Abstract
The right posterior parietal cortex (PPC) is implicated in spatial attention, but its specific role in emotional spatial attention remains unclear. In this study, we combined inhibitory transcranial magnetic stimulation (TMS) with a fear-conditioning paradigm to test the role of the right PPC in attentional control of task-irrelevant threatening distractors. In a sham-controlled within-subject design, 1-Hz repetitive TMS was applied to the left and right PPC after which participants performed a visual search task with a distractor that was either associated with a loud noise burst (threat) or not (non-threat). Results demonstrated attentional capture across all conditions as evidenced by the typical reaction time costs of the distractor. However, only after inhibitory rTMS to the right PPC reaction time cost in the threatening distractor condition was increased relative to the non-threatening distractor condition, suggesting that attention lingered longer on the threatening distractor. We propose that the right PPC is involved in disengagement of attention from emotionally salient stimuli in order to re-orient attention to task relevant stimuli and may have implications for anxiety disorders associated with difficulties to disengage from threatening stimuli.
Keywords: emotion, attentional disengagement, fear conditioning, rTMS, posterior parietal cortex
Introduction
It is crucial for our survival to prioritize threat in our environment irrespective of ongoing goals. Indeed, multiple behavioral studies indicated that threatening visual information captures attention faster and delays disengagement more than non-threatening information (Öhman et al., 2001; Fox et al., 2002; Mulckhuyse et al., 2013; Schmidt et al., 2015; Mulckhuyse and Dalmaijer, 2016; see for reviews Yiend, 2010; Carretié, 2014). Nevertheless, the neural mechanisms underlying these emotional attention processes remain unclear (LeDoux, 2003; Vuilleumier, 2005; 2015; Bishop, 2008; Vuilleumier and Huang, 2009; Pessoa and Adolphs, 2010; de Gelder et al., 2011,Tamietto and De Gelder, 2010). Current emotional attention models focus on frontal-amygdala (LeDoux, 2003; Bishop, 2008) or amygdala-sensory connections (Vuilleumier, 2005; Pourtois et al., 2013) whereas conventional spatial attention models ascribe a critical role to the right posterior parietal cortex (PPC), which is part of the dorsal fronto-parietal attention network (Corbetta and Shulman, 2002; Fox et al., 2005; Petersen and Posner, 2012) involved in shifting attention (Nobre et al., 1997; Thut et al., 2004; Mevorach et al., 2006; Hodsoll et al., 2009). However, studies that investigated the role of the right PPC in shifting attention to emotional stimuli are limited (Armony and Dolan, 2002; Keil et al., 2004; Pourtois et al., 2005; 2006; Mohanty et al., 2008, 2009; Engelmann et al., 2009; Peck et al., 2009). Therefore, the aim of this study is to examine the contributions of the right PPC in emotional spatial attention. More specifically, we tested whether right PPC is involved in attentional capture by threat (Hodsoll et al., 2009) or disengagement from threat (Chambers et al., 2004), two major components of attentional bias in emotional attention (Cisler and Koster, 2010; Clarke et al., 2013) which have not yet been disentangled in the domain of emotional attention (Armony and Dolan, 2002; Pourtois et al., 2006; Clarke et al., 2013).
To test the role of the right PPC in emotional attention, we used a sham-controlled crossover design in which inhibitory slow frequency rTMS was applied to the left and right PPC. Following inhibitory rTMS, participants performed a modified version of the additional singleton paradigm (Theeuwes, 1992), in which participants search for a target presented in a unique shape singleton while a task irrelevant salient color singleton (distractor) is presented. Typically, reaction times slow in the presence of the distractor, which has been explained as bottom-up attentional capture by the distractor and subsequent attentional dwell time on the distractor before attention disengages and shifts to the less salient target stimulus (Theeuwes, 1992, 1994, 2010). The standard approach to index attentional effects of the distractor in the additional singleton paradigm is via the interference effect, which is defined as the difference in mean reaction time in the presence versus the absence of a salient distractor. The attentional processes induced by the distractor and target in this task have been demonstrated by the activation of the dorsal fronto-parietal attention network in functional magnetic resonance imaging (fMRI) studies (de Fockert et al., 2004; Lavie and de Fockert, 2006; Talsma et al., 2010) and with the N2pc component in an electrophysiology study (Hickey et al., 2006).
A differential fear conditioning procedure (Mackintosh, 1983) was employed to associate one-colored distractor with a burst of loud noise (threatening distractor), whereas another colored distractor was not (non-threatening distractor). We hypothesized that if the right PPC is involved in bottom-up attentional control by threat, disrupting its function with rTMS should reduce the interference effect of the threatening distractor reflecting less attentional capture by the threatening distractor (Hodsoll et al., 2009). Alternatively, if the right PPC is specifically involved in top-down attentional control, then disrupting its function with rTMS should enhance the interference effect of the threatening distractor, indicative for longer attentional dwell time on the threatening distractor (Chambers et al., 2004).
Materials and methods
Participants
Twenty-six, right-handed healthy volunteers (eight males, age range 20–29 years) participated in this study. Participants were screened for contraindications for non-invasive brain stimulation (Keel et al., 2001). None of the volunteers had a history of psychiatric or neurological disease, and all had normal or corrected-to-normal vision. None of the participants was color blind. The participants were naïve as to the aim of the study, and written informed consent was obtained. The study was approved by the medical ethics committee of the University Medical Center Utrecht and Utrecht University, Utrecht, The Netherlands. Stimulation parameters were in agreement with the International Federation of Clinical Neurophysiology safety guidelines (Rossi et al., 2009) and in accordance with the standards set by the Declaration of Helsinki.
Apparatus, stimuli and design
The experimental task was run using E-prime software (release 2.0). Stimuli were displayed via a BENQ XL 2420T monitor (refresh rate: 60 Hz) and responses were recorded with a Serial Response Box (Psychology Software Tools, Sharpsburg, Inc., PA, USA). During the fear conditioning phase, a burst of white noise (200 ms, 95 dBa) was applied with a Sennheiser HD251ii headphone.
