Abstract
Background
Increasing numbers of perinatally HIV-infected children are surviving to adolescence and transitioning to adult care, yet there are scarce data on their clinical status at transfer.
Methods
We analysed prospective cohort data from the UK/Ireland national Collaborative HIV Paediatric Study (CHIPS). Clinical status at last paediatric clinic visit prior to transfer was described. Factors associated with higher CD4 cell count and viral load (VL) suppression<400c/mL among patients on ART at transfer were assessed using linear and logistic regression, respectively. Data were matched with the UK HIV Drug Resistance Database (UKHIVDRB) to assess cumulative resistance profiles at transfer.
Results
Of 1,907 children followed in CHIPS from 1996 to November 2014, 644 (34%) transferred to adult care: 53% were female, 62% born outside the UK/Ireland, 75% black African. At last paediatric follow-up, median age was 17.4 years [interquartile range 16.5,18.1], 27% had previous AIDS diagnosis, CD4 was 444 cells/mm3 [280,643], 76% were on ART, 13% off-ART and 11% ART-naive. Among patients on ART, 74% had VL<400c/mL. In multivariable analysis, higher CD4 at transfer was associated with younger age, higher CD4 at ART initiation and lower VL at transfer (p≤0.001). Predictors of viral suppression include no AIDS diagnosis and later year of transfer (p≤0.05). Of 291 patients with resistance data, 82% had resistance to ≥1 drug class, 56% to ≥2 classes and 12% had triple-class resistance.
Conclusion
Three-quarters of adolescents were on stable ART at transfer, of whom 74% were virologically suppressed. The prevalence of triple-class resistance was relatively low at 12%.
Keywords: HIV, children, adolescents, antiretroviral therapy, UK
Introduction
In 2015, there were an estimated 1.8 million children aged<15 years living with HIV, with an estimated 48% coverage of antiretroviral therapy (ART) globally [1]. As treatment programmes expand and mature, a growing proportion of perinatally HIV-infected children are surviving to adolescence and transitioning from paediatric to adult HIV care[2–4]. However, there are scarce data on their clinical status during and beyond the transition period. Such data will be critical in informing long-term clinical care of adolescents/young adults with perinatal HIV, particularly in light of recent reports highlighting HIV/AIDS as a top ten leading cause of deaths among adolescents globally [5, 6]. Due to lack of disaggregated data by mode of infection, it is unclear if the rise in HIV/AIDS-related mortality in this age-group is largely driven by perinatally-infected survivors, who have been reported to have poorer retention in care and poorer adherence on ART as compared to adults and children [7–9]
High-income countries have some most mature perinatal HIV cohorts with early access to ART, and recent surveillance reports in the UK suggest significantly lower proportions of adolescents in HIV care are on ART and achieving viral suppression as compared to adults [10], with similar trends observed in the US [11, 12]. However, these studies also combine adolescents of all modes of infection, raising questions of whether perinatally-infected adolescents are at increased risk of viremia and disease progression, particularly around the time of transition to adult care, when they may require additional support[13].
In this study, we investigate the clinical profile of adolescents at transfer to adult care in the UK and Ireland national paediatric HIV cohort, which has almost complete coverage of all HIV-infected children diagnosed. We describe the clinical, treatment status and resistance profiles and explore factors associated with improved immunological and virological status at transfer to adult care.
Methods
Our national cohort has been described elsewhere[14]. In brief, the National Study of HIV in Pregnancy and Childhood (NSHPC) collects reports of all infants born to HIV-infected women in the UK/Ireland and all children aged <16 years diagnosed with HIV-infection (regardless of country of birth). Subsequent follow-up information on HIV-infected children reported from 2000 onwards is collected annually through the Collaborative HIV Paediatric Study (CHIPS). The follow-up ceases when a patient transfers from paediatric to adult care. Both studies have NHS Research Ethics approval.
This analysis included data on HIV-infected children followed from 2000 to November 2014, with clinical data dating back to 1996.
Statistical methods
Among patients reported to have transferred to adult care, the clinical and treatment status at last paediatric clinic visit (henceforth referred to as ‘at transfer’) were described overall and compared by country of birth (UK/Ireland or abroad/unknown) using chi-square and Mann-Whitney Wilcoxon tests. Being born abroad was considered a potential confounder as it is strongly associated with older age at first presentation for HIV care in the UK/Ireland and at start of ART.
The outcomes of interest were CD4 cell count and HIV-1 RNA viral load (VL) suppression <400 copies/mL among patients on ART at transfer, excluding ART-naive patients and those off-ART (defined as stopped all antiretrovirals for ≥14 days) as lack of treatment is known to strongly affect these outcomes[2]. Factors associated with higher CD4 cell count and VL suppression on ART at transfer were assessed using linear and logistic regression, respectively. The VL<400 copies threshold was used due to varying detection levels over calendar years.
