Abstract
Type-1 regulatory T (TR1) cells are Foxp3-negative IL-10-producing CD4+ T cells with potent immune suppressive properties but their requirements for lineage development have remained elusive. Here we show that TR1 cells constitute the most abundant regulatory population after allogeneic bone marrow transplantation (BMT), express the transcription factor Eomesodermin (Eomes) and are critical for the prevention of graft-versus-host disease (GVHD). We demonstrate that Eomes is required for TR1 cell differentiation during which it acts in concert with the transcription factor B-lymphocyte-induced maturation protein-1 (Blimp-1) by transcriptionally activating IL-10 expression and repressing differentiation into other Th lineages. We further show that Eomes induction in TR1 cells requires T-bet and donor macrophage-derived IL-27. We thus define the cellular and transcriptional control of TR1 cell differentiation during bone marrow transplantation, opening new avenues to therapeutic manipulation.
Introduction
Type-1 regulatory T (TR1) cells are a FoxP3 negative, IL-10 producing T cell population, which have potent immune suppressive functions and bear alloantigen specificity (1, 2). IL-10 is the major mediator by which TR1 cells assert their immunomodulatory role. Direct and bystander-mediated T cell suppression by TGFβ and granzyme B-dependent killing of antigen presenting cells (APC) have also been described (reviewed in (3)). In addition to IL-10, TR1 cells show high expression of TGF-β, secrete intermdiate amounts of IFNγ but no IL-2 or IL-4 (3–5). Extensive studies have demonstrated the importance of TR1 cells in maintaining immune tolerance or limiting overt inflammation after transplantation, during autoimmune disease or after infections (6–9). IL-27 has been identified as a main driver of TR1 cell differentiation via the activation of transcription factors that include B-lymphocyte-induced maturation protein-1 (Blimp-1), the aryl hydrocarbon receptor (AhR) and c-Maf (5–8, 10–12). However, the function, phenotype and lineage development of TR1 cells in disease states remains poorly understood (5, 13).
Graft-versus-host disease (GVHD) is a common complication of allogeneic bone marrow transplantation (BMT), limiting survival and quality of life (14). CD4+FoxP3+ regulatory T (Treg) cells are a well defined regulatory population important for the generation of tolerance after BMT (15). Due to impaired homeostasis of Treg cells after allogenenic BMT (16), other suppressive cell populations such as TR1 cells may be imperative for the prevention and treatment of GVHD. Consistent with this idea, IL-10 deficiency in donor T cells results in more severe GVHD (17, 18). We thus developed a mouse model using a dual Il10GFP / Foxp3RFP reporter mouse strain (19, 20) to delineate regulatory T cell responses after experimental BMT. Using GVHD as a disease model, we show that TR1 cells are the most abundant IL-10 producing regulatory T cell population after experimental BMT. Further analyses demonstrate that TR1 cells that develop during GVHD express high amounts of Eomes, which is required for their development and its over-expression promotes TR1 cell development both in vivo and in vitro. Eomes acts in concert with Blimp-1, a known transcriptional regulator of TR1 cell differentiation (6–8, 21), to induce IL-10 expression. We further show that Eomes expression and TR1 cell development require T-bet and donor macrophage-derived IL-27, resulting in a T-betloEomeshi phenotype. Finally, we demonstrate that Eomes+ TR1 cells are abundant after clinical BMT indicating the applicability of our findings. Our findings open the way for new therapeutic strategies in transplantation and other clinical settings.
Results
TR1 cells represent a major regulatory T cell population in GVHD
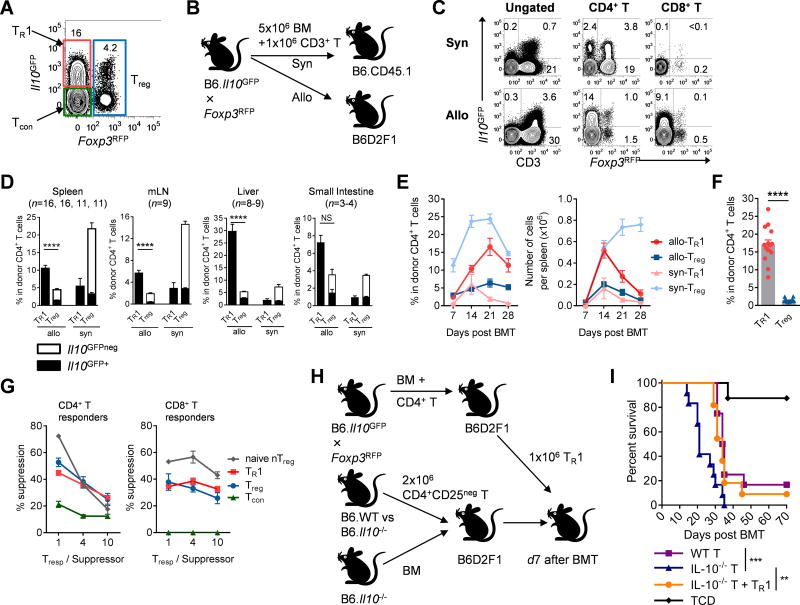
We used Il10GFP and Foxp3RFP dual reporter mice as BMT donors to define CD4+ FoxP3neg IL-10+ type-1 regulatory T (TR1) cells, CD4+ FoxP3+ regulatory T (Treg) cells and CD4+ FoxP3neg IL-10neg conventional T (Tcon) cells (Fig. 1A). T cells were the major IL-10 producers after both allogeneic and syngeneic BMT (Fig. 1B), with the highest proportion and intensity of IL-10 produced by TR1 cells (Fig. 1C). Importantly, TR1 cells were present at up to 10-fold higher frequency and number than Treg cells after allogeneic BMT in GVHD target tissues (liver, and to lesser extent small intestine), mesenteric lymph nodes (Fig. 1D) and spleen (Fig. 1D – F). TR1 cells induced under these conditions had suppressive properties in vitro equivalent to post-transplant Treg cells on a per cell basis (Figs. 1G, S1). To confirm their suppressive function in vivo, we induced GVHD with WT or Il10−/− CD4+CD25neg T cells that cannot develop into functional TR1 cells. As expected we observed enhanced GVHD in the absence of IL-10; however, adoptive transfer of limited numbers of TR1 cells at d7 after BMT (Fig. 1H) when acute GVHD was established, prolonged survival significantly (Fig. 1I), consistent with potent regulatory function. Thus, TR1 cells represent the major regulatory T cell population in GVHD induced by allogeneic BMT and contribute significantly to transplant survival.
Figure 1. TR1 cells constitute the major regulatory T cell after allogeneic BMT.
(A – E) B6 (Syn) and B6D2F1 (Allo) mice were transplanted with B6 CD3+ T (Il10GFP/Foxp3RFP). (A) Gating strategy after BMT for analysis and FACS sorting of TR1 (red), Treg (blue) and Tcon (green) cells. (B) Schema of BMT. (C) Expression of IL-10 and FoxP3 in the spleen at d14 (representative of >3 experiments). (D) Frequencies of TR1 and Treg cells at d 14 (Il10GFP+: solid bar; Il10GFPneg: open bar). (E) CD4+ T cell subsets in spleen after BMT (n = 8 – 9 per group each time point). (F) B6D2F1 mice were transplanted with B6 CD4+ T (Il10GFP and Foxp3RFP) and frequencies of TR1 and Treg cells in the spleen at d14 (n = 14). (G) Suppression of proliferation of CFSE labelled B6 CD4+ and CD8+ responder T cells in vitro by naïve Treg cells versus TR1, Treg and Tcon “suppressors” sorted from 10 transplant recipients at d14 (data combined from 2 experiments). (H) Experimental BMT schema showing adoptive transfer of sorted TR1 cells to treat established acute GVHD and (I) survival of recipients are shown (n = 8 in TCD group, others n = 11 – 12). Data represents mean ± SEM.
