Abstract
Background and Objectives:
Open conversion (OC) occurs in 5 to 10% of laparoscopic cholecystectomies (LCs) and results in suboptimal outcomes. Herein, we report our experience with OC in cholecystectomy performed with the minimally invasive (MIS) approach.
Methods:
Data from 960 minimally invasive cholecystectomies performed in the University of Illinois at Chicago (UIC) Division of General, Minimally Invasive, and Robotic Surgery were retrospectively compiled. Patient demographics and outcomes were analyzed for the major indicators that may predispose to OC.
Results:
Male gender and intraoperative diagnosis of acute or gangrenous cholecystitis were identified as statistically significant individual predictors for OC. Conversion incidence was significantly lower in every paired demographic combination when compared with the laparoscopic data.
Conclusions:
Our retrospective study identified some specific factors associated with significantly higher risk of OC in both laparoscopic and robotic cholecystectomy. The impact of these risk factors seems to be lesser in the robotic than in the laparoscopic approach. Further investigation is necessary to validate these findings.
Keywords: Cholecystectomy, Conversion to open surgery, Laparoscopy, Risk, Robotic surgery
INTRODUCTION
Cholecystectomy by a minimally invasive approach has become the most common intra-abdominal surgery performed in the United States and other Western nations.1,2 The advantages of minimally invasive over open surgery are well documented in the literature and range from decreased postoperative pain and reduced scarring to decreased length of hospital stay.3,4 Despite the aforementioned advantages of minimally invasive surgery (MIS), there are scenarios where an open procedure may be a safer choice based on perceived limitations of the minimally invasive technique. Recent studies have shown that ∼4.9% of traditional laparoscopic cholecystectomies are converted to open surgery for a variety of reasons, ranging from unclear anatomy to excessive inflammation and adhesions from prior abdominal operations.5–7 Conversion to the open technique subjects patients to increased postoperative pain, potentially higher blood loss, longer recovery time and time until return to work, and suboptimal cosmetic outcomes.8–10
Having advance knowledge of the factors that increase risk of conversion may enable surgeons to have a more individualized preoperative risk discussion with the patient. Prior evidence of adverse outcomes in patients who underwent open conversion based on certain risk factors, highlights the importance of effective preoperative risk assessment.11 Multiple scientific efforts attempted to identify factors for conversion risk; however, there is no set of unified guidelines, and those published are inherently skewed toward their center's patient population.12–18 We retrospectively reviewed the MIS experience at our institution to identify risk factors that best reflect our patient population.
MATERIALS AND METHODS
A total of 960 cases involving cholecystectomy from the University of Illinois at Chicago (UIC) Division of General, Minimally Invasive, and Robotic Surgery were retrospectively compiled between 2011 and 2015. Inclusion criteria for the study were all patients age 17 and older who underwent cholecystectomy during the study period. Patient demographics and surgical outcomes including gender, age, BMI, prior surgical history, intra-operative diagnosis, case duration, and ASA class were compiled and analyzed for the major indicators that may predispose a patient to open conversion. In total, data were compiled on 284 laparoscopic cholecystectomies (LCs) and 676 indocyanine green (ICG) fluorescence-aided robotic cholecystectomies (RCs) performed at UIC during the recruitment timeframe of 2008 to 2015. The laparoscopic data include all cases available through the electronic medical records (EMR) system that fit the inclusion criteria.
All surgeries included in the study were performed by the same surgical team at UIC, consisting of surgeons who had performed >125 robotic and laparoscopic surgeries in total. Data analysis was conducted with SAS software 9.4 (SAS Institute Inc., Cary, North Carolina, USA). No randomization of patients into either of the subgroups occurred. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Permission to use retrospective data was granted through the UIC College of Medicine Institutional Review Board (IRB) (protocol 2011-1104).
RESULTS
Results of multivariate logistic regression analysis with the outcome open conversion for the predictors of age 40+, gender, and intra-operative diagnosis resulted in statistically significant values for gender (P = .032) and intraoperative diagnosis (P = .014). Complete statistics are shown in Table 1.
Table 1.
