Abstract
Sparse data exist from sub-Saharan Africa (SSA) on the prevalence of antimicrobial resistance (AMR). A prior review of antimicrobial resistance in SSA from 1990 to 2013 showed a high prevalence of AMR to commonly used antibiotics in this setting. We reviewed the literature published since 2013. Four databases (PubMed, EMBASE, Cochrane, and African Journals Online) were searched for articles between February 2013 and March 2016 with a focus on sterile site infections (bacteremia, urinary tract infections [UTIs], and meningitis). We focused on the original World Health Organization–identified priority pathogens and antibiotics, prior to the release of the most recently updated and expanded list in 2017. There were 19 eligible studies: bacteremia (12), UTI (6), and meningitis (1). Eight studies were from Western and Central Africa, 8 from Eastern Africa, and 4 from Southern Africa. Prevalence of Escherichia coli resistance to third-generation cephalosporins ranged from 0% to 75%. No studies reported resistance to carbapenems among Klebsiella spp. Prevalence of fluoroquinolone resistance ranged from 8.3% to 100% among E. coli and 0% to 15% among Salmonella spp. Prevalence of resistance to penicillin among Streptococcus pneumoniae isolates ranged from 25% to 100%. Testing for extended-spectrum beta-lactamase was reported in 7 studies (range, 1.3–60% among tested isolates). Methods for evaluating AMR varied across studies; standardized approaches are needed in the region. Testing for mechanisms of resistance is low even in research settings, but important mechanisms of resistance such as ESBL production are present.
Keywords: antibacterial agents, antimicrobial resistance, microbial susceptibility tests, review, sub-Saharan Africa
Antimicrobial resistance (AMR) is a serious emerging public health concern. Overall, sparse data exist from sub-Saharan Africa (SSA) on the prevalence of AMR among priority pathogens causing clinical syndromes. For the World Health Organization (WHO) 2014 report on AMR surveillance, 27 (57%) of the 47 member nations in the WHO Africa region returned any information [1]. Of these, only 23 returned data sets, and 4 responded “no national data available.” A prior review suggested a high prevalence of AMR in sub-Saharan African settings in studies published between 1990 and 2013 [2]. Significant variation exists in AMR data across the continent due to a myriad of factors including poor microbiology capacity, no or weak infrastructure support (eg, trained laboratory personnel, adequate supplies, record and data systems), lack of standardization of laboratory methods where available, and lack of national or regional AMR surveillance plans. Because updates of the literature are important to guide policy and inform research and implementation strategies, we performed a systematic review of data published since 2013 on AMR in SSA.
METHODS
Search Strategy
Four databases (PubMed, Embase, Cochrane, and African Journals Online) were searched for studies investigating AMR in isolates from selected sterile sites (bloodstream infections, urinary tract infections, and meningitis). African Journals Online was included as it is a resource aimed at improving the access African researchers have to the work of other African academics in partnership with hundreds of journals based on the African continent. The full search terms were created with the assistance of a librarian and can be found in supplementary material.
Selection Criteria
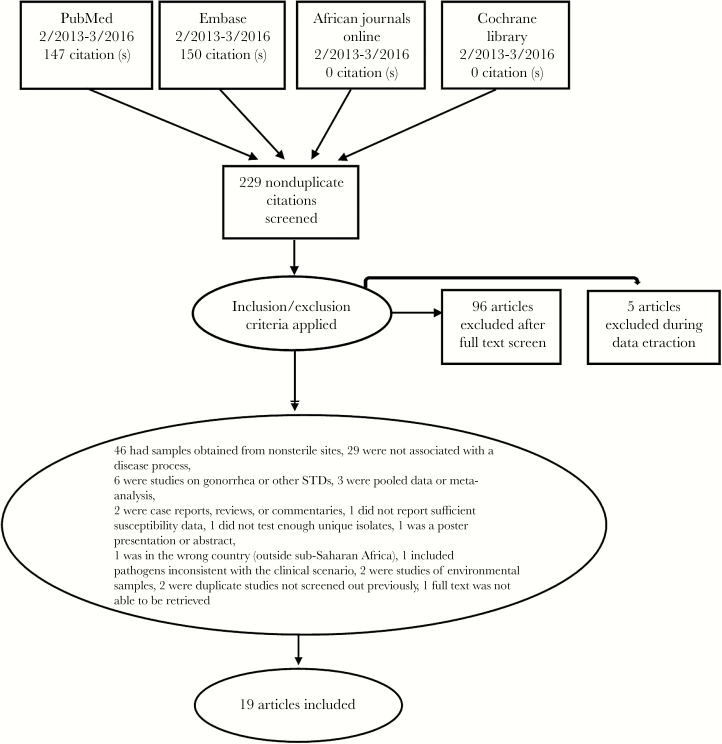
Two reviewers (MW and MK) screened titles and abstracts, and full texts where appropriate. If disagreements arose, these were discussed in person to arrive at a consensus. Studies were included if they were conducted at a clinical site between February 2013 and March 2016. Studies in adults and/or children were included. Additional inclusion criteria required studies to report antimicrobial susceptibility to the identified priority WHO pathogens [1] from the original 2014 list and to include at least 10 isolates collected from individual patients (Figure 1). We included studies that cultured specimens from sterile sites only (blood, urine, cerebrospinal fluid) and with an associated disease process or clinical syndrome.
