cells and in fibroblasts. Similarly, administration of an adenoviral vector harboring a constitutively stable β-catenin gene (Ad-catenin) reduced MI size in rats after infarction.63 Importantly, cardiomyocytes and cardiac fibroblasts displayed an antiapoptotic effect and cellular cycle activation (increasing of cells number in the S phase and cyclin D1 gene expression) after Ad-catenin injection directly into the infarction zone. Canonical Wnt signaling activity is also elevated after an induced MI in Axin2-LacZ transgenic mice,55 accompanied by β-catenin-dependent induction and expansion of many cell types, including Sca+/CD31− progenitor cells, Sca−/CD31+ endothelial cells, and also ckit+ and CD31+ cell populations. These observations suggest that canonical Wnt is involved in heart regeneration through differentiation of progenitor and endothelial cells in response to an induced MI.55 Furthermore, elevation of cytoplasmic β-catenin in cardiomyocytes leads to improvement of heart function and a decrease in the MI zone.64 Nevertheless, taken together, the precise function of β-catenin in heart regeneration is not yet fully understood. Some reports argue that up-regulation of β-catenin signaling leads to heart fibrosis and worsening of heart function. Other lines of evidence, however, suggest the opposite effect in which higher levels of β-catenin signaling are associated with greater cardiomyocyte survival, induction of CPC differentiation, and expansion of endothelial cells into the MI zone.
In contrast to MI, cardiac hypertrophy is characterized by an enlargement of cardiomyocyte size, replacement of cardiomyocytes by fibroblasts and adipocytes, and upregulation of hypertrophic or fetal genes in affected cells. The proposition that initiation of canonical Wnt signaling is a requirement for myocardium hypertrophy has been validated.65 Transgenic animals that overexpress Dvl-1 exclusively in cardiomyocytes display activation of Wnt signaling concomitant with development of hypertrophy and even lethality. Interestingly, both Wnt signaling branches were activated and there was a marked elevation of β-catenin expression and upregulation of some β-catenin downstream targets, namely Cyclin D1 and c-myc. Non-canonical Wnt signaling was evident at three months after hypertrophy induction in animals with developed pathology.65
Gain of function experiments have shown that expression of a constitutively stable form of β-catenin causes spontaneous cardiomyocyte hypertrophy in vitro.63 In knockout animals, elevation of β-catenin/TCF/LEF signaling is important for the development of stress-induced and physiological hypertrophy.66 Ablation of β-catenin in the mature myocardium did not cause premature death. Most knockout animals had a normal phenotype, and morphological changes in myocardial tissues (e.g. thinning of heart walls leading to cardiomyopathy) occurred only after chronic β-catenin deletion (32 weeks). Equally interesting is the observation that conditional cardiospecific deletion of only one β-catenin allele reduces the extent of hypertrophy after surgically-induced aortic constriction compared with the control group of mice.67 This result suggests that hypertrophy does not develop in animals with reduced myocardial β-catenin under conditions of chronic elevated blood pressure. There was also coincident increased fetal gene expression compared with the control group. In our experiments we also have observed heart growth attenuation accompanied by elevation of fetal gene expression in the β-catenin haplo insufficient heart.68 These data indicate that, at a minimum, a basal level of β-catenin is necessary for postnatal heart maturation and that its transcriptional activation is required for hypertrophy.
