Abstract
Ecdysteroids, including the biologically active hormone 20-hydroxyecdysone (20E), play essential roles in controlling many developmental and physiological events in insects. Ecdysteroid biosynthesis is achieved by a series of specialized enzymes encoded by the Halloween genes. Recently, a new class of Halloween gene, noppera-bo (nobo), encoding a glutathione S-transferase (GST) in dipteran and lepidopteran species, has been identified and characterized. GSTs are well known to conjugate substrates with the reduced form of glutathione (GSH), a bioactive tripeptide composed of glutamate, cysteine, and glycine. We hypothesized that GSH itself is required for ecdysteroid biosynthesis. However, the role of GSH in steroid hormone biosynthesis has not been examined in any organisms. Here, we report phenotypic analysis of a complete loss-of-function mutant in the γ-glutamylcysteine synthetase catalytic subunit (Gclc) gene in the fruit fly Drosophila melanogaster. Gclc encodes the evolutionarily conserved catalytic component of the enzyme that conjugates glutamate and cysteine in the GSH biosynthesis pathway. Complete Gclc loss-of-function leads to drastic GSH deficiency in the larval body fluid. Gclc mutant animals show a larval-arrest phenotype. Ecdysteroid titer in Gclc mutant larvae decreases, and the larval-arrest phenotype is rescued by oral administration of 20E or cholesterol. Moreover, Gclc mutant animals exhibit abnormal lipid deposition in the prothoracic gland, a steroidogenic organ during larval development. All of these phenotypes are reminiscent to nobo loss-of-function animals. On the other hand, Gclc mutant larvae also exhibit a significant reduction in antioxidant capacity. Consistent with this phenotype, Gclc mutant larvae are more sensitive to oxidative stress response as compared to wild-type. Nevertheless, the ecdysteroid biosynthesis defect in Gclc mutant animals is not associated with loss of antioxidant function. Our data raise the unexpected hypothesis that a primary role of GSH in early D. melanogaster larval development is ecdysteroid biosynthesis, independent from the antioxidant role of GSH.
Keywords: glutathione, Gclc, ecdysteroid, antioxidant, Drosophila melanogaster
ECDYSTEROIDS are principal insect steroid hormones, playing versatile roles in the regulation of many developmental and physiological processes, especially molting and metamorphosis (Yamanaka et al. 2013; R. Niwa and Y. S. Niwa 2014; Y. S. Niwa and R. Niwa 2014, 2016; Uryu et al. 2015). Ecdysteroids, including ecdysone and 20-hydroxyecdysone (20E), are synthesized from dietary cholesterol or phytosterols via a series of hydroxylation and oxidation steps. Over the past 15 years, a number of enzyme genes that are responsible for ecdysteroid biosynthesis have been identified and characterized, some of which are often called the Halloween genes (Gilbert 2004; Rewitz et al. 2006). The Halloween genes include noppera-bo (Chanut-Delalande et al. 2014; Enya et al. 2014), shroud (Niwa et al. 2010), spook (Namiki et al. 2005; Ono et al. 2006), phantom (Niwa et al. 2004; Warren et al. 2004), disembodied (Chávez et al. 2000; Warren et al. 2002), shadow (Warren et al. 2002), and shade (Petryk et al. 2003). The Halloween genes are characterized by typical phenotypes of their null mutants in the fruit fly Drosophila melanogaster, which display embryonic lethality, abnormalities in embryonic cuticle differentiation, and deficiencies in ecdysteroid levels (Nüsslein-Volhard et al. 1984; Chávez et al. 2000). Among them, phantom, disembodied, shadow, and shade encode cytochrome P450 monooxygenases, each of which has been biochemically characterized as enzymes essential for catalyzing a particular step of the ecdysteroid biosynthesis pathway (R. Niwa and Y. S. Niwa 2014).
Another Halloween gene, noppera-bo (nobo), belongs to a cytosolic glutathione S-transferase (GST) ε family and is well conserved, at least in dipteran and lepidopteran species (Chanut-Delalande et al. 2014; Enya et al. 2014, 2015). Nobo genes are synonymous with D. melanogaster GSTe14, Anopheles gambiae GSTe8, and Bombyx mori GSTe7. Nobo is predominantly expressed in tissues biosynthesizing ecdysteroids, including the prothoracic gland (PG) and the ovary. Notably, the nobo loss-of-function phenotype in D. melanogaster is well rescued by oral administration of cholesterol, the most upstream precursor of the ecdysteroid biosynthesis pathway (Chanut-Delalande et al. 2014; Enya et al. 2014). Therefore, Nobo seems not to catalyze any particular ecdysteroidal intermediates, but rather to regulate the transport and/or metabolism of cholesterol through unknown mechanisms.
The well-investigated reaction catalyzed by GSTs is the addition of the reduced form of L-γ-glutamyl-L-cysteinylglycine, or glutathione (GSH), to substrates. Although endogenous substrate(s) for Nobo have not yet been identified, we demonstrated that a recombinant D. melanogaster Nobo protein possesses the ability to catalyze the conjugation of reduced GSH to an artificial substrate (Fujikawa et al. 2015). It is noteworthy that the mammalian GSTA3-3 is also involved in steroid hormone biosynthesis (Raffalli-Mathieu and Mannervik 2005; Fedulova et al. 2010), while GSTA3-3 is not orthologous to nobo. Therefore, it is feasible to hypothesize that GSH itself is commonly required for steroid hormone biosynthesis across species, although this hypothesis has not yet been examined in any organisms.
GSH is biosynthesized de novo by the consecutive action of two enzymes to conjugate L-glutamine, L-cysteine, and glycine. The first enzyme is glutamate–cysteine ligase (GCL, EC 6.3.2.2), also known as γ-glutamylcysteine synthase, which generates γ-glutamylcysteine (Figure 1A). GCL is a heterodimer of a catalytic subunit (GCLc) and a modulatory subunit (GCLm), which are encoded by different genes (Griffith and Mulcahy 1999; Lu 2014). The GCLc subunit can catalyze the formation of γ-glutamylcysteine in the absence of GCLm, but its activity is increased substantially by covalent interactions with GCLm (Sies 1999; Lu 2014). The second enzyme required for de novo GSH biosynthesis is GSH synthase (GS, EC 6.3.2.3), which links glycine to γ-glutamylcysteine to form GSH (Figure 1A) (Lu 2014). It is generally thought that GCL, but not GS, is the rate-limiting enzyme in GSH biosynthesis (Lu 2014).
