Abstract
Rumination and cognitive reactivity (dysfunctional cognitions after sad mood-induction) remain high in remitted Major Depressive Disorder (MDD) and can contribute to new episodes. These factors have been linked to increased fMRI resting-state functional-connectivity within the Default-Mode Network (DMN). It remains unclear whether (I) increased DMN-connectivity persists during MDD-remission, and (II) whether sad mood-induction differentially affects DMN-connectivity in remitted-MDD vs controls. Moreover, DMN-connectivity studies in remitted-MDD were previously confounded by antidepressant-use. Sixty-two MDD-patients remitted from ≥2 episodes, psychotropic-medication free, and 41 controls, participated in two 5-min neutral and sad mood-inductions by autobiographical-recall and neutral/sad music, each followed by 8-min resting-state fMRI-scanning. We identified DMN-components using Independent Component Analysis and entered subject- and sessions-specific components into a repeated measures analysis of variance. Connectivity-differences were extracted and correlated with baseline cognitive reactivity and rumination as measures of vulnerability for recurrence. After sad vs neutral mood-induction, controls, but not remitted-MDD, showed an increase in connectivity between the posterior-DMN and a cluster consisting mostly of the hippocampus (P = 0.006). Less posterior-DMN-hippocampal connectivity was associated with higher cognitive reactivity (r = −0.21, P = 0.046) and rumination (r = −0.27, P = 0.017). After recalling sad autobiographical-memories, aberrant posterior-DMN-hippocampal connectivity, associated with cognitive reactivity and rumination, remains a neural vulnerability in MDD-remission.
Keywords: depression, mood, autobiographical memory, cognitive reactivity, remission
Introduction
One of the reasons that Major Depressive Disorder (MDD) is a highly disabling disease is its recurrent nature (Kruijshaar et al., 2005). After a first episode, MDD becomes recurrent in at least one third of patients (Eaton et al., 2008) and the risk of recurrence increases after each subsequent episode (Solomon et al., 2000). Investigation of remitted-recurrent-MDD (rrMDD), a vulnerable group at high risk of developing new episodes, might improve our knowledge about the pathophysiology of MDD vulnerability (Hetrick et al., 2008). Theory of cognitive reactivity (CR) states that, during MDD remission, sadness and stress easily activate dysfunctional cognitive schemata that further induce depressive processing-styles (Lau et al., 2004). An example of such a style is rumination, a repetitively focusing of attention to one’s sad mood, its possible causes and its negative consequences (Koster et al., 2011). Rumination has been regarded as a sub-category of CR (Raes et al., 2012) and both have been identified as risk-factors for recurrence in MDD (Segal et al., 1999, Watkins, 2008; Michalak et al., 2011; Figueroa et al., 2015).
The neurobiological mechanisms underlying persistent CR and rumination to sad mood during remission of (recurrent) MDD have not been well examined. It has been suggested that the Default-Mode Network (DMN) is important in processes as CR and rumination (Marchetti et al., 2012). The DMN is involved in spontaneous introspective thoughts during rest and becomes deactivated when tasks require external attention (Andrews-Hanna et al., 2014). The DMN is divided in anterior (aDMN) and posterior (pDMN) parts with the medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC) as main hubs, respectively (Greicius, 2007). Additional DMN-nodes include the anterior cingulate cortex, hippocampus, lateral temporal cortex and inferior parietal cortex (Mulders et al., 2015).
The aDMN is involved in self-referential processing and emotion regulation, whereas the pDMN is implicated in episodic memory retrieval through its connection with temporal structures including the hippocampus (Andrews-Hanna et al., 2010). DMN resting-state connectivity aberrations are frequently identified in depression, (Zhang et al., 2011; Davey et al., 2012; Zhu et al., 2012; Guo et al., 2013; Li et al., 2013a; Sambataro et al., 2014; Dutta et al., 2014) with most evidence pointing to increased resting-state connectivity within the aDMN and pDMN (Kaiser et al., 2015; Mulders et al., 2015). However, decreased connectivity within only the pDMN has been observed in MDD and has been suggested to underlie episodic memory retrieval impairments (Greicius, 2007; Zhu et al., 2012). Furthermore, studies have shown altered connectivity between the a/pDMN, though directions of effects are inconsistent (Mulders et al., 2015). In addition to changes of within DMN connectivity, alterations in connectivity between the a/pDMN and other resting-state networks, including frontoparietal networks and the Salience Network, have also been reported in MDD (Kaiser et al., 2015).
Rumination has consistently been associated with increased resting-state a/pDMN-connectivity in MDD (Cooney et al., 2010; Berman et al., 2011; Hamilton et al., 2011, 2015; Lemogne et al., 2012; Zhu et al., 2012; Luo et al., 2015). Moreover, DMN-abnormalities have also been identified in subjects at risk for depression (Marchetti et al., 2012) and patients in remission (Nixon et al., 2014; Zamoscik et al., 2014; Bartova et al., 2015). In remitted adolescent-onset MDD, reduced ability to de-activate DMN-areas during a working-memory task was associated with rumination (Bartova et al., 2015). Regarding CR, three studies with different paradigms reported that sad mood-induction increased connectivity in DMN-areas in remitted-MDD (Farb et al., 2011; Foland-Ross et al., 2014; Zamoscik et al., 2014). For example, Zamoscik et al. (2014) found increased connectivity between the PCC and the parahippocampal-gyri during sad-autobiographical recall.
Given evidence of DMN-abnormalities in subjects at risk for and remitted from MDD, and the association of the DMN with cognitive risk-factors for recurrence, CR and rumination, DMN-characteristics likely represent a neural MDD vulnerability marker (Marchetti et al., 2012). However, the number of studies on this subject is still sparse. To confirm this, more research, including stress/mood inductions which are thought to increase CR and rumination (Lau et al., 2004) on DMN-connectivity as a vulnerability-marker in remitted patients is needed. In addition, studies applying mood-inductions in remitted-MDD included patients on antidepressants, which might have confounded DMN-connectivity results (Li et al., 2013a; Posner et al., 2013). Last, resting-state connectivity after mood-induction has not yet been examined.
We therefore compared the effect of mood-induction by sad vs neutral autobiographical-recall on DMN resting-state functional-connectivity in patients with rrMDD, not taking antidepressants, and matched controls with no personal/familial history of MDD. We hypothesized an overall increase in within a/pDMN-connectivity in rrMDD patients vs controls, which would be further increased after the mood-induction. We expected the mood-induction to increase CR and rumination and thus hypothesized that these dimensional measures would be associated with differences in DMN-connectivity.
