Abstract
The extracellular matrix (ECM) is a dynamic environment that constantly provides physical and chemical cues to embedded cells. Much progress has been made in engineering hydrogels that can mimic the ECM, but hydrogel properties are, in general, static. To recapitulate the dynamic nature of the ECM, many reversible chemistries have been incorporated into hydrogels to regulate cell spreading, biochemical ligand presentation and matrix mechanics. For example, emerging trends include the use of molecular photoswitches or biomolecule hybridization to control polymer chain conformation, thereby enabling the modulation of the hydrogel between two states on demand. In addition, many non-covalent, dynamic chemical bonds have found increasing use as hydrogel crosslinkers or tethers for cell signalling molecules. These reversible chemistries will provide greater temporal control of adhered cell behaviour, and they allow for more advanced in vitro models and tissue-engineering scaffolds to direct cell fate.
The human body is composed of many tissues that are continually changing. Changes occur during healthy processes, such as development or the maintenance of homeostasis, and during unhealthy processes, such as disease progression1. In these tissues, the extracellular matrix (ECM) provides support to resident cells and communicates through both chemical and physical cues that change over time. For example, during adolescence, a laminin-rich form of ECM is secreted to direct the development of mammary tissue, but dysfunctional regulation of this matrix can eventually lead to tumour formation and cancer1. In another example, tissue repair after an injury proceeds in multiple stages: immediate repair relies on the deposition of a provisional matrix that is rich in fibrin and fibronectin, and later repair either modifies this matrix to restore tissue function or leads to excessive collagen secretion and eventually fibrosis2. The path leading to either healthy or diseased tissue depends on the context of the ECM; more specifically, there is a dynamic interplay involving matrix composition, biochemical molecules (for example, growth factors and cytokines) and matrix mechanics, all of which influence cellular behaviour. In addition, both the spatial orientation and the timed presentation of these cues affect how they are translated into signals within the cell3,4. As the fields of tissue engineering and regenerative medicine progress, there is a need to better understand these dynamic interactions between cells and the ECM, and a need to design more appropriate in vitro models and scaffolds to guide cell fate.
It has long been recognized that the microenvironment can influence and dictate cellular behaviour despite the cell’s genetic programming5 and that this is a continual process with no defined end point. In fact, several terms have been used to describe this concept, such as ‘dynamic reciprocity’ (REF. 5) or the ‘plasticity of the differentiated state’ (REF. 6). Efforts in tissue engineering have focused on creating stem cell niches for medical therapies7–9; however, the use of dynamic chemistries in these niches is more recent. Traditionally, these niches have been made using structural, relatively static biomaterials with predefined properties10. However, for many applications — for example, cell differentiation — more advanced materials are needed, especially those that enable various chemical and mechanical cues to be directed over time. To this end, hydrogels have emerged as a valuable platform for mimicking key aspects of the native ECM and for introducing spatiotemporally precise signals to shape cellular phenotype (FIG. 1).
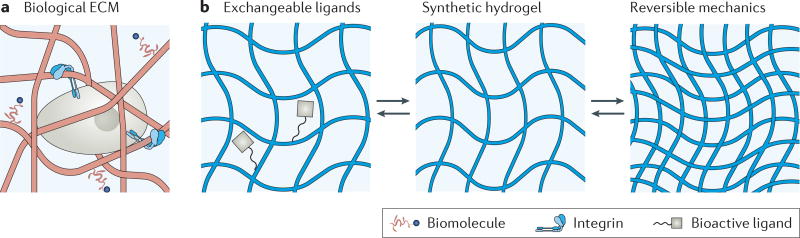
Figure 1. Biological extracellular matrix and synthetic strategies involving reversible chemistries.
a | The native extracellular matrix (ECM) is a heterogeneous fibrillar network that provides biochemical and physical cues to cells. b | Synthetic hydrogels are traditionally static, polymeric networks (middle panel). Dynamic hydrogels can capture aspects of the native ECM by temporally controlling ligand presentation (left panel) or reversibly cycling through changes in mechanics (right panel). Although these dynamic chemistries do not fully capture the complexity of the biological environment, they provide an enhanced precision over matrix cues in vitro, which will have a powerful impact on tissue engineering and regenerative medicine.
Hydrogels as matrix mimics
Composition
Hydrogels are water-swollen networks that can be formed from polymeric macromers or from small molecules that self-assemble into larger structures11,12. Depending on the chemistry of the precursors, they are held together by covalent, non-covalent or physical crosslinks. Before being widely used as cell culture platforms, these networks were utilized in several applications that interfaced with the human body, such as contact lenses and the delivery of therapeutics7,13,14. Their high water content, porosity and inherent mechanical tunability render hydrogels particularly attractive as ECM mimics, and there are a range of chemistries available to functionalize hydrogels with bioactive ligands. Furthermore, well-defined relationships (for example, between modulus and crosslinking density in rubbery elastic materials) exist to engineer desired physical properties that simulate those of soft tissues8.
Some of the first polymers used to synthesize hydro-gels included covalently bound networks of poly-(hydroxyethylmethacrylate), poly(acrylamide) and poly(ethylene glycol) (PEG)14, all of which are currently used as cell culture materials and can be found as commercially available precursors. Much of the pioneering work with these hydrogels enabled breakthroughs in understanding the effects of matrix stiffness on cell behaviour9,15–17, as well as provided a more physiologically relevant substrate from which to probe cell behaviour with soluble factors (compared with glass or plastic). However, in their most common form, these synthetic hydrogels still have several properties that are considerably different from those of the native ECM — they are typically amorphous, homogeneous materials instead of fibrillar networks. In addition, because the hydrogels are formed from non-natural polymers, there is limited or no biological activity. Cellular adhesion is typically introduced through integrin-binding peptides, such as RGD or the laminin-derived IKVAV, which are vastly simplified in binding efficiency and specificity compared with full-length matrix proteins. Traditional synthetic hydrogels are static substrates and do not recapitulate the dynamic ECM interactions that occur with the cell. To this end, synthetic strategies to create more advanced biomaterials that are capable of providing spatiotemporally defined cellular signals have been the focus of recent research18.
Responsive hydrogels
There is a rich history in the materials science community of engineering polymeric networks that respond to various stimuli19, such as pH20,21, temperature22,23, electric fields24, magnetic fields25 and light26. In addition, many ‘smart’ materials have integrated responsive moieties to specific biological targets27, particularly for drug delivery applications. Examples of these biological targets include antigen–antibody interactions28 and sugars, such as glucose29–31. However, many of these responsive technologies can have adverse or undesired effects on living cells, and careful selection of the stimuli is required when introducing dynamic properties into biomaterials such that the responses can occur under physiological conditions with minimal invasiveness10.
As progress has been made in the design of matrices for 3D cell cultures, several strategies have emerged that permit cell spreading, migration, signalling and mechanotransduction under physiological conditions. For example, passive hydrolytic degradation32–35 or cell-mediated enzymatic degradation36 of the polymer network have both been revolutionary in allowing encapsulated cells to spread, proliferate and deposit matrix. Furthermore, light-based reactions have enabled matrix mechanics to change in the presence of adhered cells, thereby altering the real-time physical cues presented to cells37. However, as many of the aforementioned degradation mechanisms are irreversible, they can lead to hydrogel erosion over time and thereby prevent long-term cell culture studies. In addition, cyclical changes in ECM remodelling cannot be captured with one-directional degradation or chemical cleavage reactions, and the effect of freely soluble cleavage products on cellular behaviour is not well understood. Some of these limitations can be addressed by reversible chemistries, which enable the spatiotemporally controlled addition and removal of biochemical signals (namely, dynamic presentation) and even repeated changes in matrix mechanics (FIG. 2).
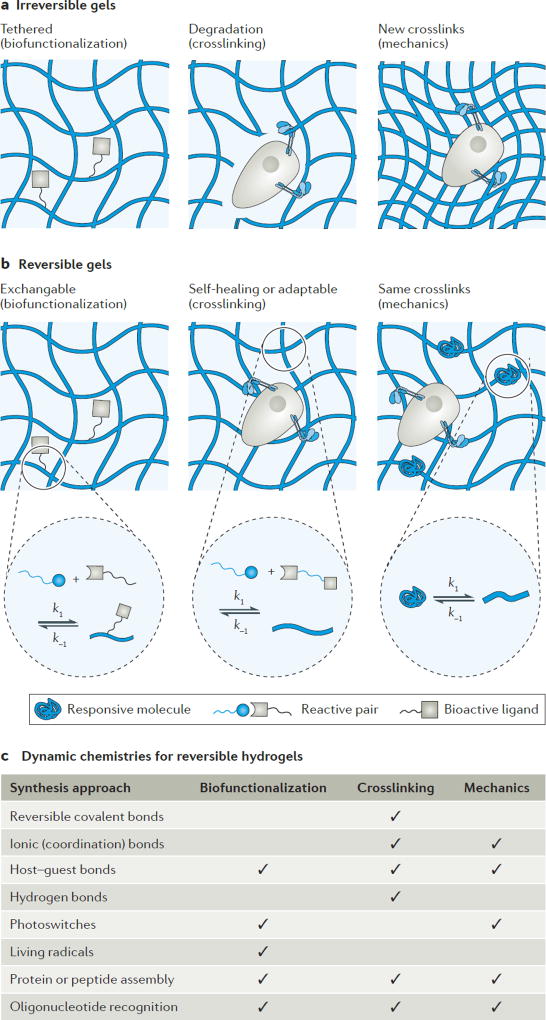
Figure 2. Irreversible and reversible chemistries for hydrogels.
a | Irreversible chemistries for hydrogels include tethered molecules for cell signalling (left panel), degradable crosslinks to mediate cell spreading (middle panel) and the generation (or degradation, which is not shown) of crosslinks to alter matrix mechanics (right panel). b | Reversible chemistries include dynamic presentation of cell signalling molecules (left panel), self-healing or adaptable crosslinks in hydrogels (middle panel) and strategies to alter the crosslinking density without changing the network connectivity of the hydrogel (right panel). c | The reversible chemistries incorporated into synthetic hydrogels and their uses for biofunctionalization, crosslinking and cyclical mechanics are shown in the table.