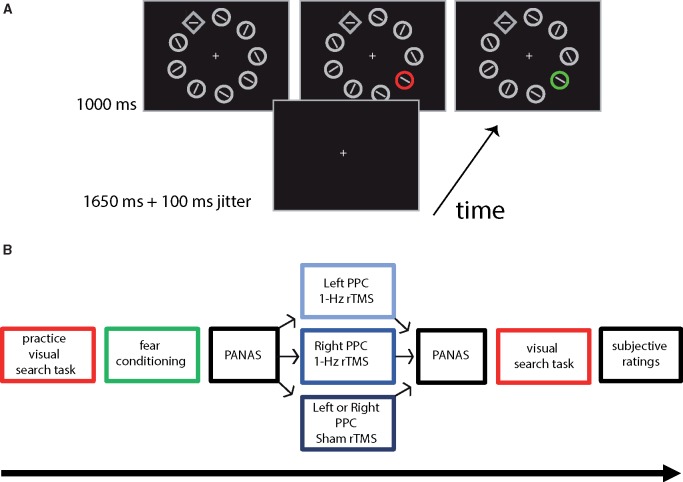
Stimuli were presented on a black background. The display consisted of nine elements equally spaced around fixation point on an imaginary circle with a radius of 6° (Figure 1A). The elements consisted of eight open circles and one open diamond, each 1.74° in diameter. In the diamond stimulus, the target was presented; either a horizontal or a vertical gray line element. In the circle stimuli, a gray line element was presented with a random orientation of 22.5° or 45° tilted to either side of the horizontal or vertical plane.
Fig. 1.
(A) From bottom to top, a sequence of events in a trial with, on the left panel, the distractor absent condition, in the middle and on the right, a CS+ and a CS− distractor condition (the colors were counterbalanced between subjects). Participants were asked to respond as fast and accurately as possible to the orientation of the line element in the unique shape (diamond) with their left (vertical line element) or right index finger (horizontal line-element). (B) Timeline of the experiment. The study consisted of three experimental sessions on three different days. Each session began with a practice block of the visual search task in which participants had to perform at 85% correct in order to continue the experiment. During the practice session, none of the distractors was reinforced. Following practice, the fear conditioning acquisition phase was applied. Colors were counterbalanced between participants and consistent for each participant among sessions. After fear conditioning, participants were asked to fill out the PANAS questionnaire. Subsequently, participants received 20 min of 1 Hz stimulation either to the left or right PPC or sham stimulation (to right or left PPC). After stimulation, they filled out the PANAS questionnaire again and subsequently, the visual search task was carried out. The experiment ended with subjective rating questionnaire.
In the color singleton absent condition, all stimuli were gray, in the color singleton present condition one of the outlines of the circles was either red or green. The red and green colors were matched for luminance.
The visual search task consisted of five blocks of 36 trials. On half of the trials, no color singleton was presented. CS + color singleton distractors and CS− color singleton distractors were presented on 25% of the trials, respectively. All trials were randomly mixed within a block. In addition, in each block one to four CS + color singleton distractor trials were added that were reinforced (in which the US was presented immediately after CS + offset) to prevent extinction (Mackintosh, 1983). These latter trials were excluded from analyses.
Each trial started with the presentation of a gray fixation dot in the center of the screen. After 1650 or 1750 ms the stimulus display was presented for 1000 ms. The diamond stimulus could appear at any of the nine locations. The color singleton distractor could also appear at any of the nine locations, but never directly next to the diamond stimulus.
Procedure
The experiment consisted of four different sessions on four different days: one intake session and three experimental sessions. During the intake session, participants performed the flicker fusion task to assess isoluminance of the red and green colors. Subsequently, participants filled out the English version or the Dutch translation of the trait anxiety inventory questionnaire (STAI-T; Spielberger et al., 1970; Van der Ploeg, 1980). Following the questionnaire, the resting motor threshold (MT) for the left and right hemisphere were determined by the thumb movement visualization method with single pulse TMS (Schutter and van Honk, 2006).
The three experimental sessions all followed the same procedure (Figure 1B). Participants started with a practice session of the visual search task of 24 trials, in which none of the trials were reinforced. They had to perform >85% correct to start with the fear-conditioning acquisition phase. Next, participants filled out the English or Dutch version of the Positive and Negative Affect Scale (PANAS) questionnaire (Watson et al., 1988; Peeters et al., 1996). The PANAS scale consists of 20 items stating a positive (10 items) or negative (10 items) adjective, such as ‘interested’ or ‘afraid’ and measures the degree of positive and negative affect using a Likert scale from 1 (not at all) to 5 (extremely). Subsequently, participants received 1 Hz rTMS stimulation of 20 min (1200 pulses). Order of left and right PPC rTMS and sham (left or right PPC) stimulation was counterbalanced across the three experimental sessions and participants. After the rTMS stimulation, participants filled out the PANAS again. Subsequently, they performed the visual search task. The time between the end of the rTMS and the start of the visual search task was about 5 min for every session. After the visual search task ratings of fear of the CS + and CS− and ratings of the intensity, unpleasantness and expectancy of the US were obtained using Likert scales (1 = ‘not at all’ to 9 = ‘extremely’) at the end of the experiment.
Visual search task
Participants were instructed to keep their eyes fixated at the fixation dot presented at the center of the screen throughout the experiment and not to make any eye movements. They were asked to respond as fast and accurately as possible to the orientation of the line element in the unique shape (diamond) with their left (vertical line element) or right index finger (horizontal line-element). In addition, they were instructed that upon presentation of the CS+, the loud noise (US) would sometimes follow. [In each block one to four additional CS + color singleton distractor trials were reinforced to avoid extinction (Mackintosh, 1983). These latter trials were excluded from analyses.] After each block, the participants received feedback about their performance.
