Abstract
Background and Aims Resin is a defence against herbivores and a floral reward in a few African and South American species whose bee pollinators collect it for nest construction. Here we describe a new role for floral resin from the Asian genus Kadsura (Schisandraceae). Kadsura tepals tightly cover a globe formed by carpels (in females) or near-fused stamens with fleshy connectives (in male flowers of most, but not all species).
Methods We carried out field observations at four sites in China and used pollinator behavioural assays, chemical analyses and time-calibrated insect and plant phylogenies to investigate the specificity of the interactions and their relationship to floral structure.
Key Results Nocturnal resin midges (Resseliella, Cecidomyiidae) walk around on the flowers’ sexual organs to oviposit, thereby transferring pollen and wounding tissues. The larvae then develop in resin-filled chambers. Male and female floral scents are dominated by α-pinene, while the resinous exudate is dominated by caryophyllene. As revealed by barcoding of multiple midge larvae per flower species, the mutualisms are species specific and appear to have evolved over the past 6–9 million years.
Conclusions Resin feeding, not pollen or ovule feeding, by midge larvae explains the abundant Kadsura exudates, highlighting the poorly known world of nocturnal flower–fly interactions.
Keywords: Basal angiosperms, coevolution, phylogenetics, pollination, resin, Diptera, molecular clocks
INTRODUCTION
Pollination mutualisms in which insects and plants trade the rearing of larvae in exchange for pollen transfer are among the most specialized insect–plant interactions known (Svensson et al., 2010). Insects involved in such systems include flies, wasps and moths (for a review, see Hembry and Althoff, 2016). In all these mutualisms, the larvae of the pollinating insects feed on the ovules or developing seeds of the plants, which involves a potentially costly trade-off for the plants between gaining pollination and losing offspring. In a few nursery pollination systems, however, flowers provide spaces in which the pollinators’ larvae develop without access to ovules or seeds. Such ‘nursery chambers’ must persist for a sufficiently long time for the pollinator’s larvae to develop, implying the retention of flowers beyond their sexual function and the provision of some alternative (not pollen or ovule) nutrition for the larvae.
We first described nursery pollination without seed consumption in Asian species of Illicium, a genus in the basal angiosperm family Schisandraceae (Luo et al., 2010), which like most ancient angiosperm lineages is adapted to wet, low-light understorey habits (Feild and Arens, 2005). The investigated species offer nursery chambers for cecidomyiid (Diptera) midges that heat up and stay above ambient temperature even after the sexual function of the bisexual flowers has ceased. The larvae develop between the carpels, feeding on floral exudates, but never ovules. The family Schisandraceae, however, also comprises two genera with unisexual flowers, Kadsura and Schisandra (Saunders, 1998, 2000). Kadsura, with 15–20 species, all in tropical Asia, has flowers with cone-like androecia of poorly understood function because the stamen’s massive connectives leave such minute spaces between adjacent thecae that it is difficult to envisage pollen export (Saunders, 1998; Endress, 2001). In female flowers, the carpels form a compact shield at the periphery of the gynoecium, with the uppermost part of each carpel’s stigma barely emerging (Endress, 2001). In K. longipedunculata, pollen tubes cross between adjacent carpels connected by mucilage (Lyew et al., 2007), and this is also true in S. sphenanthera (Wang et al., 2012). In one species, K. coccinea, the male flowers have conspicuous inner staminodes and massively enlarged inner tepals, again of unknown function.
The pollination biology of only one of the 15–20 species of Kadsura has been investigated. This species, K. longipedunculata, is pollinated by Resseliella kadsurae, a species of resin midge (Cecidomyiidae: Diptera; Yuan et al., 2008; Yukawa et al., 2011 corrected the midges’ initial wrong assignment to Megommata). Photographs prove that the midges of this species carry numerous K. longipedunculata pollen grains on their bodies. The study did not, however, address the function of any secretions, and reported the unusual finding that the midges’ only reward was pollen. Cecidomyiidae are otherwise not known to use pollen as a food source (Yuan et al., 2007, 2008; Gagné and Jaschhof, 2014), and Cecidomyiidae that feed at all as adults have mouthparts that greatly differ from those of Resseliella (Dorchin et al., 2015).
To elucidate the function of Kadsura flowers, we carried out fieldwork on four species in their natural habitats in China, including the previously investigated K. longipedunculata, in addition to three other species chosen to represent the two subgenera, one of which contains only K. coccinea (Saunders, 1998). We used gas chromatography to analyse the floral scent and the exudates, and molecular clock-dated phylogenies for both the plants and their pollinating insects to infer the specificity and geological age of the interactions. Specifically, we set out to answer the following questions. (1) Are species of Kadsura pollinated by midges and are pollinators rewarded in both flower sexes or just in the pollen-producing male flowers (as per Yuan et al., 2008)? (2) Are pollinators species specific and, if so, what is the extent of coevolution between Kadsura and its pollinators? (3) What is the function of the massively fused androecia and gynoecia, the sticky exudates just below cuticles, and the uniquely enlarged inner tepals of K. coccinea?
MATERIALS AND METHODS
Collection of material and study sites
Between 2013 and 2016, we investigated four monoecious species, namely Kadsura coccinea, K. heteroclita, K. longipedunculata and K. oblongifolia (taxonomic authorities for plant species names are given in Supplementary Data Tables S1 and S2). Kadsura has about 20 species, 16 traditionally accepted (Saunders, 1998), two placed in Kadsura by molecular data (Luo et al., 2010), but morphologically placed in Schisandra (as S. plena and S. propinqua; Saunders, 2000), and two described recently, namely S. parapropinqua (Yang and Lin, 2009) and S. macrocarpa (Lin et al., 2011). All species are twining woody vines (Saunders, 1998). Fruits are aggregate clusters of fleshy indehiscent apocarps. Herbarium vouchers and their place of deposition for all investigated species are listed in Table S1, and study locations are mapped in Supplementary Data Fig. S1.
Kadsura coccinea is distributed in southern and south-western China at (200–) 400–1400 (–1900) m altitude and is common in semi-open shrubland and forests. Observations and experiments on K. coccinea were carried out between 27 May and 26 June in 2013, between 10 and 20 June in 2014, and between 13 and 17 June 2015; day and night observations were made between 15 and 20 June 2013 and 2014, and between 14 and 16 June 2015 in Tingjingshan National Forest park (GPS co-ordinates of all study sites are given in Table S1), Guangdong Province. Within this park, we permanently marked 14 individuals near Shangshanbei Village, 15 near Dianshita Road and 12 near Yuandong for observations and as controls. The three ‘populations’ are located in different valleys, about 15 km apart from each other.
Kadsura heteroclita is widely distributed in South-east Asia at 800–2000 m altitude. Day and night observations on K. heteroclita were made in 2015 between 29 July and 2 August, and between 10 and 15 August at Pinganshan Forest Park. Kadsura longipedunculata is distributed in eastern and south-central China at 100–1200 m altitude. Day and night observations on K. longipedunculata were made between 4 and 8 August 2015 at Hengshang Nature Reserve, Hunan. Kadsura oblongifolia is distributed in Guangdong, Guangxi and Hainan Island, where it occurs at 100–900 m altitude. Day and night observations were made between 7 and 15 July 2016 near the city of Danzhou in Hainan.