Capturing matrix dynamics with reversible hydrogels
This Review highlights strategies that allow reversible modulation of scaffold properties using synthetic hydrogels (FIG. 2c). First, we cover recent approaches to both add ligands and biomolecules to, and remove them from, hydrogels to modulate the chemical context over time. Second, reversible or adaptable crosslinking chemistries are examined that enable the formation of non-degradable biomaterial platforms for cell encapsulation and cell spreading. Third, physical matrix cues are covered, with a specific focus on strategies that enable user-mediated, cyclical changes in visco-elastic moduli. Last, current challenges and emerging trends in the field are discussed. Whenever possible, recent studies in materials developed for 3D cell culture are highlighted, as there is a growing recognition by the research community of the importance of this dimension and its added complexities38. As the variety of materials for simulating the properties of the ECM increases, so will the ability to dictate cell fate and the potential to harness these materials for medical therapies.
Exchangeable ligand presentation
Challenges of dynamic ligand presentation
Beyond providing mechanical support for adhered cells, the native ECM is a porous matrix that enables and regulates the diffusion of soluble biochemical signals. Many of these soluble signals are sequestered and bound to the matrix, in which they are stored and from which can be released on demand3,4. Heparan sulfate proteoglycans bind to many growth factors (for example, fibroblast growth factors (FGFs) and vascular endothelial growth factors (VEGFs))39, and ECM proteins such as fibronectin have also been shown to have promiscuous binding sites for numerous growth factors40. As another example, ECM proteoglycans, such as decorin and betaglycan (also known as TGFR3), have been shown to sequester the inhibitory growth factor transforming growth factor β1 (TGFβ1) or TGFβ2 to regulate its signalling during skeletal muscle differentiation41. The temporal and spatial presentation of these factors to cells is inherently linked to numerous in vivo processes, and their release from the ECM is often mediated by the cell through proteolytic or mechanical forces38. TGFβ1 is sequestered in a latent form until a cell can exert a significant traction force to pull and release it into an active, soluble form, thereby mediating inflammatory processes involved in fibrosis4. Conversely, some biochemical signals are more active in their matrix form: fibronectin itself contains cellular recognition sites that are only accessible when fibronectin is assembled into fibrils42.
Recapitulating this dynamic presentation of biochemical cues in vitro presents several challenges. In fact, there has been much pioneering work with incorporating proteoglycans (for example, heparins)43 or peptides44,45 into hydrogels to present an affinity for and a dynamic association with growth factors, but these can lead to multiple interactions in serum-laden cell culture media. As a result, this approach has inspired complementary synthetic methods to select bioorthogonal chemistries, such that soluble factors can be tethered or released in the presence of adhered cells without confounding associations from other soluble factors and without rendering the environment cytotoxic or modifying the cells themselves. More precise bioorthogonal chemistries also enable the presentation of multiple cues and allow for the controlled formation of opposing gradients of signals that are often important in biology46,47.
Recent advances in bioorthogonal conjugation have enabled the generation of reversible chemistries, particularly light-based methods48–55 that photopattern, uncage and release ligands with spatiotemporal control. The ability to modulate ligand presentation in the presence of cells or even in vivo is the focus of current work52. In addition, new strategies to reversibly control the bioactivity of immobilized factors avoid the unintended effects of soluble dosing when introducing ligands for matrix modification.
Light-based strategies
Light-based chemistries are attractive for conjugating ligands to hydrogels because many light-based reactions can be carried out very rapidly under aqueous, physiologically relevant conditions in the presence of cells. Furthermore, photo-patterning provides an easy way to mimic biochemical gradients in vitro with exquisite control over spatial organization in both 2D and 3D geometries over time (FIG. 3a). To conjugate and release biomolecules in synthetic platforms, traditional masks to direct light illumination allow for control on 2D platforms; 3D patterning technologies typically rely on the use of confocal microscopes equipped with two-photon lasers56. More recently, digital light-processing techniques have allowed for the facile creation of dynamic projected light patterns for the photofabrication of hydrogels57, and ongoing work is using this technology to pattern complex 3D shapes into pre-existing matrices.
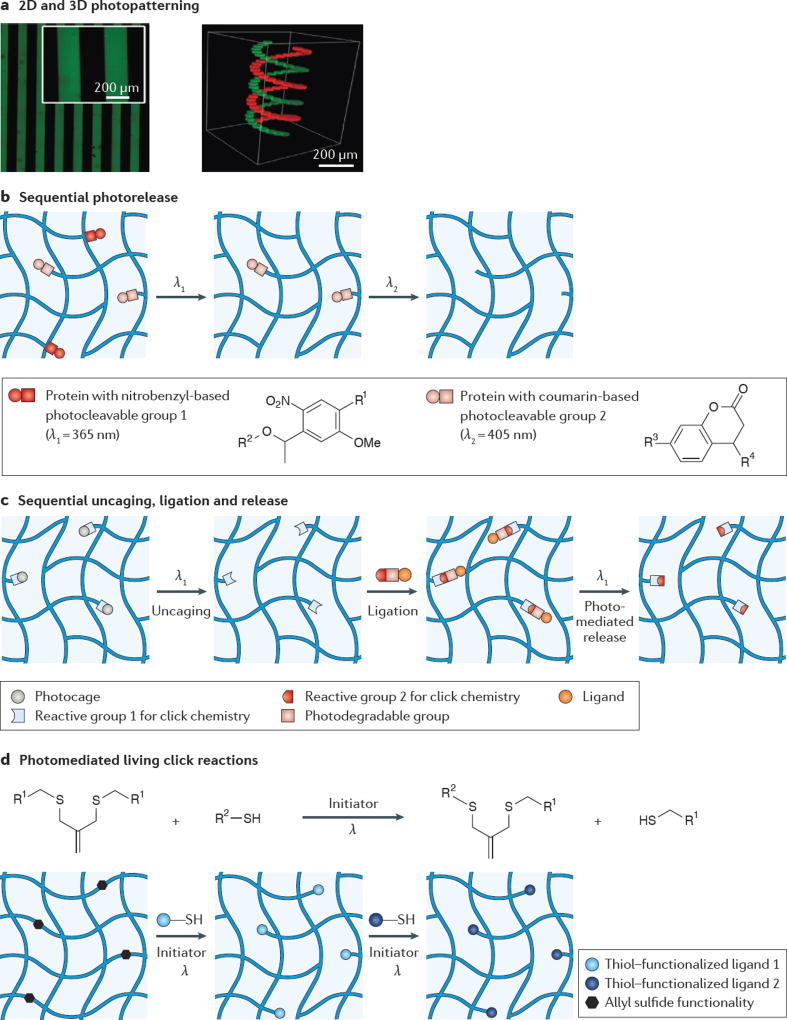
Figure 3. Light-based strategies for exchangeable ligand presentation.
a | Traditional lithographic masks are used for 2D photopatterning (left panel); however, 3D photopatterning (right panel) can access more complex shapes using a confocal laser scanning microscope. b | One strategy for the sequential release of biomolecules, such as proteins, relies on using photocleavable linkers with orthogonal absorption. For example, nitrobenzyl-based linkers cleave in response to 365 nm light and coumarin-based linkers cleave in response to 405 nm light. c | Another strategy for the reversible presentation of biomolecules first uncages a reactive group in the hydrogel, followed by ligation of the molecule (with a photocleavable linker) using click chemistry. The molecule can subsequently be removed using light, which cleaves the photodegradable linker. d | Living radical reactions, such as those mediated with an allyl sulfide functionality, provide opportunities for the reversible conjugation of biomolecules over many cycles. Panel a adapted with permission from REF. 48, Wiley-VCH. Panel b adapted with permission from REF 50, Wiley-VCH. Panel c from REF 49, Nature Publishing Group. Panel d adapted with permission from REF 54, Wiley-VCH.
Reversible light-based strategies have mainly focused on the incorporation of small molecules, such as peptides or dyes, into gels. In general, these small molecules are stable while undergoing photochemical procedures and are easy to manipulate because their rate of diffusion within a hydrogel is fast. In addition, several photosensitive functionalities allow for the selective choice of orthogonal wavelengths at which the attachment and release of different biomolecules can be carried out in sequence48,50. In one study50, both rhodamine B and fluorescein were conjugated to a hydrogel using click chemistry; however, the rhodamine B contained a nitrobenzyl-based linker, whereas the fluorescein contained a coumarin-based linker (FIG. 3b). As a consequence of the absorbance spectra of these molecules, wavelength-selective cleavage of the dyes was demonstrated: the rhodamine B was released when using ultraviolet (UV; 365 nm) light, and the fluorescein was released when using visible (405 nm) light. This strategy was also demonstrated to be successful with bone morphogenetic protein 2 (BMP2) and BMP7.
Photoreactions of larger molecules — for example, proteins — are often more difficult because they can denature or lose activity under certain conditions, and they have longer diffusion timescales in polymer matrices. Some recent approaches have focused on incorporating bioorthogonal chemistries into hydrogel matrices before swelling in a protein with a reactive group, allowing for highly specific, click-type reactions without any initiator present58. For example, one study combined photocaging and photodegradable chemistries (FIG. 3c) for the reversible patterning of vitronectin to modulate the osteogenesis of encapsulated mesenchymal stem cells49. In this study, a photocaged aldehyde was incorporated into the hydrogel, and uncaging revealed a reactive alkoxyamine on exposure to spatially defined irradiation. This alkoxyamine could subsequently react with an aldehyde linker attached to vitronectin to tether the protein into the gel. Furthermore, the vitronectin linker contained a photodegradable ortho-nitrobenzyl ester functional group that allowed for subsequent release on exposure to light. Although light-based chemistries allow for user-defined control, some proteins or biochemical ligands are less stable and may show a decrease in activity after photopatterning as a consequence of nonspecific incorporation or free radical damage from the photochemistry. One approach to circumvent this is to photoactivate an enzymatic peptide substrate in a gel and swell in the protein of interest with a counter-reactive substrate, allowing for an enzyme-catalysed reaction with the hydrogel that preserves spatiotemporal control but avoids exposing the protein to light59.
In the future, sequential light-mediated reactions could exploit the ability to introduce and remove different proteins using the same chemistry. To accomplish this goal, the progressive consumption of reactive sites in the hydrogel needs to be avoided, as this can restrict the number of cycles of ligand presentation and release. To address this issue, a photo-initiated, living strategy for the reversible exchange of ligands in a hydrogel has been developed54 (FIG. 3d). Using an allyl-sulfide-functionalized hydrogel, thiol-containing bioactive peptides could be photopatterned into the gel and removed and exchanged with another thiol-containing molecule, regenerating the allyl sulfide bond in the process. The double bond regeneration allows for numerous exchanges with additional ligands over many cycles. This strategy was shown to work in the presence of adhered cells using RGD, although it remains to be seen whether it is compatible with whole proteins and other ECM-stored growth factors.
