Abstract
Background and objective. Postural muscle responses are often impaired after stroke. We aimed to identify the contribution of deficits in very early postural responses to poorer reactive balance capacity, with a particular focus on reactive stepping as a key strategy for avoiding falls. Methods. A total of 34 chronic stroke survivors and 17 controls were subjected to translational balance perturbations in 4 directions. We identified the highest perturbation intensity that could be recovered without stepping (single stepping threshold [SST]) and with maximally 1 step (multiple stepping threshold [MST]). We determined onset latencies and response amplitudes of 7 leg muscles bilaterally and identified associations with balance capacity. Results. People with stroke had a lower MST than controls in all directions. Side steps resulted in a higher lateral MST than crossover steps but were less common toward the paretic side. Postural responses were delayed and smaller in amplitude on the paretic side only. We observed the strongest associations between gluteus medius (GLUT) onset and amplitude and MST toward the paretic side (R2 = 0.33). Electromyographic variables were rather weakly associated with forward and backward MSTs (R2 = 0.10-0.22) and with SSTs (R2 = 0.08-0.15). Conclusions. Delayed and reduced paretic postural responses are associated with impaired reactive stepping after stroke. Particularly, fast and vigorous activity of the GLUT is imperative for overcoming large sideways perturbations, presumably because it facilitates the effective use of side steps. Because people with stroke often fall toward the paretic side, this finding indicates an important target for training.
Keywords: stroke, postural muscle response, reactive stepping, postural stability
Introduction
Stroke survivors have a substantially increased fall risk compared to the general population (1.3-6.5 vs 0.65 falls per person-year1,2). These falls can have serious physical and psychosocial consequences and can even lead to long-term disability.2 Poor performance on clinical balance tests is an important risk factor for falls in daily life.2-5 Although clinical tests can identify patients with balance impairments, they do not provide insight into the underlying deficits contributing to falls. A better understanding of underlying mechanisms of postural instability after stroke is necessary to develop therapeutic interventions to improve postural stability and to prevent falls.
A critical factor for preventing falls in daily life is the ability to regain balance after a perturbation.6 After small perturbations, we can usually regain balance while keeping the feet in place. These feet-in-place strategies are not sufficient to overcome larger perturbations, which require reactive stepping or grabbing responses to avoid falling.6 In people after stroke, the quality of reactive stepping responses is impaired.7 As a result, they are more likely to fall when experiencing a balance perturbation.8
Balance perturbations evoke fast postural muscle responses with onset latencies as early as 100 ms.9 In people after stroke, these early components of the automated postural responses (APRs) are delayed and smaller in amplitude.8,10 Stroke-related deficits in APRs were more pronounced in individuals who fell in response to imposed posterior perturbations when compared with those who successfully restored balance.8 Although these findings hint at a causal relationship between defective early APRs and poorer postural stability, the strength of this relationship is unknown. Another open question is whether defective early APRs may also underlie stroke-related difficulties in sustaining lateral perturbations. This is particularly relevant toward the paretic side because it is in this direction that stroke patients are most prone to falling.2
In this study, we aimed to determine the association between stroke-related deficits in early APR components (onset latency and electromyographic [EMG] amplitude in the initial 50 ms following muscle onset) and a poorer capacity to recover from forward, backward, and sideways balance perturbations. We determined the single stepping threshold (SST; largest perturbation that can be overcome with a feet-in-place response)11-15 and the multiple stepping threshold (MST; largest perturbation that can be overcome with 1 step) as measures of balance capacity. We hypothesized that SSTs and MSTs would be reduced in people with stroke, in parallel with delayed and lower-amplitude APRs. We further expected to find the strongest associations for perturbations toward the paretic side because balance recovery in this particular direction largely depends on corrective torques generated by the paretic leg.
Methods
Participants
A total of 34 people >6 months after a unilateral supratentorial stroke and 17 healthy controls were included (Table 1). Participants had to be able to stand and walk independently or under supervision (Functional Ambulation Categories ≥3). Exclusion criteria were the presence of neurological (except stroke), cognitive (Mini Mental State Examination score <24), or musculoskeletal disorders and use of medication that affects reaction time (eg, neuroleptics and benzodiazepines). Written informed consent was obtained from all participants. The protocol was approved by the Medical Ethical Board of the region Arnhem-Nijmegen, and all procedures were conducted in accordance with the Declaration of Helsinki.
Table 1.