Figure 1.
PRISMA flow diagram of the article selection process.
Data Extraction and Analysis
Data were extracted using the Systemic Review Data Repository (SRDR) [3]. Analysis was conducted using SAS studio. (SAS Institute Inc., Cary, NC) We focused on the 2014 WHO-selected priority bacterial pathogens and antimicrobial resistance combinations [1]. These included Escherichia coli resistant to third-generation cephalosporins and fluoroquinolones, Klebsiella pneumoniae/Klebsiella spp. resistant to third-generation cephalosporins and carbapenems, Staphylococcus aureus resistant to methicillin (“MRSA”), Streptococcus pneumoniae resistant to penicillin, and nontyphoidal Salmonella vs fluoroquinolones.
RESULTS
The initial search yielded 147 citations from PubMed, 150 citations from Embase, and 0 citations from AJO and Cochrane Library (Figure 1). Of the initial 229 nonduplicate citations identified, 109 were excluded after title and abstract screen and 96 were excluded following full text screen; references and reasons for exclusion can be found in supplementary material. Nineteen studies were included in the final review.
The 19 eligible studies included 12 studies on bacteremia, 6 studies on UTI, and 1 study on meningitis (Supplementary Table 1). The meningitis study was specifically of ventriculoperitoneal (VP) shunt infections. Eight studies were from Western and Central Africa, 8 from Eastern Africa, and 4 from Southern Africa (Supplementary Figure 1). Thirty-seven percent of studies were in children only. Fever was the most common inclusion criterion used in 10 of 19 studies. For bacteremia studies, a total of 47 835 blood cultures were collected, with a range of 83 to 23 708 cultures per study. The median prevalence of positive blood cultures was 12.1% (range, 3.2%–26%). Among UTI studies, a total of 8474 cultures were processed, with a median prevalence of positive cultures of 30.9% (range, 23%–52%). In the meningitis study, 127 samples were collected, with 41.7% positive cultures.
Among bacteremic patients, the median prevalence rates of the most commonly isolated organisms were 44% for S. aureus, 11% for nontyphoidal Salmonella, 7% for Salmonella typhi, 9% for S. pneumoniae, 7% for Klebsiella spp., 7% for E. coli, and 4% for Pseudomonas aeruginosa. Among patients diagnosed with UTI, the median prevalence rates of commonly isolated organisms were 60% for E. coli, 11% for Klebsiella spp., 8% for P. aeruginosa, 4% for S. aureus, and 3% for Enterococcus spp. In the single study of meningitis/VP shunt infections, Staph species were most frequently isolated (S. aureus 24.5%, other Staph species 26%), followed by Gram-negative bacilli (40%), Streptococci (6%), and mixed infection (4%).
Resistance Profiles
All included studies reported resistance as total number of isolates by organism sensitive or resistant to a specified antibacterial agent and/or as percent of isolates by organism sensitive or resistant to a specified antibacterial agent.
Gram-Negative Pathogen Resistance to Third-Generation Cephalosporins and Carbapenems
Thirteen studies, 6 UTI and 7 bacteremia studies, reported on the median prevalence of E. coli resistance to third-generation cephalosporins, which ranged from 0% to 75%, with a median prevalence of resistance of 19.5%. Five studies, 2 UTI and 3 bacteremia studies, reported on E. coli resistance to fluoroquinolones, which ranged from 8.3% to 100%, with a median resistance prevalence of 28%. Eleven studies, 4 UTI and 7 bacteremia studies, assessed Klebsiella spp. resistance to third-generation cephalosporins, with prevalence of resistance ranging from 0% to 100%, with a median prevalence of 50%. Three studies reported on Klebsiella spp. resistance to carbapenems: 2 UTI studies and 1 bacteremia study, with all 3 studies reporting 0% incidence of resistance to carbapenems among the isolates tested [4–6].
Gram-Positive Pathogen Resistance to Beta-Lactams
Five studies, all of bacteremia, reported on S. pneumoniae resistance to penicillin. The median prevalence of S. pneumoniae resistance to penicillin among the 5 studies identified was 50% [5, 7–10]. Three studies, 2 bacteremia and 1 meningitis, reported on MRSA vs MSSA prevalence, with prevalence of MRSA ranging from 9.4% to 13.5%, with a median prevalence of 12.5% (Supplementary Table 1).