Materials and Methods
Participants
Sixty-two rrMDD patients with ≥2 depressive episodes as defined by the Structured Clinical Interview for DSM-disorders (SCID), in stable remission for ≥2 months according to DSM IV-criteria, and 41 healthy controls were scanned. Hamilton Depressive Rating Scale (HDRS) scores were ≤7 and patients were not taking antidepressant medication for ≥8 weeks. The mean time between the inclusion in the study with SCID assessment and fMRI scanning was 39.8 (SD: 24.1) days. Controls did not have any history of personal or familial psychiatric disease, as determined by the SCID. All participants were aged 35–65 years. We excluded subjects with alcohol/drug dependency; psychotic or bipolar disorder; predominant anxiety disorder; severe personality disorder; electroconvulsive therapy within 2 months before scanning; history of severe head trauma; neurological disease; severe general physical illness and no Dutch/English proficiency. rrMDD patients and controls were matched for age, sex, educational level and working class. Subjects were recruited through identical advertisements in freely available online and house-to-house papers, posters in public spaces and from previous studies in our and affiliated research centres (Mocking et al., 2016). The study was approved by the accredited Medical Ethical Committee (METC) of the Academic Medical Centre (AMC). Written informed consent was obtained from all participants.
Questionnaires
Hamilton depression rating scale-17 (HDRS-17)
The HDRS-17 is an observer rated MDD-symptom scale to assess the severity of depression. The internal consistency was high, with a previously reported Cronbach’s α of 0.80 (Rush et al., 1986)
Leiden index of depression sensitivity-revised (LEIDS-R)
Self-reported levels of CR were assessed with the LEIDS-R, a trait-measure which examines response style to sad mood on five subscales, including rumination. It instructs participants to think about the last time they felt ‘somewhat sad’, and to indicate the degree to which 34 statements describe their typical cognitions/behaviours to sad mood. In a previous study, the internal consistency ranged from 0.87 to 0.95 (Cronbach’s α) (Van der Gucht et al., 2014); Cronbach’s α was 0.93 in the current study.
Ruminative responses scale (RRS-NL)
We assessed rumination in the past week with the 26-item self-report Ruminative Responses Scale (Nolen-Hoeksema, 1991) which consists of items that describe responses to a depressed mood that are focused on the self, symptoms, or consequences of depressed mood. The internal consistency of the RRS is excellent, with a Cronbach’s alpha of 0.94 (van Rijsbergen et al., 2015) and 0.96 in the current study.
Mood-induction paradigm
In the MRI-scanner participants completed both a neutral and sad mood-induction modified from Segal et al. (2006). In preparation of the MRI-procedure, participants described a memory which they regarded as neutral (doing dishes) and one which they regarded as one of the saddest in their life (losing a job, death of a spouse). Participants were encouraged to describe as many details of the most vivid memory as possible. In addition, participants chose one neutral/sad fragment of music from 10 different fragments. See Supplementary material for more information about the music fragments and distribution of music preferences for rrMDD and controls (S1/S2). We scripted these memories in key-sentences for display on the screen in the MRI-scanner. During memory display, we played the chosen neutral or sad music. After the neutral mood-induction (before the first resting-state scan), participants were asked to rate their current mood on a scale of 0–10 (0 being extremely sad; 10 extremely happy). Participants rated their mood again after the resting-state scan. After the sad resting-state scan, the most extreme sadness was rated. In addition, subjects rated their mood before and after the sad mood-induction. The gap between the neutral and sad mood-induction in which participants completed other fMRI tasks was ±125 min, including a 30 min break. We designed the sad mood-induction to be at the end of all fMRI scanning, as it would have been too straining for participants to continue fMRI scanning and tasks after the sad mood-induction (S3).
Image acquisition and analyses
A 3 Tesla Philips Achieva XT scanner (Philips Medical Systems, Best, the Netherlands), equipped with a 32-channel SENSE head coil, was used to obtain the images. A high-resolution T1-weighted 3 D structural image was acquired using fast-field echo (FFE) for anatomical reference (220 slices; TR: 8.3 ms; TE: 3.8 ms; FOV: 240 × 188; 240 × 240 matrix; voxel size: 1 × 1 × 1 mm3). Functional images were acquired with T2*-weighted gradient echo planar imaging (EPI) sequences. Participants were instructed to close their eyes and to not fall asleep. The scans comprised 210 volumes of 37 axial-slices (TR: 2000 ms; TE: 27.6 ms; FOV: 240 × 240; 80 × 80 matrix; voxel size = 3 × 3 × 3 mm). Slices were oriented parallel to the AC-PC transverse plane and acquired in ascending order with a gap of 0.3 mm.
Preprocessing
Scans were preprocessed and analyzed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm12). First, structural and functional images were manually reoriented parallel to the AC-PC plane. Next, functional images were realigned for each subject to correct for head motion during acquisition using rigid body transformations and the mean image as reference. Then, the realigned functional images were co-registered to the structural images for each subject, segmented into CSF, WM and grey matter using the default segmentation routine of SPM12, and the functional images were normalized to MNI space. Finally, the functional data was smoothed with a smoothing kernel of 10 mm at FWHM.
ICA analysis
Preprocessed functional data were decomposed into 25 spatially independent components using the Group ICA fMRI Toolbox (GIFT v2.0a) with the Infomax algorithm (http://icatb.sourceforge.net/gift/gift_startup.php; Calhoun et al., 2001, 2004) (S4). ICA is a data driven technique which decomposes fMRI data from linear mixed signals into spatially independent components. Components consist of a time course showing the temporal fluctuations of that component and a spatial map that shows the contribution of every voxel. A strength of ICA is that it is able to separate true neural networks and noise artifacts occurring in similar frequency ranges (Calhoun et al., 2001; van de Ven et al., 2004; Beckmann et al., 2005). Further, it has been shown that group ICA is able to characterize individual variation such that might occur across sessions and participants (Calhoun et al., 2001; Allen et al., 2012). The number of components was set to 25, in line with previous reports on low-order ICA for examining robust resting-state networks (Smith et al., 2009; Zuo et al., 2010). The set of spatial maps from the group-average analysis was used to generate subject-specific versions of the spatial maps and associated time-series using spatio-temporal regression (Beckmann et al., 2009; Filippini et al., 2009). The analysis resulted in subject-specific spatial maps of two sessions per subject, and one spatial map per group-level. Components were scaled to percent signal change for comparison between subjects.
We identified neural networks of interest and discarded noise components. The ICA-components were judged independently by visual comparison by C.A.F. and S.M. according to components described by Menon (2011). After the researchers independently selected the components of interest, they were assessed for agreement, in accordance with a third researcher, G.v.W. Among neural networks of interest, we selected the aDMN and pDMN component (S5). In order to assess whether potential effects on the DMN are DMN-specific or whether similar effects are also observed in other resting-state networks affected in depression, (Damoiseaux et al., 2006; Allen et al., 2012) we additionally selected the right and left Central Executive Network (CEN) and the Salience Network (SN) (S6).
Second level analyses
The individual subject maps of relevant ICA-components were used as input for a repeated measures Analysis of Variance (rmANOVA) in SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12). The rmANOVA in SPM pools the error (unexplained variance) over the full design, instead of partitioning the error across subject effects, which in classical behavioral ANOVA’s has been argued to be the preferable approach. However, in the case of neuroimaging research, there is evidence that using a pooled error can be more powerful than using a partitioned error (Henson, (2005)), although this issue remains under debate. In order to approach this issue empirically and document the effect of partitioned variance, we additionally performed analyses using GLM flex (http://mrtools.mgh.harvard.edu), which uses a partitioned error (see S-Results in the Supplementary material).