Participant Characteristics.
| Stroke, n = 34 | Controls, n = 17 | |
|---|---|---|
| Age in years, mean (SD) | 62 (9) | 64 (5) |
| Sex (male/female) | 26/8 | 7/10a |
| Berg Balance Scale, mean (SD) | 52.3 (4.6) | 55.9 (0.5)a |
| Motricity Index Leg, mean (SD) | 76.4 (11.4) | — |
| Fugl-Meyer Score Leg, mean (SD) | 28.6 (4.6) | — |
| Type of stroke (hemorrhagic/ischemic) | 6/28 | — |
| Time since stroke in months, mean (SD) | 56 (42) | — |
P < .05 for between-group comparison.
Study Protocol
Participants were assessed on 2 separate days approximately 1 week apart. On the first day, the participants underwent a clinimetric assessment, including the Berg Balance Scale, Motricity Index, and Fugl-Meyer Scale. In addition, we determined the participant’s SST as a measure of maximum capacity of the feet-in-place response. On the second day we identified the MST as the maximum capacity for the reactive stepping response. On this occasion, we also performed EMG recordings during a series of perturbations at 2 fixed intensity levels that were equal for all participants.
Experimental Setup
Participants stood barefoot on a moveable platform (240 × 174 cm) with their feet 4.5 cm apart. The people with stroke wore an ankle brace (ASO, Medical Specialties, Wadesboro, NC) on the paretic side to prevent ankle injuries. Participants wore a safety harness that prevented them from falling in the event of balance loss but did not otherwise provide any body (weight) support. Balance perturbations were delivered by platform translations in 4 directions (forward, backward, leftward, and rightward). The perturbation direction was always unknown to the participant. The perturbation waveform consisted of a 300-ms acceleration phase, followed by 500-ms at constant velocity, and a 300-ms deceleration phase. The perturbation waveform was chosen such that individuals could complete a reactive step within the constant velocity phase, thereby preventing the deceleration from interfering with the step.16,17
Single stepping threshold: SSTs were determined for each of the 4 perturbation directions. Participants were instructed to restore balance without stepping or without grabbing the rails surrounding the platform. We defined the SST as the largest perturbation that could be sustained according to this instruction, with a maximum of 3 attempts at each perturbation intensity. We gradually increased the perturbation intensity with initial steps of 0.25 m/s2, starting at 0.25 m/s2. If a participant failed once, the intensity was decreased by 0.125 m/s2. Subsequently, the perturbation intensity was varied until the SST was determined.
Multiple stepping threshold: Participants were instructed to restore balance with a maximum of 1 step and without using the safety rails. We only considered steps that resulted in an extension of the base of support (BOS) in the perturbation direction. The MST for each direction was defined as the largest perturbation that could be sustained according to this instruction. The protocol for the MST was similar to the SST protocol, except that the starting intensity and the initial increments were set at 0.5 m/s2. The maximum value was 4.5 m/s2 because this was the maximum acceleration of the platform.
To recover from sideways perturbations, we observed different stepping strategies: (1) a side step with the leg that was loaded by the perturbation, (2) a crossover step with the leg that was passively unloaded by the perturbation, and occasionally, (3) no stepping response. In our further analyses, we distinguish between side step (1) versus no side step (2 and 3).
Four trials each were collected at 2 fixed perturbation intensities (Low, 0.5 m/s2; High, 1.5 m/s2) for each direction. These trials were used for between-subject comparison of postural responses. These intensities were chosen such that participants would be capable of using a feet-in-place recovery strategy in the vast majority of Low trials, whereas in the High trials, the participants would need to take a step.
Data Sampling and Analysis
We used surface EMG (ZeroWire by Aurion, Italy; 2000 Hz) to measure bilateral muscle activity of 7 leg muscles: gluteus medius (GLUT), biceps femoris (BFEM), rectus femoris (RFEM), peroneus longus (PER), tibialis anterior (TA), gastrocnemius medialis (GASTR), and soleus (SOL). Self-adhesive Ag-AgCl electrodes (Tyco Arbo ECG) were placed approximately 2 cm apart and longitudinally on the belly of each muscle, according to the Seniam guidelines.18 The start of the perturbation was determined with a digital trigger signal.
EMG signals were first band-pass filtered (20-450 Hz, zero-lag, second-order Butterworth filter), rectified, and low-pass filtered at 20 Hz (zero-lag, second-order Butterworth filter). EMG onset latencies and response amplitudes were determined for the prime movers for each perturbation direction (forward: BFEM, GASTR, and SOL; backward: RFEM and TA; sideways: GLUT and PER). EMG traces were aligned to the start of the perturbation and averaged for each perturbation direction and intensity. Onset latencies were determined using a semiautomatic computer algorithm that selected the instant at which the EMG activity first exceeded a threshold of 2 SDs above the mean background activity over a 500-ms period just prior to perturbation onset of all trials.19-21 After being determined by the computer algorithm, onset latencies were visually checked and corrected if needed. The mean EMG response amplitude was calculated over a period of 50 ms following the onset of muscle activity after subtraction of background EMG. This time window was chosen based on pilot experiments in healthy individuals, where we observed that EMG signals in the stepping and stance leg following High perturbations were symmetrical during the first 50 ms after muscle onset and then started to diverge depending on the role of the respective leg. In controls, values of EMG variables and balance capacity were averaged between the left and right side for comparison with the stroke group.
