Abstract
Sustained anticipatory anxiety is central to Generalized Anxiety Disorder (GAD). During anticipatory anxiety, phasic threat responding appears to be mediated by the amygdala, while sustained threat responding seems related to the bed nucleus of the stria terminalis (BNST). Although sustained anticipatory anxiety in GAD patients was proposed to be associated with BNST activity alterations, firm evidence is lacking. We aimed to explore temporal characteristics of BNST and amygdala activity during threat anticipation in GAD patients. Nineteen GAD patients and nineteen healthy controls (HC) underwent functional magnetic resonance imaging (fMRI) during a temporally unpredictable threat anticipation paradigm. We defined phasic and a systematic variation of sustained response models for blood oxygen level-dependent responses during threat anticipation, to disentangle temporally dissociable involvement of the BNST and the amygdala. GAD patients relative to HC responded with increased phasic amygdala activity to onset of threat anticipation and with elevated sustained BNST activity that was delayed relative to the onset of threat anticipation. Both the amygdala and the BNST displayed altered responses during threat anticipation in GAD patients, albeit with different time courses. The results for the BNST activation hint towards its role in sustained threat responding, and contribute to a deeper understanding of pathological sustained anticipatory anxiety in GAD.
Keywords: anticipatory anxiety, anxiety, fMRI, sustained threat responding, phasic threat responding
Introduction
Generalized anxiety disorder (GAD) patients suffer from excessive anxiety and apprehensive expectations, they are hyperresponsive to threat and have the tendency to react negatively to situations that are uncertain (Gentes and Ruscio, 2011; Buff et al., 2016). Sustained anticipatory anxiety constitutes a core symptom of GAD and appears to facilitate the development and maintenance of the disorder (Zinbarg et al., 2006).
Previous research on sustained anticipatory anxiety in rodents and nonanxious controls (HC) revealed a neural dissociation of phasic and sustained brain responses to threat anticipation (Davis et al., 2010; Forster et al., 2012; Herrmann et al., 2016). While phasic threat responding appears to be mediated by the amygdala, sustained threat responding seems related to activity in the bed nucleus of the stria terminalis (BNST) (Davis et al., 2010; Avery et al., 2016). As part of the so-called extended amygdala, the BNST seems to mediate sustained hypervigilance and preparedness in the context of prolonged threat exposure (Davis et al., 2010; Fox et al., 2015; Lebow and Chen, 2016). Emerging evidence in psychiatric patients stresses the role of the BNST in the pathophysiology of anxiety/stress disorders and accentuates it to be a marker of pathological anticipatory anxiety (Straube et al., 2007a; Münsterkötter et al., 2015; Brinkmann et al., 2017a, 2017b). Accordingly, it has been hypothesized that sustained anticipatory anxiety in GAD patients may be linked to alterations in BNST activity (Yassa et al., 2012; Hilbert et al., 2014). More specifically, pathological apprehensive expectations were hypothesized to induce hyperactivity in the BNST, through which a continuous anxious state would be maintained in patients (Hilbert et al., 2014). Following that, the BNST may be a key region underlying the maintenance and possibly also the development of maladaptive anticipatory anxiety in GAD. Surprisingly, to date, no firm evidence of BNST activity alterations in GAD patients has been reported yet. There is only preliminary evidence of deviating phasic amygdala activity in GAD patients during short-term threat anticipation and conditions of uncertainty (Nitschke et al., 2009; Yassa et al., 2012). Relative to HC, Nitschke et al. (2009) observed increased amygdala activity in GAD patients during short-term threat anticipation (between 2.5 and 4 s), but failed to replicate the respective finding in another study (Oathes et al., 2015). Yassa et al. (2012) detected suppressed amygdala activity in GAD patients relative to HC during periods of uncertainty in a monetary loss task. Yet, these studies did not investigate sustained activation to threat. Importantly, while initial evidence of deviant amygdala activity during periods of uncertainty exists, the role of BNST in sustained responses to threat anticipation in GAD remains unresolved.
When investigating BNST activity in the context of sustained anticipatory anxiety, threat anticipation paradigms proved a powerful tool, since they allow to differentiate between phasic and sustained threat responding (Forster et al., 2012). Threat anticipation paradigms parallel instructed fear conditioning tasks in that participants are made aware of the cue—stimuli pairings prior to actually experiencing it. The acquired predictive value of the cue is hold to produce an expectation of an aversive event that lasts until the delivery of the aversive event (Mechias et al., 2010). Concordantly, instructing participants that a specific cue will be followed by a threat stimulus is assumed to be sufficient to induce anticipatory anxiety responses (Mechias, 2012; Fullana et al., 2016). Moreover, previous investigations emphasized the necessity to explore different forms of sustained threat responding. While several studies on anticipatory anxiety detected sustained BNST activity starting with threat anticipation onset (Straube et al., 2007a; Herrmann et al., 2016; Brinkmann et al., 2017a, 2017b), another study observed sustained BNST activity that was delayed relative to the onset of threat anticipation (McMenamin et al., 2014). The latter finding is in line with the influential neurobiological model of sustained anxiety that postulates the BNST to show a delayed engagement relative to threat onset (Davis et al., 2010b). Another important aspect in BNST investigations constitutes the predictability of threat exposure, because it proved important to induce temporal unpredictability of threat exposure to ease detecting robust condition differences in BNST activity when present (Grupe et al., 2012; Avery et al., 2016).
