Abstract
Borderline personality disorder (BPD) patients’ hypersensitivity for emotionally relevant stimuli has been suggested be due to abnormal activity and connectivity in (para-)limbic and prefrontal brain regions during stimulus processing. The neuropeptide oxytocin has been shown to modulate activity and functional connectivity in these brain regions, thereby optimizing the processing of emotional and neutral stimuli. To investigate whether oxytocin would be capable of attenuating BPD patients’ hypersensitivity for such stimuli, we recorded brain activity and gaze behavior during the processing of complex scenes in 51 females with and 48 without BPD after intranasal application of either oxytocin or placebo. We found divergent effects of oxytocin on BPD and healthy control (HC) participants’ (para-)limbic reactivity to emotional and neutral scenes: Oxytocin decreased amygdala and insula reactivity in BPD participants but increased it in HC participants, indicating an oxytocin-induced normalization of amygdala and insula activity during scene processing. In addition, oxytocin normalized the abnormal coupling between amygdala activity and gaze behavior across all scenes in BPD participants. Overall, these findings suggest that oxytocin may be capable of attenuating BPD patients’ hypersensitivity for complex scenes, irrespective of their valence.
Keywords: oxytocin, amygdala, insula, borderline personality disorder, functional magnetic resonance imaging, eye tracking
Introduction
Borderline personality disorder (BPD) is a common mental disorder associated with substantial morbidity and mortality (Leichsenring et al., 2011), indicating a constant need for the development of novel treatment approaches (Stanley and Siever, 2010). Most, if not all, symptoms of BPD, such as self-injurious behavior, suicide attempts or aggressive outburst, manifest themselves in interpersonal situations (Bender and Skodol, 2007). As a consequence, BPD patients often experience interpersonal conflicts (e.g. Stiglmayr et al., 2005; Russell et al., 2007; Sadikaj et al., 2010), which appear to be due to a misperception of others’ feelings and intentions (Sadikaj et al., 2010). More precisely, BPD patients tend to attribute malevolence and hostility to others (e.g. Wagner and Linehan, 1999; Arntz and Veen, 2001; Domes et al., 2008; Barnow et al., 2009; Dyck et al., 2009; Arntz et al., 2011; Arntz and ten Haaf, 2012), in particular in complex situations (e.g. Arntz and Veen, 2001; Barnow et al., 2009; Arntz et al., 2011; Arntz and ten Haaf, 2012). These misperceptions are often accompanied by abnormal activity and connectivity patterns in a network of (para-)limbic and prefrontal brain regions that comprise the amygdala, insula, anterior cingulate cortex (ACC), orbitofrontal and prefrontal cortex (Mauchnik and Schmahl, 2010; Krause-Utz et al., 2014). Interestingly, these activity and connectivity patterns have been observed during the processing of a variety of stimuli, including emotional (e.g. Herpertz et al., 2001; Donegan et al., 2003; Schulze et al., 2011; Hazlett et al., 2012; Krause-Utz et al., 2012) as well as neutral ones (e.g. Donegan et al., 2003; Schulze et al., 2011; Hazlett et al., 2012; Krause-Utz et al., 2012). Apparently, BPD patients tend to perceive various stimuli as aversive and arousing, presumably due to pre-existing biases that have been shaped throughout adverse or traumatic experiences in childhood (e.g. Arntz et al., 1999; Giesen-Bloo and Arntz, 2005). As this hypersensitivity for emotionally relevant stimuli appears to drive BPD patients’ emotional and behavioral reactions in interpersonal contexts (Bender and Skodol, 2007), it has been considered to be one of the most important targets in the treatment of BPD (Gunderson, 2007; Stanley and Siever, 2010).
Recently, the neuropeptide oxytocin has been suggested to be useful for therapeutic approaches in BPD (Herpertz and Bertsch, 2015). Since oxytocin optimizes the processing of emotionally relevant stimuli by modulating activity and connectivity patterns in (para-)limbic and prefrontal brain regions (Bethlehem et al., 2013; Wigton et al., 2015), oxytocin may be capable of normalizing abnormal stimulus processing in BPD. In accordance with this notion, we recently found that oxytocin normalizes BPD patients’ abnormal gaze behavior and abnormal (para-)limbic activity during face processing (Bertsch et al., 2013). Although these findings indicate that oxytocin indeed normalizes BPD patients’ hypersensitivity for emotionally relevant stimuli (Herpertz and Bertsch, 2015), they should be treated with caution until replicated and extended in further studies to prevent an overoptimistic view of oxytocin’s therapeutic potential (Walum et al., 2016). These studies should consider that BPD patients’ hypersensitivity for emotionally relevant stimuli is particularly pronounced in complex situations (e.g. Arntz and Veen, 2001; Barnow et al., 2009; Arntz et al., 2011; Arntz and ten Haaf, 2012). It may, thus, be worthwhile to use complex rather than simple stimuli to further investigate how oxytocin normalizes abnormal stimulus processing in BPD.
In consideration of this, we aimed to replicate and extend our previous findings in an independent sample of BPD patients using scenes instead of faces. Scenes showing a large variety of social and non-social content represent a more complex class of stimuli than faces showing a restricted variety of prototypical expressions, implying that scenes may be ideally suited to reveal BPD patients’ hypersensitivity for emotionally relevant stimuli under laboratory conditions that more closely resemble real-life conditions. Of note, BPD patients are hypersensitive to all types of scenes, not only emotional (e.g. Herpertz et al., 2001; Koenigsberg et al., 2009; Niedtfeld et al., 2010; Jayaro et al., 2011; Schulze et al., 2011; Hazlett et al., 2012; Krause-Utz et al., 2012) but also neutral ones (e.g. Koenigsberg et al., 2009; Niedtfeld et al., 2010; Jayaro et al., 2011; Schulze et al., 2011; Hazlett et al., 2012; Krause-Utz et al., 2012). We, thus, employed positive, negative and neutral scenes in this study to investigate whether oxytocin would normalize abnormal stimulus processing in BPD patients. Of note, we hypothesized that oxytocin would normalize the processing of all types of scenes but nonetheless investigated both, valence-specific and, for exploratory reasons, valence-unspecific effects of oxytocin on stimulus processing. As oxytocin appears to normalize stimulus processing via gaze changes that are mediated by corresponding activity and connectivity changes in (para-)limbic brain regions (e.g. Gamer et al., 2010; Bertsch et al., 2013), we recorded BPD patients’ brain activity and gaze behavior during scene presentation. After placebo administration, BPD patients were expected to show abnormal activity and abnormal functional connectivity in (para-)limbic and prefrontal brain regions, that is, (para-)limbic hyperreactivity and prefrontal hyporeactivity to all types of scenes. We also expected BPD patients to show an abnormal coupling between (para-)limbic activity and gaze behavior during scene processing, again independent of the scenes’ valence. After oxytocin administration, BPD patients were expected to show normal activity in (para-)limbic and prefrontal brain regions, mainly due to a valence-unspecific decrease in (para-) limbic hyperactivity and functional hyperconnectivity during scene processing. Accordingly, BPD patients were expected to show a valence-unspecific normalization of the abnormal coupling between (para-)limbic activity and gaze behavior during scene processing.
