Abstract
Limb apraxia (LA) is a high-order motor disorder linked to left-hemisphere damage. It is characterized by defective execution of purposeful actions upon delayed imitation, or verbal command when the actions are performed in isolated, non-naturalistic, conditions. Whether interpersonal interactions provide social affordances that activate neural resources different from those requested by individual action execution, which may improve LA performance, is unknown. To fill this gap, we measured interaction performance, behavioral and kinematic indexes of left-brain damaged patients with/without LA in a social reach-to-grasp task involving two different degrees of spatio-temporal interactivity with an avatar. We found that LA patients’ impairment in coordinating with the virtual partner was abolished in highly interactive conditions (where patients selected their actions on-line based on the behavior of the virtual partner) with respect to low interactive conditions (where actions were selected beforehand based on abstract instructions). Voxel-based-Lesion-Symptom-Mapping indicated that impairments in low-interactive conditions were underpinned by lesions of premotor, motor and insular areas, and of the basal ganglia. Our approach expands current understanding of the behavioral and neural correlates of interactive motor performance by highlighting the important role of social affordances, and provides novel, potentially important, views on rehabilitation of higher-order motor cognition disorders.
Keywords: apraxia, on-line joint actions, social affordances, VLSM
Introduction
Limb apraxia (LA) is a high-order action representation deficit that alters gesture performance (Rothi et al., 1991) and their spatio-temporal organization and kinematic profiles (Pramstaller and Marsden, 1996; De Renzi, 1986; Leiguarda and Marsden, 2000; Hermsdorfer et al., 2013). It typically occurs after lesions to a left-lateralized cortical (fronto-parietal, premotor, insular) and subcortical (basal ganglia) neural network (Buxbaum et al., 2014). LA has been associated with defective perception (Halsband et al., 2001), evaluation (Heilman et al., 1982; Pazzaglia et al., 2008b; Canzano et al., 2014) and comprehension (Rothi et al., 1985) of observed actions, strengthening the notion that partially overlapping neural substrates may support action perception and execution (Avenanti et al., 2013; Urgesi et al., 2014; but also see Mahon and Caramazza, 2008; Stasenko et al., 2013). Since its first description, LA has been studied in ‘isolated’ conditions where the patient is asked to perform an action upon verbal command or exposition to a tool (real-use or pantomime). As an effect of the so called automatic/voluntary dissociation (De Renzi et al., 1982; Trojano et al., 2007) LA deficits may be attenuated in every-day settings, where environmental and internal cues may facilitate the transformation of the intended act into proper motor plans (Freund, 2001; Randerath et al., 2011). Crucially, naturalistic contexts not only require acting upon static objects, but also interacting with other individuals by adapting online to them (e.g. joint-actions). Accordingly, sensory-motor and cognitive systems of social species are developed in order to interact with other individuals and to efficiently couple observed actions and individual motor execution in time and space. Behavioral and kinematic studies suggest that the execution of individual movements may be radically different in inter-actions than when acting in isolation. Indeed, the kinematics of a given action is different when performed in isolation with respect to when observing another person moving (Kilner et al., 2003), when performing an action with ‘interactive’ aims (Sartori et al., 2009), or coordinating (Sacheli et al., 2012, 2013; Candidi et al., 2015) and competing with others (Naber et al., 2013). Interactive contexts also modulate brain activity in fronto-parietal areas typically recruited during action observation (Neuwman-Norlund et al., 2007, 2008) and in additional cortical and subcortical networks that may underpin the integration of individual goals with those of our partners (Kokal et al., 2009; Kokal and Keysers, 2010; Kourtis et al., 2013), a process that is essential to navigating the social world (Sebanz et al., 2006; Sacheli et al., 2015a). Using inhibitory rTMS in healthy individuals we provided the first evidence for a causal role of the left anterior Intra Parietal Sulcus in the execution of interactive actions (Sacheli et al., 2015b). Thus, because interactive and praxic functions are inherently linked to higher-order action related processes, testing apraxia in social contexts may be fundamentally important.
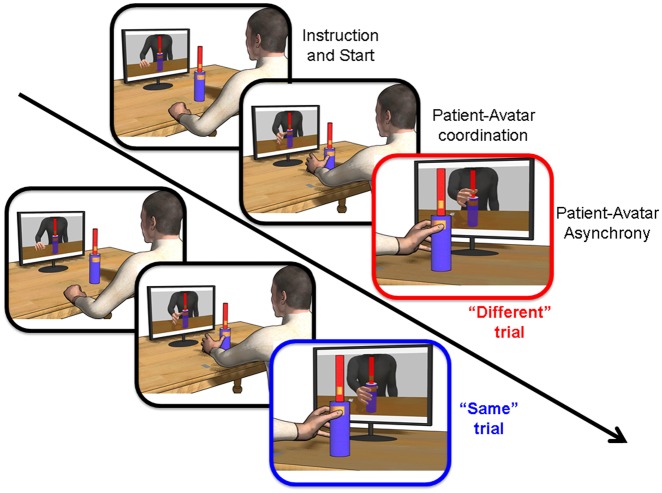
Here, we explored whether interpersonal interactions may reduce performance deficits in left brain-damaged patients with (LA+) or without (LA-) apraxia. Patients were tested in a modified version of a joint reach-to-grasp task (Sacheli et al., 2015b,c) that measures the ability to synchronize one’s own movements with those of a virtual partner (Coordination task; Figure 1) in two experimental conditions characterized by high/low interpersonal interactivity (i.e. Interactive/Instructed conditions). The Interactive condition required participants to synchronize their movements with those of the virtual partner by performing the same or a different action, without knowing in advance which individual movement was to be performed. This condition captures the essential nature of realistic interactions where coordination in space and synchronization in time with the partner is fundamental. Conversely, in the Instructed condition, participants were pre-instructed about whether a power vs precision grip was to be performed (regardless of the partner’s action) making the interaction depending only on temporal synchronization. Thus, the Interactive coordination is more demanding than the Instructed one. Yet, the presence of social affordances in the former may boost LA + performance in the more complex situation. By analyzing patients’ performance (synchrony and accuracy), behavioral and kinematic (Supplementary Material) indexes we provide a description of apraxics’ ability to overcome the challenges of realistic interactions. Voxel Lesion Symptom Mapping (VLSM) was used to search for the lesional underpinnings discriminating the behavioral difference between Interactive and Instructed coordination.
Fig. 1.
Examples of imitative ‘Same’ (patient-avatar Down-power grip) and complementary ‘Different’ (patient Down-power grip, avatar Up-precision grip) trials in the experimental set-up of the Coordination task.
