Abstract
A bottleneck for immunotherapy of cancer is the immunosuppressive microenvironment in which the tumor cells are located. Regardless of the fact that large numbers of tumor-specific T cells can be generated in patients by active immunization or adoptive transfer, these T cells do not readily translate to tumor cell killing in vivo. The immune regulatory mechanism that prevents autoimmunity may be harnessed by tumor cells for the evasion of immune destruction. Regulatory T cells, myeloid-derived suppressor cells, inhibitory cytokines and immune checkpoint receptors are the major components of the immune system acting in concert with causing the subversion of anti-tumor immunity in the tumor microenvironment. This redundant immunosuppressive network may pose an impediment to efficacious immunotherapy, thus facilitating tumor progression. Cancer progression clearly documents the failure of immune control over relentless growth of tumor cells. Detailed knowledge of each of these factors responsible for creating an immunosuppressive shield to protect tumor cells from immune destruction is essential for the development of novel immune-based therapeutic interventions of cancer. Multipronged targeted depletion of these suppressor cells may restore production of granzyme B by CD8+ T cells and increase the number of IFN-γ-producing CD4+ T cells.
Keywords: Regulatory T cells, Myeloid-derived suppressor cells, PD-1+ T cells, Immunosuppression, Hepatocellular cancer, CITIM 2015
Introduction
Emerging clinical data suggest that cancer immunotherapy is likely to become a key part of the clinical management of cancer. The major advantage of immunotherapy is that it specifically targets the tumor cells, while sparing normal healthy cells, thus preventing the debilitating side effects that are inevitable with chemo- or radiotherapy. Timely administration of immunotherapeutic strategies may eliminate minimal residual disease, thus preventing recurrence and metastasis.
A major hurdle for immunotherapy of cancer is that in most of the cases the tumor cells are located in an immunosuppressive microenvironment. Regardless of the fact that large numbers of tumor-specific T cells can be generated in patients by active immunization or adoptive transfer, these T cells cannot fully achieve their tumoricidal potential in vivo. Thus, large numbers of tumor antigen-specific T cells present in the periphery do not readily translate to tumor cell killing. Tumor cells employ various suppressive mechanisms to accomplish immune suppression, which include induction of regulatory T cells (Tregs), myeloid-derived suppressor cells [1] and expression of programmed death ligand (PD-L1).
Immunosuppression by Tregs
Regulatory T cells (Tregs) are potent inhibitors of the immune system, crucially important for maintaining peripheral tolerance as well as regulating immune responses, preventing excessive immune activation and autoimmunity. Tregs suppress effector T cells, NK cells and dendritic cells either through cell–cell contact or through secretion of immunosuppressive cytokines and indoleamine 2,3 dioxygenase (IDO). Studies have demonstrated that many cancers can induce the proliferation of Tregs and/or promote their generation from naive T cells, resulting in the accumulation of these cells in the tumor beds and in the periphery. Importantly, the elimination and/or functional inactivation of tumor-induced Tregs can promote anti-tumor immunity and enhance the efficacy of immunotherapy.
Chronic inflammation characterized by continued expression of pro-inflammatory cytokines and recruitment of immune cells to the liver contributes to hepatic carcinogenesis [2]. The inhibition of tumor-specific immune surveillance in this chronic inflammatory milieu is mediated by increased frequency of Tregs, MDSCs, changes in the expression pattern of inhibitory immune checkpoints, alterations in the function of dendritic cells (DC) and release of immunosuppressive cytokines. Decline in CD4+ T effector cell function with progressive tumor growth in HCC is related to increased Treg-mediated suppression [3, 4]. Even though the suppressive role of Tregs during the inflammation is beneficial for maintenance of normal immune homeostasis, increase in the number of Tregs during tumor progression suppresses CD8+ T effector cell-mediated anti-tumor immunity, hence creating a barrier to successful immunotherapy of cancer [5–7]. On the contrary, a paradox has been reported in colorectal cancer; while some studies concluded that high density of Tregs in the tumor microenvironment is associated with better prognosis, other studies reported their elevated numbers are associated with advanced stage tumors and poor overall survival [8]. However, the benefit or hazard of Tregs in colorectal cancer (CRC) is highly debated. It seems that Tregs have differential influences at different stages of CRC development. Excessive activation of Tregs also contributes to persistent HBV infection and progression of HCC [9], and HBV+ HCC patients have Tregs with greater suppressive potential than non-HBV+ HCC patients [10]. Negative role of Tregs through dampening favorable CD8+ T cell responses to tumor-associated antigens has been reported in breast cancer patients, and patients with low CD8+ T cells/Tregs ratio had a better survival benefit [11]. Tregs also play an important role in the progression and metastasis of lung cancer, the most lethal disease globally which has no effective therapies and expression of tumor necrosis receptor factor type II (TNRF2) by circulating Tregs in these patients correlated with poor clinical outcome [12, 13]. The inhibitory effect of Tregs on CD4+ and CD8+ T cells can be abrogated by treatment with anti-chemokine receptor type-4 (CCR4) and anti-CD56 antibody in vitro which represent a novel strategy for the elimination of Tregs in the therapeutic settings of lung cancer patients [14].
