Abstract
Empathy, the ability to understand others’ emotions, can occur through perspective taking and experience sharing. Neural systems active when adults empathize include regions underlying perspective taking (e.g. medial prefrontal cortex; MPFC) and experience sharing (e.g. inferior parietal lobule; IPL). It is unknown whether adolescents utilize networks implicated in both experience sharing and perspective taking when accurately empathizing. This question is critical given the importance of accurately understanding others’ emotions for developing and maintaining adaptive peer relationships during adolescence. We extend the literature on empathy in adolescence by determining the neural basis of empathic accuracy, a behavioral assay of empathy that does not bias participants toward the exclusive use of perspective taking or experience sharing. Participants (N = 155, aged 11.1–15.5 years) watched videos of ‘targets’ describing emotional events and continuously rated the targets’ emotions during functional magnetic resonance imaging scanning. Empathic accuracy related to activation in regions underlying perspective taking (MPFC, temporoparietal junction and superior temporal sulcus), while activation in regions underlying experience sharing (IPL, anterior cingulate cortex and anterior insula) related to lower empathic accuracy. These results provide novel insight into the neural basis of empathic accuracy in adolescence and suggest that perspective taking processes may be effective for increasing empathy.
Keywords: empathy, adolescence, perspective taking, empathic accuracy, experience sharing
Introduction
Empathy, or the ability to understand others’ emotions, is a critical skill for effective social interaction and may be a first step toward altruistic behavior (Eisenberg et al., 1991; Knafo et al., 2008). Adolescents place greater value on relationships with peers relative to those with family, which is reflected in part by an increasing proportion of time spent with peers (Brown, 2004). This change likely necessitates greater use of complex social communication skills such as empathy, as family relationships entail interactions with familiar people, whereas adolescents form new types of relationships with their peers. Research also indicates that adolescents with greater empathy have less internalizing problems and are less often the victims of bullying (Gleason et al., 2009). Thus, it is critical to understand the processes leading to successful empathy to improve adolescents’ well-being.
Zaki and Ochsner (2012) describe two processes supporting empathy: perspective taking (PT) and experience sharing (ES). Experience sharing (or affective empathy; Zahn-Waxler et al., 1992) involves an affective response whereby a perceiver vicariously feels an emotion similar to another person. Research targeting neural processes associated with ES—for example, using stimuli depicting bodily injury—indicates that this process involves activation in some of the same regions that are active during first-hand experience in adults (Singer et al., 2004; Hein and Singer, 2008; Van Overwalle and Baetens, 2009) and in children aged 7–12 years (Decety et al., 2008). Regions associated with ES include inferior parietal lobule (IPL), anterior cingulate cortex (ACC) and premotor cortex (PMC; Zaki and Ochsner, 2012), as well as anterior insula (AI; Singer et al., 2004). AI has been implicated in ES specifically for pain, and with ACC it is involved in first-hand experience of pain (Hein and Singer, 2008). Activation in PMC and IPL is associated with ES in tasks involving motor action (Chong et al., 2008), particularly when intention can be inferred (Iacoboni et al., 2005). PMC is also involved in motor planning and control (Lamm et al., 2011; Culham, 2015).
Perspective taking (or cognitive empathy) does not require an affective response but rather inferring another person’s thoughts (Ruby and Decety, 2004). Activation in regions including medial prefrontal cortex (MPFC), precuneus, superior temporal sulcus (STS) and right temporoparietal junction (RTPJ) relates to PT when adults infer a target’s thoughts based on actual or implied motion (Allison et al., 2000), written narratives and cartoon vignettes (Gallagher et al., 2000). Research in adolescents with cartoon vignettes (Sebastian et al., 2012), pictures of positive and negative social interactions (Overgaauw et al., 2014) and simulated interactions with avatars (i.e. Cyberball; Gunther Moor et al., 2012; Masten et al., 2013) has also revealed activation in MPFC, RTPJ and STS. Additionally, this network relates to cognitive processes such as autobiographical memory, prospection and task-unrelated processing (i.e. ‘default mode’ activation; Spreng et al., 2008). Thus, the neural regions associated with PT likely serve a more general function of inferring goals (Buckner and Carroll, 2007; Van Overwalle and Baetens, 2009).
These two subcomponents of empathy, PT and ES, are thus associated with activation in distinct neural networks. Notably, regions associated with PT (and, to a lesser extent, ES) undergo substantial changes during adolescence. The neural regions underlying PT mature much later than those underlying ES, and the MPFC in particular does not fully mature up to 25 years of age (Singer, 2006). There are also developmental differences between adolescents and adults in resting state (Blakemore, 2012) and task-based (Burnett and Blakemore, 2009) connectivity of regions within the network underlying PT, and with intrinsic connectivity of the ACC (Kelly et al., 2009). Moreover, behavioral data indicates adolescents perform worse than adults on a PT task (Dumontheil et al., 2010).
Given these neurodevelopmental differences and the importance of peer relationships during this period, it is important to characterize the neural correlates of empathy in adolescents. Although the studies described earlier have investigated the neural basis of PT (Masten et al., 2009; Gunther Moor et al., 2012; Sebastian et al., 2012) and witnessing others’ pain (Decety et al., 2008) in adolescents, none have utilized complex, realistic social stimuli. One exception is the study by Overgaauw et al., which included posed photographs of social scenes that provided more complex information; however, this study focused on punishment behavior (in the dictator game) and differences between positive and negative scenes, rather than the accuracy by which participants understood others’ emotions. The majority of research to date in adolescents has focused on isolating brain activation related to either ES or PT with paradigms targeting one of these two processes. For example, while Cyberball and cartoon vignettes provide valuable insight into mechanisms underlying PT, these stimuli do not permit direct access to another person’s emotional expressions and the opportunity for simulation that is a precursor of ES (Sato et al., 2013). Conversely, tasks using images of bodily injury (Decety et al., 2008) or the pictures of eyes used in the mind in the eyes paradigm (Baron-Cohen et al., 1997) might allow for mimicry but lack a narrative component that is present in PT tasks. To fully understand empathy, we need to move beyond reductive paradigms to study how these two processes support empathic behavior with realistic social stimuli.
