Abstract
Background and Aims Dioecy and sexual dimorphism occur in many terrestrial plant species but are especially widespread among the bryophytes. Despite the prevalence of dioecy in non-vascular plants, surprisingly little is known about how fine-scale sex-specific cell and leaf morphological traits are correlated with sex-specific physiology and population sex ratios. Such data are critical to understanding the inter-relationship between sex-specific morphological and physiological characters and how their relationship influences population structure. In this study, these data types were assessed to determine how they vary across three populations within one moss species and whether fine-scale morphological traits scale up to physiological and sex ratio characteristics.
Methods Twenty cell-, leaf- and canopy-level traits and two photochemical measurements were compared between sexes and populations of the dioecious moss Ceratodon purpureus. Field population-expressed sex ratios were obtained for the same populations.
Key Results Male and female plants differed in cell, leaf and photochemical measures. These sexual dimorphisms were female biased, with females having larger and thicker leaves and greater values for chlorophyll fluorescence-based, leaf photochemistry measurements than males. Female traits were also more variable than male traits. Interestingly, field population sex ratios were significantly male biased in two study populations and female biased in the third study population.
Conclusions The results demonstrate that the larger morphology and the greater physiological output of female C. purpureus gametophytes compared with males occurs across populations and is likely to have significant effects on resource allocation and biotic interactions. However, this high level of dimorphism does not explain population sex ratio variation in the three study populations tested. This research lays the groundwork for future studies on how differential sex-specific variation in cell and leaf traits influences bryophyte plant fitness.
Keywords: Ceratodon purpureus, dioecious, life history, morphology, moss, physiology, sexual dimorphism, expressed sex ratio, trait comparisons
INTRODUCTION
Separate sexes occur in > 4 % of angiosperm (Renner and Ricklefs, 1995) and in > 50 % of bryophyte species (Wyatt and Anderson, 1984; Bisang and Hedenäs, 2005), evolving independently in angiosperms and bryophytes hundreds of times (Charlesworth, 2002; McDaniel et al., 2013). When male and female functions are separated onto distinct morphs, sexual specialization may evolve, and such sexual specialization is particularly likely when differential resource needs (e.g. phosphorus vs. nitrogen demands) occur between males and females (Case and Ashman, 2005). Differential selection on males and females, whether the result of resource needs or sexual selection, may result in sexual dimorphism. In vascular plants with separate sexes, sexual dimorphism has been well documented (for reviews, see Geber et al., 1999; Barrett and Hough, 2013), with males and females differing in vegetative and reproductive morphology (e.g. Ackerly and Jasienski, 1990; Delph et al., 1996; Harris and Pannell, 2008; Teitel et al., 2016), physiology (e.g. Dawson and Bliss, 1989; Dawson and Ehleringer, 1993; Zhang et al., 2014), life history (e.g. Cipollini and Stiles, 1991; Espirito-Santo et al., 2003; Yang et al., 2014) and biotic interactions (e.g. Cornelissen and Stiling, 2005; Eppley et al., 2009; Petry et al., 2013; Varga et al., 2013; Li et al., 2015).
If male and female plants differ in size or reproductive output, it is hypothesized that there will be a sex-specific differential in energy assimilation (Dawson and Geber, 1999). In angiosperms, males and females have been found to differ substantially in gas exchange, with females often having a greater capacity for photosynthetic assimilation than males, presumably due to the greater reproductive costs for females which are associated with bearing fruit (Dawson and Ehleringer, 1993; Dawson et al., 2004). However, exceptions to this pattern have been found, with males having higher rates of assimilation than females (Marshall et al., 1993; Gehring and Monson, 1994). As many dioecious species may be spatially segregated on the landscape, understanding how physiological response varies with genotype and sex is important to understanding how differing resource availability may contribute to altering population and community structure (Dawson et al., 2004). However, only a handful of studies have quantified sexual dimorphism from several populations in a common garden experiment or across environments to determine whether genetics or environmental variation controls variation in these traits (Delph et al., 2002; Delph and Bell, 2008; Yu et al., 2011), and the few studies that have examined whether sex-specific physiology was genetically controlled in plants have generally concentrated on a single population (Wang and Griffin, 2003; Dudley and Galen, 2007).
Here, we examine whether differential selection on males and females of the cosmopolitan moss Ceratodon purpureus has led to differences in cell, leaf, canopy and physiological traits between male and female plants across three populations. Sexual dimorphism has been reported for this species (Shaw and Gaughan, 1993) and is assumed to be relatively common across moss species, yet on such small organisms these subtleties can be difficult to distinguish (Shaw and Beer, 1999). Additionally, in mosses, differences between sexes can border on the extreme (Hedenäs and Bisang, 2011) or not be present at all (Horsley et al., 2011), making mosses an excellent and globally widespread system for studying the evolutionary ecology of sexual dimorphism in plants. Sex-specific differences in life history traits have inspired investigations of bryophyte sex ratios, reproductive trade-offs and functional traits (Shaw and Gaughan, 1993; Shaw and Beer, 1999; Bowker et al., 2000; McLetchie and Puterbaugh, 2000; Bisang and Ehrlén, 2002; Bisang and Hedenäs, 2005, 2013; Stark et al., 2010; Alvarenga et al., 2013). However, in bryophytes, we know little about the breadth of between-sex diversity in morphological traits (Shaw and Beer, 1999; McLetchie and Puterbaugh, 2000; Horsley et al., 2011) or physiological traits (Stark et al., 2005; McLetchie and Stark, 2006; Groen et al., 2010), and comparatively little emphasis has been placed on understanding the underlying sex-specific physiological differences in dioecious mosses at a finer scale and their contributions to sexual dimorphisms (Stark et al., 2009). Developing this sex-specific framework is also important for understanding how moss-dominated ecosystems (the bryosphere), which occur on every continent and dominate major portions of the earth’s surface, shape the function and diversity of heterotrophic communities (Lindo and Gonzalez, 2010; Balkan, 2016) and consequently nutrient cycling (Cornelissen et al., 2007; Turetsky et al., 2012; Delgado-Baquerizo et al., 2016) in ecosystems.
Mosses are a particularly ideal study system for such analyses because population sex ratios of expressing individuals are often highly skewed, generally with a female bias (Bisang and Hedenäs, 2005; but see McDaniel et al., 2007). Additionally, while we expect selection to act rapidly on physiological traits that maximize reproductive success and minimize the differential costs of reproduction for the separate sexes, selection may in fact act differently on the gametophyte (pre-zygotic) or sporophyte (post-zygotic) generation to produce biased population sex ratios (Norrell et al., 2014). For instance, early work by Shaw and Gaughan (1993) determined that expressed sex ratios of C. purpureus populations were more female biased than male biased. Male gametophytes in these populations were significantly smaller in overall biomass and had shorter leaves than females (Shaw and Gaughan, 1993). Since mosses often lack storage organs to ameliorate interactions with the environment, mosses are often physiologically limited by water status (Ehrlén et al., 2000; Rydgren and Økland, 2003). In fact, most mosses have leaves that are only one cell thick, so altering cell or leaf size could benefit their overall water status and prolong hydration during desiccating events. Male gametophytes bear antheridia (sperm-producing reproductive structures) which along with their gametes are in fact noted for their ability to tolerate desiccation (Shortlidge et al., 2012; Stark et al., 2016). Since sexual reproduction in mosses is known to be sperm limited, this creates a scenario where sexual selection may exert pressure on morphological and physiological traits that maximize the reproductive potential of gametophytic males by maximizing their tolerance of desiccation (McLetchie, 1996; Bisang et al., 2004). If fertilization is successful, however, female gametophytes would need to stay hydrated longer than males to support the growth of the sporophyte which is physiologically dependent on the maternal gametophyte for maturation. Female gametophytes and their attached sporophytes appear to vary in their tolerance of desiccation depending on when in the maturation process drying occurs (Stark et al., 2007; Stark and Brinda, 2015). Building on these observations, here we predict that the sex having larger morphological trait values will also have been selected to have higher physiological trait values to offset reproductive costs, as has been found in angiosperms (Caruso et al., 2003).