Fear conditioning
During the fear-conditioning phase, participants viewed the color singleton distractors one-by-one at fixation and they were instructed that one of the two colors (conditioned stimulus: CS+) would sometimes be followed by the loud noise (unconditioned stimulus: US), whereas the other color (CS−) was not. The US consisted of a loud burst (95 dBa) of 200 ms white noise. In total 10 trials were presented; on half of the trials the red color singleton was presented and on the other half the green color singleton was presented. Half of the participants were presented with a red CS + and a green CS− and the other half with the reverse contingencies. Three out of five of the CS + trials were followed by the US (partial reinforcement schedule), which was presented immediately after CS + offset (Mackintosh, 1983). Colors were consistent among sessions. At the end of the acquisition phase, participants had to correctly report which color was linked to the US in order to continue the experiment.
TMS protocol
TMS was delivered with a biphasic pulse configuration using a MagVenture C-B60 Butterfly coil connected to a MagPro-X100 stimulator (MagVenture). The site of stimulation corresponded to points P3 (left parietal) and P4 (right parietal) of the 10–20 electroencephalography coordinate system. This method has been used before in TMS research stimulating PPC (Hilgetag et al., 2001; Hodsoll et al., 2009) and it has been shown that P3 and P4 correspond to regions of the left and right intraparietal sulcus, which is part of the PPC (Herwig et al., 2003). The left (M= 44.14, SD = 7.21) and right (M = 42.96, SD = 8.0) MT hemisphere were used as the reference for stimulation intensity over the left and right PPC rTMS, respectively (Schutter and van Honk, 2006). Stimulation intensity was set at 90% MT of each participant (Hodsoll et al., 2009). Participants received a 20-min 1-Hz train of pulses. In the sham condition, half of the participants received P3 (left hemisphere) sham stimulation, the other half of the participants received P4 (right hemisphere) stimulation with the coil position tilted 90° to the head.
Data analyses
For reaction time and error analyses, we performed a repeated measures ANOVA on mean reaction times and mean percentage errors, respectively. Given the presence of a significant interaction, the omnibus ANOVA on reaction time was followed up via difference scores that directly assess the magnitude of the interference effect for the threatening (CS+) and non-threatening (CS−) distractors (Hodsoll et al., 2009). We employed difference scores for two reasons: (1) because normalizing the reaction time during conditions of interest (CS + and CS−) to the distractor absent condition within each rTMS condition controls for potential confounding effects of rTMS, such as a general slowing of reaction times and (2) because assessing the impact of emotion on the interference effect was the main a priori focus of our analysis and allows comparison with prior research using this approach (e.g.de Fockert et al., 2004; Lavie and de Fockert, 2006; Hodsoll et al., 2009). Difference scores were computed by subtracting mean RT in the baseline condition (CS absent) from each condition with distractors (CS+, CS−) separately. Specifically, to assess the significant main effect of distractor, we used one-sample t-tests identifying whether interference effects were significantly larger than 0. To assess the interaction between rTMS condition and distractor, we used paired-samples t-tests to identify whether the threatening distractor had a larger impact on the interference effect than the non-threatening distractor. When appropriate, we also compared mean reaction times to further characterize the direction of the interference effect. Additional control analyses on mean reaction times are reported in Supplementary Analyses S1, and analyses of individual differences are reported in Supplementary Analyses S2. Trials in which no response was detected or in which reaction times were <150 ms (<1%) or >1500 ms (<1%) and error trials were excluded from reaction time analyses. To analyze the ratings of the subjective value of fear conditioning and the PANAS, non-parametric Friedman tests were employed, which were followed up by Wilcoxon signed-rank tests.
Results
Overall TMS was well tolerated and no serious adverse events occurred. Two participants reported mild nausea upon which we decided to stop the experiment. In addition, two participants did not receive stimulation for the full 20 min due to heating of the coil and they were excluded from the study, yielding a data set of 22 participants (7 males) in the final analysis.
Manipulation check: fear-conditioning
All participants successfully reported which color singleton (red or green) was linked with the US after the acquisition phase, indicating that participants learned the CS–US contingencies. Furthermore, collapsed across rTMS conditions, participants reported higher levels of fear of the CS + (M = 4.1, SD = 1.5) than of the CS− (M = 1.4, SD = 0.6; Wilcoxon signed-rank test, Z = 4.11, N-ties = 0, P < 0.01), and mean US expectancy ratings for the CS + (M = 5.3, SD = 1.3) was higher than for the CS− (M = 1.2, SD = 0.4; Wilcoxon signed-rank test, Z = 4.11, N-ties = 0, P < 0.01). Together with recent reports showing that US expectancy ratings are a valid proxy for fear conditioning (Boddez et al., 2013), these results indicate that we were able to successfully establish fear conditioning in all subjects.
There were no differences in the perception of different qualities of the CS + and the US (loud noise) across the rTMS conditions. Specifically, neither CS + fear ratings (χ2(2, N = 22) = 3.7, P = 0.83) nor US expectancy ratings (χ2(2, N = 22) = 4.17, P = 0.12) differed between rTMS conditions. Moreover, mean ratings of self-reported fear, intensity and unpleasantness of the US showed no significant differences between rTMS conditions (left, right and sham) in US ratings of fear (χ2(2, N = 22) = 0.86, P = 0.65), intensity (χ2(2, N = 22) = 1.07, P = 0.59) and unpleasantness (χ2(2, N = 22) = 0.09, P = 0.96). Taken together, these results suggest that our fear conditioning procedure was successful and did not differ between rTMS conditions.