Empathic accuracy paradigms provide ecological validity through the use of videos of a real person (the ‘target’) expressing actual emotions while sharing emotional memories. In the empathic accuracy task, participants have access to contextual, narrative information by way of the story told in the video, have the opportunity for simulation from the emotional facial expressions in the video and are not instructed to use a cognitive (i.e. PT) or affective (i.e. ES) strategy. Thus, participants are able to utilize either PT or ES. In this way, empathic accuracy paradigms are theory-neutral with respect to the type of process engaged during empathy. Indeed, neuroimaging research in adults demonstrates that the complex video stimuli in the empathic accuracy task elicit activation in regions involved in PT (e.g. STS, TPJ and MPFC) and ES (e.g. IPL and bilateral dorsal PMC; Zaki et al., 2009).
In addition to allowing use of either a PT or ES strategy, empathic accuracy tasks are grounded in a behavioral measure critically important for peer relations and adjustment in young adolescents. Empathic accuracy is measured from the correlation between perceiver’s ratings of a target’s emotions relative to the target’s ratings of their own emotions in the video. Adolescents (average age 12.2 years) with lower scores on this behavioral measure of empathy (empathic accuracy) were more likely to suffer from bullying and internalizing problems such as depression, while higher empathic accuracy mitigated the negative impact of poor peer relations on personal adjustment (Gleason et al., 2009). Furthermore, this study showed that self-reported empathy did not relate to outcomes such as depression and bullying (consistent with the finding that self-reported empathy is not correlated with empathic accuracy in adults; Simpson et al., 2003). Thus, empathic accuracy affords a unique behavioral measure with important consequences for young adolescents.
In this study, we expanded on prior research on empathy in adolescence to determine the neural systems supporting empathic accuracy during functional magnetic resonance imaging (fMRI) scanning. We investigated whether adolescents would have greater empathic accuracy when activating networks underlying ES and PT by using an empathic accuracy paradigm that allowed us to uniquely examine the relationship between brain activation and behavior. We hypothesized that adolescents would recruit regions related to ES (e.g. IPL, ACC, AI and PMC) when making empathically accurate responses since regions underlying PT (e.g. MPFC, RTPJ and STS) are not fully developed at this age, and behavioral research indicates that PT skills are still developing during adolescence (Dumontheil et al., 2010; Crone, 2013). Since previous research indicates empathic accuracy provides a behavioral measure of empathy that is not fully captured by self-report measures of empathy (Simpson et al., 2003; Gleason et al., 2009), we hypothesized that empathic accuracy would not be strongly related to self-reported empathic concern nor PT. Additionally, given the mixed evidence on gender differences in empathy (Ickes et al., 2000; Michalska et al., 2013; Van der Graaff et al., 2014) we did not have a specific hypothesis with regard to gender differences in empathy, and rather we capitalized on the large sample size in this study to thoroughly investigate gender differences across multiple measures of empathy in exploratory analyses.
Materials and methods
Participants
We recruited 192 healthy adolescents (average age 12.8 years ± 8.8 months, age range 11.1–15.5 years, 69 female) from the Madison, WI community online and in local print and broadcast media to participate in a study on the impact of games on behavior and brain function. Participants had to be enrolled in the 7th or 8th grade, fluent English speakers, safe for MRI scanning, not using psychotropic medications and with no current or previous diagnosis of a mental illness. The results presented here are from parcticipants’ first visit (baseline data), which was collected prior to randomization to an intervention in the larger randomized controlled trial in which this study was embedded. Participants completed other tasks as a part of the larger study to measure performance in domains we expected to be affected by the intervention, such as response inhibition measured with a Stroop task. Thirty-three participants’ data were unusable due to technical issues (21), inability to see or hear the stimuli (8) or not completing the task (4). Thus, the sample size for analysis of empathic accuracy was 159 (average age 13.3 years ± 8.3 months, age range 11.1–15.5 years, 54 female). UW-Madison’s Health Sciences Institutional Review Board (IRB) approved the protocol, and all participants provided informed assent and were given monetary compensation for participating. Legal guardians provided informed consent.
Questionnaires
Participants completed a battery of questionnaires as a part of the larger study of the impact of games on behavior and brain function, including the interpersonal reactivity index (IRI; Davis, 1983) and the prosocial norms survey (PNS; The American National Election Studies (ANES), 2015). The IRI contains four subscales, two of which measure constructs related to empathy and are relevant for the current analysis: empathic concern and PT. We also explored the personal distress subscale in a posthoc manner. The PNS provided a measure of adherence to prosocial norms in which participants select one of five responses from ‘Strongly Disagree’ to ‘Strongly Agree’ with ‘Disagree’, ‘Neither Agree nor Disagree’ and ‘Agree’ in between the two polar options. Example items from this scale include ‘it is important to help one another so that the community in general is a better place’ and ‘these days people need to look after themselves and not overly worry about others’ (the latter example being reverse scored). Following completion of the study, participants were asked to retroactively rate their pubertal development using the Tanner Scale (Taylor et al., 2001). Since participants were recontacted the sample size for the Tanner scale was further reduced but this did not affect the sample size for any of the other measures. In total, 105 participants who completed the empathic accuracy task also completed the post-study questionnaire (average age 13.4 years ± 8.5 months, 34 female).
Empathic accuracy task
The empathic accuracy task was created by Ickes (1993) to assess a participant’s ability to judge the emotions of a target person presented in a video. Participants’ (the ‘perceivers’) empathic accuracy scores are based on the targets’ ratings of their own emotions. The task used in this study was based on a version adapted by Zaki et al. (2009) for use during fMRI scanning (Figure 1). The only modifications to the task for this study were to present videos of older adolescent targets (aged 18–21 years) describing events from their adolescence to appear more relevant to the adolescent perceivers (i.e. rather than adults). Targets were videotaped while discussing emotional events such as the death of a grandparent or winning a sports competition. Targets were instructed to think of the 4 most negative and 4 most positive events from their adolescence, which they had time to recollect and write down before the videos were recorded. For each video, the targets were randomly cued to one of the events, and given a few minutes to read their recollections and put themselves in that moment before retelling on video. Targets did not read from their written responses but simply used them as a reminder. After recording, the targets watched their own videos and made ratings of their emotions as displayed in the videos, which served as the ‘correct’ response to which perceivers’ ratings were compared. The ratings were collected on a Likert scale from 1 to 9 with ‘Very Negative’ at 1 on the left and ‘Very Positive’ at 9 on the right (Figure 1A). The targets were asked to adjust their rating every time their emotion changed in the video, and ratings were collected continuously throughout the entire video.
Fig. 1.