In contrast to the results described above, deletion of β-catenin in mature myocardial tissue has been reported to cause spontaneous hypertrophy.68 After injection of angiotensin II in mice with constitutively stabilized β-catenin, there was no hypertrophic response.68 It is important to point out that this study was limited to changes only in cardiomyocyte size and the expression level of ANF as a marker for hypertrophy. A more complex approach has been typically used in other studies,65–67 including an index of heart to body weight ratio, level of expression of other hypertrophic marker genes (BNF, aMHC, bMHC), and/or expression of β-catenin target genes (Cyckin D1, c-myc, c-fos). Meta-analysis of experimental data obtained with different animal models following hypertrophic stimuli expands the marker panel to include SERCA, actin, DIF1, Axin-2, c-myc, CD1, BNP, ANP, and total protein/DNA index. On the other hand, bioinformatic analysis supports the notion that response to hypertrophy is a consequence of more global molecular mechanisms and that β-catenin is principally important as a regulator of heart adaptation to stress.69
Contradictions in the literature are probably due to the use of different experimental approaches and different observation times. For example, Wnt/β-catenin signaling is important at early time points of the hypertrophic response, a finding that is supported by several independent studies using different approaches and different hypertrophic stimuli. When pathology becomes more severe, the signaling function of β-catenin is probably repressed through a negative “feedback” or other signaling regulatory mechanisms that controls or interacts with Wnt signaling. For example, Axin2 is a β-catenin/TCF/LEF target gene and also serves as a negative regulator of β-catenin by promoting its degradation. In addition, other molecules have been described that interact directly with stabilized β-catenin or with the β-catenin/TCF/LEF complex. Thus, it is a combination of such mechanisms that ensures a negative physiological “feedback” of the canonical Wnt pathway.70
Is plakoglobin involved in the regulation of canonical Wnt signaling?
Plakoglobin (γ-catenin) is a close relative of β-catenin.69 In contrast to β-catenin, it can interact with both classical and desmosomal cadherins and maintain integrity of the adherens junctions and desmosomes. Knockout of β-catenin or plakoglobin in mice causes lethality in the embryo.71–74 The observed phenotypes are very different for β-catenin and plakoglobin mutant mice. The absence of plakoglobin is associated with compromised desmosome assembly and results in defective heart development.73,75 Mice in which β-catenin is ablated are incapable of forming dorsal structures in the developing embryos.74 These abnormalities clearly indicate the involvement of β-catenin in Wnt-mediated axis formation. It is interesting to note that β-catenin-null embryos display no detectable developmental defects in adherens junctions; there is, however, an elevated level of plakoglobin which could compensate for the adhesive role of β-catenin.73,74 Thus, this line of gene-knockout experiment reveals a structural role for plakoglobin. Other evidence, however, indicates that plakoglobin also has a signaling function through its regulation of the Wnt pathway. First, like β-catenin, elevation of plakoglobin expression leads to a duplicate axis phenotype in Xenopus laevis.72 Second, plakoglobin co-immunoprecipitates with APC and Axin.75,76 Third, upstream mediators of Wnt signaling, such as Wnt1 ligand, APC, and Axin, also regulate plakoglobin stability.77,78 And lastly, plakoglobin is able to activate β-catenin/TCF reporter constructs.79–82
Recently, plakoglobin was found to bind with LEF/TCF proteins to form a plakoglobin-containing complex that interacts with DNA, but with very low affinity83,84 (Figure 3). It can, however, potentially antagonize β-catenin/LEF/TCF-dependent signaling. Alternatively, plakoglobin-containing complexes might regulate the expression of a subset of target genes with plakoglobin-specific DNA recognition. This latter contention is supported by evidence that plakoglobin can regulate gene expression independently of β-catenin.85 Moreover, more than 5000 plakoglobin target promoters have been identified in differentiating skin keratinocytes by a ChIP–ChIP genome-wide screen.85,86 These targets include various participants of the canonical Wnt signaling pathway as well as Wnt target genes. Interestingly, the same technique uncovered only 2000 promoters for β-catenin targets with only a 38% overlap with plakoglobin target genes. This observation is consistent with distinct molecular pathways that are regulated by β-catenin and plakoglobin. Additionally, activation of WNT-3 or DVL-2 (an isoform of dishevelled) expression in transgenic mouse skin87 results in phenotypes that are very similar to those observed in mice with plakoglobin overexpresion.88 However, the phenotype is very different from that observed in mice overexpressing β-catenin. These data, therefore, support the involvement of plakoglobinin canonical Wnt signaling downstream of Wnt-3 and Dvl-2.87 Yet, Wnt-3 and Dvl-2 can efficiently elevate β-catenin levels in cultured cells.89
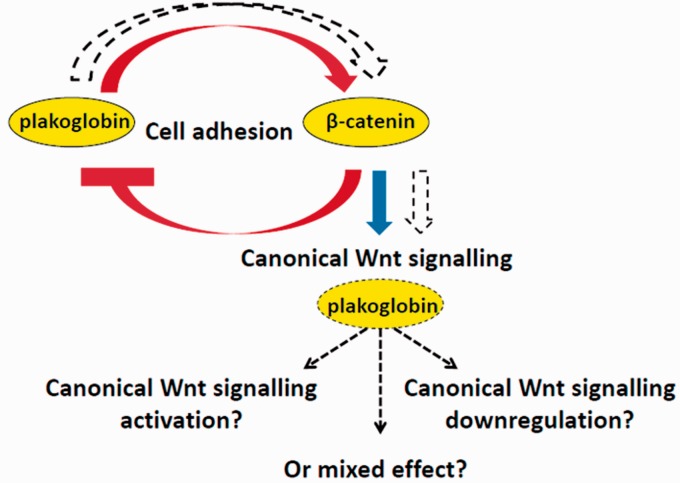
Figure 3.