Materials and methods
Patients
Twenty eight left brain damaged patients (14 males) were included in the study. They were recruited from the Neurorehabilitation Units at the IRCCS Santa Lucia (Rome) and at the Sant’Andrea hospital (Rome). The procedures were approved by the IRCCS Ethical Committee and the study was carried out in accordance with the Declaration of Helsinki. A battery of standardized tests was used for neuropsychological screening. This involved tests on general cognitive abilities (Raven et al., 1988), executive functions (non-verbal subtests of the Frontal Assessment Battery; Appollonio et al., 2005) and spatial attention (Line Bisection; Wilson et al., 1987). Verbal comprehension and denomination subtests of the Italian Version of the Aachener Aphasia Test (Luzzatti et al., 1996) were used to assess language deficits. Patients were divided in limb-apraxic (LA+, n = 12, 6 females) and non-apraxic (LA-, n = 16, 8 females) groups according to their scores on a widely used test for Upper Limb Apraxia (TULIA, Vanbellingen et al., 2010). This test consists of 48 items in which imitation and pantomime of meaningless/meaningful gestures is required. A 6-point scoring method (0 = totally incorrect action execution, 5 = perfect performance) generates performance scores ranging from 0 to 240 (pathological scores ≤ 194). All LA + patients and no LA- patient scored lower than the cut-off for upper limb apraxia (Mann–Whitney U Test P < 0. 001). One LA- patient was left out of the lesion analyses because no structural image of the lesion was retrieved (final sample for VLSM analyses: n = 12 LA+, n = 15 LA-).
Stimuli
The virtual avatar was created in Maya 2011 (Autodesk, Inc.) by a customized Python script (Prof. Orvalho V., Instituto de Telecomunicações, Porto University) and the virtual scenario was designed in 3DS Max 2011 (Autodesk, Inc.). The avatar moved according to the kinematics of a real actor’s upper body [SMART-D motion capture system, MoCAP (Bioengineering Technology & Systems, B|T|S)] (Tieri et al., 2015) recorded while the actor performed eight reach-to-grasp movements toward the upper part of the bottle (precision grip) and eight toward the lower part (power grip; see Supplementary Material Video S1 and S2). The duration of each clip (∼3 s) was the same for the different conditions (up and down movements). Each stimulus started with the avatar being still, its hand on the table. After a variable amount of time (i.e. between 200 and 500 ms) the avatar started the movement. The timing of the avatar’s hand-object contact was calculated by attaching a photodiode to the screen (where the videos were displayed) that detected the appearance of a black dot pasted on the frame where the avatar touched the bottle.
Procedure
Coordination task
Patients sat in front of a table and a bottle-shaped object was placed 45 cm to the front of them. A monitor placed behind the bottle-shaped object showed a virtual partner facing the participant. In front of this virtual partner was a virtual object identical to that of the patient (Figure 1).
Patients received a ‘go’ signal through headphones before the virtual partner started its reach-to-grasp movement toward either the upper or lower part of the bottle-shaped object. Grasping the upper part implied performing a precision grip, while grasping the lower part a power grip [factor Movement: Precision(Up)/Power(Down)]. According to trial-by-trial auditory instructions, patients were required to synchronize their reach-to-grasp actions with the movements of the virtual partner by performing either imitative or complementary interactions (factor Interaction: Same/Opposite). On top of this 2 × 2 design, in separated blocks, patients were required to: (i) on-line adapt to the partner's movement by performing the same or a different action (Interactive coordination condition), without knowing in advance whether this would imply performing a precision grip on the upper part or a power grip on the lower part of the bottle-shaped object; or (ii) grasp the upper or lower part of the bottle-shaped object regardless of what movement their partner performed (Instructed coordination condition). Patients performed 24 Same/Opposite interactions in both Interactive and Instructed conditions made of 12 Precision(Up)/Power(Down) movements in random order. In both conditions the goal of the participants was to synchronize their grasping with that of their partner. Lower asynchrony values indicate better performance. Before starting the experiments, patients became familiar with the experimental set-up by performing reach-to-grasp movements toward the upper and lower part of the bottle, as well as with the auditory instructions and the experimental request, i.e. to be synchronous with the avatar in touching the object. After the practice trials, four separate 24-trial blocks (two Interactive and two Instructed) were performed by following an across-patients counterbalanced order. In order to check for whether patients were properly able to code the instructions, a final block was always run in which patients were asked to perform six up and six down grasping movements according to randomized auditory instructions, while an immobile avatar was displayed in front of them. Thus there was no coordination between avatar and participants. This control condition ensured that any impairment in the Instructed condition could not be explained by the patients’ inability to understand the auditory instructions. RTs, Movement Times (MTs; see Supplementary Material), Accuracy of response and Performance (i.e. patient-avatar touch-time Asynchrony) were calculated by having patients release a button on the working surface and by touching the bottle-shaped object on two copper-plates targets with their index and thumb finger where two other copper-plates were fixed (as in Sacheli et al., 2012, 2013, 2015b,c; Candidi et al., 2015).
Performance (i.e. asynchrony), accuracy and inverse efficiency index (i.e. Asynchrony/Accuracy)
Asynchrony (absolute value of the difference between patient’s hand-bottle contact time and the hand-bottle contact time of the virtual partner) and Accuracy data were analyzed with non-parametric tests to compare: (i) between-groups performance (Mann–Whitney U-Test), with condition-specific differences tested by using the exact probabilities for small samples (Dinneen and Blakesley, 1973); (ii) between-conditions performance (Friedman’s ANOVA), with significance level for single comparisons between conditions (Wilcoxon sign test) Bonferroni corrected for the number of relevant comparisons. Furthermore, Asynchrony and Accuracy measures were combined together in an Inverse Efficiency index (Asynchrony/Accuracy) and analyzed via a bootstrap ANOVA procedure. Bootstrapping creates a distribution of F-values based on the resampling of the original data and allows for running an ANOVA to compare the effects observed in the original data to the null hypothesis of this new bootstrapped F-value distribution. We randomly assigned each data to each condition 10 000 times, entered the data in a mixed ANOVA with factors Group (LA+/LA-) × Coordination (Interactive/Instructed) × Interaction (Same/Opposite) × Movement [Precision(Up)/Power(Down) grip], and computed the F-value for each main effect and interaction. Then, we compared our original F-values with the distribution under the null hypothesis of the bootstrapped F-values (Berkovitset al., 2000; Panasiti et al., 2016; R Development Core Team, 2013). The bootstrap P-level was calculated as the proportion of bootstrapped F-values (included in the 95% confidence intervals) greater than the original F-value.
Lesion drawing and analyses
For each patient, lesions were drawn on the T1-weighted template MRI scan from the Montreal Neurological Institute with the MRIcron software (Rorden et al., 2007a,b). Lesion drawing was performed by an examiner unaware of patients’ clinical features and behavioral results. Superimposing each patient’s lesion onto the standard brain allowed us to estimate the total brain lesion volume (in cc). Furthermore, a lesion’s location was identified by overlaying the lesion area onto the Automated Anatomical Labeling template provided by MRIcron. LA + and LA- lesion overlap and lesion subtraction were performed to highlight the lesional pattern of patients’ profile. Only voxel lesioned in at least five patients are reported.