Potential predictors were: sex, ethnicity, born abroad; characteristics at ART start (age, CD4 percentage, initiation on combination ART (cART) (defined as ≥3 drugs across ≥2 classes or 3 Nucleoside Reverse Transcriptase Inhibitors (NRTIs) including abacavir)); and characteristics at transfer to adult care (age, CDC stage, CD4 count, VL, current ART status, exposure to triple class ART, duration since starting ART and calendar year at time of transfer). Current ART status was defined as: (i) on initial cART regimen, (ii) subsequent ART regimen (defined as change of ≥2 drugs or addition of a new drug class) or (iii) mono/dual ART. Triple class ART exposure was defined as ever received NRTIs, Non-NRTIs (NNRTI) and Protease Inhibitor (PI) drugs. Calendar years at transfer were grouped in three year intervals and groups were collapsed in analyses where there were no differences in estimates. As ethnicity and born abroad are strongly correlated; only the latter was included in multivariable models. As some patients initiated ART in older ages and may have had insufficient follow-up time to observe immune recovery before transfer, sensitivity analyses of CD4 outcome was conducted restricted to patients with ≥2 years follow-up after ART start. Variables with p<0.2 in univariable analyses were included in multivariable models, and backwards selection (exit probability p=0·1) identified those with the strongest association.
Data on patients transferred to adult care were matched to the UK HIV Drug Resistance Database (UKHDRD)[15], using an algorithm of unique patient identifiers, with resistance data up to end of December 2015. Resistance data (excluding tropism) on all tests conducted prior to transfer, or if unavailable, the closest test up to 1 year after transfer or while ≤18 years of age were analysed. Cumulative resistance was described for patients with multiple resistance tests, including all current and archived resistance mutations. Among patients who were treatment experienced at time of test, resistance was defined as the detection of ≥1 major mutation using the 2015 International Antiviral Society guidelines[16]. For patients with no previous treatment reported at time of test, resistance was defined as ≥1 mutation from the WHO 2009 surveillance list [17].
All children were included in these analyses, irrespective of mode of HIV infection. In sensitivity analyses, only children documented as perinatally-infected and those with unknown mode of infection and aged≤10 years at time of first HIV diagnosis in the UK/Ireland (proxy for vertical transmission) were included. All analyses were conducted using Stata 14, College Station, TX.
Results
Of 1,907 children enrolled in CHIPS from 1996-2014, 93% were documented as perinatally-infected. Overall, 644 (34%) had transferred to adult care by November 2014, 109 (6%) had died in paediatric care, 103 (6%) had left the country, 106 (6%) were loss to follow-up (with median age at last visit of 12.5 years (IQR, 8.8-14.7)), and 945 (50%) remained in paediatric follow-up.
Of the 644 transferred to adult care, 53% were female, 62% were born outside the UK/Ireland, and 75% were black African (Table 1). Ninety-one percent were perinatally-infected, 21 (3.3%) reported as infected through blood products, 8 (1.2%) other and 31 (4.8%) with an unknown mode of infection. The majority (95%) of children with other and unknown mode of infection were born abroad.
Table 1. Characteristics at last paediatric care visit of patients transferred to adult care.
| Characteristic | Total (N=644) |
Born in UK/Ireland (N=245) |
Born abroad* (N=399) |
P |
|---|---|---|---|---|
| n (%) or median [IQR] | ||||
| Female sex | 343 (53) | 131 (53) | 212 (53) | 0.93 |
| Ethnicity | ||||
| Black African | 480 (75) | 140 (57) | 340 (85) | <0.0001 |
| White | 86 (13) | 64 (26) | 22 (6) | |
| Other | 78 (12) | 41 (17) | 37(9) | |
| Age at first presentation in UK (years) | 6.4 [1.8, 11.1] | 1.4 [0.1,4.3] | 9.3 [5.7,12.3] | <0.0001 |
| Calendar year of first presentation in UK | ||||
| <2000 | 364 (57) | 210 (86) | 154 (39) | <0.0001 |
| ≥2000 | 280 (43) | 35 (14) | 245 (61) | |
| Ever received ART | 575 (89) | 224 (91) | 351 (88) | |
| Characteristics at ART start (n=575) | ||||
| Age (years) | 9.6 [5.4,12.8] | 6.5 [3.0,10.7] | 10.7 [7.7,13.8] | <0.0001 |
| <5 years | 116 (24) | 81 (45) | 35 (11) | |
| CDC C stage | 127 (22) | 58 (26) | 69 (20) | 0.079 |
| CD4% for age<5 yrs (n=91, 71, 20) | 15 [8,24] | 15 [8,24] | 15 [8,22] | 0.80 |
| CD4 cell count for age ≥5 yrs (n=365, 116, 249) | 210 [91,350] | 205 [100,386] | 214 [80,330] | 0.51 |
| Viral load (log10) (n=364, 133, 231) | 4.9 [4.3,5.3] | 5.0 [4.4,5.5] | 4.8 [4.3,5.2] | 0.021 |
| Initial ART regimen | ||||
| Mono/dual NRTI | 149 (26) | 85 (36) | 64 (18) | <0.0001 |
| Triple NRTI | 22 (4) | 8 (4) | 14 (4) | |
| NNRTI+NRTI | 276 (48) | 72 (32) | 204 (58) | |
| PI+NRTI | 128 (22) | 59 (26) | 69 (20) | |
| Characteristics at transfer to adult care | ||||
| Age (years) | 17.4 [16.5,18.1] | 17.4 [16.4,18.3] | 17.3 [16.5,18.1] | 0.62 |
| CDC C stage | 171 (27) | 75 (31) | 96 (24) | 0.68 |
| CD4 cell count, cells/mm3 | 444 [280,643] | 412 [250,640] | 456 [289,648] | 0.15 |
| ≤200 | 101 (16) | 50 (20) | 51 (13) | |
| 201-350 | 122 (21) | 47 (19) | 86 (22) | |
| 351-500 | 142 (22) | 56 (23) | 86 (21) | |
| >500 | 268 (42) | 92 (38) | 176 (44) | |
| Viral load (log10) (n=643) | 2.0 [1.7,3.8] | 2.0 [1.7,4.0] | 2.0 [1.7,3.7] | 0.19 |
| VL <400 copies if on ART at transfer (n=481) | 357/481 (74) | 131/178 (74) | 226/303 (75) | |
| ART status | <0.0001 | |||
| Naïve | 69 (11) | 21 (9) | 48 (12) | |
| On initial cART | 167 (26) | 43 (18) | 124 (31) | |
| On subsequent cART | 284 (44) | 114 (47) | 170 (43) | |
| On mono/dual ART | 39 (6) | 23 (9) | 16 (4) | |
| Off-ART after previous exposure | 85 (13) | 44 (18) | 41 (10) | |
| Duration since start of ART (years) (n=575) | 7.8 [4.3,12.0] | 10.8 [6.9,14.5] | 6.2 [3.4,9.8] | <0.0001 |
| Triple class exposure (n=575) | 312 (54) | 143 (64) | 169 (48) | <0.0001 |
| Calendar year of transfer | 0.52 | |||
| 2000-2002 | 21 (3) | 5 (2) | 16 (4) | |
| 2003-2005 | 68 (11) | 22 (9) | 46 (12) | |
| 2006-2008 | 126 (20) | 49 (20) | 77 (19) | |
| 2009-2011 | 226 (35) | 91 (37) | 135 (34) | |
| 2012-2014 | 203 (32) | 78 (32) | 125 (31) | |
Notes: * Includes 11 patients with unknown country of birth.