A possible limitation of sequential reaction strategies is that the molecule of interest must be solubly introduced to the network before it is tethered, and it will be present in solution after it is released from the network. If the biomolecule is active as a soluble factor, its release can lead to unintended side effects during the process of conjugation itself. Also, if the molecule is of a high molecular weight, diffusion timescales can be unreasonably long for the introduction and removal of the signal during the patterning process. An interesting alternative approach could be the incorporation of a photoswitch that controls chain conformation, such as azobenzene60 or spiropyran61, to modulate ligand bioactivity. This would allow for initial immobilization, and cell signalling would occur only on demand. In these examples, the conformational changes either expose or hide the active site of the immobilized ligand on demand.
When using light-based strategies, care must be taken to ensure that there is no phototoxicity. Many photocleavable moieties respond to UV light, which can damage DNA at high intensities and doses. Thus, chemistries with high molar absorptivity at longer wavelengths are advantageous as they allow the use of lower light intensities and lower-energy light, thereby minimizing any negative cellular responses or damage to biologics. For many in vitro settings, long-wavelength UV (365 nm) intensities in the range of 1–10 mW cm−2 and doses of 1–10 minutes have been shown to be cyto-compatible62. These intensities have even been applied to in vivo experiments at short depths52. However, for the sequential strategies proposed here, repeated UV exposure may be a problem, and in vivo applications that require deeper tissue penetration would benefit from chemistries and materials that are designed to respond to visible or near-infrared (IR) light.
Non-covalent strategies
Similar to covalent conjugation, molecular recognition between biomolecules —protein domains63, aptamers64,65 or oligonucleotides66 — provides a diverse route to achieving dynamic ligand presentation with a high degree of specificity. For example, RGD-containing peptides containing a leucine zipper domain were bound to a surface to present cell adhesion domains (FIG. 4a). The bioactivity of the peptide was reversibly turned off by introducing a PEG chain with a complementary leucine zipper, followed by a soluble, competing peptide that could remove the PEG chain from the surface. In this study, PEG (which is not bioactive) shielded the RGD, which prevented it from binding to 3T3 fibroblasts, but the RGD peptide remained bound to the surface. The constant immobilization of the RGD prevents unintended side effects from soluble RGD in the media and allows for multiple cycles of screening with PEG. This strategy has been further explored to incorporate a near-IR phototrigger to release the PEG molecules, thereby increasing spatiotemporal control67. In another example, a hydrogel was engineered to reversibly expose cell adhesion sites by folding and unfolding in response to aptamer hybridization65 (FIG. 4b). In this study, complementary molecules that unblock or recover hybridization allow for modulation of the stability of two different structural conformations. Collectively, these approaches offer an advantage in immobilizing bioactive ligands to substrates for presenting both the active and inactive states. However, they still rely on the introduction of another molecule to switch the bioactivity of the ligand. This has yet to be demonstrated in 3D cell culture matrices, in which the timescale for diffusion and transport through the gel, as well as possible cellular uptake, would need to be considered.
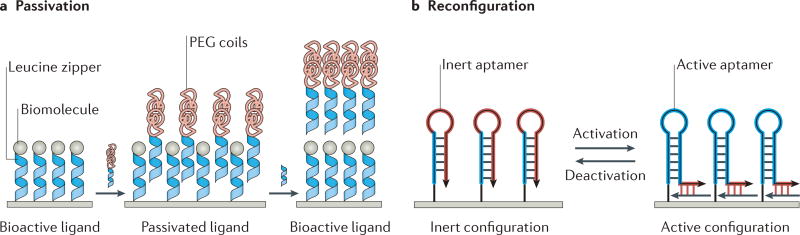
Figure 4. Non-covalent strategies for exchangeable ligand presentation.
a | Hybridization of leucine zippers allows for the passivation of immobilized biomolecules with poly(ethylene glycol) (PEG) coils. Subsequent removal of the PEG coils can be achieved using a competing leucine zipper. b | Similarly, aptamer hybridization can be utilized to reversibly expose or hide a bioactive site. Panel a adapted with permission from REF. 63, American Chemical Society. Panel b adapted with permission from REF. 65, Wiley-VCH.
Another advantage of non-covalent strategies for ligand presentation is that they allow for cell-mediated changes in the matrix to cluster the ligands68. Thus, even when the matrix is covalently bound, the bioactive ligands can still dynamically associate with cellular receptors. One such approach is host–guest chemistry, in which the ligand functions as a guest molecule to a host that is covalently bound to the matrix69–72. Another host–guest approach is to thread mobile host molecules onto linear guest polymer chains73. By using this strategy with mobile RGD-functionalized cyclodextrins and linear PEG chains, the initial adhesion of human umbilical vein endothelial cells (HUVECs) was found to occur faster on dynamic substrates than on those that had covalently bound RGD, although focal adhesion formation and spreading were suppressed73,74. As a consequence of the decreased focal adhesion and spreading, seeded mesenchymal stem cells showed less osteogenic differentiation on the dynamic substrates than on substrates that had covalently bound RGD75. These results could be of potential interest for manipulating 3D cell adhesion and matrix signalling, although these chemistries have yet to be incorporated into matrices with encapsulated cells.
Perhaps the biggest challenge to consider with the strategies detailed above is that most of them have only been demonstrated with short RGD peptides. The use of the RGD adhesive ligand is ubiquitous throughout the biomaterials community, in part because of the ease of its synthesis and its relatively low cost. RGD binds to many integrins and, as a result, its introduction is sufficient for facilitating the attachment and survival of many anchorage-dependent cell lines, primary cells and stem cells76. However, there is a large diversity of matrix proteins that cells interact with in the native matrix, such as fibronectin, laminin and fibrinogen77. Not only are these whole proteins more efficient at cellular binding78, but they are also more selective with respect to which integrins they bind to79. As the main link between the cell and its environment, integrins can initiate various intracellular pathways that can lead to diverse cell fate outcomes. Extension of these dynamic chemistries to full-length proteins should enable more direct examination of cell signalling events over time, but this approach may also require the use of site-specific modification of the protein with non-native chemical moieties80.
Reversible crosslinks for 3D hydrogels
Covalent hydrogels
As cell culture in hydrogels moved to the third dimension, one of the first challenges concerned cell spreading and morphology. When encapsulated within a static, covalently bound matrix, cells cannot spread, leading to a rounded morphology that alters function and fate81. In contrast to natural materials (for example, collagen gels) that contain bioactive motifs enabling local remodelling and cell spreading and a fibrillar architecture that can be deformed by cells, synthetic hydrogels are largely made from stable, bio-inert polymers such as PEG. Since the 1990s, the primary approach to facilitate spreading in 3D synthetic matrices has been to engineer mechanisms of degradation into the hydrogel. For example, proteolytically degradable hydrogels enable local degradation and spreading in a cell-mediated manner36,81. By using crosslinkers that degrade in response to secreted matrix metalloproteinases (MMPs), cells cleave the hydrogel network, allowing for spreading and migration in 3D geometries82–84. Although these types of gel have led to important advances in tissue-engineering applications, cell-mediated cleaving of the hydrogel translates to a change in the local mechanics in the gel and leads to bulk degradation over long culture times, which may not suit some applications.
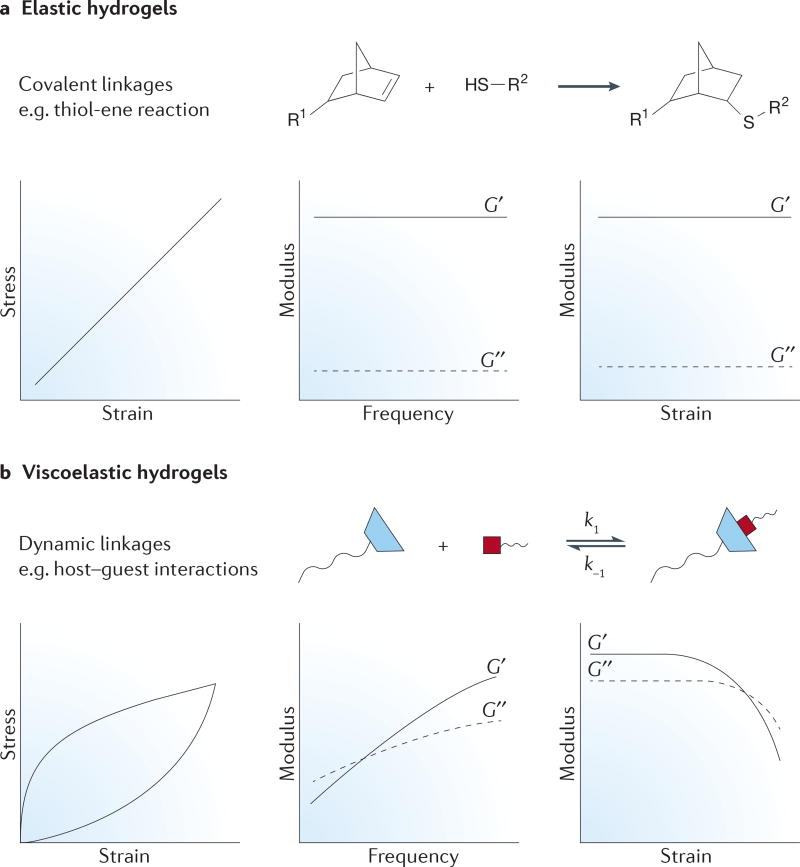
In addition to cell-mediated degradation, another aspect of the native matrix to consider is that cells often spread by locally clustering ECM fibres, proteins and other molecules near them38. In a homogeneous synthetic matrix with covalent bonds, it is difficult for a cell to effect this kind of remodelling. In general, covalent bonds constrain the mobility of flexible polymer chains in hydrogels, creating elastically active springs that lead to largely elastic material properties. This means that the gel strains linearly when a stress is applied (see the stress–strain graph in FIG. 5a) and returns to its original shape after small deformations. Thus, although a cell can exert tension on a covalently bound hydrogel, it cannot impart large rearrangements in structure. By contrast, the fibres present in the native ECM typically contain dynamic physical linkages that allow for cellular remodelling. This property allows the matrix to dissipate energy by rearranging its structure when a stress is applied, leading to a hysteresis in the bulk mechanics after stress removal (see the stress–strain graph in FIG. 5b). Recent progress in the development of reversible chemistries for hydrogels provides new opportunities to capture some of the unique material properties through dynamic covalent linkages, although most of these chemistries have been incorporated into non-fibrillar matrices until now.
