Abstract
Salicylates are among the oldest and most widely used medications, used to reduce fever, pain and inflammation. The major oral salicylates are aspirin and salsalate, both of which are rapidly metabolized to salicylate in vivo. Due to its acetyl group, aspirin irreversibly inhibits cyclo-oxygenases and thus blocks platelet aggregation, while salsalate has been used for treatment of inflammatory diseases such as rheumatoid arthritis. Recently, beneficial effects of salicylates in type 2 diabetes and cancer have been proposed. This has led to renewed interest in understanding how these simple molecules have such diverse and multifaceted effects. Here we discuss the idea that AMP-activated protein kinase (AMPK) might mediate some effects of salicylate-based drugs, particularly by modulating cellular metabolism.
Keywords: AMPK, aspirin, salicylate, inflammation, diabetes, cancer
Salicylates – origin and discovery of medicinal properties
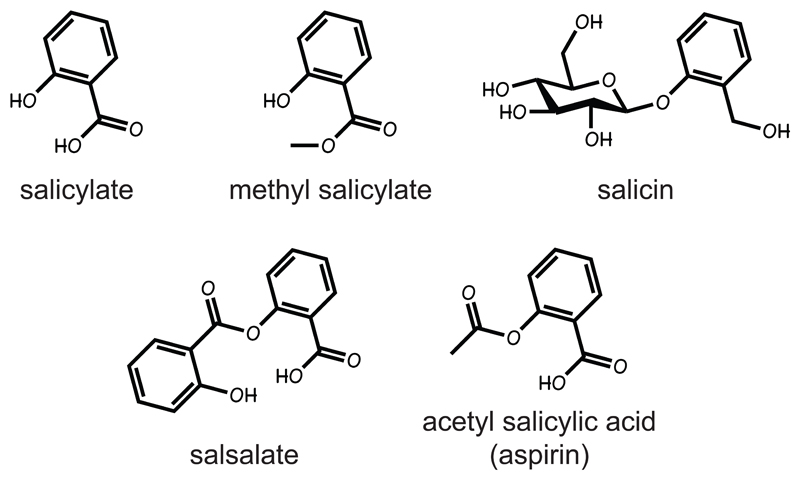
Salicylates are hormones produced by plants in response to infection, which are critical in their defence against attack by pathogens [1]. Salicylate and derivatives such as salicin (a β-glucosyl ester, Fig. 1) are transported to neighbouring tissues, triggering defensive responses; volatile derivatives such as methyl salicylate can even spread to neighbouring plants. Salicylates can therefore be regarded as the equivalent of cytokines operating in the plant version of the innate immune system. They are produced by essentially all plants, but are named after the willow Salix alba due to the high levels of salicin found in its bark. The medicinal effects of willow were known to ancient humans, making natural salicylates almost certainly the oldest drugs known to mankind (Box 1). A synthetic derivative, acetyl salicylic acid (Fig. 1) was first marketed in 1899 under the trade name of aspirin. Aspirin is rapidly broken down to salicylate in vivo by plasma and cellular esterases, and only a small proportion survives the first pass through the portal circulation to reach peripheral tissues. For example, following oral aspirin administration in rats, the peak plasma concentrations of salicylate are up to 50-fold higher than those of aspirin, while its half-life is several hours compared to only minutes for aspirin itself [2]; similar results are obtained in humans. Thus, aspirin can be regarded as a pro-drug for salicylate.
Figure 1. Structures of salicylate-based natural products (top) and synthetic derivatives (bottom).
Box 1. History of human use of salicylates as medicines.
The use of willow extracts as a medicine was recorded in the Ur III stone tablet from ancient Sumeria (now part of modern Iraq) and the Ebers papyrus from ancient Egypt. These documents can be dated to the 3rd millennium BCE. Use of willow extracts was also described by the physician Hippocrates in ancient Greece. However, this knowledge appears to have been lost to Europeans until rediscovered in the 18th century, when the use of extracts of willow bark to treat “agues” (fever) was described by an English parson, Edward Stone, in a paper published in the Proceedings of the Royal Society of London in 1763 (which did not cite his ancient predecessors!). Salicin was extracted from willow bark by the German chemist Buchner in 1829, and was used by Dr Thomas MacLagan in a trial for treatment of rheumatic fever in Dundee (home town of one of the current authors), which was reported in the Lancet in 1876. MacLagan left some patients untreated, and this is sometimes regarded as one of the first true clinical trials of any drug. Acetyl salicylic acid was synthesized by Felix Hoffman at the German company Bayer (formerly a dye manufacturer) and was marketed under the trade name aspirin in 1899. However, Bayer lost the rights to the trade name in the aftermath of the First World War, and aspirin in now regarded in most countries as the generic name for the drug. The use of aspirin became widespread following the influenza epidemic of 1918-19, when it was used to treat the associated fever. For a fascinating account of the history of aspirin and salicylates, and citations to some of the original manuscripts discussed above, see [58].