A higher proportion of children born in the UK/Ireland presented for HIV care in earlier calendar years (before 2000), with lower median age at HIV diagnosis and at ART initiation, and were more likely to have triple-class exposure at transfer as compared to those born abroad (all p<0.001).
At transfer to adult care, the median age was 17.4 years [interquartile range (IQR) 16.5, 18.1], with a median duration of follow-up in paediatric care of 10.9 years [6.4, 15.6]. The median CD4 was 444 [280, 643] cells/mm3, with no difference by country of birth (p=0.15). Twenty-seven percent of patients had a previous AIDS diagnosis and 16% were severely immunocompromised at transfer with CD4≤200cells/mm3. Eleven percent of patients (n=69) were ART naïve, 26% were on their initial cART regimen, 44% on subsequent ART regimens, 6% on mono or dual ART (approximately half on mono-PI regimens) and 13% were off-ART. The median duration since ART start was 7.8 years [4.3, 12.0] at transfer, with shorter duration among patients on their initial cART regimen (3.6 years [2.3-6.7], p<0.0001). Among those on cART at transfer, the majority were on efavirenz (32%), lopinavir (19%) and atazanavir (15%) based regimens.
Immunological and virological status by calendar year of transfer
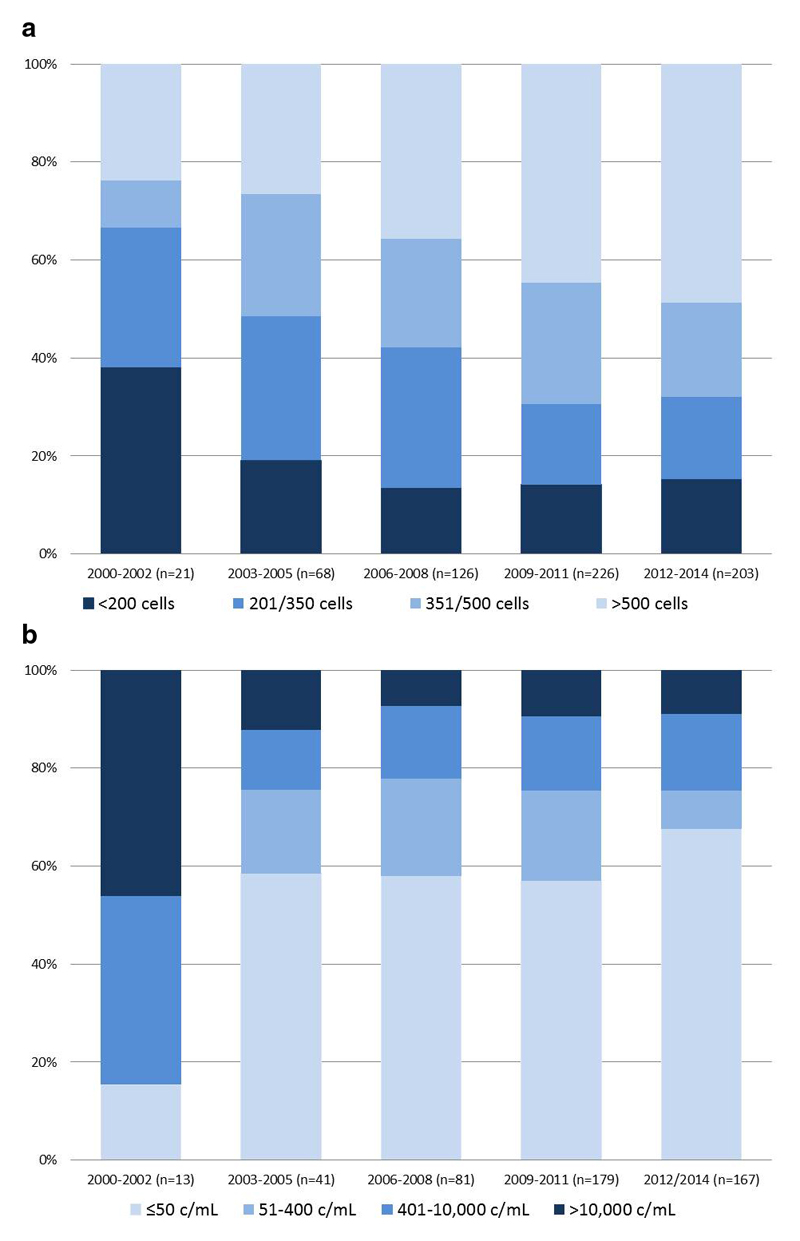
The proportion of patients with CD4≤350 cells/mm3 at transfer declined over time, from 67% in 2000-2002 to 32% in 2012-2014, while the proportion with CD4>500 cells/mm3 increased from 24% to 49% respectively (χ2 p=0.002, Figure 1a). The overall proportion of patients (irrespective of ART status) with VL<400 copies at transfer was 58%, this proportion increased over time from 14% to 65% respectively (χ2 p<0.001). When restricted to patients on ART at transfer, the proportion suppressed <400 c/mL increased from 15% in 2000-2002 to ≥75% from 2003 onwards (p<0.0.001 Figure 1b). When using the VL<50cps/mL threshold, 60%, of patients on ART had undetectable VL, with improving suppression rates over time, reaching 68% among those transferred in 2012-2014.