Proposed mechanism of β-catenin and Plakoglobin interaction. Plakoglobin is able to maintain the structural integrity of adherens junctions in the absence of β-catenin. Like β-catenin, plakoglobin has a signaling function. Plakoglobin and β-catenin likely interact in a complex manner to regulate cell fate and possibly also functions during cardiogenesis. We propose that plakoglobin has its own subset of target genes and that plakoglobin signaling activity is dependent on cell context, β-catenin gradient, and mechanism of canonical Wnt activation. (A color version of this figure is available in the online journal.)
The signaling function of plakoglobin has been studied in mouse ES cells harboring genetic knockouts of both catenins.90 The data from these ES cells suggest that a critical threshold of total catenin expression must be achieved before there is sufficient signaling-competent plakoglobin available to respond to GSK-3 inhibition and to regulate the expression of target genes. Wild type ES cells which stably overexpress plakoglobin show strong canonical Wnt signaling activation which prevents stem cells from differentiating into the three cardiac cell lineages. This response to plakoglobin mimics that which is observed when β-catenin is overexpressed. Expression of the stable form of β-catenin, however, seems to be more effective than plakoglobin overexpression for maintaining the pluripotent state of stem cells even under differentiation-inducing conditions. In cancer cells, plakoglobin compensates for reduced or absent β-catenin in adhesion junctions without affecting desmosome structure, but it does not compensate by exerting regulatory functions in Wnt signaling.91 In other carcinoma cells, plakoglobin overexpression leads to decreased cell proliferation, cell migration, and cell invasion.92 It appears that plakoglobin has ability to repress tumorigenesis and metastasis by regulating expression of a cluster of genes which orchestrate these processes. Moreover, plakoglobin can modulate gene expression in cooperation with p53 and is able to regulate the transcriptional activity of p53. This circuit would therefore account, in part, for the tumor/metastasis suppressor action of plakoglobin.93 In addition, reports suggest that APC regulates oncogenic functions not only of β-catenin but also of plakoglobin.77 The transforming activity of plakoglobin, like that of β-catenin, is dependent on Tcf/Lef function and both are able to strongly activate c-Myc expression. Therefore, β-catenin and plakoglobin may have some common activities but have dissimilar functions in regulating the canonical Wnt pathway. They may, therefore, play different roles in cancer by differently affecting downstream targets.77
To date, the signaling functions of plakoglobin during cardiogenesis are poorly explored. Key questions include whether plakoglobin function is restricted only to cell adhesion or whether it has other signaling related roles. We have previously shown that cardiospecific ablation of β-catenin in early stages of heart development is not lethal at mid-gestation but is detrimental in later embryonic and neonatal stages.94 Additionally, we found that canonical Wnt signaling is activated in new born heart heterozygous for a β-catenin knockout and where Axin2, TCF4, and c-Fos genes are overexpressed. Interestingly, we observed an elevated level of plakoglobin expression in the same samples (unpublished observations). In contrast, loss of plakoglobin in zebrafish leads to decreased heart size, reduced heart beat rate, cardiac edema, blood reflux between heart chambers, and a twisted tail.95 A reduction in expression of heart markers occurs 24 h postfertilization concomitant with activation of Wnt/β-catenin signaling. This observation indicates that plakoglobin is antagonistic to Wnt signaling. Morphants (zebrafish treated with antiplakoglobin morpholinos) display reduced numbers of desmosomes and adherens junctions in their intercalated discs. These data indicate that signaling perturbation as well as structural disruption occurs in the absence of plakoglobin, and provide