In a full factorial model in SPM12, the effect of mood (neutral/sad mood-induction) was a within-subject factor, and group (rrMDD/control) a between-subjects factor. We examined the group*mood-induction interactions and corrected for multiple comparisons with Bonferroni correction for examining five networks [P < 0.01 (0.05 divided by 5), FWE cluster corrected with an initial height threshold of P < 0.005 uncorrected]. We chose an a-priori cluster defining threshold of P < 0.005 because we were interested in diffuse whole brain effects as we (i) measured connectivity differences after the mood induction, and (ii) measured patients remitted from MDD as opposed to during the acute phase of MDD. Finally, we extracted functional-connectivity values of significant clusters (first eigenvariate) and quantified correlations between functional-connectivity differences between neutral and sad mood with RRS and LEIDS-R scores. For analyses with RRS-scores, we excluded one univariate outlier based on z-scores >3. For correlations between RRS and connectivity change, we used Spearman rank correlation because RRS-scores were not normally distributed in controls (log transformation did not lead to a normal distribution). We additionally examined for multivariate outliers based on Mahalanobis distance (De Maesschalck et al., 2000). Additionally, to examine whether correlations differed between rrMDD and controls, we tested group*RRS and group*LEIDS-R interactions in linear regression analyses with connectivity-changes as dependent variable.
Results
Sample characteristics
Seventy-two rrMDD patients and 46 controls were initially eligible of which 62 and 41 were scanned, respectively. Of these participants, we excluded five rrMDD with excessive head motion (within-scan movement >3 mm and within scan-rotation >1.5°), two rrMDD due to technical difficulties, and three rrMDD and two controls with abnormal brain anatomy (judged by a neuro-radiologist). Fifty-two rrMDD and 39 controls were included in the ICA analysis (S7). No significant differences were observed between rrMDD and controls for sex, age, education, IQ, living situation, employment status and handedness. The rrMDD showed significant higher levels of residual depressive symptoms (HDRS) P < 0.001, CR (LEIDS-R) P < 0.001 and rumination (RRS), P < 0.001 (Table 1). Comparisons between rrMDD and controls did not change when restricted to the sample selected for the ICA analyses.
Table 1.
Sample characteristics
| rrMDD | HC | Between-group statistics |
|||||
|---|---|---|---|---|---|---|---|
| (n = 62) | (n = 41) | χ2 | T | U | p | ||
| Female | N(%) | 43 (69.3%) | 28 (68.3%) | 0.01 | 0.91 | ||
| Age | Years; mean (SD) | 53.7 (7.9) | 51.8 (8.1) | 1.17 | 0.25 | ||
| Education | Levels1 | 0/0/0/4/21/23/14 | 0/0/0/1/16/17/7 | 1.49 | 0.69 | ||
| IQ | Mean (SD) | 108 (8.5) | 106 (9.9) | 878.5 | 0.14 | ||
| Living situation | Levels2 | 26/0/18/14/2/0/2 | 10/0/16/11/4/0/0 | 6.23 | 0.18 | ||
| Employment status | Levels3 | 24/23/15/0 | 21/16/4/0 | 3.7 | 0.16 | ||
| Handedness | Levels4 | 4/50/4 | 2/33/4 | 0.44 | 0.8 | ||
| Age of onset | Years; mean (SD) | 27.2 (11.2)5 | – | – | |||
| Episodes | Median (IQR) | 4.0 (2/4/7) 5 | – | – | |||
| HDRS | Mean (SD) | 2.77 (2.31) | 11.45) | −4.5 | <0.001 | ||
| LEIDS-R | Mean (SD) | 38.8 (14.1) | 15.3(15.4) | −7.5 | <0.001 | ||
| RRS | Median (IQR) | 35 (29/35/47) | 25(22.5/25/26.5) | 341.5 | <0.001 | ||
HC, healthy control; HDRS, Hamilton Depression Rating Scale; rrMDD, remitted recurrent major depressive disorder; LEIDS-R, Leiden Index Depression Sensitivity-Revised; RRS, Ruminative Response Scale. 1Level of educational attainment (Verhage: 1964): levels range from 1 to 7 (1 = primary school not finished: 7 = pre-university/university degree).
Living situation: alone/living with parents/cohabiting/cohabiting with children/single living with children/other/unknown.
Employment status: low/middle/high/never worked.
Handedness: left/right/ambidexter; IQR: Inter-quartile range; χ2: chi-square test statistic; P: P-value; U: Mann–Whitney U non-parametric test statistic; T: independent-samples T test statistic.
Mood-ratings
Both groups reported comparable neutral to positive mood after neutral mood-induction (P = 0.095). Mood-scores (±SD) decreased slightly during neutral resting state (rrMDD: −0.5 ± 0.94, controls: 0.22 ± 0.66; P = 0.002), without a group*mood interaction (P = 0.146). The sad mood-induction significantly decreased mood-scores in both groups (P < 0.001), without a group*mood-induction interaction (P = 0.58). The saddest mood reported during the second resting-state was significantly lower in rrMDD than in controls (P = 0.016; Table 2).
Table 2.
Mood-ratings before and after neutral resting state scan and sad mood induction
| rrMDD |
HC |
Between-group statistics |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | U | F | P | |
| Neutral Mood | |||||||
| Before scan | 7.28 | 0.87 | 7.62 | 0.76 | 769 | 0.095 | |
| After scan | 6.86 | 1.21 | 7.35 | 0.67 | 587 | 0.103 | |
| Difference1 | −0.5 | 0.94 | −0.2 | 0.66 | 10.8 | 0.146 | |
| Sad Mood | |||||||
| Before MIP | 6.31 | 1.24 | 6.99 | 0.79 | 511 | 0.009 | |
| After MIP | 4.04 | 1.73 | 5.1 | 1.37 | 566 | 0.001 | |
| Difference2 | −2.2 | 1.36 | −2 | 1.17 | 192 | 0.581 | |
| Lowest* | 5.1 | 1.73 | 6 | 1.37 | 532 | 0.016 | |
HC, healthy control; MIP, Mood-induction Procedure; rrMDD, remitted recurrent major depressive disorder; U, Mann–Whitney U non-parametric test statistic; F, F-statistic from repeated measures analysis; P, P-value; SD, Standard Deviation. 1Scores significantly decreased during neutral resting-state in both groups (P = 0.002).
Scores significantly decreased during sad resting-state in both groups (P < 0.001). *Subjects rated their lowest mood during the sad resting-state scan retrospectively.
Resting-state connectivity
The aDMN component showed no significant group*mood-induction interaction. There was a main effect of mood-induction with increased connectivity in the contrast sad vs neutral mood-induction in the left insula (both rrMDD and controls; peak coordinates: x = −28, y = −8, z = 18; k = 881, Z = 3.86, pFWE < 0.005).