VLSM
The VLSM analyses were performed using the Non-Parametric Mapping (NPM) software developed by Rorden et al. (2007a,b). Permutation based estimates of the non-parametric Brunner–Munzel statistics were obtained by performing 4000 permutations. In these analyses, we only included voxels that were damaged in at least five patients. We used this criterion to balance two separate requirements: to improve statistical power, achieved by testing only voxels that were damaged in a significant number of patients, and to detect the effect of regions that are reliable predictors of deficits, but lesioned in just a few patients. Colored VLSM maps were then produced and represent z statistics of the voxel-wise comparison between lesioned and non-lesioned patients. The maps indicate the voxels at which patients with a lesion performed worse than those without one. Two VLSM analyses were performed with two different behavioral predictors: (i) the difference between Interactive and Instructed performance (i.e. Interaction-Δ); (ii) patients’ apraxic score (TULIA test; see Supplementary Material). Thus, the two resulting maps represent respectively: (i) lesioned voxels that predict poorer performance in the Instructed condition as compared with the Interactive one; (ii) lesioned voxels that predict stronger apraxic deficits (lower performance in the TULIA test; see Supplementary Material). False discovery rate (FDR) correction was applied to the Brunner–Munzel values associated to damaged voxels by using an alpha level at P = 0.01 and P = 0.05 threshold for the Interaction-Δ and TULIA predictor, respectively (Nichols and Hayasaka, 2003).
Prediction task
In order to assess any perception deficit in predicting the action of the partner, patients were asked to complete a non-interactive prediction task using the same stimuli of the interactive experiment (see Supplementary Material). Participants were asked to passively observe action video clips and predict (by verbally communicating their prediction to the experimenter) whether the virtual partner intended to grasp the bottle-shaped object in the upper or lower location. In the prediction task, the video clips were interrupted at two-thirds or three-fourths of the action deployment time, thus creating short- or long-exposure stimuli.
Results
Patients
Table 1 shows LA+ and LA- patients’ demographic information, the results of neuropsychological tests and between groups comparisons.
Table 1.
Demographic and Clinical Information of the patient groups. Asterisks indicate significant between Group differences (Mann–Whitney U Test). Data are reported according to the presence of limb apraxia (LA)
| Age (years) | Education (years) | Lesion volume (cc) | Interval from lesion (days) | Raven (10 min) | TULIA | Word compreh. | Sentence compreh. | FAB tot3-6 | Line bisection | |
|---|---|---|---|---|---|---|---|---|---|---|
| LA + 1 | 53 | 13 | 245.46 | 623 | 30.5 | 122 | 27 | 22 | 2.67 | 9 |
| LA + 2 | 74 | 8 | 103.99 | 177 | 32 | 156 | 28 | 19 | 1 | 9 |
| LA + 3 | 71 | 13 | 57.11 | 227 | 19 | 190 | 26 | 21 | 1.34 | 7 |
| LA + 4 | 67 | 13 | 68.15 | 323 | 24.5 | 182 | 30 | 25 | 2 | 9 |
| LA + 5 | 40 | 13 | 243.91 | 1007 | 24 | 92 | 23 | 22 | – | – |
| LA + 6 | 79 | 13 | 3.02 | 115 | 16.5 | 93 | 28 | 19 | 1 | 7 |
| LA + 7 | 63 | 18 | 25.78 | 126 | 18 | 132 | 30 | 27 | 2.34 | 9 |
| LA + 8 | 68 | 18 | 65.35 | 175 | 31.5 | 192 | 27 | 13 | 2 | 8 |
| LA + 9 | 44 | 16 | 88.69 | 70 | 23.5 | 125 | 26 | 23 | 0.34 | 9 |
| LA + 10 | 33 | 13 | 41.23 | 598 | 31.5 | 154 | 26 | 26 | 3 | 9 |
| LA + 11 | 43 | 13 | 277.84 | 610 | 17.5 | 114 | 24 | 19 | 1.67 | 8 |
| LA + 12 | 79 | 8 | 36.78 | 175 | 24.5 | 155 | 23 | 17 | 2 | 8 |
| LA-1 | 38 | 18 | 158.69 | 665 | 26.5 | 209 | 24 | 25 | 2.67 | 9 |
| LA-2 | 47 | 13 | 20.58 | 544 | 23 | 233 | 30 | 28 | 3 | 9 |
| LA-3 | 71 | 8 | 39.22 | 45 | 25.5 | 208 | 30 | 30 | 3 | 9 |
| LA-4 | 70 | 5 | 12.11 | 74 | 29 | 234 | 28 | 30 | 2.67 | 9 |
| LA-5 | 70 | 18 | 35.07 | 266 | 35.5 | 240 | 28 | 27 | 3 | 9 |
| LA-6 | 50 | 13 | 4.37 | 36 | 32 | 240 | 30 | 30 | 3 | 9 |
| LA-7 | 67 | 13 | 4.42 | 227 | 26.5 | 227 | 30 | 30 | 2 | 8 |
| LA-8 | 62 | 13 | 16.16 | 34 | 29.5 | 238 | 30 | 24 | 3 | 9 |
| LA-9 | 86 | 13 | 18.19 | 113 | 29 | 200 | 29 | 26 | 2.34 | 9 |
| LA-10 | 38 | 13 | 16.89 | 26 | 27 | 235 | 29 | 26 | 3 | 8 |
| LA-11 | 50 | 13 | – | 105 | 31.5 | 233 | – | – | – | – |
| LA-12 | 41 | 18 | 36.52 | 187 | 31 | 231 | 26 | 26 | 3 | 8 |
| LA-13 | 68 | 13 | 39.27 | 226 | 26.5 | 205 | 26 | 27 | 1 | 8 |
| LA-14 | 57 | 13 | 62.2 | 33 | 35 | 225 | 28 | 27 | 3 | 9 |
| LA-15 | 69 | 13 | 7.5 | 109 | 32.5 | 235 | 30 | 28 | 3 | 9 |
| LA-16 | 80 | 13 | 1.82 | 949 | 26 | 228 | 30 | 22 | 3 | 9 |
| Between groups p | 0.926025 | 0.934964 | 0.004653* | 0.090089 | 0.030622* | 0.000008* | 0.022732* | 0.000308* | 0.001533* | 0.226932 |
Coordination task results
Asynchrony
Group differences in Interactive vs Instructed coordination
We were primarily interested in finding group differences related to the level of interactivity implied by the Interactive vs Instructed cooperation conditions (Figure 2). A between-groups analysis of participant-partner grasping Asynchrony showed that LA + patients were more asynchronous than LA- patients in all Instructed conditions (Mann–Whitney U, all Ps < 0.003, corrected P threshold = 0.05/8 = 0.006) except when performing Same-Power(Down) grip interactions, which differed significantly only if no statistical correction was applied (P = 0.013). Conversely, during Interactive coordination the two groups did not differ (all Ps > 0.017, corrected P threshold = 0.006).
Fig. 2.