Statistical Analysis
We compared SSTs and MSTs between people with stroke and controls with an independent-samples t-test for each perturbation direction separately. Within the stroke group, we compared balance recovery capacity on perturbations toward the paretic versus non-paretic side with a paired-samples t-test. For MSTs in sideways directions, we performed a separate analysis according to step strategy (side step/no side step). For each strategy, we compared MSTs between the paretic side, the non-paretic side, and controls using an ANOVA. P values <.05 were considered significant.
We used a linear mixed model to compare the EMG variables (as obtained at the 2 fixed perturbation intensities) between the paretic side, the non-paretic side, and controls. The dependent variables were Onset and Amplitude. Independent variables included Leg (paretic, non-paretic, control), Muscle, and Intensity. In the analysis, the factor Leg was modeled such that it included both within-subjects (between paretic and non-paretic) and between-subjects (paretic vs control and non-paretic vs control) comparisons. The interaction terms Leg × Intensity and Leg × Muscle were also included. When significant main or interaction effects were found, we used post hoc tests to compare between legs. Because EMG amplitudes demonstrated a positively skewed distribution, we applied a ln (natural logarithm) transformation to these data for the linear mixed model analysis. P values <.05 were considered significant.
To determine whether EMG variables were associated with SSTs or MSTs, we used stepwise linear regression analyses for each direction separately. The EMG variables for Low perturbations were entered in the model as determinants for the SST, whereas EMG variables for High perturbations were entered as determinants of the MST (P < .05 for entry and P > .10 for removal of variables from the model). For the stroke group, we only included EMG variables of the paretic leg because postural responses on the non-paretic side were not different from that of controls. A detailed overview of the statistical analyses can be found in Supplementary Table 1.
Results
In the stroke group, we could not determine the forward MST in one participant because of fatigue at the end of the experiment. For 3 participants in the stroke group, the High perturbations toward the paretic side were too challenging. For these participants and 2 others, this was also true for High perturbations toward the non-paretic side, whereas 1 participant could not perform the backward perturbation trials at the same intensity. The number of trials completed for the SST test was 63 ± 7 trials in the stroke group and 67 ± 4 in controls. For the MST test, stroke survivors completed 99 ± 26 trials versus 134 ± 16 trials by controls.
Balance Capacity
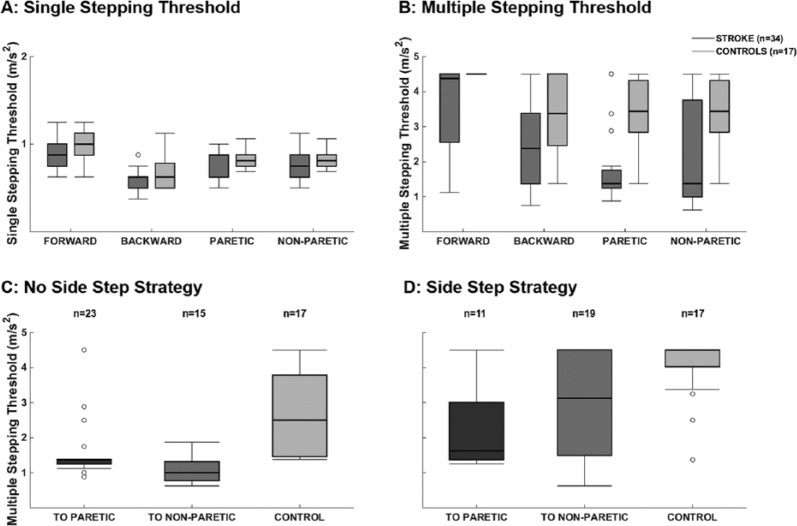
SSTs were slightly lower in the stroke group, but we observed no significant differences compared with controls (P > .05).The stroke group demonstrated substantially impaired MSTs in all directions (Figure 1, P < .01). MSTs did not differ between perturbations toward the paretic versus the non-paretic side (P = .13).
Figure 1.
Balance capacity: single and multiple stepping thresholds for people after stroke and controls. Panel A: single stepping thresholds were not different between groups (P > .05), whereas multiple stepping thresholds were smaller for people with stroke compared with controls (panel B, P < .05 for all directions). For both multiple stepping thresholds obtained without (panel C) and with a side step strategy (panel D), stroke survivors performed worse toward the paretic and non-paretic side when compared with controls (P < .05).