Empathic accuracy task. Targets were filmed describing emotional events from their adolescence. The target then watched his or her videos while continuously rating his or her emotions as expressed in the video on a scale from ‘Very Negative’ to ‘Very Positive’ (A). These videos were shown to participants in this study, the perceivers, during an fMRI scan. Perceivers made continuous ratings of the target’s emotions in the video on the same rating scale used by the targets (B). Finally, empathic accuracy scores were determined by calculating the correlation between the perceiver’s ratings with the target’s ratings of his or her emotions (C).
Eight videos were recorded of 19 different target individuals, and for each target individual there were eight videos evenly split between descriptions of negative and positive events from their adolescence. This resulted in a total of 154 videos from which we selected two sets of 18 videos for the empathic accuracy task. Thus, 36 different videos were used in total. Participants were shown one set of videos, and the set used was counterbalanced across participants. The particular videos for each set were selected based on a number of criteria. We needed to select an equal number of male and female targets from as diverse a set of ethnic backgrounds as possible. Targets needed to have at least 2 positive and 2 negative videos, so that the same targets appeared across video sets and valences. Videos could not include content deemed inappropriate for minors (e.g. swearing). Finally, we had to organize the videos into subsets for each of the three scan runs (for each of the two video sets), such that each subset of six videos (per scan run) included an equal number of videos by valence and gender and the total length for each run was equivalent.
Participants in this study (identified as ‘perceivers’ in Figure 1) completed the empathic accuracy task in three fMRI scan runs, each lasting ∼5 min. In the empathic accuracy fMRI task, a cue word was displayed for 3 s, followed by a fixation cross for 2 s and then a video, which ranged from 28 to 144 s (mean = 90 s, Figure 1B). The cue instructed participants how to rate the videos, corresponding to three different conditions, and ratings were made continuously throughout the videos. If the cue ‘OTHER’ appeared participants were instructed to rate the emotion of the target in the video from negative to positive, exactly as the targets had rated themselves. If the cue ‘SELF’ appeared participants were instructed to rate their own emotions from negative to positive using the same scale. If the cue ‘GAZE’ appeared participants were instructed to rate the direction of the target’s eye-gaze from left to right using a 1–9 Likert scale with 1 labeled ‘Left’ and 9 labeled ‘Right’. The order of conditions was pseudorandomized such that participants saw a different order of six trial blocks in each of the task runs, and targets were one-half male and one-half female. There were 18 trial blocks, six per condition, and each had a unique video stimulus. This report focuses on the ‘OTHER’ condition, which corresponded to empathic accuracy trials. Prior to the scan participants completed three practice trials with videos not used in the fMRI task. Empathic accuracy was determined from the OTHER trials by calculating the correlation between the time-course of target and perceiver ratings for each video (Figure 1C), and then r- to Z-transforming them with Fisher’s method. One trial was excluded due to large disagreement between the target’s rating and the majority of participant ratings, which resulted in extremely low average empathic accuracy scores across participants (mean r < 0.10).
Statistical analysis
See Supplementary Methods for information on MRI data acquisition and processing. We studied the relationship between blood–oxygen-level dependent (BOLD) activation and empathic accuracy across trials, within subject for ‘OTHER’ trial blocks. For the first level, time series analysis for each of 18 blocks was modeled with a separate regressor. Only the six estimates for the ‘OTHER’ trial blocks provided a measure of empathic accuracy and were considered in higher-level analyses. In a within subject, fixed effects analysis, the linear relationship between the block-based BOLD activation and within-block empathic accuracy was estimated. The group analysis then estimated the average of this linear slope across subjects in a mixed effects Flame 1 analysis. The number of ratings made per minute for each trial was an additional regressor of no interest to control for differences in the amount of ratings. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 3.1 and a (corrected) cluster significance threshold of P = 0.05 (Worsley, n.d.).
We conducted region of interest (ROI) analyses using 12 a priori ROIs previously related to PT, ES or empathic accuracy (right ventral and left dorsal MPFC—VMPFC and DMPFC, right STS and ACC, two right IPL ROIs and right and left TPJ, PMC and AI). ROIs were defined as 6 mm spheres from coordinates provided in the Supplementary Material by Zaki et al. (2009), which to our knowledge is the only study to determine the neural correlates of empathic accuracy in healthy adults. The ROI analysis allowed us to directly replicate the analysis used by Zaki et al., and to test whether ROIs related to PT vs ES differentially contributed as a set to empathic accuracy in adolescents. We extracted the mean percent signal change for each participant from the empathic accuracy-modulated contrast, and tested the mean for each ROI against zero using a two-tailed t-test. We controlled for multiple comparisons using family-wise error control over the 12 ROIs according to maximum-t based null estimated non-parametrically with a total of 5000 permutations (indicated by P*; Nichols and Holmes, 2002). All other reported statistics correspond to uncorrected results. To test whether activation in a set of regions underlying PT (VMPFC, DMPFC, STS, right and left TPJ) had a stronger relationship with empathic accuracy than regions underlying ES (two IPL ROIs, right and left AI and PMC and ACC), we computed the average of the empathic accuracy-related activations across each set. We then ran a repeated measures linear mixed effects model including the network type (ES or PT) as a fixed effect and subject number as a random effect using the lmer function from the lme4 library (Bates et al., 2015) in the statistical analysis software R (version 3.2.2; R Core Team, 2015). P-value computation used the modelSummary function of the lmSupport package, which is based on the Kenward–Rogers approximation for degrees of freedom (Kenward and Roger, 1997).
Results are reported after removing outliers based on Cook’s D using a cutoff threshold of 4/(N-P) for data points disconnected from the distribution, and outliers were removed from the corresponding figures. All results remained the same without outliers removed, except in three cases as indicated in the results. We tested for gender differences at each stage of the analysis and controlled for gender in analyses where we found gender differences in one or more variables. The results specify when gender was included as a covariate, and all the significant results remain without this covariate.
Two participants were removed from all group analyses due to extreme motion across multiple scan runs (defined as runs with a frame-wise displacement > 0.9 mm in over 25% of the time points), and two participants were removed due to extreme signal intensity based on the Cook’s D threshold across t-tests assessing whether the mean brain activation for each of the ROIs (across participants) was different from zero. Thus, the sample size for analysis with fMRI data was 155 (average age = 12.8 years ± 8.6 months, N = 53 female). Of the participants with fMRI data, 127 were right-handed, 11 were left-handed, 5 were ambidextrous and the remaining 12 did not provide handedness information. All results remain the same when limited to right-handed participants, except in one case where the effect became marginal, which is indicated in the results.