Flower development, floral exudates and mating system
Flower development was monitored at all study sites, focusing on tepal movements, presence or absence of floral odour, stigma, stamen and tepal secretions, and anther dehiscence. Stigmas were considered to be receptive if they were white and moist. We used 3-(4 5-dimethylthiazol-2-yl)-2 5-diphenyltetrazolium bromide (MTT) to test for the presence of dehydrogenase in the pollen of stamens and on stigmas (Rodriguez-Riaño and Dafni, 2000). The duration of anthesis for a single flower commenced with the unfolding of the first tepal and ended when the last tepal had dropped (for female flowers) or when the entire flower had dropped (males); the floral morphs behaved differently in this respect (see the Results). Local assistants continued to monitor marked flowering plants, taking photos of the floral stages as proof. When observation revealed an absence of nectar, but a sticky exudate at the tepal tips of the inner whorl in K. coccinea after midges had laid eggs in them, we focused on the mode of production of such exudates at all study sites (see ‘Chemical analysis of floral volatiles and resin’).
Controlled pollinations on freshly opened (unvisited) flowers were carried out in K. coccinea at peak flowering, using the following treatments: (1) randomly selected flowers were marked as controls; (2) female flowers were bagged to test for agamospermy; (3) bagged female flowers were pollinated with pollen from male flowers of the same individual to test for self-compatibility; and (4) bagged female flowers were cross-pollinated with pollen from another individual. One-way analysis of variance (ANOVA) F-tests and t-tests were carried out with the statistical package SPSS.
Measurements are reported with means and standard errors throughout.
Midge identification and behaviour experiments
Diurnal and nocturnal observations of flower visitors covered the entire period of anthesis. Types and numbers of visitors, duration of visits and insect behaviour were recorded and filmed. Although the tepals of K. coccinea never fully unfold, and the floral orifice in most species of Kadsura is small, we could see and film the midges’ activities inside flowers. Flowers visited by midges were examined for midge eggs under a stereoscope (Stemi Dv4; Zeiss, Germany), and eggs on androecia/gynoecia, inner tepals and outer tepals were counted. In K. coccinea, we bagged five male and five female flowers at the end of anthesis, and removed either the inner whorl of tepals or the outer whorl to study the effect of such mutilations on midge oviposition and larval survival. Midge eggs and/or larvae in the experimentally treated flowers as well as in controls were counted immediately, and again 2–3 d after the manipulation. In K. heteroclita and K. longipedunculata, eggs and larvae in both floral sexes were counted and observed, but no tepal removal experiments were carried out. In K. oblongifolia, we only recorded egg presence/absence.
Captured midges and stigmas were inspected for pollen under a stereoscope at high magnification, and midges collected from male and female flowers were also studied by scanning electron microscopy (SEM; Jeol JSM-6360LV, Japan). Midges were preserved in 95 % ethanol for later identification, and voucher specimens are in the first author’s collection. Some of the midges collected on 18/20 June 2013 in Tingjingshan National Forest Park on flowers of K. coccinea were sent to R. Gagné, USDA-ARS-Systematic Entomology Laboratory, Beltsville, MD, USA for identification.
A two-choice glass Y-tube olfactometer was used to test if midges were attracted to volatiles released from male and female flowers of K. coccinea. Air was drawn through Teflon tubing by an air pump and passed through a charcoal filter and distilled water, before being introduced into a glass vial containing the odour source. The total flow rate was set to 200 mL min–1, and the Y-shaped glass tube had a stem of 13·5 cm, an arm of 11·0 cm and an inner diameter of 20·0 mm, with an angle of 120 °. The room was kept dark, and the air temperature was monitored and maintained at 23 °C. Each midge was tested independently and was given 4 min to move in the olfactometer. A midge’s choice of the left or right arm of the olfactometer was noted when it went 2 cm past the Y-junction and stayed there for >30 s. Midges that did not reach the decision line after 5 min were removed and recorded as having exhibited ‘no choice’. The treatments were switched between the two arms of the Y-tube every four assays to avoid any influence of possible asymmetries in the set-up. The olfactometer was rinsed with pure ethanol and then dried after each bioassay, and for each test, one fresh open flower was placed into a glass vial and used as the scent source. A vial of identical size was used as a control for supplying clean air. The flower was replaced with a new one every 1–2 h, and tests were replicated until >10 midges had chosen one of the odour sources in the Y-tube.
Chemical analysis of floral volatiles and resin
In 2013, we performed headspace scent collection on K. coccinea flowers at the three study sites. We cut branches with freshly opened male or female flowers and collected the scent of five male and five female flowers directly in the field, using one collection vial per flower. Another ten flowers were brought to a lab, and scent there was collected in the same way. Because female flowers open 30 min after male flowers, scent collections started at 19·00 h in male flowers and at 19·30 h in female flowers, and continued for 2–3 h. We used solid phase microextraction (SPME; Sigma-Aldrich) and SPME fibre assemblies (65 µm PDMS/DVB, Sigma-Aldrich) to adsorb odour from the headspace. Ambient air was collected as a control to identify background contaminants. Scent samples were analysed by thermal desorption by gas chromatography–mass spectrometry (GC-MS), relying on a QP2010 SE GC-MS machine (Shimadzu Corporation, Japan) equipped with a SUPELCOWAX™ 10 column of 30 m × 0·25 mm × 0·25 μm (Supelco Inc., Bellefonte, PA, USA).
To test the chemistry of the resin, we used 100 mg of tepal and pedicel tissue of K. coccinea (dried and finely powdered) and extracted it in 800 μL of dichloromethane containing 5 nmol of ethyl decanoate as an internal standard using a shaker at room temperature for 12 h. The solution was then dried over anhydrous sodium sulphate, and 1 μL of the solution was subjected to GC-MS. The injector temperature was 240 °C. Splitless mode was used with a splitless time of 1 min, and helium was the carrier gas with a velocity of 1·0 mL min–1. The GC oven temperature was 60 °C for 3 min, ramp of 4 °C min–1 to 240 °C and then 240 °C for 20 min. The MS was operated with full scan mode (mass range m/z 40–200). The volatile compounds were tentatively identified by comparison with the commercial NIST spectral mass library (NIST 11; http://chemdata.nist.gov).
DNA extraction, sequence alignment and phylogenetic analyses
We generated new midge and plant phylogenies for this study. For the plant phylogeny, we amplified the nuclear ribosomal DNA region ITS1–5.8S–ITS2 and several plastid regions (trnL intron, trnL-trnF spacer and trnS-trnG), which in combination have proven useful in earlier studies of Schisandraceae (Liu et al., 2006; Morris et al., 2007; Luo et al., 2010). Primers and PCR conditions were as in Morris et al. (2007). Newly generated sequences were submitted to GenBank, and all material along with voucher information (also for the sequences from earlier studies) is shown in Supplementary Data Table S2. Sequence alignment was performed in MAFFT version 7 in the online server (http://mafft.cbrc.jp/alignment/server/) under standard parameters except for the ITS (internal transcribed spacer) region which was aligned under Q-INS-i optimization, which takes rRNA secondary structure into consideration. In the absence of statistically supported incongruence [i.e. maximum likelihood (ML) bootstrap support >75] between the plastid and nuclear data partitions, we concatenated the matrices, which yielded a final matrix of 2413 bp sequenced for 55 of the approx. 91 species of Schisandraceae. The sister group of Schisandraceae is Trimenia, for which no ITS, trnL and trnS-trnG sequences are available. We therefore rooted our trees between Illicium and Kadsura/Schisandra, a sister group relationship found in numerous studies (Hao et al., 2000, 2001; Wikstroem et al., 2001; Liu et al., 2006; Morris et al., 2007; Luo et al., 2010).
