Abstract
Background and Aims Deposition of silica in plant cell walls improves their mechanical properties and helps plants to withstand various stress conditions. Its mechanism is still not understood and silica–cell wall interactions are elusive. The objective of this study was to investigate the effect of silica deposition on the development and structure of sorghum root endodermis and to identify the cell wall components involved in silicification.
Methods Sorghum bicolor seedlings were grown hydroponically with (Si+) or without (Si−) silicon supplementation. Primary roots were used to investigate the transcription of silicon transporters by quantitative RT–PCR. Silica aggregation was induced also under in vitro conditions in detached root segments. The development and architecture of endodermal cell walls were analysed by histochemistry, microscopy and Raman spectroscopy. Water retention capability was compared between silicified and non-silicified roots. Raman spectroscopy analyses of isolated silica aggregates were also carried out.
Key Results Active uptake of silicic acid is provided at the root apex, where silicon transporters Lsi1 and Lsi2 are expressed. The locations of silica aggregation are established during the development of tertiary endodermal cell walls, even in the absence of silicon. Silica aggregation takes place in non-lignified spots in the endodermal cell walls, which progressively accumulate silicic acid, and its condensation initiates at arabinoxylan–ferulic acid complexes. Silicification does not support root water retention capability; however, it decreases root growth inhibition imposed by desiccation.
Conclusion A model is proposed in which the formation of silica aggregates in sorghum roots is predetermined by a modified cell wall architecture and takes place as governed by endodermal development. The interaction with silica is provided by arabinoxylan–ferulic acid complexes and interferes with further deposition of lignin. Due to contrasting hydrophobicity, silicification and lignification do not represent functionally equivalent modifications of plant cell walls.
Keywords: Arabinoxylan, cell wall, ferulic acid, lignin, Raman spectroscopy, root endodermis, silica, silicification, silicon, Sorghum bicolor
INTRODUCTION
Plant cell walls constitute a complex compartment that determines the mechanics of plant organs, forms conduits for water and solutes, and establishes a repository for toxic substances (Evert, 2006). By miscellaneous structural modifications the cell walls can additionally adopt highly specialized functions providing efficient adaptations to distinct environments and conditions (Houston et al., 2016). In grasses (Poaceae), the impregnation of cell walls with silica particles is a typical outcome of silicon supplementation that improves mechanical properties of their tissues and correlates with increased tolerance of various biotic and abiotic stresses (Ma, 2004; Hodson et al., 2005; Cooke and Leishman, 2011; Liang et al., 2015). Grasses are silicon-accumulating plants where the content of silicon per dry weight can reach 10 % (Epstein, 1999). Because these plants can exhibit severe growth defects under Si deprivation, Si can be considered as an essential element for them (Ma et al., 2006; Detmann et al., 2012). However, the metabolism and functions of silicon in plants are still not well understood (Exley, 2015; Liang et al., 2015).
Silicic acid is the main source of silicon taken up by roots, either passively or actively, involving silicon transporters (Richmond and Sussman, 2003; Ma and Yamaji, 2006). The passive uptake of silicic acid is conducted together with water through extracellular spaces and is restricted by the apoplasmic barriers located in the exodermis and endodermis of the roots (Aston and Jones, 1976; Ma et al., 2011). In silicon-accumulating species a cooperative system of silicon transporters is used to cross these apoplasmic barriers and allows plants to collect much higher concentration of silicon than is present in the substrate (Ma and Yamaji, 2015; Yamaji et al., 2015). Subsequently, silicic acid is transferred by conductive tissues, where it may reach concentrations far exceeding its solubility limit (Casey et al., 2003; Mitani et al., 2005). A significant portion of silicic acid is distributed to shoot epidermal tissues, where it is mineralized into amorphous hydrated silica (SiO2·nH2O) (Richmond and Sussman, 2003). As silica deposition predominates in the aboveground organs and correlates with their transpiration rates, water evaporation and dehydration are considered to be the key factors responsible for silica formation (Rudall et al., 2014; Trembath-Reichert et al., 2015). On the other hand, grass cell walls contain several unique components, such as arabinoxylans, mixed-linkage glucans and ferulic acid, that are often assumed to participate in silica deposition (Perry et al., 1987; Inanaga and Osaka, 1995; Fry et al., 2008; He et al., 2015; Zhang et al., 2015; Guerriero et al., 2016). These constituents govern some of the wall’s mechanical properties, but their roles in silicification are not clear.
Sorghum bicolor is a drought-tolerant crop that in addition to leaf silicification exhibits extensive formation of silica aggregates in the root endodermis (Sangster and Parry, 1976a, b, c). Silicification in sorghum roots is often viewed as an adaptation to drought, but the underlying mechanism is elusive (Sangster, 1978; Lux et al., 2002). Supplementation with silicic acid was correlated with improved root viscoelastic properties, hydraulic conductivity and drought resistance, but the link between these silicon-induced changes and endodermal silicification is not known (Hattori et al., 2003, 2005; Liu et al., 2014, 2015).
In this work we studied silicification as a complex process involving the uptake, distribution and deposition of silicic acid, and investigated its effect on cell wall development and composition. We selected silicification in sorghum roots as a model system in order to exclude the role of transpiration. Moreover, we tested the effect of endodermal silicification on water retention capability in roots exposed to desiccation to understand better the role of silica aggregates in the drought tolerance of sorghum plants.
MATERIALS AND METHODS
Plant material, pre-cultivation and standardized controlled conditions
Experiments were performed with seedlings of Sorghum bicolor ‘Gadambalia’. Standard pre-cultivation consisted of 15 min grain sterilization with 2·5 % sodium hypochlorite, rewashing the grains with distilled water, and 24 h imbibition in distilled water. Grains of similar morphology were selected and germinated for 72 h in a dark chamber, at 25 °C and 60 % humidity.
For standard 3-d cultivation, developed seedlings with similar morphology were selected and used for experiments. Seedlings were grown in hydroponic media containing (1) distilled water only (Si−) or (2) distilled water containing sodium silicate (Na2O(SiO2)x·xH2O) at a final concentration of 1 mmol dm−3 (Si+). The final pH of hydroponic solutions was adjusted to 5·8.
For extended 10-d cultivation, the seedlings were grown in (1) ½ Hoagland hydroponic solution only (HS/Si − treatment), or (2) ½ Hoagland hydroponic solution containing sodium silicate solution at a final concentration of 1 mmol dm−3 (HS/Si+ treatment). The pH of hydroponic solutions was adjusted to 5·8. The hydroponic solutions were changed on the fourth and seventh days of cultivation.
Cultivation was performed under standardized controlled conditions in a growth chamber with photoperiod 16 h : 8 h (light : dark), with photosynthetically active radiation (PAR) of ∼200 μmol m−2 s−1, at 25 °C : 18 °C (light : dark) and 70 % air humidity.
Preparation of peeled-off segments and longitudinal observation of silica aggregates and inner tangential cell walls
Peeled-off root segments were used in some experiments and to observe directly silica aggregates and the inner tangential cell walls (ITCWs) of the endodermis (referred to as the ‘longitudinal view’). For their preparation, the rhizodermis and outer cortical tissues of primary roots were mechanically removed to expose the inner surface of the inner tangential and anticlinal cell walls of the endodermis. The peeled-off root segments thus consisted of stele covered by endodermal ITCWs and parts of anticlinal cell walls (Supplementary Data Fig. S1) (Lux et al., 2002).
Quantitative RT–PCR analysis of silicic acid transporters
Samples were collected after standard 3-d cultivation with Si+ or Si − treatment from primary root regions 0–4 cm from the tip (apex) and 1–5 cm from the root–shoot junction (base). Lateral roots were removed and the samples were frozen in liquid nitrogen and homogenized with a pestle and mortar. Total RNA was extracted with a GeneJET™ RNA Purification Kit (Thermo Scientific, USA) and DNase treatment was carried out with a DNA-free™ kit (Thermo Scientific, USA) following the manufacturer’s instructions. Reverse transcription was performed with M-MuLV reverse transcriptase (Thermo Scientific, USA) and random hexamer primers. Primers for SbLsi1, SbLsi2 and SbLsi6 (Table 1) were designed according to corresponding homologous sequences in maize. Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, USA) together with 400 nm of each primer was used for 25 μL of real-time PCR reaction performed with an ABI Prism 7500 Sequence Detection System (Applied Biosystems, USA). Each PCR reaction was replicated three times, including no-template controls. Results were analysed with 7500 System software (Applied Biosystems, USA) and are presented as 2-ΔCt (ΔCt represents the difference between the threshold cycle values (Ct) of the target genes and the reference gene for actin).
Table 1.
List of primers used in quantitative RT-PCR analysis
| Gene ID | Gene | Primer |
|---|---|---|
| EF373651.1 | SbNIP2.1 (SbLsi1) | F 5′-AGCTCTCCTCCTTCAAGCTC-3′ R 5′-GATCATGAGCACCGACACAC-3′ |
| XM_002465971.1 | Uncharacterized (SbLsi2) | F 5′-TCCTCAAGAGCTTCGCCTAC-3′ R 5′-GAAGAAGACGAGCAGCGAGT-3′ |
| EF408053.1 | SbNIP2.2 (SbLsi6) | F 5′-GCTCTCCTCCTTCAAGCTCC-3′ R 5′-CTAGCTCTCTCCCACCACCT-3′ |
| XM_002456645.1 | Actin | F 5′-CTGGAATGGTCAAGGCAGGT-3′ R 5′-TCCATGTCATCCCAGTTGCC-3′ |
F, forward; R, reverse.