TR1 cells express Eomes and display a distinct phenotypic profile
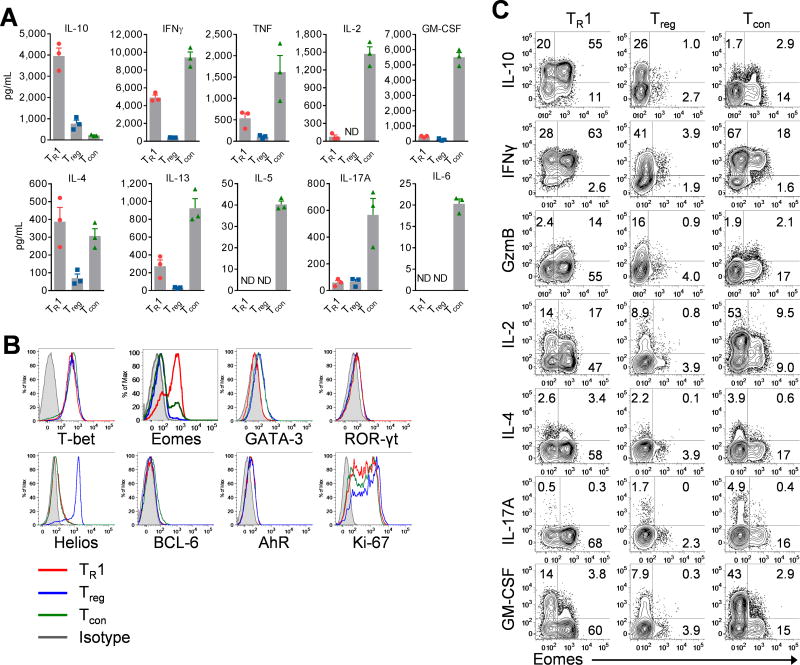
CD49b and LAG-3 co-expression can be used to identify TR1 cells in models of colitis (9), however, their expression is insufficient to identify TR1 cells after BMT (Fig. S2A). We therefore used Foxp3RFPneg and Il10GFP+ as TR1 cell markers. Thus defined TR1 cells demonstrated high expression of CD122, α4β7, LAG-3, Ly6C and TIGIT, and low expression of CD25 and CD69 relative to other CD4+ T cell subsets (Fig. S2B). Consistent with the TR1 cell phenotype (3, 5, 9), Foxp3RFPnegIl10GFP+ TR1 cells expressed high amounts of IL-10 and IFNγ but little TH2 cytokines such as IL-4, IL-13 and IL-5, or TH17 cytokines such as IL-17, IL-6 or GM-CSF (Figs. 2A, S2C).
Figure 2. TR1 cells express Eomes and display a distinct phenotypic profile.
(A – C) B6D2F1 mice were transplanted with Il10GFPFoxp3RFP B6 CD3+ T cells. CD4+ T cells from spleen were FACS sorted into TR1, Treg and Tcon cells at d14 as in Fig 1A. (Data from 3 experiments, ND = not detectable) (A) Cytokine production in culture supernatant of T cell subsets. (B) Expression of transcription factors in T cell subsets (TR1: red, Treg: blue, Tcon: green, isotype: gray). (C) Expression of cytokines and Eomes in T cell subsets. Data represents mean ± SEM.
TR1 cells have often been considered a terminally differentiated TH1 cell subset programmed to limit aberrant inflammation (5, 13, 22). Indeed, TR1 cells expressed high amounts of T-bet, the TH1 determining transcription factor, but low amounts of GATA-3, BCL-6 and ROR-γt. Strikingly, when we analysed the expression of other transcription factors related to T cell differentiation, we observed high Eomes expression, which was largely restricted to TR1 cells (Figs. 2B, S2D). Eomes expression tightly correlated with high expression of IL-10, IFNγ and granzyme B (GzmB) (Fig. 2C). In contrast, Eomes+ TR1 cells expressed low levels of IL-2, IL-17A and GM-CSF (Fig. 2C). Thus, TR1 cells that develop during allogeneic BMT specifically express Eomes.
Eomes is required for TR1 cell differentiation
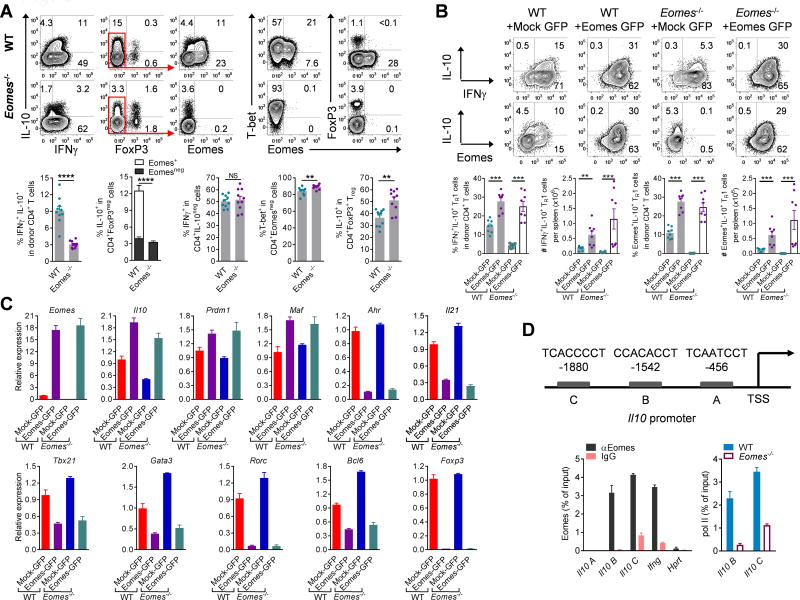
To test the role of Eomes in TR1 cell development in vivo, we used CD4+ T cells isolated from Eomesfl/flxCd4-cre donor mice in allogeneic BMT. Strikingly, TR1 cell generation was significantly reduced (by >70%) with decreased Gzmb expression in recipients of Eomes-deficient CD4+ T cells (Figs. 3A, S3A,B). Critically, the loss of Eomes did not impair the development of IL-10negIFNγ+ or T-bet+ Tcon, IFNγ+TNF+ TH1, IL-17A+ TH17 cells or IL-10 expression by Treg cells but instead favoured the expression of IL-4 and FoxP3 (Figs. 3A, S3B–F). To further elucidate the role of Eomes in the differentiation of TR1 cells and transactivation of Il10, we transplanted donor WT or Eomes−/− CD4+ T cells which constitutively expressed Eomes after retroviral transduction. Strikingly, enforced expression of Eomes rescued the development of TR1 cells from Eomes−/− CD4+ T cells after BMT and also promoted their development in WT cells (Fig. 3B). In addition, over-expression of Eomes promoted the expression of Gzmb whilst suppressing FoxP3, IL-4 and IL-17A expression (Fig. S3G). Furthermore, over-expression of Eomes upregulated the transcription of Il10 but suppressed that of other lineage defining transcription factors including Tbx21, Gata3, Rorc, Bcl6 and Foxp3 in addition to the TR1/TH17 related factors Ahr and Il21 (10, 23, 24)(Fig. 3C).
Figure 3. Eomes is required for TR1 cell differentiation.
(A – D) B6D2F1 mice were transplanted with primary or retrovirally transduced (Mock-GFP or Eomes-GFP) CD4+ T cells. (A) Expression of IL-10, IFNγ, FoxP3, Eomes and T-bet (Eomes+IL-10+: open bar; EomesnegIL-10+: solid bar, n = 10 per group) in recipients of WT or Eomes−/− CD4+ T cells at d14 (n = 10 per group). (B) Expression of IL-10, IFNγ, and Eomes in transduced WT or Eomes−/− CD4+ T cells at d7 (n = 8 per group) and (C) transcription of Il10 and related genes (data are from 4–5 pooled animals in triplicate reactions, representative of 2 independent experiments). (D) CD4+ T cells or Foxp3RFPnegIl10GFP+ TR1 cells were FACS sorted from spleen and liver at d14 (representative of 3 experiments). A schematic diagram of the mouse IL-10 promoter indicates Eomes binding sites upstream of the TSS with each sequence shown. Recruitment of Eomes to the Il10 promoter and control regions in CD4+ T cells from TR1 cells (data are from 30 pooled animals in triplicate reactions) and recruitment of RNA Pol II to the Il10 promoter in WT or Eomes−/− CD4+ T cells (data are from 10 pooled animals in triplicate reactions). Data represents mean ± SEM.