Figure 5. Crosslinking effects on bulk hydrogel properties.
a | Elastic hydrogels typically use covalent linkages, such as thiol-ene chemistry. These linkages constrain polymer conformation in the hydrogel, leading to its behaviour as an elastically active spring. Hence, the material displays a linear stress–strain curve for small deformations (left panel). The modulus is frequency independent (middle panel) and strain independent (right panel). b | Conversely, dynamic linkages (such as the host–guest chemistry) lead to viscoelastic hydrogels because the bond is in equilibrium with its precursors, which imparts liquid-like behaviour to the gels. The stress–strain curve (left panel) shows hysteresis because these linkages can rearrange to accommodate stress. In addition, the modulus displays frequency dependence (middle panel), and many of these gels show shear-thinning behaviour (the decrease in modulus upon the application of high strain; right panel). G′, the storage modulus; G″, the loss modulus.
One related consideration of covalent hydrogels is their bulk mechanical properties. Typically, these properties are measured in terms of the modulus of the hydrogel; for example, the shear modulus describes the resistance of the material to shear deformation (FIG. 5) and can be related to the Young’s modulus via the Poisson ratio. For most hydrogels with covalent linkages (for example, the thiol-ene bonds shown in FIG. 5a), the storage (elastic component) modulus, G′, is much larger than the loss (viscous) modulus, G″. In addition, the shear modulus is independent of the frequency or strain applied to the gel over a large range, meaning that cells sense the same mechanical feedback through various interactions (for example, actomyosin contractility and focal adhesion formation) with the surrounding material environment. However, dynamic linkages exhibit quite different mechanics because the fluid nature of the bonds (for example, the guest–host non-covalent linkages in FIG. 5b) imparts much more viscoelastic behaviour to the hydrogel (FIG. 5b). For example, G″ makes a larger contribution to the overall modulus, and components of both moduli show frequency-dependent behaviour. In addition, upon application of high levels of strain, these bonds can break, allowing the gels to flow as a liquid (FIG. 5b). Recent work has begun to recognize the importance of viscoelasticity in cellular behaviour; for example, the actin cytoskeleton and focal adhesion formation are sensitive to both the elastic and the viscous properties of the surrounding matrix85,86. Dynamic, reversible crosslinking strategies may enable careful tuning of the viscoelastic properties of hydrogel matrices, and unique experiments that can unravel the individual contributions of these mechanical components to cellular mechanosensing87.
Covalent adaptable gels
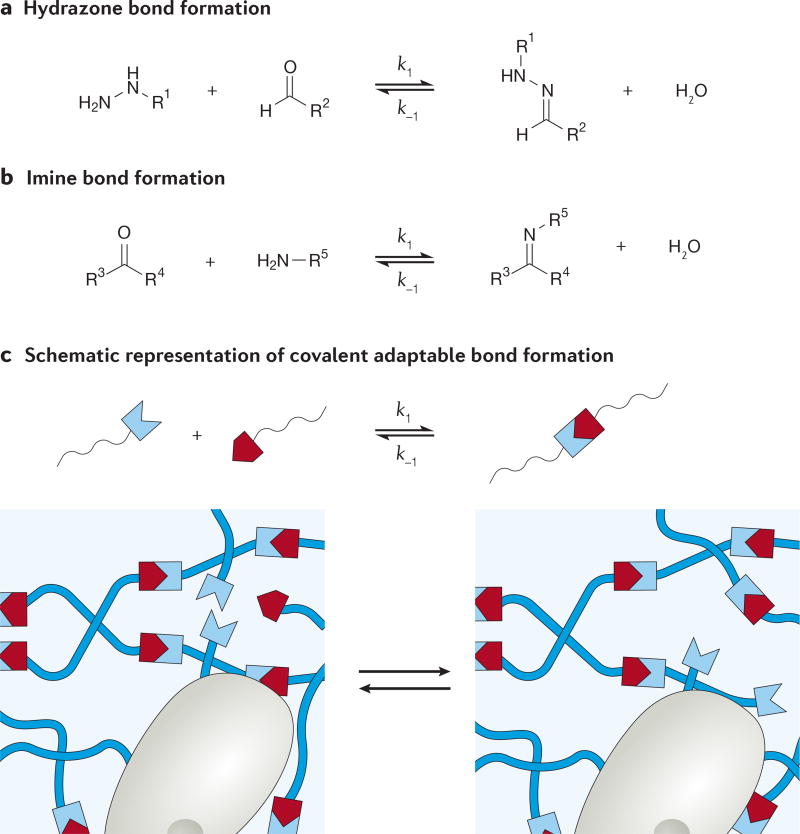
Covalent adaptable networks enable the reversible breaking and reformation of covalent bonds as a consequence of a dynamic equilibrium that maintains the total number of bonds88. The propensity to break and reform a bond is governed by the dynamic equilibrium constant (K), which translates to the permanence of elastically active crosslinks in the hydrogel. If K is low, the gel will be very permissive to changes in cell shape and motion but may have weak mechanics overall and provide minimal bulk support. Conversely, a high K may limit cell spreading and force the cells into a rounded morphology87. When considering covalent adaptable networks as cell culture matrices, the rates at which the bonds form and break (k1 and k−1, respectively; see FIG. 6) is also important, as these rates directly affect gelation and stress relaxation89. In response to an applied stress, such as a cell pulling on the matrix, local bonds can rearrange on a time-scale dictated by k1 and k−1, thereby allowing the cell to spread or move forward and the bulk properties of the gel to remain intact90. These properties lead to complex viscoelastic behaviour that is tunable with K, k1 and k−1, and, importantly, these dynamic materials allow for cell–matrix interactions together with the preservation of the bulk mechanics of the hydrogel.
Figure 6. Covalent adaptable networks.
Examples of covalent adaptable chemistries for crosslinking include hydrazone bonds (panel a) and imine bonds (panel b). Depicted schematically in panel c, these chemistries enable cells to push and pull on the matrix without degrading the polymer chains or crosslinkers.
Incorporation of covalent adaptable bonds into cell-laden hydrogels is relatively new. Hydrogels synthesized as a result of hydrazone bond formation55,90–94 (FIG. 6a) and imine bond formation95–97 (FIG. 6b) have shown cytocompatibility and maintenance of cell function during cellular encapsulation studies. In hydrazone gels functionalized with RGD, encapsulated C2C12 myoblasts were found to extend processes after as little as 24 hours and up to 10 days, depending on the kinetics of bond breakage and formation90. In another example, hydrazone gels mixed with collagen formed more robust cardiac tissue from encapsulated cardiomyocytes compared with collagen gels alone; there was less shrinkage of the tissue and greater contraction forces, which may be because of a combined effect of the signalling from collagen and the ability to maintain a robust gel during tissue deposition with reversible covalent bonds93. Studies that involve the use of biocompatible covalent adaptable networks are still ongoing, and it will be interesting to observe mechanisms of cell spreading and migration as these are completed.
Non-covalent associating networks
Non-covalent associating networks refer to matrices that are bound by linkages such as calcium coordination86,98,175, host–guest interactions99–101, hydrogen bonding102 or physical interactions (for example, those found in proteins103–106). Similar to the covalent adaptable bonds, these crosslinks are transient, leading to viscoelastic behaviour and stress-relaxing properties. Natural materials, such as alginate, exhibit some of these interactions and, as a result, there are many examples of non-covalently linked gels, although recognition of their reversible properties and viscoelastic effects has only recently emerged85,86.
When considering non-covalent networks for cell culture, many of the design rules for covalent adaptable networks apply. Calcium-crosslinked alginate hydrogels provide an attractive cell culture platform because not only can cells remodel their environment as a consequence of their dynamic ionic crosslinks, but a high calcium-binding strength also allows the gels to achieve a larger range of moduli than traditional systems such as collagen or Matrigel107. In alginate gels ranging from 2.5 to 110 kPa (as measured by unconfined compression tests), differentiation of human mesenchymal stem cells varied with the modulus, although the cells showed similar levels of low spreading across all moduli68. Specifically, cells adopted an adipogenic phenotype in soft gels (2.5–5 kPa) and an osteogenic phenotype in gels of intermediate stiffness (11–30 kPa). Interestingly, the gels at the intermediate stiffness provided the right amount of resistance for the cells to exert traction and remodel their surroundings by clustering RGD ligands for integrin binding and differentiation. Previous work on 2D platforms has shown that differentiation and cellular morphology are closely linked108,109 and, usually, cell spreading is correlated with osteogenic lineages because greater spreading occurs on stiffer substrates. However, these ionically crosslinked alginate gels provided a 3D platform to show alternative mechanisms of differentiation separate from spreading.
When using biologically inspired materials, additional control over K can be achieved by exploiting the physical interactions of biorecognition. The physical interactions typically refer to binding domains of proteins or peptides103,110–112,176, although oligonucleotides or biomimetics can also be used113. These physical interactions lead to some of the most compliant hydrogels, which correlate well with the elasticity of brain tissue and make some of these gels suitable for investigating neurite outgrowth in vitro103,114. For example, molecular recognition was used to form soft gels from proteins with repeating domains of tryptophan- and proline-rich sequences. Encapsulated adult neural stem cells were able to differentiate in these gels and, furthermore, long axon extensions (more than 100 µm) were observed after just 6 days103. Neural tissue is one of the softest tissues in the body, and further engineering of these protein-based hydrogels to access a larger range of moduli should enable their use in a broader range of applications104.
One distinguishing feature of both the covalent adaptable hydrogels and the non-covalent hydrogels is that the transient nature of the crosslinks leads to shear-thinning behaviour, which makes these materials attractive as injectable cell delivery vehicles96,111,115–117. In these materials, fast gelation kinetics are first desired for homogeneous encapsulation117. Then, under shear stress, the material exhibits liquid-like behaviour such that the cells can be locally delivered to a site of interest. Post-injection, the gel recovers its mechanical properties without an external stimulus, enabling stable delivery of cells or growth factors without leakage to the surrounding tissue. These attributes lend an advantage to reversible hydrogels over covalently bound hydrogels, which must be introduced as liquids and polymerized in situ. Depending on the end application, long-term stabilization of the hydrogel may be desired; therefore, various approaches to form secondary crosslinks have been pursued to slow erosion of the injected gel118,119. These strategies also ameliorate potential long-term cytotoxic effects of the polymer precursors, including those of certain cationic polymers120.