For the midge phylogeny, we extracted genomic DNA from ethanol-preserved larvae or adult midges collected from flowers of each Kadsura species. We used the TIANamp Tissue/Kit (Tiangen, Biotech, Beijing, China) and amplified an approx. 460 bp long fragment of the mitochondrial cytochrome oxidase subunit 1 gene (COI) using PCR and primers described by Kawakita and Kato (2006) (5′-ATAATT TTTTTT ATAGT TAT AC-3′ and 5′-ATGGGCTCATACAATAAATCCTA-3′). The PCR products were purified using a QIAquick PCR Purification Kit (Qiagen). Sequencing was performed by the Bejing Genomics Institute (Shenzhen, China) using ABI-3730XL (Applied Biosystems, USA). Alignment of sequences was straightforward and required no gaps. Supplementary Data Table S3 provides specimen information and GenBank accession numbers for all newly sequenced midges. We combined our sequences with additional COI sequences of supertribe Cecidomyiidi from GenBank, most importantly those of Yukawa et al. (2009, 2011). This supertribe has 2395 species in 11 tribes, of which we included representatives of Aphidoletini, Asphondyliini, Cecidomyiini, Lestodiplosini and Lopesiini, with three species of Lasiopteridi (genus Asteromyia) used as the outgroup based on Joy (2013) whose large midge phylogeny includes a species of Resseliella.
Maximum likelihood analyses of plant and midge matrices under the GTR + G model of substitution relied on RAxML v8.0 (Stamatakis et al., 2008), with 100 replicate heuristic searches under the same model as used in the tree searches.
Molecular clock dating of plant and midge phylogenies
Molecular clock dating was performed in BEAST 1.8.4 (Drummond et al., 2012) under an uncorrelated lognormal clock model and the GTR + G model of substitution, using a Yule tree prior, with Markov chain Monte Carlo (MCMC) chain lengths of 10–25 million trees, sampling every 1000th or 5000th generation, with chain length depending on convergence as determined by examining the log files in Tracer v. 1.6 (http://beast.bio.ed.ac.uk/) after removal of an initial burn-in proportion of 10 % of the trees (1000 of 10 000 trees). We made sure that all ESS (effective sample size) values were well above 300 as recommended in the BEAST manual. Trees were studied in FigTree v1.4.3 (http://tree.bio.ed.ac.uk/).
To calibrate the Schisandraceae phylogeny, we used three fossil constraints. First, Schisandra oregonensis seed fossils from the Middle Eocene Clarno Formation of Oregon dated to about 44 million years (Mya; Manchester, 1994), which provide a constraint for the split between the S. glabra clade (S. glabra being the only Schisandra in North America) and the remaining Schisandra species. For this constraint, we used a normal prior distribution with a range of 42–46 My. Fan et al. (2011) scored 13 morphological seed characters for 13 species of Schisandra and seven of Kadsura, and carried out a morphological cladistic analysis that placed S. oregonensis as sister to the Schisandra clade (the numbering of clades I and II in their text and fig. 2 are mixed up), and they used the fossil as a minimum constraint for the Schisandra crown node (see their fig. 2), as did we. Secondly, leaves of Illicium species 1 and 2 from the Middle Eocene Geiseltal flora near Halle, Germany, resemble those of the modern East Asian species I. simonsii, I. lanceolatum, I. henryi, I. dunnianum, I. arborescens and I. verum (Oh et al., 2003) and provide a constraint for the age of the Illicium crown group to 45 Mya. For this constraint, we used a normal prior distribution with a range of 43–47 My. Thirdly, Illicium avitum, known from seven immature fruiting axes from the Brandon Lignite of Vermont (Tiffney and Barghoorn, 1979) dated to the Early Miocene (16·4–23·8 Mya; Cohen et al., 2013), provides a minimum age for the New World Illicium clade. For this constraint, we used a lognormal prior distribution with an offset at 16·4 My and a range back to 23·5 My. Morris et al. (2007) scored 15 Illicium species for characters potentially suitable for placing I. avitum in a cladistic analysis, but could not find a sufficient number of informative characters. They therefore used the fossil in only one of their three clock models, placing it at the same node as done here.
Fig. 2.
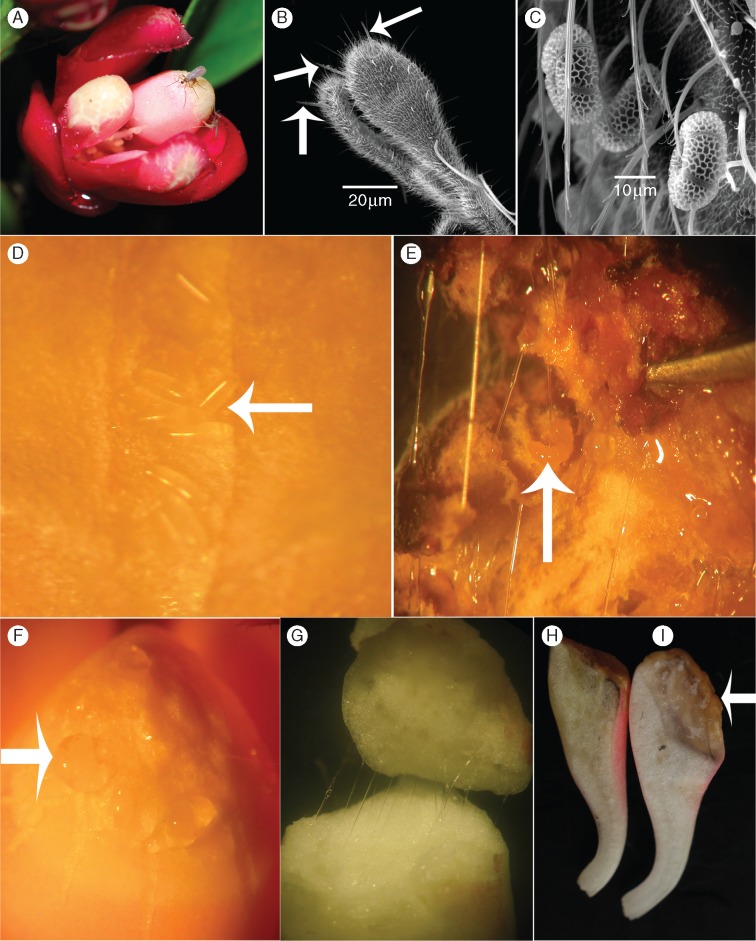
Flowers and pollinators of Kadsura coccinea in Southern China. (A) Two ovipositing Resseliella midges on the massively enlarged inner tepals. (B) Cerci of Resseliella spec_ex_K. coccinea under SEM; the arrows point to three of the four setae on the cerci of this species. (C) Pollen grains of K. coccinea on a midge’s body. (D) Eggs (arrow) of Resseliella on the apical platform of an inner tepal. (E) Longitudinal section of an inner tepal with larvae of Resseliella (arrow) feeding on the secretion. (F) Droplets of resinous exudate on the apex of an inner tepal. (G) The sticky resinous exudate of the inner tepals of first-night flowers. (H) Longitudinal sections through an inner tepal from a bagged flower not visited and wounded by midge larvae. (I) Longitudinal section through an inner tepal from a flower visited and wounded by midges, with the arrow pointing to resin droplets.
To calibrate the midge phylogeny, we removed zero-length branches, which cause problems for Bayesian molecular clock models (Lemmon et al., 2009), and thereby reduced the initial 85 sequence matrix to 47 sequences, leaving each Kadsura pollinator species with just one COI sequence. In the absence of Cecidomyiidae fossils, we assumed a relaxed clock model with a rate of 0·01 % per million years as done in other studies of arthropod COI clock studies, including for Cecidomyiidae (Stireman et al., 2010). The rate was assigned a normal distribution, with mean 0·01 and s.d. of 1·0. Using these settings, we obtained an age of 3·94 My for the Asteromyia laeviana divergence, while Stireman et al. (2010) obtained an age of 3·76 My for the same node. Also, the standard deviations of the rate estimates (ucld.stdev) were low (e.g. 0·5), suggesting that substitution accumulation in this data set is clock like.
