Abstract
Background
On August 14, 2014, the United States Food and Drug Administration approved the anti-angiogenesis drug, bevacizumab, for women with advanced cervical cancer based on a 2012 interim analysis of 271 deaths on GOG protocol 240. We now report the planned protocol-specified final analysis of the primary objective, overall survival (OS).
Methods
A phase III randomized trial using a 2×2 factorial design was conducted to determine whether intravenous chemotherapy [(cisplatin 50 mg/m2 plus paclitaxel 135 or 175 mg/m2) or (topotecan 0.75 mg/m2 days 1–3 plus paclitaxel 175 mg/m2)] with or without bevacizumab (15 mg/kg) improves OS in recurrent/persistent/metastatic cervical cancer. Patients were prospectively stratified by performance status, prior radiosensitizing platinum, and disease status. We estimated enrolling 450 patients with 346 deaths at final analysis to provide 90% power to detect a 30% reduction in risk of death.
Findings
On March 7, 2014, 348 deaths occurred among 452 patients. Regimens administering bevacizumab continued to demonstrate significant improvement in OS: 16.8 vs 13.3 mos (HR 0.77;95% CI 0.62–0.95;p=0.0068). Updated progression-free survival also favored bevacizumab (HR 0.68;95% CI 0.56–0.84;p=0.0002). Final OS among 20% (n=91) not treated with prior pelvic radiotherapy was 24.5 (bevacizumab) vs 16.8 mos (without bevacizumab). Fistula (any grade) occurred in 14.5% (n=32) receiving bevacizumab (all previously irradiated). Grade 3+ fistula developed in 5.9% (n=13) and did not result in surgical emergency, sepsis and/or death. Post-progression OS was not significantly different among those who did and did not receive bevacizumab (median 8.4 vs 7.1 mos: HR 0.32;95% CI 0.66–1.05;p=0.06).
Interpretation
The benefit conferred by incorporation of bevacizumab is sustained with extended follow-up as evidenced by the survival curves remaining separated. Following progression on bevacizumab, a negative rebound effect was not observed. This represents proof-of-concept of the efficacy and tolerability of anti-angiogenesis therapy in advanced cervical cancer.
Funding
National Cancer Institute (USA).
Keywords: Cervical cancer, angiogenesis, bevacizumab
INTRODUCTION
With an estimated annual incidence of 529,800 new cases and approximately 275,100 deaths globally, cervical cancer continues to represent a significant cause of morbidity and mortality among young and middle-aged women throughout the world.1 A clearer example in healthcare is unlikely to be found in which the lines which demarcate industrialized societies from the developing world are more readily discernible. For example, the annual incidence and mortality rates due to cervical cancer in England are 2,900 and 1,000, respectively, while in the United States, the American Cancer Society estimates there will be only 12,820 new cases and 4,210 deaths in 2017.2 In Europe, cervical cancer is the sixth most common cancer among females, with nearly 55,000 new cases diagnosed annually; rates are highest in Romania and lowest in Switzerland.1 The disease burden is greatest in sub-Saharan Africa, southeast Asia including India, and in Latin America.1
The positive impact of cervical cancer screening programs using cytology with and without high risk human papillomavirus (HPV) DNA testing remains undisputed provided members of the populations served have access to cyclical (i.e., repetitive) screening and resources to support intervention when required.3 Similarly, prophylactic HPV vaccination programs can only be effective when actively endorsed by healthcare providers, advocated by parents, and subject to robust public policy commitment designed to deliver doses to sufficient numbers of at-risk individuals.3 Patients diagnosed with early invasive disease (e.g., International Federation of Gynecology and Obstetrics (FIGO) stage IB1) may be candidates for fertility-preserving radical trachelectomy with lymphadenectomy or treated effectively with radical hysterectomy with lymphadenectomy plus tailored adjuvant therapy when indicated.3 In many cases, women who present with locally advanced disease (FIGO stage IB2 to IVA) may experience tumor eradication and long-term durable cure through platinum-based chemoradiation plus high-dose-rate intracavitary brachytherapy.3,4 However, for patients with non-resectable local recurrence and those who fail in the pelvis and at distant sites simultaneously as well as women who present with metastatic disease (i.e., FIGO stage IVB), treatment options have until most recently been palliative at best. Short-lived responses to platinum-based chemotherapy doublets followed by rapid deterioration of quality of life and early death have been the rule, with median survival ranging from seven to 12 months in the vast majority of cases.3,5
Based on clinical, pathologic, molecular, and therapeutic rationale, vascular endothelial growth factor (VEGF) has emerged as an important therapeutic target to prevent tumor angiogenesis, a process which drives HPV-mediated cervical carcinogenesis.6,7 On 7 February 2013, the National Cancer Institute issued a Press Release indicating that incorporation of the anti-VEGF monoclonal antibody, bevacizumab, with two chemotherapy doublets significantly improved overall survival (OS) at the second interim analysis of the phase III randomized clinical trial, Gynecologic Oncology Group (GOG) protocol 240.8 Within one month of formal publication of these interim results, on 10 March 2014 the United Kingdom’s Cancer Drug Fund approved bevacizumab in combination with chemotherapy for women in England with recurrent/metastatic cervical cancer. This was followed by the United States Food and Drug Administration approval on 14 August 2014 and listing of both bevacizumab-containing triplet regimens studied in GOG 240 as Category 1 in the National Comprehensive Cancer Network (NCCN) Cervical Cancer Treatment Guidelines.9–11 Following public disclosure of the final analysis, a label for bevacizumab in cervical cancer based on GOG 240 was granted by health authorities in at least 60 countries on six continents.10,11
Here we present the final protocol-specified OS analysis, post-progression survival, and final toxicology of GOG 240.
METHODS
Study Design and Participants
GOG 240 is a phase III, open-label, randomised trial performed in the United States, Canada and in Spain through the GOG and Spanish Ovarian Cancer Group (GEICO). The study is listed at www.clinicaltrials.gov (NCI identifier #NCT00803062) and the protocol is available at www.LANCET.com. Eligibility criteria included patients with metastatic, persistent, and recurrent cervical carcinoma. Patients with recurrent disease must not have been candidates for curative therapy via pelvic exenteration. All cancers had central pathology review by the Gynecologic Oncology Group (GOG) Pathology Committee. GOG performance status scores of 0 (fully active) to 1 (restricted in physically strenuous activities, but ambulatory) were required, and patients had to have adequate renal, hepatic, and bone marrow function. All patients must have had measureable disease. Patients treated with chemotherapy for recurrence, and those with non-healing wounds, active bleeding conditions, and inadequately anticoagulated thromboembolism, were ineligible. The clinical trial was approved by the NCI’s Central Institutional Review Board (IRB) and by the local IRBs of the GOG-affiliated medical centers throughout the United States and Canada, and the GEICO-affiliated hospitals in Spain where the trial was conducted. All patients provided written informed consent at enrollment.