Reaction time
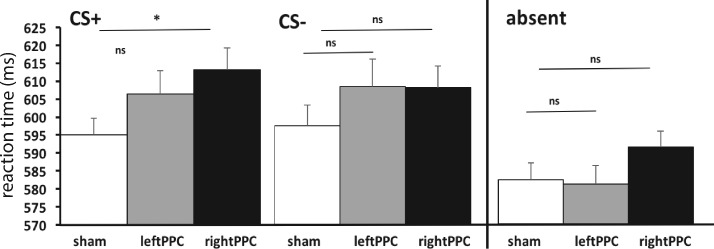
A repeated-measures omnibus ANOVA on mean reaction times (Table 1) with the factors rTMS (sham, left, right) and distractor (CS+, CS−, absent) showed a significant interaction between rTMS condition and distractor (F(4, 84) = 2.53, P < 0.05, η2p = 0.11) and a main effect of distractor (F(2, 42) = 34.1, P < 0.01, η2p = 0.62). There was no main effect of rTMS (F < 1), indicating that rTMS did not have a general effect on reaction times compared with sham. However, to ensure that rTMS indeed did not have any general slowing effects, we tested the distractor absent condition across rTMS conditions (sham, left, right) in a one-way ANOVA (Figure 3, right panel). The results showed no significant differences across the rTMS conditions in the CS absent condition: F(2, 42) = 1.039, P = 0.36, η2p = 0.047, all pairwise comparisons P ≥ 0.155. Table 1 shows the mean RTs for the rTMS (sham, left and right) and distractor conditions (CS+, CS−, absent) and the percentage of errors in each condition. Given our a priori hypothesis, in the next analysis steps we focus on the interference effect. For follow-up ANOVAs on the mean RT, see Supplementary Analyses S1.
Table 1.
Mean reaction times (and standard error of the mean) and percentage errors for the TMS (Sham, Left and Right) and distractor (absent, CS+, CS−) conditions
| TMS | Distractor |
||
|---|---|---|---|
| Absent | CS + | CS − | |
| Sham | 582 ms (9) | 595 ms (9) | 597 ms (10) |
| 3% (0.4) | 4% (0.5) | 3.4% (0.6) | |
| Left | 581 ms (8) | 607 ms (8) | 607 ms (9) |
| 3.1% (0.9) | 4.4% (1) | 4.1% (0.9) | |
| Right | 590 ms (11) | 613 ms (13) | 607 ms (13) |
| 3.9% (0.5) | 4.7% (1) | 3.2% (0.7) | |
Fig. 3.
Mean reaction time for the CS + (on the left), the CS − (in the middle) distracters and the distractor absent (on the right) for the sham, left PPC and right PPC rTMS stimulation. Error bars represent normalized standard errors (Loftus and Masson, 1994).
General interference effect observed in all conditions (main effect of distractor)
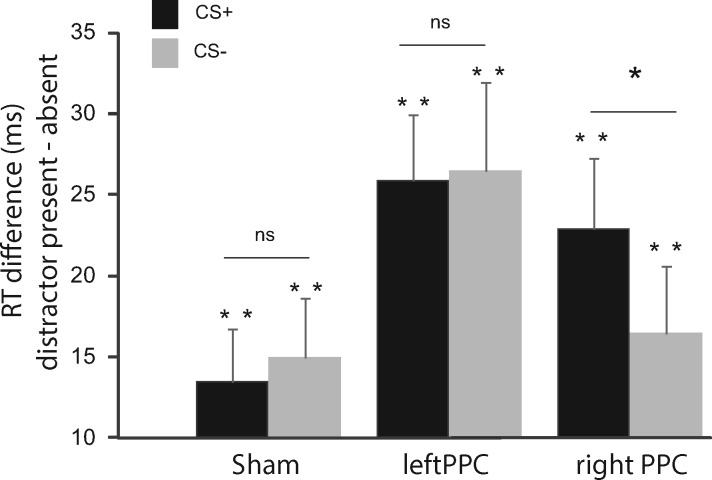
Follow-up analyses of the main effect and two-way interaction of the omnibus ANOVA focused on the interference effect by the distractor (Figure 2), operationalized as the RT difference between presence and absence of the distractor (de Fockert et al., 2004; Lavie and de Fockert, 2006; Hodsoll et al., 2009). In order to characterize the interference effect of the distractors, we performed separate one-sample t-tests for the difference scores in each rTMS condition. The results showed an interference effect in each of the rTMS conditions for both the emotional and non-emotional distractor: sham rTMS CS + (t(21) = 4.11, P < 0.01, d = 1.8), CS− (t(21) = 3.98, P < 0.01, d = 1.7), left PPC rTMS CS + (t(21) = 6.50, P < 0.01, d = 2.8), CS− (t(21) = 4.91, P < 0.01, d = 2.1), right PPC rTMS CS + (t(21) = 5.32, P < 0.01, d = 2.7), CS− (t(21) = 4.00, P < 0.01, d = 1.7). In all these cases, the observed effects were due to increased reaction times when the distractors (CS + and CS−) were presented relative to absent (all P’s survive FDR correction at a level of P < 0.000671).
Fig. 2.
Difference RT (interference effect) for the CS+ and the CS− distractors for the sham, left PPC and right PPC rTMS stimulation. The figure displays results from two specific analyses: (1) One-sample t-tests indicate significant interference effects due to increased reaction times when the distractors (CS+ and CS−) were presented relative to absent across all TMS conditions (all tests with FDR corrected P < 0.000671). Significant results of one-sample t-tests are indicated by the asterisks immediately above each bar; (2) paired-samples t-tests indicate a significantly larger interference effect for the CS+ compared with the CS− only in the right PPC rTMS, but in no other condition (both sham and the left PPC rTMS conditions with t < 1). Significant results of paired-samples t-tests are indicated by the asterisk above the lines connecting difference scores within each TMS condition. Error bars represent normalized standard errors (Loftus and Masson, 1994).