evidence that loss of plakoglobin may be one mechanism by which arhythmogenic ventricular cardiomyopathy (ARVC) may develop.95 Similarly, conditional knockout of plakoglobin in the adult murine heart produces progressive loss of cardiomyocytes, extensive fibrosis and inflammatory infiltration as well as cardiac dysfunction similar to that observed in ARVC patients.96 Conditional knockout of plakoglobin in mice produces increasing amounts of stabilized β-catenin that are associated with AKT activation and GSK-3β inhibition. It is therefore tempting to speculate that in the adult heart, plakoglobin may suppress β-catenin/TCF transcriptional activity, and that disruption of its function is one of the molecular mechanism underlying cardiac hypertrophy.96 This proposition is supported by data showing that nuclear localization of plakoglobin represses canonical Wnt signaling in CPCs and switches the transcriptional program to adipogenesis in ARVC.97 In aggregate, these experimental data suggest that plakoglobin has both structural and signaling functions and that it may interact with β-catenin during cardiogenesis and ARVC. How plakoglobin regulates heart development and remodeling remains unclear and is a subject for future research.
The literature suggests that plakoglobin and β-catenin interact in a complex way to regulate cell fate and possibly cardiogenesis. This functional redundancy in canonical WNT signaling regulation is likely weak and/or not effective at later stages of cardiogenesis (Figure 3). It is tempting to speculate that plakoglobin has a mixed effect in regulating Wnt/β-catenin downstream targets and that in the absence of β-catenin it is not able to maintain proper cardiac progenitor cell expansion in the embryonic heart, which affects heart formation and maturation. Thus, understanding the respective signaling functions of β-catenin and plakoglobin in mammalian cardiogenesis through the identification of genome-wide downstream targets of these two signaling proteins during heart development will shed light onto the mechanisms and importance of their interactions. It is also worth considering the possible interplay of plakoglobin and β-catenin in the modulation of the canonical Wnt pathway. Taken together, this information will resolve many of the gaps in our current understanding of the complex regulation of cardiogenesis and embryonic heart malformations. Moreover, it will contribute significant steps towards our appreciation of the etiologies underlying human heart disease and the mechanisms that support heart regeneration.
Remaining questions in the field
Understanding the main driving forces that give rise to normal embryonic heart development is essential for deciphering the molecular bases that underlie the frequent innate heart defects that occur in the human population and for developing new approaches for regenerating the damaged heart. In this review we have focused on the participation of canonical Wnt signaling and on a major mediator of this signaling pathway, namely β-catenin, in cardiogenesis. Heart development is an immensely complex biological process, not only in molecular terms, but also at the level of cell–cell interactions and structural morphogenesis of the organ. The embryonic heart is a three-dimensional structure which changes in cellular composition and morphology over time. Heart formation is not solely a sequence of individual events, but is a continuous process in which morphogenetic events occur concomitantly or that partially overlap. Canonical Wnt signaling is involved in retaining cardiac precursors in a proliferative and precursor state, and also participates in various aspects of cardiac differentiation (Figure 2). At the present time, however, we do not yet have a full understanding of how β-catenin and its transcriptional activities are regulated in the later stages of cardiogenesis. In addition, downstream target genes of the Wnt/β-catenin signaling pathway are largely unknown, which hinders the discovery and therapeutic application of molecular mechanisms leading to various processes in cardiogenesis.