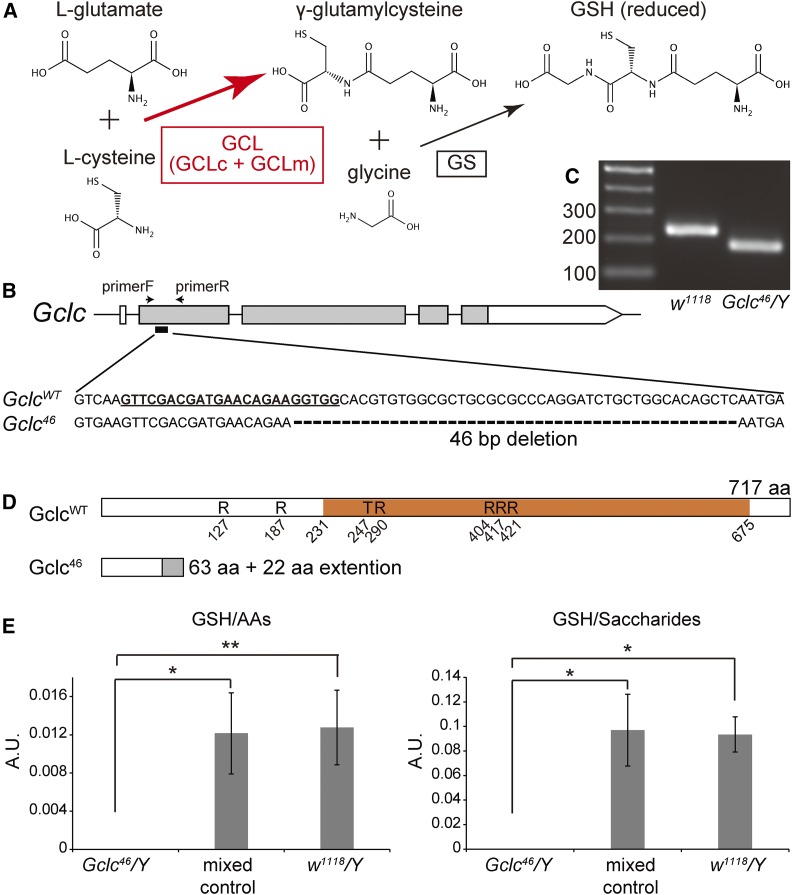
Figure 1.
Gclc is required for glutathione (GSH) biosynthesis. (A) Schematic representation of the glutathione biosynthesis pathway. GCL, γ-glutamylcysteine ligase; GCLc, catalytic subunit of GCL; GCLm, modifier subunit of GCL; and GS, glutathione synthetase. (B) The genomic structures of wild-type Gclc (GclcWT) and the Gclc46 allele. The boxes and lines indicate the exons and introns of Gclc, respectively, based on the description in FlyBase (http://flybase.org/reports/FBgn0040319.html). The Gclc46 allele lacks 46 bp in the second exon. The gray and white colors indicate the coding sequence region and untranslated regions, respectively. Bold underlined characters indicate single guide RNA target and protospacer adjacent motif sequences. (C) Genomic PCR of control (w1118) and Gclc46/Y first-instar larvae. Control larvae were a mixture of both males and females. The PCR primers used are shown in (B). 100, 200, and 300-bp size markers are displayed in the left lane. The PCR products of GclcWT and Gclc46 alleles are 236 and 190 bp, respectively. (D) The predicted primary structures of the proteins encoded by the GclcWT and Gclc46 genes. The orange box indicates the catalytic domain (231–675 aa) classified as γ-glutamylcysteine synthetase/glutamine synthetase clan (http://pfam.xfam.org/clan/CL0286) by Pfam (Finn et al. 2016). The Gclc46 gene product is thought to contain the first 63 aa of the wild-type GCLc protein fused with an additional 22-aa extension (gray), which shows no obvious homology to any known protein. The 85-aa protein lacks equivalent amino acid residues that are identified as substrate-binding determinants of GCLc in other organisms, including R127 of the human GCLc (Hamilton et al. 2003) and others of Trypanosoma brucei GCLc (Abbott et al. 2002). (E) GSH amounts in hemolymph from control and Gclc46/Y larvae at the second-instar stage, when Gclc46/Y showed developmental-arrest phenotype, as shown in Figure 2. w1118/Y male larvae and a mixed population of larvae of Gclc46/FM7 actin-GFP, FM7 act-GFP/Y, and homozygous FM7 actin-GFP (mixed control) were used as control. The arbitrary units (A.U.) were normalized by total amounts of amino acids (AAs) (left) and of saccharides (right) that were simultaneously measured in hemolymph samples. Each bar represents the mean ± SEM. N = 3. * P < 0.05 and ** P < 0.01 by Student’s t-test.
In D. melanogaster, the Gclc and Gclm genes have been reported and their products have been enzymatically characterized (Fraser et al. 2002, 2003). Moreover, Gclc overexpression exerts a beneficial effect on survival under normal and oxidative stress conditions in D. melanogaster (Orr et al. 2005; Luchak et al. 2007; Radyuk et al. 2012; Moskalev et al. 2016). Conversely, Gclc RNA interference (RNAi) animals exhibit neuronal defects, greater sensitivity to oxidative stress, and eventually reduced survival (Luchak et al. 2007; Mercer et al. 2016). All of these studies support the idea that GSH is a major antioxidant in the cell and is critical for protecting cells against reactive oxygen species in adult flies.
In contrast, the role of GSH during D. melanogaster development has not been extensively studied to date; a single genetic study has reported that a mutant animal that lacks a large fragment of the 5′ untranslated region of Gclc is developmentally lethal (Luchak et al. 2007). Therefore, the main objective of this study is to investigate the role of Gclc during development using a Gclc null mutant allele, and to examine whether GSH is involved in ecdysteroid biosynthesis and ecdysteroid-dependent developmental processes in D. melanogaster.