Procedures
Through the NCI computer-generated randomization, patients were assigned to one of four treatment regimens which were repeated at 21-day intervals. Control treatment consisted of cisplatin (50 mg/m2) plus paclitaxel (135 or 175 mg/m2). The non-platinum doublet consisted of topotecan (0.75 mg/m2 days 1–3) plus paclitaxel (175 mg/m2) (see Online Supplement). Each regimen was studied with and without bevacizumab (15 mg/kg day 1). Treatment was discontinued at the onset of disease progression, unacceptable toxic effects, voluntary withdrawal by the patient, or upon complete response (CR). See Consort Diagram (Figure 1).
Figure 1.
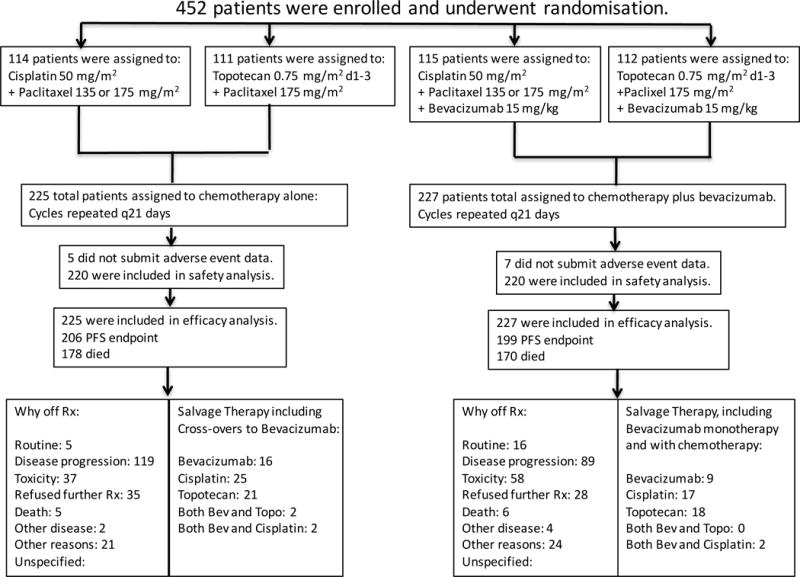
Gynecologic Oncology Group protocol 240 - Final Overall Survival Consort Diagram. Arrangements were made by the National Cancer Institute and Genentech to provide bevacizumab to patients on the chemotherapy alone arms when it was determined at the second interim analysis that the study had met its primary endpoint. These patients on the control arms who “crossed over” to receive bevacizumab were analyzed on the chemotherapy alone arm (backbones combined) for final overall survival analysis and updated progression-free survival analysis as per intent-to-treat.
Details related to disease assessment and tumor measurements using Response Evaluation Criteria in Solid Tumors (RECIST v.1) appear in the master protocol. Safety as assessed by the National Cancer Institute’s Common Terminology Criteria for Adverse Events, was monitored during each cycle. The bevacizumab dose was modified only when weight changed by more than 10%. If chemotherapy was held for low absolute neutrophil count or thrombocytopenia, bevacizumab was also held. Bevacizumab could be delayed or discontinued depending on the occurrence, duration, and severity of uncontrolled hypertension (systolic >150 mm Hg or diastolic >100 mm Hg), proteinuria (urine protein-to-creatinine ratio ≥3.5), arterial/venous thrombosis, coagulopathy, intestinal obstruction/disruption. Following discontinuation of treatment, disease was assessed every three months for two years, followed by every six months for three years until progression. Three validated and sensitive instruments were used to measure health-related quality of life.
Statistical Analysis
The statistical analysis plan is available at www.LANCET.com. Assuming absence of interaction between experimental agents, the study utilized a 2×2 factorial design to investigate the ability of either anti-VEGF therapy (bevacizumab; Factor A) or a regimen using a non-platinum chemotherapy doublet (topotecan-paclitaxel; Factor B) to significantly impact patient outcomes. The study employed the intent-to-treat principle. Patients were prospectively stratified by GOG performance status (0 vs 1), prior radiosensitizing platinum, and disease status (recurrence/persistence versus stage IV primary).
The primary endpoints were overall survival (OS) based on a pooled analysis for each of the treatment factors under investigation, and the frequency and severity of adverse events (AEs) with each regimen. OS was defined as the time of randomisation to death or date last seen. Secondary endpoints were to compare Progression-free survival (PFS) and objective tumor responses among the different treatment arms. PFS was defined as the time from randomization until the patient experienced disease progression or death, or if censored, until the date last seen. Progression was defined using modified RECIST criteria. The definition of disease progression was the observation of either a 20% increase in the sum of the target lesions’ longest dimensions, taking as reference the nadir value on study, the observation of new lesions, or a decrease in health status due to a worsening of the disease. Differences in OS and PFS by factor level were assessed primarily by the log-rank test, stratified by clinical prognostic markers and the level of the other factor. Hazard ratios (HR) were estimated with a Cox proportional hazards model.
Among the tertiary endpoints were a quality of life component, prospective validation of poor prognostic markers identified in pooled analyses from prior studies,12 the prevalence and effect of nicotine dependence on PFS and OS in this population, and several novel translational endpoints involving circulating tumor cells and VEGF isoform expression.
A sample size of approximately 450 was accrued to potentially observe 346 deaths in the final analysis to provide a study with 90% power when either factor was capable of reducing the hazard of death by at least 30%, while limiting the one-sided type I error for each to 2.5% (experiment-wise error rate no greater than 5%). An interim analysis was scheduled near 173 deaths to drop a factor or to stop the study for futility or to report regimen activity early according to the spending function in the event of dramatic improvement in survival. Since the study was designed with futility rules, the alternative hypotheses, critical regions, and p-values for the primary analyses of efficacy were listed as one-sided.
To monitor for unacceptable toxicity in the experimental arms, two 2-stage sequential toxicity analyses were embedded early in the conduct of the study (i.e., first 50 patients assigned to investigational treatment), with specific guidelines dictating when a meeting of the Data Safety Monitoring Board (DSMB) would need to be convened. Adverse events (AEs) were reported until 30 days after the last study treatment and summarized for patients who received any therapy and submitted AE information. The distribution of the number of severe adverse events was assumed to be binomial and group sequential methods were used to assess whether the probability of a severe adverse event was safe or too high. Changes in health-related quality of life (HRQoL) were evaluated using a mixed model for analysis of repeated measures.
Outcomes
There would be five principal iterations of outcome measures, four of which had been pre-specified (interim analysis, patient reported outcomes, final analysis, and toxicology), and one which emerged during the conduct of the trial (second interim analysis). Please refer to the Online Supplement for Reiteration of the 2012 Interim Analysis Report and Reiteration of the 2013 Second Interim Analysis Report.