General interference effect observed in all conditions (main effect of distractor)
Follow-up analyses of the main effect and two-way interaction focused on the interference effect by the distractor (Figure 2), operationalized as the RT difference between presence and absence of the distractor (Hodsoll et al., 2009). In order to characterize the interference main effect of the distractors, we performed separate one-sample t-tests for the difference scores in each rTMS condition. The results showed an interference effect in each of the rTMS conditions for both the emotional and non-emotional distractor: sham rTMS CS + (t(21) = 4.11, P < 0.01, d = 1.8), CS− (t(21) = 3.98, P < 0.01, d = 1.7), left PPC rTMS CS + (t(21) = 6.50, P < 0.01, d = 2.8), CS− (t(21) = 4.91, P < 0.01, d = 2.1), right PPC rTMS CS + (t(21) = 5.32, P < 0.01, d = 2.7), CS− (t(21) = 4.00, P < 0.01, d = 1.7). In all these cases, the observed effects were due to increased reaction times when the distractors (CS + and CS−) were presented relative to absent (all P’s survive FDR correction at a level of P < 0.000671).
Emotional interference effect specific for right PPC (interaction effect)
In order to identify the emotional interference effect indicated by the significant interaction between distractor and rTMS, we tested whether the presence of the threatening distractor led to a difference in the interference effect reported above relative to the non-threatening distractor. This would be reflected by a significantly larger interference effect for the CS + compared with that for the CS−. Indeed, this comparison showed a significant difference between the CS + distractor and the CS−distractor condition following right PPC rTMS (t(21) = 2.14, P < 0.05, d = 0.45), due to a larger interference effect of the CS + distractor than the CS− distractor. There were no differences between the interference effect of the CS + and the CS− distractor in the sham or in the left PPC rTMS conditions (both t < 1), demonstrating that the effect of threat on the interference effect was specific for right PPC stimulation. These analyses are supported by results from one-sample repeated measures ANOVAs showing that differences in mean RT between the CS + and the CS− distractor is significant only for the right rTMS conditions but not left and sham rTMS (see Supplementary Analyses S1 for follow-up ANOVAs on the mean RTs).
Increase of RT due to CS+ distractor after right PPC stimulation
The above analyses show that the right PPC and not the left PPC is specifically involved in attentional processing of task irrelevant emotional distracters. However, the difference in the interference effect could be due to faster reaction times (reduced attentional capture) during the CS− trials, or due to slower reaction times (delayed disengagement) during the CS + trials. To examine this possibility, we performed additional t-tests on the mean reaction time in the right PPC rTMS condition relative to the sham condition for the CS + distractor and the CS− distractor separately. We found significantly slower RTs following right PPC stimulation compared with sham stimulation for the CS + (t(21) = 2.08, P = 0.05, d = 0.44), but not for the CS− (t(21) = 1.18, P = 0.25, d = 0.25) distractor. Finally, no differences following left PPC rTMS stimulation compared with sham stimulation for the CS + (t(21) = 1.21, P = 0.24, d = 0.26) nor the CS− (t(21) = .94, P = 0.36, d = 0.2) distractor were observed (Figure 3).
These results show that right PPC rTMS significantly slows down reaction time when a CS + distractor is presented and does not speed up reaction time when a CS− distractor is presented.
TMS and visual field
To assess whether distractor presentation in the ipsilateral or contralateral visual field relative to the site of TMS stimulation affected reaction times, we performed a repeated-measures ANOVA with the factors distractor position (left, right), rTMS condition (sham, left, right) and distractor (CS+, CS−). Results showed a main effect of distractor position (F(1, 21) = 11.32, P < 0.01, η2p = 0.35), indicating that subjects were faster when the distractor was presented in the left (597 ms, SE = 9.4 ms) compared with when presented in the right visual field (610 ms, SE = 8.3 ms). However, no interactions between distractor position and rTMS were observed, indicating that rTMS stimulation did not differentially influence performance based on ipsi- and contralateral stimulation with respect to distractor position (F < 1); rTMS×CS×position (F < 1). There were also no other significant main effects.
Similarly, to assess whether target presentation in the ipsilateral or contralateral visual field relative to the site of rTMS stimulation affected reaction times, a repeated-measures ANOVA with the factors target position (left, right), rTMS condition (sham, left, right) and distractor (CS+, CS−) was performed. A main effect of target position was observed (F(1, 21) = 36.11, P < 0.01, η2p = 0.63), indicating that subjects were faster when the target was presented in the right (590 ms, SE = 9.4 ms) compared with the left visual field (613 ms, SE = 8.4 ms). There were no main effects of rTMS or distractor (both F < 1). Importantly, no interactions between target position and rTMS were observed, indicating that rTMS stimulation did not differentially influence performance based on ipsi- or contralateral stimulation with respect to target position (rTMS×position (F< 1); rTMS×CS ×position: F(2, 42) = 2.25, P = 0.12, η2p = 0.097). However, a significant interaction between rTMS and distractor was observed (F(2, 42) = 4.28, P <0.05, η2p = 0.17). This shows that the same two-way interaction obtained in our main analysis above persists in the presence of additional control variables for the location of target. Follow-up tests showed increased RT after right PPC relative to sham stimulation for the CS + distractor (t(21) = 2.25, P < 0.05), but not for the CS- distractor (all other comparisons were not significant with all t’s < 1.75). These results therefore confirm the consistency of our main results reported above.
Error rates
A repeated-measures ANOVA with the factors rTMS condition (sham, left, right) and distractor (CS+, CS−, absent) indicated a statistically non-significant trend toward a main effect of distractor (F(2, 42) = 2.63, P = 0.08, η2p = 0.11), due to slightly more errors when the CS + distractor (M = 4.35, SE = 0.61) was presented relative to no distractor (M = 3.18, SE = 0.51; P < .05). There was no difference between errors when the CS + distractor (M = 4.35, SE = 0.61) was presented relative to the CS− distractor (M = 3.59, SE = 0.53; P = 0.19), nor between the CS− distractor and no distractor (P = 0.52). No main effect of rTMS on error rates and no interactions were observed (both F’s < 1).
PANAS and STAI-T
To check whether rTMS stimulation induced mood changes, we first calculated the difference scores in affect before and after each rTMS session separately for positive and negative effect. A Friedman tests for positive and negative affect between rTMS conditions (sham, left and right rTMS) did not show a significant difference in mood change (positive: χ2(2, N = 22) = 1.68, P = 0.43; negative: χ2(2, N = 22) = 0.22, P = 0.90). Together, these results indicate that the participant’s emotional state was not influenced by rTMS. Furthermore, trait anxiety was overall low and limited in range (M = 32.1, SD = 8.8) and did not correlate with any of the findings (all rs < 0.34, all P’s > 0.13).
