Abstract
Antiphospholipid syndrome (APS) is characterized by thrombosis and/or pregnancy complications in the presence of persistent antiphospholipid antibodies (APLA). Laboratory diagnosis of APLA depends upon the detection of a lupus anticoagulant, which prolongs phospholipid-dependent anticoagulation tests, and/or anticardiolipin (aCL) and anti-β2-glycoprotein-1 (β2GPI) antibodies. APLA are primarily directed towards phospholipid binding proteins. Pathophysiologic mechanisms underlying thrombosis and pregnancy loss in APS include APLA induced cellular activation, inhibition of natural anticoagulant and fibrinolytic systems, and complement activation, among others. There is a high rate of recurrent thrombosis in APS, especially in triple positive patients (patients with lupus anticoagulants, aCL and anti-β2GPI antibodies), and indefinite anticoagulation with a vitamin K antagonist is the standard of care for thrombotic APS. There is currently insufficient evidence to recommend the routine use of direct oral anticoagulants (DOAC) in thrombotic APS. Aspirin with low molecular weight or unfractionated heparin may reduce the incidence of pregnancy loss in obstetric APS. Recent insights into the pathogenesis of APS have led to the identification of new potential therapeutic interventions, including anti-inflammatory and immunomodulatory therapies. Additional research is needed to better understand the effects of APLA on activation of signaling pathways in vascular cells, to identify more predictive biomarkers that define patients at greatest risk for a first or recurrent APLA-related clinical event, and to determine the safety and efficacy of DOACs and novel anti-inflammatory and immune-modulatory therapies for refractory APS.
Keywords: antiphospholipid syndrome, antiphospholipid antibody, thrombosis, anti-β2-glycoprotein-1, domain 1 anti-β2-glycoprotein-1
1. INTRODUCTION
The anti-phospholipid syndrome (APS) is a systemic autoimmune disorder characterized by recurrent thrombosis and/or obstetrical morbidity along with persistent anti-phospholipid antibodies (APLA), including lupus anticoagulant (LA), anti-β2-glycoprotein I (anti-β2GPI) and/or anti-cardiolipin (aCL) antibodies.1 APS is one of the most common acquired thrombophilias, and unlike most of the genetic thrombophilias, ise associated with both venous and arterial thrombosis. The deep veins of the lower extremities and the cerebral arterial circulation are the most commonly affected venous and arterial sites, respectively.2 Thrombosis can also occur in more unusual locations such as the hepatic veins, visceral veins or cerebral venous circulation; indeed development of thrombosis at unusual sites should prompt an evaluation for antiphospholipid antibodies as positive studies may inform therapy. A small number of patients (<1%) develop catastrophic anti-phospholipid syndrome (CAPS),1,3, 4 defined as small vessel thrombosis in three or more organs in less than one week in the presence of APLA, with histopathologic confirmation of small vessel thrombosis in the absence of inflammation.4 CAPS, which is often triggered by a precipitating event such as infection,5, 6 is associated with high (50%) mortality, mostly due to cerebral and cardiac thrombosis, infections and multi-organ failure.3, 7 Obstetrical morbidity in APS includes the unexplained death of one or more morphologically normal fetuses at or beyond the 10th week of gestation, the premature birth of one or more morphologically normal neonates before the 34th week of gestation because of either eclampsia or severe preeclampsia, and/or three or more unexplained, consecutive spontaneous abortions before the 10th week of gestation.1 Other ‘non-criteria’ clinical associations of APLA include thrombocytopenia, livedo reticularis, skin ulcers, and transient ischemic attacks.7 These symptoms, along with thrombosis or pregnancy loss, should alert clinicians to this diagnosis.
While much knowledge concerning the clinical manifestations of APS has been acquired, the pathogenesis of this disorder remains poorly understood. For example, it is difficult to predict who will develop APS, or why. Though specific serological characteristics of APLA provide insight into which patients with these antibodies are more likely to develop clinical manifestations, our ability to accurately risk-stratify patients with these antibodies remains limited. Finally, a number of mechanisms by which APLA may induce thrombosis have been reported, although whether these are truly distinct, reflective of antibody heterogeneity, or represent different manifestations of a single, central pathway that has not been defined has remained elusive.
2. DIAGNOSIS OF APS
The Sapporo classification criteria for APS were first proposed in 1999,8 and updated at the Eleventh International Congress on Antiphospholipid Antibodies in Sydney in 20061. While these are often used in practice as diagnostic criteria, it should be noted that they were originally developed to define a uniform cohort of APS patients for clinical studies rather than to provide a system for clinical diagnosis. Patient must have both clinical and laboratory criteria to meet a diagnosis of APS. Clinical criteria include either objectively confirmed venous, arterial or small vessel thrombosis, or obstetric morbidity including the unexplained death of one or more morphologically normal fetuses at or beyond the 10th week of gestation, the premature birth of one or more morphologically normal neonates before the 34th week of gestation, and/or three or more unexplained, consecutive spontaneous abortions before the 10th week of gestation (Table 1).1 Since these clinical manifestations are prevalent in the general population and may have a multifactorial etiology, laboratory investigations are central to the diagnosis of APS. The updated Sydney classification scheme also requires specific laboratory criteria: a lupus anticoagulant detected according to guidelines published by the International Society on Thrombosis and Hemostasis (ISTH) (Table 2),9, 10 anticardiolipin (aCL) antibodies (IgG or IgM) exceeding 40 IgG or IgM antiphospholipid units, or anti-β2GPI antibodies (IgG or IgM) at levels exceeding the 99th percentile, measured by enzyme-linked immunosorbent assay (ELISA). To minimize the risk of making a diagnosis based on transient ntiphospholipid antibodies, the recommendations are to perform assays on two separate occasions, at least twelve weeks apart.9, 10 The major changes made in the 2006 revision of the Sapporo criteria were that anti-β2GPI antibody was included for the first time and a recommendation was made to classify patients into those with only one positive APLA and those with two or three positive APLA1, based on accumulating information suggesting that positivity in more than a single assay was associated with higher thrombotic risk.11
Table 1.
Summary of the Sydney Consensus Statement on Classification of APS.*
| Antiphospholipid antibody syndrome (APS) is present if at least one of the clinical criteria and one of the laboratory criteria are met. |
|
|
| CLINICAL CRITERIA |
|
|
|
|
|
| a Investigators are strongly advised to classify subjects with obstetrical morbidity according to groups a, b, and c in populations of patients with more than one type of pregnancy morbidity. |
|
|
| LABORATORY CRITERIAb |
|
|
|
|
|
| bInvestigators are strongly urged to classify APS patients into one of the following categories: |
| I – more than one laboratory criteria present (any combination) |
| IIa – LA present alone |
| IIb – aCL present alone |
| IIc – anti- β2GPI present alone |
Adapted from Miyakis et al. J Thromb Haemost. 2006;4:295–3061
Table 2.
International Society on Thrombosis and Hemostasis criteria for the laboratory identification of lupus anticoagulant9.
Positive screening test (phospholipid-dependent coagulation assay)
|
Evidence of inhibition in mixing studies (exclude factor deficiency)
|
Evidence that inhibition is phospholipid dependent
|
Exclusion of coagulation inhibitors
|
When used as a diagnostic tool for APS, the “Sapporo”, and subsequent “Sydney” classification criteria have several shortcomings. For example, there is uncertainty as to whether patients with “non-criteria” APS manifestations other than thrombosis or pregnancy morbidity, such as immune thrombocytopenia, transient ischemic attacks, livedo reticularis, non-bacterial cardiac valve thickening and/or vegetations, and autoimmune hemolytic anemia, among others,7 and those with deep venous thrombosis but only low to moderate titers of anti-β2GPI and/or ACL antibodies should be considered as having APS. In addition, the clinical significance of IgA aCL or anti-β2GPI antibodies remain uncertain. Finally, the association of antibodies directed against antigens such as phosphatidylserine, phosphatidyethanolamine or other anionic or polar phospholipids with clinical manifestations of APS remain uncertain.
Additionally, analytical issues also add to the challenges of APLA testing. There is significant inter-laboratory variability, particularly in solid phase assays for anti-β2GPI and aCL antibodies,.12–15 Patient factors also affect testing. For example, anticoagulation with heparins or direct oral anticoagulants (DOACs) can lead to false positive tests for LA, even in the presence of a normal APTT16.17, 18 Martinuzzo et al. reported prolongation of the APTT, SCT (silica clotting time) and DRVVT in patients on rivaroxaban, dabigatran, or LMWH, who had negative tests for LA at baseline, had. LMWH was frequently associated with DRVVT prolongation (81–100%), while APTT and SCT prolongation were less prevalent (13% to 100% depending on dose).19 Warfarin variably affects clotting time ratios in LA assays. These issues have led to discrepant recommendations within different guidelines.20 Many authors advocate performance of LA studies using a 1:1 mix of patient and normal plasma, to reduce the effects of warfarin-induced reduction in clotting factor levels on LA testing (though un(der)carboxylated prothrombin may have its own anticoagulant effect). Taipan snake venom is a more “LA specific” reagent that can be used for LA diagnosis even in the presence of warfarin or rivaroxaban; however, these tests are not widely available in clinical practice.21, 22
The three types of laboratory tests for APLA detect antibodies - may have distinct or overlapping specificities. Antibodies directed against β2GPI or prothrombin are the most common cause of a positive LA test. Anti- β2GPI antibodies are associated with a higher risk of thrombosis.12, 23,24 In a recent study, an increased risk of thrombosis was strongly associated with β2GPI dependent LA [Odds Ratio (OR) 42.3; 95% confidence interval (CI) 9.9–194.3] but not with non-β2GPI dependent LA (OR 1.6; 95%CI 0.8–3.9).25 Epitope specificity also appears to be important. Emerging data suggests that APLA with specificity toward domain 1 of β2GPI may be more closely associated with thrombosis and pregnancy loss than those detected using antibodies to intact β2GPI.26 A commercial immunoassay for these antibodies is available (Quanta Flash β 2 GPI-domain1, Inova Diagnostics) but has not been widely adopted into clinical practice.
Patients that have thrombosis or fetal loss, often with other non-criteria manifestations, but with absent or sub-threshold diagnostic laboratory studies for APLA are sometimes termed as having “seronegative APS”.27, 28 This is a poorly-defined disorder with an uncertain relationship with APS, and we recommend against its routine use until its pathogenesis is better defined. Some individuals with reported ‘seronegative APS’ have IgA antibodies against aCL or β2GPI,28–32 or APLA specific for phosphatidylethanolamine and other antigens,33–35 though the frequency of these positive tests compared to normal individuals is uncertain.