Until now, reversibly crosslinked gels have shown particularly useful features for applications focused on facilitating deposition of matrix molecules by encapsulated cells (for example, cartilage tissue engineering) or those that require injectable delivery platforms. However, there are still several considerations to take into account. First, there is a lack of understanding regarding the timescale of cellular processes (such as traction generation) relative to the dynamics of the material crosslinks (such as stress relaxation). Mismatch between these factors can lead to a non-supportive gel or a gel that essentially behaves as elastic. Second, the amount of cellular spreading observed in these types of gel can be very different depending on the cell type: for example, neurons show extensive axon extension91,114, but the spreading of mesenchymal stem cells is limited68. Potential reasons for this include the nature of cellular extensions (whether the cells are pushing or pulling on the matrix), and the architecture and connectivity of the hydrogel itself. As mentioned above, the dynamic crosslinking chemistries have largely been incorporated into amorphous, non-fibrillar gels, but a recent study shows that electrospun fibrillar networks121 may integrate well with these types of dynamic chemistry and better recapitulate both the viscoelastic and structural aspects of the native ECM.
Cyclical changes in matrix mechanics
Considerations for dynamic moduli
Similar to biochemical cues, the ECM provides physical cues to resident cells that affect cell behaviour through mechanotransduction. The cell has various mechanisms to remodel the surrounding matrix, such as MMPs that can cleave the network, tissue inhibitors of metalloproteinases that inhibit MMP activity and secretory processes to generate new matrix proteins. The dynamic mechanical properties of the ECM are particularly important during cases of development and disease as the fluctuations in rigidity affect and dictate cell behaviour over time. In fact, in vitro studies have provided new insights into how both the magnitude and the duration of the physical cue affect cell fate122,123. For example, human mesenchymal stem cells cultured on a stiff substrate (~10 kPa, as measured by oscillatory shear rheology) for 10 days showed irreversible nuclear localization of the transcriptional co-activator Yes-associated protein (YAP) and biased the stem cells towards osteogenic differentiation122. However, dynamic softening of the matrix to a more compliant modulus (~2 kPa), before 10 days, led to relatively more adipogenesis. It is evident that, similar to biochemical cues, mechanical cues can be associated with a dose, but their dose effects have been difficult to isolate both in vitro and in vivo because changing matrix mechanics traditionally required replating cells from one substrate to another or altering in vivo collagen crosslinking124, which can lead to confounding effects on cellular behaviour. However, in the past 5 years, great progress has been made in the design of hydrogels with in situ changes in mechanics. By engineering reactive sites into hydrogel crosslinkers, matrix stiffening125,126 and softening37,127 have been demonstrated in the presence of cells, although these first-generation chemistries were one directional and inevitably altered the chemical composition of the gel. More recent approaches include the use of mechanically reversible substrates, which has enabled the investigation of cyclical changes in mechanics for applications related to stem cell differentiation, fibrosis and cancer.
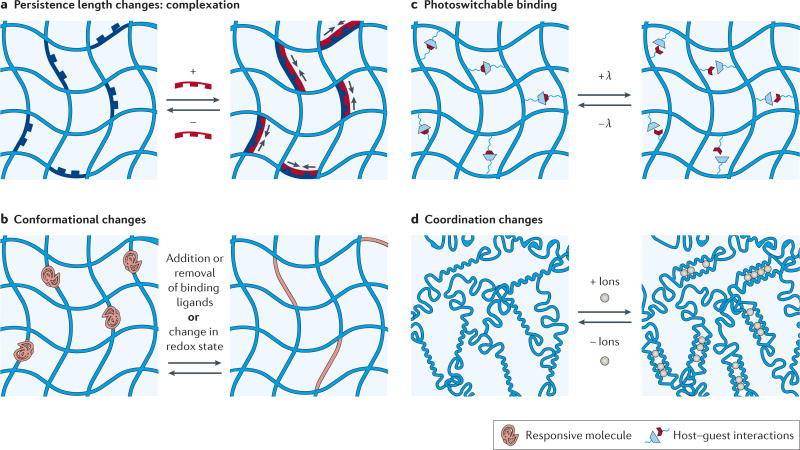
In the design of hydrogels with a reversibly tunable modulus, it is helpful to re-examine first principles in polymer physics. Classic models of hydrogel mechanics, such as rubber elasticity theory, show a direct relationship between modulus and crosslinking density and, as mentioned above, most work thus far has altered crosslinking density via chemical reactions that generate or remove crosslinks. Delving a bit deeper shows that for semi-flexible networks, the crosslinking density also depends on the contour length of the chain and, perhaps less often considered, the persistence length, which is a measure of the rigidity of the chain itself. Recent approaches for controlling hydrogel mechanics include using conformational changes to control the persistence length and contour length of the crosslinker, and we highlight some of these approaches here, as well as those that alter the number of crosslinks (FIG. 7). It should be noted that much inspiration in the field has come from mechanically dynamic polymers for sensing applications128–130.
Figure 7. Reversible control of matrix mechanics.
a | Hydrogel stiffening can be achieved by increasing the persistence length of the crosslinker using complexation (for example, with DNA hybridization), and subsequent softening can be achieved by removing the hybridizing molecule with a competing strand. b | Conformational changes in hydrogel crosslinkers, such as protein folding and unfolding in response to binding ligands or environmental changes (for example, redox state), can also be used to alter the crosslinking density (and therefore the modulus) over many cycles. c | The number of elastically active crosslinks can be modulated using photoreversible host–guest chemistry. d | Reversible crosslinking between chains can also be modulated with the introduction or removal of ion coordination (for example, calcium ions in alginate networks).
Conformational changes of biomolecules
Many biomolecules, such as proteins and DNA, undergo conformational changes upon binding or hybridization events. Some can even completely unfold, yielding a large change in the molecular length of the biomolecule. When incorporated as crosslinkers in a hydrogel, these variations in conformation lead to changes in the crosslinking density and therefore changes in the swelling and modulus of the hydrogel. One distinction between this strategy and other stiffening or softening strategies37,126 is that the length of the crosslinker is changing, whereas there is no change in the network connectivity of the hydrogel. This strategy may better preserve the compositional homogeneity of the network, leading to fewer confounding effects on the behaviour of adhered cells.
DNA is an attractive choice for reversible crosslinkers because its hybridization leads to both a length change and a relatively large change in persistence length (from 1 to 50 nm)131 (FIG. 7a). In one design, complementary strands of DNA were introduced to hybridize with DNA crosslinkers of poly(acrylamide) gels, leading to either complexation (stiffening) or removal (softening) that corresponded to a threefold change in modulus131,132. However, for studies of the effect of dynamic stiffness on cell behaviour, the hydrogel was designed to undergo changes in the number of crosslinks per chain upon hybridization, leading to larger stiffness changes ranging from ~6 to ~23 kPa. Seeded L929 fibroblasts showed an increase in aspect ratio as the gel stiffened, but these cells did not respond to exogenous DNA128. Also, neurites demonstrated changes in their outgrowth on these dynamic gels compared with static gels and had fewer focal adhesions when these gels were softened in situ129. These preliminary studies are promising in demonstrating how an in situ stiffness cue can elicit a cellular response, and ongoing work continues to refine and improve on the design of biomaterials. For example, reversible hybridization of DNA crosslinkers can be used to stiffen and soften a gel, but instead of adding or removing the complementary DNA, the DNA can be immobilized to the gel and its ability to hybridize can be inhibited by using an azobenzene molecule to control chain conformation130. Although immobilization can compromise the dynamic range of hydrogel properties (as a consequence of the constrained motion and inefficiencies in hybridization), this design avoids the addition of soluble DNA to cells.
Many proteins show reversible changes in conformation upon binding or exposure to environmental stimuli133 (FIG. 7b). Calmodulin, with changes of approximately 4 nm upon binding to phenothiazines134 or to trifluoperazines135, has been incorporated into PEG hydrogels and has shown changes in volume of up to 90%. The enzyme adenylate kinase, which collapses by approximately 1.7 nm upon binding to ATP, has also recently been incorporated into hydrogels, and this leads to a decrease in swelling of up to 15% in the gel136. In addition, changes in the oxidation state of the environment can alter protein conformation; hence, when these redox-responsive proteins are used as crosslinkers, they can endow hydrogels with tunable mechanical properties137,138. Specifically, using either dithiothreitol as a reducing agent or hydrogen peroxide as an oxidizing agent, a mutually exclusive protein was designed that completely folded and unfolded such that changes in the crosslinker length of up to 18 nm were exhibited138. This extensive length change allows for a dynamic range of elastic moduli from ~10 to ~50 kPa (as measured using tensile testing); however, it remains to be shown that this strategy is fully cytocompatible and that the changes in redox potential have no detrimental effects on cell behaviour.
Conformational changes in synthetic molecules
DNA and proteins offer highly specific responsiveness to other molecules; however, it can be costly to incorporate them as crosslinkers in hydrogels. The use of fully synthetic molecules or readily available polymers provides a more cost-efficient solution and avoids confounding effects from biomolecules on cell behaviour. For example, azobenzenes are reversible synthetic photoswitches that can alter hydrogel moduli with orthogonal, cytocompatible wavelengths of light139, or they can reversibly associate in host–guest complexes to modulate the number of crosslinks in a hydrogel140 (FIG. 7c). In another example, collagen and alginate polymers have been used to create a two-component hydrogel in which the collagen provides structural support and the alginate reversibly gels141. This design allows for stiffening upon introduction of calcium ions to crosslink the alginate and softening when the calcium ions are removed (FIG. 7d). Interestingly, the spreading and migration of encapsulated fibroblasts were restricted but not fully disrupted when the alginate was crosslinked, such that un-crosslinking allowed for movement and re-crosslinking stopped movement but did not revert the cells to a rounded morphology141. In another alginate-based 3D cell culture platform, near-IR-responsive liposomes have been incorporated into the hydrogel such that calcium or calcium chelators are released, thereby enabling spatiotemporal control of the crosslinking density142. In these gels, the spreading of 3T3 fibroblasts was inhibited as the stiffness was dynamically increased, and the cells adopted a rounded morphology.
Looking forward
As the dynamic complexity of hydrogels continues to grow, experimental control of their properties is beginning to recapitulate an increasing number of the biochemical and biophysical features of the native ECM. For example, many biological tissues are viscoelastic, and owing to the dynamic equilibrium inherent to the reversible chemistries described here, many reversible hydrogels also have viscoelastic properties. When these chemistries are used to tether ligands to the hydrogel, they are mobile, which can allow clustering or local sequestration to signal to embedded cells. In addition to allowing for more physiologically relevant in vitro culture models, many reversible hydrogels offer several advantages over their one-directional predecessors. These advantages include the avoidance of degradation products during cell spreading or matrix softening, and preventing the release of biochemical ligands into the media to further control the local bioactivity of immobilized factors. Although these properties will surely be useful for in vitro models, reversible hydrogels also offer much promise as instructive matrices to control cell fate.
