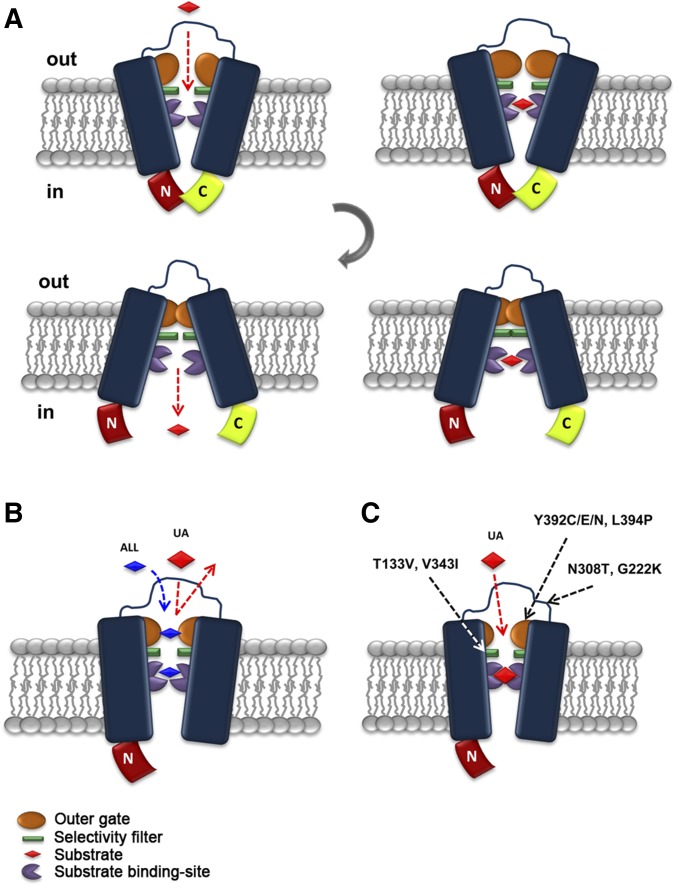
Figure 6.
Highly speculative scheme of the role of the interaction of the N- and C-terminal regions in FurE functioning. (A) In the nontruncated version found in the outward-facing conformation, an interaction of the N- and C-termini opens “fully” the external gate and thus all physiological substrates [uric acid (UA), allantoin (ALL), or uracil: red rhomboid] fit within the major substrate-binding site. Proper substrate binding triggers occlusion of the outward-facing gate (in orange). Closure of the outward-facing gate elicits a gross conformational change leading to the inward-closed structure. This will subsequently disrupt of the N- and C-termini interaction, open the internal gate, and lead to release of substrate into the cytoplasm. (B) Lack of the C- (or N-terminal) region restricts the full opening of the outer gate, in a way that allows only smaller or specific substrates (e.g., ALL or uracil) to have access to the binding site. Thus, UA is excluded either because the outer gate is not sufficiently open or because the closure of the gate does not occur properly. (C) Suppressor mutations indirectly modify the outer gate action, or other filtering or gating elements (in green), so that UA regains access to the binding site and is transported.