Results
Empathic accuracy and self-report measures
The average r value for empathic accuracy across all participants (prior to Fisher transformation) was 0.68 (standard deviation (s.d.) = 0.25, range = −0.56 to 0.99). All analyses used Fisher Z-transformed empathic accuracy to normalize the distribution of correlation values. Females (M = 1.03, s.d. = 0.18) had significantly higher empathic accuracy than males [M = 0.96, s.d. = 0.14; t(152) = −2.21, P = 0.029, b = −0.08, confidence interval (CI) = [−0.14, −0.01], 5 outliers removed; Figure 2]. The gender difference in empathic accuracy remained significant while controlling for pubertal level [t(101) = −2.67, P = 0.009, b = −0.12, CI = [−0.21, −0.03], 1 outlier removed]; however, it dropped to a trend level when controlling for age [t(151) = −1.67, P = 0.097, b = −0.06, CI = [−0.13, 0.01], 5 outliers removed]. This effect also became marginal without outliers removed (P = 0.051). There was no gender difference in age [t(153) = −0.24, P = 0.81, b = −0.31, CI = [−2.81, 2.20], 4 outliers removed].
Fig. 2.
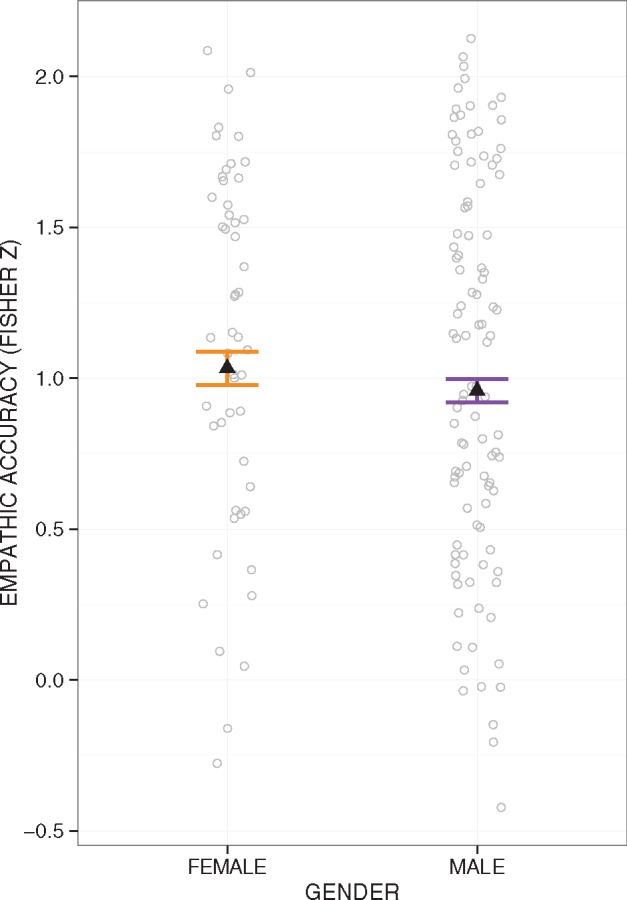
Empathic accuracy by gender. Females had greater empathic accuracy than males [t(152) = −2.21, P = 0.029]. Error bars represent 95% CIs above and below the point estimates of the means (displayed as triangles). Raw data points are overlaid in grey.
We found gender differences in the questionnaire measures, all of which had one outlier removed. Self-reported empathic concern (IRI; M = 17.27, s.d. = 4.16) was higher in females (M = 19.30, s.d. = 3.47) than males (M = 16.37, s.d. = 4.04), t(156) = −3.93, P < 0.001, b = −2.63, CI = [−3.93, −1.31] (Supplementary Figure S1A). Self-reported PT (IRI; M = 14.75, s.d. = 4.56) was higher in females (M = 15.77, s.d. = 3.89) than males (M = 14.13, s.d. = 4.67), t(156) = −2.47, P = 0.015, b = −1.87, CI = [−3.36, −0.37] (Supplementary Figure S1B). Self-reported adherence to prosocial norms (PNS; M = 31.66, s.d. = 3.79) was higher in females (M = 32.79, s.d. = 3.87) than males (M = 31.19, s.d. = 3.51), t(156) = −2.19, P = 0.030, b = −1.38, CI = [−2.63, −0.13] (Supplementary Figure S1C). All of these gender differences remained significant when controlling for either pubertal development or age.
In separate analyses, we regressed empathic accuracy on self-reported empathic concern, PT or prosocial norms while controlling for gender. Greater empathic accuracy was not associated with more empathic concern [t(149) = 1.61, P = 0.109, b = 0.01, CI = [−0.001, 0.015], 7 outliers removed; Supplementary Figure S2A]; however, this effect was significant with outliers included (P = 0.029) or if gender was not included as a covariate (P = 0.017). Empathic accuracy was not related to self-reported PT [t(150) = 1.41, P = 0.161, b = 0.005, CI = [−0.002, 0.01], 6 outliers removed; Supplementary Figure S2B] or with self-reported adherence to prosocial norms [t(152) = 0.75, P = 0.453, b = 0.004, CI = [−0.01, 0.01], 4 outliers removed; Supplementary Figure S2C] unless the outliers were included (P = 0.031).
We conducted a posthoc analysis regressing empathic accuracy on personal distress while controlling for gender (M = 10.79, s.d. = 3.62) and did not find a significant relationship [t(153) = 0.44, P = 0.661, b = 0.002, CI = [−0.01, 0.01], 3 outliers removed].