The current functional magnetic resonance imaging (fMRI) study sought to delineate temporal characteristics of the BNST and the amygdala engagement in GAD patients relative to HC during an instructed unpredictable threat anticipation paradigm. Warning cues were present during the entire anticipatory epoch, which either indicated a threatening (e.g. female screams) or neutral (e.g. water sounds) upcoming stimulus with 100% contingency. Anticipatory epochs varied in length, so that the appearance of the sounds was temporally unpredictable. Additionally, we defined phasic and a systematic variation of sustained response models for blood oxygen level-dependent responses (BOLD) during threat anticipation, to disentangle temporally dissociable involvement of the BNST and the amygdala. Given our current research focus, we concentrated on group differences in the amygdala and the BNST. Based on previous reports (Nitschke et al., 2009; Yassa et al., 2012) we expected phasic amygdala activity alterations in GAD patients relative to HC during threat anticipation. With regard to the neurobiological model described above and former preliminary considerations (Yassa et al., 2012; Hilbert et al., 2014), we expected GAD patients relative to HC to show heightened BNST activity to sustained threat anticipation.
Materials and methods
Subjects
Nineteen GAD patients (male n = 4) and 19 HC (male n = 4) matched for education, age and gender were recruited through public advertisements and an outpatient clinic (Table 1). Patients met criteria for GAD as primary diagnosis assessed by an experienced psychologist by means of the Structured Clinical Interview for Diagnostic and Statistical manual of Mental Disorders IV (DSM-IV) axis-I Disorders (Wittchen et al., 1997). The Penn-State Worry Questionnaire (PSWQ) (Meyer et al., 1990) and the Generalized Anxiety Disorder Questionnaire-IV (GAD-Q-IV) (Newman et al., 2002) supported the diagnosis. Level of depressive symptoms was assessed by the Beck Depression Inventory-II (BDI) (Beck et al., 1996). Representative of the GAD population, the patient sample had the following axis-I comorbidities (Newman et al., 2013): recurrent major depressive disorder (n = 3), posttraumatic stress disorder (PTSD) (n = 1), specific phobia (n = 1), eating disorder (n = 1). Seven GAD patients took long-term medication. Participants gave written informed consent and had normal or corrected-to-normal vision. Exclusion criteria were neurological disorders, presence or history of psychotic or bipolar disorders, current drug abuse or dependence, and fMRI contraindications. The study was approved by the local ethics committee and conformed to the latest declaration of Helsinki.
Table 1.
Demographic and clinical characterization of GAD patients and HC
| GAD patients | HC | Statistics | |
|---|---|---|---|
| Age | M = 28.26, SD = 8.93 | M = 28.00, SD = 5.28 | F[1,36] = 0.11, P = 0.913 |
| Education (years at school) | M = 12.63, SD = 1.57 | M = 12.89, SD = 0.81 | F[1,36] = −0.65, P = 0.520 |
| BDI | M = 18.00, SD = 12.67 | M = 1.11, SD = 2.00 | |
| PSWQ | M = 65.58, SD = 8.29 | ||
| GAD-Q-IV | M = 9.94, SD = 1.41 | ||
| medication intake (n) | 7 | 0 | – |
Notes and Sources. GAD, generalized anxiety disorder; HC, healthy controls; BDI, Beck-Depression Inventory-II; PSWQ, Penn State Worry Questionnaire; GAD-Q-IV, Generalized Anxiety Disorder Questionnaire-IV.
Stimuli
Representative 4-s duration sequences were taken from sound clips of the International Affective Digitized Sounds database (Bradley and Lang, 1999). Three different human screams served as threat stimuli and three different water sounds as neutral stimuli. All stimuli had a constant intensity level. Hash or percent signs presented on a screen served as cues during threat or neutral anticipation epochs.
Experimental design
An instructed, unpredictable threat anticipation paradigm, paralleling instructed fear conditioning tasks, was used during functional MRI, with 11 threat and 11 neutral trials. Each trial consisted of an anticipatory epoch and a sound being played for 4 s. Prior to scanning participants, were exposed to all sounds and informed that, for example, a hash sign indicated a threat trial at some unpredictable point followed by a scream sound (‘threat anticipation’), whereas, for example, a percent sign indicated a neutral trial (‘neutral anticipation’). Cues were counterbalanced across participants. The occurrence of the sounds was unpredictable due to the length variation of the anticipatory epoch: seven 16 s, one 10 s, one 5 s and two 3 s anticipatory epochs were used for the 11 threat and 11 neutral trials each. The limited trial number was based on previous evidence that amygdala responses rapidly habituate to conditioned or instructed fear cues (Straube et al., 2007b; Herrmann et al., 2016), leading to power decline to detect amygdala activation with further repetitions. Warning cues were present throughout the entire anticipatory epoch. During inter-stimulus intervals a white fixation cross on black background was shown for 15 s. Following scanning, participants rated cues and sounds on 9-point Self-assessment manekin scales on the dimensions of arousal (1 = not arousing at all, 9 = highly arousing), valence (1 = very negative, 5 = neutral, 9 = very positive), and anxiety (1 = not anxiety-inducing, 9 = highly anxiety-inducing) (Bradley and Lang, 1994).