RESULTS
Flower function and midge behaviour on Kadsura
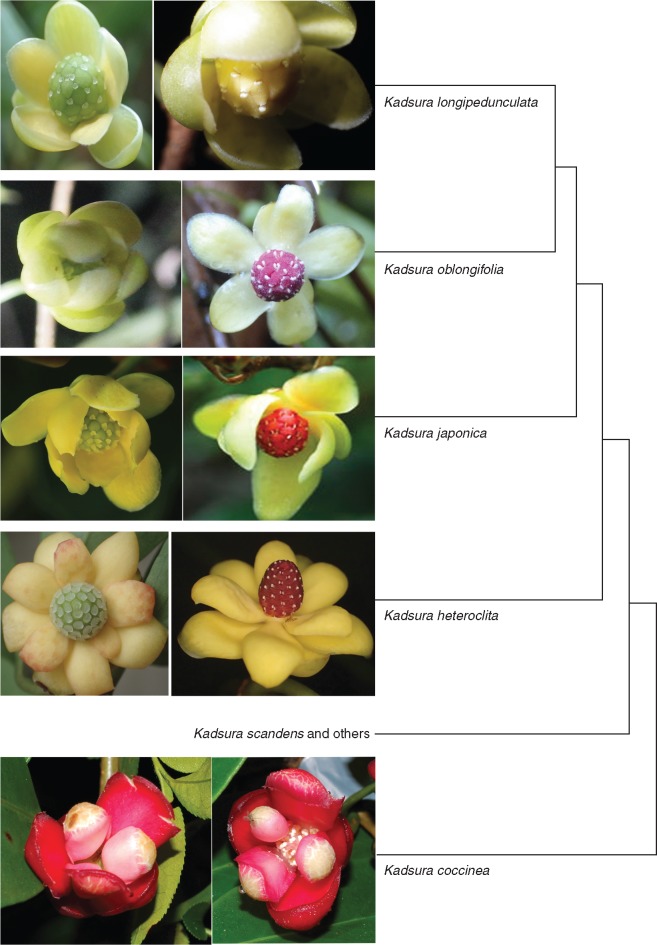
Male and female flowers of the studied species are shown in Fig. 1, and Supplementary Data Movies S1 and S2 showing the pollination of K. coccinea and K. oblongifolia are in the Supplementary Data. Male flowers of K. coccinea opened at sunset (around 19·00 h) and female flowers 30–60 min later (around 19·30–20·00 h; n = 80 of each sex). A strong odour accompanied flower opening. In both floral morphs, the tepals formed a loose chamber around the reproductive organs, and at the onset of anthesis, the flowers’ sexual organs were accessible only through narrow slits between the three huge innermost tepals (Figs 1 and 2A). In male flowers, the anthers dehisced during the afternoon before the flowers open, with the yellow pollen grains then visible at the edges of the pollen sacs. By 06·00 h the following morning, most pollen grains have dropped to the base of the flowers. Based on the MTT test, the stigmas of female flowers were receptive for an average of two nights (n = 10), which matched their visual appearance and moist surface. Open-pollinated flowers of both sexes lasted 5–7 d, but from the third day onwards they had neither pollen to disperse nor receptive stigmatic surfaces, and thus served only as nurseries for their pollinator’s larvae (below).
Fig. 1.
Female flowers (left) and male flowers (right) of the four Kadsura species studied here, with their phylogenetic relationships as inferred in this study (photos by S.-X. Luo, except for those of K. japonica, which are from asianflora.com).
Flowers of K. heteroclita, K. longipedunculata and K. oblongifolia are similar to each other in structure, but not colour (Fig. 1); all emitted a strong odour during the first two nights. In K. heteroclita, male flowers opened at around 22·30 h, and female flowers 30 min later; in K. longipedunculata, male flowers opened at around 21·00 h, and female flowers, 30–60 min later; in K. oblongifolia, both morphs opened at almost the same time, at around 20·00 h.
From the onset of flower opening (K. coccinea 19·00–19·30 h, K. heteroclita 22·30–23·00 h, K. longipedunculata 21·00–22·00 h and K. oblongifolia 20·00 h) and continuing until midnight (01·00–04·00 h), the flowers were visited by resin midges as identified by traditional morphological taxonomic characters (see the Materials and Methods) and by COI sequences matching Resseliella kadsurae sequences deposited in GenBank by Yukawa et al. (2011). Resin midges were the only insects visiting the flowers of the four species, and this was the case at all study sites (mapped in Fig. S1). They squeezed between the tepals into each flower’s centre, then out again, and in K. coccinea they also climbed on the enlarged innermost tepals to lay eggs on their fissured tops (Figs 1 and2A, D; Movie S1). During the second night, when the tepals of K. coccinea were a bit more open, midges still visited both male and female flowers, but did not lay any more eggs.
Female flowers of K. coccinea at anthesis typically contained at least one midge (n = 10), while male flowers typically contained two or three midges (n = 15). The average time midges would spend deep inside female flowers was 18·2 ± 1·94 s (n = 10), while the time spent in male flowers was 5·8 ± 0·71 s. Egg laying on top of the thickened tepals took 27·3 ± 4·94 s in male flowers and 35·2 ± 6·17 s in female flowers (t = 0·999, d.f. = 18, P = 0·33) (Fig. 2B;Movie S1).
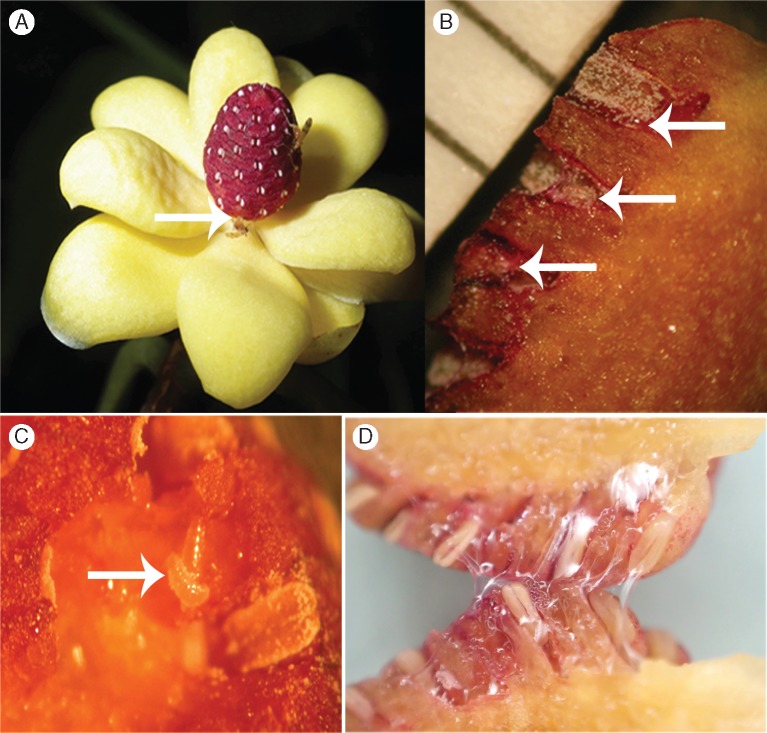
Flowers of K. heteroclita, K. longipedunculata and K. oblongifolia had a similar life cycle to those of K. coccinea, and their Resseliella pollinators had similar visiting behaviours (compare Movies S1 and S2). As soon as the tepals of male and female flowers began spreading, the first midges approached. Male flowers of K. heteroclita and K. oblongifolia usually harboured several midges, while female flowers typically harboured only one midge; in K. longipedunculata, both male and female flowers usually contained one midge. In male flowers, the midges laid eggs in the space between adjacent connectives (Fig. 3A, B), spending around 10–20 s for oviposition; during this process, pollen grains became stuck to them. In female flowers, midges always walked around on the gynoecium, contacting stigmas with their legs and abdomens and thereby transferring pollen. In both floral sexes, the wounding produced by the egg laying or young larvae caused the resin to flow out: in male flowers especially in the anther chambers (Fig. 3B–D); and in female flowers between the carpels and at the tepal bases. All Resseliella midges collected from the flowers were females, and all had Kadsura pollen grains attached to their bodies (Fig. 1C). The number of midge eggs and larvae found in flowers is shown in Table 1, and Supplementary Data Fig. S2 shows a graphic display of the results for K. coccinea.