Notably, TR1 cells generated in vitro in the presence of IL-27, a cytokine promoting TR1 cell development (8, 11, 12), did not express Eomes protein, nor did TH1, TH2, TH17, iTreg cells (Fig. S4A), indicating that short-term in vitro cultures do not replicate the conditions inducing TR1 cells after BMT. Nevertheless, Eomes mRNA was higher in TR1 than other T cell lineages in these cultures (Fig. S4B). Consistent with this observation, we did not observe a defect in TR1 differentiation in the absence of Eomes in these conditions (Fig. S5A). However, transduction of Eomes into CD4+ T cells and subsequent re-stimulation in culture dramatically promoted the differentiation of IL-10+IFNγ+ TR1 cells and the expression of granzyme B, while suppressing the expression of IL-4 and FoxP3 (Fig. S5B). Over-expression of Eomes also suppressed mRNA expression of transcription factors defining other TH lineages, including Tbx21, Gata3, Rorc and Bcl6 and other TR1/TH17 related factors, like Ahr, Maf and Il21(Fig. S5C). Collectively, we show that Eomes is required for TR1 differentiation and IL-10 secretion and repression of alternative fate differentiation
Eomes directly regulates IL-10 expression in TR1 cells
To understand the mechanism by which Eomes regulates TR1 cell differentiation, we performed chromatin immunoprecipitation (ChIP) assays on sort purified TR1 cells or CD4+ T cells 14 days after BMT. This demonstrated that Eomes is bound to multiple sites within 2kb upstream of the transcription start site (TSS) of the Il10 gene (Fig. 3D). The binding of Eomes to the Il10 promoter was similar to that observed in the Ifnγ promoter, suggesting that Eomes regulates expression of both Il10 and Ifnγ directly. Consistent with this concept, the recruitment of RNA polymerase II to the Il10 promoter, an indicator of transcriptional activity, was reduced in Eomes-deficient CD4+ T cells (Fig. 3D).
Eomes+ TR1 cells are dependent on Blimp-1, IL-27 and IL-10
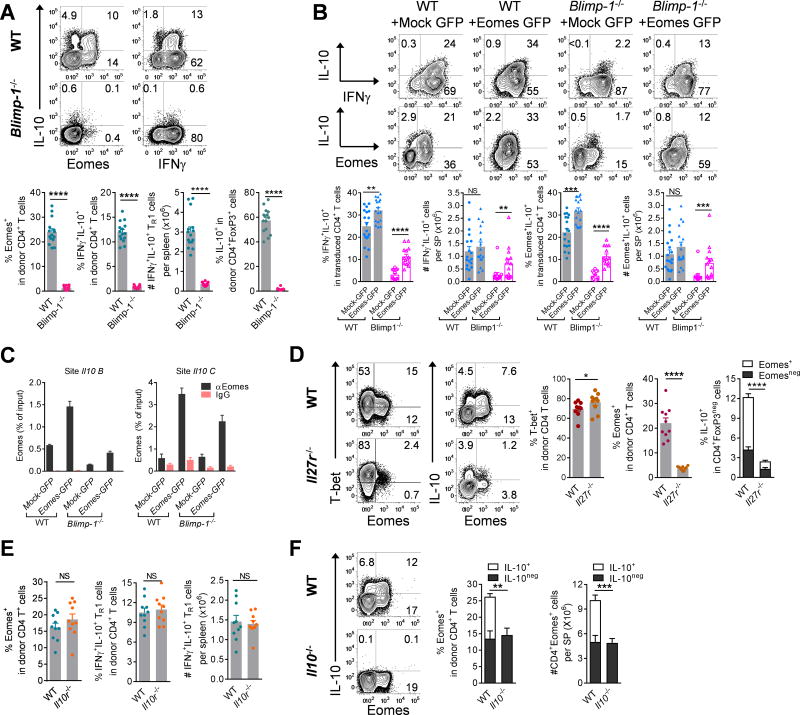
Blimp-1 is a well-defined transcriptional promoter of IL-10 in CD4+ conventional T and Treg cells (6, 11, 21). Consistent with this notion, after BMT IL-10 production in all CD4+ T cells was confined to Blimp-1 expressing cells (Fig. S6A). Critically, conditional ablation of Blimp-1 (Prdm1fl/fl × Lck-cre) in donor T cells resulted in a near complete loss of both IL-10 and Eomes expression in CD4+ T cells, demonstrating a near complete lack of TR1 cells (Fig. 4A) while the expression of T-bet was not impaired (Fig. S6B). To elucidate the relative contribution of Eomes and Blimp-1 to the expression of IL-10, we transferred Eomes-transduced WT or Blimp-1−/− CD4+ T cells into allogenic BMT recipients. Consistent with a critical role of Eomes in the differentiation of TR1 cells, over-expression of Eomes in Blimp-1-deficient CD4+ T cells partially rescued their defective expression of IL-10 and GzmB and suppressed the expression of IL-2, IL-4, IL-17A, GM-CSF and FoxP3 after BMT (Figs. 4B, S6C,D). Furthermore, Eomes transduction enhanced the recruitment of Eomes to the Il10 promoter regions both in WT and Blimp1−/− CD4 T cells (Fig. 4C).
Figure 4. Eomes+ TR1 cells are dependent on Blimp-1, IL-27 and IL-10.
(A – F) B6D2F1 mice were transplanted with primary or retrovirally transduced (Mock-GFP or Eomes-GFP) CD4+ T cells and spleen examined after BMT. (A) Expression of Eomes, IL-10 and IFNγ in WT or Blimp-1−/− CD4+ T cells at d14 (n = 14 – 15 per group). (B) Expression of Eomes, IL-10 and IFNγ (n = 18, 17 for WT; n = 13, 14 for Blimp-1−/−) in transduced CD4+ T cells at d7–10. (C) Recruitment of Eomes to Il10 promoter in transduced CD4+ T cells (WT or Blimp-1−/−) at d10 (data are from 4 animals in duplicate or triplicate reactions). (D) Expression of T-bet, Eomes and IL-10 in WT or Il27r−/− CD4+ T cells at d14 (n = 10 per group). (E) Expression of Eomes and IFNγ+IL-10+ TR1 cells in WT or Il10r−/− CD4+ T cells at d14 (n = 10 per group). (F) Expression of Eomes and IL-10 (Eomes+IL-10+: open bar; Eomes+IL-10neg: solid bar) in CD4+ T cells in recipients of WT or Il10−/− CD4+CD25neg T cells at d14 (n = 10 – 11 per group). Data represents mean ± SEM.
To test the role of IL-27 in the induction of Eomes+ TR1 cells after BMT, we transplanted Il27r−/− CD4+ T cells. Consistent with an important role for IL-27 in TR1 induction, we found substantially decreased expression of Eomes in Il27r−/− CD4+ T cells, and TR1 cells were reduced by >80% (Figs. 4D, S6E). In contrast, T-bet expression was increased in the absence of IL-27 signalling (Fig. 4D), and the development of CD4+IL-10negIFNγ+ conventional TH1 cells or IL-10 production capabilities of Treg cells were not impaired (Fig. S6E).
We next tested whether the differentiation of Eomes+ TR1 cells was dependent on IL-10 itself. The expression of Eomes, TR1 cells as well as T-bet was not reduced in Il10r–deficient CD4+ T cells (Il10rfl/fl × Lck-cre) after BMT (Figs. 4E, S6F), indicating that IL-10 signalling in T cells was not required for TR1 cell differentiation. However, when we transplanted Il10−/− CD4+CD25neg T cells, Eomes+ cells were reduced (Fig. 4F), in line with the notion that IL-10 promotes TR1 cell differentiation indirectly (22, 25). In summary, Eomes expression in TR1 cells is downstream of IL-27 and Blimp-1 but does not depend on T cell intrinsic IL-10 signalling.