Materials and methods
Participants
Forty-eight healthy females (healthy controls, HC) and 51 females with a DSM-IV diagnosis of BPD were recruited for this study. None of them had participated in our previous study investigating the effects of oxytocin on face processing in BPD (Bertsch et al., 2013). Due to technical difficulties, the data of two HC and four BPD participants could not be considered in the subsequent analyses (see Supplementary Material S1). BPD participants who also met DSM-IV diagnostic criteria for schizoaffective disorder, schizophrenia or intellectual disability were excluded from the study as well as BPD participants who received regular medication within the last 8 weeks or acute medication within the last week before study enrollment. HC participants were excluded from the study if they met DSM-IV criteria for current or lifetime diagnoses of any Axis I or Axis II disorder. Additional exclusion criteria for all participants were pregnancy, lactation, menstrual irregularities or menopause at the time of testing. Inclusion and exclusion criteria were determined by structured interviews and self-report questionnaires (see Supplementary Material S2). All participants provided written informed consent before taking part in the study, which was approved by the local ethics committee and carried out in accordance with the Declaration of Helsinki.
Experimental procedure
Participants who were not taking hormonal contraceptives were tested during the early follicular phase of their menstrual cycle, whereas those who were taking hormonal contraceptives (HC: n = 10, BPD: n = 5) were tested during their contraceptive-free period. Testing of participants was restricted to these time points to control for interactions between participants’ estrogen, progesterone and oxytocin levels during the experimental task (Domes et al., 2010; Lischke et al., 2012c). On the testing day, blood and urine samples were obtained to validate participants’ self-reports regarding pregnancy and menstrual cycle status (see Supplementary Material S3). Using a double-blind between-subjects design, participants were then randomly assigned to the placebo (HC-PLC: n = 24, BPD-PLC: n = 24) or oxytocin (HC-OXT: n = 22, BPD-OXT: n = 23) group. Following a standardized protocol (Domes et al., 2010; Lischke et al., 2012c), participants self-administered 24 IU of intranasal oxytocin (Syntocinon® Spray; Novartis, Basel, Switzerland) or placebo 45 min before the beginning of the experimental task. Substance administration increased participants’ oxytocin levels after oxytocin but not after placebo administration, without causing any side effects (see Supplementary Material S4).
Experimental task
During functional magnetic resonance imaging (fMRI), participants viewed 36 positive, 36 negative and 36 neutral scenes that were selected from the International Affective Picture System (IAPS; Lang et al., 2005; see Supplementary Material S5). Eighteen Fourier-transformed IAPS scenes were additionally presented as distractors. To ensure that participants were paying attention to the scenes, they had to indicate per button press whether the scenes depicted humans or objects. All scenes were presented in an event-related manner in a pseudo-random order, meaning that no more than two scenes of the same type followed each other. Each scene was presented for 2 s, preceded by a fixation cross period of 0.5 s and followed by a blank screen period of 2.0–20.9 s. Optseq (http://surfer.nmr.mgh.harvard.edu/optseq/) was used to optimize the stimulation order and timing. Stimulus presentation and response registration were controlled with Presentation 12.1 (Neurobehavioral Systems, Albany, CA, USA), while visual stimulation was delivered via a fiber optic goggle system (VisuaStim; Resonance Technology, Los Angeles CA, USA).
Eye-tracking
A remote infrared eye-tracker (VisuaStim; Resonance Technology) was used to record participants’ gaze behavior during scene processing (sampling rate: 60 Hz; tracking resolution: 0.15°, gaze position accuracy: 0.25°–1.0°). GazeAlyze was used for data processing and data analysis (Berger et al., 2012). After smoothing of the data, blinks were detected and interpolated whenever possible. Thereafter, fixations were coded when gaze remained for at least 100 ms within an area with a 14-pixel diameter. For each scene, the number of fixations to the entire scene and to pre-defined regions of the scene was determined. To obtain a relative index of participants’ gaze behavior during scene processing, the number of fixations to pre-defined regions was dived by the number of fixations to the entire scene. As previously described in more detail (Lischke et al., 2012c), the pre-defined regions of interest (ROIs), which were crucial for understanding the scenes’ content, were created by independent raters and empirically validated in a pilot study (see Supplementary Material S6).
Magnetic resonance imaging
MRI was performed on a 1.5-Tesla scanner (Magnetom Avanto; Siemens, Erlangen, Germany) equipped with a standard head coil. Using a T2*-sensitive gradient echo-planar imaging sequence (repetition time, 2700 ms; echo time, 40 ms; flip angel, 90°; field of view, 192 × 192 mm; in-plane resolution, 3 × 3 mm2), 36 axial slices (slice thickness, 3 mm; 1 mm gap) were acquired covering the whole brain. Additionally, isotropic high-resolution (1 × 1 × 1 mm3) structural images were recorded using a T1-weighted coronal oriented magnetization-prepared rapid gradient echo sequence (repetition time, 1500 ms; echo time, 3.9 ms; flip angle, 15°; field of view, 256 × 256 mm2) with 160 sagittal slices.
Image pre-processing and data analysis were performed with the Statistical Parametric Mapping software SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). First, slice-timing, realignment with unwarping and co-registration to the structural image of each participant was performed. Thereafter, the structural images were segmented using the unified segmentation approach. The tissue-class images for gray and white matter that were generated during the segmentation were used within the DARTEL toolbox to create structural templates across subjects as well as individual flow fields (Ashburner, 2007). These flow fields were used to normalize the functional and structural images to MNI space. Finally, the functional images were spatially smoothed (FWHM: 8 mm) and high-pass filtered (cutoff period: 128 s).