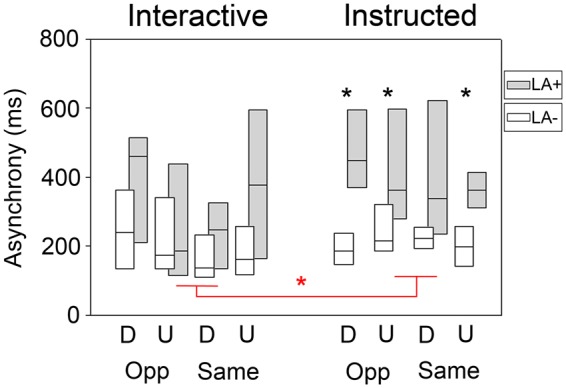
Asynchrony results of the Coordination task. Black asterisks indicate between-Group differences in each experimental condition. The comparison between Same power-grip (D, down) interactions differed significantly between groups only if no statistical correction was applied (P = 0.013016). Red asterisks indicate within-Group differences in Instructed vs Interactive experimental conditions. Red asterisks highlight the conditions that were used to create patients’ Interaction-Δ [i.e. mean asynchrony (Opposite/Up/Interactive + Same/Down/Interactive) and mean asynchrony (Opposite/Up/Instructed + Same/Down/Instructed)]. Whisker plot indicate median (smaller square), 25–75% quartiles (larger box). D, Down power-grips; U, Upper precision-grip; Opp, Opposite interaction; Same, Same interaction; LA+, apraxic; LA-, non-apraxic.
This analysis shows that LA + were as good as LA- in performing the Interactive task, while being less able to solve the Instructed task.
The two conditions that showed the smallest difference between the two groups were Same-Power(Down) grip (P = 0.174) and Opposite-Precision(Up) grip (P = 0.732). As classical null hypothesis testing is not the ideal statistical tool to make conclusions about non-significant results (Dienes, 2014), we calculated Bayes Factors (BF) for each of the eight between Groups comparisons and tested the null hypothesis that the two groups did not differ (BF10 factors bigger than 1 indicate evidence for a significant difference between conditions). We run Bayesian Independent Sample T Tests on patients Asynchrony (JASP version 0.8.12, Love et al., 2015) and found that: (i) Interactive-Opposite-Precision(Up) (BF10 = 0.477) and Interactive-Same-Power(Down) (BF10 = 0.455) showed anecdotal evidence in favor of the null hypothesis (no difference between groups); (ii) Interactive-Opposite-Power(Down) (BF10 = 3.170) and Interactive-Same-Precision(Up) (BF10 = 3.083) showed a moderate evidence of group differences; (iii) conversely, all Instructed conditions showed strong evidence for group differences [Instructed-Opposite-Precision(Up) BF10 = 178.094, Instructed-Opposite-Power(Down) BF10 = 14.605, Instructed-Same-Precision(Up) BF10 = 11.864, Instructed-Same-Power(Down) BF10 = 46.452].
Thus, the two conditions that resulted to be equally difficult for LA- and LA + when applying non-parametric tests [i.e. Interactive-Opposite-Precision(Up) (and Interactive-Same-Power(Down)] also showed no evidence of group differences with a Bayesian approach.
Across-condition differences between Interactive vs Instructed coordination
A Friedman ANOVA on participant-partner grasping Asynchrony performed on the entire sample (i.e. independently from group classification) revealed significant across-condition differences [ANOVA Chi Sqr. (N = 28, df = 7) = 25.357, P < 0.001]. Follow-up Wilcoxon Matched Pairs Tests between Interactive and Instructed conditions revealed that Instructed coordination was more difficult (i.e. higher asynchrony) than Interactive coordination only when performing Opposite-Precision(Up) grips (P = 0.009, corrected P threshold = 0.05/4 = 0.013) and Same-Power(Down) grips (P < 0.001) (all other Ps > 0.716). This result indicates that patients tended to be better at synchronizing during Interactive than Instructed coordination, showing a beneficial effect of maximally interactive conditions compared with the less interactive condition (i.e. Instructed) in which patients were not required to read the partner’s behavior in order to program their own.
Notably, however, the across-condition beneficial effect of Interactive coordination was evident only in LA + patients. Indeed, when running the across-condition analysis in the LA + and LA- groups separately, only LA + showed this pattern of impaired synchrony during Instructed compared with Interactive coordination [ANOVA Chi Sqr. (N = 12, df = 7) = 21.722, P < 0.003; significant difference between Interactive-Same-Power(Down) grips vs Instructed-Same-Power(Down) grips, P < 0.008; trend to significant Interactive-Opposite-Precision(Up) grips vs Instructed-Opposite-Precision(Up) grips difference, P = 0.015, corrected P threshold = 0.013] (Figure 2 red asterisk). Conversely, LA- did not behave differently in Interactive and Instructed coordination [ANOVA Chi Sqr. (N = 16, df = 7) = 11.521, P = 0.117].
Overall, these results show that apraxic patients improved their synchrony when acting in Interactive vs Instructed conditions, while non-apraxic patients did not.
Accuracy of performance
Group differences in Interactive vs Instructed coordination
Direct comparisons between the two Groups in the different experimental conditions showed that LA + and LA- accuracy did not differ in any condition (Mann–Whitney U, all Ps > 0.015, corrected P threshold = 0.006).
Across-condition differences between Interactive vs Instructed coordination
A Friedman ANOVA on patients’ accuracy revealed significant across-condition differences [ANOVA Chi Sqr. (N = 28, df = 7) = 25.357, P < 0.001]. Follow-up Wilcoxon Matched Pairs Tests between Interactive and Instructed conditions revealed that no Instructed vs Interactive coordination condition was significantly different after correction (all Ps > 0.018, corrected P threshold = 0.013). Although this pattern of results was also found when testing LA + (P = 0.046) and LA- patients (P = 0.001) separately, no post-hoc test was significant after correction (all Ps > 0.028).
Bootstrap ANOVA on Asynchrony/Accuracy
In order to directly test the interaction between the Group and the within subject factors, and to account for possible speed-accuracy trade-offs, we combined together the two performance measures and ran a bootstrap ANOVA on the Inverse Efficiency index (i.e. Asynchrony/Accuracy). This analysis confirmed the pattern of results found with non-parametric tests highlighting a significant Group (LA+/LA-) × Coordination (Interactive/Instructed) × Interaction (Same/Opposite) × Movement (Precision(Up)/Power(Down) grip] interaction [F(1, 26) = 5.909, bootstrapped P < 0.001). Post-hoc comparisons indicated that Interactive coordination was easier than Instructed coordination during Opposite-Power(Down) (P < 0.001) and Same-Precision(Up) (P = 0.0135) movements in LA + but not in LA- (P = 1 for both comparisons). The significant two-way interaction between Group and Interactive/Instructed factors, suggested that only the LA + group was sensitive to the interactive nature of the task, being able to perform the Interactive task as good as LA- (P = 0.269) while performing worse than LA- patients during Instructed conditions (P < 0.001). The higher-level interaction explained all significant lower level effects (Group, bootstrapped P < 0.001; Interactive/Instructed, bootstrapped P < 0.001; Group × Interactive/Instructed, bootstrapped P < 0.001; Group × Interactive/Instructed × Same/Opposite, bootstrapped P = 0.018).
Kinematic results
See Supplementary Material for results on Wrist Maximum Height, Index-Thumb Maximum Aperture, Wrist Maximum Velocity, Time of Wrist Maximum Height, Time of Maximum Index-Thumb Aperture, Time of Maximum Wrist Velocity.