Increased temporal control over cellular cues will be essential to the future of tissue engineering. Dynamic, reversible chemistries are uniquely situated for several reasons. The reversible crosslinks can break and reform but, at the same time, maintain the bulk properties of the hydrogel — a quality that is amenable to tissue deposition, such as during cartilage regeneration. This technology will enable a scaffold of high modulus to provide an appropriate physical environment for chondrocytes while also allowing space for the deposited matrix. In addition, their shear-thinning properties lend themselves to many applications in which injection is required: catheter delivery of cellular constructs, bioprinting or microfluidic platforms. Microfluidics has been used to create microgels of defined shape143–147, and shear-thinning hydrogels are particularly appealing for use in these devices because they simply reform after cessation of shear stress. With regard to the reversible exchange of biochemical ligands, these dynamic chemistries are especially important for assays that require the capture and release of cells148–150. Many existing substrates are degraded on release, but reversible technologies will enable these platforms to be used over multiple cycles, thereby enhancing their efficiency and cost-effectiveness for cellular151–154 and protein155 screening. Finally, hydrogels with reversible changes in mechanics are needed to study how cells process stiffness signals over time, especially cells involved in fibrotic disease156 as well as stem cells122,126. These dynamic chemistries will undoubtedly contribute to the repertoire of on-demand signals that can be used to recreate the stem cell niche in vitro, thereby making progress towards bridging the gap between in vitro models and in vivo studies.
Looking forward, there are several opportunities for further development to ensure the realization of the full potential of reversibly dynamic hydrogels. Perhaps the first challenge — and advantage — related to reversible hydrogels is the fact that these systems access a wide experimental space and therefore lend themselves to more detailed studies in which in situ characterization methods prove extremely useful. For example, the ability to reversibly exchange a biochemical ligand or to control its activity in the presence of adhered cells calls for ways to monitor cellular behaviour in real time. Similarly, cyclical changes in matrix stiffening highlight the need to continuously probe mechanisms of mechanotransduction. Biologists have long developed various fluorescent probes and reporter systems to monitor intracellular protein production and cytoskeletal organization157, and emerging technologies will enable expression of these probes under highly specific conditions, as well as allow for observation of multiple targets with increasingly high resolution (that is, single-molecule resolution)158. As for intracellular mechanics, microparticle tracking rheology has provided a way to measure cytoplasm mechanics in 3D matrices157. Furthermore, traction force microscopy has developed into a vastly useful tool for visualizing the traction stresses of live cells in both 2D and 3D matrices, although the cells must be sacrificed at the end of the experiment159. In addition to monitoring the cell, the field will also need better ways to measure changes to the matrix in real time. Culture in adaptable networks allows for cells to remodel their surrounding matrix, either by pushing matrix components away to migrate or by clustering matrix components for adhesion. Again, fluorescent labelling of certain matrix components will enable visualization of structural remodelling86. However, advanced techniques to quantify local mechanics will be crucial for understanding how cells interact with these dynamic matrices26. In addition to microrheological techniques160,161, force sensors that are based on fluorescence resonance energy transfer will allow quantitative measures of local moduli in hydrogels162,163. Perhaps less invasively (at least not requiring beads), magnetic resonance elastography can measure the local shear modulus and produce maps of tissue or material elasticity164,165, and it has even been used to progressively monitor the modulus of tissue constructs grown in vitro and implanted into animal models166. Although a nuclear magnetic resonance spectrometer is not readily available in most laboratories, magnetic resonance elastography is ideal because it is completely non-invasive and can be used for both in vitro and in vivo studies.
Another opportunity for the development of reversible hydrogels is to design chemistries with the time-scale of cellular processes in mind. Cells behave on timescales ranging from seconds to weeks, and future considerations need to select the appropriate reversible chemistry to influence the cellular function of choice. Moreover, multiple chemistries could be incorporated: one with a short timescale to allow local cell spreading and movement, and one with a long timescale to allow for the signalling of growth factors over several days.
Finally, there is an opportunity to borrow themes from high-throughput methods or additive manufacturing to inform the design of more intelligent tissue-engineering scaffolds. High-throughput combinatorial methods will enable the rapid investigation of multiple dynamic and temporal experimental conditions — more specifically, various time points for doses of exchangeable ligands or matrix mechanics, and their combined doses, are possible to study simultaneously167. High-content imaging techniques also allow for rapid data acquisition of these conditions in a simultaneous manner168,169. To handle the large accumulation of data, design of experimental methods167 and multivariate statistics for data analysis170 will be essential for extracting significant results and identifying synergistic effects between multiple cues. The challenge is often reading the signal of these experiments against the background noise of the biological system. This information, over a large experimental space and in real time, will enable a systems-level approach to biomaterials research using quantitative 4D analyses of 4D biology. In a similar vein, gathering inspiration from additive manufacturing or 3D printing technologies will allow for the generation of hierarchically complex constructs that are capable of providing dynamic cues to resident cells. Recently, considerable progress has been made in the 3D printing of hydrogels171,172, and these strategies can be further combined with templates that are provided by biology173 and rendered digitally174 to create materials that recapitulate properties of the native ECM for advanced tissue-engineering applications.
Acknowledgments
K.S.A. acknowledges support from the Howard Hughes Medical Institute and grants from the National Science Foundation (DMR 1408955) and the National Institutes of Health (R01 DE016523). A.M.R. gratefully acknowledges a postdoctoral fellowship from the National Heart, Lung, and Blood Institute of the US National Institutes of Health (Award Number F32HL121986) and a Postdoctoral Enrichment Program Award from the Burroughs Wellcome Fund.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004;36:1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Vlodavsky I, et al. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc. Natl Acad. Sci. USA. 1987;84:2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wipff P-J, Rifkin DB, Meister J-J, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 6.Blau H, et al. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 9.Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Acc. Chem. Res. 2010;43:419–428. doi: 10.1021/ar900226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 11.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 12.Nowak AP, et al. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417:424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 13.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 2006;18:1345–1360. [Google Scholar]
- 14.Buwalda SJ, et al. Hydrogels in a historical perspective: from simple networks to smart materials. J. Control. Release. 2014;190:254–273. doi: 10.1016/j.jconrel.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y-L, Pelham RJ., Jr . In: Methods in Enzymology. Richard BV, editor. Academic Press; 1998. pp. 489–496. [DOI] [PubMed] [Google Scholar]
- 17.Pelham RJ, Jr, Wang Y-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat. Nano. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010;35:278–301. [Google Scholar]
- 20.Zhang J, Peppas NA. Synthesis and characterization of pH- and temperature-sensitive poly(methacrylic acid)/ poly(N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules. 2000;33:102–107. [Google Scholar]
- 21.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman AS. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J. Control. Release. 1987;6:297–305. [PubMed] [Google Scholar]
- 23.Cole MA, Voelcker NH, Thissen H, Griesser HJ. Stimuli-responsive interfaces and systems for the control of protein–surface and cell–surface interactions. Biomaterials. 2009;30:1827–1850. doi: 10.1016/j.biomaterials.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Yeo W-S, Yousaf MN, Mrksich M. Dynamic interfaces between cells and surfaces: electroactive substrates that sequentially release and attach cells. J. Am. Chem. Soc. 2003;125:14994–14995. doi: 10.1021/ja038265b. [DOI] [PubMed] [Google Scholar]
- 25.Zrínyi M. Intelligent polymer gels controlled by magnetic fields. Colloid Polym. Sci. 2000;278:98–103. [Google Scholar]
- 26.Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010;22:3484–3494. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata T, Uragami T, Nakamae K. Biomolecule-sensitive hydrogels. Adv. Drug Delivery Rev. 2002;54:79–98. doi: 10.1016/s0169-409x(01)00241-1. [DOI] [PubMed] [Google Scholar]
- 28.Miyata T, Asami N, Uragami T. A reversibly antigen-responsive hydrogel. Nature. 1999;399:766–769. doi: 10.1038/21619. [DOI] [PubMed] [Google Scholar]
- 29.Miyata T, Jikihara A, Nakamae K, Hoffman AS. Preparation of reversibly glucose-responsive hydrogels by covalent immobilization of lectin in polymer networks having pendant glucose. J. Biomater. Sci. Polym. Ed. 2004;15:1085–1098. doi: 10.1163/1568562041753061. [DOI] [PubMed] [Google Scholar]
- 30.Hassan CM, Doyle FJ, Peppas NA. Dynamic behavior of glucose-responsive poly(methacrylic acid-g-ethylene glycol) hydrogels. Macromolecules. 1997;30:6166–6173. [Google Scholar]
- 31.Kost J, Langer R. Responsive polymeric delivery systems. Adv. Drug Delivery Rev. 2012;64(Suppl):327–341. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 32.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J. Biomed. Mater. Res. Part A. 2003;64A:70–79. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 33.Zustiak SP, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348–1357. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41:3993–4004. [Google Scholar]