3. PATHOGENESIS OF APS
3.1 Antiphospholipid antibodies
APLA were originally thought to react with anionic phospholipids such as cardiolipin, however, it is now known that that most APLA are directed against phospholipid binding proteins expressed on, or bound to, an appropriate surface such as a cellular membrane.36 Anti-β2GPI antibodies appear to be central to the pathogenesis of APS,12, 37 although other antigenic targets such as prothrombin have been described.12, 38, 39 Affinity-purified anti-β2GPI antibodies from patients with APS potentiate thrombosis in a mouse model,40 and or anti-β2GPI antibodies, are associated with a higher risk of thrombosis than aCL or anti-prothrombin antibodies.12, 23
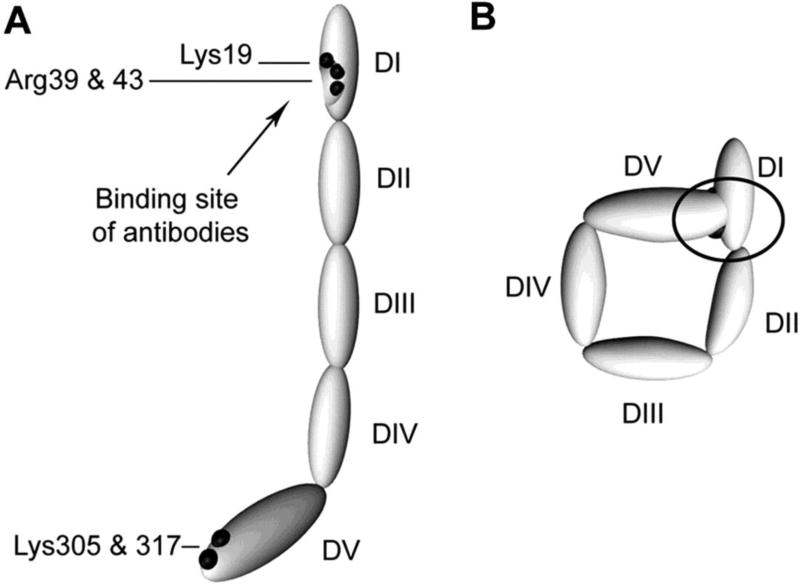
Beta-2-glycoprotein I is a plasma glycoprotein comprised of 5 ‘sushi’ domains. The first domain contains the binding site for most anti-β2GPI APLA while the fifth domain binds anionic phospholipid.41, 42 Agar et al. proposed two different conformations for β2GPI: a circulating, ‘circular’ form in which domain1 is not exposed and associates with domain 5, and an unfolded, “fishhook” conformation in which the antigenic region within domain 1 is exposed (Figure 1). Predominance of the circular form of β2GPI in the circulation may account for the absence of circulating β2GPI-containing immune complexes in patients with APLA. Unfolding of β2GPI with assumption of the “fishhook” conformation and exposure of domain 1 is likely occurs upon binding to phospholipid, though the mechanisms regulating the β2GPI conformational change are not well understood..41,41
Figure 1. Proposed structures of the open and closed forms of β2GPI.
DI-DV represent the 5 domains of β2GPI. (A) β2GPI structure in the “open” form, as identified by its crystal structure. In this conformation, often referred to the “fish hook” conformation, an epitope containing Lys19, Arg39 and Arg43 that is recognized by anti-β2GPI domain 1 antibodies is exposed. β2GPI incubated at high pH adopts this conformation, and it is proposed that binding of anionic phospholipid results in similar conformational changes. (B) The “circular” form of β2GPI. This conformation is suggested by electron microscopy of circulating plasma β2GPI. In this conformation, the epitopes recognized by anti-β2GPI domain 1 antibodies are not available, which is thought to explain the fact that circulating immune complexes are not present in patients with antiphospholipid antibodies. This conformation is proposed to be maintained by interactions between domain 1 and domain 5. Adapted from Agar et al, Blood 116:1336, 2010 with permission.
Evaluation of sera from patients with APS as well as individuals with so- called ‘seronegative APS’ has revealed autoantibodies directed against several other antigenic targets including phosphatidylserine, phosphatidyl ethanolamine, annexin II,43, 44 annexin A5,45 and vimentin/cardiolipin complexes.28, 39 However, the significance of these antibodies is not fully understood, since assays for their measurement are not standardized, their incidence in the general population is uncertain, and antibodies to some antigens such as vimentin/cardiolipin complexes may be common in patients with rheumatologic disease. Thus, measurement of such antibodies is generally discouraged outside the scope of a clinical study
3.2 Interactions with the coagulation and fibrinolytic systems
Inhibition of natural anticoagulant activity, particularly that of the protein C system, was the first identified prothrombotic mechanism of APLA.46–49 APLA impair the activation of protein C, as well as the ability of activated protein C to inactivate factors V and VIII.50, 51 These activities are mediated by antibodies to β2GPI and/or prothrombin,52–55 and phosphatidylethanolamine plays a critical role.56 APLA or associated antibodies also inhibit heparin binding and activation of antithrombin,57 and the activity of tissue factor pathway inhibitor.58 Antiphospholipid antibodies also neutralize the ability of β2GPI to stimulate the activity of tissue-type plasminogen activator, which inhibits fibrinolysis..59 Anti-β2GPI antibodies have also been reported to impair the ability of β2GPI to inhibit VWF-dependent platelet aggregation,60 and complement activation.61 Finally, elevated levels of coagulation factor XI are a risk factor for thrombosis in the general population62, and one report indicated that APS patients have higher levels of the active free thiol form of factor XI compared with age and sex matched controls.63
Annexin A5 binds to phosphatidylserine and forms a two dimensional lattice, or ‘anticoagulant shield’, over phospholipid bilayers,64 which inhibits phospholipid-mediated procoagulant activity. While this phenomenon has been primarily studied in vitro, substantial amounts of annexin A5 have been observed on the surface of placental villi,65, 66 although similar findings have not been reported with respect to vascular endothelium. In the presence of β2GPI and anti-β2GPI antibodies, the annexin V lattice is disrupted, exposing procoagulant phosphatidylserine.67, 68 In vitro experiments indicate that hydroxychloroquine protects the annexin V shield,69, 70 and has efficacy against APLA mediated thrombosis in a murine model.71 Loss of annexin A5 on placental trophoblasts and endothelial cells is a potential mechanism for APLA induced pregnancy loss.65, 66 Anti-β2GPI antibody also binds to β2GPI on placental trophoblasts resulting tissue factor and complement mediated neutrophil activation, trophoblast injury, inhibition of growth and differentiation, and fetal loss in animal models.72
3.3 Cellular activation
APLA, particularly anti-β2GPI, activate vascular cells, including endothelial cells,73–75 monocytes,76, 77 neutrophils,78 and platelets.79–81 Endothelial cell activation transforms the normally anticoagulant endothelial surface to a procoagulant phenotype.82 Activated endothelial cells demonstrate increased expression of adhesion molecules (E-selectin, VCAM-1, ICAM-1),74, 83, 84 von Willebrand Factor85, tissue factor (TF),86 and proinflammatory cytokines,87 decreased levels of endothelial cell-derived nitric oxide88 and release microparticles with pro-inflammatory and procoagulant properties89,90, 91 Monocyte activation may also be of importance in the pathogenesis of APS. Early studies demonstrated that anti-β2GPI antibodies activate monocytes in an annexin A2-dependent manner through a pathway involving Toll-like receptor 4 (TLR4) in lipid rafts.77 More recent studies have implicated other members of the TLR pathway as well, such as TLR2 and TLR7.92, 93 Like endothelial cells, activated monocytes express tissue factor and elaborate inflammatory cytokines,77 and circulating monocytes in patients with APS display an activated gene signature.94 Neutrophils are activated by APLA, releasing neutrophil extracellular traps (NETS)78, and expressing an interferon gene signature78. Interactions of β2GPI and APLA with platelets are not as well characterized. There is no convincing evidence for binding of β2GPI or anti-β2GPI antibodies to unstimulated platelets. However, under flow conditions, APLA induce adhesion of platelets to collagen in a platelet glycoprotein 1b and apoER2 dependent manner,79, 80 while in non-flow conditions, APLA potentiate platelet activation in the presence of sub-threshold thrombin.81
In vivo, mice deficient in annexin A2, TLR4, or apoER2, or treated with an NF-ĸB inhibitor are relatively protected from thrombosis following the injection of patient-derived APLA.95–98 However, no models in which mice develop spontaneous thrombosis following treatment with APLA have been described, leading some to postulate that thrombosis associated with APLA may involve a “two hit” process. For example, circulating APLA may provide the first hit by inducing a generalized procoagulant state due to inhibition of natural anticoagulant and/or fibrinolytic activities, or activation of vascular endothelial cells and circulating leukocytes leading to increased levels of microparticles, extracellular DNA, or other procoagulant materials. In this scenario, a second hit may consist of additional localized vascular injury, perhaps an infectious or inflammatory stimulus, leading to development of a thrombus. Alternatively, APLA may serve as the second hit in patients in whom the first hit involves underlying systemic hypercoagulability due to factors such as oral contraceptives, obesity, genetic thrombophilia, or others.
3.4 Complement activation
An important role of complement activation in APS was first demonstrated in murine models of APLA-associated pregnancy loss.99, 100 Complement products C3a and C5a were believed to cause placental inflammation and mice deficient in C3, C4, C5, or C5a receptors were protected from fetal loss induced by APLA IgG.101 Subsequently, it has been demonstrated that complement activation contributes to APLA mediated thrombosis in mice as demonstrated by the ability of C5 inhibitors to prevent thrombosis in animals receiving infusion of anti-β2GPI antibodies.102–104 Complement activation by APLA leads to generation of the anaphylotoxin C5a, which recruits monocytes and neutrophils, activates endothelial cells and induces expression of tissue factor.105, 106 However, while increased levels of complement activation products have been detected in patients with APS, these have not been demonstrated to correlate with thrombosis.107 Complement factor H (CFH), a homologue of β2GPI, also binds to anionic phospholipids and some patients with APS have antibodies against CFH;108 however their role in the pathogenesis of thrombosis has not been demonstrated. There is evidence supporting activation of the classical and alternative complement pathways in patients with catastrophic APS,109 and eculizumab (humanized anti-C5a monoclonal antibody) has been successfully used in CAPS and APS complicating renal transplantation.110–114
3.5 Disruption of innate immunity and generation of APLA
The origin of APLA remains enigmatic but is thought to be due to a loss of immune tolerance and perturbations of innate immunity, possibly triggered by an infectious stimulus. APLA can be induced in mice sensitized with a CMV-derived peptide,115 and protein H of Streptococcus pyogenes has been shown to bind β2GPI and expose hidden epitopes that may induce production of anti-β2GPI antibodies.116, 117 Oxidation of β2GPI by reactive oxygen and nitrosative species also increases its immunogenicity.118 In a murine model, TLR4 ligands can trigger a break in immune tolerance and induce production of APLA and a SLE like disease.119 TLR7, TLR8 and TLR9 may also contribute to the development of pathogenic APLA through influencing B cell autoreactivity.120–123 Inhibition of these receptors is a potential therapeutic target in patients with SLE and APLA.
4. THROMBOTIC RISK ASSESSMENT IN APS
Thrombotic risk prediction in APS is challenging. While sufficient information may be gleaned from antiphospholipid antibody lab testing to provide an approximate odds ratio for thrombosis prediction, direct extrapolation of these ratios to a specific individual is difficult. A major problem in the APS field is the identification of robust, easily measurable clinical and laboratory biomarkers that accurately predict the risk of cardiovascular events.
4.1 APLA profile and thrombosis
Current tests for APLA detect antibodies that may have distinct or overlapping specificities. LA positivity is more strongly associated with both arterial and venous thrombosis than either aCL or anti-β2GPI antibodies.6 However, there is significant variation in the strength of this association \in different studies that could be from differences in methodology including methods used to detect LA, and the variable inclusion of LA that were not persistently positive. Retrospective and prospective studies do not show a consistent association between thrombosis and aCL.12, 124 Since β2GPI is the primary antigen in APS, the anti-β2GPI antibody was initially proposed as the more clinically relevant and predictive APLA and several early retrospective studies showed that anti-β2GPI antibodies indeed correlate with thrombotic risk;125–127 though these results have not been universally confirmed and recent studies suggest that the thrombotic risk conferred by anti-β2GPI antibodies may be modest, with OR between 1.5–2.5.125
More definitively, patients that are have two or more types of APLA (LA, aCL, and/or anti-β2GPI) appear to be at higher risk of developing thrombosis. In the Warfarin in Antiphospholipid Syndrome (WAPS) study, patients with LA and anti-β2GPI antibodies had a significantly increased risk for thrombosis (OR 4.1, 95% CI 1.3–13.5).12 In a retrospective analysis of 160 APS patients positive for LA, aCL and anti-β2GPI - so called ‘triple positive’ patients- the cumulative incidence of recurrent thrombosis was 12.2%, 26.1% and 44.2% after 1, 5 and 10 years of follow up, respectively..11 In a multicenter prospective study of 102 ‘triple positive’ patients, the rate of first thrombosis was 5.3% per year, with a cumulative incidence of 37.1% over 10 years.128 Similarly,, the annual rate of a first thrombotic event in female APLA carriers with double or triple positivity (1.27%) was twice as high as that in women with single positivity (0.65%).129 In a prospective case control study, triple positivity was also a risk factor for pregnancy failure [OR 4.1 (95% CI 1.o-16.7) in women with primary APS.130 Such results may reflect not only the presence of the three types of antibodies, but also the fact that these patients had high levels of antibodies fully meeting positive criteria as defined by the Sydney classification scheme. Indeed, it has been suggested that triple positivity for APLA may reflect not only specificity, but high levels of antibodies with sufficient titer and activity to cause positivity in all three assays.
