Abstract
Heterozygous STAT1 gain-of-function (GOF) mutations are associated with chronic mucocutaneous candidiasis and a broad spectrum of infectious, inflammatory, and vascular manifestations. We describe therapeutic failures with the Janus Kinase (JAK) inhibitor ruxolitinib in 2 STAT1 GOF patients with severe invasive or cutaneous fungal infections.
Keywords: STAT1, gain of function, ruxolitinib, coccidiomycosis, dermatophytosis
Heterozygous signal transducer and activator of transcription 1 (STAT1) gain-of-function (GOF) mutations cause autosomal dominant chronic mucocutaneous candidiasis (CMC) [1, 2] but have also been associated with a much broader spectrum of infectious, inflammatory, vascular, and neoplastic manifestations [3–5]. These particular STAT1 mutations are considered GOF because of enhanced STAT1-dependent cellular responses to cytokines, as evidenced by high levels of phosphorylated STAT1 (pSTAT1) molecules and high levels of STAT1-dependent downstream targets such as CXCL9 (MIG) and CXCL10 (IP-10) following interferon (IFN)-γ stimulation [2, 4]. Impaired production of Th17 cells has been implicated in the pathogenesis of CMC in these and other patients [1, 2, 5].
Others have used the Janus Kinase (JAK) inhibitor ruxolitinib successfully for CMC and autoimmune phenomena in 3 patients with STAT1 GOF disease [6–8]. In contrast, we characterize ruxolitinib in vitro effects and describe ruxolitinib therapeutic failures in 2 STAT1 GOF patients with severe fungal infections other than CMC.
METHODS
Please refer to the online Supplementary Data.
RESULTS
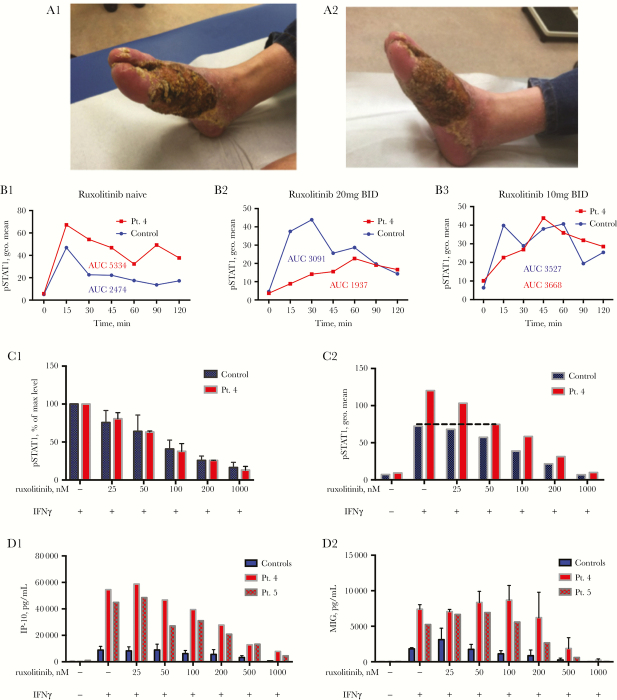
We enrolled 6 patients with 5 different STAT1 GOF mutations (3M, 3F, age range, 20–72 years) (Supplementary Table S1). Two of the 6 enrolled patients—patients 1 and 4—were treated with oral ruxolitinib. Fresh peripheral blood mononuclear cells (PBMCs) from all 6 patients were used to study ruxolitinib’s in vitro effect on STAT1 GOF mutations. Patient 1 (42-year-old man with STAT1 c.820C>T, p.R274W) suffered from severe dermatophytosis, with Trichophyton interdigitale and Trichophyton mentagrophytes, of both feet from childhood (patient 3 in [4]). He was treated in the past with itraconazole, fluconazole, voriconazole, posaconazole, amphotericin B (intravenous [IV] and topical), anidulafungin, and G-CSF, without cure. Because of persistent disease despite antifungals, ruxolitinib 20 mg orally twice daily was begun, in addition to an antifungal regimen of monthly 7 days IV anidulafungin treatment and topical amphotericin B. Beside headache, the patient did not report any other side effects. After 4 weeks of treatment, his feet were worse in comparison with stable disease in the months before the initiation of ruxolitinib treatment, and ruxolitinib was stopped (Figure 1A1–2).