To examine sex-specific patterns in morphology and physiology in C. purpureus, we concentrated our measurements on a sub-set of 20 of the cell-, leaf- and canopy-level traits that Waite and Sack (2010) demonstrated were critical to explaining moss gas exchange physiology, and that have been used to demonstrate differences across moss species in gas exchange physiology (Waite and Sack, 2010; Wang et al., 2016). We expect these traits may be important in sex-specific allocation differences within C. purpureus. However, mosses are not just scaled-down angiosperms, as area and volume scale down differentially. The efficiency of water diffusion for water transport scales with plant size, and plant–atmosphere interactions are a matter of scale, as small plants are trapped in the laminar boundary layer of the surface on which they grow, unlike larger plants (Proctor et al., 2007). These unique characteristics of small-scale moss canopies mean that the limited data we have on sexual dimorphism and sex-specific physiology in angiosperms may not translate to this system. Using the C. purpureus model system, we addressed the following questions. (1) Are there sex-specific differences in cell-, leaf- and canopy-level traits and are these observations consistent among populations of the same species? (2) How are sexual dimorphisms in C. purpureus related to measures of leaf photochemistry and/or field expressed sex ratios in this study species? To address these questions, plants from three populations were grown in a common-garden environment to evaluate the amount of phenotypic trait variation among populations that can be attributed to genetic differences, and expressed sex ratios were assessed for each of the three field populations.
MATERIALS AND METHODS
Study species and greenhouse component
Ceratodon purpureus (Hedw.) Brid. (Dicranales) is a dioecious moss that is ubiquitous across all continents and varies dramatically in its ecological tolerances (Crum and Anderson, 1981; Jules and Shaw, 1994). Previous investigations have noted discrepancies from the expected 1:1 ratio of males to females in natural populations of this species (Shaw and Gaughan, 1993), and more recent work suggests that the meiotic sex ratio apparent at spore germination can vary greatly in C. purpureus, potentially expaining some of the population sex ratio variation in this species (Norrell et al., 2014). We collected plants from three locations. Two are urban locales within Portland, OR: these will henceforth be referred to as Neuberger Hall (NH) (45º30′N, 122º41′W), on the downtown campus of Portland State University (PSU), and North East 35th (NE 35) (45º32′N, 122º37′W), in a neighbourhood 8 km north-east of downtown. A population in a third location, UCUT (45º36′N, 123º02′W), 32 km west of the Portland, OR metropolitan area, was also utilized. All three populations are assumed to be genetically distinct due to the distance between these sites. Regional climate conditions were similar between all sites, but overstorey canopy cover was noticeably different between the NE 35 and NH sites and the UCUT site. Therefore, canopy openness was measured at each site with a densiometer (NE 35, 84 % cover; NH, 98 % cover; UCUT, 0 % cover).
In order to distinguish between genetic and environmentally controlled life history traits, we grew plants from all three populations through the protonemal stage in an environmentally controlled glasshouse on the PSU campus (Shaw, 1986). Plants were collected from the field as gametophytes in clumps from disjointed areas (to decrease the likelihood of collecting ramets of the same genet) within each of the three study sites and allowed to air-dry. One shoot per clump (assumed to be an individual) was finely ground, and plant fragments were sprinkled on the soil surface of a single 10 cm2 plastic pot filled with 2:1 sand/coir mixture (Down to Earth, Eugene, OR, USA). Each individual was grown to sexual maturity for identification and then allowed to continue growth until the pot was full of ramets of that single individual (approx. 12 months). All plants received the same water regime (misted six times daily), light levels (no artificial lighting) and temperatures (ranged from 10 to 25º C) so that any field effects from the separate populations were removed. We started with about 30 individuals per population. Of those that survived and thrived, four randomly selected individuals of each sex per population provided the plant materials from which the comprehensive cell, leaf and canopy trait measurements were derived. All individuals were of reproductive age, thus allowing verification of the sex by observance of distinct sexual structures, antheridia (male) and archegonia (female), prior to analyses. In bryophytes, it is common to have gametophytes that do not produce reproductive structures even though their sex may be genetically determined. For the following trait measurements, only sexually expressing shoots of individuals are used. Determining the sex of non-sex-expressing gametophytes was beyond the scope of this study.
Leaf and cell measurements
In order to differentiate leaf and cell morphology between the sexes, two ramets per individual were selected from the same location within each pot as designated by a wire grid. Plant material was mounted fresh on a slide, then hydrated and photographed (Leica compound and dissecting microscope, Germany; Leica Application Suite 3.5.0, Germany) at × 4 and ×40 magnification to obtain images of the cells, leaves and leaf cross-sections. Lower leaves rather than parachietal leaves were measured to assess pre-reproductive sexual dimorphism (Shaw and Gaughan, 1993). Methods follow those of Waite and Sack (2010). Images were later analysed using Image-Pro Express (Media Cybernetics, MD, USA). Each leaf or cell trait was measured from two whole leaf or two leaf cross-sectional images (one leaf or cross-section per ramet) and include by category: cell traits: CLA, cell lumen area (μm2); CLL, cell lumen length (μm); CLW, cell lumen width (μm); SWT, adaxial cell wall thickness (μm); IWT, abaxial cell wall thickness (μm); CWLG, interior longitudinal cell wall thickness (μm); CWLT, interior latitudinal cell wall thickness (μm); and leaf traits: COD, costa depth (mm), COW, costa width (mm); LA, leaf area (mm2); LL, leaf length (mm); LT, lamina thickness (μm); LW, leaf width (mm); L/W, length/width (Supplementary Data Additional methods).
Canopy measurements
We quantified six canopy traits, selected for their potential association with leaf and cell traits, by dividing glasshouse-grown pots of C. purpureus into grids. From three randomly selected grid squares (repeated for each individual), three cores (4·37 cm2) of fresh plant material were collected for analysis. After a canopy projected area image was acquired with a dissecting scope, we measured the canopy height (CH) of three averaged sized gametophyte shoots of each core. One shoot, of average height, was photographed to determine the shoot projected area. Shoots were then defoliated, and leaves and stems photographed separately to allow for measurements of leaf projected area and stem projected area. All plant material was then oven-dried at 80 ºC, and dried plant weights were used to quantify canopy mass, leaf mass, stem mass and shoot mass. Canopy metrics determined from shoot and canopy images include: canopy traits: LMA, leaf mass area (g m–2); CMA, canopy mass area (g m–2); CH, canopy height (cm); CD, canopy density (g cm–3); LMF, leaf mass fraction; LAR, leaf area ratio (mm2/g). Equations and exact methods follow those of Waite and Sack (2010)(Supplementary Data Additional methods).
Leaf photochemistry measurements
Leaf photochemistry measurements [in this study chlorophyll fluorescence-based Fv/Fm and electron transport rate (ETR)] allow a non-destructive glimpse of a plant’s photosynthetic functioning under natural conditions. All photochemical measurements were made using the Junior-PAM chlorophyll fluorometer (Walz, Effeltrich, Germany), fitted with a small diameter fibre optic probe. All plants were dark adapted overnight prior to analyses. Fluorescence measurements were made in a dark room, and care was taken to ensure that plants did not desiccate.