Transfer of silicic acid to endodermal cells
After 3 d of cultivation in Si − solution, seedlings with primary roots ∼15 cm in length were selected. The rhizodermis and outer cortical tissues were mechanically removed from primary roots in the region 6–8 cm from the root–shoot junction (peeled-off region; see Supplementary Data Fig. S1). Each seedling was then arranged according to the diagram in Supplementary Data Fig. S2. The peeled-off regions overlapped joint borders of two rectangular boxes containing either (1) ½ Hoagland hydroponic solution only (HS/Si − treatment) or (2) ½ Hoagland hydroponic solution containing sodium silicate solution at a final concentration of 1 mmol dm−3 (HS/Si+ treatment). The seedlings were oriented either with root apices in HS/Si+ and root bases in HS/Si − solution (Si+/apex), or inversely (Si+/base). In order to prevent desiccation of the peeled-off regions, they were covered with wet paper roll (without contact with the hydroponic solutions). After 24 h, the samples were collected from three primary root regions: (1) root apex (from the root tip to the peeled-off region); (2) the peeled-off region; and (3) the root base (from the peeled-off region to the root–shoot junction).
Sequential analysis of ITCW development
After extended 10-d cultivation, the apical parts of primary roots (0–15 cm from the root tip) were segmented into ten regions 1.5 cm in length and used for microscopy analysis as hand cross-sections or as peeled-off segments for direct observation of silica aggregates.
In vitro cultivation of detached root segments
Seedlings were grown for 3 d in the Si − treatment. Subsequently, primary root segments 1·5 cm in length were detached sequentially from the region 0–15 cm from the root tip (total primary root length was ∼15 cm). Lateral roots emerging from the root segments were removed mechanically. Corresponding segments obtained from ten plants were placed in separate Erlenmeyer flasks with 50 mL of ½ Hoagland solution containing 2 % sucrose and sodium silicate solution at a final concentration of 1 mmol dm−3. Segments were cultured for 72 h in darkness at 25 °C, with continuous shaking at 60 rpm. Subsequently, the segments were used for microscopy analysis as hand cross-sections or as peeled segments for direct observation of silica aggregates. Additionally, the formation of silica aggregates in primary root segments with removed rhizodermis and outer cortex was tested. Seedlings were grown for 3 d in the Si − treatment. Subsequently, two types of root segments were prepared from the region 1–3 cm from the root–shoot junction: (1) segments from which the rhizodermis and the cortical tissues, including the outer parts of endodermis, had been mechanically removed (peeled-off segments); and (2) segments with preserved rhizodermis and cortical tissues (intact segments). Segments were obtained from ten plants and cultured as described above.
Digestion of roots with sulphuric acid
Seedling were grown for 3 d in the Si+ treatment and then root segments were collected from the primary root region 2–3 cm from the root–shoot junction. For scanning electron microscopy, the segments were placed on a glass slide and a drop of 96 % sulphuric acid was added. The samples were then warmed to 50 °C and gently rewashed in distilled water until oxidized organic material had been removed. For Raman analyses the root segments were placed in centrifuge tubes filled with 96 % sulphuric acid for 10 or 60 min. Some segments were after the 60 min dissolution with sulphuric acid additionally rinsed with 1 m NaOH. All segments were afterwards washed with distilled water and analysed. Polymerized sodium silicate was prepared from Na2O(SiO2)x·xH2O stock solution, air-dried at room temperature for 24 h.
Raman microspectroscopy
Seedlings were grown for 3 d in the Si+ or Si − treatment. Subsequently, hand cross-sections were prepared from the following primary root regions: (1) RL5 % (region at 5 % of the total primary root length measured from the root tip); (2) RL25 %; (3) RL50 %; and (4) RL75 %. Several cross-sections of each region were separately stained with phloroglucinol–HCl (PHG) in order to check the lignification of ITCWs. Isolated silica aggregates were collected from Si+ plants as described above. Individual samples were placed on microscopy slides, mounted in distilled water, covered with coverslips and sealed with nail polish to avoid water evaporation. Raman spectra were collected with an InVia spectrophotometer (Renishaw, UK) equipped with a 532 nm laser. Measurements were performed with 45 mW laser power, 0.1 s acquisition time and 300 accumulations per measurement. For the analysis of endodermal development, at least five spectra per region collected from each root were used. Samples were collected from four different roots. Spectral processing was performed with WIRE3.2 software (Renishaw, UK). The assignments of Raman bands used in spectral analysis are listed in Table 2.
Table 2.
List of Raman band assignments indicated with relative intensities (w, weak; m, medium; s, strong; vs, very strong). For references, see footnote
| Silica | Arabinoxylan | Cellulose | Ferulic acid | Coniferyl alcohol | Assignment | References |
|---|---|---|---|---|---|---|
| 1650–1740 w/m | 1650–1740 w/m | C=O stretching | 12, 15 | |||
| 1656 m | C=C of coniferyl and syringyl alcohols | 6, 7, 8 | ||||
| 1630 vs | C=C of phenolic esters | 7, 8, 9, 11, 13 | ||||
| 1602 vs | 1602 vs | Aromatic C–C stretch | 6, 7, 8, 9, 11, 13 | |||
| 1462 m | 1456 m | CH/CH2 wagging; CH2; COH | 1, 2, 4, 5, 11 | |||
| 1426 m | 1426 m | CH3 bend; aryl-OCH3 | 11, 13 | |||
| 1380 m | CH2 | 1, 2, 4 | ||||
| 1369 m | CH/CH2 wagging; OH; CH2 | 4, 5, 11 | ||||
| 1314 m | OH/CH; CH/CH2 wagging, CH | 4, 5, 11 | ||||
| 1271 m | 1271 m | Aromatic ring deformation; C–O(H) | 13 | |||
| 1210 w/m | 1210 w/m | Aryl-O(H); aryl-OCH3; aromatic ring deformation | 6, 7, 8 | |||
| 1172 m | 1172 m | Ring plane deformation CH, C–O(H) stretching | 5, 9, 13 | |||
| 1121 s | 1121 s | COC glycosidic, symmetrical | 5, 11 | |||
| 1093 s | 1093 s | COC glycosidic, asymmetrical | 5, 11 | |||
| 1091 s | Si–O–Si responsive to polymerization degree | 3, 10 | ||||
| 985 m | Si–OH symmetrical stretch | 3 | ||||
| 980 m | C–O stretch and ring mode region | 1, 2, 5 | ||||
| 900 m | 900 m | COC in plane; HCC and HCO bending | 11 | |||
| 855 w | C–O–C skeletal mode of α-anomers | 4, 12 | ||||
| 760–840 w | Si–O–Si bending | 3 | ||||
| 557 s | Si–O–Si in hydrated SiO2 | 10 | ||||
| 529 w | Skeletal aromatic ring deformation | 2, 6 | ||||
| 520 w | (C–H) | 1, 2 | ||||
| 480–490 s | Si–O–Si (amorphous SiO2) | 3, 10, 14 | ||||
| 494 s | (COC) glycosidic | 4, 11 | ||||
| 378 m | (CCC) ring; CCO stretching | 1, 4, 5 |
1Agarwal and Ralph (1997), 2Agarwal (2014), 3Aguiar et al. (2009), 4Gierlinger et al. (2008), 5Himmelsbach and Akin (1998), 6Larsen and Barsberg (2010), 7Lupoi and Smith (2012), 8Lupoi et al. (2015), 9Ma et al. (2014), 10McMillan and Remmele (1986), 11Piot et al. (2001), 12Prats Mateu et al. (2016), 13Ram et al. (2003), 14Scholz et al. (1997), 15Sebastian et al. (2009).
Light and fluorescence microscopy
Alkali-induced fluorescence (AIF), used for the visualization of silica aggregates (Soukup et al., 2014) and lignified cell walls (based on Harris and Hartley, 1976), was performed with 0·1 mm NaOH mounting solution. Lignin staining was performed also with PHG (Soukup, 2014) and with 1 mm neutral red (NR) at pH 5·8 (Dubrovsky et al., 2006). Visualization of suberin lamellae was carried out by staining with 0·01 % fluorol yellow 088 in lactic acid for 30 min. Fluorescence antifading solution (0·1 % FeCl3 in 50 % glycerol) was added afterwards (Brundrett et al., 1991; Lux et al., 2005). Visualization of cell wall polysaccharides was performed according to Johnsen and Krause (2014) with an 0·1 % aqueous solution of Congo red (CR) for 5 min. The samples were then washed with 0.1 m NaCl and observed. Microscopy observations were performed with a fluorescence microscope (Axioskop 2 Plus, Carl Zeiss, Germany). For fluorescence microscopy, filter set 25 (excitation filter TBP 400 + 495 + 570 nm, beamsplitter TFT 410 + 505 + 585 nm and emission filter TBP 460 + 530 + 610 nm) was used.
Scanning electron microscopy and energy-dispersive X-ray analysis
Primary roots digested with sulphuric acid (described above) were placed on a carbon stand, dried in EM-DSC 10 (JEOL, Japan) and coated with carbon vapour in a JFC-1100 machine (JEOL, Japan). Observations were performed with a JXA-840A scanning electron microscope (JEOL, Japan) with accelerating voltage 15 kV. The elemental composition of the observed samples with emphasis on silicon was determined by energy-dispersive X-ray analysis performed with a Delta III (Kevex, USA) analyser coupled with the previously mentioned electron microscope. The analysed spectra were obtained in the range of 0·1–10.1 keV collected for 100 s.
Water retention capability of primary roots
After standard pre-cultivation, seedlings were grown with primary root apices submerged in hydroponic media and the basal parts of the roots (0–4 cm from the root–shoot junction) were exposed to air during the entire experiment (Supplementary Data Fig. S3). Hydroponic media contained (1) distilled water only (Si − /AE) or (2) distilled water containing sodium silicate at a final concentration of 1 mmol dm−3 (Si+/AE). The final pH of the solutions was adjusted to 5·8. Cultivation lasted 3 d and was performed under standardized controlled conditions. Root water retention capability was determined as the rate of water evaporation from the surface of primary roots grown exposed to air. Measurements were performed with a CIRAS-2 system (PP Systems, UK) attached to an infrared gas analyser 10 min after the roots were placed in the measuring system. Root apices were kept submerged in distilled water during the entire measurement. Five roots per treatment were used for analyses.
