Abstract
Saccharomyces cerevisiae contains two genes for each core histone, which are presented as pairs under the control of a divergent promoter, i.e., HHT1-HHF1, HHT2-HHF2, HTA1-HTB1 and HTA2-HTB2. HHT1-HHF1, and HHT2-HHF2 encode histone H3 and H4 with identical amino acid sequences but under the control of differently regulated promoters. Previous mutagenesis studies were carried out by deleting one pair and mutating the other one. Here, we present the design and construction of three additional libraries covering HTA1-HTB1, HTA2-HTB2, and HHT1-HHF1 respectively. Together with the previously described library of HHT2-HHF2 mutants, a systematic and complete collection of mutants for each of the eight core S. cerevisiae histone genes becomes available. Each designed mutant was incorporated into the genome, generating three more corresponding libraries of yeast strains. We demonstrated that, although, under normal growth conditions, strains with single-copy integrated histone genes lacked phenotypes, in some growth conditions, growth deficiencies were observed. Specifically, we showed that addition of a second copy of the mutant histone gene could rescue the lethality in some previously known mutants that cannot survive with a single copy. This resource enables systematic studies of function of each nucleosome residue in plasmid, single-copy, and double-copy integrated formats.
Keywords: histone H2A, histone H2B, histone H3, histone H4, mutagenesis
IN the genomes of most eukaryotes, there are multiple genes encoding each histone protein, and these genes are often distributed throughout the chromosomes (Maxson et al. 1983; Marzluff et al. 2002, 2008), making it difficult, if not impossible, to introduce a particular mutation into all copies of a given histone gene simultaneously. Saccharomyces cerevisiae has only two copies of genes encoding each core histone, and these exist as pairs driven by bidirectional promoters, i.e., HHT1-HHF1 and HHT2-HHF2 for histone H3 and H4, and HTA1-HTB1 and HTA2-HTB2 for histone H2A and H2B (Hereford et al. 1979; Wallis et al. 1980; Choe et al. 1982; Smith and Andresson 1983; Smith and Murray 1983). More importantly, the presence of either copy can support cell viability (Rykowski et al. 1981; Kolodrubetz et al. 1982; Smith and Stirling 1988; Dai et al. 2010), making the budding yeast an ideal system for high-throughput mutagenesis studies. Several mutant libraries have been constructed and used to probe the function of histones (Hyland et al. 2005; Matsubara et al. 2007; Dai et al. 2008, 2010; Nakanishi et al. 2008; Sakamoto et al. 2009; Govin et al. 2010; Choy et al. 2011; Sen et al. 2015; Luo et al. 2016).
The expression of histone genes is tightly regulated at both the RNA and protein levels during the cell cycle (Eriksson et al. 2012). In S. cerevisiae, the two gene pairs for histone H3 and H4 are not expressed equally. HHT2-HHF2 contributes >80% of the H3–H4 mRNAs within a cell (Cross and Smith 1988). However, in the absence of HHT2-HHF2, transcription of HHT1-HHF1 can be upregulated to support cell growth, although minor defects in chromosome segregation and DNA repair have been observed in such strains (Smith and Stirling 1988; Liang et al. 2012). Although knocking out HHT1-HHF1 results in no obvious phenotypic flaw as tested (Cross and Smith 1988), previous work has shown that certain histone mutants, driven by the native promoter, were lethal when integrated at HHT2-HHF2 locus, even though they were viable when introduced as a CEN plasmid, presumably due to increased plasmid copy number (Matsubara et al. 2007; Dai et al. 2008; Nakanishi et al. 2008; Sakamoto et al. 2009). These observations suggest that such mutants are hypomorphs that can be phenotypically rescued by increasing gene dosage. These observations also suggested that complex and difficult to interpret phenotypes could be introduced during mutagenesis studies using libraries carrying only a single copy of histone genes. Specifically, such phenotype could result from some unknown combination of the mutation and the reduced gene dosage.
Here, we present the design and construction of three new libraries of histone mutants, two covering H2A/H2B and the other H3/H4, that both complete the set of all four core histones, and also can be used to restore gene dosage to the native state. As in the original H3/H4 library, we systematically changed each residue to alanine, and replaced alanine with serine. When possible, each modifiable residue was substituted with amino acids mimicking both modified and unmodified states, allowing exploration of modification state. Additional mutations were introduced to either eliminate, or even to reverse side chain charge and size. Furthermore, systematic deletion mutants at both N- and C-termini were included, generating a comprehensive library of histone H2A and H2B mutants with 592 alleles. The first library allows integration of each mutant at HTA1-HTB1 locus, and the second library targets the HTA2-HTB2 locus. Based on the previous library design, we constructed a complementary library allowing each mutant to be separately integrated at the HHT1-HHF1 locus. All three libraries are provided as bacterial stocks to allow user-defined utilization. Yeast strains with either a single copy, or two copies of the same mutants integrated at the native histone loci, were constructed and provided.
Materials and Methods
Construction of bacterial libraries of 592 H2A/H2B mutants
The mutant constructs (H2ML1, driven by the HTA1/HTB1 promoter) were synthesized and cloned into pRS416 by Epoch Life Science (http://epochlifescience.com/). The plasmids were supplied as bacterial stocks in a 96-well format. To construct the second copy of mutants (H2ML2, driven by the HTA2/HTB2 promoter), plasmids were isolated, diluted by 50-fold, and used as template to amplify mutated fragments. The purified digested PCR products were then cloned into destination plasmids pJD411 (H2A mutants, BglII/XhoI) and pJD412 (H2B mutants, ClaI/SalI) respectively. Plasmids were isolated, and sequence verified to ensure 100% accuracy. These plasmids were arrayed and stored as bacteria stocks in the same order as that of H2ML1.
Construction of the second bacterial library of 562 H3/H4 mutants
Seventy-six point mutants with charge reversal of H3 and H4 were newly designed and constructed from the WT base constructs (pJD47 for H3 and pJD62 for H4) to expand the previous H3/H4 library (Table 1) (Dai et al. 2008), generating the updated H3/H4 library with 562 alleles (H3/4ML2, Table 2).