We used Fv/Fm to measure the maximum photochemical efficiency of dark-adapted photosystem II (PSII). Four Fv/Fm measurements were taken for each individual and a mean was calculated for each. Initial chlorophyll fluorescence measurements corresponded with cell, leaf and canopy imaging dates. Subsequent measurements were repeated four times at 2 week intervals.
Photosynthetic photon flux density (PPFD) response curves followed the methods of Marschall and Proctor (2004) and were used to attain a relative measure of photosynthetic ability. The Junior-PAM chlorophyll fluorometer emits consecutive increasing intensities of light and records the instantaneous relative ETR of PSII, with 6 min at each level for equilibration. Two replicate ETR measurements were attained for each dark-acclimated individual and a mean was calculated for each.
Field population expressed sex ratio
To understand how these morphological and physiological trait investigations relate to population expressed sex ratios, we collected plants from the field in May of 2012 from the three populations mentioned above. Every 30 cm, along 152 cm transects, three 4·37 cm2 moss cores were sampled with a soil corer, placed in numbered envelopes to reduce observer bias, and stored at room temperature until analysis. Two transects per population were sampled, yielding 36 moss cores per population. In the laboratory using a dissecting microscope (Leica, Germany), we determined counts of sexually expressing male and female ramets per core, and we recorded the total number of ramets per core. We pooled the counts from all cores within each population to obtain the sex ratio per population of expressing ramets. Population sex ratio values (SRpop ) were determined by dividing the number of expressing male ramets by the total number of sexually expressing ramets (male and female). Determining the sex of non-sex-expressing ramets was beyond the scope of this study.
Statistical analyses
Analyses of variance (ANOVAs) were performed to determine the effect of sex, population and the interaction between sex and population on measured leaf, cell and canopy traits, using JMP Version 9.0 (SAS Institute, 2010). Data fit the assumptions of the ANOVA models. Because we used multiple analyses from data on the same samples, we used a Benjamini and Hochberg procedure to control for a false discovery rate (Benjamini and Hochberg, 1995). We used Tukey’s tests to determine significance among factors. We used repeated measures ANOVA to determine the effect of sex, population and the interaction between sex and population on Fv/Fm and ETR across time and light levels, respectively, using JMP Version 9.0. t-tests were performed at each time point (for Fv/Fm) and light level (for ETR) to compare the performance of males and females across populations. We used Pearson’s and Spearman’s correlation coefficients on log-transformed data to meet normality assumptions and created a correlation matrix of the 20 measured cell, leaf and canopy traits. Principal components analysis (PCA) was performed on the correlation matrix using the R package factoextra (Kassambara and Mundt, 2016; R Core Team, 2016). Three traits (LW, LL and LA) were excluded from the PCA because of their strong correlation (Waite and Sack, 2010). Using JMP Version 12.0.1 (SAS Institute, 2015), likelihood χ2 tests were used to determine whether population sex ratios varied from 1:1.
RESULTS
Cell, leaf and canopy traits
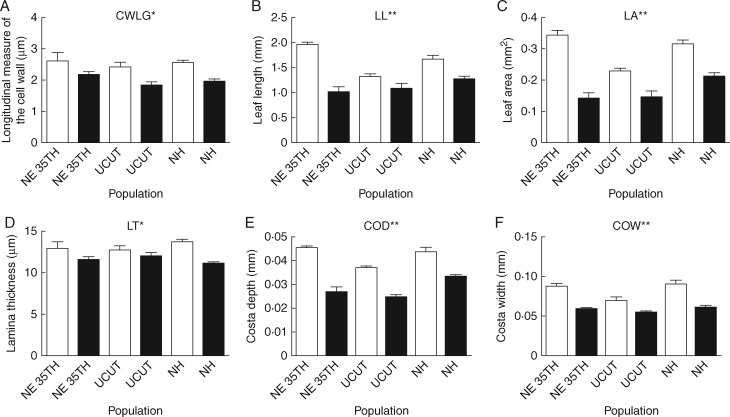
One cell trait (longitudinal measure of the cell wall; CWLG) differed significantly between the sexes, with female plants having thicker cell walls than males (Fig. 1A;Table 1). No cell traits varied among populations or in the interaction between population and sex. However, six of seven leaf traits varied significantly between expressing males and females, with greater values for females than males in each trait (Fig. 1B–F;Table 1). For four leaf traits (leaf length; LL, leaf area; LA, costa depth; COD, and costa width; COW; Table 1), population was a significant factor. For all four traits, post-hoc analyses via Tukey’s tests showed that plants from the NE 35th and NH populations had significantly larger traits than plants from the UCUT population (Supplementary Data Table S1). For two leaf traits (LL and LA), the interaction between population and sex was a significant factor indicating that the degree of sexual dimorphism of this trait differed among populations. The post-hoc analysis suggests that for leaf length the NE 35th and NH populations showed significant sexual dimorphism while the UCUT population did not (Fig. 1B). The post-hoc analysis for leaf area suggests that all three populations exhibit sexual dimorphism for this trait, but that the degree of dimorphism is significantly greater in the NE 35th population than in the other two populations (Fig. 1C). Canopy traits did not differ between the sexes or among populations, and no interactions were significant for canopy traits (Table 1).
Fig. 1.
Sex-specific comparison of six secondary reproductive traits in Ceratodon purpureus for three populations: (A) longitudinal measure of the cell wall (CWLG), (B) leaf length (LL), (C) leaf area (LA), (D) lamina thickness (LT), (E) costa depth (COD) and (F) costa width (COW). In all panels, females are represented by white bars and males are represented by black bars. Error bars indicate standard error. Asterisks indicate that the effect of sex was significant for ANOVA for each trait (*P < 0.05, **P < 0.01, ***P < 0.001; full results in Table 1).
Table 1.
Mean values and statistical analyses of leaf, cell and canopy traits from Ceratodon purpureus plants
| Trait | Symbol | Units | Mean (±s.d.) | Sex | Population | Sex × population |
|---|---|---|---|---|---|---|
| Cell traits | ||||||
| Cell lumen area | CLA | μm2 | 160·6 (50·7) | 1·42 | 0·34 | 0·17 |
| 136·1 (42·3) | ||||||
| Cell lumen length | CLL | μm | 21·09 (5·03) | 1·43 | 0·60 | 0·57 |
| 18·60 (4·80) | ||||||
| Cell lumen width | CLW | μm | 7·872 (1·11) | 0·45 | 1·90 | 2·35 |
| 7·584 (1·21) | ||||||
| Adaxial laminar cell wall thickness | SWT | μm | 2·168 (0·52) | 4·64 | 0·59 | 0·03 |
| 1·777 (0·27) | ||||||
| Abaxial laminar cell wall thickness | IWT | μm | 2·238 (0·46) | 4·76 | 0·72 | 0·15 |
| 1·862 (0·33) | ||||||
| Longitudinal measure of the cell wall | CWLG | μm | 2·537 (0·33) | 22·06* | 1·70 | 0·19 |
| 1·986 (0·23) | ||||||
| Latitudinal measure of the cell wall | CWLT | μm | 2·184 (0·32) | 5·76 | 0·48 | 0·78 |
| 1·906 (0·22) | ||||||
| Leaf traits | ||||||
| Leaf length | LL | mm | 1·651 (0·30) | 69·30** | 8·50* | 11·56** |
| 1·122 (0·20) | ||||||
| Leaf width | LW | mm | 0·311 (0·04) | 16·55* | 5·49 | 0·94 |
| 0·251 (0·04) | ||||||
| Leaf area | LA | mm2 | 0·297 (0·06) | 123·51** | 15·07** | 9·82** |
| 0·167 (0·04) | ||||||
| Lamina thickness | LT | μm | 13·13 (1·17) | 15·51* | 0·07 | 1·93 |
| 11·57 (0·73) | ||||||
| Costa depth | COD | mm | 0·042 (0·00) | 162·76** | 17·24** | 5·29 |
| 0·028 (0·00) | ||||||
| Costa width | COW | mm | 0·083 (0·01) | 81·14** | 9·90* | 2·96 |
| 0·058 (0·01) | ||||||
| Length/width | L/W | – | 5·558(1·47) | 6·50 | 1·26 | 3·80 |
| 4·494(0·71) | ||||||
| Canopy traits | ||||||
| Leaf mass area | LMA | g m–2 | 9·813 (2·41) | 1·27 | 1·72 | 0·58 |
| 14·27 (13·7) | ||||||
| Canopy mass area | CMA | g m–2 | 5·075 (0·22) | 4·14 | 0·66 | 0·20 |
| 3·948 (1·07) | ||||||
| Canopy height | CH | cm | 0·718 (0·22) | 4·77 | 0·40 | 2·24 |
| 0·556 (0·15) | ||||||
| Canopy density | CD | g cm–3 | 0·008 (0·00) | 0·27 | 0·51 | 0·89 |
| 0·008 (0·00) | ||||||
| Leaf mass fraction | LMF | – | 0·560 (0·06) | 0·15 | 0·66 | 2·15 |
| 0·573 (0·10) | ||||||
| Leaf area ratio | LAR | mm2 g–1 | 9042 (4568) | 0·53 | 2·65 | 0·18 |
| 7680 (4977) | ||||||
The means for each trait are for expressing females (upper number) and males (lower number) with the s.d. in parentheses across study populations.