Digital image analysis and statistics
Digital documentation of the microscopy images was carried out with a DP72 digital camera (Olympus, Japan). Morphological characteristics of silica aggregates were assessed by the analysis of either all detected or minimally 1500 aggregates observed in ten primary roots or segments per treatment, performed with Lucia image analysis software (v. 4.80, 2002, Laboratory Imaging, Czech Republic). Root elongation was determined as the primary root length difference between the beginning and end of treatments.
All data were analysed by one-way analysis of variance (ANOVA; Statgraphics Centurion XV, v. 15.2.05, StatPoint) and are presented as the mean ± s.d. or s.e.
RESULTS
Silicic acid uptake and transfer to root endodermis
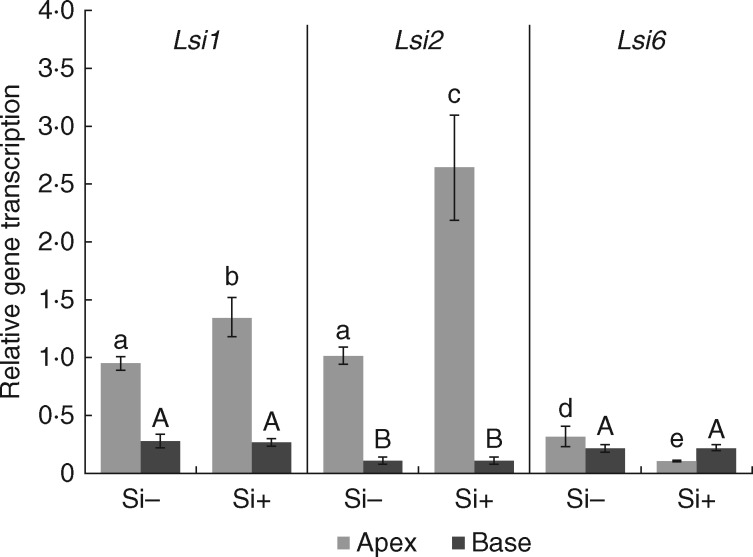
The transcription of silicon transporters SbLsi1, SbLsi2 and SbLsi6 in the apical and basal parts of primary roots was investigated by quantitative RT-PCR in seedlings grown with or without silicon supplementation. In both treatments, the transcription of silicon transporter genes dominated in the apical part of roots. In response to silicon supplementation, SbLsi1 and SbLsi2 were upregulated by 42·7 and 160·2 %, respectively. In contrast, the transcription of SbLsi6 was markedly lower compared with SbLsi1 and SbLsi2, and was downregulated by silicon treatment to 33·6 % (Fig. 1). In the root base region (active in the formation of silica aggregates), the transcription of silicon transporter genes was significantly lower and did not respond to silicon treatment.
Fig. 1.
Real-time PCR analysis of silicon transporters Lsi1, Lsi2 and Lsi6 in sorghum primary roots. Samples were collected from regions 0–4 cm from the root tip (apex) and 1–5 cm from the root–shoot junction (base) of plants grown with (Si+) or without (Si−) silicon supplementation. Results are presented as 2-ΔCt ± s.d. Different letters above columns represent statistically significant differences: ANOVA, LSD test; P < 0·05.
To investigate the pattern in which silicic acid is supplied to the endodermis in actively silicifying regions of primary root, silicon was provided only to the apical parts and the symplasmic continuum of root cortex was mechanically interrupted (Si+/apex treatment; Supplementary Data Fig. S2). After 24 h, the average diameter of aggregates in the basal part of root reached 4.82 ±0·96 μm. When only the basal part of the primary root and emerging lateral roots were exposed to the silicon source (Si+/base treatment; Supplementary Data Fig. S2), the average diameter of aggregates in the basal part of root was 5·94 ±1.54 μm. No aggregates were found in the apical part of roots, where the silicon transporters are mostly transcribed (Fig. 1), or in the peeled-off regions in any of the tested treatments.
Dynamics of endodermal ITCW development
The development of endodermal ITCWs in primary roots was observed in hydroponically grown plants and in detached primary root segments cultivated in vitro. In hydroponically grown plants the ontogenesis of root endodermal cells displayed a position effect, e.g. the progression of distinctive stages of endodermal development initiated in cells adjacent to the phloem poles of vascular tissues, continued in cells corresponding to xylem poles, and lastly occurred in passage cells. This pattern was maintained in the development of the suberin lamellae and lignified tertiary cell walls, and also in the formation of silica aggregates. Silica aggregation was observed only in cells with developed suberin lamellae and occurred simultaneously with the formation of tertiary ITCWs (Supplementary Data Fig. S4). The aggregates were usually localized along the junctions of adjacent pericycle cells and their average diameters correlated positively with the age of endodermal cells.
The in vitro cultivation of detached root segments confirmed that silica aggregation occurs only in endodermal cells with developed suberin lamellae and tertiary cell walls, as it was observed in hydroponic experiments with intact plants. Moreover, it indicated that silica aggregation occurs even if root integrity and the water transpiration stream were not preserved. The distribution of aggregates on the endodermal ITCWs was regular, but their diameters tended to decrease with the thickness of ITCWs (Supplementary Data Fig. S5). In segments where the root cortex was peeled off, no aggregates were observed.
Raman microspectroscopy of endodermal ITCWs
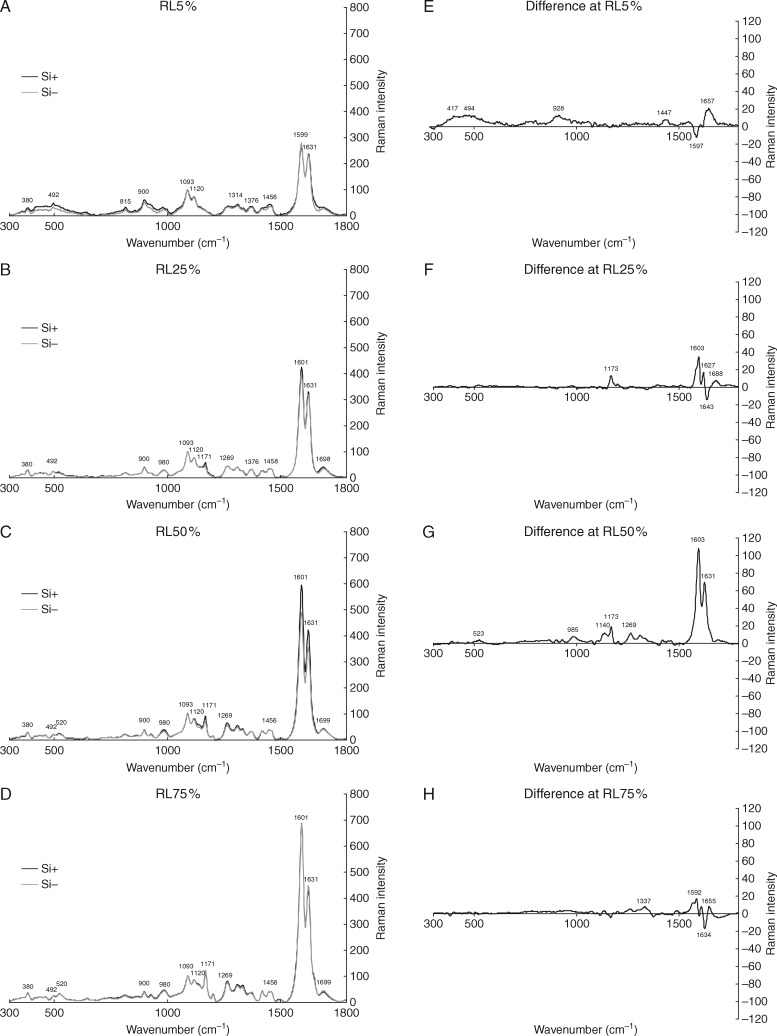
The formation of silica aggregates in detached root segments indicated that silicification is concerted rather by ITCW architecture than by systemic silicon transport. Raman microspectroscopy was thus used to investigate the composition of endodermal ITCWs during development and to identify structural changes in response to silicon supplementation.
Only minor differences were detected between Si+ and Si − treatments, without any notable qualitative transformation caused by Si supplementation (Fig. 2). Quantitative differences between treatments were observed mostly in spectral regions assigned to C=O stretching (1670–1760 cm−1), to –OCH3 and –OH groups conjugated to aromatic rings (1140–1190 cm−1) and to phenolic compounds (1550–1670 cm−1) (Fig. 2A–D). The differences were most pronounced in region RL50 %, where the spectra collected from ITCWs in the Si+ treatment indicated earlier onset of lignification, manifested by the coniferyl alcohol signal occurring as a shoulder at 1650–1660 cm−1. The result was supported by PHG staining (Supplementary Data Fig. S6).
Fig. 2.
Raman spectra of the ITCWs of endodermis in sorghum primary roots (A–D) and corresponding differences between Si+ and Si− treatments for each region (E–H). Investigated regions were located at the distances 5, 25, 50 and 75 % of the total root length measured from the root tip (RL5 %, RL25 %, RL50 % and RL75 %, respectively). Spectra presented in (A–D) were normalized against the cellulose peak at 1093 cm−1.