Materials and Methods
Fly strains
D. melanogaster flies were reared on standard agar–cornmeal medium (standard food) at 25° under a 12/12 hr light/dark cycle. w1118 was used as the wild-type (control) strain. UAS-Gclc#3 and UAS-Gclc#6 transgenic strains (Orr et al. 2005) were kindly gifted from William C. Orr (Southern Methodist University). phm-GAL4#22 (McBrayer et al. 2007) and DMef2-GAL4 (Ranganayakulu et al. 1996) were also kind gifts from Michael B. O’Connor (University of Minnesota) and Eric N. Olson (University of Texas Southwestern Medical Center at Dallas), respectively. ppl-GAL4 (Colombani et al. 2003) and byn-GAL4 (Iwaki and Lengyel 2002) were obtained from Hiroko Sano (Kurume University, Japan) and Ryutaro Murakami (Yamaguchi University, Japan), respectively. arm-GAL4 (Loncle et al. 2007), elav-GAL4 (Luo et al. 1994), tubP-GAL4 (Lee and Luo 1999), UAS-dicer2 (#24650), and hsFLP ovoD FRT19A (#23880) were provided by the Bloomington Drosophila Stock Center. p{GawB}NP3084 (Nehme et al. 2007) was provided by the Kyoto Stock Center. UAS-nobo-IR (#101884KK and #40316GD) and UAS-Gclc-IR (#108022KK and #33512GD) were obtained from the Vienna Drosophila RNAi center. y1 v1 nos-phiC31; attP40 and y2 cho2 v1; attP40{nos-Cas9}/CyO (Kondo and Ueda 2013) were obtained from the National Institute of Genetics, Japan.
Generation of Gclc loss-of-function mutant allele
Generation of the Gclc allele was carried out by the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system using the pBFv-U6.2 vector (Kondo and Ueda 2013) provided by the National Institute of Genetics, Japan. To minimize off-target effects of CRISPR/Cas9, we confirmed by BLAST search that no 15-nucleotide stretches within the selected target sequence (23 nucleotides including the protospacer adjacent motif) matched any other sequence on the X chromosome. Sense and antisense oligonucleotides corresponding to single guide RNA (sgRNA) target sequences (5′-CTTCGTTCGACGATGAACAGAAGG-3′ and 5′-AAACCCTTCTGTTCATCGTCGAAC-3′, respectively) were annealed. The annealed oligonucleotide fragment was inserted into a BbsI-digested pBFv-U6.2 vector. The Gclc sgRNA vector was injected into embryos of the y1 v1 nos-phiC31; attP40 strain, generating the U6-Gclc-sgRNA strain. Nos-Cas9-based gene targeting was carried out as previously described (Kondo and Ueda 2013). Males carrying both nos-Cas9 and U6-Gclc-sgRNA transgenes were crossed to FM7c Bar balancer flies by mass mating. Single females from their progeny carrying FM7c Bar were crossed with FM7c Bar/Y males, establishing independent isogenized strains. Among them, we surveyed strains that showed male hemizygous lethality. To confirm indel mutations at the Gclc locus in each hemizygous lethal strain, we performed the T7EI assay as previously described (Kondo and Ueda 2013). Briefly, the DNA fragment including the Cas9 target site was amplified by PCR with KOD FX Neo (Toyobo, Osaka, Japan), the extracted genome DNA from each strain, and the primers (5′-GGGTGACATATTGAAATGGGGCG-3′ and 5′-CCAGATTGAAGTGTGCCATCAGACC-3′, shown as primerF and primerR in Figure 1B, respectively). The PCR products were treated with T7 endonuclease (New England Biolabs, Beverly, MA). The reacted samples were analyzed by agarose gel electrophoresis. After these procedures, we eventually selected the Gclc46 allele. Since this allele has a large (46 bp) deletion in the coding sequence, we determined that the PCR product size difference between GclcWT and Gclc46 was easily detected without T7 endonuclease treatment (Figure 1C). The PCR product from the Gclc46 allele was subcloned into a SmaI-digested pBluescript II SK(-) plasmid (Promega, Madison, WI), then sequenced with T3 and T7 primers. The Gclc46 strain was backcrossed with the w1118 control strain five times and then balanced with the FM7 act-GFP balancer. We used this backcrossed strain—w1118 Gclc46—to which we refer throughout as “Gclc46,” for all experiments in this study. We also generated Gclc46 FRT19Aneo/FM7 act-GFP for germline clone analysis.
Mass-spectrometric quantification of glutathione
Larval bodies of control (w1118) and Gclc46/Y second-instar larvae [60 hr after egg laying (AEL)] were severed on parafilm. The body fluid (0.2 μl) that oozed from the five severed larvae was collected, then mixed with 1 μl distilled water containing 1 mM 2-mercaptoethanol (Nacalai Tesque, Kyoto, Japan). Due to the presence of 2-mercaptoethanol, glutathione disulfide (GSSG) in the sample is converted to GSH. Body-fluid proteins were removed by centrifugation at 10,000 × g for 5 min at 4° after being mixed with 1.5 μl of 0.1% formic acid (Honeywell Fluka, Morristown, NJ) in 80% methanol (Kanto Chemical, Tokyo, Japan). One microliter of the supernatant was introduced to a nanospray tip (Cellomics Tip CH-1; HUMANIX, Hiroshima, Japan), which was then set on a nano-electrospray ionization ion source. Mass-spectrometric detection of GSH was performed on a high-resolution mass spectrometer (LTQ Orbitrap Velos Pro; Thermo Fisher Scientific, Waltham, MA) as previously described (Fujii et al. 2015). The spray voltage for positive-ion detection mode was 1000 V. GSH detection was mainly performed in the range of m/z 100–1000. The spectrometer was calibrated by polytyrosine (CS Bio, Menlo Park, CA) prior to the experiments. Data analysis was conducted using Xcalibur software (Thermo Fisher Scientific). Target mass peaks were detected in the ± 5 ppm range, relative to the theoretical mass of GSH (m/z 308.091).
Mass-spectrometric quantification of 20E
For the measurement of 20E in whole bodies of control (w1118/Y) and w1118 Gclc46/Y hemizygous animals, second-instar larvae (50 hr AEL) of each genotype were collected and the wet weight of each sample was measured. The samples were frozen with liquid nitrogen and stored at −80° until measurement. Extraction of steroids, HPLC fractionation, and mass-spectrometric analyses were performed as previously described (Igarashi et al. 2011; Hikiba et al. 2013). In the mass-spectrometric analyses, the exact quantification range was 0.49–31.25 ng/ml. In this experimental condition, the limit of quantification of 20E was 3.68 pg of 20E/mg of wet weight sample.