Using a general linear model approach, two sets of random-effects analyses, one investigating differences in brain activity and one investigating differences in brain functional connectivity, were performed. The first set of analyses investigated differences in participants’ brain activity as a function of group, substance and scene type. To this end, participant-specific design matrices were constructed by modeling the onset of the scene presentation as separate regressors for each type of scene (i.e. positive, negative and neutral scenes). After convolving the regressors with a hemodynamic response function, simple contrast maps were computed for each scene type and subjected to two different random-effects analyses. In the first analysis, a two-sample t-test was used to investigate differences in brain activity between HC and BPD participants after placebo administration. In the second analysis, a 2 × 2 flexible factorial ANOVA (Group × Substance) was used to investigate how oxytocin affected these differences in brain activity. Of note, these analyses were carried out across all types of scenes due to our hypotheses of valence-unspecific differences in scene processing. However, to explore the possibility of valence-specific effects, similar analyses were carried out for each scene type (i.e. positive, negative and neutral scenes) as well as for differences between emotional and neutral scenes (i.e. negative > neutral scenes and positive > neutral scenes).
The second set of analyses investigated whether the functional connectivity between the amygdala and other, in particular prefrontal, brain regions differed as a function of group and substance. In contrast to conventional psycho–physiological interaction analyses that examine the influence of experimental manipulations on a within-subject basis, this analysis focused on the influence of between-subject factors (i.e. group and substance) on correlations of fluctuations in brain activity. Correspondingly, such analysis allows for revealing influences of these factors on the functional connectivity between brain regions. The amygdala was chosen as seed region because it is the major site for oxytocin effects on emotion processing in humans (Bethlehem et al., 2013; Wigton et al., 2015). The mean time series for each participant was extracted from a voxel in the left amygdala (−22/0/−18) that showed robust effects of group and substance in the first random effects analysis (see Results). These time series were used as regressors in participant-specific design matrices. The experimental conditions were also modeled to remove shared variance. Simple contrast maps were computed for the time series regressors and subjected to two different random-effects analyses. In the first analysis, a two-sample t-test was used to analyze differences in the functional connectivity of the amygdala and other brain regions between HC and BPD participants after placebo administration. In the second analysis, a 2 × 2 flexible factorial ANOVA (Group × Substance) was used to investigate how oxytocin affected these differences in functional connectivity.
In all random-effects analyses, participants’ estrogen and progesterone levels were used as additional regressors to account for the effects of estrogen and progesterone on brain activity and functional connectivity (Toffoletto et al., 2014).
To control for multiple comparisons, a family-wise error rate (FWE) threshold of P < 0.05, corrected for pre-defined ROIs, was used but effects that were significant at a trend level [P < 0.10] are also reported. Of the various brain regions implicated in BPD patients’ abnormal stimulus processing (Mauchnik and Schmahl, 2010; Krause-Utz et al., 2014), the amygdala, insula and ACC were of particular interest because oxytocin has repeatedly been shown to modulate activity in these brain regions during the processing of emotionally relevant stimuli (e.g. Tost et al., 2011; Striepens et al., 2012; Scheele et al., 2014). For the definition of the ROIs, an anatomical mask with a probability threshold of 50% was used for the bilateral amygdala (www.cma.mgh.harvard.edu/fsl_atlas.html) and 8 mm spheres for the bilateral insula and bilateral ACC. The spheres were centered on the peak coordinates of previous studies investigating oxytocin-induced changes in emotion processing [insula (Striepens et al., 2012): ±38/22/4; ACC (Scheele et al., 2014): ±8/26/34]. Activations outside these ROIs were reported if they survived a whole-brain correction for multiple comparisons using a false discovery rate threshold of P < 0.05 (Genovese et al., 2002) and a cluster threshold of k > 10 voxels.
For illustration purposes, the statistical parametric maps were thresholded at P < 0.005 (uncorrected) with a cluster threshold of k > 10 voxels and projected onto the mean structural image of all participants. To illustrate the interaction of group and substance, parameter estimates were extracted from the peak voxel using rfxplot (Glascher, 2009). On basis of these parameter estimates, planned comparisons were run to compare changes in brain activity between oxytocin and placebo administration within the group of HC participants as well as within the group of BPD participants. All activations are reported in x/y/z coordinates in MNI space.
Results
Participant characteristics
Several 2 × 2 ANOVAs (Group × Substance) were run to investigate differences in demographical and psychopathological characteristics between HC and BPD participants (Table 1). HC and BPD participants did not differ in general intelligence (all P> 0.31, all < 0.01) but in psychopathology, with BPD participants reporting more psychopathology than HC participants (all P< 0.001, all > 0.53). BPD participants who received oxytocin and placebo did not differ from one another in psychopathology (all P> 0.26, all < 0.01; see Supplementary Material S7). Of note, there was a slight age difference between HC and BPD participants (P < 0.03, = 0.05), but this age difference did not account for the aforementioned differences in participants’ psychopathology as indicated by a series of 2 × 2 ANCOVAs (Group × Substance) with age as a covariate.
Table 1.
Demography and psychopathology of healthy control (HC) and borderline personality disorder (BPD) participants receiving placebo (PLC) or oxytocin (OXT)
| HC |
BPD |
Test statistic |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PLC (n = 24) |
OXT (n = 22) |
PLC (n = 24) |
OXT (n = 23) |
Main effect of group | Main effect of substance | Interaction of group and substance | |||||
| m | s.d. | m | s.d. | m | s.d. | m | s.d. | F(1,89) | F(1,89) | F(1,89) | |
| Age | 25.25 | 4.41 | 23.23 | 2.27 | 26.5 | 5.52 | 26.43 | 6.24 | 4.86* | 1.07 | 0.94 |
| Intelligence (MWT-B-IQ) | 109.5 | 8.73 | 109.64 | 14.71 | 107.33 | 13.86 | 105.83 | 17.81 | 1.04 | 0.06 | 0.08 |
| General pathology (SCL-90-R-GSI) | 0.13 | 0.18 | 0.21 | 0.19 | 1.35 | 0.66 | 1.36 | 0.82 | 110.74*** | 0.14 | 0.11 |
| Borderline pathology (BSL-23) | 0.1 | 0.24 | 0.16 | 0.19 | 1.78 | 0.94 | 1.65 | 1.15 | 100.88*** | 0.06 | 0.36 |
| Post-traumatic pathology (PDS) | 0.04 | 0.09 | 0.13 | 0.28 | 1.15 | 0.72 | 1.37 | 0.63 | 128.68*** | 2.26 | 0.42 |
| Depressive pathology (BDI) | 1.71 | 3.03 | 3.41 | 3.28 | 24.38 | 12.13 | 23.91 | 11.45 | 143.72*** | 0.12 | 0.36 |
| Anxious pathology (STAI-T) | 28.79 | 4.98 | 33.41 | 6.32 | 58.13 | 12.17 | 59.17 | 7.46 | 259.54*** | 2.75 | 1.09 |
MWT-B-IQ, Multiple choice vocabulary test—Intelligence quotient; SCL-90-R-GSI, Symptom Check List 90 Revised—Global Severity Index; BSL-23, Borderline Symptom List 23; PDS, Post-traumatic Diagnostic Scale; BDI, Beck Depression Inventory; STAI-T, State Trait Anxiety Inventory—Trait Anxiety.