Correlation between apraxia scores (TULIA) and joint task index
Prediction task
The between groups analysis of patients’ Accuracy in predicting the partner's movement showed a trend to lower LA + accuracy when compared with LA-, but only for short video clips in which the action was toward the lower part of the bottle-shaped object (Mann–Whitney U, P = 0.027, all other Ps > 0.108, corrected P threshold = 0.013). An across-condition Friedman ANOVA performed on the entire sample [ANOVA Chi Sqr. (N = 26, df = 3) = 14.014, P < 0.003] showed a trend toward better performance when predicting Precision(Up) movements for long compared with short video clips both for Precision(Up) (P = 0.021, corrected P threshold = 0.013) and Power(Down) movements (P = 0.004, corrected P threshold = 0.013). Tellingly, the above pattern of results was found in the LA+ group [ANOVA Chi Sqr. (N = 11, df = 3) = 10.037, P = 0.018] but not in the LA- group [ANOVA Chi Sqr. (N = 15, df = 3) = 4.477, P = 0.214]. In sum, LA + seem less able than LA- patients to predict observed movements during a passive observation task. Notably, this pattern of results found in a passive observational task is opposite to what we found in the Interactive condition of the coordination task (see Supplementary Material for a discussion of the results of the Prediction Task).
Lesion analyses
See Supplementary Material for the: (i) Overlap map of LA + and LA- patients’ lesions; (ii) Subtraction map between LA + and LA- groups; (iii) Neural correlates of Apraxia (TULIA VLSM).
Neural underpinnings of impaired Instructed vs Interactive coordination performance
To determine the lesions that best predicted the patients’ behavioral impairment in Instructed compared with Interactive coordination conditions, we performed a VLSM analysis (Rorden et al., 2007a,b) with an Interaction-Δ as continuous predictor. The Interaction-Δ was based on the results of the Coordination Task in order to index the conditions that proved to be most difficult when performed in the Instructed condition compared with the Interactive one [i.e. Opposite-Precision(Up) and Same-Power(Down) grips]. More specifically, the Interaction-Δ was computed by using the formula: Interaction-Δ = [Interactive/Opposite/Precision(Up) + Interactive/Same/Power(Down)] - [Instructed/Opposite/Precision(Up) + Instructed/Same/Power(Down)]. The regions associated with impaired Instructed coordination performance are shown in Figure 3 and Table 2.
Fig. 3.
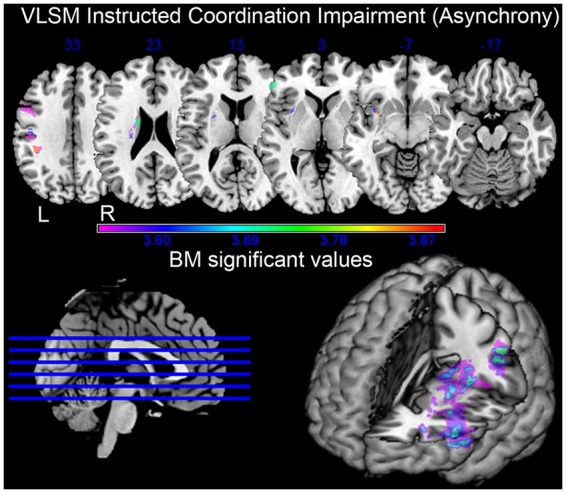
Voxel lesions predictive of patients’ Interaction-Δ in the VLSM analysis. The test compares the behavioral performance of patients on a voxel-by-voxel basis. Highlighted voxels are associated with worse performance in Instructed compared with Interactive conditions. Multi-slice representation and rendering of the entire brain. The color-code bar indicates the level of significance associated with each voxel as tested by the Brunner–Munzel statistics (FDR correction, P = 0.01).
Table 2.
Regions associated with impaired performance in Instructed compared with Interactive conditions (i.e. Interaction-Δ). For each region, the MNI coordinates of the center of mass are provided along with the maximum Brunner–Munzel (BM) z statistic obtained in each cluster and the number (n) and percentage (%) of clustering voxels that survived the threshold of P < 0.01, false discovery rate corrected
| VLSM Instructed interaction impairment (Asynchrony) | ||||||
|---|---|---|---|---|---|---|
| Area | Number of lesioned voxels | % of lesioned voxels | Max | MaxX | MaxY | MaxZ |
| Putamen_L | 226 | 3 | 3.891 | −33 | 6 | −5 |
| Insula_L | 310 | 2 | 3.891 | −34 | 13 | −12 |
| Caudate_L | 143 | 2 | 3.719 | −20 | 1 | 20 |
| Precentral_L | 422 | 2 | 3.540 | −55 | 7 | 28 |
| Frontal_Inf_Tri_L | 273 | 1 | 3.719 | −57 | 38 | 1 |
| Postcentral_L | 179 | <1 | 3.891 | −44 | −20 | 33 |
| Frontal_Inf_Oper_L | 42 | <1 | 3.540 | −47 | 5 | 29 |
| Parietal_Inf_L | 57 | <1 | 3.891 | −44 | −34 | 36 |
| Frontal_Inf_Orb_L | 22 | <1 | 3.891 | −41 | 19 | −12 |
| SupraMarginal_L | 11 | <1 | 3.891 | −44 | −35 | 34 |
VLSM showed that lesions to the left motor cortex, pars triangularis of the premotor cortex, insula and striatum (putamen and caudate) predicted poorer performance in Instructed as compared with Interactive coordination.
Discussion
Behavioral correlates of realistic cooperation
The ability of LA- patients to synchronize their movements with those of an avatar was similar during Interactive and Instructed cooperation, suggesting that the two conditions were not different per se (see Supplementary Material for a similar evidence on movement kinematics). In the coordination task, performance was overall worse in LA + than in LA- patients. Crucially, when engaged in Interactive cooperation LA + performed like LA- patients. The positive correlation between the Interaction-Δ and patients’ apraxic scores (TULIA) supports the link between apraxic deficits and impairment in performing Instructed vs Interactive coordination tasks (see Supplementary Material). It has been shown that during individual action execution, deficits of apraxics manifest when their reaching movements must adapt to increasing visuo-motor requests (Mutha et al., 2010). Thus, the reduction of apraxics’ impairments observed during the more challenging interactive condition suggests that individual action execution may benefit from the cues provided by the movements of the partner in line with the automatic/voluntary dissociation (Trojano et al., 2007; Liepmann, 1900, 1905a,b; Basso and Capitani, 1985; De Renzi et al., 1982; Pramstaller and Marsden, 1996). While coordination in the Instructed condition was based on auditory instructions specifying the target hand configuration and arm trajectory (i.e. a condition similar to standard apraxic tests), interactive coordination was based on the action of a partner (i.e. imitate and complement its actions). The improvement of LA + in the interactive coordination task may thus be explained by the ‘affordance competition hypothesis’ (Cisek, 2007; Cisek and Kalaska, 2010) according to which the brain processes sensory information to specify, in parallel, several potential actions that are currently available and compete against each other. Anatomo-functionally, the hypothesis suggests that the dorsal visual system specifies competing actions within the fronto-parietal cortex, while a variety of biasing influences are provided by prefrontal regions and the basal ganglia. Here, the concept of affordances goes beyond action specification for object interactions and refers to the action of a partner (Cisek et al., 2007).