Role of the Funding Source
The study was sponsored by the National Cancer Institute (NCI) which provided bevacizumab without charge. The study’s DSMB was comprised of NCI scientists. The DSMB conducted the first and second interim analyses and the final analysis. Following each analysis, the DSMB released the results to the NRG Oncology protocol 240 investigators. The funding source did not write the manuscript or decide where it should be submitted. The NCI’s Cancer Therapeutics Evaluation Program (CTEP) reviewed and approved the final version of the manuscript prior to submission. The following authors had access to the data: KST (first author/study chair/principle investigator), MWS (study statistician), FBS (NRG Oncology Publications Committee Chair), and BJM (senior author). The decision to submit the manuscript to The Lancet was made by the first author (KST) and approved by all co-authors and the NRG Oncology Publications Committee. The authors wrote the manuscript and take responsibility for the accuracy and completeness of the reported data and for the fidelity of the document to the protocol. All patients provided written informed consent before enrollment.
RESULTS
GOG protocol 240 was activated on 6 April 2009 and closed to patient accrual on 3 January 2012, at which point the trial had enrolled 452 patients. Patients were treated with chemotherapy alone or chemotherapy plus bevacizumab. Patients were well-matched for performance status, ethnicity, histology, disease status, and in-field pelvic recurrences (Table 1). Importantly, 75% of the entire study group had previously received platinum, and this was also evenly distributed between the 2 backbones.
Table 1.
Gynecologic Oncology Group protocol 240 clinical factors and pathologic features of patients randomized to the chemotherapy alone and chemotherapy plus bevacizumab arms.
| Characteristic | Chemotherapy Alone (n=225), (%) | Chemotherapy + Bevacizumab (n=227), (%) |
|---|---|---|
|
| ||
| Median age, years (standard deviation) | 46 (12.09) | 48 (11.73) |
|
| ||
| Histology, % | ||
| Squamous | 152 (67.6) | 158 (69.6) |
| Adenocarcinoma, unspec. | 44 (19.6) | 42 (18.5) |
| Other | 29 (12.9) | 27 (11.9) |
|
| ||
| Race, % | ||
| White | 180 (80.0) | 171 (75.3) |
| African American | 24 (10.7) | 36 (15.9) |
| Asian | 7 (3.1) | 12 (5.3) |
| Pacific Islander | 1 (0.4) | 0 (0.0) |
| Other | 13 (5.8) | 8 (3.5) |
|
| ||
| Disease presentation, % | ||
| Recurrent | 165 (73.3) | 160 (70.5) |
| Persistent | 23 (10.2) | 28 (12.3) |
| Advanced | 37 (16.4) | 39 (17.2) |
|
| ||
| Performance status, % | ||
| 0 | 131 (58.2) | 132 (58.1) |
| 1 | 94 (41.8) | 95 (41.9) |
|
| ||
| Prior platinum, % | 166 (73.8) | 171 (75.3) |
|
| ||
| Pelvic disease, % | 120 (53.3) | 123 (54.1) |
|
| ||
| Prior pelvic RT | ||
| Yes | 180 (80.0) | 181 (79.7) |
| No | 45 (20.0) | 46 (20.3) |
|
| ||
| Target Lesion in RT Zone | ||
| Yes | 94 (41.8) | 85 (37.4) |
| No | 130 (57.9) | 140 (61.7) |
| Unknown | 1 (0.4) | 2(0.9) |
RT: radiotherapy
On March 7, 2014, with 348 deaths, the protocol-specified 346 deaths had been exceeded. During the period following the second interim analysis leading up to 348 events, a total of 20 patients who had been randomly assigned to be treated with chemotherapy alone had gone on to receive salvage therapy with bevacizumab upon progression or were treated with bevacizumab when it was determined that the study had met its primary endpoint and provisions to supply bevacizumab to patients on chemotherapy alone had been made (Figure 1). In an intent-to-treat final analysis of OS, the arms administering bevacizumab continued to demonstrate a significant improvement over chemotherapy alone: 16.8 mos vs 13.3 mos; HR 0.77 (95% CI, 0.62–0.95; p=0.0068) (Figure 2A).
Figure 2.
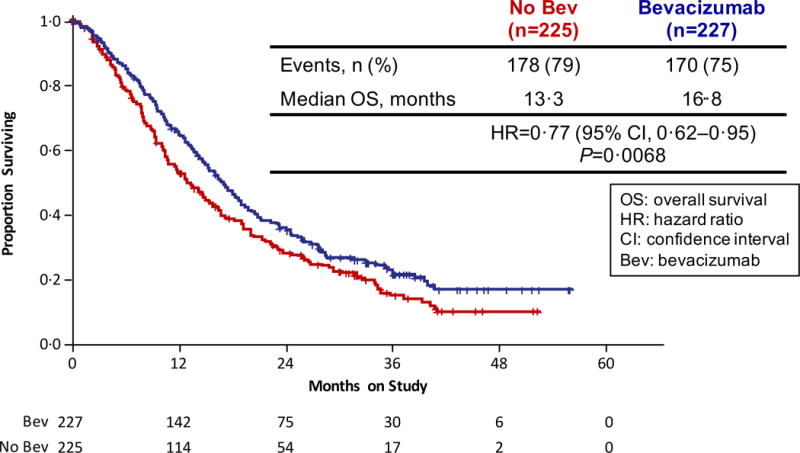
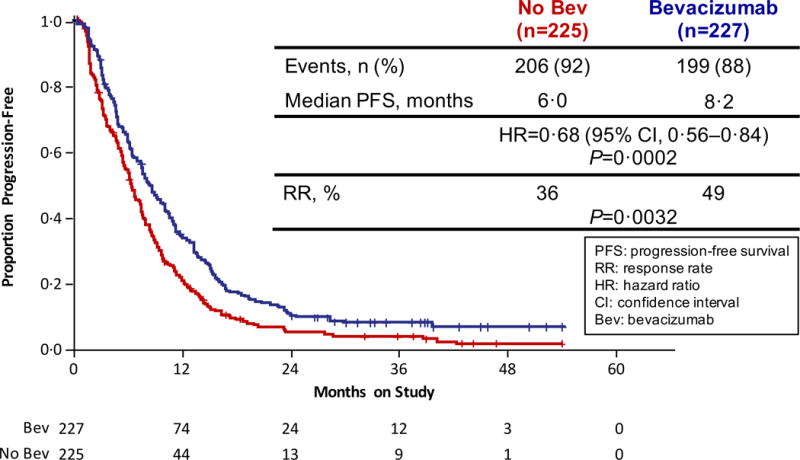
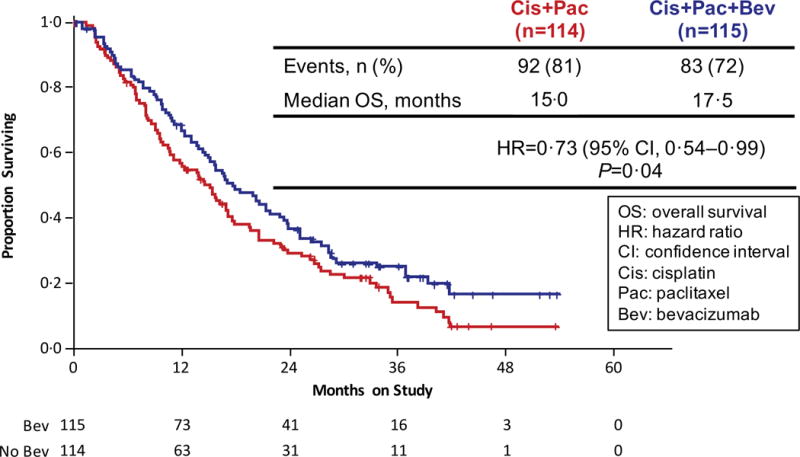
Gynecologic Oncology Group protocol 240 - Final Overall Survival and Progression-Free Survival. Panel A: Kaplan-Meier curves depicting the intent-to-treat final protocol-specified overall survival comparing chemotherapy alone (both backbones) to chemotherapy plus bevacizumab. Panel B: Kaplan-Meier curves depicting the intent-to-treat updated progression-free survival comparing chemotherapy alone (both backbones) to chemotherapy plus bevacizumab. Panel C: Kaplan-Meier curves depicting the intent-to-treat final protocol-specified overall survival comparing cisplatin plus paclitaxel with and without bevacizumab. Panel D: Kaplan-Meier curves depicting the intent-to-treat final protocol-specified overall survival comparing topotecan plus paclitaxel with and without bevacizumab. Panel E: Kaplan-Meier curves depicting the intent-to-treat final protocol-specified overall survival among patients not previously treated with pelvic radiation with and without bevacizumab.