The possible linkage between β-catenin, plakoglobin, and the TCF/LEF complex in mediating canonical Wnt signaling and in regulating cardiogenesis provides another intriguing aspect to this genetic network. There is a high degree of homology between β-catenin and plakoglobin, and both perform analogous functions in the assembly of adherens junctions. Recently, plakoglobin was shown to interact with LEF/TCF factors and form a complex with DNA, supporting evidence for a transcriptional regulatory role for plakoglobin in stem cells and cancer cells.77,90–93 The molecular mechanisms leading to transcriptional activation, however, remain unclear. One attractive prospect suggested by our data is that plakoglobin may modulate canonical Wnt signaling activity and share some target genes with β-catenin. Based on literature data as well as our own observations, we speculate that the signaling function of plakoglobin is highly dependent on the level of endogenous β-catenin, and the different biological contexts in which the Wnt/β-catenin pathway is activated. We assume that during cardiogenesis plakoglobin has a mixed effect on the regulation of canonical Wnt target genes, and that in the absence of β-catenin, plakoglobin is unable to maintain proper CPC expansion in the embryonic heart. Therefore, a complete reconstruction of the interplay between β-catenin, the TCF/LEF complex, and plakoglobin, together with identification of plakoglobin targets genes, will significantly contribute to the understanding of embryonic heart formation as well as adult heart remodeling.
Conclusion
Development of the embryonic heart is a delicately orchestrated and highly complex process that is mediated by several intersecting signaling pathways. Canonical Wnt signaling and β-catenin are critically important for proper heart development and cell specification. Disruption to β-catenin transcriptional activity has a dramatic impact on embryonic development and leads to neonatal lethality. Clarifying β-catenin interactions with new players of the canonical Wnt pathway such as plakoglobin, and identification of its target genes are crucial for the understanding of molecular regulation of heart development as well as adult heart remodeling, which is envisaged to lead to the design of future approaches to targeted heart therapy.
Author contributions
All authors participated in preparing the manuscript; OOP and CLW performed literature analysis, discussed main ideas, wrote manuscript, and prepared schemes.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
CLW is supported by the EU FP7 Grant Fishmed GA No. 316125 and Polish National Science Center (NCN) OPUS grant No. 2014/13/B/NZ2/03863. OOP is supported by the EMBO ASTF 518-2015.
References
- 1.Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol 2013; 5:a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freire AG, Resende TP, Pinto-do OP. Building and repairing the heart: what can we learn from embryonic development? Bio Med Res Int 2014; 2014:679168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signaling: diseases and therapies. Nat Rev Genet 2004; 5:691–701 [DOI] [PubMed] [Google Scholar]
- 4.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982; 31:99–109 [DOI] [PubMed] [Google Scholar]
- 5.Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol 2012; 4:a00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Virshup DM. Updating the Wnt pathways. Biosci Rep 2014; 34:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127:469–80 [DOI] [PubMed] [Google Scholar]
- 8.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 2006; 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu X, Joeng S, Nakayama KI, Nakayama K, Rajagopal J, Carroll T, McMahon A, Long F. Noncanonical Wnt signaling through G protein-linked PKC delta activation promotes bone formation. Dev Cell 2007; 12:113–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niehrs C. The complex world of WNT receptor signaling. Nat Rev Mol Cell Biol 2012; 1:767–79 [DOI] [PubMed] [Google Scholar]
- 11.Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress wingless signaling activity. Nature 1998; 395:604–8 [DOI] [PubMed] [Google Scholar]
- 12.Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destrée O, Clevers H. The Xenopus Wnt effect or XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 1998; 395:608–12 [DOI] [PubMed] [Google Scholar]
- 13.Bilić J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007; 316:1619–22 [DOI] [PubMed] [Google Scholar]