Eomes+ TR1 cells are critical for the prevention of GVHD
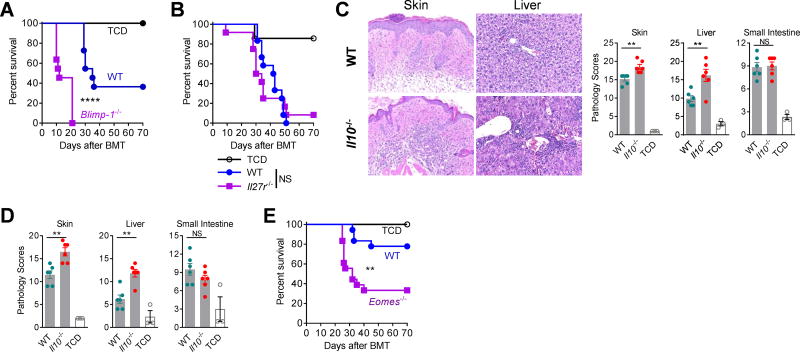
We next examined whether Blimp-1-deficient and Il27r–deficient CD4+ T cells would exacerbate GVHD due to impaired expression of Eomes and TR1 cells. Whilst Blimp1 deletion exacerbated GVHD (Fig. 5A), IL-27R deletion did not (Fig. 5B). Of note, Treg cells were increased and their IL-10 production intact in recipients of Il27r−/− CD4+ T cells (Fig. S6E, S7A), consistent with compensatory regulatory pathways in the absence of TR1. In contrast, Il10−/− CD4+ T cells sustain comparable expression of Eomes in conventional T cells and Treg cells (Figs. 4F, S7B) after BMT and thus reflect a more relevant model to define the regulatory function of TR1 cells in vivo. Consistent with the reduced frequency of TR1 cells, we observed enhanced GVHD in the skin and liver in recipients of Il10−/− CD4+CD25neg T cells (Fig. 5C). These findings were confirmed by transplanting Il10fl/fl × Lck-cre CD4+CD25neg T cells, which also led to exacerbated GVHD in the absence of IL-10 producing TR1 cells (Fig. 5D). Lastly, Eomes−/− CD4+CD25neg T cells also resulted in increased GVHD, further confirming the important regulatory role of Eomes+ TR1 cells after BMT (Fig. 5E).
Figure 5. Attenuation of GVHD by Eomes+ TR1 cells.
(A – E) B6D2F1 recipients were transplanted with CD4+ T cells and survival or histopathology examined. (A) Survival of recipients of WT or Blimp-1−/− CD4+ T cells (2×106 per mouse) (n = 11 per T cell group, n = 7 in TCD; 2 experiments). (B) Survival of recipients of WT or Il27r−/− CD4+CD25neg T cells (2×106 per mouse) (n = 12 per T cell group, n = 7 in TCD; 2 experiments). (C and D) Histology in recipients of (C) WT versus Il10−/− or (D) WT versus Il10fl/fl × Lck-cre CD4+CD25neg T cells (1×106 per mouse) at d28 (n = 6 per T cell group, n = 3 in TCD group). (E) Survival of recipients of WT or Eomes−/− CD4+CD25neg T cells (1×106 per mouse) (n = 12 per T cell group, n = 7 in TCD; 2 experiments). Histology represents mean ± SEM.
Eomes and T-bet cooperate to generate TR1 cells
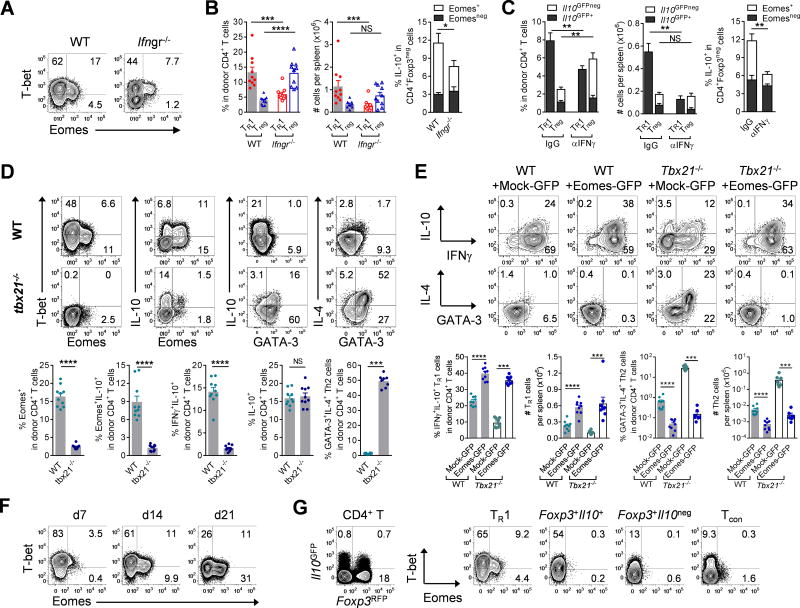
As we had observed co-expression of T-bet (encoded by Tbx21) and Eomes in TR1 cells after BMT, we wished to test the role of IFNγ signalling and T-bet in TR1 cell development. Transplanting Ifngr−/− donor T cells or neutralizing IFNγ resulted in reduced expression of T-bet and Eomes (Fig. 6A) with reduced expression of Eomes+ TR1 cells and expanded Treg cell populations (Fig. 6B, C). When we transplanted Tbx21−/− CD4+ T cells during BMT, we found that Eomes+ TR1 cells were dramatically reduced (Figs. 6D, S8A). Although overall frequencies of IL-10+CD4+ T cells were unaffected, the absolute numbers were reduced (Fig. 6D). Importantly, however, the majority of the Tbx21−/−IL-10+ CD4+ T cells did not express IFNγ but rather IL-4 and GATA3 or IL-17A, indicating that these cells had been diverted to TH2 or TH17 cells, respectively (Figs. 6D, S8B). Gene expression analysis confirmed polarization of donor CD4+ T cells to TH2 (Gata3, Il4, Il13) and TH17 (Rorc, Ahr, Il21) lineages in the absence of T-bet. The transcription of Il10 (from Th2 cells) was also increased (Fig. S8C). Notably, the residual Eomes+ population in Tbx21−/− CD4+IL-10+ cells expressed IFNγ but did not express IL-4 (Fig. S8D). Thus, T-bet and IFNγ promote Eomes expression within the TR1 lineage after BMT and, in concert with Eomes, repress alternate cell fates. To further understand the relative function of Eomes and T-bet in the differentiation of TR1 cells, we retrovirally transduced Tbx21−/− CD4+ T cells with Eomes. The over-expression of Eomes fully rescued the expression of IL-10, IFNγ, and IL-10+IFNγ+ TR1 cells and correspondingly suppressed the expression of GATA-3+IL-4+ TH2 and IL-17A+ TH17 cells (Figs. 6E, S8E,F).
Figure 6. Eomes and T-bet jointly regulate TR1 cell development.
(A and B) B6.WT or B6.Ifngr−/− CD3+ T cells were transplanted into B6D2F1 mice and splenic CD4+ T cells examined at d14. (A) Representative plots show expression of T-bet and Eomes and (B) frequencies of TR1 and Treg cells and expression of IL-10 and Eomes (n = 10 per group). (C) B6.Il10GFPFoxp3RFP CD3+ T cells were transplanted into B6D2F1 mice receiving αIFNγ or control mAb and splenic CD4+ T cells examined at d12 (n = 5 per group). Frequencies of TR1 and Treg cells and expression of Eomes and IL-10 are shown. (D) B6D2F1 mice were transplanted with WT or Tbx21−/− CD4+ T cells and expression of transcription factors and cytokines in splenic CD4+ T cells at d12 shown (n = 10 per group). (E) B6D2F1 mice were transplanted with retrovirally (Mock-GFP or Eomes-GFP) transduced WT or Tbx21−/− CD4+ T cells and expression of IL-10, IFNγ, IL-4 and GATA-3 in splenic CD4+ T cells at d7 shown (n = 8 per group). (F) Co-expression of T-bet and Eomes in CD4+ T cells over time (representative of at least 2 experiments). (G) Splenic CD4+ T cells from naïve mice FACS sorted to 4 subsets based on Il10GFP and Foxp3RFP expression and T-bet and Eomes evaluated (representative of 2 experiments). Data represents mean ± SEM.