Role of MDSCs in suppression of anti-tumor immunity
MDSCs are a heterogeneous population of immature myeloid cells that originate from the bone marrow, and normally differentiate into dendritic cells (DCs) or macrophages. However, their maturation is halted in malignancy, and they infiltrate into tumor microenvironment. MDSCs induce a state of chronic tissue inflammation and immune suppression that is characterized by the production of reactive oxygen species (ROS), nitric oxide (NO), arginase-1 (Arg-1) and cytokines IL-1, IL-6 and TNF-α. They can induce Tregs and attract suppressive tumor-associated macrophages (TAM) [15]. The expansion of both granulocytic and monocytic MDSCs in a broad spectrum of human cancers has been extensively reviewed by Stromnes et al. [16]. Abrogation of MDSC activity by targeted depletion has been shown to enhance endogenous CTL-mediated tumor cell killing, which suggests that failure of immune surveillance to cancer may be in part due to MDSCs [17]. Several strategies for therapeutic targeting of MDSCs in malignancy have been detailed, and such strategies include depletion of MDSCs, interfering with suppressive activity of MDSCs by using cyclooxygenase-2 (COX2) inhibitors and phosphodiesterase-5 (PDE-5) inhibitors [16–19]. MDSCs are recruited by IDO expression in the tumor microenvironment. In addition to attracting MDSCs, IDO is instrumental in the deprivation of tryptophan in the tumor microenvironment, thus inhibiting T cell activation [16]. IDO-mediated tryptophan depletion inhibits the kinases mammalian target of rapamycin (mTOR) and protein kinase C (PKC). The novel experimental inhibitor of IDO, 1-methyl d-tryptophan, relieves the inhibition of mTOR and PKC by acting as a tryptophan mimetic. Thus, IDO represents an attractive potential target for cancer therapy [20].
Increased levels of HLA-DRlow/neg CD14+ MDSCs detected in the circulation of patients with melanoma [21], HCC [9, 22], rectal cancer [23] and prostate cancer [24] correlated with poor prognosis; furthermore, a reciprocal relationship between Tregs and MDSCs has been demonstrated in HCC and prostate cancer patients [9, 24, 25]. In contrast, an inverse correlation between the frequency of MDSCs and the presence of functional antigen-specific T cells found in melanoma patients and breast cancer patients highlights the potent immunosuppressive role of MDSCs in cancer [11, 21]. Thus, several studies conducted in cancer patients and preclinical models suggest the pro-tumorigenic nature of MDSCs [1] and therapeutic targeting of MDSCs to maximize the efficacy of immune-based therapies are being considered in clinical trial settings [16, 26–28].