Recently, evidence has accumulated that salicylates are effective not only in their original roles of reducing pain, fever, inflammation and blood clotting, but also for treating insulin resistance and protecting against cancer. It seems unlikely that all of these beneficial effects can be explained by a single molecular target, and we discuss below the idea that some of these effects might be mediated by AMP-activated protein kinase (AMPK), a recently discovered new target [3, 4].
Mechanism of action of salicylates: inhibition of cyclo-oxygenases?
In 1971, Vane showed that aspirin inhibited prostaglandin synthesis [5]. The first two steps in this pathway are catalysed by cyclo-oxygenases that occur as two major isoforms, the ubiquitous COX1 form and COX2, whose expression is induced at sites of inflammation. Interestingly, the acetyl group of aspirin is transferred to a serine residue in the active site of both isoforms, causing irreversible inhibition [6]. Inhibition of synthesis of the pro-thrombotic prostanoid, thromboxane, elegantly explains the ability of aspirin to inhibit blood clotting triggered by platelets; because acetylation is irreversible and platelets cannot resynthesize COX1, the inhibition lasts for the full lifetime of the platelet [6]. Inhibition of COX1 may also explain effects of aspirin in reducing pain and fever. Although inhibition of COX2 is often assumed to explain the anti-inflammatory effects of aspirin, there are several findings that do not support that hypothesis. One puzzle is that salicylate, because it lacks the acetyl group, is a much less potent cyclo-oxygenase inhibitor than aspirin [5], yet appears to be equipotent in suppressing inflammation, for example in humans with rheumatoid arthritis [7]. Also, in mice where inflammation was induced in subcutaneous air pouches, the anti-inflammatory effect of aspirin was unaffected by genetic deletion of COX2 [8].
Mechanism of action of salicylates: inhibition of NF-κB signalling?
Another proposed target for salicylates is signalling to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a transcription factor activated by inflammatory stimuli and a critical modulator of inflammatory processes. The inhibitor of NF-κB (IκB) retains NF-κB in the cytoplasm, preventing its translocation to the nucleus until IκB is phosphorylated by the kinase complex containing IκKβ, thus triggering IκB degradation. High concentrations of salicylates (2-20 mM) inhibit NF-κB function in human B and T cell lines [9], and inhibition of IκKβ was proposed as the mechanism [10]. However, IκKβ inhibition by salicylate is competitive with ATP, so the effect is greatly reduced at normal physiological ATP concentrations [10]. Salicylate is, in fact, a very non-specific inhibitor of cellular kinases when they are assayed at low ATP concentrations; in a screen of 120 protein kinases, 80% of them were inhibited more potently by 10 µM salicylate than IκKβ [11]. Moreover, mice with a knockout of the p105/p50 (NF-κB1) subunit of NF-κB are not resistant to the anti-inflammatory effects of salicylate in the air pouch model described above [8].
As long ago as the 19th century, high doses of salicylates were found to reduce urine glucose in diabetics, but the significance of this was not grasped until the 1990s, when it was recognized that insulin resistance (the primary cause of type 2 diabetes) was associated with inflammation (reviewed in [12]). Treatment with salicylates, at the high doses used in rheumatoid arthritis and rheumatic fever, improves insulin sensitivity in obese mice [13] and humans with type 2 diabetes [14]. This was ascribed to inhibition of IκKβ, because mice heterozygous for IκKβ [13] or with liver-specific knockout of IκKβ [15] are protected from obesity-induced insulin resistance. Although these results do suggest a role for ΙκKβ in the development of insulin resistance during obesity, the ability of salicylates to improve insulin sensitivity in IκKβ heterozygous or liver-null mice was not examined.
To circumvent the adverse gastrointestinal effects of high aspirin doses, Shoelson initiated clinical studies with salsalate (Fig. 1), a dimer of salicylic acid that (like aspirin) is rapidly cleaved to salicylate following adsorption. Unlike aspirin, salsalate does not inhibit platelet aggregation and cause gastrointestinal bleeding, which has allowed its use at high doses (3-5 g/day) for the treatment of rheumatoid arthritis. Several small trials with salsalate have revealed a consistent ability to improve glucose homeostasis in patients with insulin resistance or type 2 diabetes (e.g. [16, 17]).
Mechanism of action of salicylates: activation of AMPK?
Unexpected findings obtained using salicylates, which could not be explained by their ability to inhibit cyclo-oxygenases or NF-κB signalling, were that they rapidly reduce circulating free fatty acids and/or triglycerides in obese rodents [13] and humans with type 2 diabetes [14], and increase fatty acid oxidation during fasting in healthy humans [18]. These changes in lipid metabolism occur before any improvements in insulin sensitivity are evident, and are consistent with early reports demonstrating inhibitory effects of salicylate on adipose tissue lipolysis [19] and liver fatty acid synthesis [20]. All of these metabolic effects can potentially be explained by recent findings that AMPK is directly activated by salicylate [3].