The statistical analyses are ANOVA for the effects of sex (male vs. female), population (UCUT, NE 35 and NH) and the interaction between sex and population on each trait, and the F statistics are provided.
Significant results are in bold;
P < 0·05
P < 0·01
P < 0·001, and are based on the Benjamini and Hochberg procedure to control for false discovery rate with multiple comparisons (Benjamini and Hochberg, 1995); n = 24.
Photochemical measures
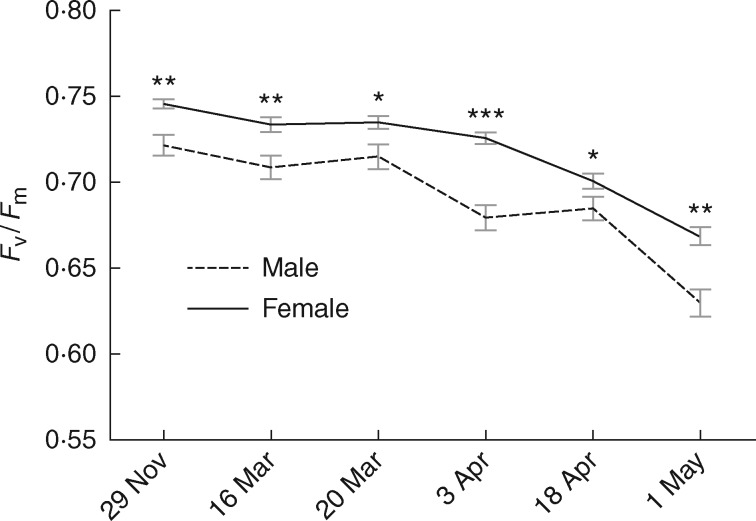
Sex and population were both significant factors in explaining dark-adapted PSII efficiency (Fv/Fm) in this moss species (F1,18 = 7·32; P = 0·02 and F2,18 = 3·47; P = 0·05, respectively). Females had significantly higher Fv/Fm values than did males (Fig. 2), and the NE 35 and NH populations had higher values than the UCUT population (data not shown). The interaction between sex and population was not significant (F2,18 = 1·67; P = 0·22). Time was also a significant factor in the model (F5,14 = 91·98; P < 0·0001), and interactions between time and sex (F5,14 = 3·99; P = 0·02) and time, sex and population were significant (F10,28 = 2·16; P = 0·05), indicating that Fv/Fm decreased overall during our sampling time period, that the overall differential in Fv/Fm between males and females increased during our sampling time period (Fig. 2), and that this increase varied among populations. The interaction between time and population was not significant (F10,28 = 0·41; P = 0·93), indicating that the difference in Fv/Fm among populations did not vary during the sampling time period but remained more or less the same.
Fig. 2.
F v/Fm values for males and females over time. Each point represents a mean of 12 Ceratodon purpureus individuals (four from each population) in three common-garden populations. Error bars indicate the s.e. A repeated measure ANOVA showed a significant effect of sex (P = 0.02) and a significant interaction between time and sex (P = 0.02); asterisks indicate significant differences between males and females for t-tests at each date (*P < 0.05, **P < 0.01, ***P < 0.001).
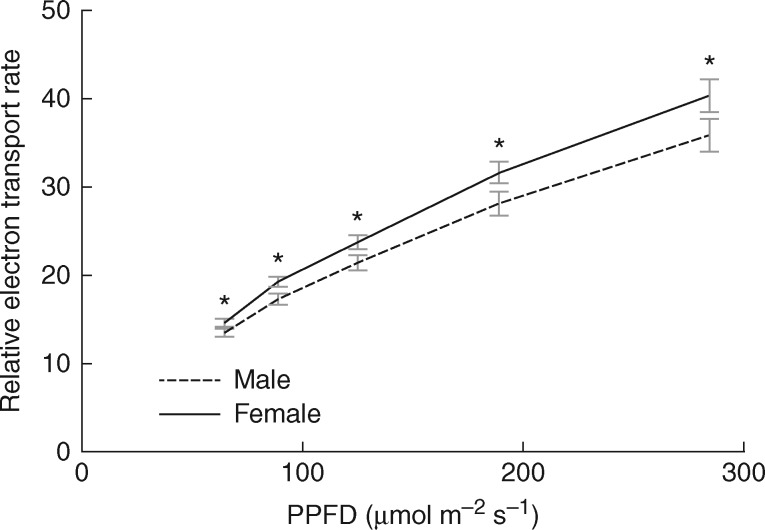
Sex and population overall did not have a significant effect on ETR (F1,18 = 2·17; P = 0·12 and F2,18 = 1·88; P = 0·18, respectively), and the interaction was only marginally significant (F2,18 = 3·22; P = 0·06). However, when we analysed our data on light levels we found that light level (F7,12 = 214·33; P < 0·0001) was significant, as ETR increased with light level, and interactions with light levels and sex (F7,12 = 3·74; P = 0·02) and light level, sex and population (F14,24 = 3·48; P = 0·004) were significant, as the greatest differences in ETR in this moss species occurred at higher light levels (Fig. 3). We did not reach saturation of electron flow in the light levels examined in this study, as the relative ETR continued to increase linearly with PPFD, and this increase corresponded with a greater divergence in values between the sexes (Proctor, 2003; Marschall and Proctor, 2004).
Fig. 3.
Photosynthetic photon flux density (PPFD) response curves comparing the relative electron transport rates (ETR) for males and females. Each point represents a mean of 12 Ceratodon purpureus individuals (four from each of three populations). Error bars indicate the s.e. A repeated measure ANOVA showed a significant interaction between light levels and sex (P = 0.02); asterisks indicate significant differences between males and females for t-tests at each light level (*P < 0.05).