Fig. 3.
Flowers and pollinators of Kadsura heteroclita. (A) Two Resseliella midges visiting and ovipositing on an androecium. (B) Resseliella eggs (arrows) in the anther chambers, with a millimetre scale to the left. (C) Resin on the connectives and feeding larvae (arrow). (D) Resin in two stamens of a flower in which midges had oviposited.
Table 1.
Resin midge eggs and larvae and the secretory organs in the studied species of Kadsura
| Species | Eggs/flower (n) | Larvae/flower (n) | Secretion organ | Oviposition site | Flowering season |
|---|---|---|---|---|---|
| K. coccinea * | 18·6 ± 1·3 (♂, 15) | 15·4 ± 1·6 (♂, 15) | Thick inner tepals (♂) | On thick inner tepals (♂) | May–June |
| 16·9 ± 1·8 (♀, 10) | 15·8 ± 1·1 (♀, 10) | Thick inner tepals (♀) | On thick inner tepals (♀) | ||
| 1·6 ± 0·7 (♂, 15) | None | Outer tepals | |||
| 2·3 ± 0·9 (♀, 10) | None | Outer tepals | |||
| K. heteroclita | 14·1 ± 3·0 (♂, 10) | 11·5 ± 2·3 (♂, 5) | Connectives (♂) | Space between adjacent connectives (♂) | June–September |
| 14·4 ± 5·2 (♀, 5) | 3 ± 1·2 (♀, 5) | Base of inner tepals (♀) | Between carpels and tepal bases (♀) | ||
| K. longipedunculata | 7·3 ± 2·3 (♂, 10) | 5·7 ± 2·2 (♂, 10) | Connectives (♂) | Space between adjacent connectives (♂) | June–September |
| 7 ± 2·3 (♀, 10) | 2·6 ± 0·8 (♀, 10) | Base of inner tepals (♀) | Between carpels and tepal bases (♀) | ||
| K. oblongifolia | N/A | N/A | Connectives (♂) | ||
| Base of inner tepals (♀) | † | July |
Numbers in parentheses are sample sizes.
means male flower.
means female flowers.
Fig. S2 shows a graphic display of the results for K. coccinea.
Movie S2 shows the ovipositioning sites in K. oblongifolia.
In three of the four species, male flowers abscised as a unit, while in female flowers, the tepals sometimes abscised individually, sometimes as a unit. The larvae continued to develop inside the resin-filled chambers on the forest floor.
Floral volatiles and bioassays on midge reaction to male and female floral scents and experimentally modified flowers
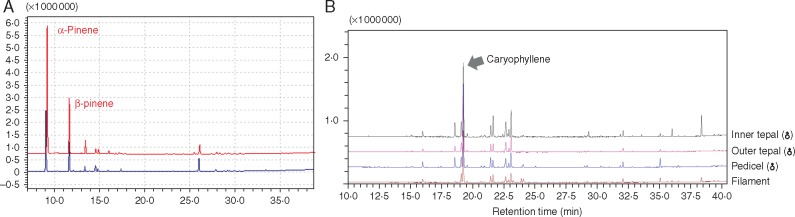
In all four species, the intensity of the floral fragrance gradually increased after the flowers opened, and remained strong for about 4–5 h, whereupon it declined. Little odour was perceptible during daytime. The GC-MS analyses of floral volatiles showed that male and female floral odours are dominated by α-pinene, with the female flowers emitting more α-pinene than the male flowers (Fig. 4A;Table 3).
Fig. 4.
Flower and resin GC-MS analyses. (A) The main chemical compounds in female (red) and male (blue) just-opened flowers of Kadsura coccinea. (B) Main chemical compounds of the resin exudate of K. coccinea. The same analysis for a female flower is shown as Fig. S5. We did not find the same peak in the blank controls.
Table 3.
Tentatively identified major volatile compounds shown in Fig. 4A
| Retention time (min) | Tentatively identified volatile compounds† |
|---|---|
| 9·033 | α-Pinene |
| 11·633 | β-Pinene |
| 13·375 | β-Myrcene |
| 14·542 | 3-Methyl-1-butanol |
| 14·817 | β-Phellandrene |
| 15·950 | 1-Methyl-4-(1-methylethyl)-1,4- cyclohexadiene |
| 16·092 | Ocimene |
| 26·008 | [1R-(1R*,4Z,9S*)]-4,11,11-trimethyl-8-methylene- bicyclo[7·2.0]undec-4-ene |
By comparison with the commercial NIST spectral mass library (NIST 11).
In the Y-tube olfactometer experiment, the Resseliella midges significantly preferred the fragrance of K. coccinea flowers to a clean air control, with 86·4 % (n = 22) and 87·5 % (n = 16) of female midges attracted by virgin (unvisited) male and female flowers, respectively. Trimming of the inner tepals in male and female flowers (Supplementary Data Fig. S3) negatively affected visiting frequencies (0·8 ± 0·25 visits per night, t = 3·56, d.f. = 20, P = 0·001), while trimming of the two outer whorls had no significant effect on visiting frequencies (2·4 ± 0·31 visits per night, t = 0·15, d.f. = 20, P = 0·89), although it significantly reduced the midges’ staying time, egg laying (t = 3·474, d.f. = 44, P = 0·001) and larval survival (Supplementary Data Figs S3 and S4). We observed midge behaviour in a virgin flower for around 3 h and then bagged this flower to study larval development, but no adults emerged, suggesting that larval development takes a long time (see the Discussion).
The production and chemistry of the floral resin exudate
A sticky exudate flowed from the midge-produced wounds in the floral tissues, and it then took about 2–5 h for exudates to cover the midge eggs and the entire apical platform of the innermost tepals (Fig. 2D–I). Transverse sections through the inner tepals of first-night flowers revealed filamentous secretions in their parenchyma (Fig. 2G). Over the 5–6 d during which midge eggs hatched and larvae began to develop, the resin-like exudate continued to accumulate, and the larvae began feeding on it (Fig. 2E). Almost no exudate was produced in bagged flowers (Fig. 2H) that had not been wounded by midges (n = 30; Fig. S2) or in hand-pollinated female flowers (n = 20), and no exudate was found in the outer tepals (n = 50). The GC-MS analyses of the extract from inner tepals, outer tepals and pedicles of K. coccinea showed that the extracts are dominated by caryophyllene (Fig. 4B;Table 4; Supplementary Data Fig. S5).
Table 4.