Side steps were less common for perturbations toward the paretic side (29% of the trials) compared with perturbations toward the non-paretic side (61%) and to sideways perturbations in controls (55%, P < .01). Overall, side steps resulted in higher MSTs than crossover steps (3.2 ± 1.4 vs 1.8 ± 1.1 m/s2, P < .01). Side steps toward both the paretic and non-paretic sides resulted in lower MSTs compared with those in controls (2.2 ± 1.3 vs 3.0 ± 1.4 m/s2 vs 4.0 ± 0.9 m/s2, P < .05); however, the difference between the paretic and non-paretic sides did not reach significance (P = .077). Similarly, MSTs achieved with crossover steps toward both the paretic and non-paretic sides were reduced compared with controls (1.5 ± 0.8 vs 1.0 ± 0.3 m/s2 vs 2.8 ± 1.1 m/s2, P < .01). Crossover steps toward the non-paretic side (ie, steps with the paretic leg) did not yield significantly lower MSTs than toward the paretic side (ie, steps taken with the non-paretic leg; P = .075).
Postural Muscle Responses
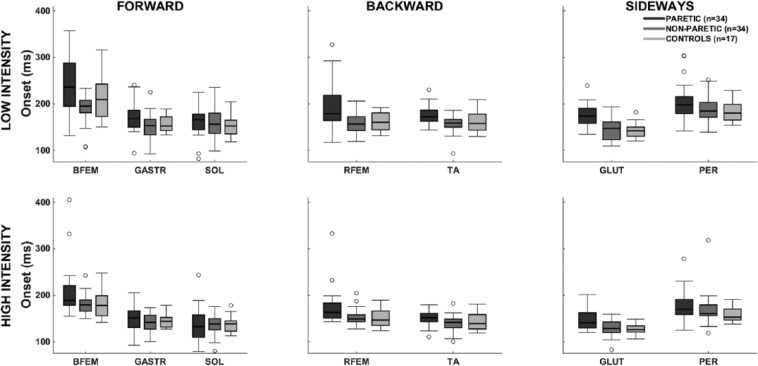
The analysis for onset latencies yielded a significant main effect of Leg (Figure 2). Paretic onset latencies exhibited an overall delay compared with the non-paretic leg (19-ms delay, P < .01) and the controls (18-ms delay, P < .01). Onset latencies in the non-paretic leg did not differ from controls (P = .86).
Figure 2.
Electromyographic onset latencies: postural response onset latencies for low- and high-intensity perturbations (0.5 and 1.5 m/s2).
Abbreviations: BFEM, biceps femoris; GASTR, gastrocnemius medialis; GLUT, gluteus medius; PER, peroneus longus; RFEM, rectus femoris; SOL, soleus; TA, tibialis anterior.
Faster muscle onsets were observed at High compared with Low perturbations. Yet this acceleration was more pronounced in the paretic leg compared with the non-paretic leg and with controls, resulting in smaller differences in onset latencies between paretic legs and controls in the high- (14 ms) compared with the low-intensity perturbations (23 ms; Leg × Intensity, P < .01).
Delays in the paretic leg compared with controls differed per muscle as indicated by a significant Leg × Muscle interaction (BFEM 29 ms, GASTR 10 ms, SOL not delayed, RFEM 27 ms, TA 12 ms, GLUT 25 ms, PER 20 ms; Leg × Muscle P < .01).
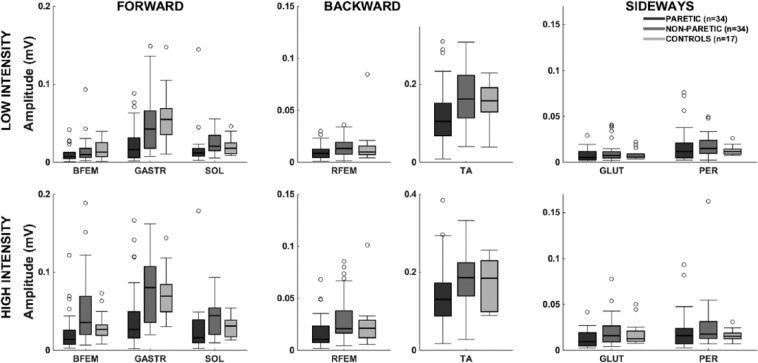
Response amplitudes (Figure 3) differed between the legs as indicated by a significant effect of Leg (P < .01). The amplitudes were smaller in the paretic leg compared with controls (0.01 mV, P < .01) and compared with the non-paretic leg (0.02 mV, P < .01). The non-paretic leg did not differ from controls (P = .06).
Figure 3.