IgG anti-β2GPI-domain1 antibodies appear to be more strongly associated with a history of thrombosis and obstetrical morbidity compared to antibodies to other regions of the protein.26, 131 Pengo et al. demonstrated that IgG anti-β2GPI domain 1 antibodies were more likely to persist at 12 weeks, were associated with triple positivity, and correlated with thrombotic risk.132 In a recent study, anti-β2GPI-domain1 antibodies predicted clinical events with an OR of 17 (95% CI, 7.1–40.5) although they did not add to the diagnostic accuracy of the standard APLA panel.133
4.2 Clinical risk scores
In addition to the APLA profile, several risk scores combining clinical and/or laboratory findings have been developed in an attempt to better identify individuals at risk of APS-related thrombosis. The antiphospholipid score (aPL-S) includes LA, aCL and anti-β2GPI positivity and titers, and was developed to predict risk of APS related clinical events in patients with autoimmune disorders,134 and subsequently validated in two independent sets of patients with autoimmune diseases.134 The global antiphospholipid syndrome score (GAPSS) was initially developed to predict both APS-related thrombosis and pregnancy loss in a cohort of patients with SLE.135 In contrast to the aPL-S, the GAPSS included conventional cardiovascular risk factors in addition to APLA profiles; points assigned on the basis of a multivariable prediction model were: 3 for hyperlipidemia, 1 for arterial hypertension, 5 for aCL IgG/IgM, 4 for anti-β2GPI IgG/IgM, 3 for anti-phosphatidylserine/prothrombin IgG/IgM and 4 for LA) and appeared to improve prediction of APS-related clinical events compared to APLA profile alone; a score GAPSS values ≥10 had the best diagnostic accuracy. The authors subsequently validated this score in a cohort of patients with primary APS.136 The score was prospectively validated in a group of patients with APS and SLE, in which a GAPSS >16 predicted thrombosis. Independent validation of the GAPSS in a Japanese cohort of patients with autoimmune disease as well as primary APS also confirmed higher scores in patients with thrombosis, with maximum diagnostic accuracy for GAPSS >6, but could not verify it’s predictive value for pregnancy loss.137 Also, the optimal cutoff on the GAPSS score was different in all of these studies, which may be attributed to differences in the baseline characteristics of the cohort. While the GAPSS score may add to the utility of APLA in predicting thrombosis in APS, it still needs to be validated in patients with asymptomatic APLA and its superiority to standard APLA testing alone requires further confirmation. Additionally, this score includes anti-phosphatidylserine/prothrombin IgG/IgM that are not routinely performed, which limits its’ widespread application.
4.3 Other thrombotic risk factors
The presence of other cardiovascular risk factors are of great importance in predicting the risk of thrombosis in patients with APS, since these may 1) increase thrombotic risk, and 2) be modifiable. While persistence and high levels of APLA are the major risk factor for thrombosis in APS,1 these patients may also have other risk factors that contribute to thrombotic risk. For example, in the RATIO study, a large multicenter study examining the risk factors for arterial thrombosis in women less than 50 years of age, the presence of LA (DRVVT ratio ≥ 1.15) was associated with an OR of 5.3 (95% CI 1.4–20.8) for myocardial infarction, and 43.1 (95% CI 12.2–152) for stroke.124 However, in women with LA taking oral contraceptives, the OR for these events increased dramatically; for MI, 21.6 (95% CI 1.9–242.0), and for stroke, 201.0 (95% CI 22.1–1828.0). Similar increases in the OR for arterial events were seen in women with LA who smoked. Multiple other risk factors such as the presence of SLE, inherited thrombophilia, immobilization, pregnancy and atherosclerotic cardiovascular disease may also increase thrombotic risk.
5. MANAGEMENT OF THROMBOSIS IN APS
Since most studies imply a high rate of recurrent thrombosis in patients with APS, long-term anticoagulation with a vitamin K antagonist (VKA) is the standard of care for patients who develop thrombosis.138 Garcia et al. conducted a careful systematic review of 8 prospective studies that evaluated patients with a first APS-related thrombotic event and reported that the rate of recurrent thrombosis after stopping anticoagulation in patients with APLA was 40% higher than in those with VTE unrelated to APLA [unadjusted relative risk 1.4 (95% CI 0.99–2.36)].139 The overall quality of the studies was poor due to widely varying inclusion and exclusion criteria, the use of non-standard cutoffs for APLA positivity and laboratory testing not meeting ISTH criteria. Still, these results support a higher rate of recurrent thrombosis after stopping anticoagulation in patients with APS. Additionally, a significant proportion of patients with APS develop recurrent thrombosis while on therapeutic anticoagulation.7, 11 Patients with thrombotic APS are at higher risk for subsequent cardiovascular mortality than patients without these antibodies138 In the Vienna APS study, a prospective observational cohort, LA positive patients, with or without thrombosis, had higher mortality at 10 years (cumulative relative survival 87%) compared with a reference population matched for age, sex and inclusion year.140
Though direct oral anticoagulants (DOAC) are increasingly used in patients with thrombosis associated with APLA, vitamin K antagonists (VKA) remain the standard of care. However, physicians should be aware that due to subtle effects of LA on the INR, VKA monitoring may be unreliable in a subset of these patients leading to inadequate anticoagulation despite apparent therapeutic INRs.141 The INR in patients with LA may be highly dependent upon the thromboplastin used in the assay,142 with the majority of LA patients having a mildly INR at baseline when tested against a panel of thromboplastins.142 One report found that approximately 10% of patients with LA treated with VKA may have a falsely prolonged INR, when therapeutic ranges were directly compared with a chromogenic factor X assay141. It has been suggested that once patients with LA treated with VKA achieve a therapeutic INR (2.0–3.0), levels of prothrombin should be checked to assure therapeutic levels in the range of 25–35%.143 Other assays including chromogenic FX assays can also be considered for monitoring VKA in patients with LA. Results of thrombin generation tests correlate with LA activity,144 and these may also prove to be a useful tool to monitor adequacy of anticoagulation. Efthymiou et al. noted that endogenous thrombin potential and peak thrombin generation correlated inversely with INR; however, one in five patients with APS had increased peak thrombin generation that exceeded that expected relative to the intensity of anticoagulation assessed by INR.145
5.1 Venous thrombosis
The standard treatment for thrombotic APS is initial anticoagulation with unfractionated heparin or low molecular weight heparin transitioned to a VKA, commonly warfarin, which is continued indefinitely.. A target international normalized ratio (INR) of 2.5 (2.0–3.0) is recommended. Because a significant subset of these patients develop recurrent thrombosis despite anticoagulation, the adequacy of this INR target has been questioned. However, two randomized trials that compared standard intensity (INR 2.0–3.0) versus high intensity (INR 3.0–4.0) anticoagulation with warfarin in patients with APS found no difference in the rates of recurrent thrombosis or major bleeding, supporting the use of standard intensity anticoagulation.146, 147 The optimal duration of anticoagulation is unclear, the current recommendation is that anticoagulation for secondary prophylaxis should be continued indefinitely.148 While the authors agree with this recommendation, we emphasize that all cases should be considered on an individual basis, and the duration of anticoagulation should be dictated by the patient-specific risk-benefit ratio, weighing the risk of recurrent thrombosis, against the likelihood of bleeding, falls and compliance. Short term (3 to 6 months) anticoagulation may be suitable for patients with APLA who develop thrombosis in the setting of a reversible risk factor, or those who subsequently test negative for APLA.148 While children can develop APLA and thrombosis, their rate of recurrent thrombosis is lower than adults and they may not require long-term anticoagulation.149 Table 3 summarized treatment recommendations for APS and APLA.
Table 3.
Summary of treatment recommendations for APS and APLA.
| Clinical setting | Recommendation | Additional comments |
|---|---|---|
|
| ||
| First venous thrombosis in APS | Anticoagulation with VKA (INR 2–3) | No clear benefit of high intensity anticoagulation |
| Insufficient data to support routine DOAC use | ||
|
| ||
| Arterial thrombosis in APS | ASA + standard intensity anticoagulation with VKA (INR 2–3), or | One study showed no benefit of ASA+VKA over ASA in APS with stroke; results are not generalizable since APLA tested only at baseline and lower target INR |
| High intensity VKA anticoagulation (INR 3–4) in high risk patients | ||
|
| ||
| Recurrent thrombosis in APS | Confirm that therapeutic INR is maintained | No clinical trial data. Based on clinical observation and expert opinion. |
| May consider LMWH or high intensity anticoagulation with VKA (INR 3–4).. | ||
| Consider adjunctive hydroxychloroquine, statin | ||
|
| ||
| Obstetric APS (without prior thrombosis) | ASA + LMWH | |
| Prophylactic dose LMWH continued until 6 weeks post-partum | ||
|
| ||
| Obstetric APS with prior thrombosis | Low dose aspirin with therapeutic dosing of LMWH. | Start LMWH (and stop VKA) at or prior to diagnosis of pregnancy. |
| After pregnancy, may be transitioned back to VKA to continue anticoagulation indefinitely. | ||
|
| ||
| Catastrophic APS | UFH plus high dose steroids (methylprednisolone 1000 mg/kg/day for 3 or more days) | Observational data supports the use of eculizumab, rituximab, plasmapheresis and defibrotide. |
|
| ||
| Asymptomatic APLA | Consider aspirin for patients with other cardiovascular risk factors | No role of primary prophylaxis with aspirin in the absence of cardiovascular risk factors |
| Hydroxychloroquine for patients with concomitant SLE | ||
| Thromboproplylaxis in high risk situations such as surgery or hospitalization | ||
| Address reversible risk factors such as obesity, smoking, combined oral contraceptives | ||
5.2 Arterial thrombosis
The Antiphospholipid Antibodies and Stroke (APASS) study, a subgroup of the Warfarin-Aspirin Recurrent Stroke Study (WARSS), evaluated warfarin versus aspirin for secondary stroke prevention in patients with APLA. The rate of recurrent stroke was similar in the warfarin (INR 1.4–2.8) and aspirin groups;150 however these results may not be generalizable to all patients with APS and stroke, since APLA were tested only once at baseline, and low titer aCL antibodies were included. A small randomized trial that reported lower rates of recurrent stroke in patients treated with aspirin plus warfarin versus aspirin alone,151 however, this study had an unexpectedly high incidence rate of recurrent stroke (8 of 11 in the aspirin arm and 3 of 11 in the combination arm) over a mean follow up of approximately 4 years. There are no other prospective trials of secondary stroke prevention in APS. The 2011 report by a task force of the 13th international congress on APLA suggested that patients with definite APS and arterial thrombosis should be treated with warfarin at an INR >3.0 or combined anti-platelet and anticoagulant (INR 2.0–3.0) therapy.148 However, there was no consensus on the use of high intensity anticoagulation and several members of the task force opined that standard intensity anticoagulation (INR 2–3) is adequate for secondary thromboprophylaxis of arterial events.148 In the setting of inadequate data concerning efficacy and safety, we believe that high intensity anticoagulation should be used very judiciously, if at all in APS patients, and reserved for those with a high-risk APLA profile and additional cardiovascular risk factors in whom the potential benefit outweighs the risk of bleeding.