Table 1. The mutagenesis strategy of the histone libraries.
| Category | Original Residue | New Residue | Rationale | H2A (No.) | H2B (No.) | H3 (No.) | H4 (No.) |
|---|---|---|---|---|---|---|---|
| Point mutant | All | Alanine(A) | Remove sidechain | 112 | 113 | 119 | 96 |
| H2A/H2B: 516 | Alanine(A) | Serine(S) | Alter sidechain | 19 | 17 | 16 | 6 |
| H3/H4: 477 | Lysine(K) | Arginine(R) | Mimic deacetyl | 11 | 19 | 16 | 11 |
| Lysine(K) | Glutamine(Q) | Mimic acetyl | 11 | 19 | 16 | 11 | |
| Arginine(R) | Lysine(K) | Mimic demethyl | 10 | 6 | 17 | 14 | |
| Serine(S) | Aspartic Acid(D) | Mimic phosphate | 8 | 19 | 10 | 6 | |
| Threonine(T) | Aspartic Acid(D) | Mimic phosphate | 5 | 11 | 9 | 6 | |
| Tyrosine(Y) | Glutamic Acid (E) | Mimic phosphate | 3 | 5 | 2 | 4 | |
| Lysine(K) | Glutamic acid(E) | Reverse charge | 11 | 19 | 16 | 11 | |
| Arginine(R) | Glutamic acid(E) | Reverse charge | 10 | 6 | 17 | 14 | |
| Aspartic Acid(D) | Arginine(R) | Reverse charge | 3 | 3 | 4 | 3 | |
| Glutamic Acid(E) | Arginine(R) | Reverse charge | 5 | 8 | 7 | 4 | |
| Histidine(H) | Glutamine(Q) | Remove charge | 3 | 2 | 2 | 2 | |
| Aspartic Acid(D) | Asparagine(N) | Remove charge | 3 | 3 | 4 | 3 | |
| Glutamic Acid(E) | Glutamine(Q) | Remove charge | 5 | 8 | 7 | 4 | |
| Asparagine(N) | Aspartic Acid(D) | Add charge | 8 | 3 | 1 | 1 | |
| Glutamine(Q) | Glutamic Acid(E) | Add charge | 6 | 4 | 8 | 2 | |
| Tyrosine(Y) | Phenylalanine(F) | Remove OH | 3 | 5 | 2 | 4 | |
| Proline(P) | Valine(V) | Block isomerization | 5 | 5 | 2 | – | |
| Deletion mutant | N Tail deletion | —— | —— | 10 | 45 | 52 | 27 |
| H2A/H2B: 68 | C Tail deletion | —— | —— | 10 | 3 | – | – |
| H3/H4: 79 | |||||||
| Comprehensive mutant | Multiple Tail Lysines(K) | Alanine(A)/Glutamine(Q)/Arginine(R) | Mimic unmodified/acetyl/deacetyl | 3 | 3 | 3 | 3 |
| H2A/H2B: 8 | Compound substitutions | —— | Mimic WT Hta1p/Htb1p | 1 | 1 | – | – |
| H3/H4: 6 | |||||||
| Total | 265 | 327 | 330 | 232 | |||
| H2A/H2B: 592 | |||||||
| H3/H4: 562 |
Bold font indicates new mutagenesis strategies, which were not included in the previous histone H3/H4 library (Dai et al. 2008).
Table 2. Formats of the histone mutant libraries (plasmids).
| Name | Histone | CEN Format Marker | Integrated Marker | Integrated Locus | E. coli Selective Marker | No. of Mutants | Published or not |
|---|---|---|---|---|---|---|---|
| H2ML1 | H2A/B | URA3 | LEU2 | HTA1-HTB1 | AmpR | 592 | This study |
| H2ML2 | H2A/B | TRP1 | NatMX4 | HTA2-HTB2 | CmR | 592 | This study |
| H3/4ML1 | H3/4 | TRP1 | HygMX4 | HHT1-HHF1 | CmR | 562 | This study |
| H3/4ML2 | H3/4 | TRP1 | URA3 | HHT2-HHF2 | AmpR | 562 | Dai et al. (2008) and this study |
To construct the second copy of H3/H4 mutants (H3/4ML1, driven by the HHT1/HHF1 promoter), the previously made plasmids (Dai et al. 2008) were isolated and diluted 50-fold as above. The purified digested PCR products were then cloned into chloramphenicol-resistant destination plasmids pJD233 (H3 mutants, SalI/ClaI) and pJD232 (H4 mutants, BglII/SphI) respectively. Plasmids were isolated, and sequence verified to ensure 100% accuracy. These plasmids were arrayed and stored as bacteria stocks in the same order as that of H3/4ML2.
Construction of yeast libraries of histone mutants
To construct the yeast H2A/H2B library, each plasmid (H2ML1) was digested by BciVI and NcoI (located within the ORF of URA3) to release the mutant constructs before they were transformed into the host strain JDY142. Correct integration was confirmed by colony PCR. PCR confirmed cells were plated onto medium containing 5-fluoroorotic acid (5-FOA) to eliminate pJD78 (HTA2-HTB2 in pRS316), the plasmid containing wild-type histone H2A and H2B genes. Viable strains were used to integrate the second copy of the same mutants (H2ML2). A BciVI digestion was done before transformations of the H2ML2 mutants.
To construct yeast strains with double-copy integrated H3/H4 mutants, viable strains with single-copy mutants in S288C background (Dai et al. 2008), and updated in this study, were used for the integration of BciVI digested mutant fragments of H3/4ML1.
Growth competition assay
Three independent colonies of each strain were inoculated into fresh YPD medium, cultured at 30° overnight, and then subcultured for an additional 5 hr from a starting A600 of ∼0.1. The cell density was determined and the cell cultures (single-copy with mCherry at the HO locus) were mixed at a 1:1 ratio. Mixed cells were inoculated into fresh YPD medium with a starting cell concentration of ∼5 × 105 cells/ml. They were cultured at 30° and diluted to 5 × 105 cells/ml every 12 hr for a total of 36 hr. Cells were collected and resuspended in PBS buffer with 0.1% Tween as FACS samples at each transfer time point and final time point. An LSR Fortessa cytometer (BD Biosciences) with a high throughput sampler (HTS) loader was used to determine the total cell number and the proportion of mCherry positive cells by analyzing 50 μl cell suspension for each sample. The fitness was calculated as described (Thompson et al. 2006).