Figure 1. (a) CD4 distribution at transfer to adult care by calendar year of transfer1 (n=644) (top); (b) Viral load at transfer among patients on ART at transfer to adult care (n=481) (bottom).
Notes: 1 includes all patients irrespective of ART status at transfer (includes ART naïve (n=69) and those off-ART (n=85))
Patients ART naïve and off-ART at transfer
Sixty-nine (11%) patients were antiretroviral naïve at transfer: 67% were female and 70% were born abroad. The median age at first presentation to HIV care was 10.6 [2.6-13.3] years and age at transfer to adult care was 17.3 [16.0-17.9] years. Seventy-two per cent were CDC stage N/A, 20% stage B and 7% (n=5) ever had a stage C event. Median CD4 at transfer was comparable to ART-experienced patients (465 cells/mm3 [340, 560] vs 434 cells/mm3 [267, 651] respectively, p=0.44). At transfer, the median viral load was 3.8 log10 copies/mL [3.4, 4.3], and ten patients (15%) had VL<1000 copies/mL. Eighteen (26%) of the naive patients had a CD4<350 cells/mm3 at transfer, of whom twelve transferred in earlier calendar years (2000-2008). The naïve patients transferred in most recent calendar years (2012-2014) all had CD4>350 cells/mm3 at last paediatric visit.
Eighty-five patients (13%) were treatment experienced but were off-ART at transfer. The proportion of patients off-ART at transfer declined over time from 24% in 2000-2002 to 9% in 2012-2014 (χ2 p<0.0001). The median duration off-ART was 16 months [6.2-29]. The median CD4 at transfer was 279 cells/mm3 [186,443], one third of patients off-ART (27/85) were severely immunocompromised with CD4<200 cells/mm3 at transfer.
Predictors of immunological and virological status among patients on ART at transfer
In multivariable analyses, predictors of improved immunological status at transfer among patients on ART were: higher CD4% and younger age at ART start and lower viral load at transfer (all p≤0.001) (Table 2). Patients initiating ART aged ≥15 years had 235 cells/mm3 (95%CI -333, -137) lower CD4 at transfer compared to those aged <5 years. In sensitivity analyses restricted to patients with ≥2 years follow-up after ART start, the age effect persisted but was less significant (p=0.044, data not shown).
Table 2. Factors associated with immunological and virological status at transfer to adult care in ART-exposed patients.