Interaction-based approaches to rehabilitation of higher-order motor disorders
Our behavioral results may provide important insights for devising interaction-based approaches for treating apraxia and possibly other higher-order motor disorders. More specifically, we show that apraxic motor deficits can be assessed by indexing the ability of patients to synchronize their actions with a virtual partner (i.e. our Instructed condition). This is radically different from how apraxia is tested in standard individual conditions that typically evaluate the ability to perform actions under verbal command, under delayed imitation or after exposition to a tool. Crucially, we show that apraxics’ impairment is reduced when the movement of the partner needs to be taken into account in order to select the individual action. This suggests that integrating ones’ own movement with that of a partner may engage additional neural resources in line with evidence showing that joint actions do not activate the very same brain regions that are activated by action observation or execution alone (Kokal et al., 2009). The Interactive condition used in the present study may possibly recreate the ecological conditions that are known to elicit the automatic/voluntary dissociation reported in apraxia studies (De Renzi et al., 1982; Trojano et al., 2007). Thus, the present data suggest that this interactional effect may be exploited for rehabilitative purposes. This seems very timely considering that current approaches to apraxia rehabilitation are based on strategies that either aim at restoring the impaired motor functions or compensate for them (i.e. restorative and compensatory strategies, Cantagallo et al., 2012) in acting-alone patients. Unfortunately, there is a general consensum on the fact that standard approaches are only partially effective (Worthington, 2016) and do not generalize, indicating that new approaches are needed (Buxbaum et al., 2008; Cantagallo et al., 2012). In their comprehensive review of rehabilitative approaches to apraxia, Buxbaum et al. (2008) list different procedures based on: (i) multiple cues, (ii) error reduction, (iii) six-stage task hierarchy, (iv) conductive education, (v) strategy training, (vi) transitive/intransitive gesture training, (vii) ‘rehabilitative training’, (viii) errorless completion + exploration training. Furthermore, Buxbaum et al. (2008) provide a list of cognitive domains that might be used for interventions (e.g. mechanical problem solving, sequence planning and organization, the ability to develop and/or retrieve optimal motor programs, knowledge of how to manipulate an object, and knowledge of optimal hand position when real-world objects provide minimal cues). Tellingly, the list seems to neglect social accounts of motor control that are the basis of the present study and that might provide a useful approach for rehabilitation. It is worth noting that an interactive approach has been used in aphasic patients who showed an increase of performance, possibly due to the mechanism of entrainment, when seeing another person producing speech while attempting to mimic the same mouth movements (Fridriksson et al., 2012, 2015). Importantly, the present pattern of results suggests that the beneficial effect of motor interactions goes beyond the possible role of on-line movement imitation as the improvement was found during both imitative and complementary interactions. From a modeling point of view, the present findings suggest that motivational factors as well as resources activated for the processing of others’ movements are intrinsic to social interactions and may improve interactive behaviors compared with individual action performance.
New technologies, such as Virtual Reality might be promising tools to implement scenarios where patients are engaged in interactions with virtual partners embodying different movement kinematics which can be modulated according to the patient’s needs. For example, exaggerating specific kinematic features (Sacheli et al., 2013; Candidi et al., 2015), slowing down the movements of the virtual partner, or even making the avatar responsive to the movement deficits of the patients might be efficient for people with different motor disabilities or in different learning stages.
Brain lesions dissociating the performance of interactive vs instructed coordination
The VLSM analyses on the entire sample of patients indicates that lesions to left motor cortex, pars triangularis of the premotor cortex, insula and striatum (putamen and caudate) were predictive of poorer performance in the Instructed condition compared with the Interactive one. The present data suggest that these regions are needed for solving the Instructed task while social interaction might be underpinned by larger brain systems.
Premotor and motor regions are well known for their role in action selection and implementation as well as in matching observed and executed actions (Rizzolatti et al., 2014; Urgesi et al., 2013; di Pellegrino et al., 1992; Gallese et al., 1996; Archer et al., 2016), a process that is fundamental in our task. Crucially, our study shows how precentral and premotor lesions, that are stable predictors of apraxia (Pazzaglia et al., 2008a,b; Buxbaum et al., 2007; Goldenberg et al., 2007), are predictive of worse performance in Instructed as compared with Interactive coordination. Importantly, while both the Interactive and Instructed coordination conditions imply predicting the timing of the partner’s movements, only the former requires integrating the spatial content of the partner’s movement into their own motor plan (e.g. only the Interactive condition requires patients to subordinate their behavior to that of their partner). Thus, premotor regions seem to be fundamental for performing actions when behaving in the Instructed task while not so for performing the Interactive task which might be based on other brain systems to scaffold performance. For example, these results are in line with our previous study showing that left parietal brain regions (the anterior intra-parietal sulcus) and not frontal regions, might play a crucial role in interpersonal coordination during motor interactions (Sacheli et al., 2015b).
Insular lesions were associated with worse performance in the Instructed as compared with the Interactive condition. It is worth noting that the anterior insula, together with prefrontal, dorsolateral prefrontal, dorsomedial superior frontal and inferior parietal lobules, is part of a ‘fronto-parietal control system’ (Spreng et al., 2009) which detects the salience of stimuli (Menon and Uddin, 2010). Thus, we propose that lesions of the insula impaired coordination in the Instructed condition since in this condition the behavior of the partner is less salient compared with the Interactive condition. This supports the idea that others’ behavior may represent a form of social affordance that facilitates the performance of individual movements.
That lesions of basal ganglia and of a portion of the premotor cortex predict impaired performance in Instructed coordination is in keeping with the notion that higher order motor cognition may be underpinned by combined cortico-subcortical circuits (Leiguarda, 2001; Bhatia and Marsden, 1994; Pramstaller and Marsden, 1998; De Renzi, 1986). While apraxic deficits may selectively regard the kinematic features of movement execution (i.e. trajectory, timing and speed; Faglioni and Basso, 1985; Denes et al., 1998) we did not find clear differences between the kinematic pattern of LA + and LA- patients in our task. This may suggest that the behavioral difference in performing the Interactive and Instructed coordination conditions between the two groups was not explained by differences in the implementation of kinematic features of the reach-to-grasp movements but rather that the role of the basal ganglia in our experimental task may have to do with signaling relevant cues that bias the fronto-parietal network toward a specific action by inhibiting unnecessary or competing ones (Cisek, 2007; Rounis and Humphreys, 2015).
Conclusion
By showing that apraxic patients are better at performing actions in an interactive context compared with isolated conditions, our study supports the notion that the social nature of action representations might be crucial for facilitating motor functions in patients suffering from higher-order motor deficits. Furthermore, by finding an interaction benefit when apraxic patients performed both complementary and imitative conditions, the present results suggest that realistic interactions may provide benefits above those of interpersonal imitation. This interaction-based approach to motor dysfunctions may thus be exploited to rehabilitate patients suffering from a variety of higher-order motor impairments.