Scoring of developmental progression of Gclc mutant animals
Gclc46/FM7 act-GFP and w1118 females were crossed with FM7 act-GFP/Y males, respectively. Eggs were laid on grape plates with yeast pastes at 25° for 4 hr. Twenty-four hours AEL, GFP-negative first-instar larvae, which correspond to Gclc46/Y and w1118/Y males, were collected under a fluorescence dissection microscope M165FC (Leica, Wetzlar, Germany). Sixty hatched GFP-negative first-instar larvae were transferred into vials with standard food (30 animals per vial) and kept at 25°. Every 24 hr, developmental stages were scored by tracheal morphology as previously described (Niwa et al. 2010).
Feeding rescue experiments with various chemicals
Two grams of standard food was kneaded with 200 μl of the following chemical solutions: 50 mg/ml cholesterol (Sigma [Sigma Chemical], St. Louis, MO) in 100% ethanol, 50 mg/ml 20E (ENZO Life Sciences, Farmingdale, NY) in 100% ethanol, 10 mg/ml L(+)-ascorbic acid in distilled water, and 50–200 mg/ml reduced form of GSH (Nacalai Tesque) in distilled water containing 1 mg/ml L(+)-ascorbic acid. For feeding experiments using ethacrynic acid (EA), standard food was kneaded with 0.01, 0.05, 0.1, 0.2, and 0.5% EA (Tokyo Kasei Kogyo, Tokyo, Japan) at final concentrations. First-instar larvae of Gclc46/Y and w1118/Y were collected as described above (see Scoring of developmental progression of Gclc mutant animals). The larvae were then transferred into vials with standard food supplemented with chemicals. Each vial contained a maximum of 30 animals. Animals were reared at 25°C and continuously fed the supplemented food throughout larval development. Developmental stages of larvae were scored by tracheal morphology.
Low-sterol food assay
A recipe for Low-sterol food (LSF) was based on Carvalho et al. (2010) with some modifications. LSF used in this study was a mixture of 10% yeast autolysate (Sigma), 10% glucose, 1% purified agar, 0.3% propionic acid, and 0.06% butyl p-hydroxybenzoate (all Nacalai Tesque). Cholesterol-supplemented LSF was prepared by adding cholesterol (Wako, Osaka, Japan) to LSF. The final concentration of cholesterol in this food was 6.2 μg/ml. In the original recipe of Eaton’s group, yeast autolysate and agarose for the lipid-depleted medium were chloroform-extracted (Carvalho et al. 2010). However, we found that even when we did not treat these materials with chloroform, the control larvae on LSF exhibited a reproducible larval-arrest phenotype (Figure 4A). More importantly, the larval-arrest phenotype on LSF was restored by adding cholesterol to LSF (Figure 4A), implying that sterol contents in our LSF were significantly reduced. We should note that the phenotype on our LSF was weaker than the phenotype on Eaton’s lipid-depleted medium (Carvalho et al. 2010), probably because our LSF recipe omitted the chloroform extraction procedure.
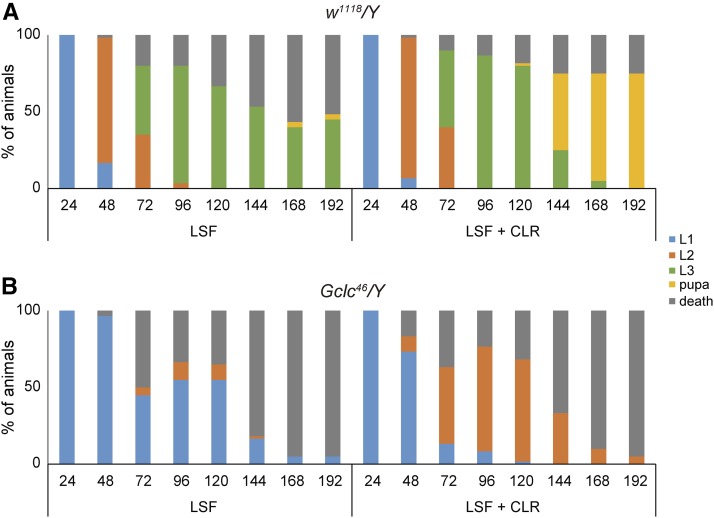
Figure 4.
Molting defect of Gclc46/Y animals is enhanced by low-sterol food (LSF). The survival rate and developmental progression of control w1118/Y (A) and Gclc46/Y (B) animals on LSF and cholesterol-supplemented LSF (LSF + CLR). L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60.
Nile red staining and quantification
Dissection of the brain-ring gland complex was performed as described in Imura et al. (2017). The brain-ring gland complexes were dissected from second-instar larvae at 60 hr AEL in phosphate-buffered saline (PBS), then fixed in 4% paraformaldehyde for 1 hr at room temperature. Fixed tissues were washed three times with PBS for 10 min and incubated in 1 μg/ml Nile Red (Wako) solution dissolved in PBS + 0.5% Tween 20 (PBST) for 30 min at room temperature. Incubated tissues were then washed three times with 0.3% PBST for 10 min and mounted using VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA). Confocal images were captured using a LSM 700 laser scanning microscope (Zeiss [Carl Zeiss], Thornwood, NY). Images of fluorescence intensity were analyzed using ImageJ (Schneider et al. 2012).
Evaluation of antioxidant capacity
For the quantitative determination of the antioxidant capacity of larval body fluids, a spectrophotometric method based on the molybdenum (Mo) blue reaction was employed as previously described (Prieto et al. 1999). The assay is based on the reduction of Mo(VI) to Mo(V) by the sample analyte and the subsequent formation of a blue-colored phosphate/Mo(V) complex at acidic pH. Larval bodies of control (w1118/Y) and Gclc46/Y second-instar larvae (48 hr AEL) were severed on parafilm, followed by dropping 20 μl distilled water on 40 dissected larvae. Ten microliters of the body-fluid–water mixture was taken and mixed with 100 μl of reagent solution [1.3% ammonium molybdate (Sigma), 0.02% dipotassium hydrogen phosphate (Wako), and 1.4 M sulfuric acid (Nacalai Tesque)]. The samples were incubated in a thermal block at 95° for 90 min. After the samples cooled to room temperature, their absorbance was measured at 695 nm against a blank. As a series (0–100 mg/ml) of solutions of L(+)-ascorbic acid (Nacalai Tesque) were used as the standard, the antioxidant capacity was expressed in ascorbic acid equivalents. Values of antioxidant capacity were also normalized by the amount of protein in larval body fluids, measured by the Bradford protein assay (Bradford 1976) with a Qubit protein assay kit and a Qubit 2.0 fluorometer (Thermo Fisher Scientific).