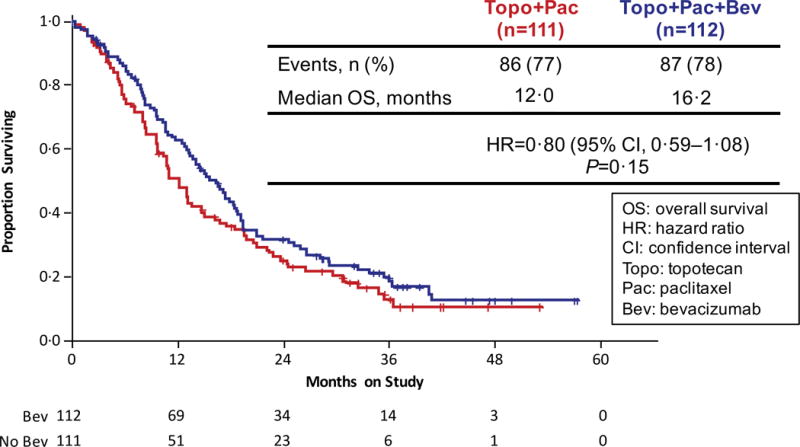
Updated PFS data in an intent-to-treat analysis also indicate that the regimens administering bevacizumab continued to significantly reduce the hazard of progression by approximately 32% (8.2 mos vs 6.0 mos; HR 0.68; 95% CI, 0.56–84;p=0.0002) (Figure 2B). Intent-to-treat OS analysis of each triplet individually also demonstrated a sustained survival benefit conferred through the incorporation of anti-angiogenesis therapy. When compared to the cisplatin-paclitaxel chemotherapy doublet, the addition of bevacizumab reduced the hazard of death by 27% (17.5 mos vs 15.0 mos; HR 0.73; 95% CI, 0.54–0.99); p=0.04) (Figure 2C). Although not statistically significant, the magnitude of survival time gained through the integration of anti-VEGF therapy to the topotecan-paclitaxel chemotherapy backbone was similar and in the same direction to that observed for the cisplatin-paclitaxel-bevacizumab triplet: 16.2 mos vs 12.0 mos (HR 0.80; 95% CI, 0.59–1.08; p=0.15) (Figure 2D).
Final analysis of response demonstrated approximately 48% of patients responding on cisplatin-paclitaxel with and without bevacizumab and topotecan-paclitaxel-bevacizumab, while those patients on topotecan-paclitaxel alone had about a 25% probability of responding (p=0.0004). Because the interaction term is significant, the impact of bevacizumab on the probability of response depends on whether the regimen contains cisplatin or topotecan. Although the administration of bevacizumab significantly impacts OS and PFS, it has a greater impact on response rate when given to topotecan-paclitaxel than when given to cisplatin-paclitaxel (Table 2). Specifically, the response rate of topotecan-paclitaxel is almost doubles when bevacizumab is incorporated into treatment.
Table 2.
Comparison of response rates among all four treatment arms at final overall survival analysis.
| Count (%) | Tumor Response over Entire Course of Treatment by Regimen | ||||
|---|---|---|---|---|---|
| Cis+Pac | Cis+Pac+Bev | Top+Pac | Top+Pac+Bev | Total | |
| Complete Response | 11 (9.65) |
18 (15.65) |
6 (5.41) |
13 (11.61) |
48 |
| Partial Response | 41 (35.96) |
40 (34.78) |
22 (19.82) |
41 (36.61) |
144 |
| Stable Disease | 45 (39.47) |
42 (36.52) |
54 (48.65) |
43 (39.39) |
184 |
| Progressive Disease | 12 (10.53) |
7 (6.09) |
21 (18.92) |
6 (5.36) |
46 |
| Indeterminate | 5 (4.39) |
8 (6.96) |
8 (7.21) |
9 (8.04) |
30 |
| Total | 114 | 115 | 111 | 112 | 452 |
Cis: cisplatin; Pac: paclitaxel; Bev: bevacizumab; Top: topotecan
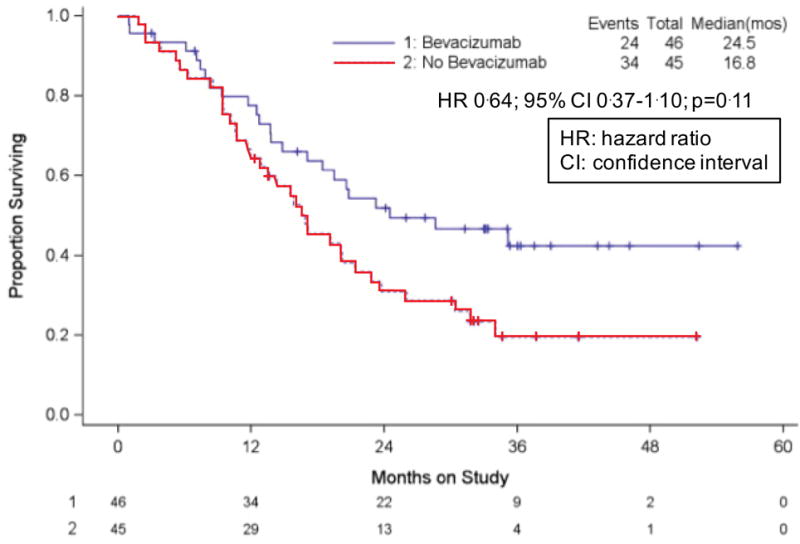
Only 20% (n=91) of the entire sample had not previously received pelvic radiotherapy, and final OS among those treated with bevacizumab (n=46) vs those who did not receive bevacizumab (n=45) was 24.5 vs 16.8 mos, respectively (HR 0.64; 95% CI 0.37–1.10; p=0.11) (Figure 2E). At final analysis the overall incidence of fistula was 14.5% (n=32) among patients treated with bevacizumab, all of whom had had prior radiotherapy (Table 3). The occurrence of clinically significant and/or severe fistula (i.e., grade 3) was 5.9% (n=13). No fistulas resulted in surgical emergencies, sepsis, or death, and in addition to pelvic irradiation, other factors associated with fistula included pelvic disease, pre-existing hypertension, and current tobacco use.