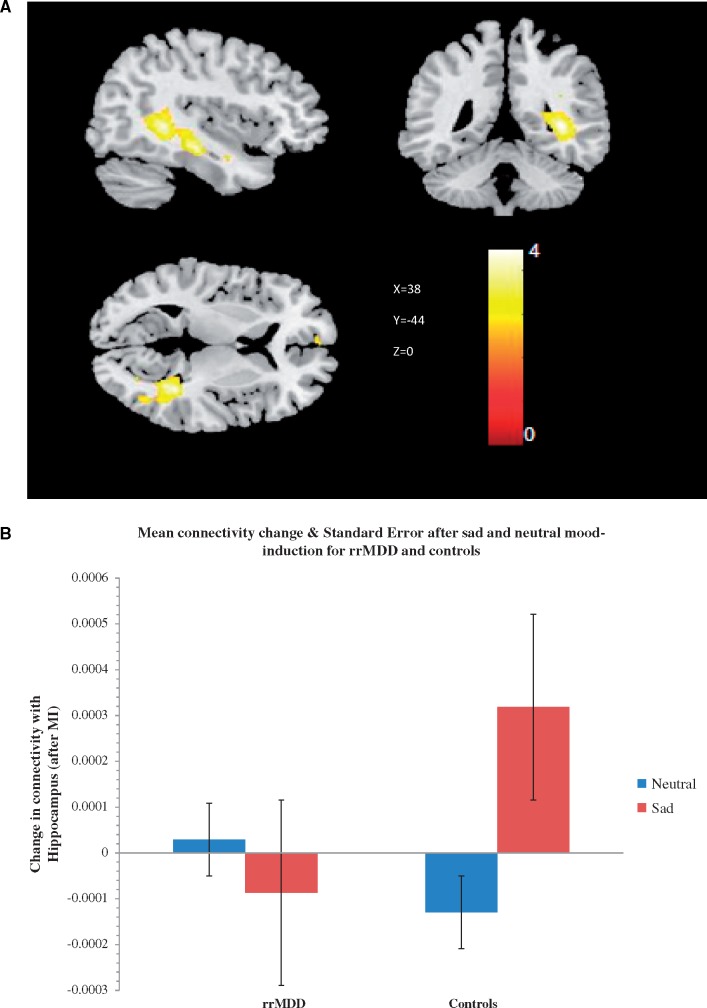
The pDMN component showed a significant group*mood-induction interaction: compared to rrMDD, controls showed increased connectivity after sad vs neutral mood-induction of the pDMN with the right medial temporal lobe including the right hippocampus [x = 38, y = −44, z = 0, and x = 40, y = −22, z = −18, k = 875, Z = 4.38, pFWE0.006, (Figure 1A and B)]. Further exploration using an xyz toolbox (http://www.gin.cnrs.fr/AAL?lang=en.; Tzourio-Mazoyer et al., 2002), revealed that 80.1% of the cluster consisted of voxels located in the hippocampus, 7.54% of the right parahippocampal gyrus, 5.71% of the right precuneus, 1.60% of the temporal inferior gyrus right, 1.60% of the right calcarine cortex and 1.03% of the right temporal medial gyrus. No significant main effect of mood-induction was observed. Post-hoc analyses stratified for group showed that in controls, sad mood-induction increased connectivity of the pDMN with the medial temporal lobe, including the right hippocampus and right medial temporal gyrus, (x = 32, y = −52, z = 6, k = 805, Z = 4.73, pFWE = 0.010) and left middle frontal gyrus (x = −36, y = 10, z = 44, k = 511, Z = 3.75, pFWE0.044). In rrMDD, there were no significant increases or decreases in connectivity after sad mood-induction (P > 0.05). In order to assess whether effects were DMN-specific we additionally examined the right and left CEN and the SN. There were no significant group*mood-induction interactions for the left and right CEN and SN (all P > 0.05).
Fig. 1.
Group x mood interaction regarding the connectivity of the posterior DMN map with the right hippocampus. (A) Cluster of 875 voxels (x = 38, y = −44, z = 0) covering the right hippocampus was more connected with the posterior DMN after sad autobiographical recall in controls, but not in remitted-patients (p(FWE corrected) = 0.006). Color scales represent t-values. (B) Mean connectivity change and standard error in the right hippocampus, plotted for every group and mood state. Patients showed no significant change in connectivity in response to sad mood-induction, while controls show an increase of connectivity.
Additional analyses by means of two sample T-tests for resting-state connectivity after neutral mood-induction showed that there were no significant differences between rrMDD and controls during neutral mood state for the a/pDMN and the left/right CEN and SN (all P’s > 0.05).
Correlation analyses
We finally assessed whether the effects of sad mood-induction on pDMN-hippocampus-connectivity were associated with CR- or rumination-scores. There were significant negative correlations between this connectivity for neutral vs sad mood-induction with LEIDS-R scores (CR) r = −0.21, P = 0.046, and RRS-scores (rumination) r = −0.26 P = 0.017 (Figure 2A and B). Because residual symptoms were not associated with pDMN-hippocampus connectivity change (P = 0.36), we did not additionally correct for residual symptomatology in our analyses. The correlations between RRS and connectivity-change remained significant after excluding one multivariate outlier (r = −0.22, P = 0.045). Group*RRS and group*LEIDS-R interactions were not significant in linear regression analyses with hippocampal connectivity-change as dependent variable (P = 0.31 and P = 0.43, respectively), indicating that the correlation-coefficients did not differ between groups. The change in pDMN-hippocampus connectivity was not associated with mood-change after sad mood-induction (P = 0.49) or lowest mood during second resting-state (P = 0.14). In the rrMDD group, we found no association between the number of previous episodes and change in pDMN-hippocampus connectivity (P = 0.126). Together, these results suggest that individuals with higher CR and rumination showed attenuated pDMN-hippocampus connectivity after sad mood-induction, regardless of whether they were in the control or rrMDD group.
Fig. 2.