Oxidative stress treatment
Methyl viologen dichloride hydrate, i.e., paraquat (PQ), was used as an oxidative stress inducer as previously described (Jünger et al. 2003; Jumbo-Lucioni et al. 2013). PQ medium was composed of 0.8% electrophoresis-grade agarose (Nacalai Tesque), 10% sucrose (Nacalai Tesque), and 20 mM PQ (Sigma) in PBS. PQ was added to the solution after cooling to 40°. Control medium was sucrose–agar without PQ. Thirty second-instar larvae at 48 hr AEL were each collected and transferred to vials containing 5 ml of PQ medium or control medium. Twelve hours after the transfer, the number of surviving and dead larvae (60 hr AEL) was counted (N = 120 for each condition).
Quantitative reverse transcription (RT)-PCR
RNA isolation, cDNA synthesis, and quantitative RT-PCR reactions were performed as previously described (Enya et al. 2014). Total RNA from several tissues of third-instar larvae and adults of the w1118 strain were used. To extract total RNA from the ring glands in the third-instar larval stage, we collected the second-instar larvae at 60 hr AEL and let them molt to the third-instar stage in 6 hr. These larvae were dissected at various hours after L2–L3 molting (hr A3L). The primers for quantifying Gclc were 5′-CTCACGCGTAACATTCGCAAGC-3′ and 5′-CAAGCAACAGCAGCCCATGC-3′. Primers amplifying neverland (nvd), nobo, and rp49 were previously described (Foley et al. 1993; Yoshiyama et al. 2006; Enya et al. 2014). Expression levels were normalized to rp49 in the same sample. Serial dilutions of a plasmid containing the ORF of each gene were used as a standard.
Transgenic rescue experiment
The GAL4/upstream activating sequence (UAS) system (Brand and Perrimon 1993) was used to overexpress genes in D. melanogaster. UAS-Gclc#3 and UAS-Gclc#6 (Orr et al. 2005) were used to overexpress Gclc genes under the control of various tissue-specific GAL4 drivers. These UAS lines carry the same pUAST construct, but the constructs in the #3 and #6 strains are located on the third and second chromosome, respectively. The following 11 strains were established and used for transgenic rescue experiments: (1) w1118; UAS-Gclc#6/CyO; TM3 Sb//TM6 Tb, (2) w1118; Roi/CyO; UAS-Gclc#3/TM3 Sb, (3) w1118; UAS-Gclc#6/CyO; tubP-GAL4/TM3 Sb, (4) w1118; UAS-Gclc#6; phm-GAL4#22/TM3 Sb, (5) w1118; phm-GAL4#22 UAS-Gclc#3/TM3 Sb, (6) w1118; arm-GAL4 UAS-Gclc#6/CyO, (7) w1118; ppl-GAL4/CyO; UAS-Gclc#3/TM3 Sb, (8) w1118: elav-GAL4 UAS-GFP/CyO; UAS-Gclc#3/TM3 Sb, (9) w1118; UAS-Gclc#6; byn-GAL4 UAS-RFP/TM3 Sb, (10) w1118; p{GawB}NP3084/CyO; UAS-Gclc#3/TM3 Sb, and (11) w1118; UAS-Gclc#6; mef2-GAL4/TM3 Sb. Parental males of these flies carrying both GAL4 and UAS constructs were crossed with parental Gclc46/FM7 act-GFP females. In the next generation, the number of adult offspring males (Gclc46/Y with GAL4 and UAS) and females (Gclc46/+ with GAL4 and UAS) was counted (Table 2).
Table 2. Viability of Gclc46/Y animals expressing Gclc.
| GAL4 driversa | Tissues/cells in which GAL4 is active | Number of adults |
|---|---|---|
| -*1 | No GAL4 | 0 (129) |
| -*2 | No GAL4 | 0 (101) |
| tubP-GAL4*1 | Ubiquitous | 35 (34) |
| phm-GAL4#22*1 | PG | 21 (104) |
| phm-GAL4#22*2 | PG | 56 (124) |
| arm-GAL4*2 | Epithelial cells | 94 (95) |
| ppl-GAL4*2 | Fat body | 48 (95) |
| elav-GAL4*2 | Nerve system | 61 (74) |
| byn-GAL4*1 | Hindgut | 32 (68) |
| NP3084*2 | Gut | 54 (55) |
| Dmef2-GAL4*1 | Muscle | 1 (165) |
The number of viable Gclc46/Y adults was scored. Transgenes were driven by several GAL4 drivers. Details of this experiment are described in the Materials and Methods. Values in parentheses indicate the number of viable control Gclc46/+ heterozygous females from the parental strains in the same experimental batches. PG, prothoracic gland.
*1, UAS-Gclc#6 was used, *2, UAS-Gclc#3 was used.
Germline mosaic clone analysis
The FLP-DFS technique was performed as previously described (Chou and Perrimon 1992). Virgin females of Gclc46 FRT19Aneo/FM7 act-GFP were crossed with males of hsFLP ovoD y1 w1118 sn3 FRT19A/C(1)DX, y1 w1 f1. By the late second/third-instar stages, the progeny were heat-shocked at 37° for 2 hr over the successive 3 days. When adults were eclosed, virgin females of Gclc46 FRT19A/hsFLP ovoD FRT19A were crossed with males of w1118. The progeny of homozygous Gclc46 germline clones completed embryogenesis and larval development.
Data availability
All strains and materials are available upon request. Supplemental Material, File S1 contains detailed descriptions of animal count data represented in Figure 2, Figure 3, Figure 4, Figure 6, and Figure 7.
Figure 2.