| CD4 cell count increase |
VL<400 copies/mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Coef (95% CI) | P | Multivariable Coef (95% CI) | P | OR (95% CI) | P | aOR (95% CI) | P | |
| Constant | 505 (479, 532) | 774 (682,867) | ||||||
| Sex - female vs male | -23.3 (-76, 29) | 0.39 | 0.87 (0.6, 1.2) | 0.41 | ||||
| Born abroad vs UK/Ireland | 25.4 (-80, 29) | 0.36 | 33 (-23, 89) | 0.25 | 1.25 (0.9, 1.8) | 0.2 | 0.9 (0.5, 1.5) | 0.60 |
| Ethnicity: Black African/Other vs Caucasian | -76 (-153, 1.4) | 0.054 | 0.85 (0.5, 1.4) | 0.522 | ||||
| Characteristics at ART start | ||||||||
| Age: <5 years | 1 | 0.033 | 1 | <0.001 | 1 | 0.04 | 1 | 0.62 |
| 5 - 9 years | -46 (-118, 27) | -38 (-112,36) | 0.6 (0.4,0.9) | 0.8 (0.5,2.3) | ||||
| 10 – 14 years | -36 (-106, 33) | -79 (-156,-2) | 0.9 (0.6, 1.4) | 1.1 (0.5, 2.3) | ||||
| ≥15 years | -178 (-274, -83) | -268 (-371, -165) | 1.2 (0.6, 2.4) | 1.6 (0.6, 4.8) | ||||
| CD4 percentage, per 1% increase | 7 (4, 10) | <0.001 | 5.1 (2.4, 7.8) | <0.001 | 1.03 (1.0, 1.05) | 0.04 | 0.98 (0.94, 1.01) | 0.211 |
| Initiation on non cART vs cART | -5.8 (-68, 56) | 0.86 | 0.7 (0.4,1.0) | 0.079 | 0.8 (0.4,1.5) | 0.43 | ||
| Characteristics at transfer | ||||||||
| Age, per year increase | -9 (-27, 9) | 0.307 | 0.92 (0.8, 1.0) | 0.19 | 1.09 (0.9, 1.3) | 0.87 | ||
| CDC stage: C vs N/A/B | 19.2 (-38, 77) | 0.514 | 0.7 (0.5, 1.0) | 0.066 | 0.43 (0.2, 0.75) | 0.003 | ||
| CD4 per 100c/mm3 increase | - | - | 1.94 (1.7, 2.2) | <0.001 | 2.1 (1.8, 2.4) | <0.001 | ||
| VL per log increase | -146 (-166, -124) | <0.001 | -139 (-162, -115) | <0.001 | - | - | ||
| ART status: Initial cART | 76 (18,132) | 0.033 | 67 (7,127) | 0.09 | 2.8 (1.7, 4.5) | <0.001 | 2.2 (1.2, 4.1) | 0.04 |
| Subsequent cART | 1 | 1 | 1 | 1 | ||||
| Mono/dual ART | 35 (-64,135) | 14 (-77,104) | 0.9 (0.4, 1.8) | 0.86 (0.3, 2.2) | ||||
| Exposure to triple class ART | -77 (-130, -24) | 0.004 | -5 (-74,64) | 0.90 | 0.6 (0.4, 0.9) | 0.006 | 0.6 (0.3, 1.3) | 0.20 |
| Duration since start ART, per year increase | 6.7 (1.1, 12) | 0.019 | 11 (-2, 24) | 0.104 | 0.98 (0.94, 1.0) | 0.176 | 0.97 (0.9, 1.03) | 0.35 |
| Calendar year: 2000-2002 | -179 (-348, -9) | 0.019 | -29 (-198,139) | 0.94 | 0.06 (0.01,0.3) | 0.002 | 0.06 (0.01,0.5) | 0.021 |
| 2003-2008 | 1 | 1 | 1 | 1 | ||||
| 2009-2014 | 40 (-20, 101) | -1 (-58,57) | 0.9 (0.6,1.2) | 0.6 (0.3, 1.2) | ||||
Notes: OR; odds ratio, aOR; adjusted odds ratio. P values <0.2 are shown in bold. Shaded variables were included in the final multivariable models.
Predictors of viral suppression <400 c/mL at transfer were CDC stage N/A/B (vs. C ,p=0.003), higher CD4 cell count at transfer (p<0.001) and being on an initial cART regimen (vs. subsequent regimen, p<0.04)). Patients who transferred in the earliest calendar years (2000-2002) had lower odds of viral suppression at transfer as compared to those transferred from 2003 onwards (p=0.021).
Resistance profile
Fifty-nine percent of adolescents transferred to adult care (n=381/644) had a linked resistance test, with a total of 841 tests. Ninety patients were ART naïve at time of their latest resistance test, conducted either at first presentation to HIV care or prior to initiation of ART (median age 14 years at test [IQR 11.4, 15.6]), of whom five (6%) had ≥1 surveillance drug resistance mutation detected (two with resistance to NRTI and NNRTI, three with NNRTI resistance). The age ranged from 11-18 years at time of test, none had prior ART reported, four patients were born abroad and presented for HIV care aged >10 years.
Among 291 patients who were ART-experienced at time of their last resistance test, the median age at test was 15.9 years [13.7, 17.3]. Eighty-two per cent of patients had cumulative IAS major resistance mutations to one or more drug class. One-quarter (26%) of patients had resistance mutations to a single drug class (16.2% NNRTI, 9.3% NRTI and 0.7% PI), 44% had resistance mutations to two drug classes, most common being NRTI+NNRTI resistance (38.1%), followed by NRTI+PI (4.1%) and NNRTI+PI (1.7%). Twelve percent of patients (n=34) had triple-class resistance. Overall, the most frequently detected mutations were to the NRTI drug class (M184V, 44% and thymidine analogue mutations, 37%) and NNRTIs drug class (K103N, 37%) with much lower prevalence of resistance mutations to the PI drug class (L90M, 6%) (Supplement Table S1). No major integrase mutations were detected among five patients tested for integrase resistance.
Of the 34 patients with triple-class resistance, 62% were male, 71% were born in the UK/Ireland and 47% initiated on mono/dual NRTIs. At transfer, the median CD4 was 310 cells [170, 472], 85% were on ART, of whom 52% had VL<400 c/mL.
Sensitivity analyses
In sensitivity analyses (n=589), restricted to children reported as perinatally–infected (n=584) and those with unknown mode of infection and aged≤10 years at first HIV diagnosis (n= 5), their characteristics were very similar to the overall cohort, with 10% of children ART naïve at transfer (n=59) and among those with resistance data (n=280), 12% (n=33) had triple class resistance (data not shown).
Discussion
To our knowledge, this is the first national study reporting characteristics of adolescents with perinatal HIV at the time of transfer to adult care. Our study benefits from high coverage of children attending paediatric HIV clinics across the UK and Ireland, the inclusion of all children diagnosed with HIV irrespective of country of birth, and low rates of loss to follow-up. One-third of our cohort had transitioned to adult care by end of 2014; three-quarters of patients were on ART at transfer, of whom 74% were virologically suppressed. However, when we include all patients, irrespective of their ART status, the proportion with VL<400c/mL reduces to 57%.