Chromatin fractionation assay
The chromatin fractionation assay was conducted following a previous paper (Liang and Stillman 1997). In brief, 50 ml cells at ∼8 × 106 cells/ml were harvested and resuspended in prespheroplasting buffer. After incubation for 10 min, cells were digested with zymolyase 100T (120493; Amsbio) at room temperature on a rotator. Then, spheroplasts were washed with ice-chilled wash buffer, and resuspended in an equal pellet volume of extraction buffer (EB). Spheroplasts were lysed by adding 1/40 vol 10% Triton X-100 (final concentration at 0.25%), and incubated on ice for 3 min with gentle mixing; the resulting sample was defined as the whole cell extract (WCE). Lysate was underlayered with 50% vol of 30% sucrose and spun at 12,000 rpm for 12 min. The upper yellow layer was the supernatant fraction. Chromatin pellet was washed and resuspended again with EBX (EB+0.25%Triton X-100). The samples of the whole cell extract, supernatant, and pellet were boiled and subjected to immunoblotting.
Data availability
The base yeast strains are available upon request, and will be deposited to the American Type Culture Collection (ATCC). The libraries will be deposited to Addgene as plasmids. Supplemental Material, File S1 contains detailed descriptions of all supplemental data, including seven supplemental figures and four supplemental tables.
Results and Discussion
Design of 592 H2A/H2B mutants
All currently available mutant libraries of histone H2A and H2B can only be used in an episomal plasmid format, which potentially limits their application in some situations, particularly when a single gene copy is strictly required. In addition, despite the fact that multiple libraries are available, the mutations are mostly limited to alanine substitutions or a few modifiable residues. A comprehensive library of histone H2A and H2B mutants would help further dissection of nucleosome function. Therefore, we designed a new collection of histone H2A and H2B mutants, systematically covering every residue in the two core histones (Table 1).
The mutants could be divided into three types (Table 1): (1) Point mutants. We substituted each amino acid residue with alanine and changed native alanine to serine, allowing us to probe the function of the side chain of every residue. To study the influence of modifications, we systematically changed any modifiable residues to mimic either modified or unmodified form. Additionally, and differently from the first library we described (Dai et al. 2008), we deliberately swapped the charge status for a charged residue. We replaced each lysine and arginine with a glutamate residue. Similarly, we replaced each glutamate and aspartate with arginine. Together, these strategies generated 516 point mutants; (2) Deletion mutants. The N- and C-termini (“tails”) of histone H2A and H2B are known to play critical roles in nucleosome function. For example, serine 128 on histone H2A is known to be phosphorylated, and phosphorylation is required to recruit important protein factors during the process of repairing damaged DNA (Downs et al. 2000; Morrison et al. 2004). A total of 68 systematic deletion mutants was designed to remove sets of four residues at either N- or C-terminal of histone H2A and H2B; (3) Multi-point mutants. Since multiple lysines are modified within the N-termini of histones H2A and H2B, we constructed six mutants that allowed us to replace multiple lysines with alanine, arginine or glutamine at each position. Therefore, potential modifications for all of these residues could be removed or mimicked simultaneously. In addition, since the two copies of genes encode slightly different amino acid sequences for both histone H2A and H2B, and the majority of the mutants were generated based on the HTA2 and HTB2 amino acid sequences, specific substitutions were made to convert the amino acid sequences to match HTA1 and HTB1. Eventually, we designed a total of 592 alleles, including 265 mutants in histone H2A and 327 mutants in histone H2B (Table 1), producing the most comprehensive histone H2A and H2B mutant library available.
We generated each mutant based on the amino acid sequences of HTA2 and HTB2 (Figure S1A in File S1), which were codon-optimized and recoded synonymously using GeneDesign (Richardson et al. 2006) to avoid potential homologous recombination between the synthetic construct and the native gene. Since HTA1-HTB1 in the genome sufficed for normal growth while HTA2-HTB2 did not (Norris and Osley 1987; Moran et al. 1990; Libuda and Winston 2006), the former locus was chosen for the integration for the synthetic cassette. To keep the expression of the histones H2A/H2B similar to that of the native form, the endogenous HTA1-HTB1 promoter was used to control expression. We swapped the native terminators of both genes with either CYC1 or ADH1 terminator, to eliminate recombination with wild-type sequences, which could potentially lead to inadvertent swapping of the histone mutant with the wild-type sequence. The synthetic histone cassette was flanked by native sequences upstream and downstream of the HTA1-HTB1 locus, allowing the construct to be integrated at this locus, replacing any DNA inbetween. For selection, a LEU2 gene was inserted between one homologous region and the 3′ end of the UTR sequences adjacent to the histone coding sequence that was mutated. Thus, the set of H2A mutants was made in one parental plasmid, and the set of H2B mutants was made in a separate one. The overall construct design is shown in Figure 1A.
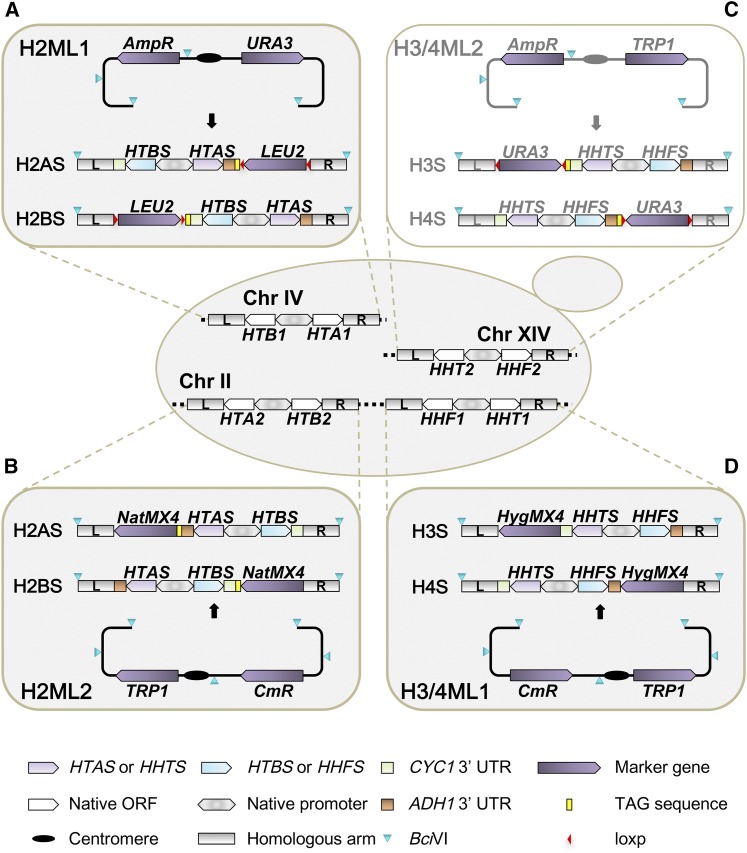
Figure 1 (A–D).