Data acquisition and analysis
Analysis of sociodemographic, clinical questionnaire and rating data was performed using IBM SPSS software (v22, Armonk, New York, USA). Rating data for threat and neutral cues and sounds on the dimensions anxiety, arousal and valence were subjected to separate 2 (cue: threat, neutral) by 2 (group: GAD, HC) or 2 (sound: threat, neutral) by 2 (group: GAD, HC) mixed model analysis of variance (ANOVA). A probability level of P < 0.05 was considered statistically significant and, Bonferroni-corrected t-tests were applied to resolve interaction effects (corrected significance level P < 0.008).fMRI data, including anatomical and functional data, were acquired using a 3 Tesla magnetic resonance scanner (‘Magnetom PRISMA’, Siemens, Erlangen, Germany) with a 20 channel head-neck coil. A high-resolution T1-weighted anatomical scan with 192 slices and functional data with a T2*-weighted echo-planar sequence (TE = 30 ms, flip angle = 90°, matrix = 92 × 92 voxels, FOV = 208 mm2, TR = 2080 ms) were acquired. Functional data consisted of 340 volumes with 36 axial slices (thickness = 3 mm, 0.3 mm gap, in plane resolution = 2.26 mm × 2.26 mm). fMRI data were preprocessed and analyzed using BrainVoyager QX (BVQX, v2.8, Brain Innovation, Maastricht, Netherlands). The first four volumes were discarded to ensure steady-state tissue magnetization, and data were corrected for slice time errors and movement artifacts. fMRI data were co-registered and normalized to Talairach space (Talairach and Tournoux, 1988), and smoothed both spatially (6 mm full-width half maximum [FWHM] Gaussian kernel) and temporally (high pass filter: 10 cycles per run; low pass filter: 2.8 s; linear trend removal). Volumes were resampled 2 × 2 × 2 mm voxel size.
Signal time course of every voxel was analyzed for each participant using multiple regression models in BrainVoyager. Brain responses during threat and neutral anticipatory epochs as well as brain responses to threat and neutral sounds were modeled by a canonical double-gamma hemodynamic response function (HRF). The processed data were analyzed using different general linear models (GLMs) to systematically explore phasic and sustained brain responses to threat anticipation. For the Phasic Response GLM, brain responses were modeled as HRFs elicited by the first second of anticipation. To systematically explore the time course of sustained brain activation during threat anticipation, we used models with a stepwise increasingly delayed onset of the HRF (Sustained Response GLMs). However, independently of delay each predictor modeled a sustained response spanning the whole remaining anticipatory interval. In the Sustained Response GLMs, onset was varied from 0 to 10 s in steps of 2 s, including trials with 16 s anticipatory epochs only. An additional modeling of the function that best described the resulting statistical values allowed to estimate the onset of the differential BNST time course between patients and controls (see Figure 3). All GLMs included additional regressors corresponding to threat and neutral sounds and six motion covariates defined as predictors of no interest. Next, %-standardized predictor estimates based on voxel-wise statistical maps were used for random effects analysis across groups. Given a-priori interest in the role of the amygdala and the BNST for the Phasic and Sustained Response GLMs, analyses were small-volume-corrected for anatomically defined regions of interest (ROIs). Based on the pathological anticipatory anxiety and GAD literature (see introduction), we were primarily interested in phasic amygdala and sustained BNST responding to threat anticipation. Furthermore, the amygdala is postulated to mediate long-lasting threat responding (Davis et al., 2010) and the BNST was recently hypothesized to be involved during brief threat exposure as well (Gungor and Paré, 2016; Shackman and Fox, 2016). For the sake of completeness, we therefore additionally explored amygdala activity in the Sustained Response GLM and BNST activity in the Phasic Response GLM, yet, without expecting differential brain activity. Since the different ROIs were used for separate hypotheses, we did not correct for the number of ROIs. The ROI for the bilateral amygdala was defined based on the Automated Anatomical Labeling (AAL) atlas included in the Wake Forest University PickAtlas software (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003; Maldjian et al., 2004) and transformed into Talairach space (Lancaster et al., 2007) using ICBM2TAL in Matlab (v8.2, The MathWorks Inc, Natick, Massachusetts, USA). The ROI for the bilateral BNST was defined based on an anatomical atlas (see Supplementary material) (Mai et al., 1997; Herrmann et al., 2016).