- 14.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 2008; 135:367–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 2012; 4:a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW, Jones DA. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 2009; 137: 623–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, Rosty C, Abal M, Marjou FE, Smits R, Louvard D, Fodde R, Robine S. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006; 131:1096–109 [DOI] [PubMed] [Google Scholar]
- 18.Obrador-Hevia A, Chin SF, González S, Rees J, Vilardell F, Greenson JK, Cordero D, Moreno V, Caldas C, Capellá G. Oncogenic KRAS is not necessary for Wnt signaling activation in APC-associated FAP adenomas. J Pathol 2010; 221:57–67 [DOI] [PubMed] [Google Scholar]
- 19.Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of Wnt receptor activation. Dev Cell 2009; 17:788–99 [DOI] [PubMed] [Google Scholar]
- 20.Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol 2009; 1:a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du C, Jaggi M, Zhang C, Balaji KC. Protein kinase D1-mediated phosphorylation and subcellular localization of beta-catenin. Cancer Res 2009; 6:1117–24 [DOI] [PubMed] [Google Scholar]
- 22.Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, Richardson ECN, Fujita Y. Case in kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Mol Cell Biol 2007; 27:3804–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archbold HC, Yang YX, Chen L, Cadigan KM. How do they do Wnt they do?: regulation of transcription by the Wnt/beta-catenin pathway. Acta Physiol 2012; 204:74–109 [DOI] [PubMed] [Google Scholar]
- 24.Daugherty RL, Gottardi CJ. Phospho-regulation of beta-catenin adhesion and signaling functions. Physiology 2007; 22:303–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of beta-catenin during axis formation in Xenopus. J Cell Biol 1996; 132:1105–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and under expression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 1994; 79:791–803 [DOI] [PubMed] [Google Scholar]
- 27.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2010; 2:a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68:3645–54 [DOI] [PubMed] [Google Scholar]
- 29.Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell 2007; 18:2013–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development 1997; 124:1631–42 [DOI] [PubMed] [Google Scholar]
- 31.Brade T, Pane LS, Moretti A, Chien KR, Laugwitz KL. Embryonic heart progenitors and cardiogenesis. Cold Spring Harb Perspect Med 2013; 3:a013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell 2006; 126:1037–48 [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Villalba A, Hoppler S, vanden Hoff MJ. Wnt signaling in the heart fields: variations on a common theme. Dev Dyn 2016; 245:294–306 [DOI] [PubMed] [Google Scholar]
- 34.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 2008; 135:789–98 [DOI] [PubMed] [Google Scholar]
- 35.Kelly RG, Buckingham ME, Moorman AF. Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med 2014; 4:a015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell 2002; 3:171–81 [DOI] [PubMed] [Google Scholar]
- 37.Chen VC, Stull R, Joo D, Cheng X, Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nature Biotechnol 2008; 26:1169–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A 2007; 104:9319–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song X, Wang S, Li L. New insights into the regulation of Axin function in canonical Wnt signaling pathway. Protein Cell 2014; 5:186–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev 2001; 15:304–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.vanden Hoff MJ, Kruithof BP, Moorman AF. Making more heart muscle. BioEssays 2004; 26:248–61 [DOI] [PubMed] [Google Scholar]
- 42.Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development 2008; 135:3185–90 [DOI] [PubMed] [Google Scholar]
- 43.Norden J, Kispert A. Wnt/Ctnnb1 signaling and the mesenchymal precursor pools of the heart. Trends Cardiovasc Med 2012; 22:118–22 [DOI] [PubMed] [Google Scholar]
- 44.Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell 2010; 18:275–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A 2007; 104:18531–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nature Cell Biol 2009; 11:951–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell 2007; 1:165–79 [DOI] [PubMed] [Google Scholar]