Versatile dosage matching libraries for all core histones. The libraries (H2ML1, H2ML2, and H3/4ML1) marked in gray were newly generated in this study; H3/4ML2 was updated from the H3/4 library reported previously (Dai et al. 2008). All libraries can be used as episomal plasmids or integrated into the genome after BciVI (marked with blue triangle) digestion. The synthetic constructs for H2A, H2B, H3, and H4 mutants are denoted as H2AS, H2BS, H3S, and H4S, respectively. The selectable markers (LEU2, URA3, NatMX4, and HygMX4) for integration are always positioned adjacent to the gene bearing the mutation(s) to prevent recombination events between the marker and the mutant (i.e., within the promoter fragment). The synthetic histone genes were driven by native promoters. The sequences flanking histone ORFs were used as homology arms. There are very minor coding sequence differences between HTA1/B1 and HTA2/B2; for H2ML1 and H2ML2, wild type Hta2-Htb2 amino acids were used as the reference sequence for recoding to HTAS and HTBS. Each mutant was labeled with unique TAG sequences (marked as yellow squares). The TAGs can be used for the identification of specific mutants using high-throughput sequencing. As in H3/4ML2, each plasmid in H2ML1 was designed with flanking loxP sites (red triangles) allowing facile “swap out” of the marker genes.
Importantly, each mutant was marked with a pair of unique DNA sequences (molecular barcodes or TAGs), taking from a subset of the molecular barcodes used in the yeast knockout collection (Winzeler et al. 1999). Each barcode was flanked by a pair of universal primers and placed adjacent to each other between the 3′ UTR of the histone mutant and the LEU2 gene. The presence of the TAGs allows pooling of the mutants and analysis of some otherwise very labor-intensive assays, such as those demonstrated using the yeast knockout collection (Smith et al. 2009, 2010, 2012; Gresham et al. 2011; Gibney et al. 2013).
The synthetic cassette was cloned into a bacteria-yeast shuttle vector pRS416, allowing propagation and amplification of each mutant in Escherichia coli. At the same time, each construct could be transformed directly into a yeast strain and used as a replication-competent episome. The synthetic cassette can also be released after a single restriction enzyme digestion, and transformed into the host strain to integrate at the native locus with high fidelity. In addition, to further increase the utility of this library, the LEU2 gene was flanked by a pair of loxP sites, allowing the LEU2 gene to be either removed or “swapped” with other selectable markers, as needed.
A second histone H2A and H2B mutant library targeting the HTA2-HTB2 locus
As described above, the initial library of histone H2A and H2B was under the control of HTA1-HTB1 promoter, and configured to integrate at HTA1-HTB1. Since the two pairs of histone genes are regulated differently in yeast (Norris and Osley 1987; Moran et al. 1990; Libuda and Winston 2006), we reasoned that we could minimize the impact of gene dosage on phenotype by integrating two copies of the same mutant at their two native loci, each under control of its endogenous promoter. Therefore, a second mutant library was designed and constructed (Figure 1B).
To construct this library, the base constructs containing wild-type histone H2A and H2B were generated by replacing the HTA1-HTB1 promoter with HTA2-HTB2 promoter, and by substituting the homologous sequences flanking HTA1-HTB1 by sequences flanking the HTA2-HTB2. We used NatMX4 (Goldstein and McCusker 1999) as a selection marker to identify the integrants, enabling selection of strains containing both copies of the mutants. We cloned this second synthetic cassette into centromeric plasmid pBC414 tagged with the TRP1 marker (Frazer and O’Keefe 2007), allowing each mutant to be supplied individually as an episome. The mutant histone sequences (with their TAGs) were amplified by PCR and cloned into the corresponding base construct (Materials and Methods), generating the second library of histone H2A and H2B mutants (H2ML2, Table 2).
A new library of histone H3 and H4 mutants
Previously, we reported the design of a versatile histone H3 and H4 mutant library under the control of the HHT2-HHF2 promoter, which could be used similarly to the H2A and H2B library described above (Figure 1C) (Dai et al. 2008). Here, we first expanded the existing H3/H4 library to include the charge reversal type mutants that were not included in the original version (H3/4ML2, Table 1 and Table 2). Since the two histone H3/H4 loci are also regulated differently, we therefore decided to construct a second library of mutants as rationalized above for histone H2A/H2B.
In the daughter library, the expression of histones H3/H4 is controlled by the native HHT1-HHF1 promoter (Figure 1D). Sequences flanking HHT1-HHF1 were used as homology arms. The selectable marker for integration, URA3 was replaced by HygMX4, and the synthetic cassette was also cloned into pBC414 (Frazer and O’Keefe 2007). The mutant histone sequences were amplified and cloned into the corresponding base construct (Materials and Methods). These manipulations produced a new library of H3/H4 mutants, comprising 562 alleles (H3/4ML1, Table 2).
Four yeast libraries of viable histone mutants
For functional studies, we integrated each histone mutant into the chromosome to generate a collection of yeast libraries. To host the histone H2A and H2B mutants, a MATα strain in the S288C background was constructed by knocking out the genomic HTA1-HTB1 and HTA2-HTB2 loci and supplied a wild-type HTA2-HTB2 on a URA3 CEN “shuffle” plasmid. All 592 mutations were integrated at HTA1-HTB1 by selecting for LEU2 and screening for loss of the resident KanMX4 marker, and subsequently confirmed by PCR. The wild-type plasmid was then removed either spontaneously by mitotic plasmid loss, or intentionally by counter-selection in medium containing 5-FOA. The viable strains lacking the wild-type plasmid constitute the yeast library with single-copy integrated H2A and H2B mutants (BY-H2ML1, Table 3). Once the above strains were constructed (BY-H2ML1, Table 3), we started to incorporate the second copy of the histone mutant (H2ML2) into the HTA2-HTB2 locus of strains that already have the same mutant integrated at the HTA1-HTB1. The strains were selected in medium containing nourseothricin, and subsequently replicated onto medium containing hygromycin B to select for strains with correct replacement of marker genes. The candidates were further confirmed by PCR to ensure that each mutant was positioned correctly. This produced the yeast library of H2A/H2B with two doses of each mutant under their native promoters (BY-H2ML1&2).