Logistic Regression of Predictive Demographics
| Odds Ratio | 95% CI | P | |
|---|---|---|---|
| Age | 1.01 | (0.97–1.06) | 0.576 |
| Female | 0.23 | (0.06–0.88) | 0.032 |
| Operative diagnosis | 7.39 | (1.51–36.11) | 0.014 |
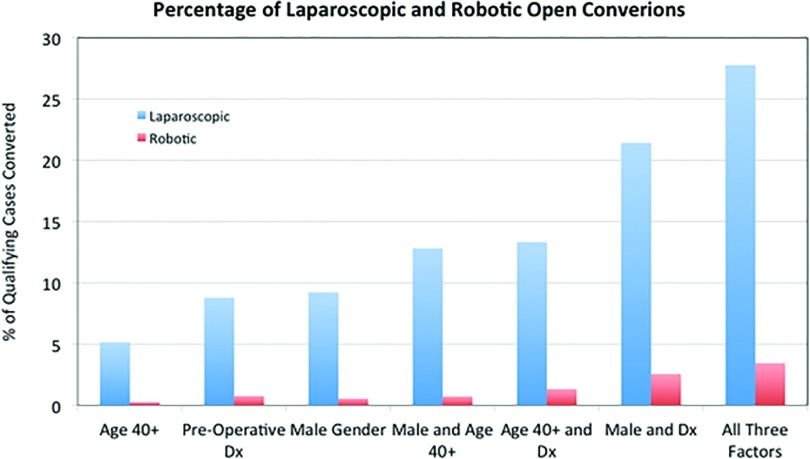
The overall conversion rate for the traditional laparoscopic and robotic groups was 3.87 and 0.15%, respectively. After dividing patients into subgroups based on the key demographics identified in the regression analysis, patients who underwent laparoscopic cholecystectomy and met 1 criterion of age over 40 (n = 136), an intra-operative diagnosis of either acute cholecystitis or gangrenous cholecystitis (n = 91), or male gender (n = 65) had an open conversion percentage of 5.15, 8.33, and 9.23%, respectively. Patients who met 2 criteria—age and gender (n = 39), age and diagnosis (n = 45), or gender and diagnosis (n = 28)—had conversion percentages of 12.82, 13.04, and 21.43%, respectively. In the subgroup of qualifying patients who met all 3 criteria (n = 18), the conversion percentage was 27.78%.
When compared with the same key demographic subsets in patients who underwent robotic procedures, a statistically significant decrease was seen in each subgroup in Z-scores calculated based on the single categorical characteristic of open conversion (Table 2). Patients who had robotic cholecystectomy and met 1 criterion of age over 40 (n = 371), an intraoperative diagnosis of either acute cholecystitis or gangrenous cholecystitis (n = 130), or male gender (n = 181) had an open conversion percentage of 0.27, 0.76, and 0.55%, respectively. Patients who met 2 criteria—age and gender (n = 138), age and diagnosis (n = 80), or gender and diagnosis (n = 39)—had conversion percentages of 0.72, 1.33, and 2.56%, respectively. In the subgroup of qualifying patients who met all 3 key demographic criteria (n = 29), the conversion percentage was 3.45%. Reasons for conversion and complete demographic information are found in Tables 3 and 4.
Table 2.
Demographic Based Conversion Comparison
| Demographic | Laparoscopic Cases: Conversions (n) | Robotic Cases: Conversions (n) | Z-Score | P |
|---|---|---|---|---|
| Age 40+ | 136:7 | 371:1 | 3.9045 | 0.0001 |
| Male gender | 65:6 | 181:1 | 3.6095 | 0.0003 |
| Preoperative diagnosis | 91:8 | 130:1 | 2.9695 | 0.00298 |
| Age and diagnosis | 45:6 | 80:1 | 2.8203 | 0.0048 |
| Gender and diagnosis | 28:6 | 39:1 | 2.4898 | 0.01278 |
| Age and gender | 39:5 | 138:1 | 3.6857 | 0.00022 |
| All three demographics | 18:5 | 29:1 | 2.4297 | 0.0151 |
Table 3.
Reasons for OC in Laparoscopic Cholecystectomy
| Reasons | Patients, n (% of Total Conversions) |
|---|---|
| Open conversion | |
| Unclear anatomy | 1 (9.09) |
| Significant inflammation | 2 (18.18) |
| Dilated cystic duct with unsuccessful cholangiogram | 1 (9.09) |
| Inflammation+adhesions | 1 (9.09) |
| Adhesions+bleeding | 3 (27.27) |
| Significant adhesions | 2 (18.18) |
| Unroofed Abscess | 1 (9.09) |
| Robotic conversion | |
| Inflammation and bleeding | 1 (100) |
Table 4.