P < 0.05.
P < 0.001.
Task performance
A series of 2 × 2 × 3 ANOVAs (Group × Substance × Valence) were run to investigate whether HC and BPD participants differed in task performance during scene presentation (Table 2). There were no differences in task performance between HC and BPD participants, neither after oxytocin nor placebo administration (group- and substance-related effects and interactions regarding response accuracy or response latency: all P > 0.11, all < 0.02). HC as well as BPD participants showed low response latencies and high response accuracies, especially during the processing of positive and neutral as compared to negative scenes (effect of valence regarding response accuracy or response latency: all P < 0.001, all > 0.12).
Table 2.
Task performance of healthy control (HC) and borderline personality disorder (BPD) participants receiving placebo (PLC) or oxytocin (OXT)
| HC |
BPD |
|||||||
|---|---|---|---|---|---|---|---|---|
| PLC (n = 24) |
OXT (n = 21)a |
PLC (n = 24) |
OXT (n = 23) |
|||||
| m | s.d. | m | s.d. | m | s.d. | m | s.d. | |
| Response accuracy (in percent) | ||||||||
| Positive scenes | 98.5 | 2.16 | 98.81 | 1.88 | 98.72 | 2.02 | 98.3 | 2.01 |
| Negative scenes | 96.99 | 2.31 | 97.48 | 2.14 | 96.16 | 4.69 | 97.56 | 1.74 |
| Neutral scenes | 97.91 | 1.89 | 98.14 | 1.83 | 97.21 | 2.47 | 97.94 | 2.4 |
| Response latency (in ms) | ||||||||
| Positive scenes | 802.23 | 177.03 | 824.05 | 173 | 792.07 | 174.21 | 763.1 | 152.81 |
| Negative scenes | 854.89 | 174.7 | 864.98 | 191.09 | 857.88 | 181.41 | 834.74 | 193.36 |
| Neutral scenes | 800.94 | 171.09 | 808.49 | 165.42 | 781.66 | 160.49 | 758.43 | 159.77 |
Data was missing for one participant of the HC-OXT group due to a recording error.
Gaze behavior
Another series of 2 × 2 × 3 ANOVAs (Group × Substance × Valence) was run to investigate whether HC and BPD participants differed in gaze behavior during scene presentation (Table 3). There were no differences in HC and BPD participants’ gaze behavior, neither after oxytocin nor after placebo administration (group- and substance-related effects and interactions regarding fixation number: all P > 0.13, all ηp2 < 0.03). HC as well as BPD participants’ number of fixations on pre-defined ROIs were high for negative, medium for positive and low for neutral scenes (effect of valence regarding fixation number: all P < 0.001, all > 0.18).
Table 3.
Gaze behavior of healthy control (HC) and borderline personality disorder (BPD) participants receiving placebo (PLC) or oxytocin (OXT)
| HC |
BPD |
|||||||
|---|---|---|---|---|---|---|---|---|
| PLC (n = 23) |
OXT (n = 22) |
PLC (n = 22) |
OXT (n = 22) |
|||||
| m | s.d. | m | s.d. | m | s.d. | m | s.d. | |
| Relative number of fixations (in percent)a | ||||||||
| Positive Scenes | 60.57 | 7.45 | 63.18 | 7.4 | 58.18 | 9.27 | 59.73 | 7.73 |
| Negative Scenes | 62.22 | 6.95 | 63.59 | 5.15 | 60.45 | 8.27 | 62.14 | 6.61 |
| Neutral Scenes | 58.43 | 8.03 | 60.55 | 7.77 | 55.41 | 7.88 | 59.41 | 7.87 |
Data was missing for one participant of the HC-PLC group, two participants of the BPD-PLC group and one participant of the BPD-OXT group due to artifacts.
Brain activity and brain functional connectivity
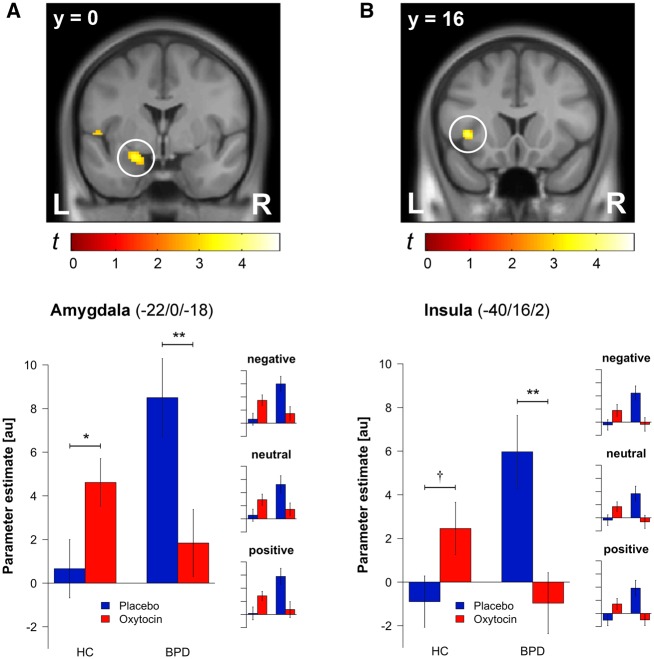
Two sets of analyses were run to investigate whether HC and BPD participants differed in brain activity and functional connectivity during scene presentation. In the first set of analyses, a two sample t-test was run to investigate differences in brain activity between HC and BPD participants after placebo administration (Table 4 and Figure 1). BPD participants, relative to HC participants, showed increased amygdala and insula reactivity to all scenes (significant effect of group). Exploratory t-tests investigating differences in brain activity between HC and BPD participants for each scene type separately revealed similar differences in (para-)limbic activity, irrespective of the scenes’ valence (Figure 1). Accordingly, there were no differences in (para-)limbic reactivity for positive or negative relative to neutral scenes as indicated by exploratory t-tests investigating differences in brain activity between HC and BPD participants for emotional as compared to neutral scenes (all P > 0.28). Thereafter, a 2 × 2 ANOVA (Group × Substance) was run to investigate how oxytocin affected the aforementioned differences in HC and BPD participants’ brain activity across all scenes (Table 4 and Figure 1). Oxytocin normalized these differences by decreasing BPD participants’ and increasing HC participants’ amygdala and insula reactivity to all scenes (significant interaction of group and substance). Exploratory 2 × 2 ANOVAs (Group × Substance) investigating how oxytocin affected differences in HC and BPD participants’ brain activity for each scene type revealed similar effects of oxytocin on (para-)limbic activity, again irrespective of the scenes’ valence (Figure 1). Accordingly, there were no differential effects of oxytocin on (para-)limbic reactivity for positive or negative relative to neutral scenes as indicated by exploratory 2 × 2 ANOVAs (Group × Substance) on contrasts in brain activity between emotional and neutral scenes (all P > 0.45).