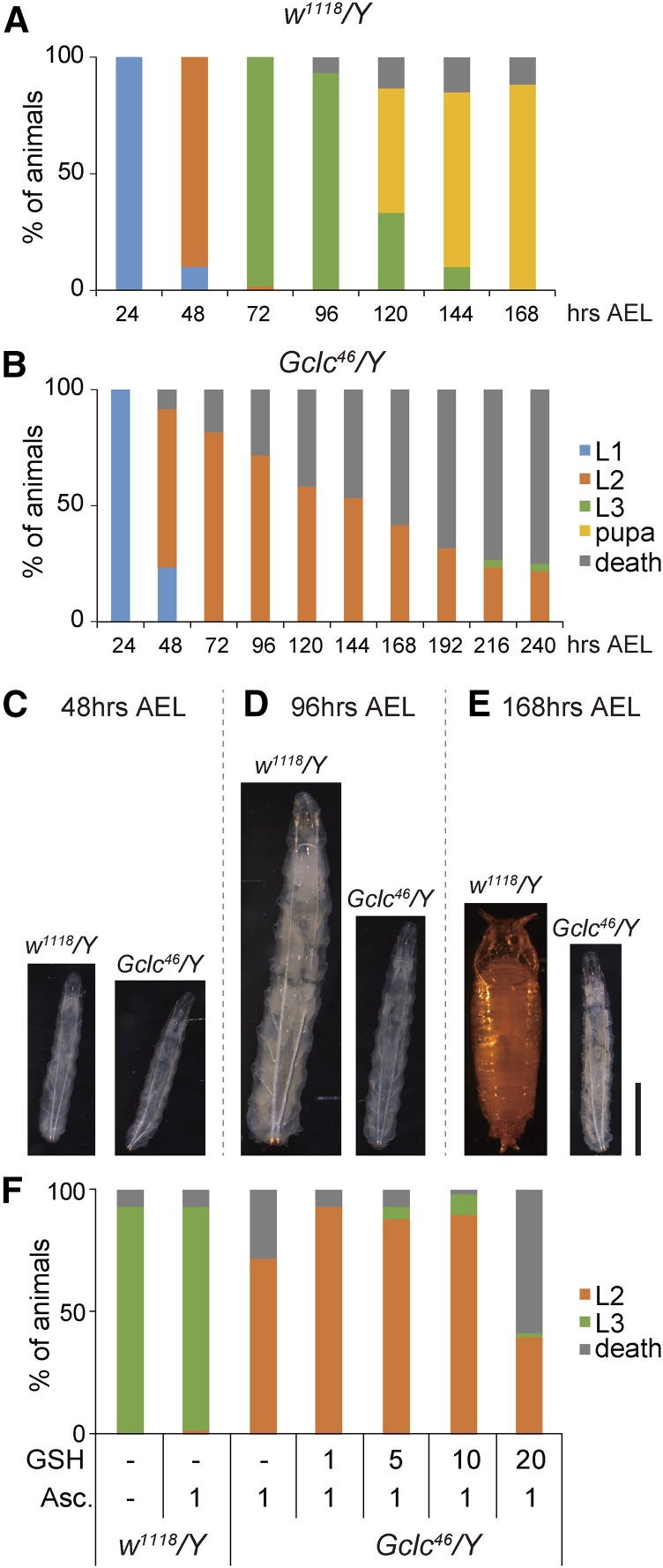
Larval lethality and developmental-arrest phenotype of Gclc46/Y larvae. (A and B) The survival rate and developmental progression of control w1118/Y (A) and Gclc46/Y (B) animals. L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60. (C–E) Comparison of body size and developmental stage between control w1118/Y (left) and Gclc46/Y (right) animals. Typically, control animals were second-instar larvae, third-instar larvae, and pupae at 48 hr (C), 96 hr (D), and 168 hr (E) after egg laying (AEL), respectively. In contrast, Gclc46/Y animals in these photos are all second-instar larvae, collected at the same time points. Bar, 0.5 mm. (F) The larval-arrest phenotype of Gclc46/Y animals was partly rescued by oral administration of glutathione (GSH) from food. The survival rate and developmental progression of w1118/Y and Gclc46/Y animals at 96 hr AEL, fed standard food supplemented with vehicle ethanol (−) with or without GSH and ascorbic acid (Asc.) just after hatching. GSH concentration in food ranged 0–20 mg/ml. Asc. concentration was 1 mg/ml. N = 60.
Figure 3.
Molting defect of Gclc46/Y animals is rescued by feeding 20-hydroxyecdysone (20E) and cholesterol. (A and B) The survival rate and developmental progression of control w1118/Y and Gclc46/Y animals that were fed standard food supplemented with vehicle ethanol (−), 20E, and cholesterol (CLR). The number of larvae was counted at 96 hr (A) and 168 hr (B) after egg laying (AEL). L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60. (C–H) Whole bodies (C and F), anterior tracheal pits (D and G), and dissected mouth hooks (E and H) of Gclc46/Y larvae at 96 hr AEL. The animals were reared on standard food supplemented with control ethanol (C–E) and 20E (F–H). Gclc46/Y larvae raised on a control diet died in the second-instar larval stage, exhibiting singular insertions of anterior tracheal pits (D) and 2–5 teeth on mouth hooks ((E); red arrowheads). In contrast, 20E-fed Gclc46/Y larvae molted in the third-instar larval stage as judged by the branched morphology of the anterior tracheal pits (G) and numerous small teeth on the mouth hook ((H); red arrowheads), typical features of third-instar larvae. Bars, 1 mm for (C and F), 148 μm for (D and G), and 125 μm for (E and H).
Figure 6.