Correlation structure among cell, leaf and canopy traits
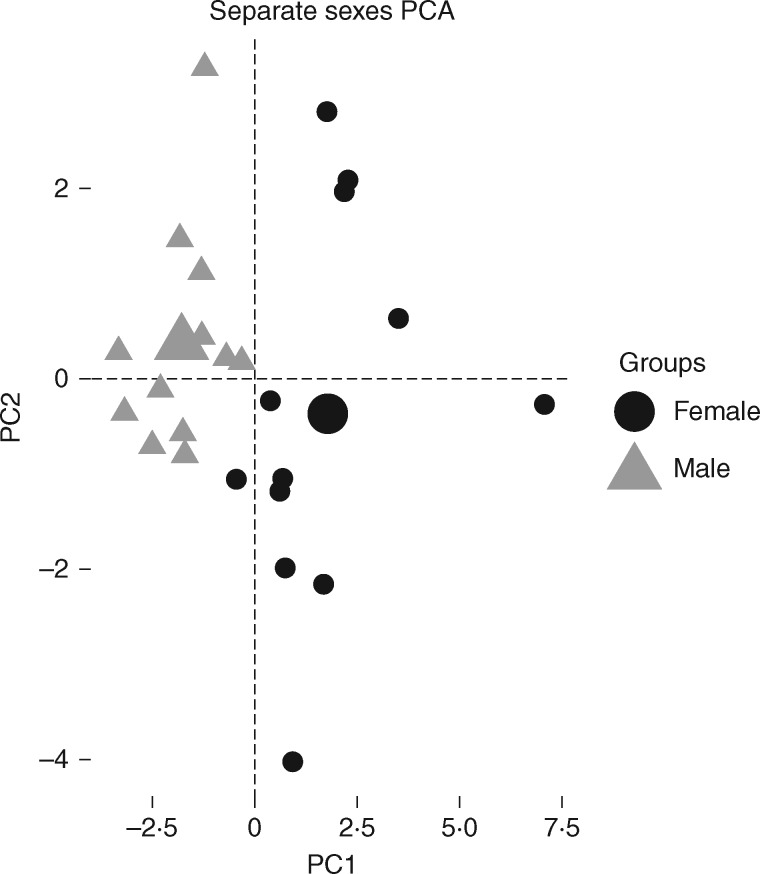
The first principal component (PC1) separated the sexes in the PCA, explained 32·9 % of the overall trait variation and pointed to a tight grouping of highly correlated cell and leaf traits which arose largely due to the allocation of females to greater leaf, costa, cell and cell wall size (Fig. 4) (Supplementary Data Fig. S1; Table S2 and S3). The second PCA axis (PC2; 15·6 % of the overall variation) primarily reflected variation in cell and canopy traits (Fig. 4). Distance between the sexes within a population on the PCA is indicative of a greater level of sexual dimorphism in life history traits. Within sex, females were more variable than males within and across populations. Populations of C. purpureus overlapped greatly in space (Supplementary Data Fig. S2). Along PC1, variation was highest in the NE 35th population. The UCUT population was the most variable along PC2 while the NE 35th population had the least variance along this axis.
Fig. 4.
Principle components analysis (PCA) of 17 cell, leaf and canopy traits. PC1 = 32.9 % of the variance; PC2 = 15.6 % of the variance (see Supplementary Data Table S2). Each point is an individual Ceratodon purpureus grown in a common garden.
Field population expressed sex ratio
The NE 35 (SRpop, 0·63; 111 males, 66 females, 26 % of shoots were fertile) and UCUT (SRpop, 0·59; 558 males, 381 females, 57 % of shoots were fertile) populations exhibited significantly male-biased expressed sex ratios (χ2 = 11·57; P = 0·0007 and χ2 = 33·57; P <0·0001, respectively). However, the NH population (SRpop, 0·08; 16 males, 182 females, 26 % of shoots were fertile) was extremely female biased in sex expression (χ2 = 163·31; P < 0·0001).
DISCUSSION
Our results present sexual dimorphism in cell, leaf and photochemical measures in the ubiquitous dioecious moss Ceratodon purpureus, with females expressing significantly larger trait values and exhibiting enhanced photochemical measures compared with males. Females had significantly larger measures for seven of 20 cell and leaf traits (including larger leaves in most measures) than males, but no canopy traits differed between sexes. Besides these sex-specific differences, our study reveals variation between the three common-garden populations in four leaf traits and in photochemical measures. In contrast to our prediction, we demonstrate that the sex with the higher trait values (in our case females) is sometimes less common in natural populations, as sexually expressing males are more common in two of our study populations than sexually expressing females. Our third study population, however, exhibits the expected female-biased sex ratio. Below, we discuss the evolutionary and ecological implications of our results with respect to sex-specific water relations, reproduction and biotic interactions.
Sex-specific differences
Female plants, in our study, have leaves that are on average 13·5 % thicker (LT; Fig. 1D) than those of male plants, a potential desiccation tolerance mechanism (Dilks and Proctor, 1979). As found in earlier studies on this species, female plants also have larger and longer leaves than males (LA, LL; Fig. 1B, C) which may prolong hydration via greater water storage in cells or by increasing the boundary layer, as conduction of water is external in most mosses, rather than internal as in angiosperms (Table 1; Shaw and Gaughan, 1993; Shaw and Beer, 1999; Proctor, 2000). Female plants in this study have wider and deeper costae than male plants (COD, COW; Fig. 1E, F), another trait in mosses often associated with water storage. The costa of C. purpureus has thick-walled cells, stereids, that are presumed to prolong leaf hydration (Frahm, 1985) and help the plant avoid desiccation. From studies on other mosses, costa size and structure has been found to vary within a species depending on the moisture conditions of a particular habitat, with larger costae being found in drier locations (Zastrow, 1934; Frahm, 1985). The increased size of these traits is likely to benefit C. purpureus females by prolonging the duration of hydration-limited physiological activity thus allowing females to assimilate carbon for longer. In contrast, cell and canopy traits were, for the most part, not sexually dimorphic in C. purpureus (with the exception of CWLG; Fig. 1A). Ceratodon purpureus cell and leaf traits did share many strong positive correlations, yet interestingly there were few correlations between cell or leaf traits and canopy traits, a pattern which deserves further investigation (Supplementary Data Table S3). Cellular desiccation poses a grave threat to the structural stability of cells, a limitation that may explain the lack of variation in cell traits between sexes or among populations in this study. Conversely, the fact that there were few correlations among cell or leaf and canopy traits could provide an opportunity for greater plasticity in canopy traits or may alternatively suggest that canopy traits are under a different selective pressure (Sultan 2000; Delph et al., 2002). Our photochemical measures suggest that females may assimilate carbon at a greater rate than males (Figs 2 and 3). Studies are currently under way to analyse more comprehensively sex-specific differences in CO2/H2O exchange and photochemistry in C. purpureus. When life histories vary between the sexes, morphological differences between the males and females are common (Geber et al., 1999). In mosses, females must remain hydrated for much longer than males to facilitate water-mediated fertilization and embryo development (Stark et al., 2007; Stark and Brinda, 2015). It seems likely that these dimorphic morphologies, particularly larger leaves, should prolong hydration and benefit females and specifically the sporophyte generation (Moore et al., 2016). Additionally, besides maximizing the amount of time spent hydrated, it follows intuitively that plasticity in these same or additional water-related traits might also ensure reproductive success in the face of environmental heterogeneity. This need for plasticity could help explain the increased trait variation measured in females vs. that of males within and across our study populations (Fig. 4).