Table 4.
Brain activity of healthy control (HC) and borderline personality disorder (BPD) participants receiving placebo (PLC) or oxytocin (OXT)
| Main effect of group among participants receiving placebo |
Interaction of group and substance among participants receiving oxytocin and placebo |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (BPDPLC > HCPLC) |
(BPDPLC > BPDOXT) > (HCPLC > HCOXT) |
|||||||||||
| MNI coordinates |
MNI coordinates |
|||||||||||
| Brain regions | x | y | z | Z | PFWE | Puncorr. | x | y | z | Z | PFWE | Puncorr. |
| Amygdala (L) | −24 | 0 | −16 | 3.99 | 0.006 | <0.001 | −22 | 0 | −18 | 3.67 | 0.016 | <0.001 |
| Insula (L) | −40 | 16 | 0 | 3.31 | 0.051 | <0.001 | −40 | 16 | 2 | 3.74 | 0.013 | <0.001 |
Note: Activations are reported when PFWE < 0.10 (small volume correction for a priori defined bilateral ROIs). L = left.
Fig. 1.
Brain regions showing activity changes as a function of group and substance. Statistical maps depict the interacting effects of group and substance on amygdala (A) and insula (B) activity pooled across scene valence. Bar plots show parameter estimates extracted from the peak voxels of amygdala and insula analyses as a function of group and substance across valence categories. Similar bar plots were also generated for each valence category to illustrate the similarity of response patterns. Significance stars (†P < 0.10, *P < 0.05, **P < 0.01) indicate results of planned comparisons between placebo and oxytocin administration within the HC group as well as within the BPD group. Error bars indicate standard errors of the mean.
In the second set of analyses, a two-sample t-test and a 2 × 2 ANOVA (Group × Substance) were run to investigate whether the functional connectivity between the amygdala and other brain regions differed between HC and BPD participants during scene processing. There were no differences in HC and BPD participant’s functional connectivity, neither after placebo nor after oxytocin administration (insignificant effect of group, insignificant interaction of group and substance).
Of note, the ACC was also selected as a ROI, but none of the aforementioned analyses revealed differences in ACC activity or ACC functional connectivity between BPD and HC participants, neither after oxytocin nor after placebo administration (insignificant effect of group, insignificant interaction of group and substance). Similarly, an exploratory whole-brain analysis revealed no differences in brain activity in other regions than the amygdala and insula (insignificant effect of group, insignificant interaction of group and substance).
Coupling between brain activity and gaze behavior
To explore the coupling between amygdala activity and gaze behavior during scene processing in HC and BPD participants, correlation analyses involving amygdala reactivity and the relative number of fixations across all scenes were run. For each participant, parameter estimates were extracted from a voxel in the left amygdala (−22/0/−18) that showed robust effects of group and substance in the above-mentioned random-effects analyses. These parameter estimates were correlated with the number of fixations on pre-defined ROIs. Among HC participants, amygdala activity and gaze behavior were uncorrelated after oxytocin [r(22) = −0.05, P = 0.83] or placebo [r(23) = −0.04, P = 0.84] administration as indicated by non-substantial and non-significant correlation coefficients. Among BPD participants, on the contrary, a different pattern of correlation coefficients emerged. Whereas amygdala activity correlated negatively with gaze behavior among BPD participants after placebo administration [r(22) = −0.49, P = 0.02], a reversed but non-significant correlation was obtained for BPD patients after oxytocin administration [r(22) = 0.17, P = 0.44]. A direct comparison of the correlation coefficients revealed a marginally significant difference between HC and BPD participants after placebo (HC-PLC vs. BPD-PLC: z = 1.52, P = 0.06) but not oxytocin (HC-OXT vs. BPD-OXT: z = 0.69, P = 0.25) administration. Moreover, there was a significant difference in correlation coefficients between BPD participants (BPD-PLC vs. BPD-OXT: z = 2.17, P =0.02) but not HC participants (HC-PLC vs. HC-OXT: z = 0.29, P = 0.39) after placebo and oxytocin administration.
Discussion
In this study, we investigated how BPD participants processed complex scenes that differed in valence after placebo and oxytocin administration. BPD participants were expected to show valence-unspecific abnormalities in scene processing after placebo administration. In particular, we expected abnormal activity and abnormal functional connectivity changes in (para-) limbic and prefrontal brain regions that are coupled with abnormal gaze changes. These abnormalities were suggested to be absent after oxytocin administration, mainly due to an oxytocin-induced normalization of BPD participants’ (para-)limbic hyperreactivity and functional hyperconnectivity during scene processing.
In our first set of analyses, we investigated whether BPD participants showed abnormal activity and abnormal functional connectivity in (para-)limbic and prefrontal brain regions that have been suggested to be related to BPD patients’ hypersensitivity for emotionally relevant stimuli (Mauchnik and Schmahl, 2010; Krause-Utz et al., 2014). After placebo administration, BPD participants showed increased amygdala and insula activity during the processing of all scenes, irrespective of the scenes’ valence. A similar increase in amygdala and insula reactivity to emotional and neutral scenes has been reported in previous studies (e.g. Herpertz et al., 2001; Koenigsberg et al., 2009; Niedtfeld et al., 2010; Schulze et al., 2011; Hazlett et al., 2012; Krause-Utz et al., 2012; Koenigsberg et al., 2014) indicating that BPD patients tend to perceive neutral scenes as emotional as positive or negative ones (Mauchnik and Schmahl, 2010; Krause-Utz et al., 2014). Interestingly, there was an inverse relationship between amygdala activity and gaze behavior during scene processing, implying that those BPD participants who were most emotionally aroused by the stimuli gazed less at scene regions that were most relevant for an understanding of the scenes’ content. It may, thus, be possible that BPD participants avoided emotional arousal during scene processing. Whether this abnormal gaze behavior was a consequence of BPD participants’ crude emotion regulation strategies remains to be determined in future studies that employ more fine-grained analyses of gaze behavior during the processing of emotionally relevant stimuli (e.g. analyses of fixation patterns during instructed up- and down-regulation of emotional reactions). Nonetheless, the valence-unspecific increase in (para-)limbic reactivity to complex scenes clearly indicates abnormalities in scene processing, which may be a reflection of BPD participants’ bias to perceive stimuli as aversive and arousing in complex situations (e.g. Arntz and Veen, 2001; Barnow et al., 2009; Arntz et al., 2011; Arntz and ten Haaf, 2012). Contrary to the abnormal activity in (para-)limbic regions, we neither found abnormal activity in prefrontal brain regions nor abnormal functional connectivity between (para-)limbic and prefrontal regions in BPD participants. Such abnormalities have previously been reported in studies investigating emotion regulation in BPD participants (e.g. Koenigsberg et al., 2009; Niedtfeld et al., 2010; Schulze et al., 2011). Whereas BPD participants in these studies performed complex tasks that required the regulation of their emotional responses (e.g. Koenigsberg et al., 2009; Niedtfeld et al., 2010; Schulze et al., 2011), BPD participants in this study performed a simple task that did not require regulatory efforts. However, complex tasks, in particular emotion regulation tasks (Ochsner et al., 2012), are more likely to engage activity and connectivity changes in prefrontal brain regions than simple tasks. This may explain why we neither found abnormal activity in prefrontal brain regions nor abnormal functional connectivity between (para-)limbic and prefrontal brain regions in BPD participants.