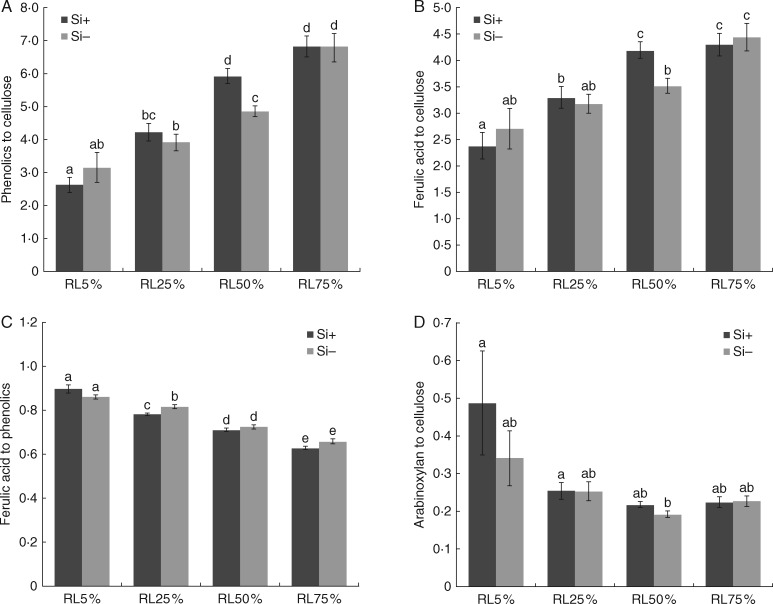
Relative contributions of selected cell wall components to ITCW composition are shown in Fig. 3. In both treatments, the content of phenolic compounds (1601 cm−1) progressively increased throughout the development of ITCWs, with a prominent contribution of ferulic acid (1631 cm−1). The ratio of arabinoxylan (492 cm−1) to cellulose was highest in the RL5 % region, declined at RL25 %, and remained almost constant in the later stages of development. In the Si+ treatment, the difference between region RL25 % (no aggregates formed yet) and region RL50 % (aggregates were already present) was predominantly attributable to ITCW lignification (Supplementary Data Fig. S7).
Fig. 3.
Relative contributions of cell wall constituents to the composition of ITCWs of sorghum root endodermis in plants grown with (Si+) or without (Si−) silicon supplementation. Phenolics were detected at 1601 cm−1 (A), ferulic acid at 1631 cm−1 (B, C) and arabinoxylan at 492 cm−1 (D). Data represent the relative intensity of individual component-specific peaks to the cellulose peak at 1093 cm−1 and are presented as mean ± s.e. Different letters above columns represent statistically significant differences; ANOVA, LSD test; P < 0·05.
Histochemical analyses of endodermal ITCWs
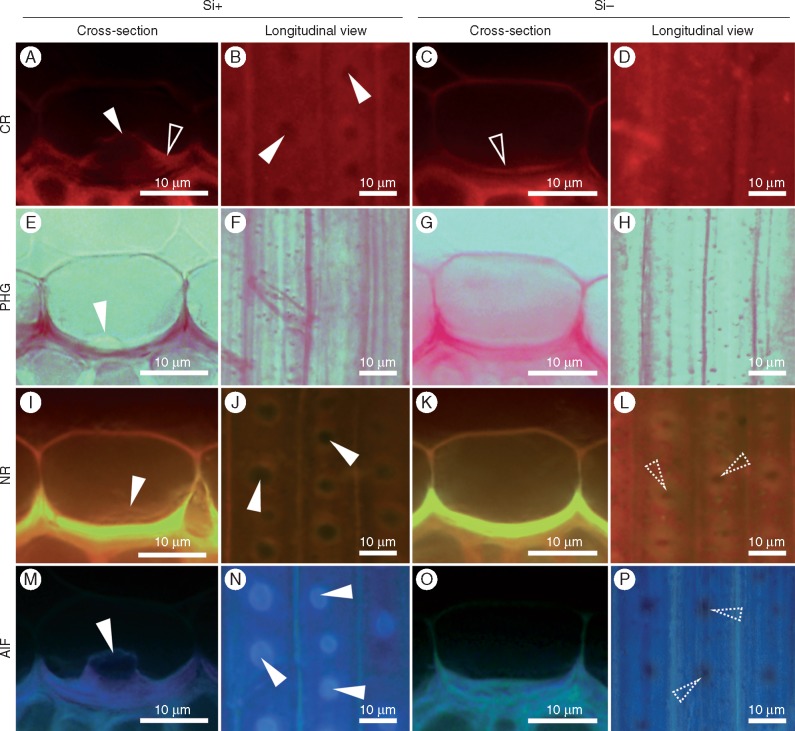
The composition of mature endodermal ITCWs was further investigated by CR staining (polysaccharides), NR staining (lignin/hydrophobic substances), the PHG reaction (lignin) and AIF (silica aggregates, lignin/ferulic acid). The CR staining showed the presence of a polysaccharide-rich layer localized on the lumen-oriented side of the tertiary ITCWs (Fig. 4A, C). Whereas in the Si+ treatment the layer appeared partially disturbed by the tips of silica aggregates (Fig. 4B), in the Si − treatment it covered the entire inner surface of ITCWs (Fig. 4D). The ITCWs exhibited intense lignification in cross-sections of samples from both Si+ and Si − treatments. Direct observation of the exposed ITCWs stained with PHG showed no or an inconclusive reaction (Fig. 4F, H), most likely due to the presence of a non-lignified polysaccharide-rich layer at the inner surface of ITCWs. Nonetheless, NR staining revealed a non-homogeneous distribution of lignin in the ITCWs. In the Si+ treatment, non-lignified spots overlapped with silica aggregate positions and in the Si − treatment they retained a distribution identical to that observed in the Si+ treatment (Fig. 4J, L). The longitudinal view of the AIF-treated samples in the Si+ treatment showed clearly the distribution of silica aggregates within the ITCWs (Fig. 4N). In the Si − treatment AIF revealed the presence of non-fluorescent spots exhibiting the same arrangement as observed by NR, indicating the absence of lignin in these positions (Fig. 4P).
Fig. 4.
Histochemical analysis of the ITCWs of endodermis in sorghum primary roots grown with (Si+) or without (Si−) silicon supplementation. Cross-sections and exposed surfaces of the ITCWs of endodermis (vertical panel labelled Longitudinal view) were investigated by CR staining for polysaccharides, the PHG reaction for lignin, NR staining for lignin and hydrophobic substances, and by the use of AIF for lignin and silica aggregates. Solid arrowheads indicate silica aggregates, open arrowheads indicate the polysaccharide-rich layer, and dotted arrowheads indicate non-lignified spots in the ITCWs of the endodermis.
Analyses of silica aggregates
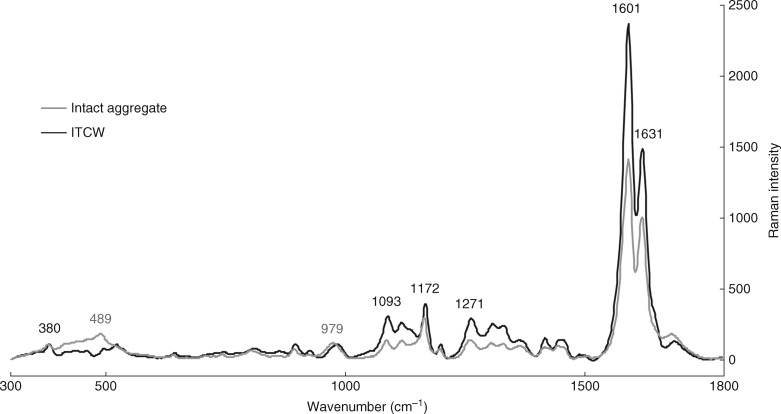
To study the silica aggregate composition, Raman spectra were collected from aggregates in the intact state, i.e. directly from the exposed ITCWs or cross-section samples. The aggregate spectra collected in the RL75 % region exhibited several differences from spectra acquired from the ITCWs in the corresponding root region of the Si+ or Si − treatment (Fig. 5). In particular, the intact aggregates showed an increased signal in the C=O stretching region (1670–1760 cm−1), an increased ratio of phenolic compounds to cellulose and a higher contribution of ferulic acid to phenolic compounds. The most notable difference was observed in the arabinoxylan/hemicellulose region at 400–500 cm−1, exhibiting a peak at 489 cm−1. Additionally, a peak shift was observed at 986 → 979 cm−1, accompanied by increased signal intensity relative to cellulose.
Fig. 5.
Representative Raman spectra of intact silica aggregate and ITCW of endodermis in sorghum primary roots grown in silicon-supplemented hydroponic solution. Spectra are normalized against 380 cm−1.
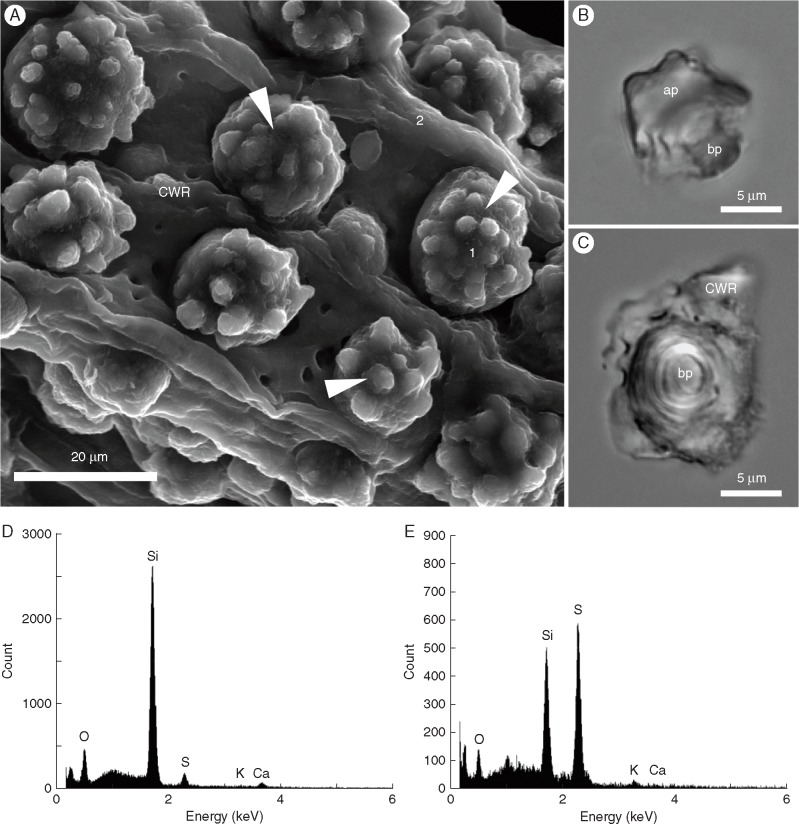
Digestion of primary roots with sulphuric acid removed most of the organic material and yielded a compact ITCW residue with attached silica aggregates and several aggregates isolated separately (Fig. 6A–C). Under light microscopy investigation the isolated aggregates exhibited a lamellar structure, recognizable mostly in their basal portion. The distribution of silicon was determined by energy-dispersive X-ray analysis and confirmed its abundance within the silica aggregates as well as in the ITCW residue, though a much lower signal was detected in the latter (Fig. 6D, E).