In addition to impacts on water relations and reproduction, differences in leaf thickness between the sexes could also influence how the sexes interact with the biotic community. In vascular plants, male and female plants have been found to interact differentially with insects and fungi (Cornelissen and Stiling, 2005; Varga and Kytöviita, 2008; Varga, 2010), and sex-specific differences in plants have been shown to lead to sex-specific heterotrophic communities (Petry et al., 2013). Thus, larger leaf traits in females compared with males are likely to lead to differences in how each sex interacts with local heterotrophs. In mosses, thicker and larger leaves may allow females greater protection against fungal pathogens, which occur at high densities in moss communities (Davey and Currah, 2006; Davey et al., 2013). Indeed, males from these same study populations, with thinner leaves, were recently found to have higher fungal biomass and richness than females (Balkan, 2016). In fact, if the higher photochemical values in females allow for greater overall net assimilation, this could provide the resources needed for fungal chemical defences and/or volatile compounds that have been hypothesized as contributing to an early ‘plant pollinator’-like syndrome in C. purpureus (Cronberg et al., 2006; Cronberg, 2012; Rosenstiel et al., 2012).
Population-level differences
While trait variation between males and females occurs more often (seven out of 20 traits) than trait variation among populations in this study (four out of 20 traits), the population-level differences help differentiate those traits that are genetically constrained from those that are more plastic. None of the cell or canopy traits measured here demonstrate population differences, suggesting that those traits and the sexual dimorphisms found for one cell trait (CWLG) may be more constrained in this species. Waite and Sack (2010) found that these same cell traits varied drastically across bryophyte species, thus perhaps cell traits are generally conserved at the species level in mosses. Leaf traits, conversely, are dimorphic between sexes and variable across these study populations. For all four leaf traits that vary by population (LL, LA, COD and COW), these traits are greatest for females in the NE 35 and NH populations where canopy cover is densest (84 and 98 % cover, respectively) and least in the UCUT population (0 % cover). Fv/Fm values follow this same trend, with the NH population having the highest values and the UCUT population the lowest values. Consideration of those traits that are consistent vs. traits that vary across populations sheds light on how natural and sexual selection interact in C. purpureus to create separate sexes with trait dimorphisms.
Although our work only considers plants from three populations with limited environmental variance, it suggests that C. purpureus may be more genetically constrained between the sexes and across populations when considering cell and canopy traits but sexually dimorphic and more plastic in its ability to modify leaf traits differentially across environments. Such remarkable fine-tuned adaptability probably contributes to the ability of this species of moss in particular to grow on every continent along almost any environmental gradient. These findings suggest that across-environment stability in C. purpureus is partially achieved by integrating its sexually selected water-related needs with environmentally mediated leaf morphologies. In addition, the fact that females express greater variability than males in this study may in part be explained by the sensitivity of the growing sporophyte to desiccation (Fig. 4; Stark et al., 2007; Stark and Brinda, 2015). Support for the effects of plasticity in leaf morphology on stabilizing fitness across environments has been found in vascular plants (Schlichting, 1986; Sultan, 2000; Pigliucci, 2001), though additional studies are needed to understand how constraints and modifications of suites of traits are influenced by environmental heterogeneity.
Leaf photochemistry and expressed sex ratios
We predicted that the sex with the larger morphology would have greater physiological output to offset potential costs. Our measures of leaf photochemistry support this prediction as females have higher Fv/Fm values across a 5 month time frame and higher relative ETRs across different light levels (Figs 2 and 3). Consistent with previous studies (Shaw and Gaughan, 1993; Shaw and Beer, 1999), C. purpureus displays biased sex ratios in our study populations; however, two of the three populations in our study were significantly male biased compared with a female bias noted in previous studies. While we cannot discount the possibility that sexual determination of non-sex-expressing shoots might alter these findings, our result is contrary to our sex ratio prediction which presumed that the sex associated with larger sized traits and greater physiological advantage would also have a fitness advantage. One explanation could be that females bear an added cost by allocating more energy to growth and that this cost is not offset sufficiently by the sex-specific difference in physiology, and thus males outcompete females. This species occurs in disturbed habitats that vary considerably in abiotic conditions. While the majority of C. purpureus populations may be female biased, males may dominate in some populations, such as our NE 35 and UCUT populations, where abiotic conditions may alter the physiological balance between the sexes to favour males, as has been found to occur in dioecious angiosperms such as Acer negundo (Ward et al., 2002). More site-specific variables would need to be examined for such an assessment.
Conclusion
Overall we find that sexual dimorphism occurs consistently for leaf traits and that this dimorphism is somewhat population specific. Females usually have larger and thicker leaves than males, and this correlates with greater photochemical output for females than males. Additionally, higher levels of variation among females within and among populations contrasts with the consistency of male traits, which vary little within or among populations. Traits that do vary among populations (LL, LA, COD and COW) should be considered with regards to how habitat heterogeneity might influence resource allocations and their corresponding dimorphic patterns in C. purpureus. Long-term studies are needed to determine how small-scale cell and leaf traits influence survival and mating differences among C. purpureus genotypes.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Additional methods. Figure S1: biplot of PCA trait scores. Figure S2: PCA of separate populations. Table S1: means (±s.d.), shown separately for each population, for traits in which population was significant in the full ANOVA model. Table S2: factor loadings of 17 traits in the first two principal components. Table S3: correlation matrix of cell leaf, and canopy traits of Ceratodon purpureus.
Supplementary Material
ACKNOWLEDGEMENTS
We thank, S. Bellush, R. Callaway, M. Duvanek, T. Hinkley, C. Piedrahita and C. Phelan for help in the lab, greenhouse, editing and with R. Two anonymous reviewers greatly improved earlier versions of this manuscript. This work was supported by a Ford Family Foundation fellowship and Montana IOE NSF EPSCoR Track-1 award [EPS-1101342] to M.L.S. and a National Science Foundation grant [IOS 128225] to T.N.R. and S.M.E.
LITERATURE CITED
- Ackerly D, Jasienski M.. 1990. Size-dependent variation of gender in high-density stands of the monoecious annual, Ambrosia artemisiifolia (Asteraceae). Oecologia 82: 474–477. [DOI] [PubMed] [Google Scholar]
- Alvarenga LDP, Porto KC, Zartman CE.. 2013. Sex ratio, spatial segregation, and fertilization rates of the epiphyllous moss Crossomitrium patrisiae (Brid.) Mull. Hal. in the Brazilian Atlantic rainforest. Journal of Bryology 35: 88–95. [Google Scholar]
- Balkan M. 2016. Sex-specific fungal communities of the dioicous moss Ceratodon purpureus. MS Thesis, Portland State University, USA.