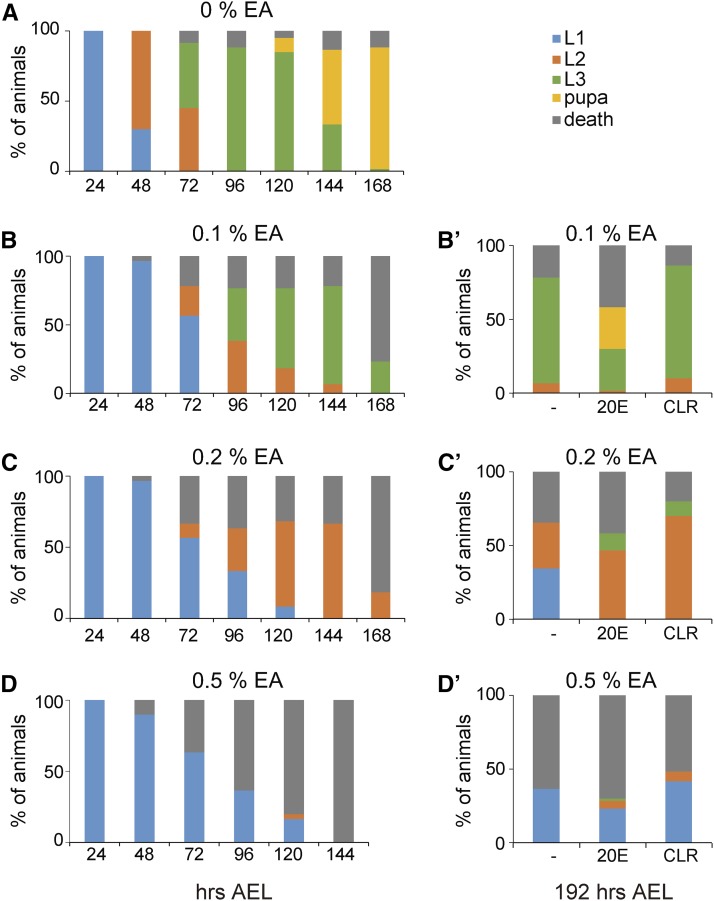
Larval-arrest phenotype induced by ethacrynic acid (EA) is rescued by feeding 20-hydroxyecdysone (20E) and cholesterol (CLR). (A–D) The survival rate and developmental progression of wild-type (w1118) animals that were fed standard food supplemented with vehicle ethanol 0%, (A), 0.1% (B), 0.2% (C), and 0.5% (D) EA. (B’–D’) The survival rate at 192 hr after egg laying (AEL) was examined when vehicle ethanol (−), 20E, and CLR were added with 0.1% (B’), 0.2% (C’), and 0.5% (D’) EA to standard food. L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60.
Figure 7.
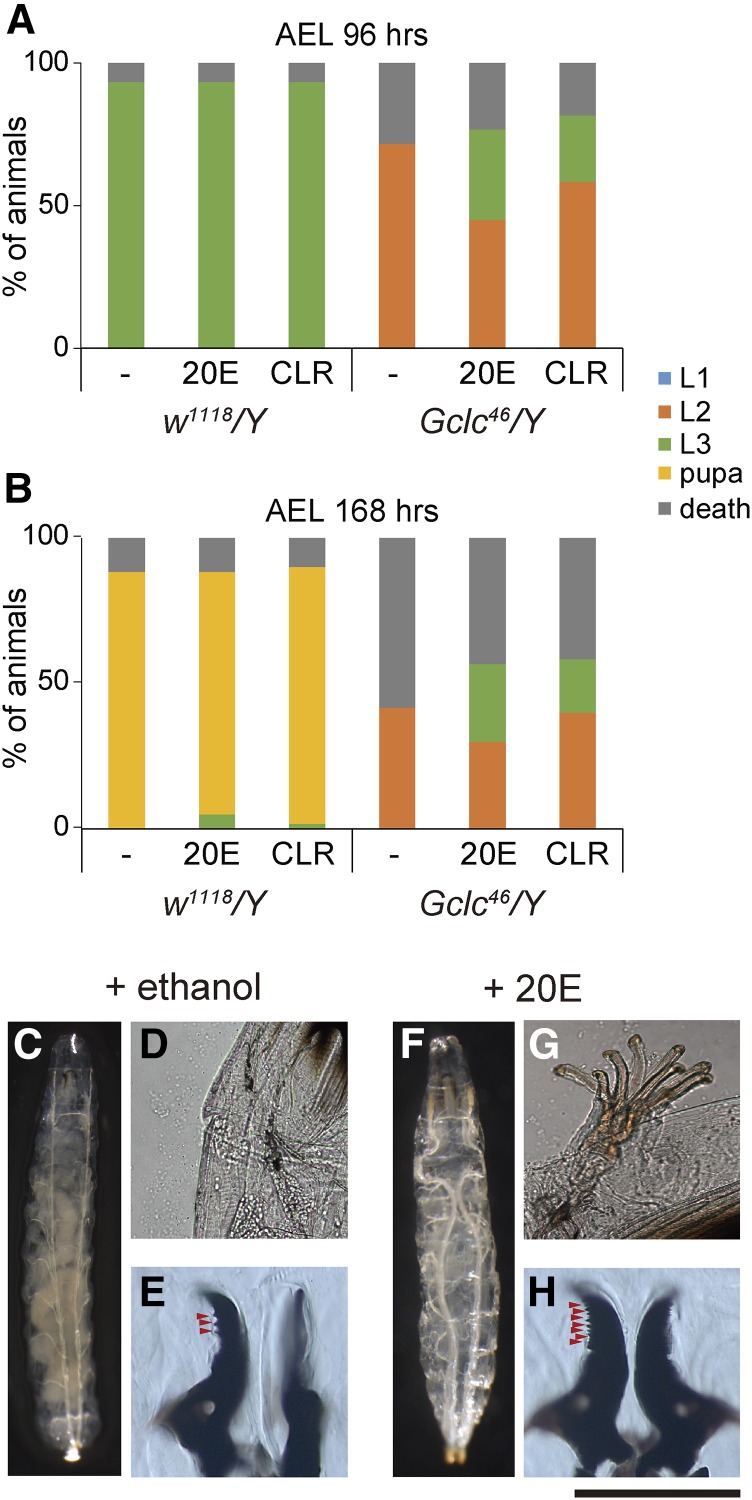
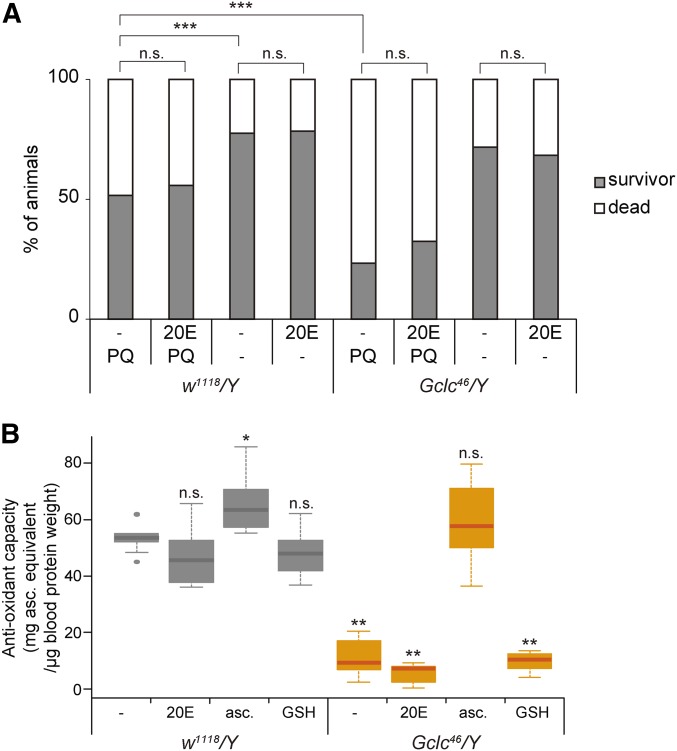
Oxidative stress response and antioxidant-capacity phenotypes of Gclc46/Y are not rescued by 20-hydroxyecdysone (20E) administration. (A) The survival rate and developmental progression of control w1118/Y and Gclc46/Y animals that were fed sucrose‐agar medium containing paraquat (PQ) and/or 20E. Second-instar larvae at 48 hr after egg laying (AEL) were transferred to the medium, then fed media for 12 hr. *** P < 0.001 chi-square test with Bonferroni correction. n.s., not significant. (B) Antioxidant capacity of body fluids isolated from control w1118/Y and Gclc46/Y second-instar larvae (60 hr AEL) that were fed standard food supplemented with vehicle ethanol (−), 20E, ascorbic acid (asc.), and glutathione (GSH). * P < 0.05 and ** P < 0.01 by Dunnett’s test.
Results
Generation of complete Gclc loss-of-function allele in D. melanogaster
We first generated a complete Gclc loss-of-function allele using CRISPR/Cas9-dependent genome-editing technology (Kondo 2014). We succeeded in isolating the allele called Gclc46, which has a 46-bp deletion 185 bp downstream of the start codon (Figure 1, B and C). The predicted protein encoded by the Gclc46 allele lacks a large portion of the C-terminus, which contains amino acid residues critical for GCL’s enzymatic activity (Abbott et al. 2002; Hamilton et al. 2003) (Figure 1D).
We next examined whether Gclc46 mutant animals exhibited GSH deficiency in vivo. We utilized Gclc46/Y hemizygous males for subsequent analyses, as Gclc is located on the X chromosome, making it technically difficult to obtain homozygous Gclc mutant females by conventional genetic crossing. Mass-spectrometric analysis revealed that hemolymph GSH in Gclc46/Y larvae was undetectable, indicating significant reduction compared to control larvae (Figure 1E). These results suggest that Gclc46 is a complete loss-of-function allele and causes drastic GSH deficiency.
Gclc mutant animals exhibit developmental arrest at the second-instar larval stage