Table 3. Yeast libraries (S288C background) of the histone mutants.
| Format | Name | Mating Type | Genotype for the Strains | Published or not |
|---|---|---|---|---|
| Single-copy | BY-H2ML1 | α | his3∆200 leu2∆0 lys2∆0 trp1∆63 ura3∆0 met15∆0 hta2-htb2::HygMX4 hta1-htb1::LEU2-HTAS-HTBS | This study |
| BY-H3/4ML2 | a | his3∆200 leu2∆0 lys2∆0 trp1∆63 ura3∆0 met15∆0 can1::MFA1pr-HIS3 hht1-hhf1::NatMX4 hht2-hhf2::URA3-HHTS-HHFS | Dai et al. (2008) and this study | |
| Double-copy | BY-H2ML1&2 | α | his3∆200 leu2∆0 lys2∆0 trp1∆63 ura3∆0 met15∆0 hta2-htb2::NatMX4-HTAS-HTBS hta1-htb1::LEU2-HTAS-HTBS | This study |
| BY-H3/4ML1&2 | a | his3∆200 leu2∆0 lys2∆0 trp1∆63 ura3∆0 met15∆0 can1::MFA1pr-HIS3 hht1-hhf1::HygMX4-HHTS-HHFS hht2-hhf2::URA3-HHTS-HHFS | This study |
The viable H3/H4 mutant strains incorporated at the HHT2-HHF2 locus in S288C background (BY-H3/4ML2, Table 3) were used for subsequent integration of the newly built histone H3/H4 mutant constructs. A similar series of steps was used to produce the new library of histone H3/H4 with two doses of each mutant (BY-H3/4ML1&2, Table 3).
Thus, a total of four yeast libraries now exists, in which there is either one or two doses of the same mutant in each strain. These libraries represent a valuable resource to study the influence of specific histone residues, tails, and surfaces on nucleosome function.
Histone dosage defect produces phenotypes
In our previous work on histones H3 and H4, we identified 27 lethal mutants in which original residues were mutated to alanine in the S288C background (Dai et al. 2008). However, another library, in which the expression of H3/H4 was driven by the same promoter on CEN plasmids, identified only 14 lethal mutants (Nakanishi et al. 2008). One possible reason for the significant difference between two studies is the dosage of histone genes. Therefore, three histone H3 mutants (I62A, L103A, and L126A), reportedly lethal when integrated but viable as episomal plasmid, were randomly chosen and tested. Consistently, all three mutants failed to propagate in the single copy integrated format. In contrast, incorporating a second copy completely rescued lethality (Figure 2A). Additionally, H3 F84A showed an obvious growth defect with only a single copy, which could be rescued when there are two copies of the mutant genes. These observations strongly support that it is important to retain both histone copies for functional analyses, and maintain the native regulation of histone gene expression (Eriksson et al. 2012) as well as gene dosage. To better understand the influence of histone copy number on phenotypes, the growth of wild type strains containing different copy numbers of synthetic H2A/H2B genes were compared in rich medium. We found no obvious difference in YPD medium by either a growth competition assay (Figure 2B) or serial dilution (Figure 2C and Figure S1B in File S1). The expression levels of histone H2A also did not show significant differences among strains, both in protein and mRNA level (Figure 2D and Figure S2 in File S1). This suggests that the proteins expressed by single-copy histone genes driven by the promoter of HTA1-HTB1 in wild-type strains are sufficient to support normal growth, consistent with previous studies (Norris and Osley 1987; Moran et al. 1990).
Figure 2.
Histone dosage defect produces phenotypes. (A) Viability test of histone mutants by plasmid shuffling. In the listed strains, histone mutants were integrated into HHT2-HHF2 site or both loci and wild-type H3/H4 were expressed from a CEN-URA3 plasmid. Cells were cultured at 30° for 3 days. (B) No significant growth advantage in strains with double-copy vs. single-copy histone genes. mCherry was integrated at the HO site in strains with single-copy histones to quantify the composition of cell populations. Cultures were inoculated by mixing equal numbers of cells (2.5 × 105 cells/ml for each type). The cells were cocultured in YPD medium at 30° and diluted to 5 × 105 cells/ml every 12 hr for a total of 36 hr. Cells were collected at corresponding time points and then subjected to flow cytometry. H2AS WT, strains with synthetic H2A base construct; H2BS WT, strains with synthetic H2B base construct. D, strains with double-copy integrated histones; S, strains with single-copy integrated histones. Data were represented as mean ± SD (C) Strains with single-copy histones were more sensitive to benomyl than those with double-copy histones (BY4742 and H2AS/H2BS WT D). Cells were diluted fourfold and spotted onto YPD or YPD with benomyl. (D) The total expression of histone H2A was measured by immunoblotting in strains at different histone dosages. (E) H2B mutants with different histone dosage showed differential sensitivity to MMS/benomyl. Cells were diluted by 10-fold, and spotted onto YPD or YPD with drugs.
Next, strains with either one or two copies of wild type H2A/B genes were tested for sensitivity to a series of stresses. All strains showed no growth difference under most conditions tested (Figure S1B in File S1). However, on plates containing benomyl, strains with single-copy histones showed slower growth than the control strain BY4742, and strains with double-copy histones (Figure 2C and Figure S1B in File S1). It suggests that the copy number of histone genes does affect phenotypes of strains, and that strains with two copies of synthetic H2A/H2B behave more like the wild-type strain. We then carried out a parallel high-throughput test using strains containing either a single copy of a histone mutant, or two copies of the same mutant upon treatment with benomyl, hydroxyurea (HU), and methyl methanesulfonate (MMS). Substantial phenotypic variations were detected. Some mutants only showed sensitivity to a drug in one strain, and some displayed differential sensitivity (Table S1 in File S1). Several mutants were randomly selected to confirm the high-throughput results (Figure 2E and Figure S4 in File S1). For H2B S67D, strains with double-copy H2A/H2B were, paradoxically, more sensitive to MMS than those with single-copy histones. In contrast, strains with H2A Y58F showed the opposite sensitivity pattern to benomyl. These results indicate that the copy number of histone mutants may contribute significantly to their phenotypes, and the effect is complex, depending on the mutation. The mutant library with two copies of the same mutant histone under the native promoters seems like the best strategy for minimizing potential artifacts related to gene dosage.