Figure 1.
Skin lesions of patient 1 before treatment (A1) and after 4 weeks of oral ruxolitinib treatment (A2). (B) Ex vivo whole blood CD14+ cells pSTAT1 level in patient 4 (red lines), at rest and up to 120’ after IFNγ stimulation, compared with 3 different healthy controls (blue lines). Assays were performed when the patient was ruxolitinib naïve (B1), treated with 20 mg ruxolitinib BID (B2), and treated with 10 mg ruxolitinib BID (B3). (C1) Patient 4’s (red bars) and a controls’ (n = 3, blue bars) CD14+ cells pSTAT1 levels with IFNγ 15’ stimulation, with and without ruxolitinib pre-incubation at increasing concentrations. pSTAT1 levels are expressed in percentages of each individual’s STAT1 phosphorylation with IFNγ stimulation with no ruxolitinib pre-incubation. (C2) Patient 4’s (red bars) and a control’s (blue bars) CD14+ pSTAT1 levels at rest and with IFNγ stimulation, with and without ruxolitinib pre-incubation. IP-10 (D1) and MIG (D2) secretion level (pg/mL) of patients 4 and 5 (red bars) and healthy controls’ (n = 3; blue bars) PBMCs stimulated with IFNγ 400 U/mL for 24 hours, after pre-incubation with ruxolitinib at increasing concentrations. Abbreviation: AUC, area under the curve.
Patient 4 (27-year-old woman with STAT1 c.1057G>A, p.E355K) had refractory, progressive disseminated coccidioidomycosis for more than 10 years, involving the brain, spine, lungs, liver, spleen, and adrenals (patient 1 in [5]). She was treated with fluconazole, itraconazole, voriconazole, posaconazole, and amphotericin B, without cure. She also received exogenous IFNγ, IFNα, S-adenosylmethionine, and interleukin (IL)-12, with appearance of new sites of disease in her liver, lung, and brain. At 25 years of age, she developed a new pleural effusion, increased pericardial effusion, and a pleural-based mass that encroached on the liver capsule. Oral ruxolitinib was begun and dosing was adjusted to normalization of STAT1 phosphorylation (Figure 1B1–3), eventually receiving 5 mg once daily. After 5 months, she had new coccidioidomycosis in her left medial rectus muscle, and ruxolitinib was stopped.
We assessed the ability of ruxolitinib to alter pSTAT1 in freshly isolated PBMCs of healthy controls and STAT1 GOF patients. Ruxolitinib had a dose-dependent effect on STAT phosphorylation in CD14+ cells in both controls and patient 4 (Figures 1C1–2). The half maximal inhibitory concentration (IC50) was 50–100 nM in both patient 4 and controls (Figure 1C1); 50 nM of ruxolitinib normalized in vitro pSTAT1 levels in patient 4 CD14+ cells (Figure 1C2), whereas 1000 nM completely blocked phosphorylation in both control and patient cells. 1000 nM ruxolitinib similarly blocked STAT1 and STAT3 phosphorylation in CD4+, CD8+, and NK cells in response to IFNγ and IL-10 stimulation, respectively (data not shown).
Patient 4’s median in vitro CD14+ cell peak pSTAT1 after IFNγ stimulation was 162% of normal (range, 131–189%; P, .0006) when she was ruxolitinib naive. We titrated her oral ruxolitinib dosage based on ex vivo pSTAT1 level after in vitro IFNγ-stimulation of CD14+ cells (Figure 1B). Two days prior to initiation of ruxolitinib, patient 4’s area under the curve (AUC) of STAT1 phosphorylation (geometric mean of fluorescence) vs time (minutes) in CD14+ cells was 2.2 times that of the control, and her peak pSTAT1 level was 1.4 times the healthy control. At 20 mg ruxolitinib twice daily, her pSTAT1 AUC level was 63% of the control, and her peak phosphorylation was 51% of normal and delayed. Ruxolitinib 10 mg twice daily made her pSTAT1 AUC similar to control (104%) with a normal peak pSTAT1. Ruxolitinib 5 mg once daily also made her pSTAT1 level similar to control (data not shown).