Voxel-wise analysis of empathic accuracy
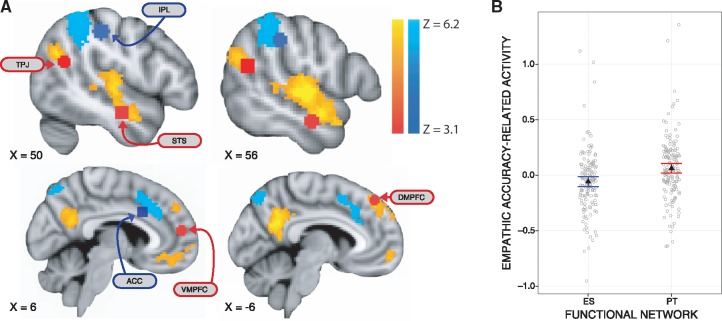
A voxel-wise analysis of the relationship between BOLD activation and empathic accuracy revealed that empathic accuracy related to greater activation in regions underlying PT, including VMPFC, DMPFC, RTPJ, precuneus and STS (depicted in orange/yellow, Figure 3A). Conversely, in regions underlying ES, including ACC and right IPL, activation was related to lower empathic accuracy (or greater empathic accuracy related to less activation in these regions; depicted in blue in Figure 3A). A summary of clusters with activation related to empathic accuracy is provided in Table 1. We ran a voxel-wise independent samples t-test between males and females and did not identify any significant gender differences in the relationship between empathic accuracy and BOLD activation.
Fig. 3.
BOLD activation related to empathic accuracy. Voxel-wise, wholebrain analysis (A) with regions where increased activation related to higher empathic accuracy are in orange/yellow, and regions where activation related to lower empathic accuracy are in light blue, thresholded using clusters determined by Z > 2.3 and a corrected threshold of P < 0.05. A priori ROIs are labeled and overlaid in red for regions related to PT and in dark blue for regions related to ES, as provided in Zaki et al. (2009), although not all 12 ROIs are depicted here. The ROIs are the same in the top of the panel. The underlay and coordinates are in MNI space. TPJ, temporal parietal junction; IPL, MNI, Montreal Neurological Institute; inferior parietal lobule; STS, superior temporal sulcus; VMPFC, ventral medial prefrontal cortex; DMPFC, dorsal medial prefrontal cortex; ACC, anterior cingulate cortex. ROI analysis (B) depicting that empathic accuracy-related activation across PT network was greater than ES network [F(1,151) = 28.8, P < 0.001]. Error bars represent 95% CIs above and below the point estimates of the means (displayed as triangles). Raw data are overlaid in grey.
Table 1.
Coordinates are provided in MNI space (mm) and Z-values were extracted from the group level analysis of the interaction of empathic accuracy (EA) with BOLD, thresholded using clusters determined by Z > 2.3 and a corrected threshold of P < 0.05
| Region | Max intensity (Z) | Peak coordinates |
Volume (mm) | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Empathic accuracy-related activation | Right STS | 6.11 | 56 | −14 | 0 | 1807 |
| Precuneus | 5.6 | −4 | −62 | 22 | 1024 | |
| Left STS | 6.05 | −58 | −16 | −8 | 1013 | |
| VMPFC | 5.7 | −2 | 56 | −12 | 516 | |
| Left temporal parietal junction | 5.47 | −46 | −56 | 26 | 485 | |
| DMPFC | 4.78 | −8 | 40 | 52 | 373 | |
| RTPJ | 4.98 | 50 | −60 | 26 | 280 | |
| Empathic inaccuracy-related activation | Right superior parietal lobule | 5.23 | 44 | −44 | 54 | 1161 |
| ACC | 5.14 | 4 | 22 | 36 | 862 | |
| Right dorsolateral prefrontal cortex | 4.46 | 44 | 34 | 30 | 513 | |
| Left lateral occipital cortex | 4.72 | −20 | −72 | 48 | 492 | |
| Left superior parietal lobule | 4.47 | −40 | −46 | 48 | 357 | |
| Right lateral occipital cortex | 4.27 | 16 | −68 | 48 | 293 | |
| Superior/middle frontal gyrus | 3.75 | 34 | 2 | 56 | 199 | |
| Left dorsolateral prefrontal cortex | 4.01 | −40 | 46 | 24 | 143 | |
| Right superior parietal lobule | 3.87 | 10 | −52 | 54 | 46 | |
ROI analysis of empathic accuracy
The results of the ROI analyses paralleled the voxel-wise results, such that higher empathic accuracy was related to greater activation in regions underlying PT: STS [t(152) = 3.49, P* = 0.006, P = 0.001, b = 0.11, 2 outliers removed] and VMPFC at a trend level [t(152) = 2.71, P* = 0.073, P = 0.008, b = 0.07, 2 outliers removed]. In contrast, greater activation in regions underlying ES related to lower empathic accuracy: ACC [t(150) = −2.48, P* = 0.042, P = 0.014, b = −0.12, 4 outliers removed], right IPL [t(153) = −2.90, P* = 0.038, P = 0.004, b = −0.11, 1 outlier removed], right AI [t(149) = −4.83, P* < 0.001, P < 0.001, b = −0.10, 5 outliers removed] and left AI [t(152) = −3.34, P* = 0.008, P = 0.001, b = −0.10, 2 outliers removed]. There were no significant gender differences in empathic accuracy-related activation in any of the ROIs.
We compared activation across ROIs in the network associated with PT to those associated with ES. Mean activation extracted from the set of PT regions was associated with greater empathic accuracy than activation from ES regions, F(1,150) = 26.8, P < 0.001, b = 0.12, CI = [0.07, 0.16] (Figure 3B). BOLD activation within the PT regions was significantly positively related to empathic accuracy [t(150) = 2.70, P = 0.008, b = 0.06, CI = [0.02, 0.11]]. Conversely, activation within the ES network was significantly negatively associated with empathic accuracy [t(150) = −2.55, P = 0.012, b = −0.06, CI = [−0.10, −0.01]]. All three results are reported with four outliers removed but remain the same if including these outliers.