To date, only three comparable studies have reported clinical status of adolescents with perinatal HIV at transfer to adult care. All were small (n<120) single clinic cohorts from Canada, Spain and Argentina [18–20]. At transfer, with a median age of 17-19 years, a comparable 42-56% of all patients, irrespective of ART status, had suppressed viral load <500 c/mL [18–20]. These studies did not report the proportion suppressed among those on ART at transfer, which was shown to have improved over calendar years in our cohort, most likely due to improved regimens available.
However, a lower proportion of adolescents in the CHIPS cohort had high CD4 ≥500 cells at transfer, at 42% as compared to 55% in the Spanish cohort [18]. This likely to be due to key differences in patient characteristics: over 85% of patients in the Spanish cohort were diagnosed at an earlier age (median 2 years vs 6.4 years in our cohort) and initiated ART earlier (median 5.6 vs 9.6 years, respectively). Indeed the strongest predictor of high CD4 among patients on ART at transfer in our cohort was younger age and higher CD4 at initiation of ART, consistent with other studies reporting that children initiating ART at older ages are less likely to or will take substantially longer to achieve immune reconstitution as compared to younger children[21, 22]. These findings support current WHO recommendations for universal ART in all HIV-infected children and adolescents, irrespective of age or CD4 [23].
In terms of drug resistance, a small number of children who were ART naïve at time of test had resistance mutations detected, most were born abroad, some presented at older ages and are likely to be due to unreported ART exposure. Among ART experienced patients with matched resistance data, 82% had resistance mutation to ≥1 drug class and 12% had triple-class resistance. This proportion with triple-class resistance was lower than other cohorts at transfer to adult care: 17.3% in Spain[18], 31.6% in Canada [19] 41% in Argentina and 18%-24% in the US paediatric and adolescent HIV cohorts [24, 25]. However the large majority of children in those cohorts were exposed to mono/dual therapy prior to availability of combination ART, as compared to one-quarter of patients in CHIPS. Furthermore, our resistance data were only matched in half of the patients transferred, this may represent an over-estimation of prevalence as those more at risk of developing resistance were more likely to have been tested. Nonetheless, this raises important questions over the risk of onward transmission of resistant viruses to partners and future offspring as an increasing proportion of adolescent with perinatal HIV become sexually active[26]. Further monitoring of resistance through adulthood is needed.
Interestingly, in our study 11% of adolescents were antiretroviral naïve at transfer. This is markedly higher than reports from other European paediatric HIV cohorts (1-3%)[2, 18]. Approximately one-third of the naïve adolescents had CD4<350 cells/mm3 at transfer, the majority of whom were transferred in earlier calendar years before the PENTA 2009 revised guidelines recommending immediate ART in children aged>5 years with CD4<350 cells/mm3[27]. The remainder were asymptomatic (72% CDC stage N/A) and 15% had low viral load (<1000 c/mL) despite never having received treatment. Some of these adolescents may be elite controllers, a unique and rare population of interest to the HIV cure agenda [28] and this is an area of ongoing study.
There are some important limitations to this study. First, analyses were based on the last paediatric care visit and findings may not accurately reflect the clinical status at entry to adult care, especially if there are gaps between paediatric and adult care. Second, there were no data on broader clinical status at transfer, related to comorbidities, neurological development or mental health, nor were there data on the socio-economic or orphan status around transition. Lastly, data on clinical outcomes after transfer to adult care are not yet available, although we are planning follow-up into adulthood with the “CHIPS+” cohort, through linkages with existing adult cohorts and national HIV surveillance to assess the long-term clinical outcomes in this unique population. This process will also allow us to assess the status of patients lost to follow-up in paediatric care.
In summary, the majority of perinatally-infected adolescents in the CHIPS national cohort were on stable ART at transfer to adult care with relatively high CD4 counts and good virological disease control; although 12% have triple-class resistance, this is one of the lowest prevalence reported for in a high-income country setting. One-quarter of patients were naïve or off-ART at transfer, the latter were more likely to be severely immunosuppressed at transfer and at risk of disease progression and will require particularly careful management. Previous studies in the UK highlighted the increased rate of mortality and hospitalisation among perinatally-infected young people in adult care as compared to younger children. [29] This along with worrying trends of poor retention among HIV-infected youth in adult care[12] highlight the importance of continued follow-up through adulthood, to assess long-term morbidity, mortality and treatment needs. Such data will be critical in informing future care in the UK/Ireland and across resource-limited settings where the first waves of adolescent survivors of perinatal HIV are emerging [4].
Supplementary Material
summary.
One-third of the UK/Ireland national paediatric HIV cohort has transferred to adult care. Three-quarters of adolescents were on ART at transfer, of whom 74% were virologically suppressed <400cps/mL. The prevalence of triple class resistance was 12%.
Acknowledgements
CHIPS Steering Committee: K Butler, K Doerholt, C Foster, DM Gibb, A Judd, N Klein, EGH Lyall, E Menson, K Prime, A Riordan, D Shingadia, PA Tookey, G Tudor-Williams, S Welch, MRC Clinical Trials Unit: IJ Collins, C Cook, DM Gibb, A Judd, L Harper, F Parrott, A Tostevin, D Dobson.