Fig. 6.
Remains of sorghum primary roots after mineralization with sulphuric acid. (A) Scanning electron micrograph of silica aggregates (arrowheads) attached to a residue of ITCWs (CWR). (B, C) Light micrographs of isolated silica aggregates showing the apical (ap) and basal (bp) portions of aggregates from side (B) and bottom (C) views. (D, E) Energy-dispersive X-ray analysis of aggregates (D) and cell wall residue (E) indicated in (A) with the numbers 1 and 2, respectively.
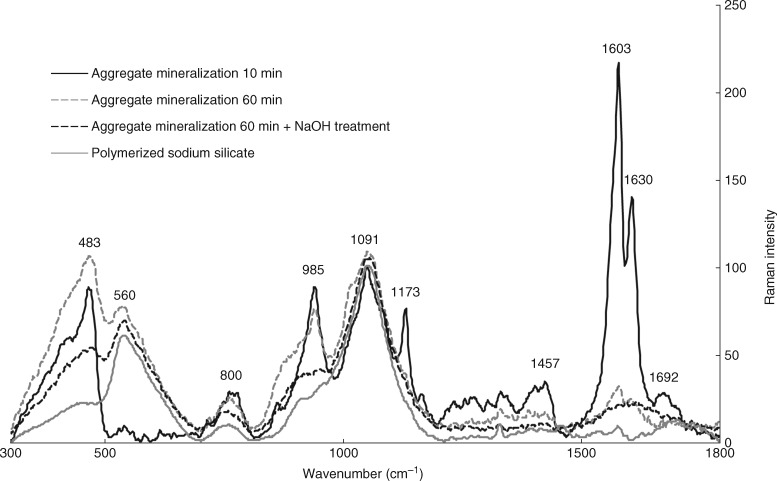
Raman analysis of aggregates isolated by 10-min sulphuric acid digestion showed residual signals of arabinoxylan/hemicellulose and ferulic acid (Fig. 7). In addition, a peak shift 489 → 483 cm−1 was detected in comparison with spectra collected from an intact aggregate. Prolonged 60-min sulphuric acid digestion eliminated the ferulic acid signal but did not affect the signal of arabinoxylan/hemicelluloses any further (Fig. 7). In samples digested with sulphuric acid for 60 min and washed in 1 m NaOH, the arabinoxylan/hemicellulose signal declined and the spectra obtained were almost identical to those collected from polymerized sodium silicate (Fig. 7).
Fig. 7.
Raman spectra of polymerized sodium silicate and of silica aggregates isolated by distinctive procedures. Basal parts of sorghum primary roots grown in silicon-supplemented solution were collected and treated with 96 % sulphuric acid for 10 min (aggregate mineralization 10 min), 60 min (aggregate mineralization 60 min) or 60 min, followed by rinsing in 1 mm NaOH (aggregate mineralization 60 min + NaOH treatment). Spectra were normalized against the peak located in region 1085 − 1095 cm−1.
Silica aggregation in roots is enhanced by drying but does not support water retention
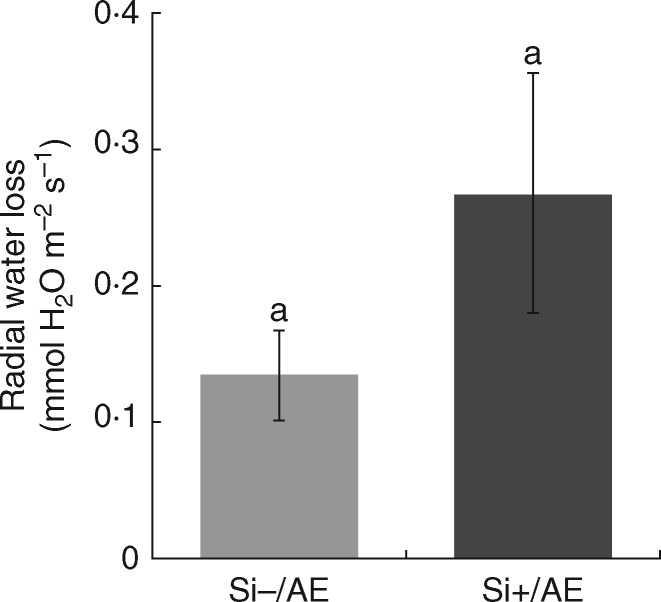
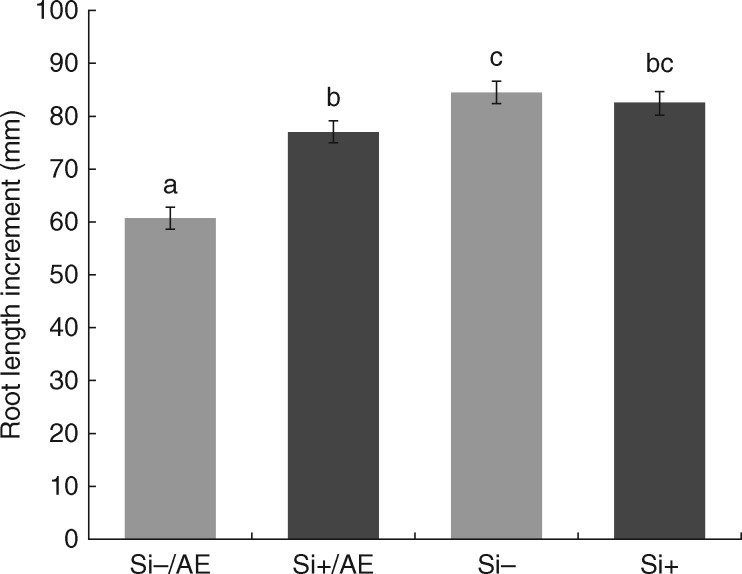
To investigate the role of endodermal silicification in the water retention capability of sorghum primary roots, seedlings were grown with root bases exposed to air and root apices submerged in hydroponic media (Supplementary Data Fig. S3) with or without silicon supply (Si+/AE and Si − /AE, respectively). The rate of water evaporation measured in the Si+/AE treatment did not show a significant difference from that in the Si − /AE treatment, but indicated rather the opposite trend (Fig. 8), i.e. reducing the ability of roots to block water leakage. Despite this result, the Si+/AE treatment exhibited significantly lower growth reduction due to root base desiccation in comparison with Si − /AE (Fig. 9). Moreover, in the air-exposed parts of Si+/AE primary roots the diameter of silica aggregates increased to 11·72 ± 2·03 µm, in comparison with 9·55 ± 1·99 µm in roots grown entirely submerged in Si+ medium (data are mean ± s.d.; P < 0·05).
Fig. 8.
Rate of radial water loss in sorghum primary root regions exposed to air-drying. Plants were grown with root apices submerged in distilled water with (Si+/AE) or without (Si−/AE) silicon supplementation. Data are presented as mean ± s.e. The same letters above columns indicate no statistically significant difference between treatments; ANOVA, LSD test; P < 0·005.
Fig. 9.
Root length increment in sorghum primary roots grown with root bases exposed to air and root apices submerged in distilled water with (Si+/AE) or without (Si−/AE) silicon supplementation, and in roots grown entirely submerged in distilled water with (Si+) or without (Si−) siicon supplementation. Data are presented as mean ± s.e. Different letters above the columns represent statistically significant differences between the treatments; ANOVA, LSD test; P < 0·05.
DISCUSSION
Because silicic acid is prone to following the movement of water, its uptake by living systems is almost unavoidable. Evaporation or selective uptake of water from the apoplast then leads to silicic acid condensation, and silica precipitation can occur. Inability to control the content and translocation of silicic acid might thus result in its uncontrolled precipitation on membranes or biomolecules and impair their functionality. Therefore, the evolutionary point of view suggests that controlled silicification evolved as a protective mechanism against the toxicity of silicic acid (Evered and O’Connor, 1986; Exley, 2009). Early mechanisms for its safe detoxification thus continually evolved into a complex adaptation providing many important benefits to silicon-accumulating plants (Hodson et al., 2005; Strömberg et al., 2016).
Silicon supply to sorghum root endodermis
The response of root silicic acid transporters to silicon supplementation differs even within the group of silicon-accumulating plants (Ma and Yamaji, 2015). Whereas in rice the expression of Lsi1 is downregulated, in maize, barley and wheat no effect of silicon supplementation was observed. On the other hand, the expression of Lsi2 was downregulated by silicon treatment in all these species. In sorghum, we observed upregulated transcription of both Lsi1 and Lsi2 transporters, which we consider to be a necessary step in providing sufficient silicon input for silica aggregation in the sorghum root endodermis. In support of this, we reported recently a positive correlation between the content of silicic acid in the substrate and the sizes of silica aggregates in sorghum roots (Soukup et al., 2014). Our results confirmed that silicic acid is actively absorbed in the root apex, is transferred basipetally via the central cylinder, and enters the aggregation sites in the endodermis by outward movement from the central cylinder, as proposed by Lux et al. (2003).