In our first set of analyses, we found abnormal (para-)limbic activity and an abnormal coupling of (para-)limbic activity with gaze behavior during scene processing in BPD participants after placebo administration, implying abnormalities in scene processing due to a hypersensitivity for emotionally relevant stimuli (Mauchnik and Schmahl, 2010; Krause-Utz et al., 2014). In our second set of analyses, we investigated whether oxytocin administration would normalize these abnormalities in scene processing. After oxytocin administration, we found a normalization of activity in (para-)limbic brain regions. BPD participants showed a valence-unspecific decrease in amygdala and insula reactivity to the scenes. Oxytocin also normalized the abnormal coupling between amygdala activity and gaze behavior in BPD participants in a valence-unspecific way. Oxytocin, thus, appeared to normalize scene processing in BPD participants. As this effect was again independent of the scenes’ valence, it may indicate an attenuation of BPD participants’ bias to perceive complex stimuli as aversive and arousing (e.g. Arntz and Veen, 2001; Barnow et al., 2009; Arntz et al., 2011; Arntz and ten Haaf, 2012). Although oxytocin affected activity in (para-)limbic regions, it neither affected activity in prefrontal brain regions nor functional connectivity between (para-)limbic and prefrontal brain regions in BPD participants. Previous studies also revealed oxytocin-induced activity changes in (para-)limbic rather than prefrontal brain regions during scene processing (Kirsch et al., 2005; Lischke et al., 2012b, c; Striepens et al., 2012), although the size and direction of effects differed between studies, possibly due to sex differences (Kirsch et al., 2005; Lischke et al., 2012b, c). Apparently, oxytocin primarily acts on (para-)limbic brain regions, such as the amygdala and insula, which is consistent with recent reviews and meta-analyses on oxytocin effects in humans (Bethlehem et al., 2013; Wigton et al., 2015).
Although we were mainly interested to investigate how oxytocin affected the processing of complex emotionally relevant stimuli in BPD participants, we also investigated the effects of oxytocin on stimulus processing in HC participants. Similarly as in BPD participants, oxytocin affected (para-)limbic activity but neither prefrontal activity nor (para-)limbic–prefrontal functional connectivity in HC participants. However, oxytocin increased rather than decreased HC participants’ amygdala and insula activity irrespective of the scenes’ valence, which is largely consistent with previous studies investigating oxytocin-induced changes in stimulus processing in healthy females (Domes et al., 2010; Lischke et al., 2012b, c; Bertsch et al., 2013). Also consistent with previous studies in healthy females (Domes et al., 2010; Lischke et al., 2012b, c; Bertsch et al., 2013), oxytocin had no effect on HC participants’ gaze behavior.
Taken together, we found that oxytocin differentially affected (para-)limbic activity as well as the coupling of (para-)limbic activity with gaze behavior during stimulus processing in HC and BPD participants, implying divergent effects of oxytocin on individuals who differ in their sensitivity for emotional and neutral stimuli (Bartz et al., 2011). Oxytocin appears to enhance the sensitivity for such stimuli in individuals who show normal to low (para-)limbic activity during stimulus processing and to attenuate sensitivity for such stimuli in individuals who show high (para-)limbic activity during stimulus processing. Accordingly, it has recently been reported that oxytocin increases (para-)limbic hyporeactivity to emotionally relevant stimuli in patients with autism spectrum disorders (ASD; Domes et al., 2013; Domes et al., 2014) but decreases (para-)limbic hyperreactivity to emotionally relevant stimuli in patients with generalized social anxiety disorder (GSAD; Labuschagne et al., 2010; Dodhia et al., 2014). These findings indicate that oxytocin is capable of normalizing abnormal activity in (para-)limbic brain regions via opposing activity changes. As a consequence, oxytocin has been suggested to be useful for the treatment of mental disorders that are characterized by hyper- as well as hyposensitivity for emotionally relevant stimuli, such as ASD, GSAD or BPD (Meyer-Lindenberg et al., 2011). However, the precise mechanism underlying the normalizing effects of oxytocin on stimulus processing remains to be elucidated (Churchland and Winkielman, 2012), indicating that further studies are warranted before oxytocin should be used for treatment purposes. Future studies should focus on the coupling between (para-)limbic activity and gaze changes because gaze changes appear be related to an oxytocin-induced normalization of stimulus processing (e.g. Gamer et al., 2010; Lischke et al., 2012a; Bertsch et al., 2013). More precisely, oxytocin may enhance or attenuate the processing of emotionally relevant stimuli via gaze changes that are mediated by activity and connectivity changes in (para-)limbic brain regions. This mechanism has so far only been demonstrated in healthy individuals (Gamer et al., 2010), but it may also work in individuals suffering from ASD, GSAD and BPD because oxytocin appears to affect both abnormal gaze behavior and abnormal (para-)limbic activity in these disorders (e.g. Andari et al., 2010; Labuschagne et al., 2010; Bertsch et al., 2013; Domes et al., 2013, 2014).