We next investigated whether there is a temporal and/or spatial collaboration between T-bet and Eomes during TR1 cell development. Firstly, Eomes expression in TR1 cells was profoundly time-dependent after BMT (Fig. S8G), and CD4+ T cells transited from a T-bethiEomeslo to a T-betloEomeshi state over time (Fig. 6F), correlating with the increasing frequency of TR1 cells (Fig. 1E). Furthermore, after repeated exposure to high levels of alloantigen in vivo, the majority of donor CD4+ T cells had acquired Eomes (>95%) and converted to TR1 cells (>70%) within four weeks of transfer into secondary BMT recipients (Fig. S8H). Consistently, over-expression of Eomes suppressed the expression of T-bet while promoting TR1 cell differentiation (Figs. 3B, S8I). TR1 cells (Foxp3RFPnegIl10GFP+), found in low frequencies in naïve mice, also exhibited higher Eomes expression. This was specific to TR1 cells as IL-10 producing Treg cells (Foxp3RFP+Il10GFP+) expressed some T-bet but not Eomes (Fig. 6G). Collectively, these data show that both T-bet and Eomes are required for TR1 cell differentiation, which is characterized by the initial up-regulation of T-bet, the acquisition of Eomes expression and the subsequent down-regulation of T-bet, resulting in a T-betloEomeshi phenotype.
Recipient DC and donor-derived IL-27 promote TR1 cell development
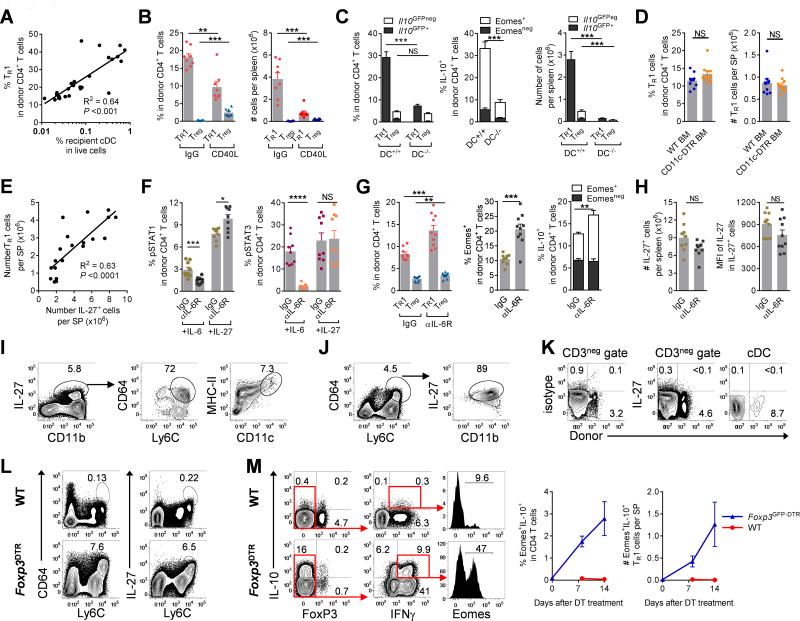
GVHD is initiated by recipient antigen presenting cells (APC) and is influenced by the intensity of conditioning, i.e. total body radiation (TBI) and chemotherapy dose-intensity, in part through inflammatory cytokine dysregulation (26, 27). We thus hypothesized that TR1 cells may also be generated in an APC and conditioning-dependent fashion. The frequency of TR1 cells in donor CD4+ T cells indeed correlated with the frequency of residual recipient conventional dendritic cells (DC) (Fig. 7A) and reduced intensity of TBI that favour the persistence of recipient DC (Fig. S9A). Blocking DC function by CD40L inhibition reduced TR1 cells whilst favouring Treg cell development (Fig. 7B). In line with this observation, depletion of both donor and recipient DC dramatically reduced the development of TR1 cells early after BMT (Fig. 7C). While the proportions of Treg cells were unaffected, absolute numbers were reduced, albeit much less dramatically than TR1 cells (Fig. 7C). In contrast, the depletion of donor DC or inactivation of donor APC function in isolation did not impair TR1 cell development (Figs. 7D, S9B,C), indicating that recipient DC are required for the development of TR1 cells early after BMT.
Figure 7. Recipient DC and macrophage-derived IL-27 promote the development of TR1 cells.
(A – K) B6D2F1 mice were transplanted with TCD BM and CD4+ T cells and spleen examined. (A) Correlation of TR1 cells (Il10GFP+Foxp3RFPneg) with proportions of recipient DC at d14 (n = 26). (B) Frequencies of Treg (Foxp3GFP+) and TR1 (IFNγ+IL-10+) cells at d14 in the presence or absence of CD40L inhibition (n = 8 per group, grafts were CD4+Foxp3GFPneg). (C) WT.B6D2F1 or CD11c-DOG × DBA/2 F1 recipients were treated with DT to deplete recipient cDC and received B6.WT or MHC-II−/− BM respectively. Expression of TR1, Treg cells, Eomes and IL-10 at d14 are shown (n = 10 and 7 respectively). (D) Recipients of WT or CD11c-DOG BM were treated with DT to deplete donor cDC with expression of TR1 and Treg cells at d10 shown (n = 10 per group). (E) Data from (A) and (B) demonstrate correlation between numbers of TR1 cells and IL-27+ cells per spleen at d14 (n = 20). (F) Recipients were treated with IL-6R and spleens analyzed at d5. Phosphorylation of STAT1 and STAT3 in response to IL-6 or IL-27 (n = 10 per group). (G and H) Recipients were treated with IL-6R and spleens analyzed at d10. (G) Expression of Foxp3RFPnegIl10GFP+ TR1, Foxp3RFP+ Treg, Eomes and IL-10 in donor CD4+ T cells and (H) numbers of IL-27+ cells with intensity (MFI) of IL-27 (n = 9 – 10 per group). (I and J) Phenotypes of CD3neg IL-27 secreting cells at d14 are shown. (K) Expression of IL-27 from recipient DC at d+1 after BMT. (L and M) B6.WT or B6.Foxp3GFP-DTR mice were treated with DT for up to 2 weeks and spleens analyzed. (L) Phenotype of IL-27 secreting macrophage in CD3neg splenocytes and (M) expression of Eomes+IL-10+ cells over time with representative plots at d14. Data represents mean ± SEM.
Consistent with the notion that Eomes+ TR1 cells are dependent on IL-27 signalling and further confirming critical role of IL-27 in promoting TR1 cell development, we found that the number of TR1 cells significantly correlated with the number of IL-27+ cells in the spleen (Fig. 7E). As IL-27R and IL-6R share and compete for the same signalling component, gp130 (28), we hypothesized that blocking IL-6R may favour IL-27R function. As expected IL-6R inhibition blocked STAT3 phosphorylation in response to IL-6 but not IL-27 (Fig. 7F). In contrast, IL-6R inhibition enhanced STAT1 phosphorylation in response to IL-27 early after BMT (Fig. 7F) and resulted in increased expression of TR1 cells and a small increase in the frequencies of Treg cells (Fig. 7G, S9D). The enhanced STAT1 phosphorylation in response to IL-27 following IL-6R inhibition was not a result of an increase in the number of cells producing IL-27 itself or IL-27 production on a per cell basis (Fig. 7H). We next sought to identify the cellular sources of IL-27 after BMT. The majority of IL-27 (70–80%) was produced by Ly6Chi donor macrophages (CD11b+, MHC II+, Ly6Chi, F4/80hi, CD64+ and CCR2+) with a more limited contribution from donor DC (CD11c+, MCH-II+) (Fig. 7I). More than 80% of all Ly6Chi donor macrophages were secreting IL-27 after BMT (Fig. 7J). Depletion of donor DC did not impair the overall frequencies or numbers of IL-27+ cells (Fig. S9B), consistent with the lack of contribution by donor DC to TR1 cell development. Lastly we demonstrated that recipient DC did not produce IL-27 early after BMT (Fig. 7K) suggesting that the requirement of recipient DC to TR1 cell development relates to their capacity for alloantigen presentation and not IL-27 production. Thus donor macrophages appear the main producers of IL-27 and, in concert with the initial stimulation by recipient DC, drive Eomes-dependent TR1 development after BMT.