In budding yeast, the two copies of histone H2A and H2B genes are nonidentical in terms of protein sequence (Figure S1A in File S1). In the library, the amino acid sequences of Hta2p and Htb2p were used as the reference to recode the DNA sequence. One potential drawback for this design is that it was possible that amino acid differences between the two copies of histone might themselves influence strain fitness. Therefore, we systematically assayed phenotypes of strains containing the two versions of the H2A or H2B sequences. We found no difference among these strains, no matter whether they were present in one or two copies (Figure S1B in File S1). These data demonstrate that HTA1-HTB1 and HTA2-HTB2 function equivalently when used as the sole histone H2A and H2B source.
Lethality profiles of H2A and H2B mutants
Previously, five alanine substitution mutants were defined as lethal including H2A Y58A, E62A, R82A, D91A, and H2B L109A (Matsubara et al. 2007), with one (H2A R82A) questionable (Nakanishi et al. 2008). Using strains containing two copies of histone mutants and a wild type HTA2-HTB2 on a centromeric plasmid, we defined a mutant as “lethal” if it failed to grow on medium containing 5-FOA at 30° for 3 days after plating. Among the 592 mutants, 22 mutants in total failed to support cell viability (Figure 3A and Table 4), including the four known lethal mutants. In agreement with Nakanishi’s work, we found H2A R82A to be viable in our library in both the integrated and episomal formats (Figure S5 in File S1). It is notable that the number of lethal mutants in H2A/H2B library is substantially lower than in the H3/4 library (22/592 of H2A/B mutants vs. 80/562 of H3/4 mutants, Table 4) (Dai et al. 2008). One possible explanation is that, overall, the function of H2A and H2B is somewhat less critical compared to that of H3 and H4. This is consistent with the fact that the bulk of H3 and H4 proteins occupy the upper half of the nucleosome where the DNA entry and exit points are, and also with the fact that the H2A/H2B amino acid sequences are less conserved. In addition, unlike H3/H4 mutants, the copy number of histone genes did not show significant effects on the viability of H2A/H2B mutants, implying that cells need more tightly regulated expression of histone H3 and H4 than that of H2A and H2B.
Figure 3.
Altering charged residues severely affects viability of histone mutants. (A) Comparison of lethal mutants between previous screens (Matsubara et al. 2007; Nakanishi et al. 2008) and results of this study. (B) Immunoblot analysis of total H2A and H2B expression in strains containing two copies of lethal histone mutants and Flag-His tagged wild-type histone plasmids. Tubulin was used as the loading control. (C) Chromatin fractionation to analyze incorporation of histone mutants (H2B Y40E, S61D) into chromatin. The same strains as in (B) were used. CP, chromatin pellets; S, supernatant; W, whole cell extracts. Histone H3 and Pgk1p were used as indicators of chromatin and cytoplasm components. 40 A600 units of cells were harvested and used for each strain. (D) Evolutionary conservation scores for viable mutants and lethal mutants were calculated using ConSurf. **** P < 0.0001. Data were represented as Mean ± SEM (E) Classification of lethal mutants based on alternation of charge state. Pink, negative charge was converted to positive charge; blue, negative charge was removed; yellow, negative charge was added; green, positive charge was swapped by negative charge; orange, other point mutation not affecting charge; gray, lethal tail deletion mutants. (F) Lethal mutants, except several buried ones and tail deletions, are marked in the nucleosome structure with the same colors used in (E). The PDB number of the nucleosome structure used in this paper is 1ID3 (yeast core nucleosome). (G) Viability test of histone mutants by plasmid shuffling. In the listed strains, histone mutants were expressed from a CEN-TRP1 plasmid, and wild-type HTA2-HTB2 were expressed from a CEN-URA3 plasmid.
Table 4. Lethal mutants in the histone libraries.
| Category | H2A | H2B | H2A&H2B | H3a | H4a | H3&H4a |
|---|---|---|---|---|---|---|
| Lethal mutants | 15 (5.7%) | 7 (2.1%) | 22 (3.7%) | 44 (13.3%) | 36 (15.5%) | 80 (14.2%) |
| Lethal point mutations | 14 | 7 | 21 | 41 | 28 | 69 |
| Lethal residues | 9 | 7 | 16 | 32 | 20 | 52 |
| Lethal mutants with changed charge | 13 | 6 | 19 | 25 | 20 | 45 |
| Lethal mutants with charge reversal | 8 | 3 | 11 | 11 | 9 | 20 |
| Mutants with charge reversal | 29 | 36 | 65 | 44 | 32 | 76 |
| Point mutations | 241 | 275 | 516 | 275 | 202 | 477 |
| Total mutants | 265 | 327 | 592 | 330 | 232 | 562 |
Indicates the results from both published data (Dai et al. 2008) and this study (Table S2 in File S1).
To rule out the possibility that a mutant is lethal because it failed to be expressed in the cell, we carried out immunoblot experiments in strains containing wild-type histones with N-terminal Flag-his tag expressed from a CEN plasmid. As shown in Figure 3B, all mutated histone proteins were detectable, suggesting that none of them has transcriptional and translational defects. One interesting observation is that, in the wild-type strains, the tagged native histones are substantially underexpressed relative to that of untagged ones, whereas in the majority of the mutants, the abundance of the tagged native protein is significantly increased (Figure 3B). One possibility is that the mutated histones are less efficiently incorporated into chromatin, leading to the over accumulation of wild-type histones and subsequent degradation of the unincorporated mutant histones (Gunjan and Verreault 2003). To test this hypothesis, chromatin fractionation was performed in two randomly selected lethal histone H2B mutants: Y40E and S61D. As shown in Figure 3C, the percentage of histones from the synthetic construct incorporated into the chromatin showed similar proportions to that in total expressed histones, with more of the tagged wild-type histones in the chromatin in both mutant strains. This result suggests that the mutant histones are less preferable as chromatin components than the wild-type histones, consistent with the above hypothesis. Further analysis confirmed that the lethal mutants were generally more conserved amino acid residues (Figure 3D).