AMPK occurs in all eukaryotes as heterotrimers composed of a catalytic α subunit and regulatory β and γ subunits [21, 22]. In mammals these are encoded by multiple genes that generate two or three isoforms of each subunit (α1, α2; β1, β2; γ1, γ2, γ3). The kinase activity of AMPK can be increased up to 100-fold by phosphorylation of a conserved threonine residue (Thr172) within the kinase domain; the major upstream kinase phosphorylating this site is a complex containing the tumor suppressor kinase, LKB1 [23]. The LKB1 complex appears to be constitutively active, since its activity is constant even under conditions where phosphorylation of Thr172 on AMPK is changing [24, 25]. The explanation for this is that the increases in AMPK phosphorylation are mediated not by activation of LKB1, but by displacement of ATP by AMP or ADP at one or more of three sites on the AMPK-γ subunit, triggering conformational changes that promote phosphorylation and inhibit dephosphorylation of Thr172 [26, 27]. Binding of AMP also causes allosteric activation, amplifying the effect a further 10-fold. AMPK is activated by many drugs (including the anti-diabetic drug metformin), by hormones (e.g. leptin, adiponectin, ciliary neurotrophic factor), by natural products of plants used in traditional medicines, and by “nutraceuticals” claimed to have health-promoting properties [21, 22]. Most drugs and xenobiotics activate AMPK indirectly, by inhibiting mitochondrial ATP synthesis and increasing the concentrations of AMP and ADP [28]. However, a synthetic activator, A-769662, has similar effects to AMP in that it causes allosteric activation and inhibits Thr172 dephosphorylation [29, 30], yet its binding site is distinct from those for AMP [28, 29]. This binding site appears to involve the β subunit, because A-769662 only activates AMPK complexes containing the β1 and not the β2 isoform [31], while a point mutation (S108A) in β1 abolishes its effects [30].
In the recent studies, salicylate was found to activate AMPK in intact human (HEK-293) cells [3]. Aspirin was without effect in these cells, although another group found that 5 mM aspirin activated AMPK in several colorectal cancer cell lines [4]; this presumably depends on expression of esterases that convert aspirin to salicylate. Although salicylate can uncouple mitochondrial respiration from ATP synthesis at higher concentrations, at concentrations below 10 mM the effects in HEK-293 cells were not due to changes in AMP/ADP and were due instead to direct binding of salicylate to AMPK [3]. Concentrations of 1-3 mM are readily reached in the plasma of humans being treated for rheumatoid arthritis with high doses of either aspirin [7] or salsalate [17], but may not be relevant in humans taking low doses of aspirin (75-80 mg/day) to inhibit platelet aggregation. Intriguingly, salicylate appears to bind at the same site as A-769662, because: (i) it caused a small allosteric activation on its own, but antagonized the larger effect caused by A-769662; (ii) salicylate binding inhibited Thr-172 dephosphorylation, and the effects of both salicylate and A-769662 on dephosphorylation were abolished by a β1-S108A mutation, and were reduced or absent in complexes containing the β2 isoform [3].
AMPK is a “master regulator” of metabolism: once activated, it phosphorylates numerous metabolic enzymes, acutely decreasing anabolic pathways that consume ATP such as fatty acid, triglyceride, phospholipid, isoprenoid and protein biosynthesis, while activating catabolic pathways that generate ATP, such as glucose uptake and fatty acid oxidation [21, 22]. In the longer term, it reinforces these effects by phosphorylating transcription factors and co-activators that regulate gene expression, for example down-regulating expression of lipogenic enzymes and up-regulating expression of enzymes of mitochondrial oxidative metabolism, as well as mitochondrial biogenesis itself. A particularly important anti-anabolic effect of AMPK is to inhibit the mechanistic target-of-rapamycin complex-1 (mTORC1). This has the effect of down-regulating translation of proteins required for rapid cell growth, including the transcription factor HIF-1α, the latter being required for the switch away from oxidative metabolism and towards glycolysis (termed the Warburg effect or aerobic glycolysis, Box 2) that is observed in most rapidly growing cells. Intriguingly, genetic loss of AMPK promotes the Warburg effect via a mechanism that requires HIF-1α [32]. There is an interesting parallel here with the role of the AMPK ortholog in yeast, see Box 2.
Box 2. The Warburg effect, aerobic glycolysis and fermentation in yeast.
In the 1920s the German biochemist Otto Warburg noticed that tumor cells exhibited a high rate of glycolysis and lactate production [59], a phenomenon that has become known as the Warburg effect. Warburg thought that this was because tumor cells had a defect in oxidative metabolism, but it is now known that this is not usually the case, exceptions being a few rare cancers caused by loss-of-function mutations in TCA cycle enzymes [60]. The Warburg effect is not restricted to tumor cells and is, in fact, displayed by most cells that undergo rapid proliferation. The high rates of glucose uptake may partly occur because glycolysis and the pentose phosphate pathway provide precursors for biosynthesis, such as serine and glycine for one-carbon metabolism, NADPH for lipid synthesis, and ribose for nucleic acid synthesis. However, another important reason may be that the TCA cycle switches from being a purely catabolic pathway to become (at least in part) an anabolic pathway that provides precursors for biosynthesis, particularly citrate for lipid biosynthesis. This inevitably means that the TCA cycle will be less productive in terms of ATP synthesis, so glycolysis may be up-regulated to provide an alternative source of ATP [37].