- Barrett S, Hough J.. 2013. Sexual dimorphism in flowering plants. Journal of Experimental Botany 64: 67–82. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y.. 1995. Controlling the false discovery rate – a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B: Methodological 57: 289–300. [Google Scholar]
- Bisang I, Ehrlén J.. 2002. Reproductive effort and cost of sexual reproduction in female Dicranum polysetum. Bryologist 105: 384–397. [Google Scholar]
- Bisang I, Hedenäs L.. 2005. Sex ratio patterns in dioicous bryophytes re-visited. Journal of Bryology 27: 207–219. [Google Scholar]
- Bisang I, Hedenäs L.. 2013. Males are not shy in the wetland moss Drepanocladus lycopodiodes. International Journal of Plant Sciences 174: 733–739. [Google Scholar]
- Bisang I, Ehrlén J, Hedenäs L.. 2004. Mate limited reproductive success in two dioicous mosses. Oikos 104: 291–298. [Google Scholar]
- Bowker MA, Stark LR, McLetchie DN, Mishler BD.. 2000. Sex expression, skewed sex ratios, and microhabitat distribution in the dioecious desert moss Syntrichia caninervis (Pottiaceae). American Journal of Botany 87: 517–526. [PubMed] [Google Scholar]
- Caruso CM, Maherali H, Jackson RB.. 2003. Gender-specific floral and physiological traits: implications for the maintenance of females in gynodioecious Lobelia siphilitica. Oecologia 135: 524–531. [DOI] [PubMed] [Google Scholar]
- Case A, Ashman T.. 2005. Sex-specific physiology and its implications for the cost of reproduction In: Reekie E, Bazzaz F, eds. Reproductive allocation in plants. Amsterdam: Elsevier, 129–157. [Google Scholar]
- Charlesworth D. 2002. Plant sex determination and sex chromosomes. Heredity 88: 94–101. [DOI] [PubMed] [Google Scholar]
- Cipollini ML, Stiles EW.. 1991. Costs of reproduction in Nyssa sylvatica: sexual dimorphism in reproductive frequency and nutrient flux. Oecologia 86: 585–593. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Lang SI, Soudzilovskaia NA, During HJ.. 2007. Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Annals of Botany 99: 987-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen T, Stiling P.. 2005. Sex-biased herbivory: a meta-analysis of the effects of gender on plant–herbivore interactions. Oikos 111: 488–500. [Google Scholar]
- Cronberg N. 2012. Animal-mediated fertilization in bryophytes – parallel or precursor to insect pollination in angiosperms? Lindbergia 35: 76–85. [Google Scholar]
- Cronberg N, Natcheva R, Hedlund K.. 2006. Microarthropods mediate sperm transfer in mosses. Science 313: 1255–1255. [DOI] [PubMed] [Google Scholar]
- Crum H, Anderson L.. 1981. Mosses of Eastern North America. New York: Columbia University Press. [Google Scholar]
- Davey ML, Currah RS.. 2006. Interactions between mosses (Bryophyta) and fungi. Canadian Journal of Botany-Revue Canadienne De Botanique 84: 1509–1519. [Google Scholar]
- Davey ML, Heegaard E, Halvorsen R, Kauserud H, Ohlson M.. 2013. Amplicon-pyrosequencing-based detection of compositional shifts in bryophyte-associated fungal communities along an elevation gradient. Molecular Ecology 22: 368–383. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Bliss LC.. 1989. Patterns of water use and the tissue water relations in the dioecious shrub, Salix arctica: the physiological basis for habitat partitioning between the sexes. Oecologia 79: 332–343. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Ehleringer JR.. 1993. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in Boxelder, Acer negundo. Ecology 74: 798–815. [Google Scholar]
- Dawson TE, Geber MA.. 1999. Sexual dimorphism in physiology and morphology In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag, 175–216. [Google Scholar]
- Dawson T, Ward J, Ehleringer J.. 2004. Temporal scaling of physiological responses from gas exchange to tree rings: a gender-specific study of Acer negundo (Boxelder) growing under different conditions. Functional Ecology 18: 212–222. [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Eldridge DJ, et al. 2016. Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytologist 209: 1540–1552. [DOI] [PubMed] [Google Scholar]
- Delph L, Bell D.. 2008. A test of the differential-plasticity hypothesis for variation in the degree of sexual dimorphism in Silene latifolia. Evolutionary Ecology Research 10: 61–75. [Google Scholar]
- Delph L, Galloway L, Stanton M.. 1996. Sexual dimorphism in flower size. American Naturalist 148: 299–320. [Google Scholar]
- Delph L, Knapczyk F, Taylor D.. 2002. Among-population variation and correlations in sexually dimorphic traits of Silene latifolia. Journal of Evolutionary Biology 15: 1011–1020. [Google Scholar]
- Dilks T, Proctor M.. 1979. Photosynthesis, respiration and water content in bryophytes. New Phytologist 82: 97–114. [Google Scholar]
- Dudley LS, Galen C.. 2007. Stage-dependent patterns of drought tolerance and gas exchange vary between sexes in the alpine willow, Salix glauca. Oecologia 153: 1–9. [DOI] [PubMed] [Google Scholar]
- Ehrlén J, Bisang I, Hedenäs L.. 2000. Costs of sporophyte production in the moss, Dicranum polysetum. Plant Ecology 149: 207–217. [Google Scholar]
- Eppley SM, Mercer CA, Haaning C, Graves CB.. 2009. Sex-specific variation in the interaction between Distichlis spicata (Poaceae) and mycorrhizal fungi. American Journal of Botany 96: 1967–1973. [DOI] [PubMed] [Google Scholar]
- Espirito-Santo MM, Madeira BG, Neves FS, Faria ML, Fagundes M, Fernandes GW.. 2003. Sexual differences in reproductive phenology and their consequences for the demography of Baccharis dracunculifolia (Asteraceae), a dioecious tropical shrub. Annals of Botany 91: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J. 1985. The ecological significance of the costal anatomy in the genus Campylopus. Abstracta Botanica 9: 159–169. [Google Scholar]
- Geber MA, Dawson TE, Delph LF.. 1999. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag. [Google Scholar]
- Gehring JL, Monson RK.. 1994. Sexual differences in gas-exchange and response to environmental-stress in dioecious Silene latifolia (Careyophyllaceae). American Journal of Botany 81: 166–174. [Google Scholar]
- Groen KE, Stieha CR, Crowley PH, McLetchie DN.. 2010. Sex-specific plant responses to light intensity and canopy openness: implications for spatial segregation of the sexes. Oecologia 162: 561–570. [DOI] [PubMed] [Google Scholar]
- Harris M, Pannell J.. 2008. Roots, shoots and reproduction: sexual dimorphism in size and costs of reproductive allocation in an annual herb. Proceedings of the Royal Society B: Biological Sciences 275: 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenäs L, Bisang I.. 2011. The overlooked dwarf males in mosses – unique among green land plants. Perspectives in Plant Ecology Evolution and Systematics 13: 121–135. [Google Scholar]
- Horsley K, Stark LR, McLetchie DN.. 2011. Does the silver moss Bryum argenteum exhibit sex-specific patterns in vegetative growth rate, asexual fitness or prezygotic reproductive investment? Annals of Botany 107: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jules ES, Shaw AJ.. 1994. Adaptation to metal-contaminated soils in populations of the moss, Ceratodon purpureus – vegetative growth and reproductive expression. American Journal of Botany 81: 791–797. [Google Scholar]
- Kassambara A, Mundt F.. 2016. Factoextra: extract and visualize the results of multivariate data analyses https://CRAN.R-project.org/package=factoextra.