Electromyographic amplitudes: postural response amplitudes for low- and high-intensity perturbations (0.5 and 1.5 m/s2).
Abbreviations: BFEM, biceps femoris; GASTR, gastrocnemius medialis; GLUT, gluteus medius; PER, peroneus longus; RFEM, rectus femoris; SOL, soleus; TA, tibialis anterior.
Response amplitudes were larger for the High compared with the Low intensity (P < .01 for main effect of Intensity). The amplitudes on the paretic side were equally reduced compared with controls for the Low and High intensity (P = .34 for Leg × Intensity). There was a significant Leg × Muscle interaction (P < .01), indicating that reduction in paretic leg response amplitudes compared with controls was different across muscles (BFEM 0.006 mV, GASTR 0.035 mV, SOL 0.004 mV, RFEM 0.006 mV, TA 0.028 mV, GLUT 0.002 mV, PER not reduced).
Postural Responses as Determinants of Balance Capacity
In general, associations between EMG variables and SSTs were weak, yet some variables reached the significance level (Table 2). For forward perturbations, delayed gastrocnemius onset latencies were associated with lower SSTs (P = .01; R2 = 0.15). For perturbations toward the paretic side, larger peroneus amplitudes were associated with lower SSTs (P = .04; R2 = 0.08). None of the EMG variables significantly predicted the backward SSTs.
Table 2.
Results of Linear Regression Analysis.
| Dependent Variable | Independent Variable | β | P | R 2 | |
|---|---|---|---|---|---|
| Single stepping threshold | Forward | Onset gastrocnemius | −0.003 | .01 | 0.15 |
| Paretic side | Amplitude peroneus | −2.459 | .04 | 0.08 | |
| Multiple stepping threshold | Forward | Amplitude gastrocnemius | 8.934 | .03 | 0.10 |
| Backward | Onset rectus femoris | −0.010 | .04 | 0.22 | |
| Amplitude rectus femoris | 26.248 | <.01 | |||
| Paretic side | Onset gluteus medius | −0.023 | <.01 | 0.33 | |
| Amplitude gluteus medius | 45.880 | <.01 | |||
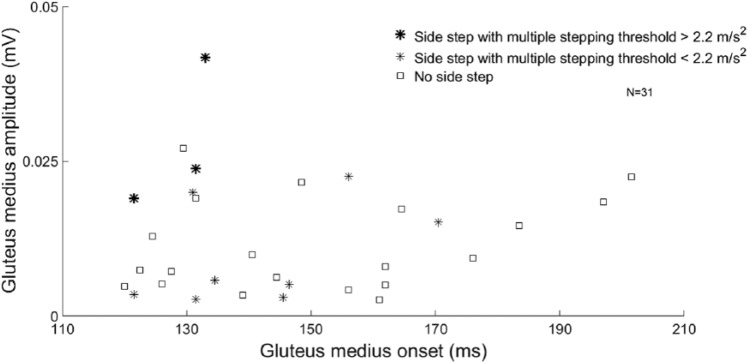
Associations between EMG variables and MSTs were strongest for perturbations toward the paretic side (R2 = 0.33). Here, delayed onsets and smaller amplitudes of the GLUT were associated with lower MSTs (P < .01). In this perturbation direction, only 3 individuals with stroke who used a side step strategy achieved MSTs within control values (Average − 2 SD = 2.2 m/s2). In the High perturbations toward the paretic side, these 3 individuals indeed demonstrated rather fast and strong paretic GLUT activity compared with the rest of the stroke group (Figure 4).
Figure 4.
Effect of gluteus medius activity and step strategy on the multiple stepping threshold toward the paretic side: amplitude and onset of the paretic gluteus medius in people with stroke using crossover steps and side steps toward the paretic side. Side steps resulted in significantly higher multiple stepping thresholds toward the paretic side than no side steps. Individuals who used a side step and reached multiple stepping thresholds within healthy control values (>2.2 m/s2) demonstrated fast and large-amplitude responses in the paretic gluteus medius. These individuals also reached good scores on the clinical tests.
Associations between EMG variables and MSTs were less pronounced for forward and backward perturbations. For forward perturbations, smaller amplitudes of the gastrocnemius were associated with lower MSTs (P = .03; R2 = 0.10). Lower backward MSTs were associated with delayed onsets and smaller amplitudes of the RFEM (P < .05; R2 = 0.22).
Discussion
We investigated to what extent deficits in very early automatic postural responses (APRs) of people with chronic stroke underlie their reduced capacity to sustain balance perturbations. Remarkably, stroke-related deficits in balance capacity were more pronounced for stepping than for feet-in-place responses. Early APR characteristics were most predictive for the MSTs toward the paretic side and, to a lesser extent, for MSTs in the forward and backward directions.