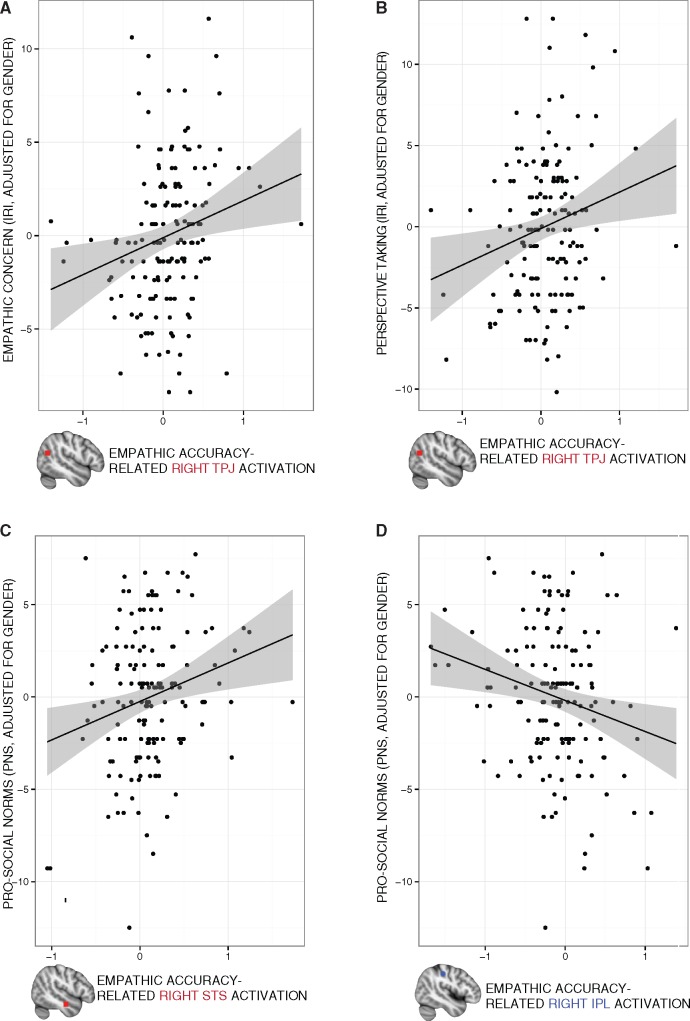
We tested whether individual differences in empathic accuracy-related activation in each of the 12 ROIs related to self-reported empathic concern, PT or adherence to prosocial norms (while controlling for gender). Greater empathic accuracy-related activation in RTPJ was associated with greater empathic concern [t(149) = 3.02, P* = 0.045, P = 0.003, b = 2.33, CI = [0.81, 3.86], 3 outliers removed; Figure 4A] and only marginally with PT [t(148) = 2.15, P* = 0.066, P = 0.034, b = 2.15, CI = [0.16, 3. 80], 4 outliers removed; Figure 4B]. Greater empathic accuracy-related activation in STS was associated with higher adherence to prosocial norms at a trend level [t(151) = 2.79, P* = 0.054, P = 0.006, b = 2.08, CI = [0.61, 3.56], 1 outlier removed; Figure 4C]. Conversely, greater empathic accuracy-related activation in right IPL was marginally associated with lower self-reported adherence to prosocial norms [t(151) = −2.74, P* = 0.066, P = 0.007, b = −1.70, CI = [−2.92, −0.47], 1 outlier removed; Figure 4D].
Fig. 4.
Empathic accuracy-related activation and questionnaire measures. Greater empathic accuracy-related activation extracted from RTPJ was associated with higher self-reported empathic concern [A; t(151) = 2.70, P = 0.008] and PT [B; t(151) = 2.59, P = 0.010]. Greater self-reported adherence to prosocial norms was associated with more empathic accuracy-related activation extracted from right STS [C; t(151) = 2.79, P = 0.006], and the opposite relationship was found for right IPL [D; t(151) = −2.74, P = 0.007]. Regression lines and data points are adjusted for gender. Precision envelopes represent 95% CIs above and below the point estimates.
We performed a posthoc analysis to separately test for relationships between empathic accuracy-related activation in the 12 ROIs and self-reported personal distress. There was no relationship between personal distress and empathic accuracy-related activation in RTPJ (P = 0.364), STS (P = 0.838) or right IPL (P = 0.151) or with any of the other ROIs.
Discussion
This study provides the first evidence that empathic accuracy in adolescents is associated with greater BOLD activation in a network implicated in PT. Previous work also implicates regions underlying PT in adolescents’ empathic processing; however, these studies used tasks lacking realistic stimuli and opportunities for ES through mimicry (Blakemore, 2008; Masten et al., 2009; Sebastian et al., 2012). This study expands upon prior work with a much larger sample and with videos depicting actual people that allow participants to utilize either PT or ES. This is in contrast to tasks that utilize avatars or cartoons, which do not provide opportunities for simulation and ES, or tasks without a narrative component such as images of bodily injury (Decety et al., 2008).
Interestingly, regions involved in ES exhibited either a non-significant or negative relationship with empathic accuracy. This contrasts with previous findings that adults engaged regions underlying PT and ES to the same degree when making empathically accurate ratings (Zaki et al., 2009). These results are also contrary to our hypothesis that adolescents would recruit regions underlying ES (e.g. IPL, PMC, ACC and AI) rather than regions underlying PT (e.g. VMPFC and DMPFC), which are still maturing at this age. However, this finding is consistent with literature showing adolescents can flexibly engage cognitive control systems in motivationally salient contexts, and at times can outperform adults (Crone and Dahl, 2012; Kleibeuker et al., 2013). Therefore, adolescents are not completely lacking in many executive functions subserved by the PFC, such as PT. However, one process in which the PFC plays a central role and that is still developing in adolescents is self-regulation (Steinberg, 2005), specifically emotion regulation. Although PT alone does not require self-regulation, as it does not necessarily entail an affective response, ES in a highly emotional context would very likely necessitate emotion regulation.
One interpretation of the negative relationship between empathic accuracy and activation in ES regions is that adolescents became immersed in their own emotions when sharing the emotional experience of the target. Although personal distress can occur with ES (Decety and Ickes, 2011), and potentially reduce empathic accuracy, we were unable to directly test this interpretation in this study. Without the ability to effectively regulate their emotions, adolescents who experience personal distress from ES may not be able to disengage with their own emotions to attend to and accurately identify the target’s emotions. Another possibility is that ES could interact with an inaccurate perspective of the target’s emotions and further degrade the perceiver’s empathic accuracy. Previous findings that adolescents have increased activation in two regions of the ES network (ACC and insula) with greater rejection-related personal distress provide additional support for this idea (Masten et al., 2009; Gunther Moor et al., 2012). Moreover, in another study adolescents had less activation in dorsolateral PFC compared with adults, which may otherwise serve to downregulate distress (Sebastian et al., 2010). However, posthoc analyses in this study did not reveal any significant relationships between self-reported personal distress (from the IRI) and empathic accuracy, or with empathic accuracy-related BOLD activation in any of the ROIs. The questionnaire measuring personal distress was administered outside the scan session, and individual differences in this measure may not have accurately reflected levels of personal distress during the empathic accuracy task. One limitation was a lack of measures of emotion regulation or personal distress following each video, which would allow us to better test the interpretation that ES caused personal distress, and led to lower empathic accuracy.