Intriguingly, >85% (19 out of 22) of the lethal mutants fell in residues with charge alterations (Figure 3E and Table 4), which is a higher proportion than that of H3/4 lethal mutants (45/80, Table 4) (Dai et al. 2008). And 11 of those lethal mutants came from the new charge reversal strategy (Table 4). When we mapped the position of these mutants to nucleosome structure, several features were revealed. (1) All three alleles swapping from positive to negative residues (K76E, R78E, and R30E) were positioned at surfaces that contact DNA (green, Figure 3F). The negative charge probably affects the nucleosome structure by repelling the DNA. In support of this, we found that neutralizing the charges with alanine substitution would not impair cellular viability at all three positions. (2) Several lethal mutant residues, such as H2A E57, E62, E65, D91, and E93, clustered at the “acidic patch,” which interacts with positively charged residues within the histone H4 N terminal and is reportedly crucial for formation of higher order nucleosome arrays (Luger et al. 1997; Dorigo et al. 2004; Zhou et al. 2007; Song et al. 2014). Lethal mutations in these residues, such as E/D to R (in pink, Figure 3F) may cause lethality by compromising this important interaction. Remarkably, every mutation tested at positions E62 (A/Q/R) and D91 (A/N/R) on histone H2A causes lethality, highlighting that these two positions are so critical that a wide variety of perturbation types cannot be tolerated. Since all mutants changed the charge status at both locations, we hypothesized that the negative charge might be pivotal. To test this hypothesis, additional mutants E62D and D91E, which contain slightly altered side chains but similar negative charges, were constructed. As expected, H2A E62D was able to support cell viability (Figure 3G, upper panel). On the other hand, H2A D91E was lethal, indicating that charge is not the sole determinant of lethality at this particular position. For other mutants described in groups 1 and 2 above, it is notable that the charge neutralization mutants at the same residues are viable. Therefore, we conclude that active repulsion of charge is substantially more disruptive than simple charge neutralization. (3) In addition, some lethal mutants were scattered among histone–histone interaction surfaces (Figure 3F and Figure S6 in File S1). These mutants might affect nucleosome structure integrity or chromatin assembly by disrupting histone–histone interactions. (4) We previously identified several N-terminal tail deletions in histones H3 and H4 as lethal. However, among all of the deletion mutants, only one of them (H2A del118-127) was lethal (Figure 3B), indicating that the tails on H2A and H2B are almost dispensable.
Compared to the lethal mutants in histone H3 and H4, the distribution of histone H2A and H2B lethal mutants was quite different. Most of the H2A/H2B mutants were located at the nucleosome surface (such as the acid patch, Figure 3F and Figure S7 in File S1) whereas many H3/H4 lethal mutants were buried in the nucleosome core, besides those mutants tracking the DNA-binding surface (Figure S7, Table S2 in File S1, and Table 4) (Dai et al. 2008). Additionally, the buried lethal residues of H3/H4 are mostly located at the H3/H4 interface, or the H3/H3 interface involved in the formation of H3/H4 tetramers, rather than the interface with H2A or H2B. The above features are reminiscent of the spatial and temporal order of nucleosome assembly in which H3/H4 tetramer is deposited to DNA first and H2A/H2B heterodimers are subsequently loaded to complete the core particles (Worcel et al. 1978; MacAlpine and Almouzni 2013). Therefore, the lethality of histone H3/H4 mutants are more likely due to defects in local nucleosome structure or assembly, whereas H2A/H2B lethal mutants might interfere more with the interactions between nucleosomes and other protein factors or adjacent nucleosomes.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300450/-/DC1.
Acknowledgments
We are grateful for financial support from the National Key Research and Development Program of China (2017YFA0505103); Research Fund for the Doctoral Program of Higher Education of China 20120002110022 and National Natural Science Foundation of China (31471254) to J.D. This work was also supported by the National Institutes of Health (NIH) grant U54GM103520 to J.D.B.
Note added in proof: See Jiang et al. 2017 (pp. 3857–3866) in G3: Genes|Genomes|Genetics for a related work.
Footnotes
Communicating editor: O. Rando
Literature Cited
- Choe J., Kolodrubetz D., Grunstein M., 1982. The two yeast histone H2A genes encode similar protein subtypes. Proc. Natl. Acad. Sci. USA 79: 1484–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy J. S., Acuna R., Au W. C., Basrai M. A., 2011. A role for histone H4K16 hypoacetylation in Saccharomyces cerevisiae kinetochore function. Genetics 189: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Smith M. M., 1988. Comparison of the structure and cell cycle expression of mRNAs encoded by two histone H3–H4 loci in Saccharomyces cerevisiae. Mol. Cell. Biol. 8: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Hyland E. M., Yuan D. S., Huang H., Bader J. S., et al. , 2008. Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell 134: 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Hyland E. M., Norris A., Boeke J. D., 2010. Yin and Yang of histone H2B roles in silencing and longevity: a tale of two arginines. Genetics 186: 813–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo B., Schalch T., Kulangara A., Duda S., Schroeder R. R., et al. , 2004. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science 306: 1571–1573. [DOI] [PubMed] [Google Scholar]
- Downs J. A., Lowndes N. F., Jackson S. P., 2000. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 408: 1001–1004. [DOI] [PubMed] [Google Scholar]
- Eriksson P. R., Ganguli D., Nagarajavel V., Clark D. J., 2012. Regulation of histone gene expression in budding yeast. Genetics 191: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer L. N., O’Keefe R. T., 2007. A new series of yeast shuttle vectors for the recovery and identification of multiple plasmids from Saccharomyces cerevisiae. Yeast 24: 777–789. [DOI] [PubMed] [Google Scholar]
- Gibney P. A., Lu C., Caudy A. A., Hess D. C., Botstein D., 2013. Yeast metabolic and signaling genes are required for heat-shock survival and have little overlap with the heat-induced genes. Proc. Natl. Acad. Sci. USA 110: E4393–E4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Govin J., Dorsey J., Gaucher J., Rousseaux S., Khochbin S., et al. , 2010. Systematic screen reveals new functional dynamics of histones H3 and H4 during gametogenesis. Genes Dev. 24: 1772–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham D., Boer V. M., Caudy A., Ziv N., Brandt N. J., et al. , 2011. System-level analysis of genes and functions affecting survival during nutrient starvation in Saccharomyces cerevisiae. Genetics 187: 299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunjan A., Verreault A., 2003. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell 115: 537–549. [DOI] [PubMed] [Google Scholar]