- 35.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutolf MP, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker BM, Chen CS. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hynes RO. Extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 41.Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 2006;25:332–341. doi: 10.1016/j.matbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc. Natl Acad. Sci. USA. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldwin AD, Kiick KL. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers. 2010;94:128–140. doi: 10.1002/bip.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin C-C, Anseth KS. Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv. Funct. Mater. 2010;19:2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCall JD, Lin C-C, Anseth KS. Affinity peptides protect transforming growth factor β during encapsulation in poly(ethylene glycol) hydrogels. Biomacromolecules. 2011;12:1051–1057. doi: 10.1021/bm101379v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azagarsamy MA, Anseth KS. Bioorthogonal click chemistry: an indispensable tool to create multifaceted cell culture scaffolds. ACS macro Lett. 2013;2:5–9. doi: 10.1021/mz300585q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimmo CM, Shoichet MS. Regenerative biomaterials that “click”: simple, aqueous-based protocols for hydrogel synthesis, surface immobilization, and 3D patterning. Bioconjugate Chem. 2011;22:2199–2209. doi: 10.1021/bc200281k. [DOI] [PubMed] [Google Scholar]
- 48.DeForest CA, Anseth KS. Photoreversible patterning of biomolecules within click-based hydrogels. Angew. Chem. Int. Ed. Engl. 2012;51:1816–1819. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 50.Azagarsamy MA, Anseth KS. Wavelength-controlled photocleavage for the orthogonal and sequential release of multiple proteins. Angew. Chem. Int. Ed. Engl. 2013;52:13803–13807. doi: 10.1002/anie.201308174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sur S, Matson JB, Webber MJ, Newcomb CJ, Stupp SI. Photodynamic control of bioactivity in a nanofiber matrix. ACS Nano. 2012;6:10776–10785. doi: 10.1021/nn304101x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee TT, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 2015;14:352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen S, et al. Phototriggering of cell adhesion by caged cyclic RGD peptides. Angew. Chem. Int. Ed. Engl. 2008;47:3192–3195. doi: 10.1002/anie.200704857. [DOI] [PubMed] [Google Scholar]
- 54.Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Adv. Mater. 2014;26:2521–2526. doi: 10.1002/adma.201304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts MC, Hanson MC, Massey AP, Karren EA, Kiser PF. Dynamically restructuring hydrogel networks formed with reversible covalent crosslinks. Adv. Mater. 2007;19:2503–2507. [Google Scholar]
- 56.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv. Mater. 2006;18:2679–2684. [Google Scholar]
- 57.Soman P, Chung PH, Zhang AP, Chen S. Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol. Bioeng. 2013;110:3038–3047. doi: 10.1002/bit.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wylie RG, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 59.Mosiewicz KA, et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013;12:1072–1078. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- 60.Auernheimer J, Dahmen C, Hersel U, Bausch A, Kessler H. Photoswitched cell adhesion on surfaces with RGD peptides. J. Am. Chem. Soc. 2005;127:16107–16110. doi: 10.1021/ja053648q. [DOI] [PubMed] [Google Scholar]
- 61.Li W, et al. Noninvasive and reversible cell adhesion and detachment via single-wavelength near-infrared laser mediated photoisomerization. J. Am. Chem. Soc. 2015;137:8199–8205. doi: 10.1021/jacs.5b03872. [DOI] [PubMed] [Google Scholar]
- 62.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 63.Liu B, Liu Y, Riesberg JJ, Shen W. Dynamic presentation of immobilized ligands regulated through biomolecular recognition. J. Am. Chem. Soc. 2010;132:13630–13632. doi: 10.1021/ja1054669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Chen N, Li S, Battig MR, Wang Y. Programmable hydrogels for controlled cell catch and release using hybridized aptamers and complementary sequences. J. Am. Chem. Soc. 2012;134:15716–15719. doi: 10.1021/ja307717w. [DOI] [PubMed] [Google Scholar]
- 65.Li S, Gaddes ER, Chen N, Wang Y. Molecular encryption and reconfiguration for remodeling of dynamic hydrogels. Angew. Chem. Int. Ed. Engl. 2015;54:5957–5961. doi: 10.1002/anie.201500397. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Li S, Chen N, Yang C, Wang Y. Programmable display of DNA–protein chimeras for controlling cell–hydrogel interactions via reversible intermolecular hybridization. Biomacromolecules. 2013;14:1174–1180. doi: 10.1021/bm400096z. [DOI] [PubMed] [Google Scholar]
- 67.Yang J, et al. A near-infrared light-controlled system for reversible presentation of bioactive ligands using polypeptide-engineered functionalized gold nanorods. Chem. Commun. 2015;51:2569–2572. doi: 10.1039/c4cc09516b. [DOI] [PubMed] [Google Scholar]
- 68.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boekhoven J, Rubert Pérez CM, Sur S, Worthy A, Stupp SI. Dynamic display of bioactivity through host-guest chemistry. Angew. Chem. Int. Ed. Engl. 2013;52:12077–12080. doi: 10.1002/anie.201306278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neirynck P, et al. Carborane-β-cyclodextrin complexes as a supramolecular connector for bioactive surfaces. J. Mater. Chem. B. 2015;3:539–545. doi: 10.1039/c4tb01489h. [DOI] [PubMed] [Google Scholar]
- 71.Cabanas-Danés J, et al. A supramolecular host-guest carrier system for growth factors employing VHH fragments. J. Am. Chem. Soc. 2014;136:12675–12681. doi: 10.1021/ja505695w. [DOI] [PubMed] [Google Scholar]
- 72.Brinkmann J, et al. About supramolecular systems for dynamically probing cells. Chem. Soc. Rev. 2014;43:4449–4469. doi: 10.1039/c4cs00034j. [DOI] [PubMed] [Google Scholar]
- 73.Seo J-H, et al. Inducing rapid cellular response on RGD-binding threaded macromolecular surfaces. J. Am. Chem. Soc. 2013;135:5513–5516. doi: 10.1021/ja400817q. [DOI] [PubMed] [Google Scholar]
- 74.Kakinoki S, et al. Mobility of the Arg–Gly–Asp ligand on the outermost surface of biomaterials suppresses integrin-mediated mechanotransduction and subsequent cell functions. Acta Biomater. 2015;13:42–51. doi: 10.1016/j.actbio.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Seo J-H, Kakinoki S, Yamaoka T, Yui N. Directing stem cell differentiation by changing the molecular mobility of supramolecular surfaces. Adv. Healthcare Mater. 2015;4:215–222. doi: 10.1002/adhm.201400173. [DOI] [PubMed] [Google Scholar]
- 76.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 77.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 78.Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J. Biol. Chem. 1989;264:1437–1442. [PubMed] [Google Scholar]
- 79.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 80.Foley TL, Burkart MD. Site-specific protein modification: advances and applications. Curr. Opin. Chem. Biol. 2007;11:12–19. doi: 10.1016/j.cbpa.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 81.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 82.Kraehenbuehl TP, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 83.Kyburz KA, Anseth KS. Three-dimensional hMSC motility within peptide-functionalized PEG-based hydrogels of varying adhesivity and crosslinking density. Acta Biomater. 2013;9:6381–6392. doi: 10.1016/j.actbio.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 85.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 86.Chaudhuri O, et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, Heilshorn SC. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015;27:3717–3736. doi: 10.1002/adma.201501558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bowman CN, Kloxin CJ. Covalent adaptable networks: reversible bond structures incorporated in polymer networks. Angew. Chem. Int. Ed. Engl. 2012;51:4272–4274. doi: 10.1002/anie.201200708. [DOI] [PubMed] [Google Scholar]
- 89.McKinnon DD, Domaille DW, Cha JN, Anseth KS. Bis-aliphatic hydrazone-linked hydrogels form most rapidly at physiological pH: identifying the origin of hydrogel properties with small molecule kinetic studies. Chem. Mater. 2014;26:2382–2387. [Google Scholar]
- 90.McKinnon DD, Domaille DW, Cha JN, Anseth KS. Hydrogels: biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 2014;26:865–872. doi: 10.1002/adma.201303680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKinnon DD, et al. Measuring cellular forces using bis-aliphatic hydrazone crosslinked stress-relaxing hydrogels. Soft Matter. 2014;10:9230–9236. doi: 10.1039/c4sm01365d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan S, et al. Injectable in situ self-cross-linking hydrogels based on poly(l-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules. 2014;15:4495–4508. doi: 10.1021/bm501313t. [DOI] [PubMed] [Google Scholar]
- 93.Dahlmann J, et al. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials. 2013;34:940–951. doi: 10.1016/j.biomaterials.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30:6076–6085. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang B, et al. Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier. Polym. Chem. 2012;3:3235–3238. [Google Scholar]
- 96.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weng L, Romanov A, Rooney J, Chen W. Non-cytotoxic, in situ gelable hydrogels composed of N-carboxyethyl chitosan and oxidized dextran. Biomaterials. 2008;29:3905–3913. doi: 10.1016/j.biomaterials.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X, Huebsch N, Mooney DJ, Suo Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys. 2010;107:063509. doi: 10.1063/1.3343265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodell CB, Wade RJ, Purcell BP, Dusaj NN, Burdick JA. Selective proteolytic degradation of guest-host assembled, injectable hyaluronic acid hydrogels. ACS biomater. Sci. Eng. 2015;1:277–286. doi: 10.1021/ab5001673. [DOI] [PubMed] [Google Scholar]
- 100.Liao X, Chen G, Jiang M. Hydrogels locked by molecular recognition aiming at responsiveness and functionality. Polym. Chem. 2013;4:1733–1745. [Google Scholar]
- 101.Park KM, et al. In situ supramolecular assembly and modular modification of hyaluronic acid hydrogels for 3D cellular engineering. ACS Nano. 2012;6:2960–2968. doi: 10.1021/nn204123p. [DOI] [PubMed] [Google Scholar]
- 102.Dankers PYW, Harmsen MC, Brouwer LA, Van Luyn MJA, Meijer EW. A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat. Mater. 2005;4:568–574. doi: 10.1038/nmat1418. [DOI] [PubMed] [Google Scholar]
- 103.Wong Po Foo CTS, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl Acad. Sci. USA. 2009;106:22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015;25:1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sathaye S, et al. Engineering complementary hydrophobic interactions to control β-hairpin peptide self-assembly, network branching, and hydrogel properties. Biomacromolecules. 2014;15:3891–3900. doi: 10.1021/bm500874t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glassman MJ, Chan J, Olsen BD. Reinforcement of shear thinning protein hydrogels by responsive block copolymer self-assembly. Adv. Funct. Mater. 2013;23:1182–1193. doi: 10.1002/adfm.201202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 109.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ito F, et al. Reversible hydrogel formation driven by protein-peptide-specific interaction and chondrocyte entrapment. Biomaterials. 2010;31:58–66. doi: 10.1016/j.biomaterials.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 111.Lu HD, Charati MB, Kim IL, Burdick JA. Injectable shear-thinning hydrogels engineered with a self-assembling dock-and-lock mechanism. Biomaterials. 2012;33:2145–2153. doi: 10.1016/j.biomaterials.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 112.Shen W, Kornfield JA, Tirrell DA. Dynamic properties of artificial protein hydrogels assembled through aggregation of leucine zipper peptide domains. Macromolecules. 2007;40:689–692. doi: 10.1039/b610986a. [DOI] [PubMed] [Google Scholar]