Consistent with prior work (Gleason et al., 2009), we did not find significant relationships between empathic accuracy and self-report measures of empathy (e.g. empathic concern or PT). Thus, empathic accuracy provides a behavioral measure of empathic ability that is not captured by questionnaires. We also replicated research showing gender differences in self-reported empathy and empathic accuracy (Van der Graaff et al., 2014). Females scored higher on empathy measures in addition to self-reported adherence to prosocial norms. However, similar to Michalska et al. (2013) there were no gender differences in empathic accuracy-related brain activation. Given that gender differences in self-reported empathy in adults may be due to motivational factors (Ickes et al., 2000), similar motivational factors based on gender roles may have contributed to gender differences in this study. However, we are unable to adequately test this interpretation and future work will be needed to disentangle factors contributing to gender differences in adolescents’ empathic ability.
This study utilized videos of late adolescents in the empathic accuracy task. Although younger adolescent targets would be preferable, it was not possible to obtain videos of minors due to confidentiality considerations and timeline constraints related to IRB approval. The younger appearance of the targets made them more relatable to adolescents, in contrast to the videos of adults used by Zaki et al. (2009). The content of the targets’ stories and their emotional reactions were also more vivid, as these events from their adolescence occurred within the past few years. Future work should aspire to include videos from same-aged peers to compare with the present results, as results of this study may not generalize to adolescents’ empathic abilities with regard to same-aged peers. Moreover, this study captured empathic accuracy processing during early- to mid-adolescence, and the underlying brain regions may be differentially recruited at different points during this developmental period. Thus, future work with a longitudinal design, combined with videos of targets matched for the age of the perceiver at each point, would provide the strongest test of development of empathic accuracy and the underlying neural networks during adolescence.
This study also lacked measures to determine the strategy participants used during the empathic accuracy task (i.e. PT, ES or another strategy). The empathic accuracy task provides stimuli that allow utilization of ES or PT strategies; however, participants may have been more likely to engage in one strategy over another. Although activation in regions underlying these two processes was differentially related to empathic accuracy, we cannot determine whether activation was caused by engagement with PT or ES. These regions are involved in other processes that may be related to but distinct from PT or ES. However, the fact that activation across the set of regions associated with PT, both as a group and individually, consistently related to empathic accuracy and activation in the set of regions associated with ES consistently related to lower empathic accuracy lends support to this inference. Furthermore, participants who had greater self-reported empathy and adherence to prosocial norms also made more empathically accurate responses when they had greater activation in PT regions (RTPJ and STS, respectively), and less activation in right IPL, a region underlying ES. Taken together, the results of this study suggest that adolescents may be more empathically accurate when engaged in PT than ES.
The pattern of findings uncovered in this study has important implications for how to effectively train empathy in adolescents (possibly through PT). In conclusion, PT networks appear to play a key role in empathic accuracy in adolescents and could be potentially harnessed in programs seeking to cultivate empathy (e.g. social emotional learning, compassion-based training methods; Durlak et al., 2011; Mascaro et al., 2012; Reddy et al., 2012). Additional research is needed to directly assess the strategy adolescents engage when they are empathically accurate to fully characterize effective empathic responding during this important developmental period.
Funding
This work was supported by the Bill & Melinda Gates Foundation (grant number OPP1033728 to R.J.D.); a core grant to the Waisman Center from the National Institute of Child Health and Human Development (NICHD) (grant number P30 HD003352 to Albee Messing) and the National Institute of Mental Health (NIMH) (grant numbers R01MH043454, P50MH100031 to R.J.D.). T.R.A.K. was supported by the National Institute of Mental Health (grant number T32MH018931).
Supplementary Material
Acknowledgements
We thank Michael Anderle, Ron Fisher, Jeanne Harris, Chris Harty, Lisa Angelos and Abigail Freeman for assistance with data collection. We also thank Steven Loria and Nate Vack for assistance in planning and programming the task and Andy Schoen, David Perlman, Daniel Levinson, Cory Burghy, Diane Bussan, Reza Farajian, Nagesh Adluru, Steve Kecskemeti and Sasha Sommerfeldt for technical assistance and their helpful feedback on the study design and analysis.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. Dr R.J.D. is the founder, president and serves on the board of directors for the non-profit organization, Healthy Minds Innovations, Inc. In addition, Dr R.J.D. serves on the board of directors for the Mind and Life Institute.
References
- Allison T., Puce A., McCarthy G. (2000). Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences, 4, 267–78. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Jolliffe T., Mortimore C., Robertson M. (1997). Another advanced test of theory of mind: evidence from very high functioning adults with autism or Asperger syndrome. Journal of Child Psychology and Psychiatry, 38, 813–22. [DOI] [PubMed] [Google Scholar]
- Bates D., Machler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar]
- Blakemore S.-J. (2008). The social brain in adolescence. Nature Reviews. Neuroscience, 9, 267–77. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. (2012). Imaging brain development: the adolescent brain. NeuroImage, 61, 397–406. [DOI] [PubMed] [Google Scholar]
- Brown B.B. (2004). Adolescents’ relationships with peers In: Lerner R.M., Steinberg L., editors. Handbook of Adolescent Psychology, pp. 363–94, Hoboken, New Jersey: John Wiley & Sons. [Google Scholar]
- Buckner R.L., Carroll D.C. (2007). Self-projection and the brain. Trends in Cognitive Sciences, 11, 49–57. [DOI] [PubMed] [Google Scholar]
- Burnett S., Blakemore S.-J. (2009). Functional connectivity during a social emotion task in adolescents and in adults. The European Journal of Neuroscience, 29, 1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T.T.-J., Cunnington R., Williams M.A., Kanwisher N., Mattingley J.B. (2008). fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Current Biology, 18, 1576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A. (2013). Considerations of fairness in the adolescent brain. Child Development Perspectives, 7, 97–103. [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews. Neuroscience, 13, 636–50. [DOI] [PubMed] [Google Scholar]