A phenomenon very similar to the Warburg effect occurs in the budding yeast Saccharomyces cerevisiae. When they are maintained in high glucose they grow rapidly, mainly use fermentation (glycolysis) to produce ATP (one difference is that yeast generate ethanol as the end-product, rather than lactate). As glucose in the medium starts to run out, their growth slows and they switch to the use of other fermentable sugars such as sucrose or, failing that, to oxidative metabolism. The latter, of course, generates much more ATP per molecule of glucose utilized than glycolysis. Intriguingly, the yeast ortholog of AMPK (the SNF1 complex) is not required for growth in high glucose, but is absolutely required for the various metabolic switches, including the switch to oxidative metabolism, that occur when glucose runs low [61].
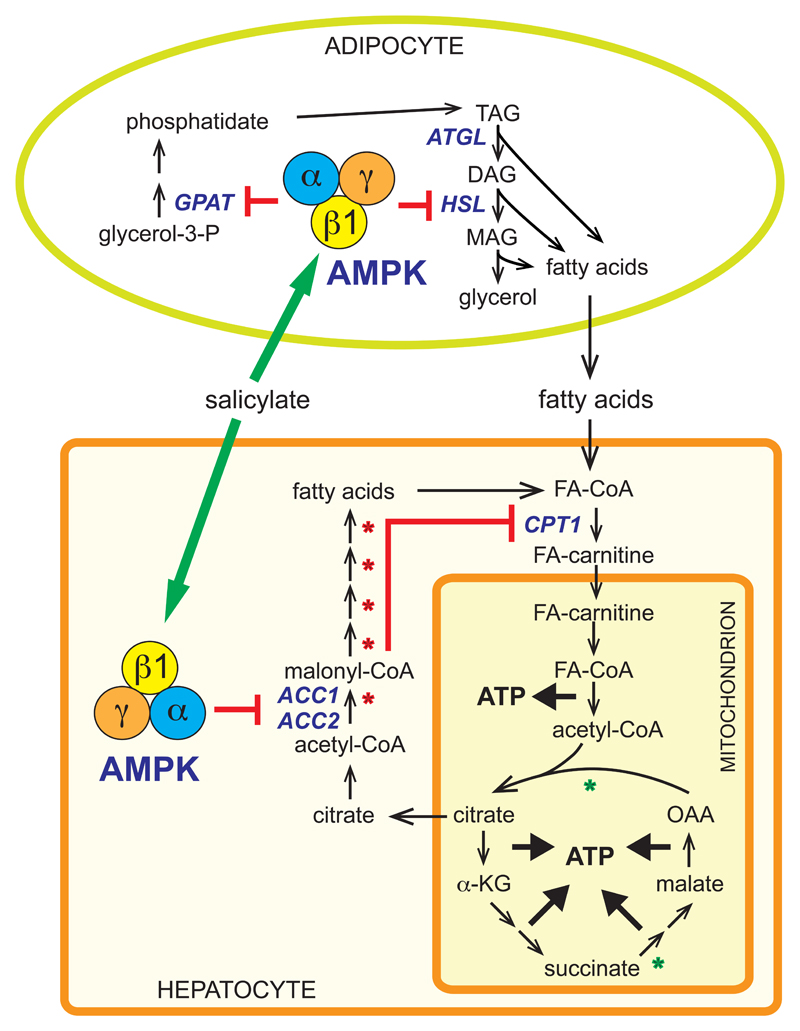
AMPK is also believed to be important for enhancing insulin sensitivity, because its activity is increased by exercise, as well as by hormones and therapeutics (including metformin) that improve insulin sensitivity [21, 22]. One long-established target for AMPK is acetyl-CoA carboxylase (ACC), whose phosphorylation reduces the cellular concentration of its product, malonyl-CoA, a key intermediate in fatty acid synthesis as well as an inhibitor of fatty acid oxidation. AMPK also inhibits adipose tissue lipolysis through phosphorylation of hormone-sensitive lipase [33, 34]. Consistent with these findings, treatment of mice with salicylate at doses that generated plasma concentrations of 1-2 mM activated AMPK and increased ACC phosphorylation in tissues expressing the β1 isoform, including liver and adipose tissue. This was associated with a reduction in circulating free fatty acids as well as in the respiratory exchange ratio [3], the latter signifying a metabolic shift from carbohydrate to fatty acid oxidation. Importantly, these effects were eliminated in AMPK-β1 null mice, showing that AMPK activation is required for these effects of salicylate on lipid metabolism. The overall effects on lipid metabolism triggered by salicylate activation of AMPK are summarized in Fig. 2.
Figure 2. Effects of salicylate on lipid metabolism mediated by AMPK.