Inhibitory checkpoint receptors and “T cell exhaustion”
PD-1 is a checkpoint receptor expressed on activated T cells that down-modulates effector T cell function and prevents the formation of immune memory. Interaction of activated PD-1+ T cells with PD-L1-expressing tumor cells, immune cells such as DCs, macrophages, NK cells, monocytes and B cells results in suppression of T cells. Upregulation of PD-L1 by neoplastic cells allows tumors to escape the anti-tumor effector T cell responses. Studies conducted in different types of human cancers have shown that PD-L1 expression on tumor cells reflects an immune reactive microenvironment, and responsiveness to anti-PD-1 therapy has strong correlation with tumor PD-L1 expression [29]. Impressive clinical responses have been achieved by therapeutic targeting of T cell inhibitory pathways using monoclonal antibodies directed against immune checkpoints PD-1, PD-L1 and CTLA-4. While therapeutic efficacy of PD-1/PD-L1 blockade has been shown to be promising with accomplishment of better survival and durable remission reported in different types of solid malignancies [30–32], this represents a potential strategy to target immune suppression in a variety of hematological malignancies [33]. Soluble CD80-mediated intervention of PD-1/PD-L1 axis can restore the activation of both CD4+ and CD8+ T cells with IFN-γ production [34, 35]. Virotherapy using oncolytic viruses has been reported to reduce the systemic resistance to PD-1 immunotherapy in an experimental model of liver cancer, thus providing the rationale for combining oncolytic viruses and immune checkpoint blockade in clinical trial [36]. Immune checkpoint receptors are recognized as important players of immune suppression in HCC [37, 38]. We have reported the increased frequency of exhausted T cells expressing PD-1 in HCC patients with advanced stage of the disease, which contributes to incompetent T effector cell function; selective in vitro depletion of the immunosuppressive cells resulted in partial restoration of T effector cell function in HCC [25, 39].
CTLA-4 is another inhibitory receptor pathway hindering T cell function in cancer; it competes with CD28 for binding to the co-stimulatory receptors CD80 and CD86 on antigen-presenting cells, thus impairing initial stages of T cell activation and proliferation. While PD-1 plays a central role in downregulating activated T cells in the periphery, CTLA-4 predominantly regulates early stages of T cell activation. Ipilimumab, an anti-CTLA-4 monoclonal antibody, augmented anti-CD3-driven T cell proliferation in vitro and enhanced bispecific antibody-directed cytolysis of tumor cells by activated T cells; this is implicated to attenuation of Treg-mediated suppression by ipilimumab [40]. Ipilimumab has been administered for the treatment of melanoma in combination with bevacizumab, and improvement in overall survival was recorded in melanoma patients [41]. In a recent study, combination therapy using ipilimumab and anti-PD-1 antibody, nivolumab, has been shown to produce significant improvement in the progression-free survival of metastatic melanoma patients as compared to ipilimumab alone. This combination therapy was found to be more effective in PD-L1-negative tumors [42].
Immune suppression in HCC
HCC is an inflammation-associated malignancy, and majority of the patients suffer from cirrhosis of viral etiology or chronic alcohol consumption. Immunogenicity of HCC renders it a suitable candidate for immunotherapeutic approaches. Alfa-fetoprotein (AFP), glypican-3 (GPC-3), New York esophageal squamous cell cancer-1 (NY-ESO-1) and melanoma antigen-A (MAGE-A) are the prominent and most studied antigens in HCC. Spontaneous cellular or humoral responses against NY-ESO1 antigen have been reported in 50 % of the patients [43]. Infiltration of tumor-associated antigen-specific CD8+ T cells was reported in 50 % of HCC patients and antigen-specific CD8+ T cell responses correlated with progression-free survival [44]. Nevertheless, HCC exploits multiple immunosuppressive pathways to effectively evade the host’s anti-tumor immune responses. The immune regulatory mechanism that prevents autoimmunity may be harnessed by tumor cells to accomplish immune escape or the evasion of immune destruction. Regulatory T cells (Tregs), myeloid-derived suppressor cells [1] and PD-1+ T cells are major components of the immune system acting in concert with causing the subversion of anti-tumor immunity in the tumor microenvironment [25, 39, 44, 45]. This redundant immunosuppressive network in HCC poses an important obstacle to success of immunotherapy. Detailed knowledge of these factors responsible for creating an immunosuppressive milieu is essential for the development of novel immune-based therapeutic modalities.
Several studies have reported that dysregulated immune function poses an impediment to immunotherapeutic approaches in HCC. While many of these studies have focused on investigating one single aspect of dysfunctional immunity in HCC the impact of either Tregs [4] or MDSCs [22] on abrogation of anti-tumor immunity, we were the first to perform a global analysis of immune dysfunction in this patient population [25]. HCC exploits multiple immunosuppressive mechanisms to evade active immune surveillance of the host; this includes induction of Tregs, recruitment of MDSCs, accumulation of exhausted T effector cells, defective antigen presentation by dendritic cells (DCs) and overproduction of inhibitory cytokines such as IL-10 and TGF-β1 [25, 37, 38, 44].