- Culham J. (2015). Cortical areas engaged in movement: Neuroimaging methods A2 – Wright, James D., in: International Encyclopedia of the Social and Behavioral Sciences (Second Edition) pp. 21–9, Oxford: Elvesier. [Google Scholar]
- Davis M.H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44, 113–26. [Google Scholar]
- Decety J., Ickes W. (2011). The Social Neuroscience of Empathy. Cambridge, Massachusetts: The MIT Press.. [Google Scholar]
- Decety J., Michalska K.J., Akitsuki Y. (2008). Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia, 46, 2607–14. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Apperly I.A., Blakemore S.-J. (2010). Online usage of theory of mind continues to develop in late adolescence. Developmental Science, 13, 331–8. [DOI] [PubMed] [Google Scholar]
- Durlak J.A., Weissberg R.P., Dymnicki A.B., Taylor R.D., Schellinger K.B. (2011). The impact of enhancing students’ social and emotional learning: a meta-analysis of school-based universal interventions. Child Development, 82, 405–32. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Miller P.A., Shell R., McNalley S., Shea C. (1991). Prosocial development in adolescence: a longitudinal study. Developmental Psychology, 27, 849–57. [Google Scholar]
- Gallagher H.L., Happé F., Brunswick N., Fletcher P.C., Frith U., Frith C.D. (2000). Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia, 38, 11–21. [DOI] [PubMed] [Google Scholar]
- Gleason K.A., Jensen-Campbell L.A., Ickes W. (2009). The role of empathic accuracy in adolescents’ peer relations and adjustment. Personality & Social Psychology Bulletin, 35, 997–1011. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B., Güroğlu B., Op de Macks Z.A., Rombouts S.A.R.B., Van der Molen M.W., Crone E.A. (2012). Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage, Neuroergonomics: The Human Brain in Action and at Work, 59, 708–17. [DOI] [PubMed] [Google Scholar]
- Hein G., Singer T. (2008). I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology, Cognitive Neuroscience, 18, 153–8. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Molnar-Szakacs I., Gallese V., Buccino G., Mazziotta J.C., Rizzolatti G. (2005). Grasping the intentions of others with one’s own mirror neuron system. PLOS Biology, 3, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes W. (1993). Empathic accuracy. Journal of Personality, 61, 587–610. [Google Scholar]
- Ickes W., Gesn P.R., Graham T. (2000). Gender differences in empathic accuracy: differential ability or differential motivation? Personal Relationships, 7, 95–109. [Google Scholar]
- Kelly A.M.C., Martino A.D., Uddin L.Q., et al. (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex, 19, 640–57. [DOI] [PubMed] [Google Scholar]
- Kenward M.G., Roger J.H. (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics, 53, 983–97. [PubMed] [Google Scholar]
- Kleibeuker S.W., De Dreu C.K.W., Crone E.A. (2013). The development of creative cognition across adolescence: distinct trajectories for insight and divergent thinking. Developmental Science, 16, 2–12. [DOI] [PubMed] [Google Scholar]
- Knafo A., Zahn-Waxler C., Van Hulle C., Robinson J.L., Rhee S.H. (2008). The developmental origins of a disposition toward empathy: genetic and environmental contributions. Emotion, 8, 737–52. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–502. [DOI] [PubMed] [Google Scholar]
- Mascaro J.S., Rilling J.K., Negi L.T., Raison C. (2012). Compassion meditation enhances empathic accuracy and related neural activity. Social Cognitive and Affective Neuroscience, nss095. doi:10.1093/scan/nss095. [DOI] [PMC free article] [PubMed]
- Masten C.L., Eisenberger N.I., Borofsky L.A., et al. (2009). Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience, 4, 143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Pfeifer J.H., Colich N.L., Dapretto M. (2013). Associations among pubertal development, empathic ability, and neural responses while witnessing peer rejection in adolescence. Child Development, 84, 1338–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska K.J., Kinzler K.D., Decety J. (2013). Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Developmental Cognitive Neuroscience, 3, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaauw S., Güroğlu B., Rieffe C., Crone E.A. (2014). Behavior and neural correlates of empathy in adolescents. Developmental Neuroscience, 36, 210–9. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Reddy S.D., Negi L.T., Dodson-Lavelle B., et al. (2012). Cognitive-based compassion training: a promising prevention strategy for at-risk adolescents. Journal of Child and Family Studies, 22, 219–30. [Google Scholar]
- Ruby P., Decety J. (2004). How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience, 16, 988–99. [DOI] [PubMed] [Google Scholar]
- Sato W., Fujimura T., Kochiyama T., Suzuki N. (2013). Relationships among facial mimicry, emotional experience, and emotion recognition. PLoS One, 8, e57889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., Fontaine N.M.G., Bird G., et al. (2012). Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Social Cognitive and Affective Neuroscience, 7, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., Roiser J.P., Tan G.C.Y., Viding E., Wood N.W., Blakemore S.-J. (2010). Effects of age and MAOA genotype on the neural processing of social rejection. Genes, Brain, and Behavior, 9, 628–37. [DOI] [PubMed] [Google Scholar]
- Simpson J.A., Minda M., Ickes W. (2003). When accuracy hurts, and when it helps: a test of the empathic accuracy model in marital interactions. Journal of Personality and Social Psychology, 85, 881–93. [DOI] [PubMed] [Google Scholar]
- Singer T. (2006). The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neuroscience and Biobehavioral Reviews, 30, 855–63. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303, 1157–62. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. (2008). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Steinberg L. (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9, 69–74. [DOI] [PubMed] [Google Scholar]
- Taylor, S.J., Whincup, P.H., Hindmarsh, P.C., Lampe, F., Odoki, K., Cook, D.G. (2001). Performance of a new pubertal self-assessment questionnaire: a preliminary study. Pediatric and Perinatal Epidemiology, 15, 88–94. [DOI] [PubMed] [Google Scholar]
- The American National Election Studies (ANES). (2015). ANES 2008 time series study (No. ICPSR25383-v3). MI: Inter-university Consortium for Political and Social Research, Ann Arbor, MI.
- Van der Graaff J., Branje S., De Wied M., Hawk S., Van Lier P., Meeus W. (2014). Perspective taking and empathic concern in adolescence: gender differences in developmental changes. Developmental Psychology, 50, 881–8. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. NeuroImage, 48, 564–84. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. (2001). Statistical analysis of activation images. In: Functional magnetic resonance imaging: An introduction to methods Oxford: Oxford University Press. [Google Scholar]
- Zahn-Waxler C., Robinson J.L., Emde R.N. (1992). The development of empathy in twins. Developmental Psychology, 28, 1038–47. [Google Scholar]
- Zaki J., Ochsner K.N. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15, 675–80. [DOI] [PubMed] [Google Scholar]
- Zaki J., Weber J., Bolger N., Ochsner K. (2009). The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America, 106, 11382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.