Tentatively identified major compounds of the resin shown in Fig. 4B
| Retention time (min) | Tentatively identified volatile compounds† |
|---|---|
| 15·972 | Copaene |
| 18·542 | 4,11,11-Trimethyl-8-methylene-bicyclo[7·2.0]undec-4-ene |
| 19·059 | [1S-(1α,2β,4β)]-1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane |
| 19·221 | Caryophyllene |
| 19·535 | (–)-Aristolene |
| 21·415 | Humulene |
| 21·607 | 2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4α,5,6,7-octahydronaphthalene |
| 22·63 | [1S-(1α,7α,8aα)]-1,2,3,5,6,7,8,8α-Octahydro-1,8a-dimethyl-7-(1-methylethenyl)-naphthalene |
| 22·877 | [1S-(1α,2β,4β]-1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane |
| 23·056 | [2R-(2α,4aα,8αβ)]-1,2,3,4,4a,5,6,8α-Octahydro-4α,8-dimethyl-2-(1-methylethenyl)-naphthalene, |
| 24·025 | (1S-cis)-1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene |
| 25·035 | α-Muurolene |
| 28·959 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 29·156 | Cubedol |
| 30·258 | Caryophyllene oxide |
| 31·807 | 3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol |
| 32·031 | 1-Hydroxy-1,7-dimethyl-4-isopropyl-2,7-cyclodecadiene |
| 35·013 | 2-Isopropyl-5-methyl-9-methylene-bicyclo[4·4.0]dec-1-ene |
| 36·466 | α-Cadinol |
| 38·331 | Heneicosane |
By comparison with the commercial NIST spectral mass library (NIST 1).
The mating system of Kadsura coccinea
The experiments on K. coccinea showed that bagged flowers never set fruit (no agamospermy), and that this species is self-compatible (Table 2), with statistically identical fruit set rates for experimental cross- and self-pollination.
Table 2.
Fruit set in natural (resin midge-pollinated) and manipulated flowers of Kadsura coccinea
| Treatment | No. of flowers (n) | No. of fruits | Average apocarp no. per flower (mean ± s.e., range) | Average seed no. per flower (mean ± s.e., range) | Fruit set ratio (mean ± s.e., %) |
|---|---|---|---|---|---|
| Natural pollination | 18 (6) | 9 | 18·7 ± 4·9 (20–56) | 47·9 ± 13·4 (31–136) | 40·9 ± 0·10 |
| Bagged | 15 (6) | 0 | 0 | 0 | 0 |
| Assisted self-pollination | 19 (7) | 16 | 37·4 ± 4·5 (25–60) | 108·5 ± 13·7 (70–180) | 83·9 ±0·09 |
| Assisted cross-pollination | 16 (5) | 15 | 45·7 ± 3·6 (37–63) | 136·9 ± 11·7 (102–204) | 97·3 ± 0·03 |
n = sample size.
Molecular phylogenies for the obligate mutualists in Resseliella and Kadsura
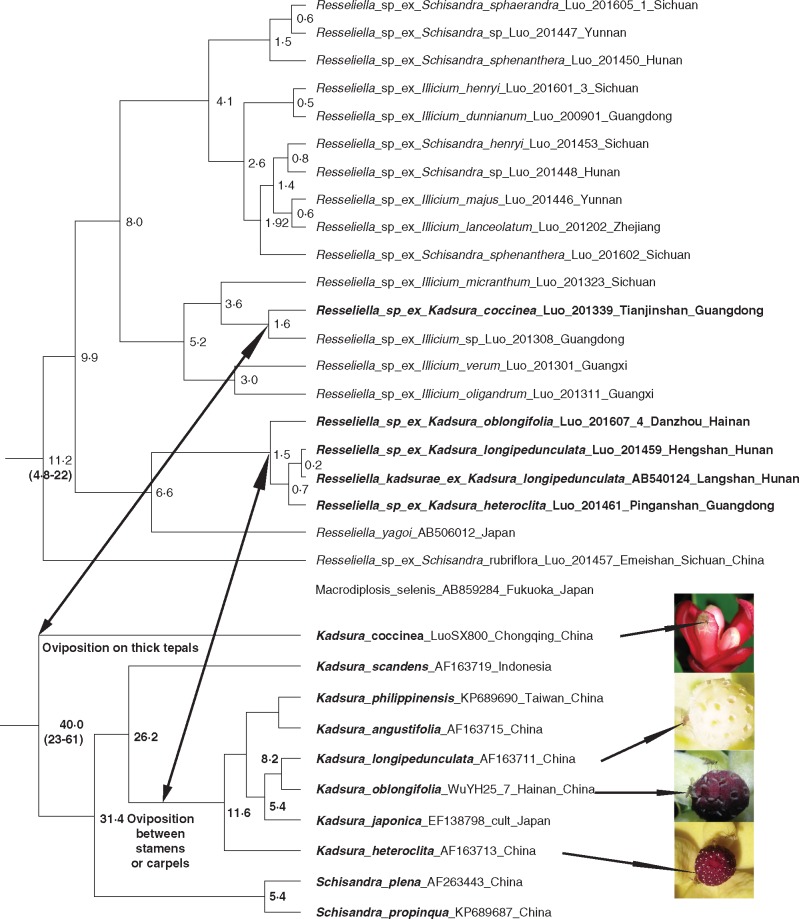
Our plant chronogram includes ten of the approx. 20 species of Kadsura, and with this sampling, K. heteroclita, K. longipedunculata and K. oblongifolia are relatively closely related, while K. coccinea is sister to all remaining species (Fig. 5; Supplementary Data Fig. S6 shows errors around node estimates). We inferred a Kadsura crown age of 40 (23–61) Mya, this being the time of the K. coccinea divergence from the remaining species, and an age of 11·6 (4·8–22) Mya for the clade containing K. heteroclita and its relatives. A midge phylogeny with multiple midge samples per plant species is shown as Supplementary Data Fig. S7, and a chronogram with just one midge species per plant species is shown in Fig. 5 (Supplementary Data Fig. S8 shows errors around node estimates). Each of the studied species of Kadsura has its own Resseliella pollinator, with the midge species that pollinate K. heteroclita, K. longipedunculata and K. oblongifolia forming a clade about 1·5 (0·4–3·7) My old and the midge pollinating K. coccinea belonging to a different group about 1·5 (0·4–3·6) My old (Fig. 5). All these estimates need to be regarded with caution because of the sparse species sampling.
Fig. 5.
Chronograms for the Resseliella midges (top) and the Kadsura species they pollinate (bottom), with 95 % error ranges for the time estimates of nodes discussed in the text. See the Materials and Methods for fossil and rate calibrations, and Figs S6 and S8 for errors around time estimates on nodes with >96 % posterior probability. A phylogeny with multiple midge samples per Kadsura species is shown as Fig. S7.
DISCUSSION
Floral resin exudates as the sole reward in Kadsura
This study set out to resolve the pollination mechanism of Kadsura, its pollinator rewards, the function of the fused androecia and gynoecia, and the function of the massively enlarged inner tepals of K. coccinea. Our results show that species-specific resin midges of the genus Resseliella are the sole pollinators of the studied Kadsura, with each species having its own midge species and with the sole reward being resin-filled larval nurseries in which the larvae develop and feed on the resin. Similar numbers of eggs were laid in male and female flowers, and larvae developed in both morphs.
The genus Resseliella has about 50 species that lay eggs in species from a dozen plant families, half of them Pinaceae and a third Rosaceae (Sanui and Yukawa, 1985; Skuhravá and Skuhravy, 2009; Yukawa et al., 2009). Larvae typically develop in resin under the bark (Sanui and Yukawa, 1985; Yukawa et al., 2009), either pupating inside resin exudates or else leaving and pupating elsewhere, depending on the species (Gagné and Jaschhof, 2014). Resins are lipophilic materials secreted by plants and are a mixture of volatile and non-volatile terpenes, including caryophyllene (Fahn, 1988). Caryophyllene is a main compound of the K. coccinea resin (Fig. 4B;Table 4; Fig. S5) and of other resins, such as that of pine (Ulukanli et al., 2014). The stickiness of the Kadsura exudate is conspicuous (Figs 2E, G and 3D). Prior to this study, floral resin production was only known from a few neotropical Clusiaceae and Orchidaceae, and a genus of Madagascan Euphorbiaceae. In all these cases, it is bee pollinators that use the resins in nest construction (Armbruster, 1984; Bittrich and Amaral, 1997; Porto et al., 2000; Parra-Tabla et al., 2000). No resinous floral exudates were known from any Asian species.