Demographics of Patients Who Undergo Laparoscopic Cholecystectomy
| Demographic | Laparoscopic Conversion (n = 11) | Laparoscopic Nonconversion (n = 278) | Robotic Conversion (n = 1) | Robotic Nonconversions (n = 675) |
|---|---|---|---|---|
| Average age, y | 42.54 | 40.69 | 63 | 43.88 |
| Average ASA class | 2.27 | 1.96 | 2 | 2.06 |
| Average BMI | 28.47 | 31.29 | 36.80 | 32.27 |
| Prior abdominal surgery, n (%) | 2 (18.18) | 62 (22.30) | 0 (0) | — |
| Male patients, n (%) | 6 (54.45) | 58 (20.86) | 1 (100) | 179 (26.52) |
DISCUSSION
Numerous studies have attempted to identify a set of risk factors for open conversion in laparoscopic cholecystectomy. By identifying data points that may be incorporated into a scoring algorithm, patients could be risk stratified before surgery to decide which approach is ideal.19 Many of the studies identify similar demographics including gender, patient age, preoperative diagnosis, previous abdominal surgery, or other comorbidities that are easily compiled with a simple history, yet they often differ on specifics. For example, one study suggested that patients >50 years of age are at increased risk for conversion.6 Another study suggests higher risk at 60 years of age.15 Some research has also suggested 65 years of age as the appropriate cutoff, presumably based on the patient populations sampled in each study.20 In our study, we found that the percentage of conversions was highest in patients older than 40, with slight variations when the data were stratified upward at 5-year intervals (Table 5). Other studies have cited risk factors that require laboratory testing and further resource utilization, including ultrasound parameters, bilirubin and liver enzyme levels, white cell count, and inflammation assessment before surgery.13,15,21 The range of study results makes it difficult to set definitive guidelines; however, working within ranges may allow for effective stratification.
Table 5.
Laparoscopic Patient Age Ranges and Open Conversions
| Patient Age Range (y) | Open Conversions/Qualifying Patients (%) |
|---|---|
| 70+ | 1/20 (5) |
| 65+ | 1/31 (3.22) |
| 60+ | 1/42 (2.38) |
| 55+ | 2/57 (3.51) |
| 50+ | 4/83 (4.82) |
| 45+ | 5/102 (4.90) |
| 40+ | 7/136 (5.15) |
Our study concludes that some specific risk factors for open conversion are being identified simultaneously and across both patient populations (laparoscopic and robotic). In addition, by retrospectively matching patients on risk factors in both laparoscopic and robotic groups we were able to identify possible scenarios where the robotic approach may be better suited for a particular patient to reduce the risk of open conversion. This specific and additional finding is in line with evidence from recent literature indicating that robot-assisted procedures may reduce the risk of open conversion across multiple specialties.22–25 Regarding cholecystectomy specifically, we do not suggest that the robotic approach is superior in all cases—merely, that consideration of individual patient risk factors in light of conversion rates observed in multiple studies may improve outcomes in certain scenarios. As medicine continues to move toward greater individualization of care, it is prudent for surgical interventions to mirror this individualized approach to provide optimal care whenever possible.
Although not within the scope of this article to discuss at length, the economic factors at play when comparing the robotic and laparoscopic techniques for cholecystectomy are important to address. Although the robotic cholecystectomy remains more expensive on a per-case basis, there is evidence from other specialties to show that large centers that use robotic capability to capacity may reduce per-case costs to a level comparable to that of laparoscopic procedures.26 In addition, the lowered risk of open conversion in robotic cholecystectomy may further even out surgical costs by eliminating the estimated $8500 additional cost for open conversion, primarily because of increased length of hospital stay.27 Larger, economics-focused studies are needed to thoroughly investigate the costs associated with robotic and laparoscopic cholecystectomy, taking into account variables such as volume and capacity.