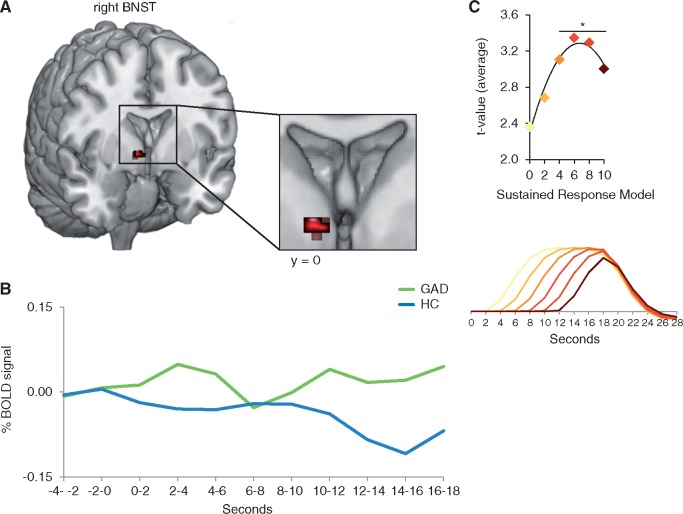
Fig. 3.
Elevated bed nucleus of the stria terminalis activity to threat minus neutral anticipation in patients relative to healthy controls. (A) Generalized anxiety disorder (GAD) patients relative to healthy controls (HC) responded with elevated sustained, but delayed bed nucleus of the stria terminalis (BNST) activity to threat minus neutral anticipation for all Sustained Response General Linear Models (GLM) with a delayed onset of at least 4 s in a highly overlapping cluster. (B) Time courses of the blood oxygen level-dependent (BOLD) signal change, extracted from the overlapping BNST cluster (of the 4, 6, 8 and 10 s Sustained Response GLMs) and averaged across time points and participants per group, are displayed for the anticipatory epoch (threat minus neutral anticipation) starting 4 s before cue onset. (C) Quadratic regression analyses on the average t-values for the contrast threat minus neutral anticipation in GAD patients as compared to HC extracted from the overlapping cluster of all significant Sustained Response GLMs, revealed a significant quadratic relationship. The vertex of the function was at 6.69 s, suggesting a delayed onset of about 6–7 s of the differential sustained BNST effect. The lower graph displays all Sustained Response Models. *P < 0.05.
Correction for multiple comparisons was performed using a cluster-based permutation (CBP) approach (Eklund et al., 2016). The non-parametric method of CBP requires no assumption about the distribution of the test statistic, and results in precise false discovery rates (Eklund et al., 2016). Voxel-level threshold was set to P < 0.005. We investigated pairwise group (GAD, HC) by anticipation epoch (threat, neutral) interactions. Permutation tests were performed with 1000 permutations. For each permutation, individual beta maps (including the anticipation effect: threat anticipation minus neutral anticipation) were randomly assigned without replacement to one of the two groups. Cluster mass was assessed by summing all t-values (or in case of correlational analyses Fisher’s z-transformed correlation coefficients) in neighboring significant voxels. Next, the observed cluster mass was compared with the distribution of the maximum cluster mass observed in each of the 1000 permutations. Finally, cluster masses larger or equal to the 95th percentile of the permutation distribution were considered as statistically significant cluster (i.e. P(cluster) < 0.05). Correlation analyses were performed for BDI, PSWQ, subjective ratings and medication intake in patients. To examine whether differential brain effects were maintained after inclusion of subjective ratings as covariates, 2 (anticipation: threat vs neutral anticipation) × 2 (group: GAD patients vs HC) analyses of covariance (ANCOVA) were performed separately for each rating as a covariate (anxiety-inducing, arousal, valence).
Results
Rating data
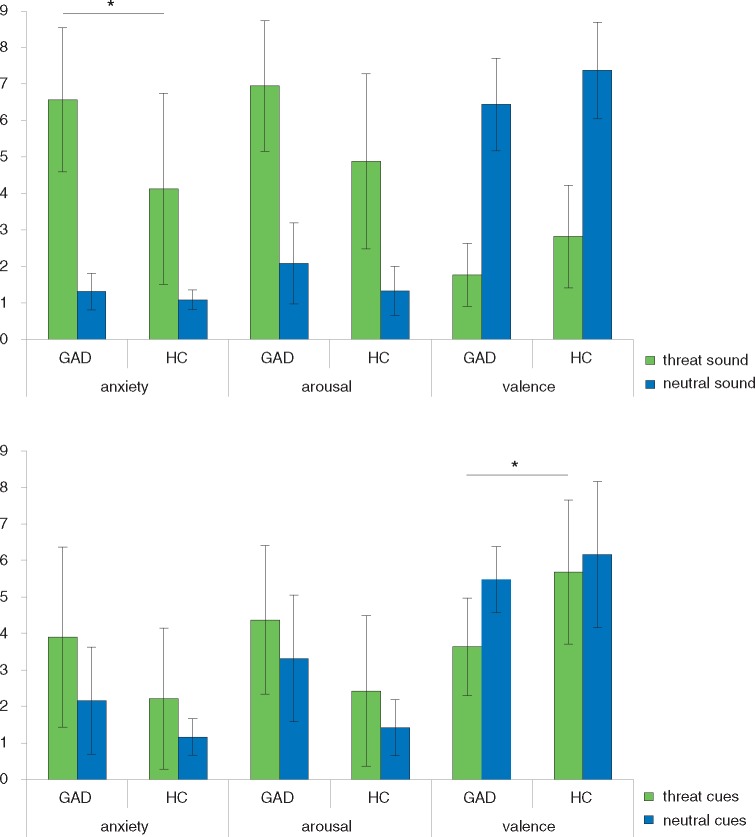
Figure 1 displays mean ratings for (threat/neutral) sounds and (threat/neutral) cues. Collapsed over groups, threat sounds were rated as more arousing, more negative and more anxiety-inducing than neutral sounds (main effect sound: arousal F(1,36) = 160.29, P < 0.001; valence: F(1,36) = 408.00, P < 0.001; anxiety: F(1,36) = 129.36, P < 0.001). GAD patients rated both threat and neutral sounds as more arousing, more negative and more anxiety-inducing than HC (main effect group: arousal: F(1,36) = 11.74, P = 0.002; valence: F(1,36) = 9.17, P = 0.005, anxiety: F(1,36) = 11.21, P = 0.002). There was a significant interaction effect on the dimension of anxiety (group × sound interaction: F(1,36) = 9.21, P = 0.004). More specifically, GAD patients rated threat sounds as more anxiety-inducing as compared to HC, while there were no rating differences between groups regarding neutral sounds (threat sounds: t(36) = 3.24, P = 0.003, neutral sound: n.s.).