Discussion
The aim of this study was to examine if and how the right PPC is involved in emotional spatial attention. The contribution of our findings to the literature is 2-fold. First, we demonstrate that the right PPC plays a significant role in emotional spatial attention. This is consistent with previous neuroimaging studies showing a modulation of PPC activity by emotional stimuli in spatial attention tasks (Armony and Dolan, 2002; Keil et al., 2004; Pourtois et al., 2005, 2006; Mohanty et al., 2008, 2009; Engelmann et al., 2009; Peck et al., 2009). Second, we show that its role in emotional attention is specific for top-down attentional control of emotional stimuli. In contrast to left and sham rTMS, inhibitory rTMS to the right PPC increased the interference effect of a threatening relative to a non-threatening distractor. This larger interference effect was due to an increase in mean reaction time in the presence of the threatening distractor, reflecting delayed disengagement relative to the non-threatening distractor. Moreover, the difference was not due to a decrease in reaction time in the presence of a non-threatening distractor, which would have reflected less attentional capture of the non-threatening distractor relative to the threatening distractor.
Note that inhibitory rTMS to the right PPC did not induce a general slowing effect of reaction times, which would have been evidenced by a main effect of rTMS. In addition, when the distractor was absent, there were no differences in reaction time between the three rTMS conditions. Since the response selection in this task is the same for distractor absent and distractor present conditions (e.g. Mortier et al., 2005; Theeuwes, 2010), differences in reaction time between the conditions are due to the presence of the distractor. Accordingly, our results support the specific involvement of the right PPC in attentional control of a task-irrelevant emotionally salient stimulus (see also Pourtois et al., 2006).
Increased reaction times of responses to emotional stimuli relative to neutral stimuli have been observed previously after inhibitory rTMS to right dorsolateral prefrontal cortex (dlPFC) (Zwanzger et al., 2014), suggesting that disruption of the right dlPFC also interferes with attentional processing of emotional stimuli. Similarly, de Raedt et al. (2010) showed delayed disengagement of threatening faces in an exogenous cueing task when right dlPFC was disrupted with high frequency rTMS (HF–rTMS). In this study, fMRI results showed that HF–rTMS to right dlPFC decreased activity in that area. More importantly, delayed disengagement of emotional stimuli was associated with decreased activity in right dlPFC, which was interpreted by the authors as suggesting a specific role of the right dlPFC in attentional control of emotional information (see also d’Alfonso et al., 2000; Leyman et al., 2009). Our findings, showing specific involvement of right PPC in emotional attention, extend these previous findings and suggest that interactions between parietal and frontal regions underlie to the control of emotional attention (Corbetta and Shulman, 2002; Fox et al., 2005; Petersen and Posner, 2012).
Furthermore, our results suggest that bottom-up attentional capture by emotional stimuli does not rely on the right PPC. Instead, we show an interference effect of the distractors across all rTMS conditions, which indicate that the distractors captured attention irrespective of emotional value and rTMS session. These results are inconsistent with a previous study showing a reduction of attentional capture by salient (non-emotional) distractors following inhibitory rTMS to the right PPC (Hodsoll et al., 2009). As opposed to Hodsoll et al. (2009) we may have observed attentional capture across all conditions in our experiment due to the enhanced behavioral relevance of both distractor stimuli as they signaled either the presence of threat (CS+), or safety (CS−). Attentional capture by behaviorally relevant stimuli might be mediated more by the ventral attention network. For example, it has been suggested that the right temporal parietal junction (TPJ), which is part of the ventral network and acts as a so-called ‘circuit breaker’ is especially activated by behaviorally relevant salient stimuli (Corbetta and Shulman, 2002; Fox et al., 2005, 2006; Vossel et al., 2014). Therefore, inhibiting right PPC might not influence processing of right TPJ responsible for orienting toward behaviorally relevant stimuli. Importantly, our findings show an emotional valence-dependent effect of rTMS on attentional top-down control, such that rTMS applied to right PPC disrupted disengagement only for the threatening distractor.
Finally, our observation of a right lateralized effect is consistent with previous notions of a right hemispheric dominance for visual spatial attention (Rafal, 1994; Schutter et al., 2001; Vuilleumier and Schwartz, 2001; Corbetta and Shulman, 2002; Muri et al., 2002; de Raedt et al., 2010; Zwanzger et al., 2014) and in line with previous neuroimaging research on spatial attention with non-emotional stimuli (Rushworth et al., 2001; Chambers et al., 2004; Rushworth and Taylor, 2006; Capotosto et al., 2012; see for improved ipsilateral orienting Hilgetag et al., 2001; Heinen et al., 2011). Note, that the lateralized effect is not likely to be explained by higher stimulation output for the right hemisphere as the mean MT was lower for the right than the left hemisphere. Even though the 10–20 EEG coordinate system shows a reasonable correspondence with the underlying cortical areas, we cannot rule out the possibility that P3 and P4 stimulation might have targeted adjacent regions (Herwig et al., 2003; Sack et al., 2009).
A limitation of our study is that we did not observe the expected increased interference effect for the threatening relative to the non-threatening distractor in the sham and left PPC rTMS conditions, which was previously shown in a behavioral study by Schmidt et al. (2015). However, analyses of individual differences show clearly that the enhancement of the emotional interference effect after right PPC rTMS occurs generally for our sample of participants and is not driven by a small subset of subjects showing relatively extreme results, or very different behavioral patterns during the sham compared to the TMS conditions (see Supplementary Analyses S2). Therefore, the modulation of the interference effect following inhibitory right PPC stimulation reveals that the threatening distractor was processed as such: Attention lingered longer on the CS + distractor than on the CS− distractor. Consequently, our results suggest that once attention is captured, the right PPC mediates the disengagement from an emotional salient stimulus in order to reorient to a task relevant stimulus (Chambers et al., 2004).