A clear limitation of our study is the single-institution retrospective design and the inherent biases that accompany it. However, it is still useful to consider the statistically significant outcomes that were found. This study may provide a framework from which a randomized controlled trial could be designed to validate and expand upon the findings presented here or provide a useful stimulus for other institutions to analyze their own unique patient population–based data. Additional criticisms may include the surgeon's preference and selection bias, as well as experience bias regarding the cases involved. Although a potentially valid consideration, it is important to reiterate that the surgeons in the study have extensive experience in each approach and continue to perform both as part of daily surgical practice. Publications vary in their definition of an “expert” or “high-volume” surgeon in laparoscopic cholecystectomy based on number of cases performed, but consensus seems to be >100 cases overall; each UIC surgeon included in the study has performed well over that number.6,28,29 There are also no dedicated robotic and traditional laparoscopic teams at UIC. In addition, the UIC general surgery team performs only the traditional laparoscopic cholecystectomy at affiliated institutions, because of the lack of a robotic platform at these community sites; however, information from these cases could not be included within the study because of a lack of IRB oversight at these sites. The limitation of sample size discrepancy is also valid; however, the study includes all available laparoscopic cases from the ERM available to the researchers.
CONCLUSIONS
Our retrospective study identified specific risk factors (age 40+ years, male gender, and preoperative diagnosis of acute or gangrenous cholecystitis) that are associated with a significantly higher risk of open conversion in both laparoscopic and robotic cholecystectomy. However, in our own center's experience, the impact of the identified risk factors seem to be lesser in the robotic versus the laparoscopic approach (Figure 1).
Figure 1.
Paired conversion percentages in laparoscopic versus robotic cholecystectomy.
Further investigation, ideally through prospective randomized trials, is necessary to validate our retrospective findings before considering the possibility of introducing a more individualized preoperative risk discussion and surgical plan in this specific subset of patients. Until then, definitive conclusions cannot be drawn as to which approach is best suited for the individual patient.
Contributor Information
Antonio Gangemi, Division of General, Minimally Invasive, and Robotic Surgery, University of Illinois at Chicago, Chicago, Illinois, USA..
Richard Danilkowicz, Division of General, Minimally Invasive, and Robotic Surgery, University of Illinois at Chicago, Chicago, Illinois, USA..
Francesco Bianco, Division of General, Minimally Invasive, and Robotic Surgery, University of Illinois at Chicago, Chicago, Illinois, USA..
Mario Masrur, Division of General, Minimally Invasive, and Robotic Surgery, University of Illinois at Chicago, Chicago, Illinois, USA..
Pier Cristoforo Giulianotti, Division of General, Minimally Invasive, and Robotic Surgery, University of Illinois at Chicago, Chicago, Illinois, USA..
References:
- 1. Comitalo JB. Laparoscopic cholecystectomy and newer techniques of gallbladder removal. JSLS. 2012;16:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaillard M. New minimally invasive approaches for cholecystectomy: review of literature. World J Gastrointest Surg. 2015;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2005;92:44–49. [DOI] [PubMed] [Google Scholar]
- 4. Glavic Z, Begic L, Simlesa D, Rukavina A. Treatment of acute cholecystitis: a comparison of open vs laparoscopic cholecystectomy. Surg Endosc. 2001;15:398–401. [DOI] [PubMed] [Google Scholar]
- 5. Coffin SJ, Wrenn SM, Callas PW, Abu-Jaish W. Three decades later: investigating the rate of and risks for conversion from laparoscopic to open cholecystectomy. Surg Endosc. 2017, in press. [DOI] [PubMed] [Google Scholar]
- 6. Sakpal SV, Bindra SS, Chamberlain RS. Laparoscopic cholecystectomy conversion rates two decades later. JSLS. 2010;14:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205–211. [DOI] [PubMed] [Google Scholar]