Table 3.
Updated toxicology observed on the chemotherapy alone and chemotherapy plus bevacizumab arms at final overall survival analysis of Gynecologic Oncology Group protocol 240.
| Adverse Event | Chemotherapy Alone n=220 (%) | Chemotherapy + Bevacizumab n=220 (%) | Risk Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Genitourinary fistula grade 2 | 1 (0.45) | 8 (3.64) | 8 (1.01 ~ 63.4) | 0.037 |
| Genitorurinary fistula grade 3 | 1 (0.45) | 6 (2.7) | 6 (0.73 ~ 49.4) | 0.12 |
| GI fistula, grade 2 | 1 (0.45) | 11 (5.00) | 11 (1.43 ~ 84.5) | 0.006 |
| GI fistula, grade 3 | 0 | 7 (3.2) | n/a | 0.015 |
| Hypertension, grade ≥ 2 | 4 (1.8) | 55 (25) | 13.8 (5.07~ 37.3) | <0.001 |
| Neutropenia, grade ≥ 4 | 58 (26) | 80 (36.4) | 1.37 (1.04 ~ 1.83) | 0.0308 |
| Febrile neutropenia, grade ≥ 3 | 12 (5.5) | 12 (5.5) | 1.0 (0.46 ~ 2.18) | 1.000 |
| GI bleeding grade ≥ 3 | 1 (0.45) | 4 (1.8) | 4.0 (0.45 ~ 35.5) | 0.37 |
| Proteinuria, grade ≥ 3 | 0 | 5 (2.3) | n/a | 0.06 |
| Thrombosis/embolism, gr ≥ 3 | 4 (1.8) | 18 (12.7) | 4.5 (1.55 ~ 13.1) | 0.004 |
| Pain grade ≥ 2 | 63 (28.7) | 72 (32.7) | 1.1 (0.86 ~ 1.51) | 0.41 |
Following this page, materials for the Online Supplement appear.
When examining bevacizumab-related toxicology with an exploratory post-hoc landmark OS analysis among those treated with anti-angiogenesis therapy, the occurrence of grade 2 or higher neutropenia could be associated with improved OS (HR 0.75; 95% CI, 0.60–0.93; p=0.0089) (Online Supplement Table 1, Online Supplement Figure 1), suggesting that neutropenia may represent a surrogate biomarker for survival. Conversely, the development of grade 3 or higher thromboembolism and grade 3 or higher fistula may be associated with a survival disadvantage (Online Supplement Table 1). The protocol stipulated that patients who developed fistula or thromboembolism were taken off study and not treated further with anti-angiogenesis therapy.
Following progression on protocol-specified therapy, the post-progression OS was 8.4 vs 7.1 mos among patients treated with and without bevacizumab, respectively (HR 0.83; 95% CI 0.659–1.052; p=0.06) (Figure 3A). For patients treated on the cisplatin-paclitaxel chemotherapy backbone, post-progression survival was 8.3 mos (with bevacizumab) vs 6.2 mos (without bevacizumab) with a hazard of death of 0.83; 95% CI 0.595–1.153; p=0.13 (Figure 3B). Post-progression survival for patients assigned to the topotecan-paclitaxel chemotherapy backbone with and without bevacizumab was 8.7 vs 7.5 mos, respectively, with a hazard of death of 0.84; 95% CI 0.602–1.165; p=0.14 (Figure 3B).
Figure 3.
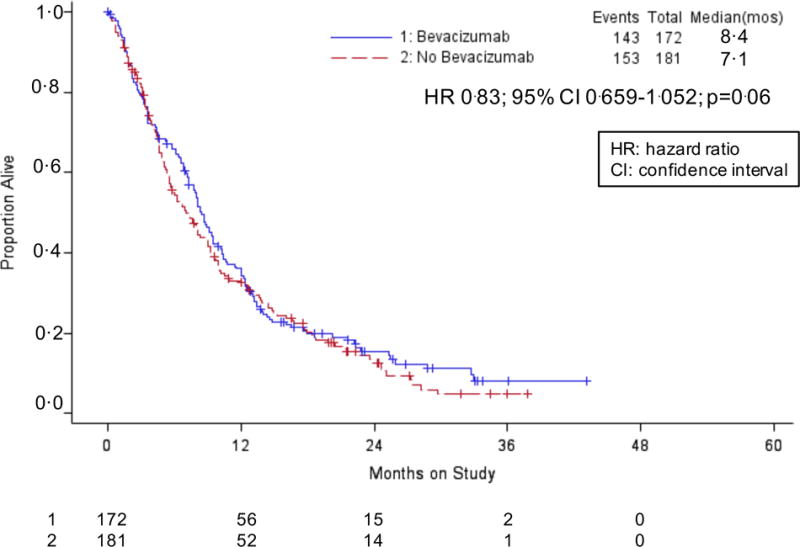
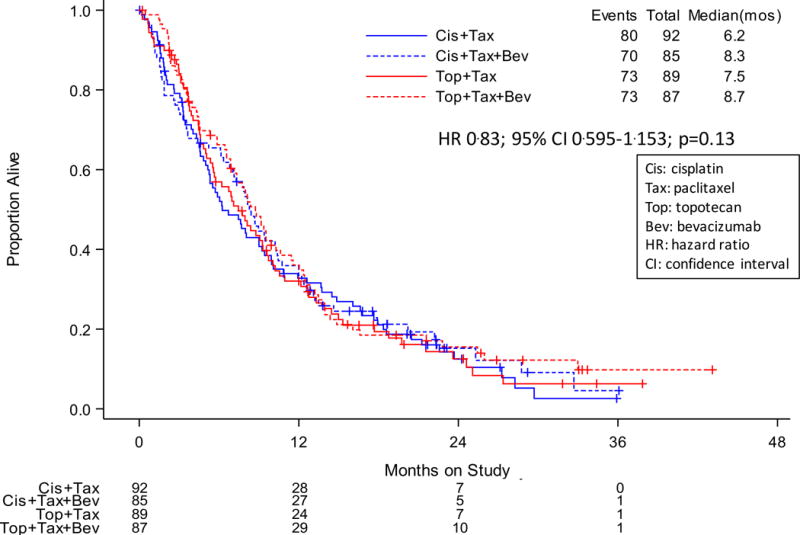
Gynecologic Oncology Group protocol 240 – Post-progression survival after protocol treatment with and without bevacizumab. Panel A: Kaplan-Meier curves demonstrating post-progression survival among patients treated with chemotherapy alone (both backbones) vs chemotherapy plus bevacizumab. Panel B: Kaplan-Meier curves demonstrating post-progression survival for each of the four arms of the protocol, specifically, cisplatin-paclitaxel with and without bevacizumab and topotecan-paclitaxel with and without bevacizumab.