Since CD25 is expressed on both activated T effector cells and Tregs, this marker alone is incapable of accurately discriminating Tregs from activated effector T cells. Expression of Foxp3 alone is also not a reliable marker, as this protein is also expressed on non-immunosuppressive Tregs. Therefore, we have used multiple markers (CD3+CD4+Foxp3+CD127−) for the identification and characterization of Tregs and detected significantly elevated frequency and absolute number of this phenotype in HCC patients [25]. Following a recent workshop study, a consensus has been reached over the controversy regarding the markers that should be used for the identification of Tregs, and the same markers used in our study have been validated and proposed as essential marker set for the identification of Tregs from PBMC [46]. Furthermore, intracellular localization of Foxp3 protein precludes its use for therapeutic targeting and depletion of suppressive Tregs using specific antibodies. In order to identify highly immunosuppressive Tregs bearing markers that can be therapeutically targeted, we evaluated the expression of CTLA-4 and glycoprotein A repetition predominant (GARP) on the surface of Foxp3+ Tregs. High prevalence of this phenotype in patients with advanced HCC highlighted the severe immune dysfunction in these patients and provided the rationale for therapeutic depletion of immunosuppressive Tregs.
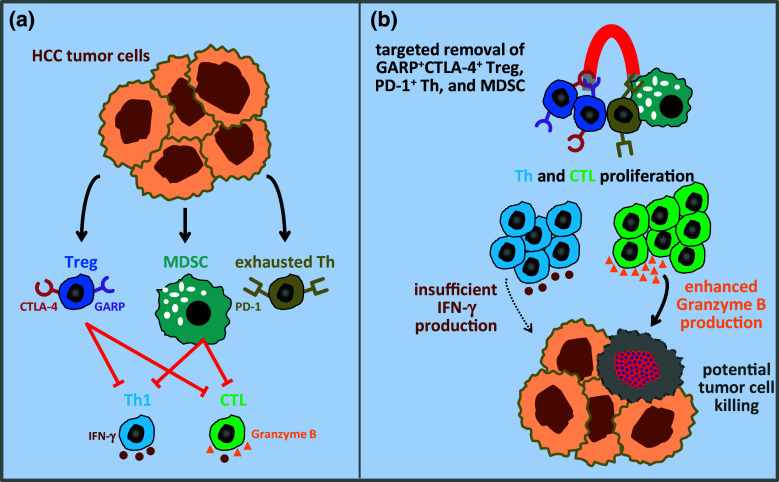
The central role of MDSCs in dampening anti-tumor immunity in HCC has not been appreciated before, despite its high importance as a potent immunosuppressive cell subset in other cancers. Recognizing the potential relationship between MDSCs and induction of Tregs during the progression of malignancy, we therefore evaluated the frequency of CD14−HLA-DR−CD11b+CD33+ MDSCs. In conjunction with elevated levels of Tregs, the frequency and absolute number of MDSCs were significantly high in HCC patients. Additionally, the frequency of MDSCs showed excellent correlation with frequency of Tregs, indicating that the accumulation of MDSC is in concurrence with Treg accumulation and the interplay between these immunosuppressive cell subsets is very critical in the establishment of tolerogenic tumor microenvironment in HCC (Fig. 1a, Copyright permission of [39]). Thus, depletion of one of these subsets is unlikely to have a beneficial impact on anti-tumor immune responses in HCC, as one of these cell types can induce the generation of other by creating a feedback loop. This provided the rationale for the combined depletion of Tregs and MDSCs, which resulted in restoration of T effector cell functions, enhancement in T cell proliferation and granzyme B production (Fig. 1b).
Fig. 1.
Targeted depletion of Tregs, exhausted PD-1+ T helper cells and myeloid-derived suppressor cells may restore effective anti-tumor T cell function. a Tumor cell-mediated induction of exhausted effector T helper cells and immunosuppressive GARP+CTLA-4+ Tregs and myeloid-derived suppressor cells diminish the anti-tumor capacity of Th1 and CTL by inhibition of IFN-γ and granzyme B production. b The restoration of effector T cell proliferation and granzyme B production (but not IFNγ secretion) by the combined removal of all these immunosuppressive cells may restore anti-tumor Th1 and CTL responses (adapted from [39])
Heightened immunosuppression in HCC is additionally reflected by elevated levels of Treg-derived cytokines IL-10 and TGF-β1 with concomitant downregulation of IFN-γ, a potent anti-tumor cytokine whose diminished levels could be attributed to high prevalence of suppressive Tregs in HCC. Serum interferon-γ levels may reflect host’s anti-tumor immunity and may be potential marker of HCC recurrence after curative therapy in patients [47].