To conclude, results of combining rTMS with a well-controlled fear conditioned distractors in a visual search task showed that inhibitory rTMS to the right PPC increased reaction times in the presence of a threatening distractor relative to a non-threatening distractor. This finding offers evidence for the role of the right PPC in attentional control of disengagement from task irrelevant emotional stimuli.
Supplementary Material
Acknowledgements
This research was funded by a VENI grant from NWO (Netherlands Organization for Scientific Research) to M.M. K.R. was supported by a VICI grant (#453-12-001) from the Netherlands Organization for Scientific Research (N.W.O.) and a starting grant from the European Research Council (ERC_StG2012_313749). J.E. was supported by a Radboud Excellence Fellowship from the RU, Nijmegen. We would like to thank Poppy Sharp for help with data collection.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Armony J.L., Dolan R.J. (2002). Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia 40(7), 817–26. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. (2008). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences 1129(1), 141–52. [DOI] [PubMed] [Google Scholar]
- Boddez Y., Baeyens F., Luyten L., Vansteenwegen D., Hermans D., Beckers T. (2013). Rating data are underrated: validity of US expectancy in human fear conditioning. Journal of Behavior Theraphy and Experimental Psychiatry 44(2), 201–6. [DOI] [PubMed] [Google Scholar]
- Capotosto P., Babiloni C., Romani G.L., Corbetta M. (2012). Differential contribution of right and left parietal cortex to the control of spatial attention: a simultaneous EEG–rTMS study. Cerebral Cortex 22(2), 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L. (2014). Exogenous (automatic) attention to emotional stimuli: a review. Cognitive, Affective & Behavioral Neuroscience 14(4), 1228–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.D., Payne J.M., Stokes M.G., Mattingley J.B. (2004). Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience 7(3), 217–8. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Koster E.H.W. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review 30, 203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P.J.F., MacLeod C., Guastella A.J. (2013). Assessing the role of spatial engagement and disengagement of attention in anxiety-linked attentional bias: a critique of current paradigms and suggestions for future research directions. Anxiety Stress Coping 26(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience 3(3), 201–15. [DOI] [PubMed] [Google Scholar]
- d’Alfonso A.A.L., van Honk J., Hermans E., Postma A., de Haan E.H.F. (2000). Laterality effects in selective attention to threat after repetitive transcranial magnetic stimulation at the prefrontal cortex in female subjects. Neuroscience Letters 280, 195–8. [DOI] [PubMed] [Google Scholar]
- de Fockert J., Rees G., Frith C., Lavie N. (2004). Neural correlates of attentional capture in visual search. Journal of Cognitive Neuroscience 16(5), 751–9. [DOI] [PubMed] [Google Scholar]
- de Gelder B., van Honk J., Tamietto M. (2011). Emotion in the brain: of low roads, high roads and roads less travelled. Nature Reviews Neuroscience 11, 773–83. [DOI] [PubMed] [Google Scholar]
- de Raedt R.D., Leyman L., Baeken C., et al. (2010). Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biological Psychology 85(3), 487–95. [DOI] [PubMed] [Google Scholar]
- Engelmann J.B., Damaraju E., Padmala S., Pessoa L. (2009). Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience 3, 4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E., Russo R., Dutton K. (2002). Attentional bias for threat: evidence for delayed disengagement from emotional faces. Cognition & Emotion 16(3), 355–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the USA 102, 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the USA 103, 10046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K., Ruff C.C., Bjoertomt O., et al. (2011). Concurrent TMS–fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. European Journal of Neuroscience 33(5), 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U., Satrapi P., Schonfeldt-Lecuona C. (2003). Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography 16, 95–9. [DOI] [PubMed] [Google Scholar]
- Hickey C., McDonald J.J., Theeuwes J. (2006). Electrophysiological evidence of the capture of visual attention. Journal of Cognitive Neuroscience 18(4), 604–13. [DOI] [PubMed] [Google Scholar]
- Hilgetag C.C., Théoret H., Pascual-Leone A. (2001). Enhanced visual spatial attention ipsilateral to rtms-induced ′virtual lesions′ of human parietal cortex. Nature Neuroscience 4(9), 953–7. [DOI] [PubMed] [Google Scholar]
- Hodsoll J., Mevorach C., Humphreys G.W. (2009). Driven to less distraction: RTMS of the right parietal cortex reduces attentional capture in visual search. Cerebral Cortex 19(1), 106–14. [DOI] [PubMed] [Google Scholar]
- Keel J.C., Smith M.J., Wassermann E.M. (2001). A safety screening questionnaire for transcranial magnetic stimulation. Clinical Neurophysiology 112(4), 720. [DOI] [PubMed] [Google Scholar]
- Keil A., Moratti S., Sabatinelli D., Bradley M.M., Lang P.J. (2004). Additive effects of emotional content and spatial selective attention on electrocortical facilitation. Cerebral Cortex 15(8), 1187–97. [DOI] [PubMed] [Google Scholar]
- Lavie N., de Fockert J. (2006). Frontal control of attentional capture in visual search. Visual Cognition 14(4–8), 863–76. [Google Scholar]
- LeDoux J. (2003). The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology 23(4–5), 727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman L., de Raedt R., Vanderhasselt M.A., Baeken C. (2009). Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychological Medicine 39(6), 1019–28. [DOI] [PubMed] [Google Scholar]
- Loftus G.R., Masson M.E.J. (1994). Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review 1(4), 476–90. [DOI] [PubMed] [Google Scholar]
- Mackintosh N.J. (1983) Conditioning and Associative Learning. New York: Oxford University Press. [Google Scholar]
- Mevorach C., Humphreys G.W., Shalev L. (2006). Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nature Neuroscience 9(6), 740–2. [DOI] [PubMed] [Google Scholar]