- 48.Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 2004; 6:685–98 [DOI] [PubMed] [Google Scholar]
- 49.Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development 2012; 139:1931–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain R, Li D, Gupta M, Manderfield LJ, Ifkovits JL, Wang Q, Liu F, Liu Y, Poleshko A, Padmanabhan A, Raum JC, Li L, Morrisey EE, Lu MM, Won KJ, Epstein JA. Heart development. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science 2015; 348:aaa6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.vanden Berg G, Abu-Issa R, de Boer BA, Hutson MR, deBoer PAJ, Soufan AT, Ruijter JM, Kirby ML, vanden Hoff MJB, Moorman AFM. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res 2009; 104:179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Wijk B, vanden Hoff M. Epicardium and myocardium originate from a common cardiogenic precursor pool. Trends Cardiovasc Med 2010; 20:1–7 [DOI] [PubMed] [Google Scholar]
- 53.Klaus A, Müller M, Schulz H, Saga Y, Martin JF, Birchmeier W. Wnt/beta-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc Natl Acad Sci U S A 2012; 109:10921–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech 2011; 4:469–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinus IFJ, Oerlemans MJG, van Middelaar B, Clevers H, Doevendans PA, Sluijter JPG. Active Wnt signaling in response to cardiac injury. Basic Res Cardiol 2010; 105:631–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res 2010; 107:1198–208 [DOI] [PubMed] [Google Scholar]
- 57.Daskalopoulos EP, Janssen BJ, Blankesteijn WM. Targeting Wnt signaling to improve wound healing after myocardial infarction. Methods Mol Biol 2013; 1037:355–80 [DOI] [PubMed] [Google Scholar]
- 58.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Lionel L, Moreau C, Dare D, Duplàa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice over expressing FrzA. Circulation 2003; 108:2282–9 [DOI] [PubMed] [Google Scholar]
- 59.He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, Pratt RE, Dzau VJ. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A 2010; 107:21110–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A 2007; 104:1643–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zelarayán LC, Noack C, Sekkali B, Kmecova J, Gehrke C, Renger A, Zafiriou MP, vander Nagel R, Dietz R, de Windt LJ, Balligand JL, Bergmann MW. Beta-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proc Natl Acad Sci U S A 2008; 105:19762–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan J, Gherghe C, Liu D, Eric H, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, Majesky M, Deb A. Wnt1/beta catenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J 2012; 31:429–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim K, Park KW, Koo BK, Chae IH, Shin CS, Oh BH, Choi YS, Park YB, Kim HS. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J Biol Chem 2006; 281:30979–89 [DOI] [PubMed] [Google Scholar]
- 64.Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3 beta/beta-catenin promotes angiogenic and antiapoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol 2006; 40:138–47 [DOI] [PubMed] [Google Scholar]
- 65.Malekar P, Hagenmueller M, Anyanwu A, Buss S, Streit MR, Weiss CS, Wolf D, Riffel J, Bauer A, Katus HA, Hardt SE. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension 2010; 55:939–45 [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, Kerkelä R, Molkentin JD, Liao R, Salomon RN, Patten R, Force T. The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol 2006; 26:4462–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu J, Zhou J, Ping XY, Dong B, Zheng H, Miller LM, Wang X, Schneider MD, Li F. Cardiac-specific haplo insufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol 2007; 43:319–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin down regulation is required for adaptive cardiac remodeling. Circ Res 2007; 100:1353–62 [DOI] [PubMed] [Google Scholar]
- 69.Butz S, Stappert J, Weissig H, Kemler R. Plakoglobin and beta-catenin: distinct but closely related. Science 1992; 257:1142–4 [DOI] [PubMed] [Google Scholar]
- 70.Filipovich A, Gehrke I, Poll-Wolbeck SJ, Kreuzer KA. Physiological inhibitors of Wnt signaling. Eur J Haematol 2011; 86:453–65 [DOI] [PubMed] [Google Scholar]
- 71.Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol 1996; 180:780–5 [DOI] [PubMed] [Google Scholar]