Supplementary Material
Acknowledgments
We would like to thank Dr Enea Francesco Pavone at BrainTrends for developing the experimental set-up. BrainTrends has no financial or intellectual conflict of interest in connection with the manuscript. We would like to also thank Arran T. Reader for reviewing and editing the draft of this paper.
Funding
MC was supported by Sapienza University (Progetti Medi 2016). SMA was funded by PRIN grant (Progetti di Ricerca di Rilevante Interesse Nazionale, Edit. 2015, Prot. 20159CZFJK) and from H2020-SESAR-2015-1 (MOTO: The embodied reMOte Tower, Project Number 699379).
Author contribution
Conceptualization, M.C., L.M.S. and S.M.A.; Methodology, M.C., L.M.S. and S.M.A.; Resources, L.C. and G.T.; Investigation, M.C., L.M.S. and V.E.; Formal Analysis, M.C., L.M.S., V.E.; Writing—Original Draft, M.C., L.M.S., V.E. and S.M.A.; Funding Acquisition, M.C., S.M.A.; Supervision, SMA.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Appollonio I., Leone M., Isella V., Piamarta F., Consoli T., Villa M.L. (2005). The frontal assessment battery (FAB): normative values in an Italian population sample. Neurological Sciences, 26(2), 108e116. [DOI] [PubMed] [Google Scholar]
- Archer D.B., Misra G., Patten C., Coombes S.A. (2016). Microstructural properties of premotor pathways predict visuomotor performance in chronic stroke. Human Brain Mapping, 37(6), 2039–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A., Candidi M., Urgesi C. (2013). Vicarious motor activation during action perception: beyond correlational evidence. Frontiers in Human Neuroscience, 7, 185.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A., Capitani E. (1985). Spared musical abilities in a conductor with global aphasia and ideomotor apraxia. Journal of Neurology, Neurosurgery and Psychiatry, 48(5), 407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia K.P., Marsden C.D. (1994). The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain, 117(4), 859–76. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J., Kyle K., Grossman M., Coslett H.B. (2007). Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex, 43(3), 411–23. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J., Haaland K.Y., Hallett M., et al. (2008). Treatment of limb apraxia: moving forward to improved action. American Journal of Physical Medicine & Rehabilitation, 87(2), 149–61. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J., Shapiro A.D., Coslett H.B. (2014). Critical brain regions for tool-related and imitative actions: a componential analysis. Brain, 137(Pt 7), 1971–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candidi M., Curioni A., Donnarumma F., Sacheli L.M., Pezzulo G. (2015). Interactional leader-follower sensorimotor communication strategies during repetitive joint actions. Journal of the Royal Society Interface, 110, 0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagallo A., Maini M., Rumiati R.I. (2012). The cognitive rehabilitation of limb apraxia in patients with stroke. Neuropsychological Rehabilitation, 22(3), 473–88. [DOI] [PubMed] [Google Scholar]
- Canzano L., Scandola M., Pernigo S., Aglioti S.M., Moro V. (2014). Anosognosia for apraxia: experimental evidence for defective awareness of one's own bucco-facial gestures. Cortex, 61, 148–57. [DOI] [PubMed] [Google Scholar]
- Cisek P. (2007). Cortical mechanisms of action selection: the affordance competition hypothesis. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 362(1485), 1585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. (2010). Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience, 33, 269–98. [DOI] [PubMed] [Google Scholar]
- D'Ausilio A., Badino L., Cipresso P., Chirico A., Ferrari E., Riva G. (2015). Automatic imitation of the arm kinematic profile in interacting partners. Cognitive Processing, 16(Suppl. 1), 197–201. [DOI] [PubMed] [Google Scholar]
- De Renzi E. (1986). The apraxias. Diseases of the nervous system In: Asbury A.K., McKhann G.M., McDonald W.I., editors. Clinical Neurobiology. Philadelphia: W.B. Saunders; p. 848–54. [Google Scholar]
- De Renzi E., Faglioni P., Sorgato P. (1982). Modality specific and supramodal mechanisms of apraxia. Brain, 105(Pt 2), 301–12. [DOI] [PubMed] [Google Scholar]
- Denes G., Mantovan M.-C., Gallana A., Cappelletti J.Y. (1998). Limb-kinetic apraxia. Movement Disorders, 13(3), 468–76. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G., Fadiga L., Fogassi L., Gallese V., Rizzolatti G. (1992). Understanding motor events: a neurophysiological study. Experimental Brain Research, 91(1), 176–80. [DOI] [PubMed] [Google Scholar]
- Dienes Z. (2014). Using Bayes to get the most out of non-significant results. Frontiers in Psychology, 5, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faglioni P., Basso A. (1985). Historical perspective on neuroanatomical correlates of limb apraxia In: Roy E.A., editor. Neuropsychological Studies of Apraxia and Related Disorders. Amsterdam: North-Holland Press; p. 3–44. [Google Scholar]
- Freund H.J. (2001). The parietal lobe as a sensorimotor interface: a perspective from clinical and neuroimaging data. Neuroimage, 14(1 Pt 2), S142–6. [DOI] [PubMed] [Google Scholar]
- Fridriksson J., Hubbard H.I., Hudspeth S.G., et al. (2012). Speech entrainment enables patients with Broca's aphasia to produce fluent speech. Brain, 135(Pt 12), 3815–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Basilakos A., Hickok G., Bonilha L., Rorden C. (2015). Speech entrainment compensates for Broca's area damage. Cortex, 69, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. (1996). Action recognition in the premotor cortex. Brain, 119(2), 593–609. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Hermsdorfer J., Glindemann R., Rorden C., Karnath H.O. (2007). Pantomime of tool use depends on integrity of left inferior frontal cortex. Cerebral Cortex, 17(12), 2769–76. [DOI] [PubMed] [Google Scholar]
- Halsband U., Schmitt J., Weyers M., Binkofski F., Grützner G., Freund H.J. (2001). Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: a perspective on apraxia. Neuropsychologia, 39(2), 200–16. [DOI] [PubMed] [Google Scholar]
- Heilman K.M., Rothi L.J., Valenstein E. (1982). Two forms of ideomotor apraxia. Neurology, 32(4), 342–6. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J., Li Y., Randerath J., Roby-Brami A., Goldenberg G. (2013). Tool use kinematics across different modes of execution. Implications for action representation and apraxia. Cortex, 49(1), 184–99. [DOI] [PubMed] [Google Scholar]
- Kilner J.M., Paulignan Y., Blakemore S.J. (2003). An interference effect of observed biological movement on action. Current Biology, 13(6), 522–5. [DOI] [PubMed] [Google Scholar]
- Kokal I., Gazzola V., Keysers C. (2009). Acting together in and beyond the mirror neuron system. Neuroimage, 47(4), 2046–56. [DOI] [PubMed] [Google Scholar]
- Kokal I., Keysers C. (2010). Granger causality mapping during joint actions reveals evidence for forward models that could overcome sensory-motor delays. PLoS ONE, 5(10), e13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis D., Sebanz N., Knoblich G. (2013). Predictive representation of other people's actions in joint action planning: an EEG study. Society for Neuroscience, 8(1), 31–42. [DOI] [PubMed] [Google Scholar]
- Leiguarda R., Marsden C.D. (2000). Limb apraxias: higher-order disorders of sensorimotor integration. Brain, 123(5), 860–79. [DOI] [PubMed] [Google Scholar]
- Leiguarda R. (2001). Limb apraxia: cortical or subcortical. Neuroimage, 14(1 Pt 2), S137–41. [DOI] [PubMed] [Google Scholar]
- Liepmann H. (1900). Das Krankheitsbild der Apraxie (motorische Asymbolie) auf Grund eines Falles von einseitiger Apraxie. Monatschrift Fur Psychiatrie Und Neurologie, 8(3), 15–44. 102–132, 182–197. [Google Scholar]
- Liepmann H. (1905a). Der weitere Krankheitsverlauf bei dem einseitig Apraktischen und der Gehirnbefund auf Grund von Serienschnitten. Monatschrift Fur Psychiatrie Und Neurologie, 17, 289–311. [Google Scholar]
- Liepmann H. (1905b). Der weitere Krankheitsverlauf bei dem einseitig Apraktischen und der Gehirnbefund auf Grund von Serienschnitten. Monatschrift Fur Psychiatrie Und Neurologie, 19, 217–43. [Google Scholar]
- Love J., Selker R., Marsman M., Jamil T., Dropmann D., Verhagen A.J., et al. JASP (Version 0.6.6). [Computer software]. 2015.