Dynamics of endodermal ITCW development
The development of the sorghum root endodermis followed a pattern typical of monocotyledonous species, including the formation of Casparian bands (data not shown), the deposition of suberin lamellae (considered as the secondary cell wall) and an asymmetrical deposition of a thick lignocellulosic tertiary wall on the inner tangential side of the endodermal cells (White, 2001; Enstone et al., 2003). Our results further confirmed that silica aggregation in silicon-supplied plants appeared simultaneously with the formation of tertiary ITCWs, as suggested by Sangster and Parry (1976b) and Hodson and Sangster (1989). Throughout the entire development of endodermal ITCWs, we observed a progressive increase in the content of phenolic compounds, especially ferulic acid. In grasses, ferulic acid serves to tether cell wall arabinoxylans in order to improve the stiffness of growing cell walls (Kamisaka et al., 1990). Moreover, it establishes binding sites for subsequent deposition of lignin (Jacquet et al., 1995; Ralph et al., 1995). In sorghum roots, we detected earlier onset of endodermal lignification in response to silicon supplementation. Similar results were observed in rice (Fleck et al., 2011) and maize (Lukačová et al., 2013).
Specific roles of cell-wall components in the formation of silica aggregates
The participation of non-cellulosic polysaccharides in the silicification of plant cell walls was identified in several plant species (Perry et al., 1987; Gierlinger et al., 2008; Currie and Perry, 2009; Law and Exley, 2011; Leroux et al., 2013; He et al., 2015). In grasses, arabinoxylans and mixed-linkage glucans are the predominant non-cellulosic cell wall polysaccharides forming the grass cell wall matrix (Carpita, 1996; Fry et al., 2008; Kozlova et al., 2014; Mikkelsen et al., 2015). Enhanced deposition of arabinose and xylose during the initial stage of silicification in lemma hairs of Phalaris canariensis (Perry et al., 1987) suggests their direct involvement in silica deposition. In the later stage of hair silicification, the synthesis of arabinose and xylose declined, whereas the synthesis of mixed-linkage glucans was enhanced and affected the structure of subsequently deposited silica. However, the mixed-linkage glucans seem not to be responsible for silicification, as their absence did not stop the deposition of silica in rice leaves but instead resulted in its ectopic distribution (Kido et al., 2015).
Several studies reported that cell wall phenolics might contribute to silicification as well (Scurfield et al., 1974; Inanaga and Okasaka, 1995; Fang and Ma, 2006; Zhang et al., 2013; Fleck et al., 2015). Ferulic acid is one of the main phenolic substances in grass cell walls (Harris and Hartley, 1976; Buanafina, 2009), and it is capable of interaction with silica surfaces through several functional groups (Kulik et al., 2009). Moreover, in view of the fact that ferulic acid is the main source of cell wall autofluorescence in grasses (Lichtenthaler and Schweiger, 1998), the fluorescent properties of grass silica phytoliths (Dabney et al., 2016), including sorghum root aggregates (Soukup et al., 2014), suggest its participation in silica deposition. Ferulic acid binds to arabinoxylans (Ishii, 1997; Buanafina, 2009) and anchors coniferyl alcohol, which initiates the deposition of lignin in grasses (Jacquet et al., 1995; Ralph et al., 1995; Grabber et al., 2002; Barros et al., 2015). Since silica aggregation sites were predetermined by a local absence of lignification, we propose a model for silica aggregate formation that involves both arabinoxylan and ferulic acid.
As the movement of silicic acid follows that of water, their apoplasmic routes can be modified or blocked by hydrophobic substances, such as lignin or suberin. The non-homogeneous deposition of lignin in the ITCWs can thus restrict and concentrate the flow of silicic acid from the central cylinder directly to the silicification sites. The non-lignified silicification sites in the ITCWs contain arabinoxylan–ferulic acid complexes that exhibit a high density of lone electron pairs (ester bond, aromatic ring, hydroxyl groups). Such negative dipoles can interact with the hydroxyl groups of silicic acid and facilitate its condensation to silica. Our model thus predicts that arabinoxylan–ferulic acid complexes serve as nucleation sites for silicification and remain trapped inside the aggregates. We supported this prediction by showing that the aggregates contain ferulic acid and hemicelluloses by Raman and fluorescence microscopy. Since the endodermal ITCWs constitute the silicification sites independently of the presence of silicon, they are able to trap and polymerize silicic acid even if supplied at later stages of development (Lux et al., 2003) (Supplementary Data Fig. S5).
To complement the model, we are suggesting also a role for mixed-linkage glucans. This group of polysaccharides forms a cell wall matrix that embeds arabinoxylans (Kozlova et al., 2014; Mikkelsen et al., 2015). Regarding the fact that mixed-linkage glucans are clearly not responsible for silicification (Kido et al., 2015), we suggest that their role is to prevent the ectopic interaction between silicic acid and arabinoxylan–ferulic acid complexes. Consequently, mixed-linkage glucans allow the movement of silicic acid through non-lignified portions of cell walls without spontaneous precipitation. The organized distribution of mixed-linkage glucans and lignin can thus regulate the apoplasmic movement of silicic acid and establish specific sites for silica deposition.
Silica–lignin cross-talk in plant cell walls
Plants supplied with silicon can often exhibit alterations in the quantitative composition of organic cell wall constituents (Zhang et al., 2015). A structural trade-off between silica and lignin was reported in rice (Suzuki et al., 2012; Yamamoto et al., 2012) and in a number of wetland species (Schoelynck et al., 2010). As the deposition of silica has 20-fold lower metabolic costs than lignification whilst providing comparable compression resistance, silicification is at least to some extent preferable (Raven, 1983). So far, the mechanism of silica–lignin trade-off is not clear, but the interaction between silicic acid and arabinoxylan–ferulic acid complexes suggests a model explaining this phenomenon. As the silicic acid binds to the arabinoxylan–ferulic acid complex and condensates, steric modification of the complex occurs and this can hinder further attachment of monolignols to ferulic acid.
Regarding the silica-lignin trade-off, it is also necessary to consider the contrasting properties of these two components in their relationship to water. Whereas the nature of silica is predominantly hydrophilic, lignin exhibits a hydrophobic character. Silicification of sorghum roots was suggested to improve their water retention capability (Sangster and Parry, 1976a, b, c; Lux et al., 2002; Hattori et al., 2005). However, the rate of water loss from the air-exposed roots indicated that the silicification of the endodermis did not support its function as a water leakage barrier. On the other hand, silicon supplementation reduced the root growth inhibition imposed by desiccation.
The physiological roles of lignin and silica cannot be considered as identical and their trade-off must be investigated in more detail. Even though only minor differences in the ratio between silica and lignin contents occur, changes in their distribution in specific tissues can markedly affect the balance of water movement in the plant.
Conclusions
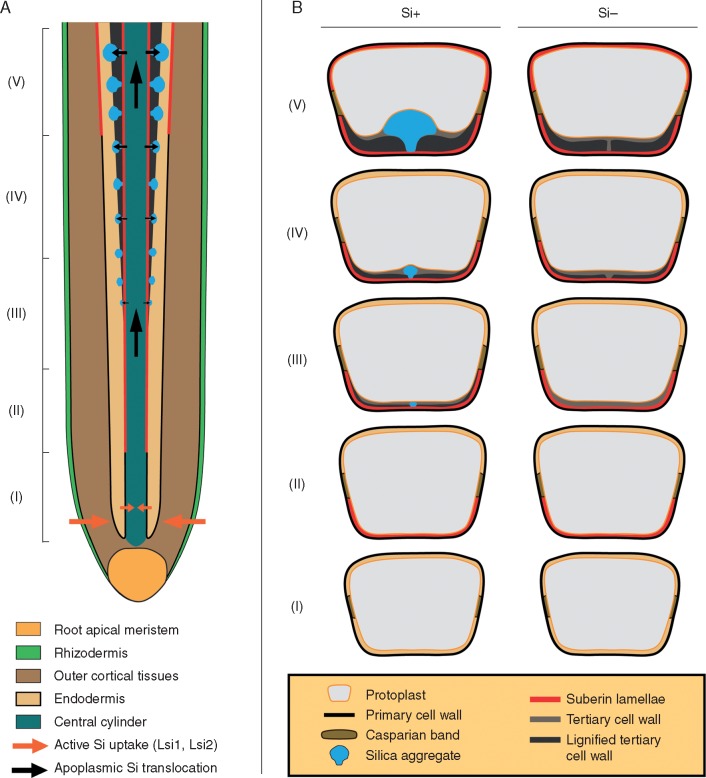
We propose a model of silica aggregate formation in sorghum roots, controlled by the architecture and development of endodermal cell walls (Fig. 10). Silica aggregation sites are established during the development of tertiary endodermal cell walls by non-homogeneous lignification. As the movement of silicic acid follows that of water, their flow is directed through the non-lignified spots in the cell wall, where silicic acid accumulates and condensates at arabinoxylan–ferulic acid complexes. Since the aggregation sites are established independently of the presence of silicon, the formation of silica aggregates in sorghum roots exhibits the attributes of a genetically predetermined adaptation.
Fig. 10.