5.3 Direct oral anticoagulants
The use of VKAs is problematic in some patients because of the need for frequent monitoring and numerous food and drug interactions, as well as the direct effects of LA on accurate monitoring of the INR. Hence, in theory, the direct oral anticoagulants (DOACs) present an attractive alternative. Until recently, published data regarding their use in APS was limited to anecdotal reports in case studies and case series with variable results.152–155 Three case series including a total of 69 (35, 26, and 8) patients with thrombotic APS reported acceptable safety profiles for DOACs with low rates of recurrent thrombosis.156–158 However, other series reported high rates of recurrent thrombosis including arterial events in patients with thrombotic VTE switched to a DOAC, raising major concerns about efficacy. These series represented a selected population of patients who were unable to maintain therapeutic INRs on VKA or failed VKA therapy, and a many of those with recurrent thrombosis had high-risk APS with triple positive APLA or a history of arterial events.152, 153, 159 The recently reported Rivaroxaban in APS (RAPS) study was a phase 2/3, non-inferiority trial that randomized patients with thrombotic APS who were on warfarin to either rivaroxaban or continued warfarin.160 The primary endpoint of the study was a laboratory evaluation of efficacy of anticoagulation through measurement of the endogenous thrombin potential from randomization to day 42. Rivaroxaban did not meet the non-inferiority threshold for the primary endpoint; in fact, the, endogenous thrombin potential was higher in the rivaroxaban group. However, there were no thrombotic events and no clinically significant bleeding in either group over 6 months of follow up.160 Based on this, the authors suggested that rivaroxaban is a safe and effective alternative to warfarin in thrombotic APS. While this data is promising, it must be noted that six months is a relatively short follow-up time for these patients. Also, with only with 54 and 56 patients in the rivaroxaban and warfarin groups, respectively, and the lack of a clinical endpoint, this study was not powered to evaluate clinical efficacy. Additional clinical trials, currently underway, will be required to evaluate the efficacy of DOAC in patients with APS. In the meantime, cannot be generally recommended in these patients, and their use should be limited to individuals who either fail or are intolerant of a VKA or low molecular weight heparin.
5.4 Treatment of refractory thrombotic APS
Rates of recurrent thrombosis in excess of 30% have been reported even in anticoagulated patients with APLA.7 The management of these cases is difficult and there is insufficient evidence to prescribe standardized approaches. The first step is to ensure that the patient is adequately anticoagulated with a target INR in the therapeutic range, as well as a therapeutic factor X level.148, 161 Higher intensity anticoagulation is sometimes used in patients with recurrent venous thromboses. Limited data support the use of low molecular weight heparin as an effective alternative to warfarin in APS;162 this may be used as an alternative in patients who are unable to achieve a therapeutic INR or have recurrent thrombosis despite high intensity anticoagulation (INR >3). For still refractory patients, adjunctive non-anticoagulant therapies such as hydroxychloroquine, statins, and rituximab may be considered;148 however, there are no well-designed clinical studies supporting their use. These agents are discussed below.
6. TREATMENT OF CATASTROPHIC APS
CAPS can be a challenging diagnosis to make in patients without a known history of APLA. A delays in diagnosis can have lethal consequences Early diagnosis is essential to rescue patients with this rapidly progressive, potentially fatal condition. Prospective trials for treatments of CAPS have not been conducted, and are not expected for this rare disorder. The optimal treatment of CAPS is unknown. Based on observational data and expert opinion, anticoagulation with heparin and high dose steroids (methylprednisolone 1000 mg daily for 3 days or longer) are the mainstay of therapy. Additional recommendations include searching for and treating any precipitating factor such as infection and debriding/amputating any necrotic tissues to limit inflammation. Plasma exchange has been shown to improve mortality in the CAPS registry.3 Intravenous immunoglobulin alone does not appear to be beneficial in patients with CAPS.3
Eculizumab has been reported to successfully treat patients with refractory CAPS,110–114 and an ongoing phase 2 study is evaluating the efficacy of eculizumab in preventing recurrent CAPS after renal transplantation (clinicaltrials.gov #NCT01029587). Eculizumab increases the risk of meningococcal infection and patients should be vaccinated before starting treatment.163 The adenosine receptor antiagonist, Defibrotide, has antithrombotic, profibrinolytic, and anti-inflammatory effects on vascular endothelial cells and also blocks neutrophil tissue factor expression. Defibrotide is approved for the treatment of hepatic sinusoidal obstruction syndrome after stem cell transplant. It is believed to act by decreasing endothelial inflammation and has been used to treat refractory CAPS.164
7. MANAGEMENT OF OBSTETRIC APS
The management of obstetrical APS remains controversial, largely because numerous observational and randomized studies used different definitions of recurrent miscarriage, pre-eclampsia or placental insufficiency, and different APLA were tested, with variability in cut-off values for positive tests and in APLA profiles. A prospective observational study reported live births in 71% of pregnancies treated with aspirin in combination with either heparin or LMWH.165 Two randomized studies comparing aspirin have demonstrated an increased rate of live births with aspirin and unfractionated heparin compared with aspirin alone, though different doses of heparin were employed.166, 167 However, two prospective studies evaluating the combination of low molecular weight heparin with aspirin versus aspirin alone could not replicate these results, due primarily to the better than expected outcomes in the aspirin alone group, which had 72% live births.168, 169 A recent Cochrane review concluded that aspirin alone has not been demonstrated to improve pregnancy outcomes in APS.170 A subsequent meta-analysis concluded that the combination of aspirin and unfractionated heparin improves outcomes in obstetric APS while the efficacy of aspirin with LMWH is not proven.171 The most common approach, endorsed by the American College of Chest Physicians guidelines is the combination of heparin (unfractionated or low molecular weight; prophylactic or intermediate dose) and low dose aspirin (75–100 mg) daily for women who fulfill the clinical and serologic criteria for obstetric APS.172 In women with APS and prior thrombosis, aspirin and therapeutic dose LMWH should be employed. Since warfarin has been linked to fetal malformations, women who are anticoagulated with VKA should be switched to LMWH prior to, or as soon as pregnancy is diagnosed. Evidence on how to manage women who meet serological criteria for APLA but do not meet clinical criteria for obstetric APS, or alternatively those with three or more miscarriages and low titer APLA not meeting serologic criteria for APLA is limited to observational studies with conflicting results.173, 174 Therapy with aspirin and LMWH could be considered, on an individual basis, for women with a high-risk APLA profile, concomitant SLE, or pre-eclampsia. Further clinical studies are needed to determine the optimal management of APLA-positive women.
8. NON-ANTICOAGULANT THERAPEUTIC APPROACHES
Insights into the pathogenic mechanisms involved in APS have identified novel targeted therapeutic approaches including inhibition of specific signaling pathways, and immunomodulatory therapies, which may be particularly useful in patients with refractory thrombotic APS and underlying SLE. Case reports of CAPS as well as studies in animal models suggest that plasma exchange, defibrotide and complement directed therapies may also be effective.
8.1 Hydroxychloroquine
Hydroxychloroquine is an established therapy for SLE due to its anti-inflammatory and immunomodulatory effects. It has been reported to recduce the risk of both arterial and venous thrombosis In patients with SLE, with and without APLA.175 Potential mechanisms include a decrease in lupus activity as well as modulation of APLA effects. Hydroxychloroquine has been shown to inhibit the prothrombotic effects of APLA injected into mice71 and was reported to inhibit the expression of GPIIb/IIIa by platelets exposed to a thrombin receptor agonist in the presence of human IgG APLA (though activation-dependent antibodies were not employed).176 Rand et al. demonstrated that hydroxychloroquine reduces binding of APLA and restores annexin V expression by cultured human syncytiotrophoblasts.177 One retrospective study suggests that HCQ treatment may reduce APLA levels in patients with APS, though differences in treated and non-treated patients were minimal.178
A small prospective study reported that adding hydroxychloroquine to comparing anticoagulation with a VKA (fluindone) reduced VTE in patients with primary APS - 30% patients in the monotherapy group but none in the hydroxychloroquine group developed VTE over 36 months of follow up.179 A retrospective cohort study of pregnant women with APLA reported marginally higher rates of live births (67% versus 57%; P = .05) and a lower prevalence of APLA-related pregnancy morbidity (47% versus 63%; P = .004) in women who received hydroxychloroquine for 6 months prior to and continued throughout pregnancy.180 Hydroxychloroquine is advised for APLA positive patients with SLE; however, there is currently no high quality that supports the use of hydroxychloroquine in patients with primary APS. Unfortunately, a multicenter, randomized trial of hydroxychloroquine for primary thrombosis prevention in patients with primary APS (clinicaltrials.gov #NCT01784523) was terminated due to poor accrual.
8.2 Statins
Statins have long been known to have anti-inflammatory properties. Statins block APLA-induced activation of endothelial cells in vitro181, and inhibit tissue factor expression induced by APLA.182 Simvastatin and pravastatin inhibit APLA-induced fetal loss in a murine model.72 A small prospective study demonstrated that fluvastatin treatment for 3 months reversibly reduced the levels of inflammatory biomarkers and thrombosis in patients with APLA.183 Based on results of the JUPITER trial, that demonstrated that rosuvastatin reduced the incidence of venous thrombosis in healthy individuals without hypercholesterolemia,181 \ it has been suggested that statins should be considered in patients with APLA and a history of arterial events and hyperlipidemia.184 Further clinical studies are needed to establish their role in primary thromboprophylaxis and as adjuvant therapy in APS.
8.3 B cell directed therapy
Anecdotal reports have reported a beneficial effect of Rituximab on APLA titers.185 A recent open-label pilot study of Rituximab in primary APS reported that Rituximab had some efficacy in controlling non-criteria manifestations such as thrombocytopenia, hemolytic anemia and skin ulcers though it did not substantially change the APLA profile.186 There is equivocal data concerning the use of ritixumab in CAPS. Fifteen of twenty (75%) patients from the CAPS registry that received rituximab in combination with first line treatment because of severe presentations or associated lymphoproliferative disorders, or as second line therapy due to refractory CAPS recovered from the acute episode.187 Other B cell-directed therapies have shown activity in murine models. Co-stimulatory blockade with cytotoxic T lymphocyte antigen 4 (CTLA4) Ig prevented B cell activation and APLA production if administered prior to APLA development although it could not diminish levels of established APLA. In another approach, inhibition of B cell activating factor (BAFF) prevented APS onset and prolonged survival in autoimmune mice.188, 189 These pathways are particularly attractive targets since a CTLA4 blocker (Abatacept) and BAFF antagonist (Belimumab) are approved for use in other autoimmune disorders.
8.4 Modulation of intracellular signaling pathways
As noted above, several intracellular signaling pathways are involved in the pathogenic effects of APLA and present potential therapeutic targets. For example, NFĸB inhibition inhibits APLA induced cellular activation and thrombosis,98 and TLR4 inhibition appears to inhibit anti-β2GPI/β2GPI-induced tissue factor (TF) expression.190 However, interactions between multiple signaling pathways implicated in APS have not been defined, and further studies are needed in order to identify the optimal targets for prevention of pathologic signaling responses to APLA.