The resin-filled nurseries for midge larvae formed after the midges had wounded a flower’s tissues. The exudate was then produced for 2–6 d after the flowers’ sexual function was over and until tepals (in females) or entire flowers (in males) dropped. At least one species, K. longipedunculata, has thermogenic flowers, but temperatures were only measured from 22·00 h until 18·00 h the next day (Yuan et al., 2008). Midge larvae continue to develop in the dropped floral parts, and in cultivated Illicium verum shrubs, their maturation takes 12 months (Dr Li Su, Guangxi University, pers. comm. to S.X.L., November 2016).
None of the midges was observed feeding on pollen, and their mouthparts did not contain pollen grains. This matches the view of midge taxonomists that adult Resseliella feed on liquids or not at all (Gagné and Jaschhof, 2014; R. Gagné, pers. comm. to S.S.R. of 7 December 2009), and the finding that the few other Cecidomyiidae that do feed as adults have sturdy mouthparts (Dorchin et al., 2015). Another aspect is that sporopollenin pollen walls are indigestible to most insects and must be voided through the anus (Luo et al., 2011).
Coevolution between resin midges and species of Kadsura
The dominant odour component of K. longipedunculata and K. coccinea is α-pinene (Yuan et al., 2008; this study) and, given that larvae of Resseliella develop in resin exudates and feed on resin, these midges may be ancestrally attracted by resin-like scents. (The attractiveness of the scent to these midges was proven by our Y-tube olfactometer experiments.) Resseliella midges are species specific to particular species of Kadsura (Fig. S7), and the interactions between Kadsura and Resseliella have gone on for several million years (Fig. 5). Our molecular clock dating, however, is based on just 460 bp of the COI marker, and therefore needs to be taken with a pinch of salt. Nevertheless, it is clear that the Kadsura Resseliella association is several million years old and probably involved turnover of interacting species (i.e. extinction and speciation, both local and global), which may explain the discrepant plant and midge ages.
Kadsura coccinea is the only species in the entire Illiciales with both inner staminodes and thickened tepals (Fig. 2A, H; Fig. S3A, B), and it has been suggested that it may have retained an ancestral androecial structure (Saunders, 1998). In both the morphological cladistic analysis of Saunders (1998) and in our molecular phylogeny, K. coccinea is sister to the remaining species. An isolated phylogenetic position matches our discovery that K. coccinea is the only species in which some tepals have specialized as exclusive nurseries, forcing midge egg laying away from stamens and carpels.
Conclusion
Our study illustrates a novel pollination mechanism that consists of resin-filled nurseries in flowers that coevolved with resin midges whose larvae develop in, and feed on, resinous exudates. The resin-filled chambers only exist after midge oviposition, and the mutualism is obligate and species specific. The Schisandraceae, with at least 91 species in three genera, are the largest family among the oldest surviving angiosperm lineages (Nymphaeaceae have 70 species), and it is gradually becoming clear that their pollination involves highly specialized mutualisms with nocturnal midges (Yuan et al., 2007, 2008; Luo et al., 2010; Fan et al., 2011; Du et al., 2012). Notably, Kadsura achieves species-specific reliable pollination without paying with nectar, pollen or ovules/seeds.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: herbarium vouchers and study sites; the study locations are also shown in Fig. S1. Table S2: plant material included in this study, with authors of species names, vouchers and their collecting site, natural species range and GenBank accession numbers. Table S3: newly generated midge sequences included in this study with GenBank accession numbers. Figure S1: map of the study sites. Figure S2: number of eggs and larvae on the inner and outer tepals of Kadsura coccinea. Figure S3: flower mutilation experiments in Kadsura coccinea. Figure S4: mean number of midge eggs or larvae per inner tepal of male or female flowers of K. coccinea under two treatments shown in Figure S3. Figure S5: main chemical compounds of the resin exudate of Kadsura coccinea. The same analysis for a male flower is shown as Fig. 4B. Figure S6: Schisandraceae chronogram with error bars around nodes with >96 % posterior probability. Figure S7: maximum likelihood tree showing that all sequenced midge larvae from each Kadsura species have the same COI sequence and probably each belong to a different species. Figure S8: midge chronogram with error bars around nodes with >96 % posterior probability. Movie S1: Resseliella midges visiting and ovipositing in a male flower of Kadsura coccinea filmed by the first author in 2013 at Tingjingshan National Forest. Movie S2: Resseliella midges visiting and ovipositing in a male flower of Kadsura oblongifolia filmed by the first author in 2016 near Danzhou on Hainan.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Raymond J. Gagné (Systematic Entomology Laboratory, US Department of Agriculture) for midge identification in May 2015 (samples deposited in the USDA entomological collections) and again in 2016, from scanning electron micrographs and photos. For help in the field, we thank Ziwei Wang, Lianjie Zhang and Qinggong Mao (South China Botanical Garden), Zhifa Liu and Huagui Peng (Tianjinshan Natural Reserve) and Rongtao Li (Hainan Branch Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences). Martin Silber submitted all sequences, and two anonymous reviewers provided critical comments. This project was supported by grants from the National Science Foundation of China (grant nos 31170217 and 31370268) to S.X.L.
LITERATURE CITED
- Armbruster WS. 1984. The role of resin in angiosperm pollination: ecological and chemical considerations. American Journal of Botany 71: 1149–1160. [Google Scholar]
- Bittrich V, Amaral MCE.. 1997. Flower biology of some Clusia species from Central Amazonia. Kew Bulletin 52: 617–635. [Google Scholar]
- Cohen KM, Finney SC, Gibbard PL, Fan J-X.. 2013. The ICS International Chronostratigraphic Chart. Episodes 36: 199–204. [Google Scholar]
- Dorchin N, Astrin JJ, Bodner L, Harris KM.. 2015. Morphological and molecular revision of the genus Ozirhincus (Diptera: Cecidomyiidae) – long-snouted seed-feeding gall midges on Asteraceae. PLoS One 10: e0130981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A.. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7 Molecular Biology And Evolution 29: 1–12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Huang L-J, Wang X-F.. 2012. Deceit pollination and the effect of deforestation on reproduction in dioecious Schisandra sphenanthera (Schisandraceae) in central China. Journal of Systematics and Evolution 50: 36–44. [Google Scholar]
- Endress PK. 2001. The flowers in extant basal angiosperms and inferences on ancestral flowers. International Journal of Plant Sciences 162: 1111–1140. [Google Scholar]
- Fahn A. 1988. Secretory tissues in vascular plants. New Phytologist 108: 229–257. [DOI] [PubMed] [Google Scholar]
- Fan J-H, Thien LB, Luo Y-B.. 2011. Pollination systems, biogeography, and divergence times of three allopatric species of Schisandra in North America, China, and Japan. Journal of Systematics and Evolution 49: 330–338. [Google Scholar]
- Feild TS, Arens NC.. 2005. Form, function and environments of the early angiosperms: merging extant phylogeny and ecophysiology with fossils. New Phytologist 166: 383–408. [DOI] [PubMed] [Google Scholar]
- Gagné RJ, Jaschhof M.. 2014. A catalog of the Cecidomyiidae (Diptera) of the world, 3rd edn. Digital version 2. http://www.ars.usda.gov/SP2UserFiles/Place/12454900/Gagne_2014_World_Cecidomyiidae_Catalog_3rd_Edition.pdf.