DISCUSSION
The rationale to target angiogenesis in advanced cervical cancer has been reviewed previously. Specifically, clinical, histopathologic, and molecular evidence supports this therapeutic strategy.3,13–16 Sequestration of VEGF using bevacizumab when combined with chemotherapy led to a statistically significant and clinically meaningful survival advantage among women with advanced cervical cancer in this phase III randomized clinical trial. Final analysis of this study is noteworthy in having taken place nearly 26 months following randomization of the last patient on the trial. The continued separation of the OS curves at final analysis indicates that the survival benefit conferred by the incorporation of bevacizumab identified at the second interim analysis was not transient. At greater than 50 months of maximal follow-up, many patients continue to benefit from stable disease, and some have been cured with no evidence of clinical and radiologic disease. Clearly first-line therapy using bevacizumab in the recurrent/persistent and metastatic setting has clinical value, as the curves do not converge at final intent-to-treat analysis, despite having over 20 patients initially randomized to the chemotherapy alone arms crossing over to receive salvage therapy with bevacizumab. Given that this is a disease in which OS has been typically measured in months (e.g., 7 to 12 months at best), these data indicate that anti-angiogenesis therapy can have clinically meaningful therapeutic benefit in this population.
Clinically significant fistula (i.e., grade 3, requiring intervention) occurred in 5.9% (n=13) of patients receiving bevacizumab, all of whom had been previously irradiated. The magnitude of risk conferred by additional clinical factors (e.g., concurrent tobacco use, pre-existing hypertension, and pelvic tumor) is underway. Unlike the surgical emergencies that manifest when gastrointestinal perforation occurs among women with ovarian cancer who receive bevacizumab, as stated earlier, none of the fistulae required urgent surgical intervention or resulted in sepsis or death. Importantly, the prospective validation of the Moore clinical criteria12 in GOG 240 provides oncologists with the first scoring system in cervical cancer which can be used to estimate response and survival and potentially identify patients who are best treated without bevacizumab (e.g., a pre-irradiated patient at risk for fistula for whom the survival benefit attributable to adding bevacizumab is negligible).17 It should be noted that the late injury of radiotherapy is microvascular and therefore preirradiated patients being considered for treatment with chemotherapy plus bevacizumab should be counseled that the risk of vascular complications may be increased.
Through highly stringent and rigid eligibility criteria not previously employed by the GOG in previous therapeutic trials in advanced cervical cancer, the study population was effectively sanitized. Specifically, by restricting performance status to 0–1, requiring normal renal function, correction of malnutrition, optimization of medical co-morbidities, and control of tumor-related pain, the GOG 240 population represented the ‘healthiest’ cohort of a population with a very poor prognosis. This was felt to be necessary to effectively position the factors under investigation (bevacizumab and substitution of topotecan for cisplatin) for the best chance for success. Although clearly conjecture at this point, it is possible that these restrictions on eligibility for trial participation contributed to the development of a control arm which did not underperform and quality of life (QoL) scores which, based on three independent previously validated QoL instruments, did not deteriorate significantly when anti-angiogenesis therapy was added to either chemotherapy backbone.18 Clearly the message that emerges is one of medicine-first/oncology-second in that women with advanced cervical cancer are best served when physicians emphasize medical management before dispatching the oncologic armamentarium.
Interestingly, as per the first interim analysis findings, the substitution of topotecan for cisplatin did not circumvent suspected acquired drug resistance to platinum that may have occurred when patients received platinum-based chemoradiation prior to recurrence. This suggests that the issue is not one of platinum resistance per se but rather chemotherapy resistance in general. However, as much as GOG 240 is seen as an anti-angiogenesis therapy trial, it is also a study which serves as a proof of principle and value of systemic therapy.19,20 The magnitude of efficacy benefit conferred by bevacizumab is similar regardless of which chemotherapy doublet it is combined with. With the recent reporting of the Japanese Clinical Oncology Group phase III non-inferiority trial of cisplatin-paclitaxel vs carboplatin-paclitaxel in which significant non-inferiority was demonstrated (JCOG protocol 0505),21 several centers have considered a carboplatin-paclitaxel backbone to combine with bevacizumab. Based on subset analyses from the Japanese trial, a survival advantage in the cisplatin arm was observed among women who had not previously received cisplatin as part of primary chemoradiation for locally advanced disease. Therefore, the cisplatin-paclitaxel-bevacizumab triplet may be preferable for patients who are cisplatin-naïve, as well as the elderly and those who have received prior extended field irradiation for whom diminished bone marrow reserves would render carboplatin prohibitive.
Following this line of reasoning, it was remarkable that on analysis of prognostic factors that the significant survival benefit conferred by bevacizumab was sustained even when the disease was in the previously irradiated pelvis. Clearly it was possible to deliver antibody to an anatomic region with presumed radiation-induced vascular compromise. Together with the observations at final analysis that OS and PFS remain significantly improved among those randomized to the arms administering bevacizumab, GOG 240 stands as a proof of concept of anti-angiogenesis therapy in this disease.22 This in turn suggests that bevacizumab monotherapy deserves some consideration, particularly among patients intolerable to anti-neoplastic therapy despite several dose reductions ultimately requiring the peeling back of chemotherapy.
Anti-angiogenesis therapy has also been shown to be effective in other gynecologic cancers. Interestingly, there have been eight phase III randomized trials evaluating five different agents in newly diagnosed and recurrent ovarian cancer, all of which met their primary endpoint (i.e., PFS), and a ninth trial recently reported that met its secondary endpoint (i.e., PFS).23 Many investigators hypothesize that the inability to demonstrate OS with anti-angiogenesis therapy in ovarian cancer is due to the fact that the disease is chemosensitive and because post-progression therapy cannot be controlled, the OS question concerning anti-angiogenesis therapy becomes diluted. Conversely, cervical cancer is relatively chemoresistant and therefore it may be easier to demonstrate OS with a therapy (e.g., anti-angiogenesis treatment) that has activity in the advanced cervix population because these patients are unlikely to receive multiple lines of chemotherapy. However, an alternative explanation has emerged recently which suggests that because ovarian cancer is characterized by genomic instability, it is a more heterogeneous disease than cervical cancer. Cervical cancer is driven by HPV infection resulting in a more homogeneous tumor characterized by viral oncogene-driven angiogenesis.11 Although clearly conjecture, it is possible that VEGF inhibition may result in an OS benefit in cervical cancer and not in ovarian cancer because bevacizumab is more effective (i.e., works better) in cervical cancer. In point of fact, Gourley and colleagues from the UK have recently identified an immune subgroup in ovarian cancer that does not respond to bevacizumab and at least two proangiogenic subgroups for which a trend towards PFS is evident with bevacizumab therapy.24
Two important issues are generated by the final OS analysis. Although the drug has been approved for the advanced cervical cancer indication in many countries, it is not being provided by various governments and ministries of health for all patients who are candidates for treatment. In most countries, only those who can afford bevacizumab are able to receive it. This is also true in those countries which are part of the “developing world”, which in fact is not developing, but rather remains the same. Perhaps if one were to superimpose a map of nations with clean water, paved roads, reliable electricity, and women’s rights, then those factors could be correlated with death from cervical cancer.