Stroke-related deficits in reactive steps are in line with previous studies that included forward and backward perturbations.7,22,23 This study significantly adds to previous work by demonstrating that reactive stepping responses in both lateral directions are also profoundly impaired after stroke, which most likely increases the risk of falling sideways. Sideways falls have a 2.5 greater odds of resulting in hip fractures compared with other fall directions.24 Our finding of lower lateral MSTs in the people with stroke may partially explain why they are at a much higher risk of sustaining a hip fracture than healthy persons.25
Another main finding was that deficits in early APR components—as the first line of defense against perturbations—were associated with impaired reactive stepping performance. These associations were strongest for perturbations toward the paretic side (R2 = 33%). When perturbed sideways, the most beneficial strategy for recovering balance is making a side step, as demonstrated by the overall higher MSTs that we observed for side steps compared with crossover steps. Yet this side-step strategy involves stepping with the leg that is passively loaded by the perturbation26 and thus requires rapid active unloading to enable the leg to step. In stroke survivors, rapid unloading of the paretic leg following perturbations toward this side is presumably hampered by their poorer APRs in paretic GLUT, which may, consequently, affect their side-stepping ability. Indeed, side steps were 52% less prevalent toward the paretic compared with the non-paretic side. Moreover, MSTs resulting from side steps with the paretic leg were substantially lower compared with side steps in controls, indicating that these paretic side steps were often of poor quality. In fact, only 3 people with stroke who took paretic side steps achieved the MSTs within the range of the control group. These individuals had relatively good clinical scores and demonstrated fast and strong GLUT activity compared with the other participants with stroke. Our findings, therefore, suggest that the ability to make effective paretic side steps following sideways balance perturbations greatly depends on fast and vigorous activity of the paretic hip abductor.
Interestingly, MSTs were almost equally affected for perturbations toward the paretic versus the non-paretic side. MSTs toward the non-paretic side were particularly affected when people with stroke used a (paretic) crossover step to recover balance. Despite this poor cross-stepping performance with the paretic leg, as many as 44% of the stroke participants used this strategy. It remains an open question whether these participants would still have the ability to use a non-paretic side-stepping strategy instead. Hence, for future intervention studies aimed at improving balance control after stroke, we propose retraining of reactive side stepping as a key target, not only toward the paretic, but also toward the non-paretic side.
The finding that postural response deficits after stroke were associated with impaired backward MSTs confirms previous findings that individuals who fell in response to a balance perturbation demonstrated delayed postural responses compared with those who did not fall.8 Our results add to these previous findings by quantifying the strength of this association, which appeared to be rather weak (R2 = 22%). It may be that greater non-paretic APRs possibly compensated for the impaired paretic APRs. Our finding that APR amplitudes in the non-paretic leg were not increased compared with controls does not support this idea, but compensatory activity in the non-paretic leg may occur in later phases of the postural response.
For forward perturbations, the association between APR characteristics and MSTs was even weaker (R2 = 10%). In this direction, we observed a ceiling effect in the MST, with all the controls and 16 participants with stroke reaching the maximum score (4.5 m/s2). To identify the impact of this ceiling effect on the strength of the association, we performed an additional regression analysis without these participants, yielding no significant associations between APR characteristics and forward MSTs either. This finding negates defective APRs being a key determinant of poor reactive stepping in the forward direction.
Instead, factors associated with the execution and postlanding phase of the step may be more important determinants of the MST in the sagittal plane. Previous studies in healthy individuals have found that successful balance recovery in the forward and backward directions can be predicted by the length of the step relative to the center-of-mass (COM) excursions.27-29 In addition, eccentric knee extensor torques are important in reducing further forward displacement of the COM after landing of the stepping foot following forward perturbations.30 Future studies may, therefore, focus on these mechanisms in explaining stroke-related deficits in MSTs in the sagittal plane.
Surprisingly, SSTs were not significantly reduced in our stroke group, despite substantially impaired paretic leg postural responses. Additionally, we found no strong relationships between impaired muscle responses and reduced SSTs. This seems to contrast with the previous observations that impaired postural responses on the paretic side were related to larger body sway.8 This discrepancy may be explained by the notion that people with stroke are reluctant to take a step. Indeed, this study, in addition to previous articles, has demonstrated that stepping performance is greatly impaired in people with stroke. Consequently, they may perceive a greater need to rely on their feet-in-place responses and, therefore, allow the COM to more closely approach the boundaries of the BOS, before eventually taking a step. In contrast, healthy individuals may prefer to step at lower perturbation intensities—even if instructed not to do so—resulting in an underestimation of their maximum feet-in-place capacity. Future studies should, therefore, include a measure of the COM-BOS relationship to determine whether the boundary conditions for stepping are indeed different between healthy individuals and people with stroke.