Although we assume that oxytocin differentially affected the processing of complex scenes in BPD and HC participants via the proposed mechanism, it has to be acknowledged that oxytocin-induced changes in scene processing were more pronounced on the neural than behavioral level. First of all, oxytocin did not affect participants’ task performance during scene processing, which was probably due to the simplicity of the task. Task performance was almost perfect across participants, indicating that ceiling effects may have prevented behavioral changes in scene processing. Although more complex tasks may have been better suited to reveal such changes, they are less likely to engage activity and connectivity changes in (para-) limbic brain regions than less complex tasks (e.g. Hariri et al., 2003; Taylor et al., 2003; Grimm et al., 2006). As (para-)limbic brain regions represent the major site for oxytocin effects on stimulus processing (Bethlehem et al., 2013; Wigton et al., 2015), we were interested to challenge (para-)limbic rather than prefrontal brain regions during scene processing. For this reason, we deliberately employed a simple and not a complex task in this study. However, future studies should also use complex tasks to address other aspects of scene processing than the present task. Rating tasks asking for the perceived valence and arousal of the scenes may be of particular interest because BPD participants often report abnormal valence and abnormal arousal ratings for emotional and neutral scenes (e.g. Koenigsberg et al., 2009; Jayaro et al., 2011; Schulze et al., 2011; Krause-Utz et al., 2012). As methodological reasons precluded the use of rating tasks in this study (e.g. reducing patient burden, restricting study duration to time window of oxytocin effects, restraining interactions between behavioral ratings and brain activity), these tasks should be used in future studies to elucidate how oxytocin affects valence and arousal perception during scene processing. Second, oxytocin did not affect participants’ gaze behavior during scene processing per se, but modulated the coupling between amygdala activity and gaze behavior. Oxytocin-induced changes in gaze behavior have so far, if at all, only been observed during the processing of faces but not scenes (Guastella et al., 2008; Domes et al., 2010; Lischke et al., 2012a, b, c; Bertsch et al., 2013). It may, thus, be possible that the processing of scenes is accompanied by more complex gaze patterns than the processing of faces (i.e. fixations tend to cluster around two regions in faces but are distributed across multiple regions in scenes), making it more difficult to reveal oxytocin-induced changes in gaze behavior during scene than face processing. In this respect, it is noteworthy that we found an oxytocin-induced normalization of the abnormal coupling between amygdala activity and gaze behavior, which has also been reported previously (Gamer et al., 2010). Neural measures may, thus, be more sensitive than behavioral measures to detect oxytocin-induced changes in stimulus processing. Future studies investigating the coupling between (para-)limbic activity and gaze changes with more advanced methods, like, for example, methods that are capable to differentiate between reflexive and sustained attention processes (Gamer et al., 2013), may help to elucidate how oxytocin affects gaze behavior during stimulus processing. In this respect, it may be particularly interesting to use faces as well as scenes to determine whether the behavioral and neural effects of oxytocin are comparable between different classes of stimuli.
Overall, we found that oxytocin differentially affected (para-) limbic activity as well as the coupling of (para-)limbic activity with gaze behavior during the processing of emotional and neutral scenes in HC and BPD participants, implying that oxytocin attenuated sensitivity for such stimuli in BPD participants and enhanced sensitivity for such stimuli in HC participants. Our findings, which were consistent with previous studies, were based on robust analyses of behavioral and neural data provided by a large sample of HC and BPD participants that were well matched with respect to possible confounds (i.e. differences in estrogen or progesterone levels). Nonetheless, future studies are warranted that further investigate how oxytocin affects the processing of complex scenes on the neural and behavioral level, preferably after repeated and prolonged administration. These studies, which should include male and female participants, will help to determine the therapeutic potential of oxytocin for attenuating or enhancing the processing of emotionally relevant stimuli in mental disorders (Meyer-Lindenberg et al., 2011).
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
The authors would like to thank Annegret Herfort, Gisela Irmisch, Anne Grau and Martin Winkels for research assistance and Andreas Löw for statistical advice.
Funding
Funding for this study was provided by grants from the German Federal Ministry of Education and Research to S.C.H. (BMBF 01GW0784), from the European Research Council to M.G. (ERC-2013-StG-336305) and from the German Research Foundation to A.L. (DFG LI 2517/2-1). The funding sources had no further role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Conflict of interest. None declared.
References
- Andari E., Duhamel J.R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Science of the United States of America 107(9), 4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntz A., Dietzel R., Dreessen L. (1999). Assumptions in borderline personality disorder: specificity, stability and relationship with etiological factors. Behaviour Research and Therapy 37(6), 545–57. [DOI] [PubMed] [Google Scholar]
- Arntz A., ten Haaf J. (2012). Social cognition in borderline personality disorder: evidence for dichotomous thinking but no evidence for less complex attributions. Behaviour Research and Therapy 50(11), 707–18. [DOI] [PubMed] [Google Scholar]
- Arntz A., Veen G. (2001). Evaluations of others by borderline patients. The Journal of Nervous and Mental Disease 189(8), 513–21. [DOI] [PubMed] [Google Scholar]
- Arntz A., Weertman A., Salet S. (2011). Interpretation bias in Cluster-C and borderline personality disorders. Behaviour Research and Therapy 49(8), 472–81. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Barnow S., Stopsack M., Grabe H.J., et al. (2009). Interpersonal evaluation bias in borderline personality disorder. Behaviour Research and Therapy 47(5), 359–65. [DOI] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Science 15(7), 301–9. [DOI] [PubMed] [Google Scholar]
- Bender D.S., Skodol A.E. (2007). Borderline personality as a self-other representational disturbance. Journal of Personality Disorders 21(5), 500–17. [DOI] [PubMed] [Google Scholar]
- Berger C., Winkels M., Lischke A., Hoppner J. (2012). GazeAlyze: a MATLAB toolbox for the analysis of eye movement data. Behaviour Research and Therapy 44(2), 404–19. [DOI] [PubMed] [Google Scholar]
- Bertsch K., Gamer M., Schmidt B., et al. (2013). Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. The American Journal of Psychiatry 170(10), 1169–77. doi: 10.1176/appi.ajp.2013.13020263. Erratum in: Am J Psychiatry 70(10), 1218. [DOI] [PubMed] [Google Scholar]
- Bethlehem R.A., van Honk J., Auyeung B., Baron-Cohen S. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38(7), 962–74. [DOI] [PubMed] [Google Scholar]
- Churchland P.S., Winkielman P. (2012). Modulating social behavior with oxytocin: how does it work? What does it mean? Hormones and Behavior 61(3), 392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodhia S., Hosanagar A., Fitzgerald D.A., et al. (2014). Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 39(9), 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Czieschnek D., Weidler F., Berger C., Fast K., Herpertz S.C. (2008). Recognition of facial affect in borderline personality disorder. Journal of Personality Disorders 22(2), 135–47. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Kumbier E., Grossmann A., Hauenstein K., Herpertz S.C. (2013). Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry 74(3), 164–71. [DOI] [PubMed] [Google Scholar]