- Li Z, Wu N, Liu T, Chen H, Tang M.. 2015. Sex-related responses of Populus cathayana shoots and roots to AM fungi and drought stress. PLos One 10: e0128841. doi:10.1371/journal.pone.0128841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindo Z, Gonzalez A.. 2010. The bryosphere: an integral and influential component of the Earth’s biosphere. Ecosystems 13: 612–627. [Google Scholar]
- Marschall M, Proctor MCF.. 2004. Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Annals of Botany 94: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Dawson T, Ehleringer J.. 1993. Gender-related differences in gas-exchange are not related to host quality in the xylem-tapping mistletoe, Phoradendron juniperinum (Viscaceae). American Journal of Botany 80: 641–645. [Google Scholar]
- McDaniel SF, Willis JH, Shaw AJ.. 2007. A linkage map reveals a complex basis for segregation distortion in an interpopulation cross in the moss Ceratodon purpureus. Genetics 176: 2489–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel SF, Atwood J, Burleigh JG.. 2013. Recurrent evolution of dioecy in bryophytes. Evolution 67: 567–572. [DOI] [PubMed] [Google Scholar]
- McLetchie DN. 1996. Sperm limitation and genetic effects on fecundity in the dioecious liverwort Sphaerocarpos texanus. Sexual Plant Reproduction 9: 87–92. [Google Scholar]
- McLetchie D, Puterbaugh M.. 2000. Population sex ratios, sex-specific clonal traits and tradeoffs among these traits in the liverwort Marchantia inflexa. Oikos 90: 227–237. [Google Scholar]
- McLetchie DN, Stark LR.. 2006. Sporophyte and gametophyte generations differ in their thermotolerance response in the moss Microbryum. Annals of Botany 97: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J, Kollar L, McLetchie D.. 2016. Does selection for gamete dispersal and capture lead to a sex difference in clump water-holding capacity? American Journal of Botany 103: 1449–1457. [DOI] [PubMed] [Google Scholar]
- Norrell TE, Jones KS, Payton AC, McDaniel SF.. 2014. Meiotic sex ratio variation in natural populations of Ceratodon purpureus (Ditrichaceae). American Journal of Botany 101: 1572–1576. [DOI] [PubMed] [Google Scholar]
- Petry WK, Perry KI, Fremgen A, et al. 2013. Mechanisms underlying plant sexual dimorphism in multi-trophic arthropod communities. Ecology 94: 2055–2065. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. 2001. Phenotypic plasticity. Baltimore, MD: John Hopkins University Press. [Google Scholar]
- Proctor MCF. 2000. Physiological ecology In: Shaw AJ, Goffinet B, eds. Bryophyte biology. Cambridge: Cambridge University Press. [Google Scholar]
- Proctor MCF. 2003. Experiments on the effect of different intensities of desiccation on bryophyte survival, using chlorophyll fluorescence as an index of recovery. Journal of Bryology 25: 201–210. [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, et al. 2007. Desiccation-tolerance in bryophytes: a review. Bryologist 110: 595–621. [Google Scholar]
- R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Available at https://www.r-project.org. [Google Scholar]
- Renner S, Ricklefs R.. 1995. Dioecy and its correlates in the flowering plants. American Journal of Botany 82: 596–606. [Google Scholar]
- Rosenstiel TN, Shortlidge EE, Melnychenko AN, Pankow JF, Eppley SM.. 2012. Sex-specific volatile compounds influence microarthropod-mediated fertilization of moss. Nature 481: 431–433. [DOI] [PubMed] [Google Scholar]
- Rydgren K, Økland RH.. 2003. Short-term costs of sexual reproduction in the clonal moss Hylocomium splendens. Bryologist 106: 212–220. [Google Scholar]
- SAS Institute. 2010. JMP for Windows. Release 9.0. Cary, NC: SAS Institute. [Google Scholar]
- SAS Institute. 2015. JMP for Windows. Release 12.0.1. Cary, NC: SAS Institute [Google Scholar]
- Schlichting CD. 1986. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics 17: 667–693. [Google Scholar]
- Shaw AJ, Gaughan JF.. 1993. Control of sex-ratios in haploid populations of the moss, Ceratodon purpureus. American Journal of Botany 80: 584–591. [DOI] [PubMed] [Google Scholar]
- Shaw J. 1986. A new approach to the experimental propagation of bryophytes. Taxon 35: 671–675. [Google Scholar]
- Shaw J, Beer SC.. 1999. Life history variation in gametophyte populations of the moss Ceratodon purpureus (Ditrichaceae). American Journal of Botany 86: 512–521. [PubMed] [Google Scholar]
- Shortlidge EE, Rosenstiel TN, Eppley SM.. 2012. Tolerance to environmental desiccation in moss sperm. New Phytologist 194: 741–750. [DOI] [PubMed] [Google Scholar]
- Stark LR, Brinda JC.. 2015. Developing sporophytes transition from an inducible to a constitutive ecological strategy of desiccation tolerance in the moss Aloina ambigua: effects of desiccation on fitness. Annals of Botany 115: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LR, Nichols L, McLethie DN, Bonine ML.. 2005. Do the sexes of the desert moss Syntrichia caninervis differ in desiccation tolerance? A leaf regeneration assay. International Journal of Plant Sciences 166: 21–29. [Google Scholar]
- Stark LR, Oliver MJ, Mishler BD, McLetchie DN.. 2007. Generational differences in response to desiccation stress in the desert moss Tortula inermis. Annals of Botany 99: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LR, McLetchie DN, Roberts SP.. 2009. Gender differences and a new adult eukaryotic record for upper thermal tolerance in the desert moss Syntrichia caninervis. Journal of Thermal Biology 34: 131–137. [Google Scholar]
- Stark LR, McLetchie DN, Eppley SM.. 2010. Sex ratios and the shy male hypothesis in Bryum argenteum (Bryaceae). Bryologist 113: 788–797. [Google Scholar]
- Stark LR, McLetchie DN, Greenwood JL, Eppley SM.. 2016. Moss antheridia are desiccation tolerant: rehydration dynamics influence sperm release in Bryum argenteum. American Journal of Botany 103: 856–864. [DOI] [PubMed] [Google Scholar]
- Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–542. [DOI] [PubMed] [Google Scholar]
- Teitel Z, Pickup M, Field DL, Barrett SCH.. 2016. The dynamics of resource allocation and costs of reproduction in a sexually dimorphic, wind-pollinated dioecious plant. Plant Biology 18: 98–103. [DOI] [PubMed] [Google Scholar]
- Turetsky MR, Bond-Lamberty B, Euskirchen E, et al. 2012. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytologist 196: 49–67. [DOI] [PubMed] [Google Scholar]
- Varga S. 2010. Review. Effects of arbuscular mycorrhizas on reproductive traits in sexually dimorphic plants. Spanish Journal of Agricultural Research 8: S11–S24. [Google Scholar]
- Varga S, Kytöviita MM.. 2008. Sex-specific responses to mycorrhiza in a dioecious species. American Journal of Botany 95: 1225–1232. [DOI] [PubMed] [Google Scholar]
- Varga S, Vega-Frutis R, Kytöviita MM.. 2013. Transgenerational effects of plant sex and arbuscular mycorrhizal symbiosis. New Phytologist 199: 812–821. [DOI] [PubMed] [Google Scholar]
- Waite M, Sack L.. 2010. How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytologist 185: 156–172. [DOI] [PubMed] [Google Scholar]
- Wang X, Griffin KL.. 2003. Sex-specific physiological and growth responses to elevated atmospheric CO2 in Silene latifolia Poiret. Global Change Biology 9: 612–618. [Google Scholar]
- Wang Z, Liu X, Bao WK.. 2016. Higher photosynthetic capacity and different functional trait scaling relationships in erect bryophytes compared with prostrate species. Oecologia 180: 359–369. [DOI] [PubMed] [Google Scholar]
- Ward JK, Dawson TE, Ehleringer JR.. 2002. Responses of Acer negundo genders to interannual differences in water availability determined from carbon isotope ratios of tree ring cellulose. Tree Physiology 22: 339–346. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Anderson LE.. 1984. Breeding systems in bryophytes In: Dyer AF, Duckett JG, eds. The experimental biology of bryophytes. London: Academic Press, 39–64. [Google Scholar]
- Yang S, Wang B-X, Xu X, Huan H-H, Qin F, Chen M-H.. 2014. Sex-specific responses of flowering phenology and floral morphology of Humulus scandens to drought. Plant Diversity and Resources 36: 653–660. [Google Scholar]
- Yu Q, Ellen ED, Wade MJ, Delph LF.. 2011. Genetic differences among populations in sexual dimorphism: evidence for selection on males in a dioecious plant. Journal of Evolutionary Biology 24: 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrow E. 1934. Experimentelle Studien aber die Anpassung von Wasser-und Sumpfmoosen. Pflanzenborschung 17: 1–70. [Google Scholar]
- Zhang S, Jiang H, Zhao HX, Korpelainen H, Li CY.. 2014. Sexually different physiological responses of Populus cathayana to nitrogen and phosphorus deficiencies. Tree Physiology 34: 343–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.