A limitation of this study is that SSTs were determined on a separate testing day. Between-day variations in performance may result in weaker relationships between SSTs and EMG variables. It seems more likely, however, that the weaker relationships result from the absence of stroke-related impairments in SSTs. A second limitation of this study may be that only the cardinal (anteroposterior and mediolateral) perturbation directions were investigated. Previous studies suggested that postural responses are controlled by a low-dimensional set of muscle synergies, which often show their largest activation in diagonal perturbation directions.31 In people with stroke, some of these postural synergies are defective and some are intact.32 Possibly, relationships between postural response deficits and balance performance are stronger for perturbation directions that specifically involve the recruitment of those muscle synergies that are often defective after stroke. Moreover, diagonal perturbations toward the non-paretic side increase the number of paretic steps, resulting in different step characteristics.33 We, therefore, suggest that future studies include diagonal perturbations as well. A third limitation is the large number of trials performed by participants. This may have induced both learning and fatigue effects, particularly affecting those participants who achieved greater perturbation intensities and, thus, had a larger number of trials. A limitation of using EMG amplitude as an outcome is that the signal can be influenced by factors other than muscle activation (ie, background noise, skin impedance). Although we corrected our amplitudes for background activity, these factors may still have had some influence on the outcomes. In addition, stroke induces muscle atrophy, specific loss of type 2 muscle fibers,34 and a smaller number of motor units35 on the paretic side, which influence EMG amplitude36 and may, thus, affect amplitude differences between the paretic and non-paretic and control legs. Finally, the deceleration of the platform (800 ms after perturbation onset) may have helped participants regain balance when it occurred during the first reactive step. However, in 95% of the trials collected at the MST, the step was completed within 800 ms, indicating that the deceleration only had a minor effect on group performance.
In conclusion, stroke survivors show impaired reactive stepping capacity in all perturbation directions, whereas feet-in-place capacity remains unaffected. Poorer stepping performance can partially be explained by impaired early postural muscle responses. Particularly, fast and vigorous GLUT activity appears critical for overcoming large sideways perturbations, presumably by facilitating the effective use of side steps. There is some evidence that in people with stroke, both early APRs and reactive stepping can be improved with agility or perturbation-based training.37,38 Based on our findings, we specifically recommend including exercises for enhancing paretic side steps as a primary target for training and for preventing falls in people with stroke.
Supplementary Material
Footnotes
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
Authors’ Note: The fourth and fifth authors contributed equally to the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a Veni Grant given to Dr. Vivian Weerdesteyn.
References
- 1. Simpson LA, Miller WC, Eng JJ. Effect of stroke on fall rate, location and predictors: a prospective comparison of older adults with and without stroke. PLoS One. 2011;6:e19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195-1213. [PubMed] [Google Scholar]
- 3. Mackintosh SF, Hill KD, Dodd KJ, Goldie PA, Culham EG. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil. 2006;87:1583-1589. [DOI] [PubMed] [Google Scholar]
- 4. Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83:165-170. [DOI] [PubMed] [Google Scholar]
- 5. Belgen B, Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87:554-561. [DOI] [PubMed] [Google Scholar]
- 6. Maki BE, McIlroy WE. Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age Ageing. 2006;35(suppl 2):ii12-ii18. [DOI] [PubMed] [Google Scholar]
- 7. Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27:526-533. [DOI] [PubMed] [Google Scholar]
- 8. Marigold DS, Eng JJ. Altered timing of postural reflexes contributes to falling in persons with chronic stroke. Exp Brain Res. 2006;171:459-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marigold DS, Eng JJ, Inglis JT. Modulation of ankle muscle postural reflexes in stroke: influence of weight-bearing load. Clin Neurophysiol. 2004;115:2789-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirker SG, Simpson DS, Jenner JR, Wing AM. Stepping before standing: hip muscle function in stepping and standing balance after stroke. J Neurol Neurosurg Psychiatry. 2000;68:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sturnieks DL, Menant J, Delbaere K, et al. Force-controlled balance perturbations associated with falls in older people: a prospective cohort study. PLoS One. 2013;8:e70981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crenshaw JR, Grabiner MD. The influence of age on the thresholds of compensatory stepping and dynamic stability maintenance. Gait Posture. 2014;40:363-368. [DOI] [PubMed] [Google Scholar]