- 72.Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the arm adillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol 1995; 128:959–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development 1995; 121:3529–37 [DOI] [PubMed] [Google Scholar]
- 74.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-cateninin anterior-posterior axis formation in mice. J Cell Biol 2000; 148:567–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kodama S, Ikeda S, Asahara T, Kishida M, Kikuchi A. Axin directly interacts with plakoglobin and regulates its stability. J Biol Chem 1999; 274:27682–88 [DOI] [PubMed] [Google Scholar]
- 76.Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J Biol Chem 1995; 270:5549–55 [DOI] [PubMed] [Google Scholar]
- 77.Kolligs FT, Kolligs B, Hajra KM, Hu G, Tani M, Cho KR, Fearon ER. Gamma-catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of beta-catenin. Genes Dev 2000; 14:1319–31 [PMC free article] [PubMed] [Google Scholar]
- 78.Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol 1996; 16:2128–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, Kusugami K, Sekido Y. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene 2004; 23:964–72 [DOI] [PubMed] [Google Scholar]
- 80.Shimizu M, Fukunaga Y, Ikenouchi J, Nagafuchi A. Defining the roles of beta-catenin and plakoglobin in LEF/T-cell factor-dependent transcription using beta-catenin/plakoglobin-null F9 cells. Mol Cell Biol 2008; 28:825–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams BO, Barish GD, Klymkowsky MW, Varmus HE. A comparative evaluation of beta-catenin and plakoglobin signaling activity. Oncogene 2000; 19:5720–8 [DOI] [PubMed] [Google Scholar]
- 82.Zhou J, Qu J, Yi XP, Graber K, Huber L, Wang X, Geres AM, Li F. Upregulation of gamma-catenin compensates for the loss of beta-cateninin adult cardiomyocytes. Am J Physiol Heart Circ Physiol 2007; 292:H270–6 [DOI] [PubMed] [Google Scholar]
- 83.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci 2000; 113:3127–39 [DOI] [PubMed] [Google Scholar]
- 84.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol Cell Biol 2000; 20:4238–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swope D, Cheng L, Gao E, Li J, Radice GL. Loss of cadherin-binding proteins beta-catenin and plakoglobinin the heart leads to gap junction remodeling and arrhythmogenesis. Mol Cell Biol 2012; 32:1056–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, Bolli R, Hunziker T, Suter MM, Muller EJ. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J 2006; 25:3298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol 1999; 207:133–49 [DOI] [PubMed] [Google Scholar]
- 88.Charpentier E, Lavker RM, Acquista E, Cowin P. Plakoglobin suppresses epithelial proliferation and hair growth in vivo. J Cell Biol 2000; 149:503–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem 1999; 274:21464–70 [DOI] [PubMed] [Google Scholar]
- 90.Mahendram S, Kelly KF, Paez-Parent S, Mahmood S, Polena E, Cooney AJ, Doble BW. Ectopic gamma-catenin expression partially mimics the effects of stabilized beta-catenin on embryonic stem cell differentiation. PloS One 2013; 8:e65320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wickline ED, Du Y, Stolz DB, Kahn M, Monga SP. Gamma-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after beta-catenin knockdown. Neoplasia 2013; 15:421–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aktary Z, Pasdar M. Plakoglobin represses SATB1 expression and decreases in vitro proliferation, migration and invasion. PloS One 2013; 8:e78388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aktary Z, Kulak S, Mackey J, Jahroudi N, Pasdar M. Plakoglobin interacts with the transcription factor p53 and regulates the expression of 14-3-3 sigma. J Cell Sci 2013; 126:3031–42 [DOI] [PubMed] [Google Scholar]
- 94.Piven OO, Kostetskii IE, Macewicz LL, Kolomiets YM, Radice GL, Lukash LL. Requirement for N-cadherin-catenin complex in heart development. Exp Biol Med 2011; 236:816–22 [DOI] [PubMed] [Google Scholar]
- 95.Martin ED, Moriarty MA, Byrnes L, Grealy M. Plakoglobin has both structural and signalling roles in zebrafish development. Dev Biol 2009; 327:83–96 [DOI] [PubMed] [Google Scholar]
- 96.Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL. Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol 2011; 31:1134–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lombardi R, da Graca Cabreira-Hansen M, Bell A, Fromm RR, Willerson JT, Marian AJ. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res 2011; 109:1342–53 [DOI] [PMC free article] [PubMed] [Google Scholar]