- 8. Keus F, de Jong JAF, Gooszen HG, van Laarhoven CJHM. Laparoscopic versus small-incision cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;CD006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coccolini F, Catena F, Pisano M, et al. Open versus laparoscopic cholecystectomy in acute cholecystitis: systematic review and meta-analysis. Int J Surg. 2015;18:196–204. [DOI] [PubMed] [Google Scholar]
- 10. Berggren U, Gordh T, Grama D, Haglund U, Rastad J, Arvidsson D. Laparoscopic versus open cholecystectomy: hospitalization, sick leave, analgesia and trauma responses. Br J Surg. 1994;81:1362–1365. [DOI] [PubMed] [Google Scholar]
- 11. Wolf AS, Nijsse BA, Sokal SM, Chang Y, Berger DL. Surgical outcomes of open cholecystectomy in the laparoscopic era. Am J Surg. 2009;197:781–784. [DOI] [PubMed] [Google Scholar]
- 12. Peters JH, Krailadsiri W, Incarbone R, et al. Reasons for conversion from laparoscopic to open cholecystectomy in an urban teaching hospital. Am J Surg. 1994;168:555–559. [DOI] [PubMed] [Google Scholar]
- 13. Liu CL, Fan ST, Lai EC, Lo CM, Chu KM. Factors affecting conversion of laparoscopic cholecystectomy to open surgery. Arch Surg. 1996;131:98–101. [DOI] [PubMed] [Google Scholar]
- 14. Atmaram DC, Lakshman K. Predictive factors for conversion of laparoscopic cholecystectomy. Indian J Surg. 2011;73:423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simopoulos C, Botaitis S, Polychronidis A, Tripsianis G, Karayiannakis AJ. Risk factors for conversion of laparoscopic cholecystectomy to open cholecystectomy. Surg Endosc Other Interv Tech. 2005;19:905–909. [DOI] [PubMed] [Google Scholar]
- 16. Shapiro AJ, Costello C, Harkabus M, North JH., Jr Predicting conversion of laparoscopic cholecystectomy for acute cholecystitis. JSLS. 1999;3:127–130. [PMC free article] [PubMed] [Google Scholar]
- 17. Tang B, Cuschieri A. Conversions during laparoscopic cholecystectomy: risk factors and effects on patient outcome. J Gastrointest Surg. 2006;10:1081–1091. [DOI] [PubMed] [Google Scholar]
- 18. Kaafarani HMA, Smith TS, Neumayer L, Berger DH, Depalma RG, Itani KMF. Trends, outcomes, and predictors of open and conversion to open cholecystectomy in Veterans Health Administration hospitals. Am J Surg. 2010;200:32–40. [DOI] [PubMed] [Google Scholar]
- 19. Kama NA, Kologlu M, Doganay M, Reis E, Atli M, Dolapci M. A risk score for conversion from laparoscopic to open cholecystectomy. Am J Surg. 2001;181:520–525. [DOI] [PubMed] [Google Scholar]
- 20. van der Steeg HJJ, Alexander S, Houterman S, Slooter GD, Roumen RMH. Risk factors for conversion during laparoscopic cholecystectomy: experiences from a general teaching hospital. Scand J Surg. 2011;100:169–173. [DOI] [PubMed] [Google Scholar]
- 21. Lipman JM, Claridge JA, Haridas M, et al. Preoperative findings predict conversion from laparoscopic to open cholecystectomy. Surgery. 2007;142:556–565. [DOI] [PubMed] [Google Scholar]
- 22. Gangemi A, Danilkowicz R, Elli FE, Bianco F, Masrur M, Giulianotti PC. Could ICG-aided robotic cholecystec tomy reduce the rate of open conversion reported with laparoscopic approach? A head to head comparison of the largest single institution studies. J Robot Surg. 2017, in press. [DOI] [PubMed] [Google Scholar]
- 23. Bhama AR, Wafa AM, Ferraro J, et al. Comparison of risk factors for unplanned conversion from laparoscopic and robotic to open colorectal surgery using the Michigan Surgical Quality Collaborative (MSQC) Database. J Gastrointest Surg. 2016;20:1223–1230. [DOI] [PubMed] [Google Scholar]
- 24. Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18:816–830. [DOI] [PubMed] [Google Scholar]
- 25. Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ. Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot Comput Assist Surg. 2012;8:360–370. [DOI] [PubMed] [Google Scholar]
- 26. Wright JD, Ananth CV, Tergas AI, et al. An Economic analysis of robotically assisted hysterectomy. Obstet Gynecol. 2014;123:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lengyel BI, Panizales MT, Steinberg J, Ashley SW, Tavakkoli A. Laparoscopic cholecystectomy: what is the price of conversion? Surgery. 2012;152:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore MJ, Bennett CL. The learning curve for laparoscopic cholecystectomy. Am J Surg. 1995;170:55–59. [DOI] [PubMed] [Google Scholar]
- 29. McMahon AJ, Fischbacher CM, Frame SH, MacLeod MC. Impact of laparoscopic cholecystectomy: a population-based study. Lancet. 2000;356:1632–1637. [DOI] [PubMed] [Google Scholar]