9. ASYMPTOMATIC ANTIPHOSPHOLIPID ANTIBODIES
Asymptomatic APLA are present in 1% to 5% of healthy individuals without a history of thrombotic events and as many as 11% to 86% of individuals with SLE; these wide estimates reflect in part the use of different assays and non-standardized approaches to APLA detection. It is difficult to predict the rate of first thrombosis in these patients although a prospective study of triple-positive APLA carriers reported a 5.3% annual incidence of thromboembolism that was not diminished by the use of aspirin administered in a non-controlled manner.128 A randomized trial addressing the use of aspirin in thrombosis-naïve patients, the APLASA trial, was terminated early due to an unexpectedly low rate of thrombosis and thus was underpowered, but did not detect a reduction in thrombosis in aspirin-treated patients.191 While there is currently no strong evidence supporting the use of aspirin for APLA carriers without other cardiovascular risk factors192, the level of evidence is poor, and the 13th Congress on Antiphospholipid Antibodies task force gave low dose aspirin a grade 2C recommendation for primary thromboprophylaxis in individuals with a high risk APLA profile, especially in the presence of other cardiovascular risk factors.148 A recent systematic review of 5 studies involving 154 pregnancies also concluded that primary prophylaxis with aspirin does not improve obstetric outcomes in otherwise healthy women who are asymptomatic APLA carriers.193 Prophylaxis with LMWH or aspirin may be considered in high-risk periods such as surgery and hospitalization, though anticoagulation in such settings would likely be more effective.194 Estrogen-containing oral contraceptives are a major risk factor for arterial thrombotic events in young women with APLA as demonstrated by the RATIO trial and should be avoided.124.
10. CONCLUSIONS AND FUTURE DIRECTIONS
APS is an autoimmune, inflammatory disorder associated with a substantial incidence of thrombosis, pregnancy morbidity, and potentially devastating complications such as catastrophic APS. APLA are common in the general population and identifying individuals at greatest risk of clinical events remains very challenging. Anti-β2GPI-Domain1 antibodies and β2GPI specific LA are potentially more specific markers of thrombotic risk but are not widely available outside of the research setting. The current standard of care for thrombotic APS is long term anticoagulation with a VKA and for obstetric APS, aspirin with LMWH (or UFH), though these recommendations are based on generally low quality data. There are important questions that need to be addressed in clinical trials of patient with APLA, such as which, if any, patients can be safely treated with shorter duration anticoagulation, the significance, if any, of APLA other than aCL and anti-β2GPI antibodies, and the optimal management of patients with non-criteria manifestations of APS and asymptomatic APLA, and the role of DOAC in secondary thrombosis prevention in patients with APS. There is little evidence to guide the management of patients who develop recurrent thrombosis while on standard anticoagulation. Innovative therapeutic approaches focused on immune modulation, and defining inflammatory signaling pathways are being studied and may provide much needed therapeutic advances for this devastating disease.
PRACTICE POINTS.
“Sydney” classification criteria for APS classification require the persistent presence of an antiphospholipid antibody accompanied by thrombosis or pregnancy morbidity.
Laboratory evaluation of APLA can be challenging; APLA assays are affected by patient factors (inflammation, anticoagulants) and inter-laboratory variability.
Long-term anticoagulation with a vitamin K antagonist is the standard of care for patients with thrombotic APS.
The optimal management of patients with APLA who develop thrombosis in the setting of a reversible risk factor or those who subsequently test negative for APLA is unknown.
Low dose aspirin in conjunction with unfractionated heparin or low molecular weight heparin is the current standard of care to prevent pregnancy loss in obstetric APS.
RESEARCH AGENDA.
Continued efforts toward universal standardization of APLA assays.
Development of enhanced biomarkers with better predictive value for primary and recurrent thrombosis, and adverse pregnancy outcomes in patients with APLA.
Improved characterization of the mechanisms and signaling pathways underlying altered cellular functions in APS, including defining interactions between diverse signaling pathways, and characterization of the roles of multiple receptors and how they may interact.
Further investigation of the safety and efficacy of DOACs and novel anti-inflammatory and immune-modulatory therapies for refractory APS.
Establishment of optimal guidelines for the management of asymptomatic APLA.
Acknowledgments
SC and KRM drafted and edited the manuscript and both authors approved the final version submitted for publication. This work was supported in part by HL123416 and an ASH Bridge grant (to KRM) and an ASH RTAF (to SC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–27. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 3.Cervera R, Espinosa G. Update on the catastrophic antiphospholipid syndrome and the "CAPS Registry". Semin Thromb Hemost. 2012;38:333–8. doi: 10.1055/s-0032-1304718. [DOI] [PubMed] [Google Scholar]
- 4.Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530–4. doi: 10.1191/0961203303lu394oa. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Pinto I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, Espinosa G, et al. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun Rev. 2016 doi: 10.1016/j.autrev.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Asherson RA. The catastrophic antiphospholipid syndrome, 1998. A review of the clinical features, possible pathogenesis and treatment. Lupus. 1998;7(Suppl 2):S55–62. doi: 10.1177/096120339800700214. [DOI] [PubMed] [Google Scholar]
- 7.Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramon E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Annals of the rheumatic diseases. 2015;74:1011–8. doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 8.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost. 1995;74:1185–90. [PubMed] [Google Scholar]
- 10.Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737–40. doi: 10.1111/j.1538-7836.2009.03555.x. [DOI] [PubMed] [Google Scholar]
- 11.Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost. 2010;8:237–42. doi: 10.1111/j.1538-7836.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 12.Galli M, Borrelli G, Jacobsen EM, Marfisi RM, Finazzi G, Marchioli R, et al. Clinical significance of different antiphospholipid antibodies in the WAPS (warfarin in the antiphospholipid syndrome) study. Blood. 2007;110:1178–83. doi: 10.1182/blood-2007-01-066043. [DOI] [PubMed] [Google Scholar]
- 13.Willis R, Lakos G, Harris EN. Standardization of antiphospholipid antibody testing--historical perspectives and ongoing initiatives. Semin Thromb Hemost. 2014;40:172–7. doi: 10.1055/s-0033-1364207. [DOI] [PubMed] [Google Scholar]
- 14.Kutteh WH, Franklin RD. Assessing the variation in antiphospholipid antibody (APA) assays: comparison of results from 10 centers. Am J Obstet Gynecol. 2004;191:440–8. doi: 10.1016/j.ajog.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Audrain MA, Colonna F, Morio F, Hamidou MA, Muller JY. Comparison of different kits in the detection of autoantibodies to cardiolipin and beta2glycoprotein 1. Rheumatology (Oxford, England) 2004;43:181–5. doi: 10.1093/rheumatology/keh029. [DOI] [PubMed] [Google Scholar]
- 16.Eby C. Novel anticoagulants and laboratory testing. Int J Lab Hematol. 2013;35:262–8. doi: 10.1111/ijlh.12065. [DOI] [PubMed] [Google Scholar]
- 17.Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost. 2011;105:385–6. doi: 10.1160/TH10-08-0511. [DOI] [PubMed] [Google Scholar]
- 18.Tripodi A, Chantarangkul V, Clerici M, Mannucci PM. Laboratory diagnosis of lupus anticoagulants for patients on oral anticoagulant treatment. Performance of dilute Russell viper venom test and silica clotting time in comparison with Staclot LA. Thromb Haemost. 2002;88:583–6. [PubMed] [Google Scholar]
- 19.Martinuzzo ME, Barrera LH, MA Da, Otaso JC, Gimenez MI, Oyhamburu J. Frequent false-positive results of lupus anticoagulant tests in plasmas of patients receiving the new oral anticoagulants and enoxaparin. Int J Lab Hematol. 2014;36:144–50. doi: 10.1111/ijlh.12138. [DOI] [PubMed] [Google Scholar]
- 20.Moore GW. Recent guidelines and recommendations for laboratory detection of lupus anticoagulants. Semin Thromb Hemost. 2014;40:163–71. doi: 10.1055/s-0033-1364185. [DOI] [PubMed] [Google Scholar]
- 21.Parmar K, Lefkou E, Doughty H, Connor P, Hunt BJ. The utility of the Taipan snake venom assay in assessing lupus anticoagulant status in individuals receiving or not receiving an oral vitamin K antagonist. Blood Coagul Fibrinolysis. 2009;20:271–5. doi: 10.1097/mbc.0b013e3283256037. [DOI] [PubMed] [Google Scholar]
- 22.van Os GM, de Laat B, Kamphuisen PW, Meijers JC, de Groot PG. Detection of lupus anticoagulant in the presence of rivaroxaban using Taipan snake venom time. J Thromb Haemost. 2011;9:1657–9. doi: 10.1111/j.1538-7836.2011.04395.x. [DOI] [PubMed] [Google Scholar]
- 23.Devreese K, Peerlinck K, Hoylaerts MF. Thrombotic risk assessment in the antiphospholipid syndrome requires more than the quantification of lupus anticoagulants. Blood. 2010;115:870–8. doi: 10.1182/blood-2009-09-244426. [DOI] [PubMed] [Google Scholar]
- 24.De Laat B, Derksen RH, Reber G, Musial J, Swadzba J, Bozic B, et al. An international multicentre-laboratory evaluation of a new assay to detect specifically lupus anticoagulants dependent on the presence of anti-beta2-glycoprotein autoantibodies. J Thromb Haemost. 2011;9:149–53. doi: 10.1111/j.1538-7836.2010.04068.x. [DOI] [PubMed] [Google Scholar]
- 25.de Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood. 2004;104:3598–602. doi: 10.1182/blood-2004-03-1107. [DOI] [PubMed] [Google Scholar]
- 26.de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–5. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Garcia JL, Bertolaccini ML, Cuadrado MJ, Sanna G, Ateka-Barrutia O, Khamashta MA. Clinical manifestations of antiphospholipid syndrome (APS) with and without antiphospholipid antibodies (the so-called 'seronegative APS') Annals of the rheumatic diseases. 2012;71:242–4. doi: 10.1136/annrheumdis-2011-200614. [DOI] [PubMed] [Google Scholar]
- 28.Nayfe R, Uthman I, Aoun J, Saad Aldin E, Merashli M, Khamashta MA. Seronegative antiphospholipid syndrome. Rheumatology (Oxford, England) 2013;52:1358–67. doi: 10.1093/rheumatology/ket126. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Garcia R, Serrano M, Martinez-Flores JA, Mora S, Morillas L, Martin-Mola MA, et al. Isolated IgA anti- beta2 glycoprotein I antibodies in patients with clinical criteria for antiphospholipid syndrome. J Immunol Res. 2014;2014:704395. doi: 10.1155/2014/704395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cousins L, Pericleous C, Khamashta M, Bertolaccini ML, Ioannou Y, Giles I, et al. Antibodies to domain I of beta-2-glycoprotein I and IgA antiphospholipid antibodies in patients with 'seronegative' antiphospholipid syndrome. Annals of the rheumatic diseases. 2015;74:317–9. doi: 10.1136/annrheumdis-2014-206483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattia E, Ruffatti A, Tonello M, Meneghel L, Robecchi B, Pittoni M, et al. IgA anticardiolipin and IgA anti-beta2 glycoprotein I antibody positivity determined by fluorescence enzyme immunoassay in primary antiphospholipid syndrome. Clin Chem Lab Med. 2014;52:1329–33. doi: 10.1515/cclm-2014-0039. [DOI] [PubMed] [Google Scholar]