Fig. 1.
Rating data. Mean ratings for (threat/neutral) sounds and (threat/neutral) cues on a 9-point Self-assessment manekin scale on the dimensions arousal (1 = not arousing at all, 9 = highly arousing), valence (1 = very negative, 5 = neutral, 9 = very positive), and anxiety (1 = not anxiety-inducing, 9 = highly anxiety-inducing). Generalized anxiety disorder (GAD) patients relative to healthy controls (HC) rated threat sounds as more anxiety-inducing and threat cues as more negative. *P < 0.008.
Collapsed over groups, threat cues were rated as more arousing, more negative and more anxiety-inducing than neutral cues (main effect cues: arousal: F(1,36) = 10.08, P = 0.003; valence: F(1,36) = 15.91, P < 0.001; anxiety: F(1,36) = 20.37, P < 0.001). GAD patients rated cues as more arousing, more negative and more anxiety-inducing than HC (main effect group: arousal: F(1,36) = 17.49, P < 0.001; valence: F(1,36) = 9.69, P = 0.004; anxiety: F(1,36) = 7.97, P = 0.008). There was a significant interaction effect on the dimension of valence (group × cues interaction: F(1,36) = 5.56, P = 0.024). More precisely, threat cues were rated by GAD patients as more negative as compared to HC, while there were no rating differences between groups regarding neutral cues (threat cue: t(36) = −3.75, P = 0.001, neutral cue: n.s.).
fMRI analysis
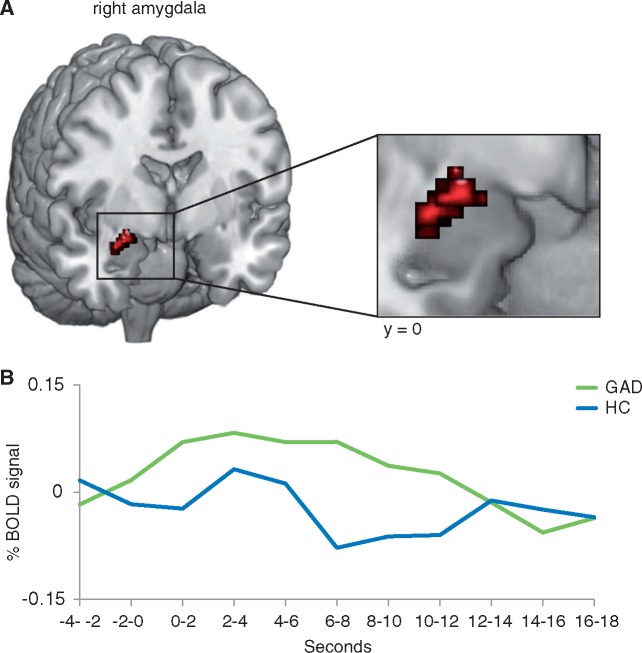
Phasic Response GLM: GAD patients relative to HC responded to threat minus neutral anticipation with increased phasic activation in the right amygdala (peak voxel Talairach coordinates: x = 29, y = −1, z = −19; size: 296 mm³; maximal t-value: 3.64 average t-value: 3.06; P < 0.05 corrected) (Figure 2). Activity in the functionally defined amygdala cluster showed no correlation with medication intake, subjective ratings, PSWQ or BDI scores in patients. There were no significant effects in the BNST. The influence of subjective ratings were tested by means of ANCOVAs and unveiled maintained interaction effects (all F-values ≥ 12.45, all p ≤ .001) and no significant effects of the covariates (all P > 0.05).
Fig. 2.
Elevated amygdala activity to threat minus neutral anticipation in patients relative to healthy controls. (A) Generalized anxiety disorder (GAD) patients relative to healthy controls (HC) responded with elevated phasic activity in the right amygdala. (B) Time courses of the blood oxygen level-dependent (BOLD) signal change, extracted from the amygdala cluster and averaged across time points and participants per group, are displayed for the anticipatory epoch (threat minus neutral anticipation) starting 4 s before cue onset.