- 113.Um SH, et al. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006;5:797–801. doi: 10.1038/nmat1741. [DOI] [PubMed] [Google Scholar]
- 114.Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9:5590–5599. doi: 10.1016/j.actbio.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodell CB, Kaminski AL, Burdick JA. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules. 2013;14:4125–4134. doi: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parisi-Amon A, Mulyasasmita W, Chung C, Heilshorn SC. Protein-engineered injectable hydrogel to improve retention of transplanted adipose-derived stem cells. Adv. Healthcare Mater. 2013;2:428–432. doi: 10.1002/adhm.201200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haines-Butterick L, et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl Acad. Sci. USA. 2007;104:7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J, et al. Physically associated synthetic hydrogels with long-term covalent stabilization for cell culture and stem cell transplantation. Adv. Mater. 2011;23:5098–5103. doi: 10.1002/adma.201103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodell CB, et al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 2015;25:636–644. doi: 10.1002/adfm.201403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Newcomb CJ, et al. Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat. Commun. 2014;5:3321. doi: 10.1038/ncomms4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baker BM, et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015;14:1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr. Biol. 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 124.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, et al. Spatiotemporally controllable and cytocompatible approach builds 3D cell culture matrix by photo-uncaged-thiol michael addition reaction. Adv. Mater. 2014;26:3912–3917. doi: 10.1002/adma.201306061. [DOI] [PubMed] [Google Scholar]
- 126.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 127.He M, Li J, Tan S, Wang R, Zhang Y. Photodegradable supramolecular hydrogels with fluorescence turn-on reporter for photomodulation of cellular microenvironments. J. Am. Chem. Soc. 2013;135:18718–18721. doi: 10.1021/ja409000b. [DOI] [PubMed] [Google Scholar]
- 128.Jiang FX, Yurke B, Schloss RS, Firestein BL, Langrana NA. The relationship between fibroblast growth and the dynamic stiffnesses of a DNA crosslinked hydrogel. Biomaterials. 2010;31:1199–1212. doi: 10.1016/j.biomaterials.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 129.Jiang FX, Yurke B, Schloss RS, Firestein BL, Langrana NA. Effect of dynamic stiffness of the substrates on neurite outgrowth by using a DNA-crosslinked hydrogel. Tissue Eng. Part A. 2010;16:1873–1889. doi: 10.1089/ten.TEA.2009.0574. [DOI] [PubMed] [Google Scholar]
- 130.Peng L, et al. Macroscopic volume change of dynamic hydrogels induced by reversible DNA hybridization. J. Am. Chem. Soc. 2012;134:12302–12307. doi: 10.1021/ja305109n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin DC, Yurke B, Langrana NA. Inducing reversible stiffness changes in DNA-crosslinked gels. J. Mater. Res. 2005;20:1456–1464. [Google Scholar]
- 132.Lin DC, Yurke B, Langrana NA. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J. Biomech. Eng. 2004;126:104–110. doi: 10.1115/1.1645529. [DOI] [PubMed] [Google Scholar]
- 133.Murphy WL. Emerging area: biomaterials that mimic and exploit protein motion. Soft Matter. 2011;7:3679–3688. doi: 10.1039/C0SM01351J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ehrick JD, et al. Genetically engineered protein in hydrogels tailors stimuli-responsive characteristics. Nat. Mater. 2005;4:298–302. doi: 10.1038/nmat1352. [DOI] [PubMed] [Google Scholar]
- 135.Murphy WL, Dillmore WS, Modica J, Mrksich M. Dynamic hydrogels: translating a protein conformational change into macroscopic motion. Angew. Chem. Int. Ed. Engl. 2007;46:3066–3069. doi: 10.1002/anie.200604808. [DOI] [PubMed] [Google Scholar]
- 136.Yuan W, Yang J, Kopečková P, Kopeček J. Smart hydrogels containing adenylate kinase: translating substrate recognition into macroscopic motion. J. Am. Chem. Soc. 2008;130:15760–15761. doi: 10.1021/ja805634x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang S, Glassman MJ, Li S, Socrate S, Olsen BD. Oxidatively responsive chain extension to entangle engineered protein hydrogels. Macromolecules. 2014;47:791–799. doi: 10.1021/ma401684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kong N, Peng Q, Li H. Rationally designed dynamic protein hydrogels with reversibly tunable mechanical properties. Adv. Funct. Mater. 2014;24:7310–7317. [Google Scholar]
- 139.Rosales AM, Mabry KM, Nehls EM, Anseth KS. Photoresponsive elastic properties of azobenzene-containing poly(ethylene-glycol)-based hydrogels. Biomacromolecules. 2015;16:798–806. doi: 10.1021/bm501710e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tamesue S, Takashima Y, Yamaguchi H, Shinkai S, Harada A. Photoswitchable supramolecular hydrogels formed by cyclodextrins and azobenzene polymers. Angew. Chem. Int. Ed. Engl. 2010;49:7461–7464. doi: 10.1002/anie.201003567. [DOI] [PubMed] [Google Scholar]
- 141.Gillette BM, Jensen JA, Wang M, Tchao J, Sia SK. Dynamic hydrogels: switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv. Mater. 2010;22:686–691. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 142.Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl Acad. Sci. USA. 2015;112:1953–1958. doi: 10.1073/pnas.1421897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seiffert S, Weitz DA. Microfluidic fabrication of smart microgels from macromolecular precursors. Polymer. 2010;51:5883–5889. [Google Scholar]
- 144.Shah RK, Kim J-W, Agresti JJ, Weitz DA, Chu L-Y. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter. 2008;4:2303–2309. [Google Scholar]
- 145.Das M, Zhang H, Kumacheva E. Microgels: old materials with new applications. Annu. Rev. Mater. Res. 2006;36:117–142. [Google Scholar]
- 146.Panda P, et al. Stop-flow lithography to generate cell-laden microgel particles. Lab. Chip. 2008;8:1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu S, et al. Generation of monodisperse particles by using microfluidics: control over size, shape, and composition. Angew. Chem. Int. Ed. Engl. 2005;44:724–728. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- 148.Shen Q, et al. Specific capture and release of circulating tumor cells using aptamer-modified nanosubstrates. Adv. Mater. 2013;25:2368–2373. doi: 10.1002/adma.201300082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deng Y, et al. An integrated microfluidic chip system for single-cell secretion profiling of rare circulating tumor cells. Sci. Rep. 2014;4:7499. doi: 10.1038/srep07499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao W, et al. Bioinspired multivalent DNA network for capture and release of cells. Proc. Natl Acad. Sci. USA. 2012;109:19626–19631. doi: 10.1073/pnas.1211234109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu H, et al. Dual-responsive surfaces modified with phenylboronic acid-containing polymer brush to reversibly capture and release cancer cells. J. Am. Chem. Soc. 2013;135:7603–7609. doi: 10.1021/ja401000m. [DOI] [PubMed] [Google Scholar]
- 152.Ouyang J, et al. Morphology controlled poly(aminophenylboronic acid) nanostructures as smart substrates for enhanced capture and release of circulating tumor cells. Adv. Funct. Mater. 2015;25:6122–6130. [Google Scholar]
- 153.Pan G, et al. Dynamic introduction of cell adhesive factor via reversible multicovalent phenylboronic acid/ cis-diol polymeric complexes. J. Am. Chem. Soc. 2014;136:6203–6206. doi: 10.1021/ja501664f. [DOI] [PubMed] [Google Scholar]
- 154.Li W, Wang J, Ren J, Qu X. 3D graphene oxide–polymer hydrogel: near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv. Mater. 2013;25:6737–6743. doi: 10.1002/adma.201302810. [DOI] [PubMed] [Google Scholar]
- 155.Hyun J, Lee W-K, Nath N, Chilkoti A, Zauscher S. Capture and release of proteins on the nanoscale by stimuli-responsive elastin-like polypeptide “switches”. J. Am. Chem. Soc. 2004;126:7330–7335. doi: 10.1021/ja049721e. [DOI] [PubMed] [Google Scholar]
- 156.Mabry KM, Lawrence RL, Anseth KS. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials. 2015;49:47–56. doi: 10.1016/j.biomaterials.2015.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsien RY. Constructing and exploiting the fluorescent protein paintbox (Nobel lecture) Angew. Chem. Int. Ed. Engl. 2009;48:5612–5626. doi: 10.1002/anie.200901916. [DOI] [PubMed] [Google Scholar]
- 158.Dean KM, Palmer AE. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat. Chem. Biol. 2014;10:512–523. doi: 10.1038/nchembio.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Legant WR, et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Meth. 2010;7:969–971. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schultz KM, Anseth KS. Monitoring degradation of matrix metalloproteinases-cleavable PEG hydrogels via multiple particle tracking microrheology. Soft Matter. 2013;9:1570–1579. [Google Scholar]
- 161.Bloom RJ, George JP, Celedon A, Sun SX, Wirtz D. Mapping local matrix remodeling induced by a migrating tumor cell using three-dimensional multiple-particle tracking. Biophys. J. 2008;95:4077–4088. doi: 10.1529/biophysj.108.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 163.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Manduca A, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med. Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 165.Othman SF, Xu H, Royston TJ, Magin RL. Microscopic magnetic resonance elastography (µMRE) Magn. Reson. Med. 2005;54:605–615. doi: 10.1002/mrm.20584. [DOI] [PubMed] [Google Scholar]
- 166.Othman SF, Xu H, Mao JJ. Future role of MR elastography in tissue engineering and regenerative medicine. J. Tissue Eng. Regener. Med. 2015;9:481–487. doi: 10.1002/term.1801. [DOI] [PubMed] [Google Scholar]
- 167.Ranga A, Lutolf MP. High-throughput approaches for the analysis of extrinsic regulators of stem cell fate. Curr. Opin. Cell Biol. 2012;24:236–244. doi: 10.1016/j.ceb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 168.Zanella F, Lorens JB, Link W. High content screening: seeing is believing. Trends Biotechnol. 2010;28:237–245. doi: 10.1016/j.tibtech.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 169.Megason SG, Fraser SE. Imaging in systems biology. Cell. 2007;130:784–795. doi: 10.1016/j.cell.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 170.Chen WLK, Likhitpanichkul M, Ho A, Simmons CA. Integration of statistical modeling and high-content microscopy to systematically investigate cell–substrate interactions. Biomaterials. 2010;31:2489–2497. doi: 10.1016/j.biomaterials.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 171.Highley CB, Rodell CB, Burdick JA. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015;27:5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 172.Kolesky DB, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 173.Culver JC, et al. Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv. Mater. 2012;24:2344–2348. doi: 10.1002/adma.201200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tumbleston JR, et al. Continuous liquid interface production of 3D objects. Science. 2015;347:1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 175.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012;41:6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 176.Cheng E, et al. A pH-triggered, fast-responding DNA hydrogel. Angew. Chem. Int. Ed. Engl. 2009;48:7660–7663. doi: 10.1002/anie.200902538. [DOI] [PubMed] [Google Scholar]