National Study of HIV in Pregnancy & Childhood, UCL Institute of Child Health: PA Tookey, H Peters, C Thorne
UK HIV Drug Resistance Database Steering Committee: C Aitken, D Asboe, A Pozniak, P Cane, D Chadwick, D Churchill, D Clark, S Collins, V Delpech, S Douthwaite, D Dunn, E Fearnhill, K Porter, A Tostevin, E White, C Fraser, AM Geretti, A Hale, S Hué, S Kaye, P Kellam, L Lazarus, A Leigh-Brown, T Mbisa, N Mackie, S Moses, C Orkin, E Nastouli, D Pillay, A Phillips, C Sabin, E Smit, K Templeton, P Tilston, I Williams, H Zhang.
Funding: The Collaborative HIV Paediatric Study is funded by the NHS (London Specialised Commissioning Group) and has received additional support from the PENTA Foundation as well as Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen and Roche.
Footnotes
Conflict of interest: No conflict of interest.
We thank the staff, families & children from the following hospitals who participate in CHIPS (in alphabetical order):
Republic of Ireland: Our Lady's Children’s Hospital Crumlin, Dublin: K Butler, A Walsh. UK: Birmingham Heartlands Hospital, Birmingham: S Scott, Y Vaughan, S Welch; Blackpool Victoria Hospital, Blackpool: N Laycock; Bristol Royal Hospital for Children, Bristol: J Bernatoniene, A Finn, L Hutchison; Calderdale Royal Hospital, Halifax: G Sharpe; Central Middlesex Hospital, London: A Williams; Chelsea and Westminster Hospital, London: EGH Lyall, P Seery; Coventry & Warwickshire University Hospital, Coventry: P Lewis, K Miles; Derbyshire Children’s Hospital, Derby: B Subramaniam; Derriford Hospital, Plymouth: L Hutchinson, P Ward; Ealing Hospital, Middlesex: K Sloper; Eastbourne District General Hospital, Eastbourne: G Gopal; Glasgow Royal Hospital for Sick Children, Glasgow: C Doherty, R Hague, V Price; Great Ormond St Hospital for Children, London: H Bundy, M Clapson, J Flynn, DM Gibb, N Klein, A Bamford, D Shingadia; Halliwell Children’s Centre, Bolton: P Ainsley-Walker; Harrogate District Hospital, Harrogate: P Tovey; Homerton University Hospital, London: D Gurtin; Huddersfield Royal Infirmary, Huddersfield: JP Garside; James Cook Hospital, Middlesbrough: A Fall; John Radcliffe Hospital, Oxford: D Porter, S Segal; King's College Hospital, London: C Ball, S Hawkins; Leeds General Infirmary, Leeds: P Chetcuti, M Dowie; Leicester Royal Infirmary, Leicester: S Bandi, A McCabe; Luton and Dunstable Hospital, Luton: M Eisenhut; Mayday University Hospital, Croydon: J Handforth; Milton Keynes General Hospital, Milton Keynes: PK Roy; Newcastle General Hospital, Newcastle: T Flood, A Pickering; Newham General Hospital, London: S Liebeschuetz; Norfolk & Norwich Hospital, Norwich: C Kavanagh; North Manchester General Hospital, Manchester: C Murphy, K Rowson, T Tan; North Middlesex Hospital, London: J Daniels, Y Lees; Northampton General Hospital, Northampton: E Kerr, F Thompson; Northwick Park Hospital Middlesex; M Le Provost, A Williams; Nottingham City Hospital, Nottingham: L Cliffe, A Smyth, S Stafford; Queen Alexandra Hospital, Portsmouth: A Freeman; Raigmore Hospital, Inverness: T Reddy; Royal Alexandra Hospital, Brighton: K Fidler; Royal Belfast Hospital for Sick Children, Belfast: S Christie; Royal Berkshire Hospital, Reading: A Gordon; Royal Children’s Hospital, Aberdeen: D Rogahn; Royal Cornwall Hospital, Truro: S Harris, L Hutchinson; Royal Devon and Exeter Hospital, Exeter: A Collinson, L Hutchinson; Royal Edinburgh Hospital for Sick Children, Edinburgh: L Jones, B Offerman; Royal Free Hospital, London: V Van Someren; Royal Liverpool Children’s Hospital, Liverpool: C Benson, A Riordan; Royal London Hospital, London: A Riddell; Royal Preston Hospital, Preston: R O’Connor; Salisbury District General Hospital, Salisbury: N Brown; Sheffield Children's Hospital, Sheffield: L Ibberson, F Shackley; Southampton General Hospital, Southampton: SN Faust, J Hancock; St George's Hospital, London: K Doerholt, S Donaghy, K Prime, M Sharland, S Storey; St Luke’s Hospital, Bradford: S Gorman; St Mary’s Hospital/Imperial College Healthcare NHS Trust, London: C Foster, EGH Lyall, C Monrose, S Raghunanan, P Seery, G Tudor-Williams, S Walters; St Thomas’ Hospital (Evelina Children’s Hospital), London: R Cross, E Menson; Torbay Hospital, Torquay: J Broomhall, L Hutchinson; University Hospital Lewisham, London: D Scott, J Stroobant; University Hospital of North Staffordshire, Stoke On Trent: A Bridgwood, P McMaster; University Hospital of Wales, Cardiff: J Evans, T Gardiner; Wexham Park, Slough: R Jones; Whipps Cross Hospital, London: K Gardiner;
References
- 1.UNAIDS. Children and HIV: Fact Sheet. [Accessed Access date: 25 July 2016]; Available at: http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf.