Salicylate activates AMPK in both hepatocytes and adipocytes. In hepatocytes, AMPK activation causes phosphorylation of the ACC1 and ACC2 isoforms of acetyl-CoA carboxylase, lowering malonyl-CoA. This inhibits fatty acid synthesis, while also promoting fatty acid oxidation by relieving inhibition of carnitine-palmitoyl-CoA transferase-1 (CPT1), thus helping to generate ATP. In the longer term, AMPK promotes oxidative metabolism by increasing expression of oxidative enzymes (e.g. those marked with green asterisks), while down-regulating lipogenic enzymes (red asterisks). In adipocytes, AMPK activation inhibits fatty acid synthesis (not shown) by phosphorylation of ACC1, and triacylglycerol (TAG) synthesis by inactivation of glycerol phosphate acyl transferase (GPAT), while inhibiting TAG breakdown by phosphorylation of hormone-sensitive lipase (HSL), the enzyme that converts diacylglycerol (DAG) to monoacylglycerol (MAG). The latter effect reduces release of fatty acids into the bloodstream (lipolysis). The net effect of these changes is that salicylate reduces the accumulation of lipids in hepatocytes, enhancing insulin sensitivity.
It is also worth noting that there are numerous papers suggesting that the function of the NF-κB signalling pathway is down-regulated in response to AMPK activation, in various cell types ranging from macrophages to endothelial cells and muscle cells [35, 36]. There is no consensus about which downstream targets of AMPK are responsible for these effects, and their detailed molecular mechanisms remain unclear. However, these findings raise the possibility that inhibition of NF-κB signalling by salicylates (see previous section) might also be secondary to AMPK activation.
Anti-inflammatory effects of AMPK activation
There is increasing evidence that AMPK exerts anti-inflammatory effects, and that this arises in part from its effects on cell metabolism [37]. Unactivated cells of the immune system, including dendritic cells, neutrophils and naïve T cells, utilize mainly oxidative metabolism (including fat oxidation) to generate ATP. However, once activated by ligands for Toll-like receptors (TLRs) such as lipopolysaccharide (LPS), by pro-inflammatory cytokines or by antigen presentation, they switch to aerobic glycolysis (Box 2). In T cells, where antigen presentation stimulates rapid proliferation, this may occur because the TCA cycle is being used as a source of biosynthetic precursors and is less able to supply reducing equivalents to the respiratory chain [37]. In other cases such as dendritic cells, where stimulation by TLR agonists does not cause proliferation, the switch may occur because the respiratory chain is inhibited by the inflammatory mediator nitric oxide, thus generating reactive oxygen species rather than ATP [38, 39]. The switch to glycolysis in dendritic cells is associated with reduced Thr172 phosphorylation on AMPK, and is inhibited by pharmacological activation of AMPK, and promoted by AMPK down-regulation [39]. Very similar effects are observed in macrophages: classically activated (M1) macrophages with a pro-inflammatory role utilize aerobic glycolysis, whereas alternatively activated (M2) macrophages, more involved in the resolution of inflammation, utilize oxidative metabolism instead. In a recent study, induction of macrophage polarisation towards the M1 phenotype by the TLR4 ligand LPS was associated with enhanced glycolysis and increases flux into the pentose phosphate pathway, the latter effect associated with down-regulation of the sedoheptulose kinase, CARKL [40]. Studies using macrophages where AMPK has been down-regulated suggested that it normally attenuates production of inflammatory cytokines in response to fatty acids and LPS [41, 42]. The anti-inflammatory actions triggered by the metabolic effects of AMPK were revealed even more clearly by genetic deletion in mice of the AMPK-β1 subunit (the predominant β isoform in macrophages), which markedly reduced both ACC phosphorylation and cellular mitochondrial content [43]. This resulted in reduced rates of macrophage fatty acid oxidation that promoted the accumulation of pro-inflammatory diacylglycerols, leading to increased c-Jun kinase (JNK) activation and M1 skewing, in macrophages derived from both bone marrow and adipose tissue. This reduction in macrophage AMPK activity led to the development of a pro-inflammatory environment that promoted the development of insulin resistance when the mice were fed a high-fat diet [43]. Notably, pharmacological activation of AMPK with A-769662 increased fatty acid oxidation and reduced JNK activation in wild type but not β1-deficient macrophages [43]. Importantly, the effects of A-769662 on suppressing inflammation were impaired if fatty acid oxidation was blocked. These findings suggest that AMPK is critical for controlling macrophage polarization and, in turn, insulin sensitivity and glucose homeostasis during obesity. They also suggest that a major component of the anti-inflammatory action of AMPK is mediated by its effects on fatty acid oxidation.
There are also important metabolic effects of AMPK in T cells. AMPK is rapidly activated via a Ca2+-mediated mechanism, and ACC phosphorylated, when mouse or human T cells are stimulated via cross-linking of the T cell receptor [44, 45]. CD8+ T cells lacking AMPK-α1 (the sole catalytic subunit isoform expressed in these cells [44]), proliferate normally in response to stimulation both in vitro and in vivo [46], although they do display increased levels of secreted interferon-γ and IL-17A, a higher glycolytic rate, and increased phosphorylation of mTORC1 targets [45]. They also demonstrate defects in their responses to glucose deprivation and treatment with the glycolytic inhibitor 2-deoxyglucose, and reduced viability when cultured in the presence of the latter [44, 45]. When the infection that triggers a T cell response is resolved, most of the activated T cells undergo apoptosis, but a small proportion survive as memory T cells that can rapidly respond to a second bout of infection involving the same antigen; this is the phenomenon underlying immunization. In mice with a T cell-specific knockout of AMPK the proliferative response of the cells to antigen challenge appears normal. However, if the T cells are transferred to a naïve mouse not treated with antigen, the numbers surviving as memory cells are greatly reduced [47]. Memory T cells switch from aerobic glycolysis back to the use of oxidative metabolism, and it is tempting to speculate that the cells find this switch difficult to achieve in the absence of AMPK. This is equivalent to yeast cells that lack the AMPK ortholog being unable to switch back to oxidative metabolism when glucose runs low (Box 2).