Because JAK inhibition affected pSTAT1 formation, we examined the ruxolitinib effect on downstream gene expression. We measured MIG and IP-10 levels in supernatants of IFNγ-stimulated PBMCs from STAT1 GOF patients and controls, along with increasing concentrations of ruxolitinib (25–1000 nM). Patients 4 and 5 had elevated IP-10 and MIG levels compared with controls (5.6× and 3.5× higher, respectively) (Figure 1D1–2). However, doses of ruxolitinib that led to normalization of IFNγ-stimulated pSTAT1 levels did not normalize IFNγ-stimulated IP-10 or MIG secretion in patients 4 and 5. The amount of ruxolitinib required to normalize IP-10 secretion from patients 4 and 5 PBMCs was 10× higher than that required to normalize pSTAT1 formation in patient 4’s CD14+ cells (50 vs 500 nM) (Figure 1D1). Paradoxically, in the low to medium concentration range (25–100 nM), in both controls and patients, ruxolitinib increased MIG secretion levels, but to a greater extent in the patients than controls (Figure 1D2). Only high concentrations (200/500 nM) of ruxolitinib could normalize MIG secretion in the PBMCs of patients 5 and 4, respectively.
We looked at the effect of ruxolitinib on IL-17 production and Th17 cell number in patients 1 and 4. Cells from patient 1 did not show any IL-17 production in response to C. albicans, regardless of the presence of ruxolitinib in vitro, either at baseline or 2 weeks into ruxolitinib treatment. Patient 4’s baseline Th17 levels in PBMCs were comparable with controls. On day 13 of treatment with ruxolitinib 20 mg daily, IL-17A+ CD4+ T cells were 1.4%, and 3 months into treatment they were 1.7%, similar to controls (average of 3 controls, 1.55%; range, 1.34–1.84%). These levels were comparable with her Th17 levels prior to treatment (1.27%) [4]. We also asked whether ruxolitinib itself might impair Th17 function in our patients and controls. We stimulated the PBMCs of controls and 2 patients with CD3/CD28 antibodies for 72 hours, along with increasing concentrations of ruxolitinib (0–1000 nM) and measured supernatant IL-17. Patients 4 and 6 produced lower levels of supernatant IL-17 than controls (Supplementary Figure 1). Ruxolitinib had a dose-dependent negative effect on IL-17 secretion in both patients and controls.
We looked at the immune phenotypes of the patients (Supplementary Table S1). Patient 4 had low total lymphocytes, CD3+ lymphocytes, CD4+, and CD8+ T cells. Patients 3, 4, and 6 had low NK cells. Patient 2 had low CD19+ cells. Patients 5 and 6 had low IgA levels. Patient 4 had a nonprotective level of diphtheria antibodies.
DISCUSSION
Ruxolitinib did not improve the clinical condition of either of our 2 STAT1 GOF–treated patients, despite clear inhibition of STAT1 phosphorylation in cells from patients with STAT1 GOF mutations. Despite the ability of ruxolitinib to normalize the level of pSTAT1 in vitro and ex vivo, it did not normalize the downstream activities of pSTAT1 at the doses used clinically. To achieve ruxolitinib normalization of chemokine production in vitro, levels of ruxolitinib may need to be much higher than those currently approved for use [9]. Extrapolating from pharmacokinetic data in healthy volunteers, achieving plasma levels of ruxolitinib above 500 nM might require oral doses as high as 100 mg twice daily, 5× higher than the current maximum recommended dosage. Patient 4 was treated with posaconazole, which might inhibit ruxolitinib metabolism by cytochrome P450 3A4, and thus may have increased ruxolitinib exposure in this specific patient [10]. It is possible that patient 4 was exposed to supra-therapeutic levels of ruxolitinib during the first weeks of therapy. However, we adjusted oral ruxolitinib dose to a level that normalized this patient’s ex vivo CD14+ pSTAT1 level, but did not suppress it below normal level. Ruxolitinib dosing was reduced first to 10 mg twice daily and eventually to 5 mg per day, meaning that the patient was treated with doses that were 50% to 12.5% of the daily-recommended dose of 40 mg.