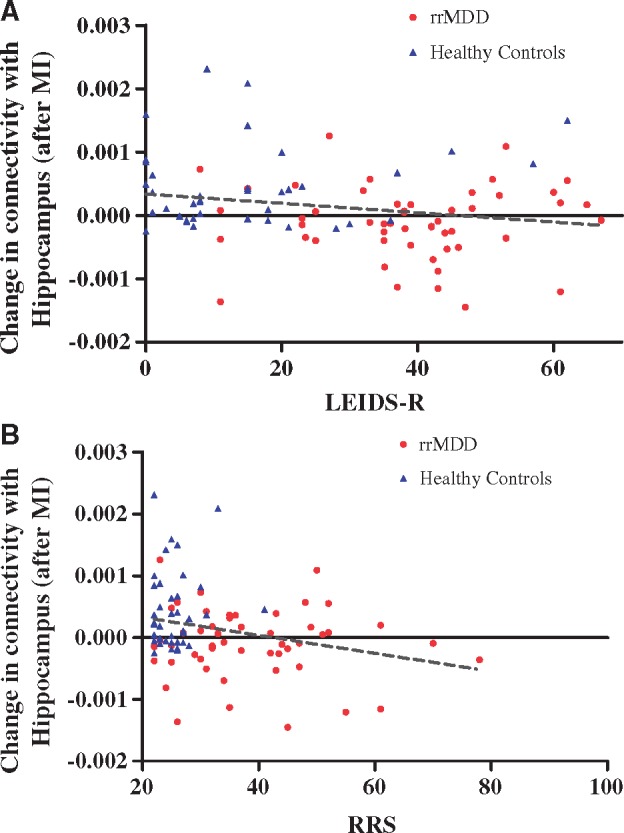
Correlations between change in hippocampus connectivity after sad vs neutral mood-induction and rumination and cognitive reactivity. (A) Significant negative correlation between the decrease in hippocampal connectivity for neutral vs sad mood-induction with LEIDS-R scores (CR) r = −0.21, P = 0.046. (B) Significant negative correlation between the decrease in hippocampal connectivity for neutral vs sad mood-induction with RRS-scores r = −0.27; P = 0.017. The effect remained significant after excluding one multivariate outlier in the rrMDD group. LEIDS-R, Leiden Index Depression Sensitivity-Revised; RRS, Ruminative Response Scale; rrMDD, remitted-recurrent Major Depressive Disorder; MI, Mood-induction.
Discussion
This study examined DMN resting-state connectivity after recalling sad autobiographical memories in medication-free rrMDD patients vs controls, and the association with CR and rumination. Contrary to the a-priori hypothesized increased overall DMN-connectivity in rrMDD, after sad vs neutral autobiographical-recall, controls showed increased pDMN connectivity to a cluster consisting mostly of the hippocampus, and additionally of temporal/occipital regions, which was not present in rrMDD. Moreover, less pDMN-hippocampus connectivity was associated with two MDD-recurrence risk-factors: higher CR and rumination. This suggests that aberrant connectivity between the pDMN and the hippocampus and temporal/occipital areas in remitted-MDD after sad autobiographical recall represents a neural MDD-vulnerability factor. There were no interaction-effects of group*mood-induction on the salience and central executive network, suggesting that effects were specific for the DMN. Moreover, there were no baseline connectivity differences (after neutral mood-induction) between rrMDD and controls.
We hypothesize that the aberrant pDMN-hippocampus connectivity in rrMDD, thus the failure to show increased connectivity between the pDMN and hippocampus after sad autobiographical recall, might be related to the phenomenon of over-general autobiographical memory (OAM), persisting in rrMDD (Spinhoven et al., 2006). OAM refers to the observation that individuals retrieve autobiographical memories in a less detailed, more generic form, referring to series of events and general self-knowledge, which occurs more in MDD-vulnerable subjects (Sumner, 2012). OAM has been associated with a poor MDD-prognosis and is maintained by rumination (Sumner, 2012). Indeed, during resting-state, connectivity of the hippocampus and other temporal areas with (posterior)-DMN areas is increased during autobiographical thoughts (Andrews-Hanna et al., 2010). Task-based studies show that the hippocampus is recruited more during retrieval of specific compared to generic memories (Maguire and Mummery, 1999) and that hippocampus activity is associated with the vividness of memories (Addis et al., 2004; Ford and Kensinger, 2016). In depression, hippocampus connectivity was reduced during autobiographical recall, even when specificity of memories was matched (Hach et al., 2014). In addition, it has been suggested that individuals who spontaneously invoke more specific memories have a stronger connectivity of the hippocampus to the DMN during resting-state (Yang et al., 2012). Thus, our result of aberrant connectivity between the pDMN and hippocampus might indicate that controls were more engaged in details of their specific memory after autobiographical recall than remitted-MDD, leading to an increase in hippocampal involvement within the DMN, which was absent in remitted-MDD.
Further supportive of this hypothesis, detailed memories are thought to be stored in and retrieved by the hippocampus, whereas memories that are generic are incorporated in neocortical networks of overlapping memories (Preston and Eichenbaum, 2013). The storage and retrieval of memories is influenced by pre-existing schemas; self-learned knowledge about the world, the self and others. For instance, van Kesteren et al. (2010) found that the existence of prior schemas was associated with reduced connectivity between cortical areas and the hippocampus during memory encoding (van Kesteren et al., 2010). This pattern persisted during 15 min resting-state, suggesting that when memories fit into schemas, they are also less processed by the hippocampus during rest. In rrMDD, dysfunctional schemas have been found to persist and, corroborative with the theory of CR, can be (further) activated by sad mood (Lau et al., 2004). For rrMDD patients, negative autobiographical memories will better fit with prior (depressive) schemas and might thus be retrieved by other neural structures than the hippocampus. In a post-hoc analysis we examined the correlation between change in connectivity between de pDMN and cluster consisting mostly of the hippocampus, and a measure of dysfunctional schemas; the Dysfunctional Attitudes Scale (DAS); see SDiscussion in Supplementary material. DAS-scores were trendwise negatively correlated with pDMN-hippocampus connectivity-change (r = −0.22, P = 0.059). This indicates that subjects with increased pDMN-hippocampus connectivity might also have lower levels of dysfunctional schemas, corroborative with our hypothesis (S9).
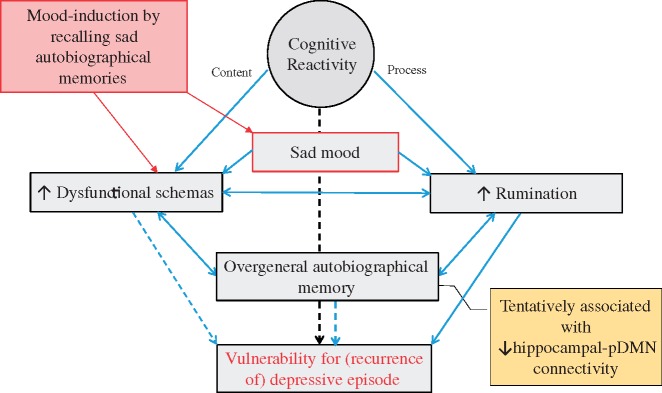
Evidence from behavioral studies suggests that CR, dysfunctional schemas, rumination and OAM are related constructs (Figure 3). Importantly, CR, dysfunctional schemas and rumination have all been identified as risk-factors for recurrence, (Segal et al., 1999; Michalak et al., 2011; Jarrett et al., 2012; van Rijsbergen et al., 2013; Figueroa et al., 2015) although not unequivocally for CR (Jarrett et al., 2012; van Rijsbergen et al., 2013) and dysfunctional schemas (Figueroa et al., 2015). Therefore, we propose that the observed abberant pDMN-hippocampus connectivity, through its association with CR, rumination and dysfunctional schemas, represents a neural vulnerability marker, possibly contributing to new MDD-episodes.
Fig. 3.