Interestingly, in a study of mice with a T cell deficiency in TRAF6, it was found that the cells exhibited a reduced rate of fatty acid oxidation, correlating with a reduced expression of AMPK-α1, when stimulation by interleukin-2 was withdrawn. However, the rate of fatty acid oxidation could be restored by adding metformin, which promoted ACC phosphorylation. The TRAF6-deficient mice also displayed a severe defect in the formation of memory T cells, which could be rescued by treatment with metformin in vivo [48].
The effects of salicylate on AMPK signalling in cells of the immune system, such as dendritic cells, macrophages and T cells, has not yet been studied in detail. However, the findings that AMPK tends to exert anti-inflammatory effects in these cells, most likely through its effects on metabolism, suggest that this is an area that is ripe for investigation.
Salicylate and cancer chemoprevention
Many observational studies have found that daily aspirin reduces the risk of developing colon, breast and prostate cancers (reviewed in [49]). Based on analysis of randomised trials originally designed to examine effects of aspirin on vascular events, treatment with daily aspirin for 5 years or more was found to reduce the subsequent risk of colorectal cancer and some other solid cancers, particularly adenocarcinomas; this protective effect only seemed to require low aspirin doses (<100 mg/day) [50]. The issue of dose is important, because high doses of aspirin carry a greatly increased risk of gastrointestinal bleeding, while low doses may be insufficient to activate AMPK in tissues other than the intestine. Inhibition of COX2 is usually cited as the mechanism for cancer chemoprevention by aspirin, particularly in colon cancer. Indeed, in the APCmin mouse model that is prone to this cancer, knockout of COX2 or treatment with a COX2-specific inhibitor reduced the number of intestinal polyps [51]. However, human studies do not uniformly support the idea that COX inhibition is the sole mechanism for protective effects in cancer. For example, use of aspirin was found to be associated with a reduced risk of melanoma in post-menopausal women, but use of other non-steroidal anti-inflammatory drugs (NSAIDs), which are also COX inhibitors, was not [52]. A trial of a low dose aspirin (100 mg every second day, which should fully inhibit platelet COX1) found no effects on prevention of breast cancer [53]. Aspirin use is also associated with a lower risk of prostate cancer in men, while use of other NSAIDs, such as ibuprofen, is not [54, 55].
These results suggest that the protective effects of aspirin in cancer may not be fully explained by COX inhibition. Recent evidence suggests that AMPK (like its upstream kinase LKB1) is a tumour suppressor [32], and use of another known AMPK activator, metformin, is associated with reduced incidence of cancer [56]. Indeed, metformin suppresses the formation of intestinal polyps in the APCmin mouse [57], and in a small trial of non-diabetic humans with aberrant crypt foci in the colon (believed to represent precursors of adenocarcinoma), one month treatment with low doses of metformin reduced the number of such foci [57]. Many of the known effects of AMPK activation on cells would exert a cytostatic effect and thus oppose tumorigenesis (Fig. 3). All of these observations suggest that the anti-cancer effects of salicylates could be mediated in part by AMPK activation. In a recent study there was evidence that AMPK was activated and/or mTORC1 inhibited in colon biopsies from both mice and humans treated with aspirin [4]. However, further studies of animal models will be required to test whether loss of AMPK affects the development of colorectal cancer, and whether any protective effects of metformin and/or salicylates might be mediated by AMPK.
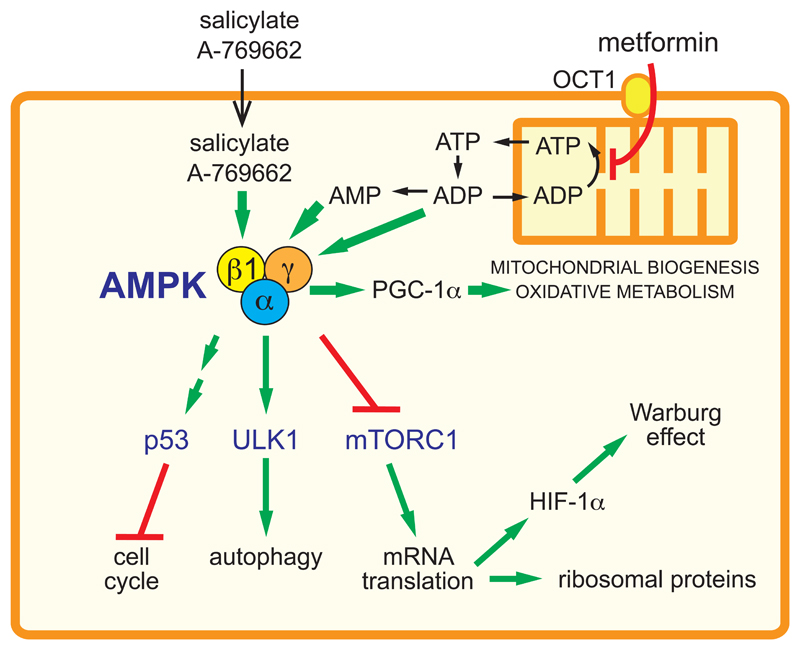
Figure 3. Proposed mechanisms by which AMPK activators exert a cytostatic effect and oppose the metabolic changes occurring in proliferating cells.