- 32.Conti F, Capozzi A, Truglia S, Lococo E, Longo A, Misasi R, et al. The mosaic of "seronegative" antiphospholipid syndrome. J Immunol Res. 2014;2014:389601. doi: 10.1155/2014/389601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berard M, Chantome R, Marcelli A, Boffa MC. Antiphosphatidylethanolamine antibodies as the only antiphospholipid antibodies. I. Association with thrombosis and vascular cutaneous diseases. The Journal of rheumatology. 1996;23:1369–74. [PubMed] [Google Scholar]
- 34.Sanmarco M, Bardin N. The contribution of antiphosphatidylethanolamine antibodies in the diagnosis of the antiphospholipid syndrome. Lupus. 2012;21:727–8. doi: 10.1177/0961203312437272. [DOI] [PubMed] [Google Scholar]
- 35.Gris JC, Quere I, Sanmarco M, Boutiere B, Mercier E, Amiral J, et al. Antiphospholipid and antiprotein syndromes in non-thrombotic, non-autoimmune women with unexplained recurrent primary early foetal loss. The Nimes Obstetricians and Haematologists Study--NOHA. Thromb Haemost. 2000;84:228–36. [PubMed] [Google Scholar]
- 36.Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RF. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb Haemost. 1991;66:629–32. [PubMed] [Google Scholar]
- 37.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci U S A. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertolaccini ML, Sciascia S, Murru V, Garcia-Fernandez C, Sanna G, Khamashta MA. Prevalence of antibodies to prothrombin in solid phase (aPT) and to phosphatidylserine-prothrombin complex (aPS/PT) in patients with and without lupus anticoagulant. Thromb Haemost. 2013;109:207–13. doi: 10.1160/TH12-07-0527. [DOI] [PubMed] [Google Scholar]
- 39.Ortona E, Capozzi A, Colasanti T, Conti F, Alessandri C, Longo A, et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood. 2010;116:2960–7. doi: 10.1182/blood-2010-04-279208. [DOI] [PubMed] [Google Scholar]
- 40.Arad A, Proulle V, Furie RA, Furie BC, Furie B. beta(2)-Glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2011;117:3453–9. doi: 10.1182/blood-2010-08-300715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agar C, van Os GM, Morgelin M, Sprenger RR, Marquart JA, Urbanus RT, et al. Beta2-glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–43. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 42.Hunt JE, Simpson RJ, Krilis SA. Identification of a region of beta 2-glycoprotein I critical for lipid binding and anti-cardiolipin antibody cofactor activity. Proc Natl Acad Sci U S A. 1993;90:2141–5. doi: 10.1073/pnas.90.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salle V, Maziere JC, Smail A, Cevallos R, Maziere C, Fuentes V, et al. Anti-annexin II antibodies in systemic autoimmune diseases and antiphospholipid syndrome. J Clin Immunol. 2008;28:291–7. doi: 10.1007/s10875-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 44.Cesarman-Maus G, Rios-Luna NP, Deora AB, Huang B, Villa R, Cravioto Mdel C, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–82. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becarevic M. The IgG and IgM isotypes of anti-annexin A5 antibodies: relevance for primary antiphospholipid syndrome. J Thromb Thrombolysis. 2016;42:552–7. doi: 10.1007/s11239-016-1389-5. [DOI] [PubMed] [Google Scholar]
- 46.Urbanus RT, de Laat B. Antiphospholipid antibodies and the protein C pathway. Lupus. 2010;19:394–9. doi: 10.1177/0961203309360841. [DOI] [PubMed] [Google Scholar]
- 47.Rossetto V, Spiezia L, Franz F, Salmaso L, Pozza LV, Gavasso S, et al. The role of antiphospholipid antibodies toward the protein C/protein S system in venous thromboembolic disease. Am J Hematol. 2009;84:594–6. doi: 10.1002/ajh.21466. [DOI] [PubMed] [Google Scholar]
- 48.Wahl D, Membre A, Perret-Guillaume C, Regnault V, Lecompte T. Mechanisms of antiphospholipid-induced thrombosis: effects on the protein C system. Curr Rheumatol Rep. 2009;11:77–81. doi: 10.1007/s11926-009-0011-7. [DOI] [PubMed] [Google Scholar]
- 49.Esmon CT. The anticoagulant and anti-inflammatory roles of the protein C anticoagulant pathway. J Autoimmun. 2000;15:113–6. doi: 10.1006/jaut.2000.0400. [DOI] [PubMed] [Google Scholar]
- 50.Marciniak E, Romond EH. Impaired catalytic function of activated protein C: a new in vitro manifestation of lupus anticoagulant. Blood. 1989;74:2426–32. [PubMed] [Google Scholar]
- 51.Borrell M, Sala N, de Castellarnau C, Lopez S, Gari M, Fontcuberta J. Immunoglobulin fractions isolated from patients with antiphospholipid antibodies prevent the inactivation of factor Va by activated protein C on human endothelial cells. Thromb Haemost. 1992;68:268–72. [PubMed] [Google Scholar]
- 52.de Laat B, Eckmann CM, van Schagen M, Meijer AB, Mertens K, van Mourik JA. Correlation between the potency of a beta2-glycoprotein I-dependent lupus anticoagulant and the level of resistance to activated protein C. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2008;19:757–64. doi: 10.1097/MBC.0b013e32830f1b85. [DOI] [PubMed] [Google Scholar]
- 53.Galli M, Willems GM, Rosing J, Janssen RM, Govers-Riemslag JW, Comfurius P, et al. Anti-prothrombin IgG from patients with anti-phospholipid antibodies inhibits the inactivation of factor Va by activated protein C. Br J Haematol. 2005;129:240–7. doi: 10.1111/j.1365-2141.2005.05438.x. [DOI] [PubMed] [Google Scholar]
- 54.Nojima J, Kuratsune H, Suehisa E, Iwatani Y, Kanakura Y. Acquired activated protein C resistance associated with IgG antibodies against beta2-glycoprotein I and prothrombin as a strong risk factor for venous thromboembolism. Clin Chem. 2005;51:545–52. doi: 10.1373/clinchem.2004.043414. [DOI] [PubMed] [Google Scholar]
- 55.Izumi T, Pound ML, Su Z, Iverson GM, Ortel TL. Anti-beta(2)-glycoprotein I antibody-mediated inhibition of activated protein C requires binding of beta(2)-glycoprotein I to phospholipids. Thromb Haemost. 2002;88:620–6. [PubMed] [Google Scholar]
- 56.Smirnov MD, Triplett DT, Comp PC, Esmon NL, Esmon CT. On the role of phosphatidylethanolamine in the inhibition of activated protein C activity by antiphospholipid antibodies. J Clin Invest. 1995;95:309–16. doi: 10.1172/JCI117657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shibata S, Harpel PC, Gharavi A, Rand J, Fillit H. Autoantibodies to heparin from patients with antiphospholipid antibody syndrome inhibit formation of antithrombin III-thrombin complexes. Blood. 1994;83:2532–40. [PubMed] [Google Scholar]
- 58.Liestol S, Sandset PM, Jacobsen EM, Mowinckel MC, Wisloff F. Decreased anticoagulant response to tissue factor pathway inhibitor type 1 in plasmas from patients with lupus anticoagulants. Br J Haematol. 2007;136:131–7. doi: 10.1111/j.1365-2141.2006.06385.x. [DOI] [PubMed] [Google Scholar]
- 59.Bu C, Gao L, Xie W, Zhang J, He Y, Cai G, et al. beta2-glycoprotein i is a cofactor for tissue plasminogen activator-mediated plasminogen activation. Arthritis Rheum. 2009;60:559–68. doi: 10.1002/art.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hulstein JJ, Lenting PJ, de Laat B, Derksen RH, Fijnheer R, de Groot PG. beta2-Glycoprotein I inhibits von Willebrand factor dependent platelet adhesion and aggregation. Blood. 2007;110:1483–91. doi: 10.1182/blood-2006-10-053199. [DOI] [PubMed] [Google Scholar]
- 61.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. The New England journal of medicine. 2013;368:1033–44. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 62.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. The New England journal of medicine. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 63.Giannakopoulos B, Gao L, Qi M, Wong JW, Yu DM, Vlachoyiannopoulos PG, et al. Factor XI is a substrate for oxidoreductases: enhanced activation of reduced FXI and its role in antiphospholipid syndrome thrombosis. J Autoimmun. 2012;39:121–9. doi: 10.1016/j.jaut.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Irman S, Miha S, Igor M, Rozman B, Bozic B. In vitro model of annexin A5 crystallization on natural phospholipid bilayers observed by atomic force microscopy. Autoimmunity. 2009;42:414–23. doi: 10.1080/08916930902785371. [DOI] [PubMed] [Google Scholar]
- 65.Rand JH, Wu XX, Andree HA, Lockwood CJ, Guller S, Scher J, et al. Pregnancy loss in the antiphospholipid-antibody syndrome--a possible thrombogenic mechanism. The New England journal of medicine. 1997;337:154–60. doi: 10.1056/NEJM199707173370303. [DOI] [PubMed] [Google Scholar]
- 66.Rand JH, Wu XX, Guller S, Gil J, Guha A, Scher J, et al. Reduction of annexin-V (placental anticoagulant protein-I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171:1566–72. doi: 10.1016/0002-9378(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 67.Rand JH, Wu XX, Quinn AS, Chen PP, McCrae KR, Bovill EG, et al. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. The American journal of pathology. 2003;163:1193–200. doi: 10.1016/S0002-9440(10)63479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rand JH, Wu XX, Quinn AS, Chen PP, Hathcock JJ, Taatjes DJ. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112:1687–95. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rand JH, Wu XX, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood. 2010;115:2292–9. doi: 10.1182/blood-2009-04-213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu XX, Guller S, Rand JH. Hydroxychloroquine reduces binding of antiphospholipid antibodies to syncytiotrophoblasts and restores annexin A5 expression. Am J Obstet Gynecol. 2011;205:576, e7–14. doi: 10.1016/j.ajog.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96:4380–4. doi: 10.1161/01.cir.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 72.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118:3453–61. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Del Papa N, Guidali L, Sala A, Buccellati C, Khamashta MA, Ichikawa K, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40:551–61. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 74.Simantov R, LaSala JM, Lo SK, Gharavi AE, Sammaritano LR, Salmon JE, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96:2211–9. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2005;105:1964–9. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 76.Lambrianides A, Carroll CJ, Pierangeli SS, Pericleous C, Branch W, Rice J, et al. Effects of polyclonal IgG derived from patients with different clinical types of the antiphospholipid syndrome on monocyte signaling pathways. J Immunol. 2010;184:6622–8. doi: 10.4049/jimmunol.0902765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–97. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 78.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pennings MT, Derksen RH, van Lummel M, Adelmeijer J, VanHoorelbeke K, Urbanus RT, et al. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2'. Journal of thrombosis and haemostasis : JTH. 2007;5:369–77. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 80.Shi T, Giannakopoulos B, Yan X, Yu P, Berndt MC, Andrews RK, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis and rheumatism. 2006;54:2558–67. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 81.Vega-Ostertag M, Harris EN, Pierangeli SS. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004;50:2911–9. doi: 10.1002/art.20434. [DOI] [PubMed] [Google Scholar]
- 82.Williams FM, Parmar K, Hughes GR, Hunt BJ. Systemic endothelial cell markers in primary antiphospholipid syndrome. Thromb Haemost. 2000;84:742–6. [PubMed] [Google Scholar]
- 83.Espinola RG, Liu X, Colden-Stanfield M, Hall J, Harris EN, Pierangeli SS. E-Selectin mediates pathogenic effects of antiphospholipid antibodies. J Thromb Haemost. 2003;1:843–8. doi: 10.1046/j.1538-7836.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Meng H, Ma R, He Z, Wu X, Cao M, et al. Circulating Microparticles, Blood Cells and Endothelium Induce Procoagulant Activity in Sepsis Through Phosphatidylserine Exposure. Shock. 2015 doi: 10.1097/SHK.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 85.McCrae KR, DeMichele A, Samuels P, Roth D, Kuo A, Meng QH, et al. Detection of endothelial cell-reactive immunoglobulin in patients with anti-phospholipid antibodies. Br J Haematol. 1991;79:595–605. doi: 10.1111/j.1365-2141.1991.tb08087.x. [DOI] [PubMed] [Google Scholar]