Sustained Response GLMs: We detected elevated BNST activity to threat minus neutral anticipation in GAD patients as compared to HC in the 4, 6, 8 and 10 s Sustained Response GLMs in the right BNST in an highly overlapping cluster with maximal t-values varying between 3.14 and 3.46, average t-values between 2.92 and 3.12, and cluster sizes between 96 and 144 mm3 (Table 2 and Figure 3). There was no differential BNST activity in the 0, and 2-s Sustained Response GLM. As indicated in Figure 3, the mean statistical values for the contrast threat minus neutral anticipation in GAD patients as compared to HC extracted from the overlapping cluster of all significant Sustained Response GLMs, followed a highly significant quadratic function with the delay of the sustained response predictor (function: y = −0.022x2 + 0.297x + 2.291, R2 = 0.96, P < 0.007). The vertex of the function was at 6.69 s, suggesting a delayed onset of about 6–7 s of the differential sustained BNST effect. Activity in the functionally defined BNST cluster showed no correlation with medication intake, subjective ratings, PSWQ or BDI scores in patients. The influence of subjective ratings were tested by means of ANCOVAs and unveiled maintained interaction effects (all F-values ≥ 10.49, all P≤ 0.003) and no significant effects of the covariates (all P > 0.05). There were no significant effects in the amygdala.
Table 2.
Significant effects for the contrast threat minus neutral anticipation in GAD patients as compared to HC in the right bed nucleus of the stria terminalis
| Delayed-Sustained Response GLM | x | y | z | Average t-value | Maximal t-value | mm3 |
|---|---|---|---|---|---|---|
| 4-s | 6 | 3 | −2 | 2.97 | 3.22 | 120 |
| 6-s | 4 | −1 | −2 | 3.12 | 3.46 | 144 |
| 8-s | 4 | −1 | −2 | 3.06 | 3.44 | 144 |
| 10-s | 4 | −1 | −2 | 2.92 | 3.14 | 96 |
Notes and Sources: GAD, generalized anxiety disorder; HC, healthy controls; GLM, general linear model; x, y, z, Talairach coordinates of maximally activated voxel (P < 0.05 corrected).
Discussion
The present study explored phasic and sustained brain responses to threat anticipation in GAD patients relative to HC in an instructed threat anticipation paradigm with temporally unpredictable threat exposure. As predicted, GAD patients compared to HC responded with heightened phasic activity in the amygdala. Furthermore, we detected enhanced BNST activity in GAD patients relative to HC that emerged delayed, relative to threat anticipation onset. Rating data showed that GAD patients rated threat anticipation as more negative and threat sounds as more anxiety-inducing than HC.
Our findings provide first evidence of a temporal dissociation of BNST and amygdala activity during threat anticipation in GAD patients. Importantly, GAD patients showed significantly heightened delayed-sustained BNST activity during unpredictable threat anticipation. Enhanced BNST activity appears to reflect elevated physiological and subjective emotional responses to uncertainty (for review see Avery et al., 2016; Lebow and Chen, 2016). Also, BNST engagement seems to be related to the ability to differentiate between safe and non-safe-contexts (for review see Lebow and Chen, 2016). Thus, elevated sustained BNST activity to threat anticipation in GAD patients suggests that upcoming threat exposure may have been particularly aversive to them, which is in line with results of rating data. This fits with hypersensitivity and exaggerated apprehensive responding to threat in GAD patients, shown with a startle paradigm (Grillon et al., 2016). Yet, former studies did not report significant differential BNST activity in GAD patients relative to HC (Nitschke et al., 2009; Oathes et al., 2015). Depending on study design and analysis, absent differential BNST activity may be attributable to short anticipatory epochs, predictable onset of threat, unsuited HRF models, or reduced differentiation between aversive and neutral stimuli, for example in (not instructed) fear conditioning designs (Greenberg et al., 2013). Nevertheless, our results support previous findings of delayed-sustained BNST engagement (McMenamin et al., 2014), postulations of an influential neurobiological model (Davis et al., 2010), and encourage future studies to consider different temporal aspects of BNST activation during threat anticipation.
GAD patients responded also with exaggerated amygdala activity to threat anticipation onset, which is in line with a previous study (Nitschke et al., 2009). Amygdala activity appears to be essential for phasic attention-vigilance aspects of threat processing and immediate threat detection (Etkin, 2010). Phasic hyperreactive amygdala responding may indicate that GAD patients were particular vigilant and attentive towards upcoming threat (Grupe and Nitschke, 2013), which fits with behavioral evidence from GAD patients showing an attentional bias towards threatening information (for review see Hayes and Hirsch, 2007). Such an information processing bias eases the perception of danger, and possibly contributes to heightened threat expectancies (Hayes and Hirsch, 2007; Grupe and Nitschke, 2013). Biased threat expectancies under conditions of uncertainty are proposed to initiate a vicious circle that may result in prolonged anxious threat apprehension in GAD patients (Grupe and Nitschke, 2013), which may be reflected by enhanced BNST engagement.