To further understand the requirement of Eomes in TR1 cell development, we investigated the expression of Eomes+ TR1 cells in other models of immune pathology. To this end we used Foxp3GFP-DTR mice to temporarily deplete Treg cells, thereby causing autoimmunity (29–31). Indeed, depletion of Treg cells from adult mice resulted in a dramatic increase in IL-27 producing Ly6Chi macrophages (Fig. 7L) and critically, induced large numbers of Eomes+ TR1 cells (Fig. 7M). Thus, our data show that different inflammatory conditions result in the development of Eomes+ TR1 cells. Furthermore, our results demonstrated that defects in Treg cells are associated with compensatory increases in Eomes+ TR1 cells.
Identification of TR1 cells in humans
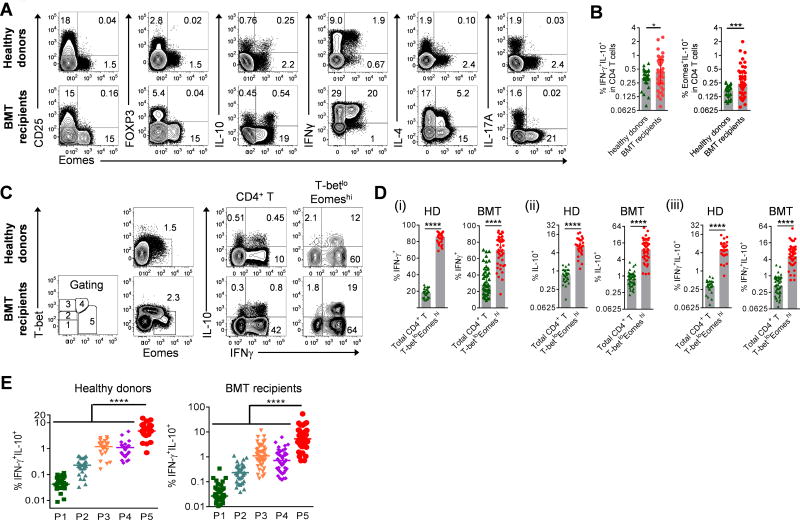
To validate whether our findings from experimental BMT can be translated into humans, we analyzed the expression of Eomes, IL-10 and other markers in CD4+ T lymphocytes collected from healthy donors and BMT recipients. Eomes+ CD4+ cells from healthy individuals as well as BMT recipients were CD25lo, FOXP3neg, IFNγhi, IL-4lo and IL-17Aneg and a proportion secreted IL-10 (Fig. 8A). Thus, human Eomes+IL-10+ cells show the characteristics of TR1 cells. Of note, compared to currently utilized IL-10+IFNγ+ staining methods, the use of Eomes in defining IL-10 positive TR1 cells (Eomes+IL-10+) provides better discrimination of TR1 cells between healthy donors and BMT recipients (Fig. 8B). Furthermore, the use of T-bet and Eomes expression defines populations with increasing proportions of IL-10+IFNγ+ TR1 cells (Fig. 8C – E) consistent with the requirement for these transcription factors at different stages of differentiation both in steady state and after clinical BMT. IL-10+IFNγ+ TR1 cells were enriched (>10 fold) in the T-betloEomeshi population, which exhibited an effector memory (CD45RAnegCCR7neg) phenotype (Figs. 8C – E, S10). Thus, consistent with the findings in the mouse model, after clinical BMT high Eomes and low T-bet expression in CD4+ T cells can be used to identify a population that is enriched for TR1 cells.
Figure 8. Co-expression of T-bet and Eomes identifies a TR1 cells enriched population in human CD4+ T cells.
(A) Representative plots show the correlation of Eomes to CD25, FOXP3 and cytokines in CD4+ T cells in healthy individuals and at d60 after clinical allo-BMT. (B) Frequencies of TR1 cells defined as IFNγ+IL-10+ or Eomes+IL-10+ in CD4+ T cells in healthy donors (n = 27) or d60 after clinical allo-BMT (n = 43). (C – E) Expression of cytokines in the T-betloEomeshi population relative to total CD4+ T cells or subpopulations defined with differential expression of Eomes and T-bet in healthy individuals (HD, n = 27) and at d60 after allo-BMT (BMT, n = 43). Data represents median ± interquartile range.
Discussion
We demonstrate that Eomes acts together with Blimp-1 and specifically drives the development of TR1 cells. Based on our data and published results (8, 32), we propose a model for the differentiation of TR1 cells after BMT as illustrated in Figure S11. In this model, antigen presentation by recipient DC and macrophages-derived IL-27 provide the cellular and molecular cues for the development of TR1 cells, inducing Blimp-1 expression, which initiates the transcription of Il10. Blimp-1 is also required for Eomes expression, and both factors act in concert, enabling stable IL-10 production and TR1 cell differentiation. Concurrently, T-bet is required to suppress GATA3 and RORγt whilst driving IFNγ and Eomes expression ultimately leading to a T-betloEomeshi phenotype, which can reliably identify TR1 cells after BMT as well as in steady state in mouse and man. There are some limitations to this study. Our preclinical studies utilized predominantly a single transplant model, although clinical data were congruent. In addition, while Treg depletion results in TR1 generation in vivo, it is not yet clear how important TR1 cells are in other disease settings. Finally, the relative in vivo suppressive activity of TR1 versus Treg remain to be fully explored.
There is still debate whether TR1 cells constitute an independent lineage or simply represent IL-10 producing TH1 cells. In particular, the lack of a master transcriptional factor for TR1 cells has made progression of the field difficult (5, 13, 33). Multiple transcription factors, including Blimp-1, AhR and c-Maf are induced by IL-27 and have been shown to be critical for TR1 cell differentiation (5–8, 10); however, none of them appear to be specific to the TR1 lineage. Eomes is a T-box transcription factor which is more often than not coupled with T-bet in the biology of CD8+ T cells and NK cells (34, 35). Its role in regulating functions of CD4+ T cells (36, 37) and suppressing Treg and TH17 cells differentiation have been described recently (38, 39). Here we demonstrate that IL-10+IFNγ+ TR1 cells are uniquely dependent on Eomes. We found that Eomes bound to the Il10 and Ifnγ promoters. Similarly, it has been shown that Eomes also binds to the promoter of Gzmb (35), expression of which is another feature of TR1 cells. Eomes over-expression was sufficient to promote IL-10 and GzmB and suppress other lineage-characteristic transcription factors (e.g. FoxP3, GATA-3, RORγt and BCL-6) and cytokines (e.g. IL-2, IL-4, IL-13, GM-CSF and IL-17A). Therefore, expression of Eomes and IL-10 within CD4+ T cells defines the TR1 cell lineage.
Increasing data has suggested a close relationship between TR1 and TH17 cells linked via AhR, c-Maf and IL-21 (10, 23, 24, 40). However, TR1 and TH17 cells require different cytokines for their respective differentiation, IL-27/IL-10 for the former and IL-6/TGF-β/IL-23 for the later (12, 41–43). Multiple groups have independently shown IL-27 opposed the functions of IL-6/IL-23 in TH17 differentiation (8, 28, 44). Our data demonstrate that inhibition of IL-6R signaling favors IL-27 function and subsequent development of Eomes+ TR1 cells. We further show that Eomes distinguishes TR1 cells from other TH lineages including TH17 cells and its over-expression represses polarization to TH17 cells. This is in line with the notion that Eomes suppresses TH17 cell differentiation by directly inactivating Rorc and Il17a promoters (39). A role for IL-27 in inhibiting Treg reconstitution after BMT has also recently been reported (45), consistent with the counter-balanced TR1 expansion seen here. There appears to be significant interplay between IL-6 and IL-27 (28), an effect also seen during GVHD. IL-6 inhibition has an intriguing capacity to enhance IL-27 responses and thereby to promote TR1 cell differentiation, an effect likely contributing to clinical efficacy (46).