Two weeks of ruxolitinib treatment did not increase Th17 number or IL-17 secretion in patient 1. Ruxolitinib also had a negative in vitro effect on IL-17 secretion from PBMCs of both patients and healthy controls, a possible concern for ruxolitinib therapy.
The progression of infections during ruxolitinib treatment in our patients despite continued aggressive antifungal therapy is of concern. Indeed, infections are possible complications of ruxolitinib therapy [11]. It is remarkable that severe CMC appears to respond so well to ruxolitinib therapy [6–8], which is in sharp contrast to our experience in severe or disseminated disease.
The immune defect and infection that were initially associated with GOF mutations were impaired Th17 immunity and CMC [1, 2]. However, there is a solid body of evidence that GOF mutations are associated with other infectious diseases and immune defects [3–5]. Sampaio et al. reported invasive fungal infections in 5 patients with GOF mutations [3]. In a cohort of 274 STAT1 GOF patients, CMC was observed in 98% of the patients [5]. However, the number of patients with documented Candida mucocutaneous infection was lower than the number of patients with documented viral infections: 140 (51%) vs 162 (59%) patients, respectively. Ninety-nine (36%) of the patients had documented bacterial infections, 34 (12%) patients had documented invasive fungal infections, and 17 (6%) had documented mycobacterial infections. Of the tested patients in the same cohort, 19% had lymphopenia, 28% CD4 lymphopenia, 16% CD8 lymphopenia, 19% low B cells, 49% low memory B cells, 25% low NK cells, 82% low CD3+ IL-17+ or CD4+IL-17+ cells, 32% low T cell proliferation, 38% low IgG2, and 50% low IgG4 [5]. Tabellini et al. demonstrated impaired NK cell function in GOF mutations [12]. In our cohort, 1 patient had low CD3+, CD4+, and CD8+ cells. Three patients had low NK cells. One patient had low B cells. Two other patients had low IgA levels.
The mechanisms behind STAT1 GOF immunodeficiency are not yet fully explained. However, it is reasonable to assume that the immune defects in GOF mutations are wider and more complicated than believed initially. Hence the increased susceptibility of the majority of these patients to bacterial, viral, and fungal infections probably reflects broader impairment of the immune system than just Th17 immunity.
There are limited data on coccidiomycosis and primary immune deficiency (PID). Odio et al. recognized 8 patients with PID among 370 cases of disseminated coccidiomycosis [13]. Two had STAT3 loss-of-function mutations, 1 had IFN-γ receptor1 deficiency, 3 had IL-12 receptor loss-of-function mutations, and 2 had STAT1 gain-of-function mutations. These findings indicate that the IL-12/IFN-γ axis and STAT3-mediated immunity are central to protection against coccidiomycosis. Patient 4’s Th17 levels were normal prior to initiation of ruxolitinib treatment. Her CD4+, CD8+, and NK cells levels were low. The patient never had CMC, but she did have chronic dermatophytosis in childhood and young adulthood.
The failure to improve Th17 activity in our case (patient 1) and to fully recover it in previously published cases [6–8] implies that ruxolitinib may be exerting effects on mucocutaneous candidiasis through mechanisms other than or in addition toTh17. Ruxolitinib has a theoretical direct anticandida activity. Staurosporine, which is a nonspecific kinase inhibitor, was found to have direct and indirect anticandida activity [14–16]. Omura et al. demonstrated a direct anticandida activity of Staurosporine [14]. Lafayette et al. demonstrated that Staurosporine enhanced the efficacy of antifungals targeting the cell wall, micafungin, and those targeting the cell membrane, including fluconazole and the morpholine fenpropimorph, which inhibits Erg2 and Erg24 [16]. Staurosporine also reduced azole resistance in clinical isolates [16]. We could not find similar evidence of staurosporine activity against coccidiomycosis.