Elevated phasic amygdala and sustained BNST activity to threat anticipation was unveiled in previous studies in specific phobia, panic disorder or PTSD patients (Straube et al., 2007a; Münsterkötter et al., 2015; Brinkmann et al., 2017a, 2017b, 2017c). Interestingly, the above reported studies detected elevated BNST activity to threat anticipation onset, while in GAD patients elevated BNST activity emerged delayed relative to threat anticipation onset. Together, these observations lend support for two notions: First of all, it appears that elevated phasic amygdala and sustained BNST activity during anxious apprehension constitute translational pathological brain response patterns cutting across anxiety/stress disorders. Interestingly, both the amygdala and the BNST received prominent attention within the addiction disorder literature as well (for review see Stamatakis et al., 2014; Avery et al., 2016). More specifically, it is hold that both structures mediate the stage of withdrawal/negative affect that is defined by lasting increases in anxiety (Gilpin et al., 2015). It may be speculated that anticipation of exposure to addiction-related stimuli might evoke altered amygdala and BNST activity in addiction disorder patients too. Second of all, the unique delayed time course of heightened BNST activity to threat anticipation in GAD might represent a disorder-specific response pattern. Yet, it remains to note that former investigations did not apply the here-reported analysis, leaving it to call into question whether this pattern is disorder-specific or not. Whether or not the pattern of delayed-sustained BNST engagement to threat anticipation is unique to GAD patients would require a direct comparison between psychiatric disorders.
Our findings of elevated amygdala and BNST engagement during unpredictable threat anticipation in GAD patients should be considered in light of the studýs strengths and limitations. As major strengths, we consider our instructed threat paradigm, and the methods of data analysis used (Grupe et al., 2012; McMenamin et al., 2014; Herrmann et al., 2016; Brinkmann et al., 2017b). A limitation is that patients were medicated at time of testing and had comorbidities, which possibly constitute confounds. We carefully ensured that GAD was the main diagnosis for patients and since comorbidities are frequent in GAD, our sample is likely to be representative of the GAD population (Newman et al., 2013). Also, correlation analyses showed no relationship between medication intake and increased amygdala or BNST activity in GAD patients. Nonetheless, investigations with patients that are off psychopharmacological medication and without comorbidities would be beneficial in future studies. Another drawback concerns absent correlational effects between differential brain responses and behavioral measures (e.g. subjective ratings, PSWQ) that could have strengthened the interpretations and provide further insight into the functional role of the BNST and the amygdala during pathological anticipatory anxiety. In addition to that, we recommend exploring the involvement of additional brain regions (e.g. anterior insula, anterior cingulate cortex, nucleus accumbens) in future studies, as these proved to be relevant during threat anticipation and instructed fear conditioning (Klucken et al., 2009; Alvarez et al., 2011; Grupe et al., 2012; Somerville et al., 2013; Herrmann et al., 2016b).
To conclude, unpredictable threat anticipation induced heightened phasic amygdala and delayed-sustained BNST engagement in GAD patients relative to HC. Heightened phasic amygdala activity may point towards exaggerated attention-vigilance processing during early phases of threat anticipation. Information-processing biases may lead to heightened threat expectancies and initiate a vicious circle, resulting in prolonged anxious threat apprehension. Respective sustained threat anticipation may be reflected by heightened delayed-sustained BNST activity in GAD patients. Overall, the present results suggest that BNST and amygdala activity alterations may be useful neurobiological markers of anticipatory anxiety in GAD patients, albeit with different time courses.
Supplementary Material
Acknowledgment
This work was supported by the German Research Foundation (DFG: SFB/TRR 58: C06, C07).
Supplementary data
Supplementary data are available at SCAN online.
Funding
This work was supported by the German Research Foundation (DFG: SFB/TRR 58: C06, C07). The funding body had no role in study design, data collection and data analysis, decision to publish, or preparation of the manuscript.
Conflict of interest. None declared.
References
- Alvarez R.P., Chen G., Bodurka J., Kaplan R., Grillon C. (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery S.N., Clauss J.A., Blackford J.U. (2016). The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology, 41, 126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Beck-Depression-Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bradley M.M, Lang P.J. (1999). International affective digitized sounds (IADS): Stimuli, instruction manual and affective ratings. (Tech. Rep. No. B-2). Gainesville, FL: Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Bradley M.M., Lang P.J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25, 49–59. [DOI] [PubMed] [Google Scholar]
- Brinkmann L., Buff C., Feldker K., et al. (2017a). Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychological Medicine, 1–14, doi: 10.1017/S0033291717001192. [DOI] [PubMed] [Google Scholar]
- Brinkmann L., Buff C., Neumeister P., et al. (2017b). Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post‐traumatic stress disorder patients. Human Brain Mapping, 38(4), 2190–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L., Poller H., Herrmann M.J., Miltner W., Straube T. (2017c). Initial and sustained brain responses to threat anticipation in blood-injection-injury phobia. NeuroImage: Clinical, 13, 320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff C., Brinkmann L., Neumeister P., et al. (2016). Specifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder. NeuroImage: Clinical, 12, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35, 105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113, 7900–5 .. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. (2010). Functional neuroanatomy of anxiety: a neural circuit perspective. Current Topics in Behavioral Neurosciences, 2, 251–77. [DOI] [PubMed] [Google Scholar]