Proposed model of silica aggregate formation in Sorghum bicolor root endodermis. (A) Diagram of longitudinal primary root section showing silicic acid uptake and translocation associated with the formation of silica aggregates at the inner tangential walls of the endodermis. Silicic acid is taken up in the apical part of the primary root and transferred basipetally through the central cylinder. In the basal part of the root, silicic acid enters the endodermis by outward radial flow. (B) Cross-section diagrams of individual endodermal cells depicted according to the gradual ontogenetic development observed in plants grown with (Si+) or without (Si−) silicon supplementation. (I) Endodermal cell with primary cell wall and Casparian band. (II) Suberin lamellae are deposited at the inner tangential wall. (III) Formation of tertiary inner tangential wall initiates. In the Si+ treatment it is already accompanied by non-homogeneous deposition of lignin with a preserved non-lignified spot predetermined for silica aggregation. As the silicic acid enters the non-lignified cell wall matrix, its condensation and polymerization start to occur at arabinoxylan–ferulic acid complexes. (IV) Development of tertiary cell wall continues. In the Si+ treatment the silica aggregate grows in the non-lignified portion of the cell wall. In the Si− treatment the delayed lignification of tertiary cell wall initiates, following a pattern identical to that observed in the Si+ treatment. (V) Final stage of endodermal development. Suberin lamellae are deposited around the entire cell wall. Silica aggregate is incorporated within the tertiary wall and protrudes towards the cell lumen. In the Si− treatment the non-lignified spot is preserved, allowing the formation of silica aggregates after silicon supplementation is provided even in the later stages of development.
Therefore, we suggest that the specific organization of cell wall components can direct the apoplasmic movement of silicic acid and establish the locations favouring silica deposition. Our results indicate that silica–cell wall interaction in grasses can be provided by arabinoxylan–ferulic acid complexes. This interaction implies that silicification can hinder further deposition of lignin and explain the mechanism of the structural trade-off between these two components. Even though silica and lignin can provide comparable stiffness, they cannot be considered as structural equivalents due to contrasting hydrophobicity. On the other hand, their specific distribution in cell walls can thus affect the balance of water movement in plant tissues and help to sustain adverse environmental conditions by more sophisticated means.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: cross-section diagram of intact primary root (left panel) and of peeled-off root segment with removed rhizodermis and outer cortical tissues (right panel). Figure S2: experimental setup to investigate silicic acid uptake and transfer to silica aggregation sites in the endodermis. Figure S3: experimental setup to investigate the effects of root base desiccation on silica aggregation in root endodermis. Figure S4: dynamics of endodermal development in the extended 10-d hydroponic cultivation in HS/Si+ treatment. Figure S5: dynamics of endodermal development in detached root segments cultivated in vitro. Figure S6: phloroglucinol–HCl staining of primary root samples collected in distinct regions along the root axis. Figure S7: Raman spectra representing the structural difference between the inner tangential walls of primary root endodermal cells in regions RL50 % and RL25 % of Si+ treated plants.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank to Dr Notburga Gierlinger and Dr Batirtze Prats Mateu for advices and help with Raman microspectroscopy, and Monika Bathóová for help with experiments. This work was supported by the Scientific Grant Agency (VEGA 1/0755/16), the Slovak Research and Development Agency (SK-SRB-2013-0021), Serbian Ministry of Education, Science and Technological Development (OI173005), and the Israel Science Foundation (534/14).
LITERATURE CITED
- Agarwal UP. 2014. 1064 nm FT-Raman spectroscopy for investigations of plant cell walls and other biomass materials. Frontiers in Plant Science 5: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal UP, Ralph SA.. 1997. FT-Raman spectroscopy of wood: identifying contributions of lignin and carbohydrate polymers in the spectrum of black spruce (Picea mariana). Applied Spectroscopy 51: 1648–1655. [Google Scholar]
- Aguiar H, Serra J, González P, León B.. 2009. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. Journal of Non-Crystalline Solids 355: 475–480. [Google Scholar]
- Aston MJ, Jones MM.. 1976. A study of the transpiration surfaces of Avena sterilis L. var. Algerian leaves using monosilicic acid as a tracer for water movement. Planta 130: 121–129. [DOI] [PubMed] [Google Scholar]
- Barros J, Serk H, Granlund I, Pesquet E.. 2015. The cell biology of lignification in higher plants. Annals of Botany 115: 1053–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA.. 1991. Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in polyethylene glycol-glycerol. Biotechnic & Histochemistry 66: 111–116. [DOI] [PubMed] [Google Scholar]
- Buanafina MMDO. 2009. Feruloylation in grasses: current and future perspectives. Molecular Plant 2: 861–872. [DOI] [PubMed] [Google Scholar]
- Carpita NC. 1996. Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology 47: 445–476. [DOI] [PubMed] [Google Scholar]
- Casey WH, Kinrade SD, Knight CTG, Rains DW, Epstein E.. 2003. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant, Cell & Environment 27: 51–54. [Google Scholar]
- Cooke J, Leishman MR.. 2011. Is plant ecology more siliceous than we realise? Trends in Plant Science 16: 61–68. [DOI] [PubMed] [Google Scholar]
- Currie HA, Perry CC.. 2009. Chemical evidence for intrinsic ‘Si’ within Equisetum cell walls. Phytochemistry 70: 2089–2095. [DOI] [PubMed] [Google Scholar]
- Dabney C, Ostergaard J, Watkins E, Chen C.. 2016. A novel method to characterize silica bodies in grasses. Plant Methods 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmann KC, Araújo WL, Martins SCV, et al. 2012. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytologist 196: 752–762. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Guttenberger M, Saralegui A, et al. 2006. Neutral red as a probe for confocal laser scanning microscopy studies of plant roots. Annals of Botany 97: 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma F.. 2003. Root endodermis and exodermis: structure, function, and responses to the environment. Journal of Plant Growth Regulation 21: 335–351. [Google Scholar]
- Epstein E. 1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50: 641– 664. [DOI] [PubMed] [Google Scholar]
- Evered D, O’Connor M.. 1986. Silicon biochemistry. Ciba Foundation Symposium 121. Chichester: John Wiley. [Google Scholar]
- Evert RF. 2006. Esau's Plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development, 3rd edn.New Jersey: John Wiley. [Google Scholar]
- Exley C. 2009. Silicon in life: whither biological silicification? In: Müller WEG, Grachev MA. eds. Biosilica in evolution, morphogenesis, and nanobiotechnology .Berlin: Springer, 173–184. [DOI] [PubMed] [Google Scholar]
- Exley C. 2015. A possible mechanism of biological silicification in plants. Frontiers in Plant Science 6: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Ma X.. 2006. In vitro simulation studies of silica deposition induced by lignin from rice. Journal of Zhejiang University. Science B 7: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK.. 2011. Silicon enhances suberization and lignification in roots of rice (Oryza sativa). Journal of Experimental Botany 62: 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck AT, Schulze S, Hinrichs M, et al. 2015. Silicon promotes exodermal Casparian band formation in Si-accumulating and Si-excluding species by forming phenol complexes. PLoS One 10: e0138555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Nesselrode BHWA, Miller JG, Mewburn BR.. 2008. Mixed-linkage (1→3,1→4)-β-D-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytologist 179: 104–115. [DOI] [PubMed] [Google Scholar]
- Gierlinger N, Sapei L, Paris O.. 2008. Insights into the chemical composition of Equisetum hyemale by high resolution Raman imaging. Planta 227: 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabber JH, Ralph J, Hatfield RD.. 2002. Model studies of ferulate–coniferyl alcohol cross-product formation in primary maize walls: implications for lignification in grasses. Journal of Agricultural and Food Chemistry 50: 6008–6016. [DOI] [PubMed] [Google Scholar]
- Guerriero G, Hausman J-F, Legay S.. 2016. Silicon and the plant extracellular matrix. Frontiers in Plant Science 7: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PJ, Hartley RD.. 1976. Detection of bound ferulic acid in cell walls of the Graminae by ultraviolet fluorescence microscopy. Nature 259: 501–507. [Google Scholar]
- Hattori T, Inanaga S, Tanimoto E, Lux A, Luxová M, Sugimoto Y.. 2003. Silicon-induced changes in viscoelastic properties of Sorghum root cell walls. Plant and Cell Physiology 44: 743–749. [DOI] [PubMed] [Google Scholar]
- Hattori T, Inanaga S, Araki H, et al. 2005. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiologia Plantarum 123: 459–466. [Google Scholar]
- He C, Ma J, Wang L.. 2015. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytologist 206: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Himmelsbach DS, Akin DE.. 1998. Near-infrared Fourier-transform Raman spectroscopy of flax (Linum usitatissimum L.) stems. Journal of Agricultural and Food Chemistry 46: 991–998. [Google Scholar]
- Hodson MJ, Sangster AG.. 1989. X-ray microanalysis of the seminal root of Sorghum bicolor with particular reference to silicon. Annals of Botany 64: 659–667. [Google Scholar]
- Hodson MJ, White PJ, Mead A, Broadley MR.. 2005. Phylogenetic variation in the silicon composition of plants. Annals of Botany 96: 1027–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston K, Tucker MR, Chowdhury J, Shirley N, Little A.. 2016. The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Frontiers in Plant Science 7: 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanaga S, Okasaka A.. 1995. Calcium and silicon binding compounds in cell walls of rice shoots. Soil Science and Plant Nutrition 41: 103–110. [Google Scholar]