Eomes can be regulated by T-bet in a Runx-3 dependent manner and the differential expression of these two T-box transcription factors is critical for the differentiation of CD8+ T cells (47, 48). In line with this notion, we show that IFNγ signalling and T-bet expression were required for Eomes expression, demonstrating an important role of T-bet in the early phase of TR1 cell development. Downstream of IL-27, Blimp-1 is critical for the expression of IL-10 in CD4+ T cells in various models (6–8, 21, 49). Here we show Eomes+ TR1 cells were regulated by both Blimp-1 and T-bet, consistent with a recent report that demonstrated close collaboration between Blimp-1 and T-bet in CTL generation (50). In addition, binding of Blimp-1 to the Eomes promoter in CD8+ T cells during viral infection has been described (32), suggesting that Blimp-1 not only regulates IL-10 expression directly but also contributes to the induction or maintenance of Eomes expression in TR1 cells. Notably, both Blimp-1 (6) and Eomes bind to the Il10 locus, and the activity of both is required to promote efficient TR1 differentiation and Il10 expression. Interestingly, similar to Eomes, Blimp-1 is not only required for IL-10 expression but also for granzyme B (51). We also confirmed that IL-10 itself contributes to TR1 cell differentiation, a T cell extrinsic effect likely via myeloid cells (22, 25). Overall these data suggest that the functional interactions between Blimp-1, T-bet and Eomes are important for the differentiation of CD4+ T cells and TR1 lineage in particular.
Identification of the bona fide transcriptional and cellular control of TR1 cell development should allow for therapeutic utilization of TR1 cells in transplantation and other diseases where excessive and aberrant immunity results in immune pathology.
Materials and Methods
Study design
Female C57BL/6 (B6.WT, H-2b, CD45.2), B6.SJL-Ptprca (PTPrca, H-2b, CD45.1) and B6D2F1 (H-2b/d, CD45.2) mice were purchased from the Animal Resource Center (Perth, WA, Australia). B6 Il27r−/− and Tbx21−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). B6 Blimp-1GFP(52), Il10GFP × Foxp3RFP(19, 20), Foxp3GFP, Foxp3GFP-DTR, Il10−/−, Ifnγr−/−, MHC-II−/−, Il10rfl/fl × Lck-cre(53), Il10fl/fl × Lck-cre(54), Prdm1fl/fl × Lck-cre (Blimp-1−/−)(51), CD11cDOG and DBA2 × B6.CD11cDOG mice were bred at the QIMR Berghofer Medical Research Institute animal facility. B6 Eomesfl/fl mice were derived from the Eomesfloxed/mcherry mice previously generated by GTB and described in (55). The Eomesfloxed/mcherry mice were crossed to the B6.129S4-Gt(ROSA)26Sortm2(FLP*)Sor/J line which induces FLP-mediated recombination to remove the mCherry/Amp cassette to generate the Eomesfloxed line. Removal of the Frt sites (and hence the IRES-Cherry cassette) was detected using primers (a) 5’-ggacttggggagccaaaa-3’ (forward) and (b) 5’-cacatctgtaaccgcagcat-3’ (reverse) (deleted allele, 306 bp). The primers (c) 5’-agtcggtttgagctggtgac-3’ (forward), (d) 5’-tttggaacagcctccaaatc-3’ (reverse) were used to detect the wild-type (339 bp) and floxed allele (421 bp) while primer (e) 5’-AAGGGGAAGGGTGGTTAGAA-3’ (reverse) was used to detect the floxed allele (1941 bp) and germline deletion (587 bp). This Eomesfloxed line was subsequently crossed with Cd4-cre or Lck-cre mice to generate T cell restricted Eomes−/− offspring. All recipient mice were used between 6 and 10 week of age and age matched female donor mice were used. Mice were housed in microisolator cages and received acidified autoclaved water (pH 2.5) after BMT. All animal studies were performed in accordance with the QIMR Berghofer Medical Research Institute Animal Ethics Committee. We chose sample sizes based on estimates from initial and previously published results in order to ensure appropriate power. As stated in Figure legends and wherever possible, n values were derived from individual mice from replicated experiments.
Bone marrow transplantation
BM (B6.CD45.1+ or where indicated) was T cell depleted and splenocytes processed to CD3+ or CD4+ T cells as described previously (56). On day −1, recipient mice received 1100 cGy (B6D2F1), 1000 cGy (B6), 900 cGy (CD11c-DOG × DBA/2 F1) or otherwise specified doses of total body irradiation ([137Cs] source at 108 cGy/min), split into two doses separated by 3 h. On day 0, recipients were transplanted with 5–10×106 BM cells with or without 1–2×106 T cells (CD3+ or CD4+). Intraperitoneal injections of rat-anti-mouse IFNγ (XMG1.2, produced in house, 1mg/dose, 3 times per week), hamster-anti-mouse CD40L (MR1, BioXcell, 500ug/dose, d0, +2, +4, +6), rat-anti-mouse IL-6R (MR16-1, Chugai Pharmaceutical Co, Japan, 500ug/dose, d-1, +3, +7) and control mAb were administered to recipients. In some experiments, CD11c-DOG mice (in which diphtheria toxin (DT) receptor is driven off the CD11c promoter) were used as BM donors. Recipients were given intraperitoneal injections of DT (160ng/dose, 3 times per week) after BMT to deplete donor DC. For depletion of recipient DC, B6.CD11c-DOG × DBA/2 F1 mice were used as recipients and treated with DT on d-3, −1, 0, +1, +3, +5, +7.
Treg depletion
For depletion of Treg, age-matched recipients (B6.WT or B6.Foxp3GFP-DTR) were given intraperitoneal injections of DT (160ng/dose, 3 times per week) for up to 2 weeks.
Histology
GVHD target tissues (skin, liver and small intestine) were taken, preserved in 10% formalin, embedded in paraffin, processed to 5-mm-thick sections and H&E-stained. The sections were examined in a blinded fashion using a semi-quantitative scoring system and images acquired as previously described (56, 57).
Flow Cytometry
Single cell suspensions were processed and stained, cells were analyzed on a LSR Fortessa cytometer (Beckman Dickinson) and data were processed using FlowJo Version 9.0 (TreeStar). Cell sorting was performed using a FACSAria or Moflo.
Clinical analysis
Peripheral blood was collected from healthy donors (n = 27) or patients (d60 after BMT) of an observational study (n = 18) and a phase III clinical trial (ACTRN12614000266662) (n = 25). All studies were approved by the institutional ethics committee and all subjects signed informed consent. Peripheral blood mononuclear cells (PBMC) were purified from whole blood using Ficoll-paque centrifugation and stained immediately.
Gene expression analysis
Total RNA was extracted with the RNeasy Micro kit (Qiagen, Netherlands) and gene expression determined using TaqMan GE assays (Applied Biosystems, MA, USA). All measurements were run in parallel with the housekeeping gene Hprt. All primers/probe mixtures were purchased from Applied Biosystems.
Statistics
Results from mouse experiments are presented as mean ± SEM and the Mann–Whitney U test used for comparisons. Results from clinical samples are presented as median ± interquartile range and Mann–Whitney U test used for comparisons. Survival is estimated and plotted using Kaplan–Meier methods, and the difference between subgroups is estimated using log-rank methods. Ordinary least squares method is used in the linear or semi-log regression analysis. A two-sided p value 0.05 is considered statistically significant. Statistical analyses are performed using Prism Version 6 software (GraphPad). NS = not significant, *P <0.05; **P <0.01; ***P <0.001; ****P <0.0001.
Supplementary Material
Table S2, Tabulated data for Figures.
Materials and Methods
Fig S1, TR1 cells are suppressive in vitro. Data related to Fig 1G.
Fig S2, TR1 cells display a distinct profile of markers. Data related to Fig 2.
Fig S3, Eomes is required for the development of TR1 cells after BMT. Data related to Fig 3.
Fig S4, Expression of Eomes during in vitro culture.
Fig S5, Role of Eomes in the generation of TR1 cells in vitro.
Fig S6, Eomes+TR1 cells are dependent on Blimp-1, IL-27 and IL-10. Data related to Fig 4.
Fig S7, Treg development in Il27r−/− and Il10−/− T cells after BMT. Data related to Fig 5.
Fig S8, Both T-bet and Eomes are required for TR1 cell generation. Data related to Fig 6.