Salicylate or A-769662 enter the cell and activate AMPK complexes containing the β1 subunit. Metformin, a cation, enters by transport catalyzed by the organic cation transporter 1 (OCT1), and accumulates in mitochondria due to the electrical gradient across the inner membrane. Here metformin inhibits Complex I and hence ATP synthesis, causing a build-up of ADP and AMP in the cytoplasm. This is detected by the AMPK-γ subunit, leading to increased Thr172 phosphorylation and allosteric activation. The activated AMPK then triggers cell cycle checkpoints through increased p53 activity, activates autophagy via ULK1 phosphorylation, and inhibits the mechanistic target-of-rapamycin complex-1 (mTORC1). The latter effect inhibits translation of proteins required for rapid cell growth, including ribosomal proteins and hypoxia-inducible factor-1α (HIF-1α), with the latter being required for rapid glucose uptake and glycolysis (the Warburg effect). At the same time, AMPK activates peroxisomal proliferator-activated receptor-γ co-activator-1α (PGC-1α), increasing mitochondrial biogenesis and expression of mitochondrial oxidative enzymes. Thus, the cell switches from the glycolytic metabolism typical of a proliferating cell to the oxidative metabolism more typical of a quiescent cell.
Concluding remarks and future perspectives
Although aspirin is a much more potent inhibitor of cyclo-oxygenases, salicylate generated by aspirin breakdown is likely to be the active component that promotes oxidative metabolism and thus suppresses inflammation. While salicylate is a small molecule and is perhaps unlikely to bind to any single protein target with high affinity, the finding that AMPK is activated by salicylate (rather than inhibited like most other kinases) makes it less likely that this is a non-specific effect. Salicylates have been used for millennia without their action being understood, but it now seems important to identify the detailed molecular mechanisms. The long-term use of aspirin, particularly at high doses, is limited by the risk of gastrointestinal bleeding. However, this risk is much lower for other orally available forms of salicylate, such as salsalate. If the protective effects of aspirin on some types of cancer could be shown to be due to activation of AMPK rather than inhibition of cyclo-oxygenases, then it might make sense to treat individuals at risk of those cancers with salsalate, instead of aspirin. Future research could be aimed at testing these ideas in animal models, for example using models of inflammation or tumorigenesis in which the cell types under investigation express AMPK mutants resistant to salicylate activation. Results of these studies could then be used to guide the design of clinical trials in humans.
Outstanding questions.
Is the inhibition of inflammation and NF-κB signalling by salicylates mediated by IκKβ inhibition, by AMPK activation or by a combination of these effects?
Are the beneficial effects of long-term salicylate treatment on insulin resistance mediated by inhibition of NF-κB signalling, by AMPK activation, or by a combination of these effects?
How important is the activation of AMPK by salicylates for the prevention of cancer and/or inhibition of tumor cell proliferation?
Could the protective effects of aspirin in cancer be mimicked by salsalate, which carries a much lower risk of gastrointestinal bleeding?
Glossary Box.
| Aerobic glycolysis | another term for the Warburg effect (see below); it tends to be used when discussing cells other than tumor cells, such as cells of the immune system |
| Insulin resistance | a condition, strongly associated with obesity and excessive lipid stores, whereby organs or tissues such as liver, muscle and adipose tissue become less sensitive to the anabolic effects of insulin |
| LPS | lipopolysaccharide, a component of the outer membrane of Gram-negative bacteria that is sensed by the Toll-like receptor TLR4 |
| NSAIDs | non-steroidal anti-inflammatory drugs, including salicylates; most are thought to act by inhibiting COX1 and/or COX2 |
| Portal circulation | the part of the circulatory system via which blood passes directly from the gut to the liver, without first returning to the heart: it ensures that the liver is the organ that first encounters molecules absorbed from the gut |
| Prostanoids | extracellular mediators derived from long chain unsaturated fatty acids, especially arachidonic acid; they include the prostaglandins as well as other prostanoids such as thromboxane, which promotes platelet aggregation and hence blood clotting |
| Toll-like receptors | receptors related to the product of the Drosophila gene Toll, which activate cells of the innate immune system when they bind ligands derived from microbial infection |
| Warburg effect | the high rate of glucose uptake, glycolysis and lactate production observed in many rapidly proliferating cells, especially tumor cells |
salicylates, which include aspirin, are among the oldest drugs known to man
their known targets, COX1/2, cannot explain all anti-inflammatory actions
salicylate also activates AMPK by direct binding involving the β1 subunit
by modulating metabolism, this may account for some anti-inflammatory effects
it might also explain beneficial effects of salicylates in diabetes and cancer
Acknowledgements
GRS is supported by a Canada Research Chair in Metabolism and Obesity and grants from the Canadian Institutes of Health Research and the Canadian Diabetes Association. DGH is supported by a Senior Investigator Award from the Wellcome Trust and a Programme Grant from Cancer Research UK, and by the pharmaceutical companies supporting the Division of Signal Transduction Therapy at Dundee (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck KgaA, Janssen Pharmaceutica and Pfizer). MD was supported by a Clinical PhD studentship from the Wellcome Trust.