- 86.Branch DW, Rodgers GM. Induction of endothelial cell tissue factor activity by sera from patients with antiphospholipid syndrome: a possible mechanism of thrombosis. Am J Obstet Gynecol. 1993;168:206–10. doi: 10.1016/s0002-9378(12)90915-1. [DOI] [PubMed] [Google Scholar]
- 87.Hamid C, Norgate K, D'Cruz DP, Khamashta MA, Arno M, Pearson JD, et al. Anti-beta2GPI-antibody-induced endothelial cell gene expression profiling reveals induction of novel pro-inflammatory genes potentially involved in primary antiphospholipid syndrome. Annals of the rheumatic diseases. 2007;66:1000–7. doi: 10.1136/ard.2006.063909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mineo C. Inhibition of nitric oxide and antiphospholipid antibody-mediated thrombosis. Current rheumatology reports. 2013;15:324. doi: 10.1007/s11926-013-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Betapudi V, Lominadze G, Hsi L, Willard B, Wu M, McCrae KR. Anti-beta2GPI antibodies stimulate endothelial cell microparticle release via a nonmuscle myosin II motor protein-dependent pathway. Blood. 2013;122:3808–17. doi: 10.1182/blood-2013-03-490318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91:667–73. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 91.Wu M, Barnard J, Kundu S, McCrae KR. A novel pathway of cellular activation mediated by antiphospholipid antibody-induced extracellular vesicles. J Thromb Haemost. 2015;13:1928–40. doi: 10.1111/jth.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prinz N, Clemens N, Strand D, Putz I, Lorenz M, Daiber A, et al. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood. 2011;118:2322–32. doi: 10.1182/blood-2011-01-330639. [DOI] [PubMed] [Google Scholar]
- 93.Gladigau G, Haselmayer P, Scharrer I, Munder M, Prinz N, Lackner K, et al. A role for Toll-like receptor mediated signals in neutrophils in the pathogenesis of the anti-phospholipid syndrome. PLoS One. 2012;7:e42176. doi: 10.1371/journal.pone.0042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bernales I, Fullaondo A, Marin-Vidalled MJ, Ucar E, Martinez-Taboada V, Lopez-Hoyos M, et al. Innate immune response gene expression profiles characterize primary antiphospholipid syndrome. Genes Immun. 2008;9:38–46. doi: 10.1038/sj.gene.6364443. [DOI] [PubMed] [Google Scholar]
- 95.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Annals of the rheumatic diseases. 2007;66:1327–33. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, Papalardo E, Vargas G, Deora AB, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–83. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus RT, Derksen RH, Pennings MT, Papalardo E, et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117:1408–14. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montiel-Manzano G, Romay-Penabad Z, Papalardo de Martinez E, Meillon-Garcia LA, Garcia-Latorre E, Reyes-Maldonado E, et al. In vivo effects of an inhibitor of nuclear factor-kappa B on thrombogenic properties of antiphospholipid antibodies. Ann N Y Acad Sci. 2007;1108:540–53. doi: 10.1196/annals.1422.057. [DOI] [PubMed] [Google Scholar]
- 99.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 101.Salmon JE, Girardi G, Holers VM. Activation of complement mediates antiphospholipid antibody-induced pregnancy loss. Lupus. 2003;12:535–8. doi: 10.1191/0961203303lu397oa. [DOI] [PubMed] [Google Scholar]
- 102.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–6. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 103.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52:2120–4. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 104.Agostinis C, Durigutto P, Sblattero D, Borghi MO, Grossi C, Guida F, et al. A non-complement-fixing antibody to beta2 glycoprotein I as a novel therapy for antiphospholipid syndrome. Blood. 2014;123:3478–87. doi: 10.1182/blood-2013-11-537704. [DOI] [PubMed] [Google Scholar]
- 105.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 106.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–31. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breen KA, Seed P, Parmar K, Moore GW, Stuart-Smith SE, Hunt BJ. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb Haemost. 2012;107:423–9. doi: 10.1160/TH11-08-0554. [DOI] [PubMed] [Google Scholar]
- 108.Foltyn Zadura A, Memon AA, Stojanovich L, Perricone C, Conti F, Valesini G, et al. Factor H Autoantibodies in Patients with Antiphospholipid Syndrome and Thrombosis. The Journal of rheumatology. 2015;42:1786–93. doi: 10.3899/jrheum.150185. [DOI] [PubMed] [Google Scholar]
- 109.Barratt-Due A, Floisand Y, Orrem HL, Kvam AK, Holme PA, Bergseth G, et al. Complement activation is a crucial pathogenic factor in catastrophic antiphospholipid syndrome. Rheumatology (Oxford, England) 2016;55:1337–9. doi: 10.1093/rheumatology/kew040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zikos TA, Sokolove J, Ahuja N, Berube C. Eculizumab Induces Sustained Remission in a Patient With Refractory Primary Catastrophic Antiphospholipid Syndrome. J Clin Rheumatol. 2015;21:311–3. doi: 10.1097/RHU.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 111.Shapira I, Andrade D, Allen SL, Salmon JE. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719–23. doi: 10.1002/art.34440. [DOI] [PubMed] [Google Scholar]
- 112.Lonze BE, Zachary AA, Magro CM, Desai NM, Orandi BJ, Dagher NN, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459–65. doi: 10.1111/ajt.12540. [DOI] [PubMed] [Google Scholar]
- 113.Wig S, Chan M, Thachil J, Bruce I, Barnes T. A case of relapsing and refractory catastrophic anti-phospholipid syndrome successfully managed with eculizumab, a complement 5 inhibitor. Rheumatology (Oxford, England) 2016;55:382–4. doi: 10.1093/rheumatology/kev371. [DOI] [PubMed] [Google Scholar]
- 114.Kronbichler A, Frank R, Kirschfink M, Szilagyi A, Csuka D, Prohaszka Z, et al. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine (Baltimore) 2014;93:e143. doi: 10.1097/MD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256–63. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 116.van Os GM, Meijers JC, Agar C, Seron MV, Marquart JA, Akesson P, et al. Induction of anti-beta2 - glycoprotein I autoantibodies in mice by protein H of Streptococcus pyogenes. J Thromb Haemost. 2011;9:2447–56. doi: 10.1111/j.1538-7836.2011.04532.x. [DOI] [PubMed] [Google Scholar]
- 117.de Laat B, van Berkel M, Urbanus RT, Siregar B, de Groot PG, Gebbink MF, et al. Immune responses against domain I of beta(2)-glycoprotein I are driven by conformational changes: domain I of beta(2)-glycoprotein I harbors a cryptic immunogenic epitope. Arthritis Rheum. 2011;63:3960–8. doi: 10.1002/art.30633. [DOI] [PubMed] [Google Scholar]
- 118.Ioannou Y, Zhang JY, Qi M, Gao L, Qi JC, Yu DM, et al. Novel assays of thrombogenic pathogenicity in the antiphospholipid syndrome based on the detection of molecular oxidative modification of the major autoantigen beta2-glycoprotein I. Arthritis Rheum. 2011;63:2774–82. doi: 10.1002/art.30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rauch J, Dieude M, Subang R, Levine JS. The dual role of innate immunity in the antiphospholipid syndrome. Lupus. 2010;19:347–53. doi: 10.1177/0961203310361492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–72. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 121.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–7. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 123.Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, et al. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J Immunol. 2013;190:4982–90. doi: 10.4049/jimmunol.1202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. 2009;8:998–1005. doi: 10.1016/S1474-4422(09)70239-X. [DOI] [PubMed] [Google Scholar]
- 125.Urbanus RT, de Groot PG. Antiphospholipid antibodies--we are not quite there yet. Blood Rev. 2011;25:97–106. doi: 10.1016/j.blre.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Devreese K, Hoylaerts MF. Challenges in the diagnosis of the antiphospholipid syndrome. Clin Chem. 2010;56:930–40. doi: 10.1373/clinchem.2009.133678. [DOI] [PubMed] [Google Scholar]
- 127.de Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost. 2005;3:1993–7. doi: 10.1111/j.1538-7836.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 128.Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood. 2011;118:4714–8. doi: 10.1182/blood-2011-03-340232. [DOI] [PubMed] [Google Scholar]
- 129.Mustonen P, Lehtonen KV, Javela K, Puurunen M. Persistent antiphospholipid antibody (aPL) in asymptomatic carriers as a risk factor for future thrombotic events: a nationwide prospective study. Lupus. 2014;23:1468–76. doi: 10.1177/0961203314545410. [DOI] [PubMed] [Google Scholar]
- 130.Ruffatti A, Tonello M, Visentin MS, Bontadi A, Hoxha A, De Carolis S, et al. Risk factors for pregnancy failure in patients with anti-phospholipid syndrome treated with conventional therapies: a multicentre, case-control study. Rheumatology (Oxford) 2011;50:1684–9. doi: 10.1093/rheumatology/ker139. [DOI] [PubMed] [Google Scholar]
- 131.de Laat B, Pengo V, Pabinger I, Musial J, Voskuyl AE, Bultink IE, et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J Thromb Haemost. 2009;7:1767–73. doi: 10.1111/j.1538-7836.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 132.Pengo V, Ruffatti A, Tonello M, Cuffaro S, Banzato A, Bison E, et al. Antiphospholipid syndrome: antibodies to Domain 1 of beta2-glycoprotein 1 correctly classify patients at risk. J Thromb Haemost. 2015;13:782–7. doi: 10.1111/jth.12865. [DOI] [PubMed] [Google Scholar]
- 133.De Craemer AS, Musial J, Devreese KM. Role of anti-domain 1-beta2 glycoprotein I antibodies in the diagnosis and risk stratification of antiphospholipid syndrome. J Thromb Haemost. 2016;14:1779–87. doi: 10.1111/jth.13389. [DOI] [PubMed] [Google Scholar]
- 134.Otomo K, Atsumi T, Amengual O, Fujieda Y, Kato M, Oku K, et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum. 2012;64:504–12. doi: 10.1002/art.33340. [DOI] [PubMed] [Google Scholar]
- 135.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology (Oxford, England) 2013;52:1397–403. doi: 10.1093/rheumatology/kes388. [DOI] [PubMed] [Google Scholar]
- 136.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. The global anti-phospholipid syndrome score in primary APS. Rheumatology (Oxford, England) 2015;54:134–8. doi: 10.1093/rheumatology/keu307. [DOI] [PubMed] [Google Scholar]
- 137.Oku K, Amengual O, Bohgaki T, Horita T, Yasuda S, Atsumi T. An independent validation of the Global Anti-Phospholipid Syndrome Score in a Japanese cohort of patients with autoimmune diseases. Lupus. 2015;24:774–5. doi: 10.1177/0961203314561284. [DOI] [PubMed] [Google Scholar]
- 138.Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332–8. doi: 10.1016/s0002-9343(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 139.Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood. 2013;122:817–24. doi: 10.1182/blood-2013-04-496257. [DOI] [PubMed] [Google Scholar]
- 140.Gebhart J, Posch F, Koder S, Perkmann T, Quehenberger P, Zoghlami C, et al. Increased mortality in patients with the lupus anticoagulant: the Vienna Lupus Anticoagulant and Thrombosis Study (LATS) Blood. 2015;125:3477–83. doi: 10.1182/blood-2014-11-611129. [DOI] [PubMed] [Google Scholar]
- 141.Rosborough TK, Shepherd MF. Unreliability of international normalized ratio for monitoring warfarin therapy in patients with lupus anticoagulant. Pharmacotherapy. 2004;24:838–42. doi: 10.1592/phco.24.9.838.36102. [DOI] [PubMed] [Google Scholar]
- 142.Moll S, Ortel TL. Monitoring warfarin therapy in patients with lupus anticoagulants. Ann Intern Med. 1997;127:177–85. doi: 10.7326/0003-4819-127-3-199708010-00001. [DOI] [PubMed] [Google Scholar]