- Hereford L., Fahrner K., Woolford J., Jr., Rosbash M., Kaback D. B., 1979. Isolation of yeast histone genes H2A and H2B. Cell 18: 1261–1271. [DOI] [PubMed] [Google Scholar]
- Hyland E. M., Cosgrove M. S., Molina H., Wang D., Pandey A., et al. , 2005. Insights into the role of histone H3 and histone H4 core modifiable residues in Saccharomyces cerevisiae. Mol. Cell. Biol. 25: 10060–10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Rykowski M. C., Grunstein M., 1982. Histone H2A subtypes associate interchangeably in vivo with histone H2B subtypes. Proc. Natl. Acad. Sci. USA 79: 7814–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Stillman B., 1997. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 11: 3375–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Burkhart S. L., Singh R. K., Kabbaj M. H., Gunjan A., 2012. Histone dosage regulates DNA damage sensitivity in a checkpoint-independent manner by the homologous recombination pathway. Nucleic Acids Res. 40: 9604–9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libuda D. E., Winston F., 2006. Amplification of histone genes by circular chromosome formation in Saccharomyces cerevisiae. Nature 443: 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K., Mader A. W., Richmond R. K., Sargent D. F., Richmond T. J., 1997. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260. [DOI] [PubMed] [Google Scholar]
- Luo J., Deng X., Buehl C., Xu X., Kuo M. H., 2016. Identification of tension sensing motif of histone H3 in Saccharomyces cerevisiae and its regulation by histone modifying enzymes. Genetics 204: 1029–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlpine D. M., Almouzni G., 2013. Chromatin and DNA replication. Cold Spring Harb. Perspect. Biol. 5: a010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff W. F., Gongidi P., Woods K. R., Jin J., Maltais L. J., 2002. The human and mouse replication-dependent histone genes. Genomics 80: 487–498. [PubMed] [Google Scholar]
- Marzluff W. F., Wagner E. J., Duronio R. J., 2008. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat. Rev. Genet. 9: 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Sano N., Umehara T., Horikoshi M., 2007. Global analysis of functional surfaces of core histones with comprehensive point mutants. Genes Cells 12: 13–33. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T., 1983. Expression and organization of histone genes. Annu. Rev. Genet. 17: 239–277. [DOI] [PubMed] [Google Scholar]
- Moran L., Norris D., Osley M. A., 1990. A yeast H2a-H2b promoter can be regulated by changes in histone gene copy number. Genes Dev. 4: 752–763. [DOI] [PubMed] [Google Scholar]
- Morrison A. J., Highland J., Krogan N. J., Arbel-Eden A., Greenblatt J. F., et al. , 2004. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119: 767–775. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Sanderson B. W., Delventhal K. M., Bradford W. D., Staehling-Hampton K., et al. , 2008. A comprehensive library of histone mutants identifies nucleosomal residues required for H3K4 methylation. Nat. Struct. Mol. Biol. 15: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D., Osley M. A., 1987. The two gene pairs encoding H2A and H2B play different roles in the Saccharomyces cerevisiae life cycle. Mol. Cell. Biol. 7: 3473–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. M., Wheelan S. J., Yarrington R. M., Boeke J. D., 2006. GeneDesign: rapid, automated design of multikilobase synthetic genes. Genome Res. 16: 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rykowski M. C., Wallis J. W., Choe J., Grunstein M., 1981. Histone H2B subtypes are dispensable during the yeast cell cycle. Cell 25: 477–487. [DOI] [PubMed] [Google Scholar]
- Sakamoto M., Noguchi S., Kawashima S., Okada Y., Enomoto T., et al. , 2009. Global analysis of mutual interaction surfaces of nucleosomes with comprehensive point mutants. Genes Cells 14: 1271–1330. [DOI] [PubMed] [Google Scholar]
- Sen P., Dang W., Donahue G., Dai J., Dorsey J., et al. , 2015. H3K36 methylation promotes longevity by enhancing transcriptional fidelity. Genes Dev. 29: 1362–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. M., Heisler L. E., Mellor J., Kaper F., Thompson M. J., et al. , 2009. Quantitative phenotyping via deep barcode sequencing. Genome Res. 19: 1836–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. M., Heisler L. E., St Onge R. P., Farias-Hesson E., Wallace I. M., et al. , 2010. Highly-multiplexed barcode sequencing: an efficient method for parallel analysis of pooled samples. Nucleic Acids Res. 38: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. M., Durbic T., Kittanakom S., Giaever G., Nislow C., 2012. Barcode sequencing for understanding drug-gene interactions. Methods Mol. Biol. 910: 55–69. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Andresson O. S., 1983. DNA sequences of yeast H3 and H4 histone genes from two non-allelic gene sets encode identical H3 and H4 proteins. J. Mol. Biol. 169: 663–690. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Murray K., 1983. Yeast H3 and H4 histone messenger RNAs are transcribed from two non-allelic gene sets. J. Mol. Biol. 169: 641–661. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Stirling V. B., 1988. Histone H3 and H4 gene deletions in Saccharomyces cerevisiae. J. Cell Biol. 106: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Chen P., Sun D., Wang M., Dong L., et al. , 2014. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science 344: 376–380. [DOI] [PubMed] [Google Scholar]
- Thompson D. A., Desai M. M., Murray A. W., 2006. Ploidy controls the success of mutators and nature of mutations during budding yeast evolution. Curr. Biol. 16: 1581–1590. [DOI] [PubMed] [Google Scholar]
- Wallis J. W., Hereford L., Grunstein M., 1980. Histone H2B genes of yeast encode two different proteins. Cell 22: 799–805. [DOI] [PubMed] [Google Scholar]
- Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., et al. , 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906. [DOI] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L., 1978. Assembly of newly replicated chromatin. Cell 15: 969–977. [DOI] [PubMed] [Google Scholar]
- Zhou J., Fan J. Y., Rangasamy D., Tremethick D. J., 2007. The nucleosome surface regulates chromatin compaction and couples it with transcriptional repression. Nat. Struct. Mol. Biol. 14: 1070–1076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The base yeast strains are available upon request, and will be deposited to the American Type Culture Collection (ATCC). The libraries will be deposited to Addgene as plasmids. Supplemental Material, File S1 contains detailed descriptions of all supplemental data, including seven supplemental figures and four supplemental tables.