- 13. Crenshaw JR, Kaufman KR. The intra-rater reliability and agreement of compensatory stepping thresholds of healthy subjects. Gait Posture. 2014;39:810-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mille ML, Rogers MW, Martinez K, et al. Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol. 2003;90:666-674. [DOI] [PubMed] [Google Scholar]
- 15. de Kam D, Kamphuis JF, Weerdesteyn V, Geurts ACH. The effect of weight-bearing asymmetry on dynamic postural stability in healthy young individuals. Gait Posture. 2016;45:55-61. [DOI] [PubMed] [Google Scholar]
- 16. Carpenter MG, Thorstensson A, Cresswell AG. Deceleration affects anticipatory and reactive components of triggered postural responses. Exp Brain Res. 2005;167:433-445. [DOI] [PubMed] [Google Scholar]
- 17. Tokuno CD, Cresswell AG, Thorstensson A, Carpenter MG. Age-related changes in postural responses revealed by support-surface translations with a long acceleration-deceleration interval. Clin Neurophysiol. 2010;121:109-117. [DOI] [PubMed] [Google Scholar]
- 18. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361-374. [DOI] [PubMed] [Google Scholar]
- 19. Nonnekes J, Arrogi A, Munneke MA, et al. Subcortical structures in humans can be facilitated by transcranial direct current stimulation. PLoS One. 2014;9:e107731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nonnekes J, Oude Nijhuis LB, de Niet M, et al. StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci. 2014;34:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nonnekes J, Scotti A, Oude Nijhuis LB, et al. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience. 2013;245:109-120. [DOI] [PubMed] [Google Scholar]
- 22. Lakhani B, Mansfield A, Inness EL, McIlroy WE. Compensatory stepping responses in individuals with stroke: a pilot study. Physiother Theory Pract. 2011;27:299-309. [DOI] [PubMed] [Google Scholar]
- 23. Inness EL, Mansfield A, Bayley M, McIlroy WE. Reactive stepping after stroke: determinants of time to foot off in the paretic and non-paretic limb. J Neurol Phys Ther. 2016;40:196-202. [DOI] [PubMed] [Google Scholar]
- 24. Wei TS, Hu CH, Wang SH, Hwang KL. Fall characteristics, functional mobility and bone mineral density as risk factors of hip fracture in the community-dwelling ambulatory elderly. Osteoporos Int. 2001;12:1050-1055. [DOI] [PubMed] [Google Scholar]
- 25. Ramnemark A, Nilsson M, Borssen B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31:1572-1577. [DOI] [PubMed] [Google Scholar]
- 26. King LA, Horak FB. Lateral stepping for postural correction in Parkinson’s disease. Arch Phys Med Rehabil. 2008;89:492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carty CP, Cronin NJ, Lichtwark GA, Mills PM, Barrett RS. Mechanisms of adaptation from a multiple to a single step recovery strategy following repeated exposure to forward loss of balance in older adults. PLoS One. 2012;7:e33591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsiao ET, Robinovitch SN. Elderly subjects’ ability to recover balance with a single backward step associates with body configuration at step contact. J Gerontol A Biol Sci Med Sci. 2001;56:M42-M47. [DOI] [PubMed] [Google Scholar]
- 29. Weerdesteyn V, Laing AC, Robinovitch SN. The body configuration at step contact critically determines the successfulness of balance recovery in response to large backward perturbations. Gait Posture. 2012;35:462-466. [DOI] [PubMed] [Google Scholar]
- 30. Madigan ML, Lloyd EM. Age-related differences in peak joint torques during the support phase of single-step recovery from a forward fall. J Gerontol A Biol Sci Med Sci. 2005;60:910-914. [DOI] [PubMed] [Google Scholar]
- 31. Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol. 2007;98:2144-2156. [DOI] [PubMed] [Google Scholar]
- 32. de Kam D, Weerdesteyn V, Torres-Oviedo G. Missing Muscle Synergies for Balance Control in Paretic Side After Stroke (Program No 52009). Washington, DC: Society for Neuroscience; 2015. [Google Scholar]
- 33. Martinez KM, Mille ML, Zhang Y, Rogers MW. Stepping in persons poststroke: comparison of voluntary and perturbation-induced responses. Arch Phys Med Rehabil. 2013;94:2425-2432. [DOI] [PubMed] [Google Scholar]
- 34. Toffola ED, Sparpaglione D, Pistorio A, Buonocore M. Myoelectric manifestations of muscle changes in stroke patients. Arch Phys Med Rehabil. 2001;82:661-665. [DOI] [PubMed] [Google Scholar]
- 35. Arasaki K, Igarashi O, Ichikawa Y, et al. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006;250:27-32. [DOI] [PubMed] [Google Scholar]
- 36. de Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135-163. [Google Scholar]
- 37. Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. J Am Geriatr Soc. 2005;53:416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mansfield A, Inness EL, Komar J, et al. Training rapid stepping responses in an individual with stroke. Phys Ther. 2011;91:958-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.