- Domes G., Kumbier E., Heinrichs M., Herpertz S.C. (2014). Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with Asperger syndrome. Neuropsychopharmacology 39(3), 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Lischke A., Berger C., et al. (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Donegan N.H., Sanislow C.A., Blumberg H.P., et al. (2003). Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry 54(11), 1284–93. [DOI] [PubMed] [Google Scholar]
- Dyck M., Habel U., Slodczyk J., et al. (2009). Negative bias in fast emotion discrimination in borderline personality disorder. Psychological Medicine 39(5), 855–64. [DOI] [PubMed] [Google Scholar]
- Gamer M., Schmitz A.K., Tittgemeyer M., Schilbach L. (2013). The human amygdala drives reflexive orienting towards facial features. Current Biology 23(20), R917–8. [DOI] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Buchel C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Science of the United States of America 107(20), 9400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15(4), 870–8. [DOI] [PubMed] [Google Scholar]
- Giesen-Bloo J., Arntz A. (2005). World assumptions and the role of trauma in borderline personality disorder. Journal of Behavior Therapy and Experimental Psychiatry 36(3), 197–208. [DOI] [PubMed] [Google Scholar]
- Glascher J. (2009). Visualization of group inference data in functional neuroimaging. Neuroinformatics 7(1), 73–82. [DOI] [PubMed] [Google Scholar]
- Grimm S., Schmidt C.F., Bermpohl F., et al. (2006). Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. Neuroimage 30(1), 325–40. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Mitchell P.B., Dadds M.R. (2008). Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry 63(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Gunderson J.G. (2007). Disturbed relationships as a phenotype for borderline personality disorder. The American Journal of Psychiatry 164(11), 1637–40. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry 53(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Hazlett E.A., Zhang J., New A.S., et al. (2012). Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biological Psychiatry 72(6), 448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz S.C., Bertsch K. (2015). A new perspective on the pathophysiology of borderline personality disorder: a model of the role of oxytocin. The American Journal of Psychiatry 172(9), 840–51. [DOI] [PubMed] [Google Scholar]
- Herpertz S.C., Dietrich T.M., Wenning B., et al. (2001). Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry 50(4), 292–8. [DOI] [PubMed] [Google Scholar]
- Jayaro C., De La Vega I., Bayon-Palomino C., et al. (2011). Depressive-type emotional response pattern in impulsive -aggressive patients with borderline personality disorder. Journal of Affective Disorders 135(1-3), 37–42. [DOI] [PubMed] [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience 25(49), 11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Denny B.T., Fan J., et al. (2014). The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. The American Journal of Psychiatry 171(1), 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Fan J., Ochsner K.N., et al. (2009). Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological Psychiatry 66(9), 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A., Oei N.Y., Niedtfeld I., et al. (2012). Influence of emotional distraction on working memory performance in borderline personality disorder. Psychological Medicine 42(10), 2181–92. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A., Winter D., Niedtfeld I., Schmahl C. (2014). The latest neuroimaging findings in borderline personality disorder. Current Psychiatry Reports 16(3), 438.. [DOI] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35(12), 2403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2005) International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-6. University of Florida, Gainesville, FL.
- Leichsenring F., Leibing E., Kruse J., New A.S., Leweke F. (2011). Borderline personality disorder. Lancet 377(9759), 74–84. [DOI] [PubMed] [Google Scholar]
- Lischke A., Berger C., Prehn K., Heinrichs M., Herpertz S.C., Domes G. (2012a). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology 37(4), 475–81. [DOI] [PubMed] [Google Scholar]
- Lischke A., Gamer M., Berger C., et al. (2012b). Oxytocin increases amygdala-dependent threat-processing in females. European Journal of Psychotraumatology 3(Suppl), p 46–7. [Google Scholar]
- Lischke A., Gamer M., Berger C., et al. (2012c). Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 37(9), 1431–8. [DOI] [PubMed] [Google Scholar]
- Mauchnik J., Schmahl C. (2010). The latest neuroimaging findings in borderline personality disorder. Current Psychiatry Reports 12(1), 46–55. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neurosciences 12(9), 524–38. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I., Schulze L., Kirsch P., Herpertz S.C., Bohus M., Schmahl C. (2010). Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biological Psychiatry 68(4), 383–91. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.J., Moskowitz D.S., Zuroff D.C., Sookman D., Paris J. (2007). Stability and variability of affective experience and interpersonal behavior in borderline personality disorder. Journal of Abnormal Psychology 116(3), 578–88. [DOI] [PubMed] [Google Scholar]
- Sadikaj G., Russell J.J., Moskowitz D.S., Paris J. (2010). Affect dysregulation in individuals with borderline personality disorder: persistence and interpersonal triggers. Journal of Personality Assessment 92(6), 490–500. [DOI] [PubMed] [Google Scholar]
- Scheele D., Striepens N., Kendrick K.M., et al. (2014). Opposing effects of oxytocin on moral judgment in males and females. Human Brain Mapping 35(12), 6067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L., Domes G., Kruger A., et al. (2011). Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biological Psychiatry 69(6), 564–73. [DOI] [PubMed] [Google Scholar]
- Stanley B., Siever L.J. (2010). The interpersonal dimension of borderline personality disorder: toward a neuropeptide model. The American Journal of Psychiatry 167(1), 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglmayr C.E., Grathwol T., Linehan M.M., Ihorst G., Fahrenberg J., Bohus M. (2005). Aversive tension in patients with borderline personality disorder: a computer-based controlled field study. Acta Psychiatrica Scandinavica 111(5), 372–9. [DOI] [PubMed] [Google Scholar]
- Striepens N., Scheele D., Kendrick K.M., et al. (2012). Oxytocin facilitates protective responses to aversive social stimuli in males. Proceedings of the National Academy of Science of the United States of America 109(44), 18144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Phan K.L., Decker L.R., Liberzon I. (2003). Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 18(3), 650–9. [DOI] [PubMed] [Google Scholar]
- Toffoletto S., Lanzenberger R., Gingnell M., Sundstrom-Poromaa I., Comasco E. (2014). Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology 50, 28–52. [DOI] [PubMed] [Google Scholar]
- Tost H., Kolachana B., Verchinski B.A., et al. (2011). Neurogenetic effects of OXTR rs2254298 in the extended limbic system of healthy Caucasian adults. Biological Psychiatry 70(9), e37–9. author reply e41–32. [DOI] [PubMed] [Google Scholar]
- Wagner A.W., Linehan M.M. (1999). Facial expression recognition ability among women with borderline personality disorder: implications for emotion regulation? Journal of Personality Disorders 13(4), 329–44. [DOI] [PubMed] [Google Scholar]
- Walum H., Waldman I.D., Young L.J. (2016). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological Psychiatry 79(3), 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigton R., Radua J., Allen P., et al. (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. Journal of Psychiatry & Neuroscience 40(1), E1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.