Salmonella Resistance to Fluoroquinolones
Eight studies, all of bacteremia, reported on the prevalence of fluoroquinolone resistance among Salmonella spp., which ranged from 0% to 15%. Five of those studies reported on nontyphoidal Salmonella resistance specifically, with a median resistance of 4.3%, and the remaining 3 reported on overall Salmonella spp. or Salmonella typhi resistance, with a median resistance of 0%.
Extended-Spectrum Beta-Lactamase Production
Testing for extended-spectrum beta-lactamase (ESBL) was reported in 7 studies (range, 1.3%–60% among tested isolates). Two of the studies were UTI studies, while 5 were bacteremia studies. Details of testing methodology for ESBL production, as well as organisms identified as ESBL producers, can be found in supplementary Table 1.
Resistance Patterns
E. coli resistance to both third-generation cephalosporins and fluoroquinolones is notable, particularly among UTIs (Supplementary Figure 2a/b). Median prevalence of resistance to fluoroquinolones among E. coli in the 2014 Leopold et al. review was 12.7% compared with 37.5% in our review and with third-generation cephalosporins, Leopold et al. showed a 5% resistance rate among E. coli–causing UTIs while our study showed 40.5% resistance [2]. The older review grouped studies of bacteremia under an overarching category of community-acquired febrile illness. Among studies of bacteremia in our review, E. coli resistance to fluoroquinolones was 70% compared with only 8% among studies of community-acquired febrile illness in the Leopold et al. review [2]. Resistance rates to third-generation cephalosporins were lower in our review at 8% compared with 13% in the older review [2]. K. pneumoniae resistance to third-generation cephalosporins had median prevalence of resistance rates ranging from 22% in Eastern Africa to 15% in Central/Southern Africa, and no data available from Western Africa in the prior Leopold et al. review [2], compared with median prevalence of 50% among the studies done in East Africa in our review, 19% in Central and Southern Africa, and 50% in the Western Africa region. Unfortunately, our single study of meningitis did not yield any S. pneumoniae isolates as it was specifically a study of VP shunt infections. Median prevalence of penicillin resistance to S. pneumoniae in our review was 50% compared with 10% in the Leopold et al. review [2] among those presenting with community-acquired febrile illness (Supplementary Figure 2c).
DISCUSSION
The results of this review show that AMR is a growing problem in SSA, consistent with other global trends [11]. E. coli resistance to third-generation cephalosporins in our data presents a particularly concerning picture, especially among UTIs. A previous review of the literature up to 2013 showed a 5% resistance rate to cephalosporins among E. coli–causing UTIs [2] while our study showed 40% resistance. Fluoroquinolone resistance among E. coli also appeared to be trending up among UTIs (12.7% [2] to 28%), as well as among studies of community-acquired febrile illness/bacteremia (8% [2] to 43%), although no formal tests of significance were performed given significant variation between the studies.
Our study did not distinguish between health care–associated and community-acquired infections. We attempted to exclude studies with small numbers of isolates in order to avoid biasing our results. A cutoff of 10 isolates was selected arbitrarily. Despite this exclusion criterion, when looking at isolates by organism, several of the studies had very small numbers. The majority of studies were hospital- and/or laboratory-based. In addition, blood cultures are not routinely obtained in SSA. Therefore, it is possible that there was sampling bias toward sicker patients with longer durations of hospitalization and who may have been more likely to have resistant organisms.
Heterogeneity in resistance patterns was noted, including occasionally within countries. Notably, among 3 studies conducted in Ethiopia, Wasihun et al., Endris et al., and Kibret et al., resistance rates to third-generation cephalosporins in E. coli were reported as 75%, 0%, and 34%, respectively [12–14]. Endris et al. conducted their study among septic patients with visceral leishmaniasis and most commonly isolated gram-positive organisms like Staphylococcus aureus [13]. There was only 1 E. coli isolate tested in this study that was not resistant to ceftriaxone but was resistant to ciprofloxacin. Wasihun et al. conducted their study among bacteremic patients and collected 12 E. coli isolates, of which 9 (75%) were resistant to ceftriaxone. Kibret et al. was a study of UTIs that tested 56 E. coli isolates against ceftriaxone, of which 34% were resistant (actual number of isolates not reported), and 81 E. coli isolates against ciprofloxacin, of which 34% were resistant [14]. All 3 studies were conducted in regional city hospitals and laboratories, with the Endris et al. study conducted in a university teaching hospital (University of Gondar).