Proposed relations between recalling sad autobiographical memories, cognitive risk factors and risk of recurrence in remitted MDD. Lines and arrows indicate directions of relations, dotted lines represent directions for which evidence is not unequivocal. Recalling sad autobiographical memories leads to induction of sad mood, which increases dysfunctional schemas and activates rumination [cognitive reactivity (CR); Koster et al., 2011; Lau et al., 2004]. As negative autobiographical memories fit with dysfunctional schemas, sad autobiographical memory recall itself also leads to increase in dysfunctional schemas (Raes et al., 2012). The activation of dysfunctional schemas facilitates a ruminative-state: individuals are unable to disengage from negative, ruminative thoughts, as these are congruent with activated schemas (Koster et al., 2011). Rumination is a causal factor of Over-general autobiographical memory (OAM): it interferes with the retrieval of specific memories (Sumner, 2012). OAM can also strengthen rumination (Sumner, 2012). Further, OAM, aggravated by rumination, reinforces the completion of thinking patterns by abstract representations, as dysfunctional schemas (Belzung et al., 2015). CR refers to both the content of thoughts (dysfunctional schema's) as well as the process (rumination) invoked by sad mood (Lau et al., 2004; Raes et al., 2012). CR and dysfunctional schema's have been identified as risk-factors for recurrence, although evidence is mixed (Figueroa et al., 2015, van Rijsbergen et al., 2013). Rumination has been identified as an important risk-factor for recurrence (Michalak et al., 2011). OAM has been associated with new episodes (Sumner, 2012) though reports of OAM as a risk-factor for recurrence vary (Spinhoven et al., 2006). We hypothesize that all these processes act together, and are associated with abberant hippocampal-DMN connectivity, to increase risk of depressive recurrence.
In addition to the hippocampus and other temporal areas, the cluster in which we observed aberrant connectivity in rrMDD contained occipital areas (calcarine cortex). Occipital regions are responsible for visual perception, higher order visual association and attention, and processing of mental imagery and visual memory (Slotnick et al., 2012). Interestingly, Farb et al. (2011) found that during a sad mood-induction by playing sad film clips, reactivity of the calcarine cortex was protective of relapse within 18 months in remitted MDD patients, and that this reactivity was associated with a self-report tendency towards adaptive (non-judgmental) cognition during dysphoric feeling states. In addition, a previous study, Piguet et al. (2014), found that a lower tendency to ruminate correlated with increased visual cortical connectivity during resting-state, and decreased occipital lobe resting-state connectivity (Zou et al., 2016) and regional homogeneity (Peng et al., 2011) have been reported in MDD. Furthermore, it has been argued that deficits in the occipital lobes may be an initiating factor for depression onset (Li et al., 2013b). We therefore hypothesize that aberrant occipital connectivity may be related to cognitive vulnerability in rrMDD after sad mood-induction.
We could not confirm the a priori hypothesized increased DMN-connectivity in rrMDD compared to controls after sad autobiographical-recall. Several explanations are possible. First, DMN-connectivity in antidepressant-free remitted rMDD might differ from acutely depressed-subjects. Although in contrast to previous theory, (Marchetti et al., 2012) DMN increased-connectivity might represent a state effect of MDD more than a vulnerability during remission. Second, although most evidence points to increased pDMN connectivity, decreased pDMN connectivity has been observed before in MDD and was associated with over-general memory (Greicius, 2007; Zhu et al., 2012). Thus, aberrant DMN resting-state connectivity might indeed represent a neural vulnerability, as aDMN/pDMN connectivity can be increased or decreased, according to different DMN-functions. Future studies should examine how DMN sub-divisions are affected in rrMDD after different mood-induction paradigms. Importantly, we did not observe any baseline DMN-connectivity differences between rrMDD and controls after neutral mood-induction. Two previous studies using ICA to examine resting-state differences between patients with mild depression or remitted-MDD and controls also found no differences in within DMN connectivity (Veer et al., 2010; Sexton et al., 2012). That we only found connectivity differences after sad mood-induction, highlights the importance of stressful triggers, such as sad autobiographical memories, to unmask MDD-vulnerability (Lau et al., 2004). However, differences between groups even after sad mood-induction were more subtle than we initially expected.
Zamoscik et al. (2014) used a comparable mood-induction with sad autobiographical-recall and identified increased connectivity between the PCC (pDMN) and bilateral para-hippocampal gyri (PHG) in rrMDD compared to controls (Zamoscik et al., 2014). These results appear contradictory to our observed decreased connectivity between the pDMN and hippocampus. Differences between Zamoscik et al. and our study might be explained by the fact that Zamoscik et al. did not contrast sad- to neutral mood-induction and included patients on antidepressants. An alternative explanation of differences between our study and Zamoscik et al., is that we examined resting-state connectivity after autobiographical recall, instead of during. During the task, subjects might be more intensely engaged in their memory, resulting in increased pDMN-PHG connectivity, implicated in memory processing. We suggest that after sad mood-induction, when subjects are no longer required to focus on details of their negative memory, OAM might become more pronounced, characterized by a failure to increase pDMN-hippocampus-connectivity rrMDD. Indeed, some behavioral studies suggests that, in rrMDD, OAM has to first be activated by a state of rumination (such as a mood-induction; Raes et al., 2012). Both studies highlight the role of dysfunctional memory processes in association with neural substrates, as a vulnerability in remitted MDD.
A strength of this study is that we examined a large rrMDD sample in stable remission and not taking medication. This allowed us to examine effects of vulnerability instead of depressive-state, and without confounding effects of antidepressants. A second strength is that we used a repeated-measures design to contrast sad- with neutral autobiographical-recall, increasing the likelihood that our effects are due to sad mood-induction. A first limitation is that we used a relatively lenient initial threshold for cluster-wise correction of P < 0.005 uncorrected, because we were interested in diffuse effects after mood-induction in a group of patients remitted from depression. Because a recent study reported that using lenient initial cluster based thresholds can result in too many false positive findings (Eklund et al. 2016), we also examined results when using an initial threshold of p < 0.001 (FWE cluster corrected P < 0.05). When using this more stringent threshold, only a cluster containing occipital and temporal areas (right calcarine cortex 98.26%, right fusiform gyrus, 0.69, precuneus 0.35%) remained significant (FWE P = 0.023). With this more stringent threshold of P < 0.001, we also observe a cluster which includes right hippocampus 44.14%, right parahippocampal gyrus 30.63%, right temporal inferior gyrus 21.62% and right fusiform gyrus 1.80% (x = 40, y = −22, z = −18, k = 111), which is however no longer significant (FWE P = 0.30). Supplementary Figure S10 shows how the spatial extent of the cluster changes for P < 0.005 and P < 0.001. Thus, our result of increased pDMN-hippocampus connectivity is likely a subtle effect which does not survive a more stringent threshold. To investigate the reproducibility of our findings, results of this study should be replicated by future studies, and should be included by meta-analyses. Second, the nature of resting-state precludes certainty about subjects’ thoughts during the scan. Thus, we cannot be certain that rrMDD-subjects were engaged in over-general autobiographical memories. Nevertheless, considering the function of the hippocampus and the associations of attenuated hippocampal-pDMN connectivity with rumination, and CR, it is likely that our findings are representative of dysfunctional (memory) processing. Future studies using a comparable paradigm should also include measures of (trait) OAM. Finally, although examining patients free of antidepressants precludes confounding medication-effects, this might have led to inclusion of a sample of subjects not representative of the general rrMDD-population.