- 2.Dollfus C, Le Chenadec J, Faye A, et al. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10) Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(2):214–24. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
- 3.Foster C, Judd A, Tookey P, et al. Young people in the United Kingdom and Ireland with perinatally acquired HIV: the pediatric legacy for adult services. AIDS Patient Care STDS. 2009;23(3):159–66. doi: 10.1089/apc.2008.0153. [DOI] [PubMed] [Google Scholar]
- 4.Slogrove A, A J, Leroy A, Collaboration CIfPHEaRCGC . AIDS 2015. Durban, South Africa: 2016. The epidemiology of perinatally HIV-infected adolescents: a CIPHER cohort collaboration global analysis (WEACO301) [Google Scholar]
- 5.Kyu HH, Pinho C, Wagner JA, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA pediatrics. 2016;170(3):267–87. doi: 10.1001/jamapediatrics.2015.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahy M. Explaining global adolescent mortality data: where do the numbers come from and what do they mean? (Abstract TUSY0902). International AIDS Conference 2016; Durban, South Africa. 2016. [Google Scholar]
- 7.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28(13):1945–56. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18579. doi: 10.7448/IAS.16.1.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–53. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 10.Delpech V. Quality of HIV care in the United Kingdom is excellent and improving: over 80% of diagnosed patients are virologically suppressed. 22nd Annual Conference of British HIV Association (BHIVA); Manchester. 2016. [Google Scholar]
- 11.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28(3):128–35. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agwu AL, Lee L, Fleishman JA, et al. Aging and loss to follow-up among youth living with human immunodeficiency virus in the HIV Research Network. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2015;56(3):345–51. doi: 10.1016/j.jadohealth.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judd A, Sohn AH, Collins IJ. Interventions to improve treatment, retention and survival outcomes for adolescents with perinatal HIV-1 transitioning to adult care: moving on up. Curr Opin HIV AIDS. 2016 doi: 10.1097/COH.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 14.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clinical infectious diseases. 2007;45(7):918–24. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty R, Smith CJ, Dunn D, et al. HIV-1 drug resistance in HIV-1-infected children in the United Kingdom from 1998 to 2004. Pediatr Infect Dis J. 2008;27(5):457–9. doi: 10.1097/INF.0b013e3181646d6f. [DOI] [PubMed] [Google Scholar]
- 16.Wensing AM, Calvez V, Gunthard HF, et al. 2015 Update of the Drug Resistance Mutations in HIV-1. Top Antivir Med. 2015;23(4):132–41. [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Mulder M, Yebra G, Navas A, et al. High drug resistance prevalence among vertically HIV-infected patients transferred from pediatric care to adult units in Spain. PLoS One. 2012;7(12):e52155. doi: 10.1371/journal.pone.0052155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Linden D, Lapointe N, Kakkar F, et al. The young and the resistant: HIV-infected adolescents at the time of transfer to adult care. J Ped Infect Dis. 2012:1–4. doi: 10.1093/jpids/pis106. [DOI] [PubMed] [Google Scholar]
- 20.Cordova E, Yanez J, Rodriguez Ismael CG. Safety and efficacy of antiretroviral therapy in perinatally HIV-1 infected patients following transition to an adult HIV-care hospital with virological failure in Buenos Aires, Argentina. Enferm Infecc Microbiol Clin. 2015;33(2):134–5. doi: 10.1016/j.eimc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Lewis J, Walker AS, Castro H, et al. Age and CD4 Count at Initiation of Antiretroviral Therapy in HIV-Infected Children: Effects on Long-term T-Cell Reconstitution. Journal of Infectious Diseases. 2012;205(4):548–56. doi: 10.1093/infdis/jir787. [DOI] [PubMed] [Google Scholar]
- 22.Patel K, Hernan MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(11):1751–60. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. [Accessed Accessed 10 January 2016]; Available at: Mhttp://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. [PubMed]
- 24.Van Dyke RB, Patel K, Kagan RM, et al. Antiretroviral Drug Resistance Among Children and Youth in the United States With Perinatal HIV. Clinical Infectious Diseases. 2016;63(1):133–7. doi: 10.1093/cid/ciw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Contreras GA, Bell CS, Del Bianco G, et al. Incidence and predictors of antiretroviral resistance in perinatally HIV-1 infected children and adolescents. Journal of Infection. 72(3):353–61. doi: 10.1016/j.jinf.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Croucher AP, Jose S, McDonald S, Foster C, Fidler S. Sexual and reproductive health in a UK cohort of young adults perinatally infected with HIV. Sexually transmitted infections. 2013;89(5):392–4. doi: 10.1136/sextrans-2012-050831. [DOI] [PubMed] [Google Scholar]
- 27.Committee PS. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV medicine. 2009;10(10):591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 28.Hammer SM. Baby Steps on the Road to HIV Eradication. New England Journal of Medicine. 2013;369(19):1855–7. doi: 10.1056/NEJMe1309006. [DOI] [PubMed] [Google Scholar]
- 29.Fish R, Judd A, Jungmann E, O'Leary C, Foster C, Network HIVYP Mortality in perinatally HIV-infected young people in England following transition to adult care: an HIV Young Persons Network (HYPNet) audit. HIV medicine. 2014;15(4):239–44. doi: 10.1111/hiv.12091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.