Since HCC has strong association with chronic viral infections (HBV/HCV), appearance of exhausted T cells expressing PD-1 may be considered as a hallmark of HCC patients with viral etiology. Thus, abundance of PD-1+ T cells detected in HCC patients is an indication of yet another level of immune dysregulation that would need to be circumvented, in order to accomplish efficacious anti-tumor immune responses. Functional defects in both CD4+ and CD8+ T cells reflected by decreased proliferation and diminished granzyme B or IFN-γ secretion in response to anti-CD3/CD28/mitogenic stimulation is not surprising; this could be due to the cumulative effect of underlying immunosuppressive burden in these patients (Fig. 1a). Multi-pronged depletion of suppressive cell subsets restored the proliferation of both CD4+ T helper cells and CD8+ CTL, but not to the levels equivalent to T cells from healthy controls. Nonetheless, combined depletion of suppressive cells fully restored granzyme B production by CD8+ T cells to the levels equivalent to those observed with T cells from healthy donors (Fig. 1b). IFN-γ production by T cells also improved but to a lesser extent. Thus, elevated levels of immunosuppressive cells in HCC patients compromise CTL function, partly by inhibiting granzyme B production, resulting in attenuated tumor cell killing.
Conclusions
In conclusion, even though there is abundant in vitro evidence for human T cell reactivity against tumors, such responses are often ineffective in vivo as tumor cells exploit multiple mechanisms to avoid immune cell recognition and anti-tumor effector cell function, thereby limiting the clinical benefits of immunotherapeutic strategies. Mitigation of immunosuppressive cells to augment Th1 and CTL response is critical for improving the efficacy of immunotherapy of cancer, which may impact the survival of patients.
Acknowledgments
This research in the Thanavala Lab was supported in part through discretionary funds available to Dr. Thanavala. We gratefully acknowledge Dr. Paul Wallace and Earl Timm for their help in designing flow cytometry experiments. The patient samples were obtained through the Roswell Park Cancer Institute Data Bank and Biorepository which is a Cancer Center Support Grant (CCSG) Shared Resource supported by National Institute of Health P30 CA016056-27.
Abbreviations
- AFP
Alpha-fetoprotein
- Arg-1
Arginase-1
- CCR4
Chemokine receptor type-4
- COX2
Cyclooxygenase-2
- CRC
Colorectal cancer
- GARP
Glycoprotein A repetition predominant
- GPC-3
Glypican-3
- HCC
Hepatocellular carcinoma
- MAGE-A
Melanoma antigen-A
- mTOR
Mammalian target of rapamycin
- NO
Nitric oxide
- NY-ESO-1
New York esophageal squamous cell cancer-1
- PDE-5
Phosphodiesterase-5
- PKC
Protein kinase C
- ROS
Reactive oxygen species
- TAM
Tumor-associated macrophages
- Th1
T helper 1
- Tregs
Regulatory T cells
Compliance with ethical standards
Conflict of interest
None of the authors have any conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fourth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2015), held in Ljubljana, Slovenia, 27th–30th April 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Schrader J. The role of MDSCs in hepatocellular carcinoma—in vivo veritas? J Hepatol. 2013;59:921–923. doi: 10.1016/j.jhep.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera R, Szabo G. Another armed CD4(+) T cell ready to battle hepatocellular carcinoma. Hepatology. 2013;58:1–3. doi: 10.1002/hep.26377. [DOI] [PubMed] [Google Scholar]
- 4.Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58:139–149. doi: 10.1002/hep.26054. [DOI] [PubMed] [Google Scholar]
- 5.Pastille E, Bardini K, Fleissner D, Adamczyk A, Frede A, Wadwa M, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014;74:4258–4269. doi: 10.1158/0008-5472.CAN-13-3065. [DOI] [PubMed] [Google Scholar]
- 6.Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62:1420–1429. doi: 10.1016/j.jhep.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z, et al. Intrahepatic interleukin-17+ T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:851–859. doi: 10.1111/jgh.12418. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Kelaria S, Kerstetter J, Wang J. The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J Gastrointest Oncol. 2015;6:307–313. doi: 10.3978/j.issn.2078-6891.2015.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo Y, Shimosegawa T. Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs. Int J Mol Sci. 2015;16:3307–3322. doi: 10.3390/ijms16023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Khosla R, David P, Rastogi A, Vyas A, Singh D, et al. CD4+CD25+CD127 (low) regulatory T cells play predominant anti-tumor suppressive role in hepatitis B virus-associated hepatocellular carcinoma. Front Immunol. 2015;6:49. doi: 10.3389/fimmu.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailur JK, Gueckel B, Derhovanessian E, Pawelec G. Presence of circulating Her2-reactive CD8+ T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. 2015;17:34. doi: 10.1186/s13058-015-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Chen Z, Wang DC, Wang X. Regulatory T cells and potential inmmunotherapeutic targets in lung cancer. Cancer Metastasis Rev. 2015;34:277–290. doi: 10.1007/s10555-015-9566-0. [DOI] [PubMed] [Google Scholar]
- 13.Yan F, Du R, Wei F, Zhao H, Yu J, Wang C, et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol Immunother. 2015;64:1475–1485. doi: 10.1007/s00262-015-1751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurose K, Ohue Y, Sato E, Yamauchi A, Eikawa S, Isobe M, et al. Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760) J Thorac Oncol. 2015;10:74–83. doi: 10.1097/JTO.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 15.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stromnes IM, Greenberg PD, Hingorani SR. Molecular pathways: myeloid complicity in cancer. Clin Cancer Res. 2014;20:5157–5170. doi: 10.1158/1078-0432.CCR-13-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63:1769–1781. doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao Y, Poschke I, Wennerberg E, de Pico CY, Egyhazi BS, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 19.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–38. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20:1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 22.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, et al. Increase in CD14+ HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Napolitano M, D’Alterio C, Cardone E, Trotta AM, Pecori B, Rega D, et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget. 2015;6:8261–8270. doi: 10.18632/oncotarget.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idorn M, Kollgaard T, Kongsted P, Sengelov L, Thor SP. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177–1187. doi: 10.1007/s00262-014-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina-Echeverz J, Eggert T, Han M, Greten TF. Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol Immunother. 2015;64:931–940. doi: 10.1007/s00262-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Hawkins WG, DeNardo DG. Regramming myeloid responses to improve cancer immunotherapy. Oncoimmunology. 2015;4:e974399. doi: 10.4161/2162402X.2014.974399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014;28:1784–1792. doi: 10.1038/leu.2014.108. [DOI] [PubMed] [Google Scholar]
- 34.Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191:2829–2836. doi: 10.4049/jimmunol.1202777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. 2014;193:3835–3841. doi: 10.4049/jimmunol.1401572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woller N, Gurlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, et al. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther. 2015;23:1630–1640. doi: 10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt N, Flecken T, Thimme R. Tumor-associated antigen specific CD8 T cells in hepatocellular carcinoma—a promising target for immunotherapy. Oncoimmunology. 2014;3:e954919. doi: 10.4161/21624011.2014.954919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lugade AA, Kalathil S, Miller A, Iyer R, Thanavala Y. High immunosuppressive burden in advanced hepatocellular carcinoma patients: Can effector functions be restored? Oncoimmunology. 2013;2:e24679. doi: 10.4161/onci.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yano H, Thakur A, Tomaszewski EN, Choi M, Deol A, Lum LG. Ipilimumab augments antitumor activity of bispecific antibody-armed T cells. J Transl Med. 2014;12:191. doi: 10.1186/1479-5876-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 44.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao HQ, Li WM, Lu ZQ, Yao YM. Roles of Tregs in development of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20:7971–7978. doi: 10.3748/wjg.v20.i24.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother. 2015;64:1271–1286. doi: 10.1007/s00262-015-1729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee IC, Huang YH, Chau GY, Huo TI, Su CW, Wu JC, et al. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer. 2013;133:2895–2902. doi: 10.1002/ijc.28060. [DOI] [PubMed] [Google Scholar]