References
- 1.Vlot AC, et al. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 2.Higgs GA, et al. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci USA. 1987;84:1417–1420. doi: 10.1073/pnas.84.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawley SA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Din FV, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR, et al. Cyclooxygenases 1 and 2. Ann Rev Pharm Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 7.Preston SJ, et al. Comparative analgesic and anti-inflammatory properties of sodium salicylate and acetylsalicylic acid (aspirin) in rheumatoid arthritis. Br J Clin Pharmacol. 1989;27:607–611. doi: 10.1111/j.1365-2125.1989.tb03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronstein BN, et al. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci USA. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 10.Yin MJ, et al. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 11.International Centre for Kinase Profiling. Kinase Inhibitor Database. MRC Protein Phosphorylation and Ubiquitylation Unit; 2013. http://www.kinase-screen.mrc.ac.uk/kinase-inhibitors. [Google Scholar]
- 12.Shoelson SE, et al. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 14.Hundal RS, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 16.Goldfine AB, et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013 doi: 10.1007/s00125-012-2819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischman A, et al. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meex RC, et al. Stimulation of human whole-body energy expenditure by salsalate is fueled by higher lipid oxidation under fasting conditions and by higher oxidative glucose disposal under insulin-stimulated conditions. J Clin Endocrinol Metab. 2011;96:1415–1423. doi: 10.1210/jc.2010-1816. [DOI] [PubMed] [Google Scholar]
- 19.Vik-Mo H, Mjos OD. Mechanisms for inhibition of free fatty acid mobilization by nicotinic acid and sodium salicylate in canine subcutaneous adipose tissue in situ. Scandinavian journal of clinical and laboratory investigation. 1978;38:209–216. doi: 10.3109/00365517809108413. [DOI] [PubMed] [Google Scholar]
- 20.Beynen AC, et al. Inhibition of hepatic lipogenesis by salicylate. Toxicology. 1982;24:33–43. doi: 10.1016/0300-483x(82)90060-9. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 22.Hardie DG, et al. AMP-activated protein kinase: a target for drugs both ancient and modern. Chemistry & Biology. 2012;19:1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto K, et al. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lizcano JM, et al. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakhill JS, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 27.Xiao B, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goransson O, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders MJ, et al. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 31.Scott JW, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Faubert B, et al. AMPK Is a negative regulator of the Warburg effect and suppresses tumor growth In vivo. Cell Metab. 2012;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daval M, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 34.Garton AJ, et al. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- 35.Salminen A, et al. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green CJ, et al. Counter-modulation of fatty acid-induced pro-inflammatory nuclear factor kappaB signalling in rat skeletal muscle cells by AMP-activated protein kinase. Biochem J. 2011;435:463–474. doi: 10.1042/BJ20101517. [DOI] [PubMed] [Google Scholar]
- 37.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 38.Everts B, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haschemi A, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sag D, et al. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, et al. Macrophage {alpha}1-AMP-activated protein kinase ({alpha}1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galic S, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamas P, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacIver NJ, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rolf J, et al. AMPKalpha1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolf J, et al. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013 doi: 10.1002/eji.201243008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuzick J, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 50.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 51.Oshima M, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 52.Gamba CA, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: The Women's Health Initiative. Cancer. 2013;119:1562–1569. doi: 10.1002/cncr.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook NR, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 54.Veitonmaki T, et al. Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer. 2013;49:938–945. doi: 10.1016/j.ejca.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Shebl FM, et al. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer. 2012;107:207–214. doi: 10.1038/bjc.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soranna D, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomimoto A, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99:2136–2141. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeffreys D. Aspirin: the remarkable story of a wonder drug. Bloomsbury Publishing; 2004. [Google Scholar]
- 59.Warburg O, et al. Uber den Stoffwechsel der Tumoren. Biochem Z. 1924;152:319–344. [Google Scholar]
- 60.Pollard PJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 61.Young ET, et al. Multiple pathways are co-regulated by the protein kinase Snf1 and the transcription factors Adr1 and Cat8. J Biol Chem. 2003;278:26146–26158. doi: 10.1074/jbc.M301981200. [DOI] [PubMed] [Google Scholar]