The direct effect of JAK inhibitors on Candida has not been tested to the best of our knowledge. However, mouse models show conflicting data on the effect of JAK inhibitors in invasive candidiasis [17, 18]. In 1 study, tofacitinib was associated with reduced survival [17]. In a different study, administration of ruxolitinib 50 mg/kg/d starting a day before Candida injection was associated with reduced survival, but administration of 6.25 mg/kg/d beginning on the second day of the infection was associated with increased survival [18]. Despite their relative specificity to JAK proteins in humans, JAK inhibitors might have a similar effect as staurosporine in eukaryotes. This is an area that requires further research.
The mechanisms of CMC in STAT1 GOF are clearly associated with impaired Th17 immunity. However, the critical final effectors of mucosal Candida immunity remain unclear. Other possible mechanisms that might account for the frequent CMC in STAT1 GOF disease include IFNγ tachyphylaxis [4] and overexpression of chemokines and cytokines, leading to other forms of immune dysfunction [2, 3]. The differences between our patients’ experiences with ruxolitinib and the relative successes reported in patients with CMC or autoimmunity may reflect that the immune defects that make patients susceptible to CMC are repaired by ruxolitinib, but the immune defects that lead to susceptibility to invasive fungal disease are not. Our experience with a small number of patients is too limited to draw general conclusions about the role of ruxolitinib in STAT1 GOF disease. The reported benefits of ruxolitinib in STAT1 GOF patients with either autoimmune phenomena or mucosal candidiasis suggest that the underlying processes that govern invasive infections, autoimmunity, and CMC in STAT1 GOF may not all be amenable to the same intervention.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online (ofid.oxfordjournals.org). Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Financial support. This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. FvdV was supported by the European Research Area (ERA)-Net for Research Programs on Rare Diseases “EURO-CMC.” MGN was supported by an European research Council (ERC) Consolidator Grant (#310372).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. van de Veerdonk FL, Plantinga TS, Hoischen A et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011; 365:54–61. [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Okada S, Kong XF et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 2011; 208:1635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uzel G, Sampaio EP, Lawrence MG et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol 2013; 131:1611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sampaio EP, Hsu AP, Pechacek J et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 2013; 131:1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toubiana J, Okada S, Hiller J et al. ; International STAT1 Gain-of-Function Study Group Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 2016; 127:3154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins E, Al Shehri T, McAleer MA et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol 2015; 135:551–3. [DOI] [PubMed] [Google Scholar]
- 7. Weinacht KG, Charbonnier LM, Alroqi F et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol 2017; 139:1629–40.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mössner R, Diering N, Bader O et al. Ruxolitinib induces Interleukin 17 and ameliorates chronic mucocutaneous Candidiasis caused by STAT1 gain-of-function mutation. Clin Infect Dis 2016; 62:951–3. [DOI] [PubMed] [Google Scholar]
- 9. Shi JG, Chen X, McGee RF et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol 2011; 51:1644–54. [DOI] [PubMed] [Google Scholar]
- 10. Shi JG, Chen X, Emm T et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol 2012; 52:809–18. [DOI] [PubMed] [Google Scholar]
- 11. Heine A, Brossart P, Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis?Blood 2013; 122:3843–4. [DOI] [PubMed] [Google Scholar]
- 12. Tabellini G, Vairo D, Scomodon O et al. Impaired natural killer cell functions in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol 2017; 140:553–64.e4. [DOI] [PubMed] [Google Scholar]
- 13. Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 2017; 23:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Omura S, Iwai Y, Hirano A et al. A new alkaloid AM-2282 OF Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J Antibiot (Tokyo) 1977; 30:275–82. [DOI] [PubMed] [Google Scholar]
- 15. Xie JL, O’Meara TR, Polvi EJ, Robbins N, Cowen LE. Staurosporine induces filamentation in the human fungal pathogen Candida albicans via signaling through Cyr1 and Protein Kinase A. mSphere 2017; 2:pii: e00056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. LaFayette SL, Collins C, Zaas AK et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 2010; 6:e1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Gong FY, Li ZJ et al. A study on the risk of fungal infection with tofacitinib (CP-690550), a novel oral agent for rheumatoid arthritis. Sci Rep 2017; 7:6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsirigotis P, Papanikolaou N, Elefanti A et al. Treatment of experimental Candida sepsis with a Janus kinase inhibitor controls inflammation and prolongs survival. Antimicrob Agents Chemother 2015; 59:7367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.