- Hao G, Saunders RMK, Chye ML.. 2000. A phylogenetic analysis of the Illiciaceae based on sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. Plant Systematics and Evolution 223: 81–90. [Google Scholar]
- Hao G, Chye ML, Saunders RMK.. 2001. A phylogenetic analysis of the Schisandraceae based on morphology and nuclear ribosomal ITS sequences. Botanical Journal of the Linnean Society 135: 401–411. [Google Scholar]
- Hembry DH, Althoff DM.. 2016. Diversification and coevolution in brood pollination mutualisms: windows into the role of biotic interactions in generating biological diversity. American Journal of Botany 103: 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy JB. 2013. Symbiosis catalyses niche expansion and diversification. Proceedings of the Royal Society B: Biological Sciences 280: 20122820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita A, Kato M.. 2006. Assessment of the diversity and species specificity of the mutualistic association between Epicephala moths and Glochidion trees. Molecular Ecology 15: 3567–3581. [DOI] [PubMed] [Google Scholar]
- Lemmon AR, Brown JM, Stanger-Hall K, Lemmon EM.. 2009. The effect of ambiguous data on phylogenetic estimates obtained by maximum likelihood and Bayesian inference. Systematic Biology 58: 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Shui Y-M, Yang Z-R.. 2011. Schisandra macrocarpa (Schisandraceae), a new species from Yunnan Province, China. Systematic Botany 36: 595–599. [Google Scholar]
- Liu Z, Hao G, Luo YB, et al. . 2006. Phylogeny and androecial evolution in Schisandraceae, inferred from sequences of nuclear ribosomal DNA ITS and chloroplast DNA trnL-F regions. International Journal of Plant Sciences 167: 539–550. [Google Scholar]
- Luo S-X, Chaw S, Zhang D, Renner SS.. 2010. Flower heating following anthesis and the evolution of gall midge pollination in Schisandraceae. American Journal of Botany 97: 1220–1228. [DOI] [PubMed] [Google Scholar]
- Luo S-X, Li Y-Q, Chen S, Zhang D-X, Renner SS.. 2011. Gelechiidae moths are capable of chemically dissolving the pollen of their Phyllanthaceae hosts. PLoS One 6: e19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyew J, Li Z, Yuan L-C, Luo Y-B, Sage TL.. 2007. Pollen tube growth in association with a dry-type stigmatic transmitting tissue and extragynoecial compitum in the basal angiosperm Kadsura longipedunculata (Schisandraceae). American Journal of Botany 94: 1170–1182. [DOI] [PubMed] [Google Scholar]
- Manchester SR. 1994. Fruits and seeds of the Middle Eocene Nut Beds flora, Clarno Formation, Oregon. Palaeontographica Americana 58: 1–205. [Google Scholar]
- Morris AB, Bell CD, Clayton JW, Judd W, Soltis DE, Soltis PS.. 2007. Phylogeny and divergence time estimation in Illicium with implications for New World biogeography. Systematic Botany 32: 236–249. [Google Scholar]
- Oh I-C, Denk T, Friis EM.. 2003. Evolution of Illicium (Illiciaceae): mapping morphological characters on the molecular tree. Plant Systematics and Evolution 240: 175–209. [Google Scholar]
- Parra-Tabla V, Vargas CF, Magaña-Rueda S, Navarro J.. 2000. Female and male pollination success of Oncidium ascendens Lindey (Orchidaceae) in two contrasting habitat patches: forest vs agricultural field. Biological Conservation 94: 335–340. [Google Scholar]
- Porto ALM, Machado SMF, de Oliviera CMA, Bittrich V, Amaral MCE, Marsaioli AJ.. 2000. Polyisoprenylated benzophenones from Clusia floral resins. Phytochemistry 55: 755–768. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Riaño T, Dafni A.. 2000. A new procedure to assess pollen viability. Sexual Plant Reproduction 12: 241–244. [Google Scholar]
- Sanui T, Yukawa J.. 1985. A new gall midge of the genus Resseliella (Diptera_ Cecidomyiidae) inhabiting resin of the Japanese Cedar, Cryptomeria japonica (Taxodiaceae). Applied Entomological Zoology 20: 27–33. [Google Scholar]
- Saunders RMK. 1998. Monograph of Kadsura (Schisandraceae). Systematic Botany Monographs 54: 1–106. [Google Scholar]
- Saunders RMK. 2000. Monograph of Schisandra (Schisandraceae). Systematic Botany Monographs 58: 1–146. [Google Scholar]
- Skuhravá M, Skuhravy V.. 2009. Species richness of gall midges (Diptera: Cecidomyiidae) in Europe (West Palaearctic): biogeography and coevolution with host plants. Acta Societatis Zoologicae Bohemicae 73: 87–156. [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J.. 2008. A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758–771. [DOI] [PubMed] [Google Scholar]
- Stireman JO, Devlin H, Carr TG, Abbot P.. 2010. Evolutionary diversification of the gall midge genus Asteromyia (Cecidomyiidae) in a multitrophic ecological context. Molecular Phylogenetics and Evolution 54: 194–210. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Okamoto T, Kawakita A, Goto R, Kato M.. 2010. Chemical ecology of obligate pollination mutualisms: testing the ‘private channel’ hypothesis in the Breynia–Epicephala association. New Phytologist 186: 995–1004. [DOI] [PubMed] [Google Scholar]
- Tiffney BH, Barghoorn ES.. 1979. Flora of the Brandon Lignite. IV. Illiciaceae. American Journal of Botany 66: 321–329. [Google Scholar]
- Ulukanli Z, Karabörklü S, Bozok F, et al. . 2014. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chinese Journal of Natural Medicines 12: 901–910. [DOI] [PubMed] [Google Scholar]
- Wang X-F, Armbruster WS, Huang S-Q.. 2012. Extra-gynoecial pollen-tube growth in apocarpous angiosperms is phylogenetically widespread and probably adaptive. New Phytologist 193: 253–260. [DOI] [PubMed] [Google Scholar]
- Wikström N, Savolainen V, Chase MW.. 2001. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society B: Biological Sciences 268: 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZR, Lin Q.. 2009. Schisandra parapropinqua (Schisandraceae), a new species from southwest China. Annales Botanici Fennici 46: 138–142. [Google Scholar]
- Yuan LC, Luo YB, Thien LB, Fan JH, Xu HL, Chen ZD.. 2007. Pollination of Schisandra henryi (Schisandraceae) by female, pollen-eating Megommata species (Cecidomyiidae, Diptera) in South-central China. Annals of Botany 99: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LC, Luo YB, Thien LB, et al. , 2008. Pollination of Kadsura longipedunculata (Schisandraceae), a monoecious basal angiosperm, by female, pollen-eating Megommata sp. (Cecidomyiidae: Diptera) in China. Biological Journal of the Linnean Society 93: 1–12.. [Google Scholar]
- Yukawa J, Sato S, Harris KM, et al. . 2009. A new species of Resseliella (Diptera: Cecidomyiidae), infesting Japanese pear, Pyrus pyrifolia (Rosaceae). Applied Entomology and Zoology 44: 655–666. [Google Scholar]
- Yukawa J, Sato S, Xu H-L, Tokuda M.. 2011. Description of a new species of the genus Resseliella (Diptera: Cecidomyiidae), a pollinator of Kadsura longipedunculata (Schisandraceae) in China, with comments on its flower-visiting habit. Entomological Science 14: 297–303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.