- Mohanty A., Gitelman D.R., Small D.M., Mesulam M.M. (2008). The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex 18(11), 2604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Egner T., Monti J.M., Mesulam M.M. (2009). Search for a threatening target triggers limbic guidance of spatial attention. Journal of Neuroscience 29(34), 10563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier K., Theeuwes J., Starreveld P. (2005). Response selection modulates visual search within and across dimensions. Journal of Experimental Psychology: Human Perception and Performance 31(3), 542–57. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M., Crombez G., Van der Stigchel S. (2013). Conditioned fear modulates visual selection. Emotion 13(3), 529–36. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M., Dalmaijer E.S. (2016). Distracted by danger: temporal and spatial dynamics of visual selection in the presence of threat. Cognitive, Affective, & Behavioral Neuroscience 16(2), 10.. [DOI] [PubMed] [Google Scholar]
- Muri R.M., Buhler R., Heinemann D., et al. (2002). Hemispheric asymmetry in visuospatial attention assessed with transcranial magnetic stimulation. Experimental Brain Research 143(4), 426–30. [DOI] [PubMed] [Google Scholar]
- Nobre A.C., Sebestyen G.N., Gitelman D.R., Mesulam M.M., Frackowiak R.S., Frith C.D. (1997). Functional localization of the system for visuospatial attention using positron emission tomography. Brain 120(3), 515–33. [DOI] [PubMed] [Google Scholar]
- Öhman A., Flykt A., Esteves F. (2001). Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General 130(3), 466–78. [DOI] [PubMed] [Google Scholar]
- Peck C.J., Jangraw D.C., Suzuki M., Efem R., Gottlieb J. (2009). Reward modulates attention independently of action value in posterior parietal cortex. Journal of Neuroscience 29(36), 11182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters F.P.M.L., Ponds R.W.H.M., Vermeeren M.T.G. (1996). Affectiviteit en zelfbeoordeling van depressie en angst. Tijdschr Psychiatr 38, 240–50. [Google Scholar]
- Pessoa L., Adolphs R. (2010). Emotion processing and the amygdala: from a ′low road′ to ′many roads′ of evaluating biological significance. Nature Reviews Neuroscience 11(11), 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience 35(1), 73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G., Thut G., de Peralta R.G., Michel C., Vuilleumier P. (2005). Two electrophysiological stages of spatial orienting towards fearful faces: early temporo-parietal activation preceding gain control in extrastriate visual cortex. Neuroimage 26(1), 149–63. [DOI] [PubMed] [Google Scholar]
- Pourtois G., Schwartz S., Seghier M.L., Lazeyras F., Vuilleumier P. (2006). Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. Neuroimage 31(2), 920–33. [DOI] [PubMed] [Google Scholar]
- Pourtois G., Schettino A., Vuilleumier P. (2013). Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biological Psychology 92(3), 492–512. [DOI] [PubMed] [Google Scholar]
- Rafal R.D. (1994). Neglect. Current Opinion in Neurobiology 4(2), 231–6. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A.. The safety of TMS consensus group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology 120(12), 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F., Ellison A., Walsh V. (2001). Complementary localization and lateralization of orienting and motor attention. Nature Neuroscience 4(6), 656–61. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F.S., Taylor P.C.J. (2006). TMS in the parietal cortex: updating representations for attention and action. Neuropsychologia 44(13), 2700–16. [DOI] [PubMed] [Google Scholar]
- Sack A.T., Cohen Kadosh R., Schuhmann T., Moerel M., Walsh V., Goebel R. (2009). Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. Journal of Cognitive Neuroscience 21(2), 207–21. [DOI] [PubMed] [Google Scholar]
- Schmidt L.J., Belopolsky A.V., Theeuwes J. (2015). Attentional capture by signals of threat. Cognition and Emotion 29(4), 687–94. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L., Putman P., Hermans E., van Honk J. (2001). Parietal electroencephalogram beta asymmetry and selective attention to angry facial expressions in healthy human subjects. Neuroscience Letters 314(1–-2), 13–6. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G., van Honk J. (2006). A standardized motor threshold estimation procedure for transcranial magnetic stimulation research. Journal of ECT 22(3), 176–8. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. (1970). The State-Trait Anxiety Inventory (Test Manual. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Tamietto M., Coe B. (2010). Neural bases of the non-conscious perception of emotional signals. Nature Reviews Neuroscience 11(10), 697–709. [DOI] [PubMed] [Google Scholar]
- Talsma D., Coe B., Munoz D.P., Theeuwes J. (2010). Brain structures involved in visual search in the presence and absence of color singletons. Journal of Cognitive Neuroscience 22(4), 761–74. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. (1992). Perceptual selectivity for color and form. Perception & Psychophysics 51(6), 599–606. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. (2010). Top–down and bottom–up control of visual selection. Acta Psychologica 135(2), 77–99. [DOI] [PubMed] [Google Scholar]
- Thut G., Nietzel A., Pascual-Leone A. (2004). Dorsal posterior parietal rtms affects voluntary orienting of visuospatial attention. Cerebral Cortex 15(5), 628–38. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg H.M. (1980). Validatie van de Zelf Beoordelings Vragenlijst. Nederlands Tijdschr Psychol 35, 243–9. [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. (2014). Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20(2), 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Schwartz S. (2001). Beware and be aware: capture of spatial attention by fear-related stimuli in neglect. NeuroReport 12(6), 1119–22. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Science 9(12), 585–94. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Huang Y.M. (2009). Emotional attention uncovering the mechanisms of affective biases in perception . Current Directions in Psychological Science 18(3), 148–52. [Google Scholar]
- Vuilleumier P. (2015). Affective and motivational control of vision. Current Opinion in Neurology 28(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Yiend J. (2010). The effects of emotion on attention: a review of attentional processing of emotional information. Cognition and Emotion 24(1), 3–47. [Google Scholar]
- Zwanzger P., Steinberg C., Rehbein M.A., et al. (2014). Inhibitory repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex modulates early affective processing. Neuroimage 101, 193–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.