We acknowledge that regulatory approval of a drug for a specific indication by a country’s health agency does not indicate that the drug will be provided to all affected citizens. This is particularly true when considering drugs deemed to be costly. Interestingly, US FDA approval was actually preceded by Cancer Drug Fund approval in England. Unlike the National Institute of Health and Care Excellence (NICE), the Cancer Drug Fund focuses on making drugs available that are determined to not be cost-effective. A Markov analysis using these trial data indicated that bevacizumab becomes cost-effective with a 75% reduction in cost.25 This suggests that part of the solution to providing anti-angiogenesis therapy may lie in the upcoming expiration of the bevacizuamb patent from 2019 to 2022 and the introduction of biosimilars. It should be noted, however, that biosijilars to monoclonal antibodies may be difficult to generate.
The second issue created by GOG 240 is that of a new patient population, specifically, women with advanced cervical cancer who progress following treatment with bevacizumab. Although a rebound effect was not observed in our analysis of post-progression survival, there still remains the question of what therapies to study in the second-line setting. Other anti-angiogenesis agents such as cediranib should be studied, particularly in light of the activity recently reported by Symonds et al in this population.26,27 Non-VEGF-dependent angiogenesis inhibitors (e.g., the angiopoietin axis inhibitor, trebananib) and vascular disrupting agents that target existing tumor vasculature may also be considered. Given the immunologic dysfunction that prevents viral clearance in women who develop cervical cancer, immunologic-based therapies are promising, and currently undergoing investigation is a Listeria monocytogenes-based HPV 16 E7 therapeutic vaccine (ADXS-HPV), autologous T-cell therapy, and the anti-programmed cell death 1 (PD-1) immunomodulators, nivolumab, and pembrolizumab.28,29 Signal transduction pathways relevant to cervical carcinogenesis that can be targeted include the PI3K/Akt/mTOR pathway, homologous recombination deficiency pathways through which to exploit synthetic lethality, and the Notch binary cell-fate decision pathway.28,30 Finally, adenoviral-directed gene therapy to reconstitute wild type p53 function or suicide genes, as well as the identification and targeting of cervical cancer stem cells may also represent viable therapeutic options in the future.
To be able to even discuss novel therapies in this disease is a statement that some progress has finally been made. The GOG has now conducted nine phase III randomized trials over three decades in this population and with final OS analysis of the ninth study (i.e., GOG 240) we have at last placed the proverbial foot in the door. With some ground gained, there is the challenge to identify tolerable treatments that can extend survival further. Upcoming trials will likely emphasize different classes of anti-angiogenesis agents, immune modulators and chemotherapy-free combinations, and translational science.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Based on the activity of the anti-angiogenesis drug bevacizumab in colorectal cancer and other solid tumors, vascular endothelial growth factor (VEGF) had emerged in the first decade of the 21st Century as an important molecule to target to prevent tumor angiogenesis. The rationale to target angiogenesis in advanced cervical cancer is based on four observations: 1) clinically, women with abnormal Papanicolaou cervical cytologic screening tests will often be demonstrated to have vascular markings upon cervical colposcopy which represent harbors of angiogenesis and the beginnings of microinvasive disease; 2) pathologically, a high microvessel intratumoral density of the endothelial cell antigen, CD31, portends a poor prognosis among women diagnosed with invasive cervical cancer; 3) molecularly, the consequences of viral integration into host DNA activates a cascade through which human papilloma viral oncoproteins E6 and E7 degrade/inactivate cellular tumor suppressor gene products p53, and retinoblastoma, respectively, ultimately leading to increased hypoxia-inducible factor alpha expression, and increased VEGF production which promotes angiogenesis; 4) therapeutically, evidence of anti-angiogenesis activity in women with advanced cervical cancer can be found in a phase I trial of Aspergillus fumigatus fresenius-derived angio-inhibitory molecule, TNP-470, and phase II trials of the anti-VEGF fully humanized monoclonal antibody, bevacizumab, and the oral small molecule VEGF-receptor tyrosine kinase inhibitor, pazopanib. Following a second interim analysis of the phase III randomized trial of chemotherapy with and without bevacizumab for women with recurrent/persistent and metastatic cervical carcinoma, the National Cancer Institute (NCI) reported that the study had met one of its primary endpoints in that the incorporation of bevacizumab with two distinct chemotherapy doublets resulted in a significant survival advantage compared to those patients treated with chemotherapy alone (17.0 vs 13.3 mos: HR 0.71; 98% CI, 0.54–0.95; p=0.004).
Added value of this study
The study’s primary analysis at the second interim analysis concluded that bevacizumab prolonged OS. This was an important observation, yet concerns remained about whether these survival differences were robust, clinically meaningful, and would persist in final analysis. This final protocol-specified intent-to-treat analysis was conducted with 348 pre-specified deaths and confirms that the survival advantage observed at interim analysis has been sustained, despite a number of women in the control arms being provided bevacizumab following the NCI press release. Importantly, responding to bevacizumab was not associated with early death following progression as evidenced by the detailed post-progression survival analysis. Finally, longer follow-up has provided for greater precision in the characterization of the adverse event profile allowing for an accurate estimation of the occurrence of venous thromboembolism and permits the separation of gastrointestinal (GI) perforation events (a surgical emergency) from development of GI- and genitourinary-vaginal fistula.
Implications of all the available evidence
As a consequence of the integration of bevacizumab with chemotherapy in GOG 240, the clinical, pathologic, molecular, and therapeutic rationale to target tumor angiogenesis in advanced cervical cancer has resulted in a significant survival advantage without concomitant deterioration of quality of life. These results were sustained through the final protocol-specified survival analysis and did not leave patients vulnerable to early death following progression on bevacizumab. The incorporation of bevacizumab in the treatment of recurrent/persistent, and metastatic cervical is practice-changing as evidenced by adoption and endorsement of this therapy by multiple regulatory agencies throughout the world. Cervical cancer remains a major health problem globally and, while screening and prevention (i.e., vaccination) programs need to be established in the developing world, for the first time in decades, a small therapeutic window of opportunity has been identified in the management of advanced disease through which patients deriving benefit from chemotherapy plus bevacizumab may be treated with other active novel agents prior to progression. Toxicity requires continued evaluation, particularly in cases of fistula formation.