Overall, studies performed in children and studies on bacteremia are the most common. Despite the relative ease of obtaining urine cultures, published studies of bacteremia outnumbered studies of UTIs 2:1; this observation was also noted in a prior review [15]. The majority of studies were from Eastern Africa. Central and Southern Africa had the fewest published studies (Supplementary Figure 1).
Standardization of surveillance methods will be required to gather applicable data that can be used to inform policy and treatment. The Global AMR Surveillance System (GLASS), developed by the WHO, is expected to release its first report in the last quarter of 2017, and it will address some of the identified gaps [16]. We found that methods for evaluation of AMR varied across studies and testing for mechanisms of resistance is low even in research settings. Lack of microbiologic capacity continues to be a significant hurdle to surveillance efforts, and urgent interventions are needed to build laboratory capacity across the continent. Building microbiology capacity should be the cornerstone of strategies to document and track AMR in low-resource settings. Research and development should include non-culture-based microbiologic tests that are both rapid and cost-effective for low- and middle-income country settings, especially in rural and semirural settings where resources and a trained laboratory workforce are scarce. Other diagnostic tools that may help to decrease the use of antibiotics include biomarkers such as C-reactive protein, which may distinguish between viral and bacterial infection and thus reduce antibiotic prescribing [17]. Focused and synchronized surveillance efforts at both the hospital and community levels to understand the extent and drivers of AMR in the sub-Saharan African setting will be helpful in guiding the path forward.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgements
Author contributions. YCM conceived the idea for the study. MW, SEC, YCM, and MK designed the study. MW searched published work. MW and MK reviewed and made the selection of eligible studies. MW and MK extracted and compiled the data. MW analyzed the data and prepared the first draft of the paper. All authors contributed to the writing of the paper and have seen and approved the final version.
Funding. This work was supported by the National Institute of Health grant T32 AI007291-27 to MW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Antimicrobial resistance: global report on surveillance 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1.
- 2. Leopold SJ, van Leth F, Tarekegn H, Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother 2014; 69:2337–53. [DOI] [PubMed] [Google Scholar]
- 3. Systematic Review Data Repository Retrieved from https://srdr.ahrq.gov/ Accessed 15 September 2016.
- 4. Irenge LM, Kabego L, Vandenberg O et al. . Antimicrobial resistance in urinary isolates from inpatients and outpatients at a tertiary care hospital in South-Kivu Province (Democratic Republic of Congo). BMC Res Notes 2014; 7:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Preziosi M, Zimba TF, Lee K et al. . A prospective observational study of bacteraemia in adults admitted to an urban Mozambican hospital. S Afr Med J 2015; 105:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ochada NS, Nasiru IA, Thairu Y et al. . Antimicrobial susceptibility pattern of urinary pathogens isolated from two tertiary hospitals in Southwestern Nigeria. Afr J Clin Exp Micro 2015; 16:12–22. [Google Scholar]
- 7. Isendahl J, Manjuba C, Rodrigues A et al. . Prevalence of community-acquired bacteraemia in Guinea-Bissau: an observational study. BMC Infect Dis 2014; 14:3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahende C, Ngasala B, Lusingu J et al. . Bloodstream bacterial infection among outpatient children with acute febrile illness in north-eastern Tanzania. BMC Res Notes 2015; 8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Musiime V, Cook A, Bakeera-Kitaka S et al. ; ARROW Trial Team Bacteremia, causative agents and antimicrobial susceptibility among HIV-1-infected children on antiretroviral therapy in Uganda and Zimbabwe. Pediatr Infect Dis J 2013; 32:856–62. [DOI] [PubMed] [Google Scholar]
- 10. Moon TD, Silva WP, Buene M et al. . Bacteremia as a cause of fever in ambulatory, HIV-infected Mozambican adults: results and policy implications from a prospective observational study. PLoS One 2013; 8:e83591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmes AH, Moore LS, Sundsfjord A et al. . Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387:176–87. [DOI] [PubMed] [Google Scholar]
- 12. Wasihun AG, Wlekidan LN, Gebremariam SA et al. . Diagnosis and treatment of typhoid fever and associated prevailing drug resistance in Northern Ethiopia. Int J Infect Dis 2015; 35:96–102. [DOI] [PubMed] [Google Scholar]
- 13. Endris M, Takele Y, Woldeyohannes D et al. . Bacterial sepsis in patients with visceral leishmaniasis in Northwest Ethiopia. Biomed Res Int 2014; 2014:361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kibret M, Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed 2014; 4:164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Call for participation: Global Antimicrobial Resistance Surveillance System (GLASS) 2015. http://www.who.int/drugresistance/survei llance/glass-enrolment/en/.
- 17. Aabenhus R, Jensen JU, Jorgensen KJ et al. . Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014; 11:CD010130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.