Fig S9, Recipient DC and donor IL-27 promote TR1 cell development after experimental BMT. Data related to Fig 7.
Fig S10, Eomes and T-bet can be used to identify TR1 cells after clinical BMT. Data related to Fig 8.
Fig S11, Proposed cellular and transcriptional regulation of TR1 cell development after BMT.
Table S1, Primer sets used for ChIP assays.
Acknowledgments
We thank Paula Hall, Michael Rist and Grace Chojnowski for expert cell sorting, and the animal facilities of QIMR Berghofer Medical Research Institute and Madeleine Flynn for expert graphical work.
Funding: This work was supported by research grants held by G.R. Hill from the National Health and Medical Research Council (NHMRC, Australia). B. Blazar is supported by funds from National Institutes of Health R01 AI34495, R01 HL56067 and AI 11879. C.R. Engwerda is an NHMRC Senior Research Fellow. G.T. Belz is an Australian Research Council Future Fellow. A. Kallies is a fellow of the Sylvia and Charles Viertel Foundation. M.A. Degli-Esposti is an NHMRC Principal Research Fellow. G.R. Hill is an NHMRC Senior Principal Research Fellow and QLD Health Senior Clinical Research Fellow.
Footnotes
Author contributions: P.Zhang designed, performed all experiments, performed the data analysis, statistical analysis and wrote the paper; J.S.Lee performed the ChIP assays and data analysis; K.H.Gartlan, I.S. Schuster, I. Comerford, A.Varelias, M.A.Ullah, S.Vuckovic and M.Koyama helped perform research; R.D.Kuns, K.R.Locke, K.J. Beckett, M.Montes de Oca and F.de Labastida Rivera helped perform experiments and take care of animals; S.D.Olver and L.D.Samson helped process clinical samples; A.D.Clouston performed histology analysis; C.R.Engwerda and G.T.Belz supplied transgenic mice, plasmids, provided critical review and edited paper; A.Kallies supplied transgenic mice, helped experimental design, provided critical review and edited paper; B.R.Blazar, K.P.MacDonald, R.Thomas, M.A. Degli-Esposti, S.R. McColl, and S-K. Tey helped experimental design, provided critical review and edited paper; G.R.Hill designed research and wrote the paper.
Competing interests: GRH has received funding from Roche for clinical studies of IL-6 inhibition..
References
- 1.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 2.Bacchetta R, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. The Journal of experimental medicine. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregori S, Goudy KS, Roncarolo MG. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Frontiers in immunology. 2012;3:30. doi: 10.3389/fimmu.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 5.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Seminars in immunology. 2011;23:202–208. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann C, et al. Role of Blimp-1 in programing Th effector cells into IL-10 producers. The Journal of experimental medicine. 2014;211:1807–1819. doi: 10.1084/jem.20131548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montes de Oca M, et al. Blimp-1-Dependent IL-10 Production by Tr1 Cells Regulates TNF-Mediated Tissue Pathology. PLoS pathogens. 2016;12:e1005398. doi: 10.1371/journal.ppat.1005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinemann C, et al. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nature communications. 2014;5:3770. doi: 10.1038/ncomms4770. [DOI] [PubMed] [Google Scholar]
- 9.Gagliani N, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 10.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature immunology. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasanthakumar A, Kallies A. IL-27 paves different roads to Tr1. European journal of immunology. 2013;43:882–885. doi: 10.1002/eji.201343479. [DOI] [PubMed] [Google Scholar]
- 12.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 13.Cope A, Le Friec G, Cardone J, Kemper C. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends in immunology. 2011;32:278–286. doi: 10.1016/j.it.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews. Immunology. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka K, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. The Journal of clinical investigation. 2010;120:1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blazar BR, et al. Interleukin-10 dose-dependent regulation of CD4+ and CD8+ T cell-mediated graft-versus-host disease. Transplantation. 1998;66:1220–1229. doi: 10.1097/00007890-199811150-00018. [DOI] [PubMed] [Google Scholar]
- 18.Morris ES, et al. Donor treatment with pegylated G-CSF augments the generation of IL-10-producing regulatory T cells and promotes transplantation tolerance. Blood. 2004;103:3573–3581. doi: 10.1182/blood-2003-08-2864. [DOI] [PubMed] [Google Scholar]
- 19.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 22.Gabrysova L, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. The Journal of experimental medicine. 2009;206:1755–1767. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 24.Mascanfroni ID, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nature medicine. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zigmond E, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Hill GR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 27.Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124:354–362. doi: 10.1182/blood-2014-02-514745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stumhofer JS, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nature immunology. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 30.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahl K, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. The Journal of experimental medicine. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin HM, et al. Epigenetic modifications induced by Blimp-1 Regulate CD8(+) T cell memory progression during acute virus infection. Immunity. 2013;39:661–675. doi: 10.1016/j.immuni.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nature reviews. Immunology. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 34.Gordon SM, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 36.Raveney BJ, et al. Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation. Nature communications. 2015;6:8437. doi: 10.1038/ncomms9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran MA, et al. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. The Journal of experimental medicine. 2013;210:743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupar E, et al. Eomesodermin Expression in CD4+ T Cells Restricts Peripheral Foxp3 Induction. Journal of immunology. 2015;195:4742–4752. doi: 10.4049/jimmunol.1501159. [DOI] [PubMed] [Google Scholar]
- 39.Ichiyama K, et al. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-beta is mediated by suppression of eomesodermin. Immunity. 2011;34:741–754. doi: 10.1016/j.immuni.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Gagliani N, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahern PP, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature immunology. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 43.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal immunology. 2010;3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 45.Belle L, et al. Blockade of interleukin 27 signaling reduces GVHD in mice by augmenting Treg reconstitution and stabilizing FOXP3 expression. Blood. 2016 doi: 10.1182/blood-2016-02-698241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy GA, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. The Lancet. Oncology. 2014;15:1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 47.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nature reviews. Immunology. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of experimental medicine. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwasaki Y, et al. Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. European journal of immunology. 2013;43:1063–1073. doi: 10.1002/eji.201242942. [DOI] [PubMed] [Google Scholar]
- 50.Xin A, et al. A molecular threshold for effector CD8(+) T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nature immunology. 2016;17:422–432. doi: 10.1038/ni.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Kallies A, et al. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. The Journal of experimental medicine. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pils MC, et al. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. European journal of immunology. 2010;40:443–448. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- 54.Roers A, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. The Journal of experimental medicine. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kara EE, et al. CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nature communications. 2015;6:8644. doi: 10.1038/ncomms9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang P, et al. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. Journal of immunology. 2013;191:5291–5303. doi: 10.4049/jimmunol.1301181. [DOI] [PubMed] [Google Scholar]
- 57.Burman AC, et al. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110:1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 58.Koyama M, et al. Donor colonic CD103+ dendritic cells determine the severity of acute graft-versus-host disease. J Exp Med. 2015;212:1303–1321. doi: 10.1084/jem.20150329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2, Tabulated data for Figures.
Materials and Methods
Fig S1, TR1 cells are suppressive in vitro. Data related to Fig 1G.
Fig S2, TR1 cells display a distinct profile of markers. Data related to Fig 2.
Fig S3, Eomes is required for the development of TR1 cells after BMT. Data related to Fig 3.
Fig S4, Expression of Eomes during in vitro culture.
Fig S5, Role of Eomes in the generation of TR1 cells in vitro.
Fig S6, Eomes+TR1 cells are dependent on Blimp-1, IL-27 and IL-10. Data related to Fig 4.
Fig S7, Treg development in Il27r−/− and Il10−/− T cells after BMT. Data related to Fig 5.
Fig S8, Both T-bet and Eomes are required for TR1 cell generation. Data related to Fig 6.
Fig S9, Recipient DC and donor IL-27 promote TR1 cell development after experimental BMT. Data related to Fig 7.
Fig S10, Eomes and T-bet can be used to identify TR1 cells after clinical BMT. Data related to Fig 8.
Fig S11, Proposed cellular and transcriptional regulation of TR1 cell development after BMT.
Table S1, Primer sets used for ChIP assays.