Acknowledgments
The authors would like to acknowledge Juliet Wolford, MD for her assistance with editing the survival curve figures so that the legends and color schemes remained consistent across Figures 2, 3, and Online Supplement Figures 1–3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Summary of Authors’ Disclosures of Potential Conflicts of Interest
Dr(s) Tewari, Brady, and Monk report that their institutions and the Gynecologic Oncology Group received grants from Genentech to conduct this clinical trial. Dr(s). Tewari, Burger, and Monk report that they have participated on an Advisory Board and/or served as a speaker on a Round Table Discussion of Bevacizumab for Genentech. Dr(s) Monk and Thigpen have served on a Genentech Speaker’s Bureau. Dr. Penson reported that he received clinical trial funding from Genentech/Roche. All other co-authors, including Dr(s) Sill, Huang, Ramondetta, Look, Landrum, Oaknin, Reid, Leitao, Michael, DiSaia, Copeland, Creasman, Stehman, Birrer, Waggoner, Moore, and Koh report that they have nothing to disclose.
Statement of Author Contributions
Dr. Tewari (first and corresponding author) served as the Study Chair and Principle Investigator of this clinical trial and Dr. Monk (senior author) was the disease site chair (i.e., Cervical Cancer Committee Chair of NRG Oncology). He along with Dr(s) Sill, Brady, Penson, DiSaia, Copeland, Creasman, Stehman, Burger, Thigpen, Birrer, Waggoner, Moore, Koh, and Monk were responsible for the design and conduct of the study. Dr. Huang analyzed quality of life data and Dr. Michael served as the pathologist for this clinical trial. Dr(s) Tewari, Monk, Ramondetta, Landrum, Oaknin, Reid, and Leitao were the assigned investigators at institutions that enrolled the highest numbers of patients onto this clinical trial. The original and subsequent manuscript revisions were written by Dr Tewari and reviewed by all co-authors.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Small W, Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017 May 2; doi: 10.1002/cncr.30667. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25:2952–65. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 5.Hirte H, Kennedy EB, Elit L, Fung Kee Fung M. Systemic therapy for recurrent, persistent, or metastatic cervical cancer: a clinical practice guideline. Curr Oncol. 2015;22:211–9. doi: 10.3747/co.22.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano-Olvera A, Cetina L, Coronel J, Duenas-Gonzaelez A. Emerging drugs for the treatment of cervical cancer. Expert Opin Emerg Drugs. 2015;20:165–82. doi: 10.1517/14728214.2015.1002768. [DOI] [PubMed] [Google Scholar]
- 7.Fisher CM, Schefter TE. Profile of bevvacizumab and its potential in the treatment of cervical cancer. Onco Targets Ther. 2015;8:3425–31. doi: 10.2147/OTT.S73251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bizzarri N, Ghirardi V, Alessandri F, et al. Bevacizmab for the treatment of cervical cancer. Expert Opin Biol Ther. 2016;16:407–19. doi: 10.1517/14712598.2016.1145208. [DOI] [PubMed] [Google Scholar]
- 10.Liu FW, Cripe J, Tewari KS. Anti-angiogenesis therapy in gynecologic malignancies. Oncology. 2015;29:350–60. [PubMed] [Google Scholar]
- 11.Sugiyama T, Mizuno M, Aoki Y, et al. A single-arm study evaluating bevacizumab, cisplatin, and paclitaxel followed by single-agent bevacizumab in Japanese patients with advanced cervical cancer. Jpj J Clin Oncol. 2017;47:39–46. doi: 10.1093/jjco/hyw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DH, Tian C, Monk BJ, et al. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116:44–9. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, et al. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endoethelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19:4611–20. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 14.Kudelka AP, Verschraegen CF, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med. 1998;338:991–2. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- 15.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–9. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 17.Tewari KS, Sill MW, Monk BJ, et al. Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with and without bevacizumab: A NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res. 2015;21:5480–7. doi: 10.1158/1078-0432.CCR-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penson RT, Huang HQ, Wenzel LB, et al. Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240) Lancet Oncol. 2015;16:301–11. doi: 10.1016/S1470-2045(15)70004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervial Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015;13:395–404. doi: 10.6004/jnccn.2015.0055. [DOI] [PubMed] [Google Scholar]
- 20.Rosen VM, Guerra I, McCormack M, et al. Systematic review and network meta-analysis of bevacizumab plus first-line topotecan-pacclitaxel or cisplatin-paclitaxel versus non-bevacizumab-containing therapies in persistent, recurrent, or metastatic cervical cancer. Int J Gynecol Cancer. 2017 Apr 26; doi: 10.1097/IGC.0000000000001000. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: The open-label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33:1–9. doi: 10.1200/JCO.2014.58.4391. [DOI] [PubMed] [Google Scholar]
- 22.Gadducci A, Lanfredini N, Sergiampietri C. Antiangiogenic agents in gynecologic cancer: State of art and perspectives of clinical research. Crit Rev Oncol Hematol. 2015;96:113–28. doi: 10.1016/j.critrevonc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Wei XW, Zhang ZR, Wei YQ. Anti-angiogenic drugs currently in phase II clinical trials for gynecological cancer treatment. Expert Opin Investig Drugs. 2013;22:1181–92. doi: 10.1517/13543784.2013.812071. [DOI] [PubMed] [Google Scholar]
- 24.Thanapprasapasr D, Hu W, Sood AK, Coleman RL. Moving beyond VEGF for anti-angiogenesis strategies in gynecologic cancer. Curr Pharm Des. 2012;18:2713–9. doi: 10.2174/138161212800626201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minion LE, Bai J, Monk BJ, et al. A Markov model to evaluate cost-effectiveness of antiangiogenesis therapy using bevacizumab in advanced cervical cancer. Gynecol Oncol. 2015;137:490–6. doi: 10.1016/j.ygyno.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Symonds P, Gourley C, Davidson S, et al. Cedirinib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervix cancer (CIRCCa): a randomised, double blind, placebo controlled phase 2 trial. Lancet Oncol. 2015;16:1515–24. doi: 10.1016/S1470-2045(15)00220-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luvero D, Plotti F, Lopez S, et al. Antiangiogenics and immunotherapies in cervical cancer: an update and future’s view. Med Oncol. 2017;34:115. doi: 10.1007/s12032-017-0972-8. Epub 2017 May 5. [DOI] [PubMed] [Google Scholar]
- 28.Dizon DS, Mackay HJ, Thomas GM, et al. State of the science in cervical cancer: where we are today and where we need to go. Cancer. 2014;120:2282–8. doi: 10.1002/cncr.28722. [DOI] [PubMed] [Google Scholar]
- 29.McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol (R Coll Radiol) 2017;29:153–60. doi: 10.1016/j.clon.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Crafton SM, Salani R. Beyond chemotherapy. An overview and review of targeted therapy in cervical cancer. Clin Ther. 2016;38:449–58. doi: 10.1016/j.clinthera.2016.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