- Luzzatti C., Willmes K., De Bleser R. (1996). Aachener Aphasie Test (AAT). Firenze: Organizzazioni Speciali. [Google Scholar]
- Mahon B.Z., Caramazza A. (2008). A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology – Paris, 102(1–3), 59–70. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha P.K., Sainburg R.L., Haaland K.Y. (2010). Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia, 48(13), 3855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber M., Vaziri Pashkam M., Nakayama K. (2013). Unintended imitation affects success in a competitive game. Proceedings of the National Academy of Sciences of the United States of America, 110(50), 20046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T., Hayasaka S. (2003). Controlling the family wise error rate in functional neuroimaging: a comparative review. Statistical Methods in Medical Research, 12(5), 419–46. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M., Pizzamiglio L., Pes E., Aglioti S.M. (2008). The sound of actions in apraxia. Current Biology, 18(22), 1766–72. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M., Smania N., Corato E., Aglioti S.M. (2008). Neural underpinnings of gesture discrimination in patients with limb apraxia. Journal of Neuroscience, 28(12), 3030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramstaller P.P., Marsden C.D. (1996). The basal ganglia and apraxia. Brain, 119(1), 319–40. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from: http://www.r-project.org [Google Scholar]
- Randerath J., Goldenberg G., Spijkers W., Li Y., Hermsdörfer J. (2011). From pantomime to actual use: how affordances can facilitate actual tool-use. Neuropsychologia, 49(9), 2410–6. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Cattaneo L., Fabbri-Destro M., Rozzi S. (2014). Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiological Reviews, 94(2), 655–706. [DOI] [PubMed] [Google Scholar]
- Rorden C., Bonilha L., Nichols T.E. (2007). Rank-order versus mean based statistics for neuroimaging. Neuroimage, 35(4), 1531–7. [DOI] [PubMed] [Google Scholar]
- Rorden C., Karnath H.O., Bonilha L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–8. [DOI] [PubMed] [Google Scholar]
- Rothi L.J.G., Ochipa C., Heilman K.M. (1991). A cognitive neurophychological model of limb praxis. Cognitive Neuropsychology, 8, 443–8. [Google Scholar]
- Rothi L.J., Heilman K.M., Watson R.T. (1985). Pantomime comprehension and ideomotor apraxia. Journal of Neurology, Neurosurgery, and Psychiatry, 48(3), 207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounis E., Humphreys G. (2015). Limb apraxia and the “affordance competition hypothesis”. Frontiers in Human Neuroscience, 9, 429.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli L.M., Candidi M., Pavone E.F., Tidoni E., Aglioti S.M. (2012). And yet they act together: interpersonal perception modulates visuo-motor interference and mutual adjustments during a joint-grasping task. PLoS ONE, 7(11), e50223.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli L.M., Tidoni E., Pavone E.F., Aglioti S.M., Candidi M. (2013). Kinematics fingerprints of leader and follower role-taking during cooperative joint actions. Experimental Brain Research, 226(4), 473–86. [DOI] [PubMed] [Google Scholar]
- Sacheli L.M., Aglioti S.M., Candidi M. (2015a). Social cues to joint actions: the role of shared goals. Frontiers in Psychology, 6, 1034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli L.M., Candidi M., Era V., Aglioti S.M. (2015b). Causative role of left aIPS in coding shared goals during human-avatar complementary joint actions. Nature Communications, 6, 7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli L.M., Christensen A., Giese M.A., Taubert N., Pavone E.F., Aglioti S.M., et al. (2015c). Prejudiced interactions: implicit racial bias reduces predictive simulation during joint action with an out-group avatar. Scientific Reports, 5, 8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori L., Becchio C., Bulgheroni M., Castiello U. (2009). Modulation of the action control system by social intention: unexpected social requests override preplanned action. Journal of Experimental Psychology: Human Perception & Performance, 35(5), 1490–500. [DOI] [PubMed] [Google Scholar]
- Sebanz N., Bekkering H., Knoblich G. (2006). Joint action: bodies and minds moving together. Trends in Cognitive Sciences, 10(2), 70–6. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Stasenko A., Garcea F.E., Mahon B.Z. (2013). What happens to the motor theory of perception when the motor system is damaged? Language and Cognitive Processes, 5(2–3), 225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieri G., Tidoni E., Pavone E.F., Aglioti S.M. (2015). Mere observation of body discontinuity affects perceived ownership and vicarious agency over a virtual hand. Experimental Brain Research, 233(4), 1247–59. [DOI] [PubMed] [Google Scholar]
- Trojano L., Labruna L., Grossi D. (2007). An experimental investigation of the automatic/voluntary dissociation in limb apraxia. Brain and Cognition, 65(2), 169–76. [DOI] [PubMed] [Google Scholar]
- Urgesi C., Candidi M., Avenanti A. (2014). Neuroanatomical substrates of action perception and understanding: an anatomic likelihood estimation meta-analysis of lesion-symptom mapping studies in brain injured patients. Frontiers in Human Neuroscience, 8, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbellingen T., Kersten B., Van Hemelrijk B., et al. (2010). Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). European Journal of Neurology, 17(1), 59e66. [DOI] [PubMed] [Google Scholar]
- Worthington A. (2016). Treatments and technologies in the rehabilitation of apraxia and action disorganisation syndrome: a review. NeuroRehabilitation, 39(1), 163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadikoff C., Lang A.E. (2005). Apraxia in movement disorders. Brain, 128(Pt 7), 1480–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.