- 143.Kasthuri RS, Roubey RA. Warfarin and the antiphospholipid syndrome: does one size fit all? Arthritis Rheum. 2007;57:1346–7. doi: 10.1002/art.23111. [DOI] [PubMed] [Google Scholar]
- 144.Devreese K, Peerlinck K, Arnout J, Hoylaerts MF. Laboratory detection of the antiphospholipid syndrome via calibrated automated thrombography. Thromb Haemost. 2009;101:185–96. [PubMed] [Google Scholar]
- 145.Efthymiou M, Lawrie AS, Mackie I, Arachchillage D, Lane PJ, Machin S, et al. Thrombin generation and factor X assays for the assessment of warfarin anticoagulation in thrombotic antiphospholipid syndrome. Thromb Res. 2015;135:1191–7. doi: 10.1016/j.thromres.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 146.Crowther MA, Ginsberg JS, Julian J, Denburg J, Hirsh J, Douketis J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. The New England journal of medicine. 2003;349:1133–8. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 147.Finazzi G, Marchioli R, Brancaccio V, Schinco P, Wisloff F, Musial J, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS) J Thromb Haemost. 2005;3:848–53. doi: 10.1111/j.1538-7836.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 148.Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, Brey R, Crowther M, Derksen R, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. 2011;20:206–18. doi: 10.1177/0961203310395803. [DOI] [PubMed] [Google Scholar]
- 149.Hunt BJ. Pediatric antiphospholipid antibodies and antiphospholipid syndrome. Semin Thromb Hemost. 2008;34:274–81. doi: 10.1055/s-0028-1082271. [DOI] [PubMed] [Google Scholar]
- 150.Levine SR, Brey RL, Tilley BC, Thompson JL, Sacco RL, Sciacca RR, et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. Jama. 2004;291:576–84. doi: 10.1001/jama.291.5.576. [DOI] [PubMed] [Google Scholar]
- 151.Okuma H, Kitagawa Y, Yasuda T, Tokuoka K, Takagi S. Comparison between single antiplatelet therapy and combination of antiplatelet and anticoagulation therapy for secondary prevention in ischemic stroke patients with antiphospholipid syndrome. Int J Med Sci. 2009;7:15–8. doi: 10.7150/ijms.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Son M, Wypasek E, Celinska-Lowenhoff M, Undas A. The use of rivaroxaban in patients with antiphospholipid syndrome: A series of 12 cases. Thromb Res. 2015;135:1035–6. doi: 10.1016/j.thromres.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 153.Schaefer JK, McBane RD, Black DF, Williams LN, Moder KG, Wysokinski WE. Failure of dabigatran and rivaroxaban to prevent thromboembolism in antiphospholipid syndrome: a case series of three patients. Thromb Haemost. 2014;112:947–50. doi: 10.1160/TH14-03-0272. [DOI] [PubMed] [Google Scholar]
- 154.Win K, Rodgers GM. New oral anticoagulants may not be effective to prevent venous thromboembolism in patients with antiphospholipid syndrome. Am J Hematol. 2014;89:1017. doi: 10.1002/ajh.23797. [DOI] [PubMed] [Google Scholar]
- 155.Haladyj E, Olesinska M. Rivaroxaban - a safe therapeutic option in patients with antiphospholipid syndrome? Our experience in 23 cases. Reumatologia. 2016;54:146–9. doi: 10.5114/reum.2016.61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Betancur JF, Bonilla-Abadia F, Hormaza AA, Jaramillo FJ, Canas CA, Tobon GJ. Direct oral anticoagulants in antiphospholipid syndrome: a real life case series. Lupus. 2016;25:658–62. doi: 10.1177/0961203315624555. [DOI] [PubMed] [Google Scholar]
- 157.Noel N, Dutasta F, Costedoat-Chalumeau N, Bienvenu B, Mariette X, Geffray L, et al. Safety and efficacy of oral direct inhibitors of thrombin and factor Xa in antiphospholipid syndrome. Autoimmun Rev. 2015;14:680–5. doi: 10.1016/j.autrev.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 158.Sciascia S, Breen K, Hunt BJ. Rivaroxaban use in patients with antiphospholipid syndrome and previous venous thromboembolism. Blood Coagul Fibrinolysis. 2015;26:476–7. doi: 10.1097/MBC.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 159.Signorelli F, Nogueira F, Domingues V, Mariz HA, Levy RA. Thrombotic events in patients with antiphospholipid syndrome treated with rivaroxaban: a series of eight cases. Clin Rheumatol. 2016;35:801–5. doi: 10.1007/s10067-015-3030-y. [DOI] [PubMed] [Google Scholar]
- 160.Cohen H, Hunt BJ, Efthymiou M, Arachchillage DR, Mackie IJ, Clawson S, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426–36. doi: 10.1016/S2352-3026(16)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Espinosa G, Cervera R. Current treatment of antiphospholipid syndrome: lights and shadows. Nat Rev Rheumatol. 2015;11:586–96. doi: 10.1038/nrrheum.2015.88. [DOI] [PubMed] [Google Scholar]
- 162.Dentali F, Manfredi E, Crowther M, Ageno W. Long-duration therapy with low molecular weight heparin in patients with antiphospholipid antibody syndrome resistant to warfarin therapy. J Thromb Haemost. 2005;3:2121–3. doi: 10.1111/j.1538-7836.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 163.Crum-Cianflone N, Sullivan E. Meningococcal Vaccinations. Infect Dis Ther. 2016;5:89–112. doi: 10.1007/s40121-016-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burcoglu-O'Ral A, Erkan D, Asherson R. Treatment of catastrophic antiphospholipid syndrome with defibrotide, a proposed vascular endothelial cell modulator. The Journal of rheumatology. 2002;29:2006–11. [PubMed] [Google Scholar]
- 165.Backos M, Rai R, Baxter N, Chilcott IT, Cohen H, Regan L. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low dose aspirin and heparin. Br J Obstet Gynaecol. 1999;106:102–7. doi: 10.1111/j.1471-0528.1999.tb08208.x. [DOI] [PubMed] [Google Scholar]
- 166.Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies) Bmj. 1997;314:253–7. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol. 1996;174:1584–9. doi: 10.1016/s0002-9378(96)70610-5. [DOI] [PubMed] [Google Scholar]
- 168.Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100:408–13. doi: 10.1016/s0029-7844(02)02165-8. [DOI] [PubMed] [Google Scholar]
- 169.Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. The Journal of rheumatology. 2009;36:279–87. doi: 10.3899/jrheum.080763). [DOI] [PubMed] [Google Scholar]
- 170.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD002859.pub2. Cd002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ziakas PD, Pavlou M, Voulgarelis M. Heparin treatment in antiphospholipid syndrome with recurrent pregnancy loss: a systematic review and meta-analysis. Obstet Gynecol. 2010;115:1256–62. doi: 10.1097/AOG.0b013e3181deba40. [DOI] [PubMed] [Google Scholar]
- 172.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e691S–736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arachchillage DR, Machin SJ, Mackie IJ, Cohen H. Diagnosis and management of non-criteria obstetric antiphospholipid syndrome. Thromb Haemost. 2015;113:13–9. doi: 10.1160/TH14-05-0416. [DOI] [PubMed] [Google Scholar]
- 174.Del Ross T, Ruffatti A, Visentin MS, Tonello M, Calligaro A, Favaro M, et al. Treatment of 139 pregnancies in antiphospholipid-positive women not fulfilling criteria for antiphospholipid syndrome: a retrospective study. The Journal of rheumatology. 2013;40:425–9. doi: 10.3899/jrheum.120576. [DOI] [PubMed] [Google Scholar]
- 175.Petri M. Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep. 2011;13:77–80. doi: 10.1007/s11926-010-0141-y. [DOI] [PubMed] [Google Scholar]
- 176.Espinola RG, Pierangeli SS, Gharavi AE, Harris EN. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost. 2002;87:518–22. [PubMed] [Google Scholar]
- 177.Wu XX, Guller S, Rand JH. Hydroxychloroquine reduces binding of antiphospholipid antibodies to syncytiotrophoblasts and restores annexin A5 expression. Am J Obstet Gynecol. 2011;205:576.e7–14. doi: 10.1016/j.ajog.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nuri E, Taraborelli M, Andreoli L, Tonello M, Gerosa M, Calligaro A, et al. Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol Res. 2016 doi: 10.1007/s12026-016-8812-z. [DOI] [PubMed] [Google Scholar]
- 179.Schmidt-Tanguy A, Voswinkel J, Henrion D, Subra JF, Loufrani L, Rohmer V, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927–9. doi: 10.1111/jth.12363. [DOI] [PubMed] [Google Scholar]
- 180.Sciascia S, Hunt BJ, Talavera-Garcia E, Lliso G, Khamashta MA, Cuadrado MJ. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. 2016;214:273 e1–8. doi: 10.1016/j.ajog.2015.09.078. [DOI] [PubMed] [Google Scholar]
- 181.Meroni PL, Raschi E, Testoni C, Tincani A, Balestrieri G, Molteni R, et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-beta2-glycoprotein I) antibodies: effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 2001;44:2870–8. doi: 10.1002/1529-0131(200112)44:12<2870::aid-art475>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 182.Ferrara DE, Swerlick R, Casper K, Meroni PL, Vega-Ostertag ME, Harris EN, et al. Fluvastatin inhibits up-regulation of tissue factor expression by antiphospholipid antibodies on endothelial cells. J Thromb Haemost. 2004;2:1558–63. doi: 10.1111/j.1538-7836.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 183.Erkan D, Willis R, Murthy VL, Basra G, Vega J, Ruiz-Limon P, et al. A prospective open-label pilot study of fluvastatin on proinflammatory and prothrombotic biomarkers in antiphospholipid antibody positive patients. Annals of the rheumatic diseases. 2014;73:1176–80. doi: 10.1136/annrheumdis-2013-203622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Erkan D, Aguiar CL, Andrade D, Cohen H, Cuadrado MJ, Danowski A, et al. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev. 2014;13:685–96. doi: 10.1016/j.autrev.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 185.Kumar D, Roubey RA. Use of rituximab in the antiphospholipid syndrome. Curr Rheumatol Rep. 2010;12:40–4. doi: 10.1007/s11926-009-0074-5. [DOI] [PubMed] [Google Scholar]
- 186.Erkan D, Vega J, Ramon G, Kozora E, Lockshin MD. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis Rheum. 2013;65:464–71. doi: 10.1002/art.37759. [DOI] [PubMed] [Google Scholar]
- 187.Berman H, Rodriguez-Pinto I, Cervera R, Morel N, Costedoat-Chalumeau N, Erkan D, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev. 2013;12:1085–90. doi: 10.1016/j.autrev.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 188.Kim KS, Denton MD, Chandraker A, Knoflach A, Milord R, Waaga AM, et al. CD28-B7-mediated T cell costimulation in chronic cardiac allograft rejection: differential role of B7-1 in initiation versus progression of graft arteriosclerosis. Am J Pathol. 2001;158:977–86. doi: 10.1016/S0002-9440(10)64044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, et al. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58:2824–34. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xie H, Zhou H, Wang H, Chen D, Xia L, Wang T, et al. Anti-beta(2)GPI/beta(2)GPI induced TF and TNF-alpha expression in monocytes involving both TLR4/MyD88 and TLR4/TRIF signaling pathways. Molecular immunology. 2013;53:246–54. doi: 10.1016/j.molimm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 191.Erkan D, Harrison MJ, Levy R, Peterson M, Petri M, Sammaritano L, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum. 2007;56:2382–91. doi: 10.1002/art.22663. [DOI] [PubMed] [Google Scholar]
- 192.Chaturvedi S, McCrae KR. The antiphospholipid syndrome: still an enigma. Hematology Am Soc Hematol Educ Program. 2015;2015:53–60. doi: 10.1182/asheducation-2015.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Amengual O, Fujita D, Ota E, Carmona L, Oku K, Sugiura-Ogasawara M, et al. Primary prophylaxis to prevent obstetric complications in asymptomatic women with antiphospholipid antibodies: a systematic review. Lupus. 2015 doi: 10.1177/0961203315578765. [DOI] [PubMed] [Google Scholar]
- 194.Giron-Gonzalez JA, Garcia del Rio E, Rodriguez C, Rodriguez-Martorell J, Serrano A. Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: prospective analysis of 404 individuals. The Journal of rheumatology. 2004;31:1560–7. [PubMed] [Google Scholar]