Conclusion
After sad autobiographical recall, connectivity between the pDMN and a cluster consisting mostly of the hippocampus was increased in healthy controls, whereas unmedicated remitted-MDD patients failed to show this greater pDMN-hippocampal connectivity. The observed reduced connectivity was associated with known risk-factors for recurrence; CR and rumination. Aberrant pDMN-hippocampus connectivity after sad autobiographical recall in remitted MDD might represent an MDD vulnerability marker. This study adds evidence that the DMN is an important neural-network that is persistently dysfunctional during remission in patients at high risk for recurrence, but only in the presence of stressful triggers.
Supplementary Material
Acknowledgements
Role of the Funder/sponsor: None of the supporting organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for submission.
Funding
This study is supported by unrestricted personal grants from the Academic Medical Centre to C.A. Figueroa (AMC MD-PhD Scholarship) and R.J. Mocking (AMC PhD Scholarship) and a dedicated grant from the Dutch Brain Foundation (Hersenstichting The Netherlands: 2009(2)-72). Dr. H.G. Ruhé is supported by a NWO/ZonMW VENI-Grant #016.126.059.
Additional contributions
First of all, we would like to thank the study subjects that participated in this research. Second, we acknowledge the thoughtful comments of Prof. Z. Segal1 and Prof. C.L. Bockting2 regarding our in-scanner mood-induction procedure and thank Prof. R. de Raedt3 and Dr. I. Marchetti3 for their suggestions regarding the analyses and interpretation of results.
Third, we would like to thank the following persons who helped collect/process data: Eline Meijer4, Lisa Bouma, Bsc4, Gelera Mahmoud, Msc4 helped with collection of data; Henk Hallie5 helped with input and checking of questionnaire data; Michelle Servaas, PhD5, helped with pre-processing of fMRI data; Jan-Bernard Marsman, PhD5, and Maaike Rive, MD4 gave advice regarding the ICA analysis; Egill Axfjord Fridgeirsson helped to conduct the GLM Flex analysis. No one that helped collect/process data received financial compensation for their contributions.
These persons are affiliated with: 1University of Toronto, Toronto, Canada, 2University of Ghent, Ghent, Belgium, 3Univeristy of Utrecht, Utrecht, the Netherlands, 4the Academic Medical Center/University of Amsterdam, Amsterdam, the Netherlands; 5University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Addis D.R., Moscovitch M., Crawley A.P., McAndrews M.P. (2004). Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus, 14, 752–62. [DOI] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Wei Y., Eichele T., Calhoun V.D. (2012). Capturing inter-subject variability with group independent component analysis of fMRI data: a simulation study. NeuroImage, 59, 4141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Huang C., Buckner R.L. (2010). Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology, 104, 322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova L., Meyer B.M., Diers K., Rabl U., Scharinger C., et al. (2015). Reduced default mode network suppression during a working memory task in remitted major depression. Journal of Psychiatric Research, 64, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 360, 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. (2009). Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage, 47(Suppl 1), S148. [Google Scholar]
- Belzung C., Willner P., Philippot P. (2015). Depression: from psychopathology to pathophysiology. Current Opinion in Neurobiology, 30, 24–30. [DOI] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6, 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pekar J.J. (2004). A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magnetic Resonance Imaging, 22, 1181–91. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugene F., Dennis E.L., Gotlib I.H. (2010). Neural correlates of rumination in depression. Cognitive, Affective, and Behavioral Neuroscience, 10, 470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., et al. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103, 13848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Harrison B.J., Yucel M., Allen N.B. (2012). Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological Medicine, 42, 2071–81. [DOI] [PubMed] [Google Scholar]
- De Maesschalck R., Jouan-Rimbaud D., Massart D.L. (2000). The Mahalanobis distance. Chemometrics and Intelligent Laboratory Systems, 50, 1–18. [Google Scholar]
- Dutta A., McKie S., Deakin J.F. (2014). Resting state networks in major depressive disorder. Psychiatry Research 224, 139–51. [DOI] [PubMed] [Google Scholar]
- Eaton W.W., Shao H., Nestadt G., Lee B., Bienvenu O., Zandi P. (2008). POpulation-based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry, 65, 513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Bloch R.T., Segal Z.V. (2011). Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry, 70, 366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa C.A., Ruhe H.G., Koeter M.W., Spinhoven P., Van der Does W., et al. (2015). Cognitive reactivity versus dysfunctional cognitions and the prediction of relapse in recurrent major depressive disorder. The Journal of Clinical Psychiatry, 76, e1306–12. [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106, 7209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Cooney R.E., Joormann J., Henry M.L., Gotlib I.H. (2014). Recalling happy memories in remitted depression: a neuroimaging investigation of the repair of sad mood. Cognitive, Affective & Behavioral Neuroscience, 14, 818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.H., Kensinger E.A. (2016). Effects of internal and external vividness on hippocampal connectivity during memory retrieval. Neurobiology of learning and memory, 134, Part A, 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62, 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Liu F., Zhang J., Zhang Z., Yu L., et al. (2013). Dissociation of regional activity in the default mode network in first-episode, drug-naive major depressive disorder at rest. Journal of Affective Disorders, 151, 1097–101. [DOI] [PubMed] [Google Scholar]
- Hach S., Tippett L.J., Addis D.R. (2014). Neural changes associated with the generation of specific past and future events in depression. Neuropsychologia, 65, 41–55. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological psychiatry, 78(4), 224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. (2011). Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry, 70, 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R.N. (2015). Analysis of variance (ANOVA). Brain Mapping: an encyclopedic reference. Elsevier, 477–81. [Google Scholar]
- Hetrick S.E., Parker A.G., Hickie I.B., Purcell R., Yung A.R., McGorry P.D. (2008). Early identification and intervention in depressive disorders: towards a clinical staging model. Psychotherapy and Psychosomatics, 77, 263–70. [DOI] [PubMed] [Google Scholar]
- Jarrett R.B., Minhajuddin A., Borman P.D., Dunlap L., Segal Z.V., et al. (2012). Cognitive reactivity, dysfunctional attitudes, and depressive relapse and recurrence in cognitive therapy responders. Behaviour Research and Therapy, 50, 280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72, 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster E.H.W., De Lissnyder E., Derakshan N., De Raedt R.. 2011. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clinical Psychology Review, 31, 138–45 [DOI] [PubMed] [Google Scholar]
- Kruijshaar M.E., Barendregt J., Vos T., de Graaf R., Spijker J., Andrews G. (2005). Lifetime prevalence estimates of major depression: an indirect estimation method and a quantification of recall bias. Europeon Journal of Epidemiology, 20, 103–11. [DOI] [PubMed] [Google Scholar]