- Ishii T. 1997. Structure and functions of feruloylated polysaccharides. Plant Science 127: 111–127. [Google Scholar]
- Jacquet G, Pollet B, Lapierre C, Mhamdi F, Rolando C.. 1995. New ether-linked ferulic acid-coniferyl alcohol dimers identified in grass straws. Journal of Agricultural and Food Chemistry 43: 2746–2751. [Google Scholar]
- Johnsen H, Krause K.. 2014. Cellulase activity screening using pure carboxymethylcellulose: application to soluble cellulolytic samples and to plant tissue prints. International Journal of Molecular Sciences 15: 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisaka S, Takeda S, Takahashi K, Shibata K.. 1990. Diferulic and ferulic acid in the cell wall of Avena coleoptiles — their relationships to mechanical properties of the cell wall. Physiologia Plantarum 78: 1–7. [Google Scholar]
- Kido N, Yokoyama R, Yamamoto T, et al. 2015. The matrix polysaccharide (1;3,1;4)-β-D-glucan is involved in silicon-dependent strengthening of rice cell wall. Plant & Cell Physiology 56: 268–276. [DOI] [PubMed] [Google Scholar]
- Kozlova LV, Ageeva MV, Ibragimova NN, Gorshkova TA.. 2014. Arrangement of mixed-linkage glucan and glucuronoarabinoxylan in the cell walls of growing maize roots. Annals of Botany 114: 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik TV, Lipkovska NA, Barvinchenko VN, et al. 2009. Interactions between bioactive ferulic acid and fumed silica by UV–vis spectroscopy, FT-IR, TPD MS investigation and quantum chemical methods. Journal of Colloid And Interface Science 339: 60–68. [DOI] [PubMed] [Google Scholar]
- Larsen KL, Barsberg S.. 2010. Theoretical and Raman spectroscopic studies of phenolic lignin model monomers. Journal of Physical Chemistry B 114: 8009–8021. [DOI] [PubMed] [Google Scholar]
- Law C, Exley C.. 2011. New insight into silica deposition in horsetail (Equisetum arvense). BMC Plant Biology 11: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux O, Leroux F, Mastroberti AA, et al. 2013. Heterogeneity of silica and glycan-epitope distribution in epidermal idioblast cell walls in Adiantum raddianum laminae. Planta 237: 1453–1464. [DOI] [PubMed] [Google Scholar]
- Liang Y, Nikolic M, Bélanger R, Gong H, Song A.. 2015. Silicon in agriculture: from theory to practice, 1st edn Dordrecht: Springer. [Google Scholar]
- Lichtenthaler HK, Schweiger J.. 1998. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. Journal of Plant Physiology 152: 272–282. [Google Scholar]
- Liu P, Yin L, Deng X, Wang S, Tanaka K, Zhang S.. 2014. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. Journal of Experimental Botany 65: 4747–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yin L, Wang S, et al. 2015. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environmental and Experimental Botany 111: 42–51. [Google Scholar]
- Lukačová Z, Švubová R, Kohanová J, Lux A.. 2013. Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regulation 70: 89–103. [Google Scholar]
- Lupoi JS, Smith EA.. 2012. Characterization of woody and herbaceous biomasses lignin composition with 1064 nm dispersive multichannel Raman spectroscopy. Applied Spectroscopy 66: 903–910. [DOI] [PubMed] [Google Scholar]
- Lupoi JS, Singh S, Parthasarathi R, Simmons BA, Henry RJ.. 2015. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renewable and Sustainable Energy Reviews 49: 871–906. [Google Scholar]
- Lux A, Luxová M, Hattori T, Inanaga S, Sugimoto Y.. 2002. Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiologia Plantarum 115: 87–92. [DOI] [PubMed] [Google Scholar]
- Lux A, Luxová M, Abe J, Tanimoto E, Hattori T, Inanaga S.. 2003. The dynamics of silicon deposition in the sorghum root endodermis. New Phytologist 158: 437–441. [DOI] [PubMed] [Google Scholar]
- Lux A, Morita S, Abe J, Ito K.. 2005. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany 96: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhou X, Ma J, Ji Z, Zhang X, Xu F.. 2014. Raman microspectroscopy imaging study on topochemical correlation between lignin and hydroxycinnamic acids in Miscanthus sinensis. Microscopy and Microanalysis 20: 956–963. [DOI] [PubMed] [Google Scholar]
- Ma JF. 2004. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Science and Plant Nutrition 50: 11–18. [Google Scholar]
- Ma JF, Yamaji N.. 2006. Silicon uptake and accumulation in higher plants. Trends in Plant Science 11: 392–397. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N.. 2015. A cooperative system of silicon transport in plants. Trends in Plant Science 20: 435–442. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, et al. 2006. A silicon transporter in rice. Nature 440: 688–691. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani-Ueno N.. 2011. Transport of silicon from roots to panicles in plants. Proceedings of the Japan Academy, Series B, Physical and Biological Sciences 87: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan PF, Remmele RL.. 1986. Hydroxyl sites in SiO2 glass: a note on infrared and Raman spectra. American Mineralogist 71: 772–778. [Google Scholar]
- Mikkelsen D, Flanagan BM, Wilson SM, Bacic A, Gidley MJ.. 2015. Interactions of arabinoxylan and (1,3)(1,4)-β-glucan with cellulose networks. Biomacromolecules 16: 1232–1239. [DOI] [PubMed] [Google Scholar]
- Mitani N, Ma JF, Iwashita T.. 2005. Identification of the silicon form in xylem sap of rice (Oryza sativa L.). Plant & Cell Physiology 46: 279–283. [DOI] [PubMed] [Google Scholar]
- Perry C, Williams R, Fry SC.. 1987. Cell wall biosynthesis during silicification of grass hairs. Journal of Plant Physiology 126: 437–448. [Google Scholar]
- Piot O, Autran J, Manfait M.. 2001. Investigation by confocal Raman microspectroscopy of the molecular factors responsible for grain cohesion in the Triticum aestivum bread wheat. Role of the cell walls in the starchy endosperm. Journal of Cereal Science 34: 191–205. [Google Scholar]
- Prats Mateu B, Hauser MT, Heredia A, Gierlinger N.. 2016. Waterproofing in Arabidopsis: following phenolics and lipids in situ by confocal Raman microscopy. Frontiers in Chemistry 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J, Grabber JH, Hatfield RD.. 1995. Lignin-ferulate cross-links in grasses: active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydrate Research 275: 167–178. [Google Scholar]
- Ram MS, Dowell FE, Seitz LM.. 2003. FT-Raman spectra of unsoaked and NaOH-soaked wheat kernels, bran, and ferulic acid. Cereal Chemistry 80: 188–192. [Google Scholar]
- Raven JA. 1983. The transport and function of silicon in plants. Biological Reviews 58: 179–207. [Google Scholar]
- Richmond KE, Sussman M.. 2003. Got silicon? The non-essential beneficial plant nutrient. Current Opinion in Plant Biology 6: 268–272. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Prychid CJ, Gregory T.. 2014. Epidermal patterning and silica phytoliths in grasses: an evolutionary history. Botanical Review 80: 59–71. [Google Scholar]
- Sangster AG. 1978. Silicon in the roots of higher plants. American Journal of Botany 65: 929–935. [Google Scholar]
- Sangster AG, Parry DW.. 1976a. Endodermal silicification in mature, nodal roots of Sorghum bicolor (L.) Moench. Annals of Botany 40: 373–379. [Google Scholar]
- Sangster AG, Parry DW.. 1976b. Endodermal silicon deposits and their linear distribution in developing roots of Sorghum bicolor (L.) Moench. Annals of Botany 40: 361–371. [Google Scholar]
- Sangster AG, Parry DW.. 1976c. The ultrastructure and electron-probe microassay of silicon deposits in the endodermis of the seminal roots of Sorghum bicolor (L.) Moench. Annals of Botany 40: 447–459. [Google Scholar]
- Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E.. 2010. Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytologist 186: 385–391. [DOI] [PubMed] [Google Scholar]
- Scholz SM, Dutta J, Hofmann H.. 1997. Raman spectroscopic study of silicon nanopowders. Journal of Material Science and Technology 13: 327–332. [Google Scholar]
- Scurfield G, Anderson CA, Segnit ER.. 1974. Silica in woody stems. Australian Journal of Botany 22: 211–229. [Google Scholar]
- Sebastian S, Sundaraganesan N, Manoharan S.. 2009. Molecular structure, spectroscopic studies and first-order molecular hyperpolarizabilities of ferulic acid by density functional study. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy 74: 312–323. [DOI] [PubMed] [Google Scholar]
- Soukup A. 2014. Selected simple methods of plant cell wall histochemistry and staining for light microscopy In: Žárský V, Cvrčková F. eds. Plant cell morphogenesis. Heidelberg: Springer, 24–40. [Google Scholar]
- Soukup M, Martinka M, Cigáň M, Ravaszová F, Lux A.. 2014. New method for visualization of silica phytoliths in Sorghum bicolor roots by fluorescence microscopy revealed silicate concentration-dependent phytolith formation. Planta 240: 1365–1372. [DOI] [PubMed] [Google Scholar]
- Strömberg CAE, Di Stilio VS, Song Z.. 2016. Functions of phytoliths in vascular plants: an evolutionary perspective. Functional Ecology 30: 1286–1297. [Google Scholar]
- Suzuki S, Ma JF, Yamamoto N, Hattori T, Sakamoto M, Umezawa T.. 2012. Silicon deficiency promotes lignin accumulation in rice. Plant Biotechnology 29: 391–394. [Google Scholar]
- Trembath-Reichert E, Wilson JP, Mcglynn SE, Fischer WW.. 2015. Four hundred million years of silica biomineralization in land plants. Proceedings of the National Academy of Sciences of the USA 112: 5449–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. 2001. The pathways of calcium movement to the xylem. Journal of Experimental Botany 52: 891–899. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Sakurai G, Mitani-Ueno N, Ma JF.. 2015. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proceedings of the National Academy of Sciences of the USA 112: 11401–11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nakamura A, Iwai H, et al. 2012. Effect of silicon deficiency on secondary cell wall synthesis in rice leaf. Journal of Plant Research 125: 771–779. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang L, Zhang W, Zhang F.. 2013. Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis? Plant and Soil 372: 137–149. [Google Scholar]
- Zhang J, Zou W, Li Y, et al.2015. Silica distinctively affects cell wall features and lignocellulosic saccharification with large enhancement on biomass production in rice. Plant Science 239: 84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.