- Forster G.L., Novick A.M., Scholl J.L., Watt M.J. (2012). The Role of the Amygdala in Anxiety Disorders, The Amygdala - A Discrete Multitasking Manager. In: Ferry, B., editor. InTech. DOI: 10.5772/50323. Available: https://www.intechopen.com/books/the-amygdala-a-discrete-multitasking-manager/the-role-of-the-amygdala-in-anxiety-disorders. [Google Scholar]
- Fox A.S., Oler J.A., Tromp D.P., Fudge J.L., Kalin N.H. (2015). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38, 319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana M.A., Harrison B.J., Soriano-Mas C., et al. (2016). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21, 500–8. [DOI] [PubMed] [Google Scholar]
- Gentes E.L., Ruscio A.M. (2011). A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive–compulsive disorder. Clinical Psychology Review, 31, 923–33. [DOI] [PubMed] [Google Scholar]
- Gilpin N.W., Herman M.A., Roberto M. (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biological Psychiatry, 77, 859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T., Carlson J.M., Cha J., Hajcak G., Mujica-Parodi L.R. (2013). Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depression and Anxiety, 30, 242–50. [DOI] [PubMed] [Google Scholar]
- Grillon C., O’Connell K., Lieberman L., et al. (2016). Distinct responses to predictable and unpredictable threat in anxiety pathologies: effect of panic attack. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, doi: 10.1016/j.bpsc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Oathes D.J., Nitschke J.B. (2012). Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex, 23(8), 1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor N.Z., Paré D. (2016). Functional heterogeneity in the bed nucleus of the stria terminalisfunctional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience, 36, 8038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S., Hirsch C.R. (2007). Information processing biases in generalized anxiety disorder. Psychiatry, 6, 176–82. [Google Scholar]
- Herrmann M.J., Boehme S., Becker M.P., et al. (2016). Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping, 37, 1091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert K., Lueken U., Beesdo-Baum K. (2014). Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: A systematic review. Journal of Affective Disorders, 158, 114–26. [DOI] [PubMed] [Google Scholar]
- Klucken T., Kagerer S., Schweckendiek J., Tabbert K., Vaitl D., Stark R. (2009). Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture–picture conditioning paradigm. Neuroscience, 158, 721–31. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas‐Gutiérrez D., Martinez M., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28, 1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow M., Chen A. (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21, 450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J., Assheuer J., Paxinos G. (1997). Atlas of the Human Brain. San Diego, CA: Academic Press. [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21, 450–5. [DOI] [PubMed] [Google Scholar]
- McMenamin B.W., Langeslag S.J., Sirbu M., Padmala S., Pessoa L. (2014). Network organization unfolds over time during periods of anxious anticipation. The Journal of Neuroscience, 34, 11261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M.-L. (2012). Cognitive emotion regulation through reappraisal in an anticipatory anxiety paradigm (Doctoral dissertation). Available: http://ediss.sub.uni-hamburg.de/volltexte/2012/5752.
- Mechias M.L., Etkin A., Kalisch R. (2010). A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage, 49, 1760–8. [DOI] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. (1990). Development and validation of the Penn state worry questionnaire. Behaviour Research and Therapy, 28, 487–95. [DOI] [PubMed] [Google Scholar]
- Münsterkötter A.L., Notzon S., Redlich R., et al. (2015). Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety, 32, 656–63. [DOI] [PubMed] [Google Scholar]
- Newman M.G., Zuellig A.R., Kachin K.E., et al. (2002). Preliminary reliability and validity of the generalized anxiety disorder questionnaire-IV: a revised self-report diagnostic measure of generalized anxiety disorder. Behavior Therapy, 33, 215–33. [Google Scholar]
- Newman M.G., Llera S.J., Erickson T.M., Przeworski A., Castonguay L.G. (2013). Worry and generalized anxiety disorder: A review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annual Review of Clinical Psychology, 9, 275–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke J.B., Sarinopoulos I., Oathes D.J., et al. (2009). Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. American Journal of Psychiatry, 166, 302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oathes D.J., Hilt L.M., Nitschke J.B. (2015). Affective neural responses modulated by serotonin transporter genotype in clinical anxiety and depression. PLoS One, 10, e0115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Fox A.S. (2016). Contributions of the central extended amygdala to fear and anxietycontributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience, 36, 8050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Wagner D.D., Wig G.S., Moran J.M., Whalen P.J., Kelley W.M. (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex, 23, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A.M., Sparta D.R., Jennings J.H., McElligott Z.A., Decot H., Stuber G.D. (2014). Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology, 76, 320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T., Mentzel H.J., Miltner W.H. (2007a). Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. NeuroImage, 37, 1427–36. [DOI] [PubMed] [Google Scholar]
- Straube T., Weiss T., Mentzel H.-J., Miltner W.H. (2007b). Time course of amygdala activation during aversive conditioning depends on attention. NeuroImage, 34, 462–9. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Wunderlich U., Gruschwitz S., Zaudig M. (1997). SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. Z Fur Klin Psychol Psychother, 28, 68–70. [Google Scholar]
- Yassa M.A., Hazlett R.L., Stark C.E.L., Hoehn-Saric R. (2012). Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research, 46, 1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg R.E., Craske M.G., Barlow D.H. (2006). Mastery of Your Anxiety and Worry (MAW): Therapist Guide, Vol. 1, New York: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.