- Lau M.A., Segal Z.V., Williams J.M. (2004). Teasdale's differential activation hypothesis: implications for mechanisms of depressive relapse and suicidal behaviour. Behaviour Research andTherapy, 42, 1001–17. [DOI] [PubMed] [Google Scholar]
- Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. (2012). Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders 136, e1–e11. [DOI] [PubMed] [Google Scholar]
- Li B., Liu L., Friston K.J., Shen H., Wang L., et al. (2013a). A treatment-resistant default mode subnetwork in major depression. Biological Psychiatry, 74, 48–54. [DOI] [PubMed] [Google Scholar]
- Li J., Xu C., Cao X., Gao Q., Wang Y., et al. (2013b). Abnormal activation of the occipital lobes during emotion picture processing in major depressive disorder patients. Neural Regeneration Research, 8, 1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Kong F., Qi S., You X., Huang X. (2015). Resting-state functional connectivity of the default mode network associated with happiness. Social Cognitive and Affective Neuroscience, 11(3), 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Mummery C.J. (1999). Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus, 9, 54–61. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H., Sonuga-Barke E.J., De Raedt R. (2012). The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review, 22, 229–51. [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Michalak J., Hölz A., Teismann T. (2011). Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression. Psychology and Psychotherapy, 84, 230–6. [DOI] [PubMed] [Google Scholar]
- Mocking R.J.T., Figueroa C.A., Rive M.M., Geugies H., Servaas M.N., et al. (2016). Vulnerability for new episodes in recurrent major depressive disorder: protocol for the longitudinal DELTA-neuroimaging cohort study. BMJ Open, 6, e009510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. (2015). Resting-state functional connectivity in major depressive disorder: a review. Neuroscience and Biobehavioral Reviews, 56, 330–44. [DOI] [PubMed] [Google Scholar]
- Nixon N.L., Liddle P.F., Nixon E., Worwood G., Liotti M., Palaniyappan L. (2014). Biological vulnerability to depression: linked structural and functional brain network findings. The British Journal of Psychiatry: The Journal of Mental Science, 204, 283–9. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100, 569–82. [DOI] [PubMed] [Google Scholar]
- Peng D.H., Jiang K.D., Fang Y.R., Xu Y.F., Shen T., et al. (2011). Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chinese Medical Journal, 124, 369–73. [PubMed] [Google Scholar]
- Posner J., Hellerstein D.J., Gat I., Mechling A., Klahr K., et al. (2013). Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry, 70, 373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A.R., Eichenbaum H. (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23, R764–R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes F., Schoofs H., Griffith J.W., Hermans D. (2012). Rumination relates to reduced autobiographical memory specificity in formerly depressed patients following a self-discrepancy challenge: the case of autobiographical memory specificity reactivity. Journal of Behavior Therapy and Experimental Psychiatry, 43, 1002–7. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Giles D.E., Schlesser M.A., Fulton C.L., Weissenburger J., Burns C. (1986). The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Research, 18, 65–87. [DOI] [PubMed] [Google Scholar]
- Sambataro F., Wolf N.D., Pennuto M., Vasic N., Wolf R.C. (2014). Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological Medicine, 44, 2041–51. [DOI] [PubMed] [Google Scholar]
- Segal Z.V., Gemar M., Williams S. (1999). Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. Journal of Abnormal Psychology, 108, 3–10. [DOI] [PubMed] [Google Scholar]
- Segal Z.V., Kennedy S., Gemar M., Hood K., Pedersen R., Buis T. (2006). Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry, 63, 749–55. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., Allan C.L., Le Masurier M., McDermott L.M., Kalu U.G., et al. (2012). Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Archives of General Psychiatry, 69, 680–9. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Thompson W.L., Kosslyn S.M. (2012). Visual memory and visual mental imagery recruit common control and sensory regions of the brain. Cognitive Neuroscience, 3, 14–20. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., et al. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106, 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D.A., Keller M.B., Leon A.C., Mueller T.I., Lavori P.W., et al. (2000). Multiple recurrences of major depressive disorder. American Journal of Psychiatry, 157, 229–33. [DOI] [PubMed] [Google Scholar]
- Spinhoven P., Bockting C.L., Schene A.H., Koeter M.W., Wekking E.M., Williams J.M. (2006). Autobiographical memory in the euthymic phase of recurrent depression. Journal of Abnormal Psychology, 115, 590–600. [DOI] [PubMed] [Google Scholar]
- Sumner J.A. (2012). The mechanisms underlying overgeneral autobiographical memory: an evaluative review of evidence for the CaR-FA-X model. Clinical Psychology Review, 32, 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- van de Ven V.G., Formisano E., Prvulovic D., Roeder C.H., Linden D.E. (2004). Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Human Brain Mapping, 22, 165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gucht K., Takano K., Van Broeck N., Raes F. (2015). A mindfulness-based intervention for economically disadvantaged people: effects on symptoms of stress, anxiety, and depression and on cognitive reactivity and overgeneralization. Mindfulness, 6, 1042–52. [Google Scholar]
- van Kesteren M.T., Fernandez G., Norris D.G., Hermans E.J. (2010). Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences of the United States of America, 107, 7550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijsbergen G.D., Bockting C.L., Burger H., Spinhoven P., Koeter M.W., et al. (2013). Mood reactivity rather than cognitive reactivity is predictive of depressive relapse: a randomized study with 5.5-year follow-up. Journal of Consulting and Clinical Psychology, 81, 508–17. [DOI] [PubMed] [Google Scholar]
- van Rijsbergen G.D., Kok G.D., Elgersma H.J., Hollon S.D., Bockting C.L. (2015). Personality and cognitive vulnerability in remitted recurrently depressed patients. Journal of Affective Disorders, 173, 97–104. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J., Ferrarini L., Milles J., et al. (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E.R. (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134, 163–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-F., Bossmann J., Schiffhauer B., Jordan M., Immordino-Yang M.H. (2012). Intrinsic default mode network connectivity predicts spontaneous verbal descriptions of autobiographical memories during social processing. Frontiers in Psychology, 3, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoscik V., Huffziger S., Ebner-Priemer U., Kuehner C., Kirsch P. (2014). Increased involvement of the parahippocampal gyri in a sad mood predicts future depressive symptoms. Social Cognitive and Affective Neuroscience, 9, 2034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., et al. (2011). Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biological Psychiatry, 70, 334–42. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., Liao J., Zhong M., et al. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry, 71, 611–7. [DOI] [PubMed] [Google Scholar]
- Zou K., Gao Q., Long Z., Xu F., Sun X., et al. (2016). Abnormal functional connectivity density in first-episode, drug-naive adult patients with major depressive disorder. Journal of Affective Disorders, 194, 153–8. [DOI] [PubMed] [Google Scholar]
- Zuo X.-N., Kelly C., Adelstein J.S., Klein D.F., Castellanos F.X., Milham M.P. (2010). Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. NeuroImage, 49, 2163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.