Similar to a phenotype of mutants lacking a large fragment of the 5′ untranslated region of Gclc (Luchak et al. 2007), Gclc46/Y hemizygous mutant animals were lethal. We examined the lethal phase for Gclc46/Y animals in more detail. Gclc46/Y animals completed embryogenesis, hatched normally, and showed no apparent morphological defects in the first- and second-instar larval stages (Figure 2, A–C). However, most Gclc46/Y animals showed arrested development in the second-instar larval stage (Figure 2, B and D), and never became third-instar larvae, pupae, or adults (Figure 2, B and E). As described later, we confirmed that the lethality of Gclc46/Y animals was rescued by transgenic expression of Gclc, suggesting that the observed lethality did not result from off-target mutations by CRISPR/Cas9.
Oral administration of GSH to partly rescue second-instar larval-arrest phenotype of Gclc mutants
To clarify that the second-instar larval-arrest phenotype of Gclc46/Y was caused by loss of GSH, we conducted a feeding rescue experiment to examine whether oral administration of GSH rescued the phenotype. Feeding standard food mixed with GSH did not rescue the second-instar larval-arrest phenotype of Gclc46/Y animals (data not shown). Since GSH is easily oxidized in the environment and converted into GSSG, which loses the antioxidant capacity (Sies 1999; Aquilano et al. 2014), we speculated that a large portion of GSH mixed with food might convert to the inactive GSSG. Thus, we next conducted feeding rescue experiments using standard food containing not only GSH but also ascorbic acid, a well-known reducing agent, to prevent the conversion of GSH to GSSG. Oral administration of ascorbic acid alone did not rescue the second-instar larval-arrest phenotype of Gclc46/Y animals (Figure 2E). In contrast, we found that a small but significant number of Gclc46/Y animals developed to third-instar larvae on standard food supplemented with both 5–20 mg/ml GSH and 1 mg/ml ascorbic acid (Figure 2E). These results suggest that, at least in part, the phenotype observed upon Gclc loss-of-function is due to loss of GSH in vivo.
The second-instar larval-arrest phenotype of the Gclc mutant is due to loss of ecdysteroids
The larval-arrest phenotype of the Gclc46/Y mutant phenotype was reminiscent of that of partial loss-of-function animals of several ecdysteroidogenic genes, such as shroud RNAi and nobo RNAi animals (Niwa et al. 2010; Enya et al. 2014). Thus, we next examined whether the larval-arrest phenotype of Gclc46/Y animals was due to the loss of ecdysteroids. We first examined ecdysteroid titer in second-instar larvae (50 hr AEL) of control (w1118/Y) and Gclc46/Y animals by mass-spectrometric analysis. In control larvae, we detected 9.87 ± 2.82 pg of 20E equivalent/mg of wet weight (mean ± SEM, N = 5). In contrast, among five independent samples of Gclc46/Y larvae, ecdysteroid titer in three samples was below the quantifiable limit under the same experimental conditions (See Materials and Methods). The titer in the two other samples also showed a significantly lower amount (6.22 ± 1.62 pg of 20E/mg of wet weight).
We also found that a considerable number of Gclc46/Y animals were able to grow to the third-instar larval stage when they were fed standard food containing 20E just after hatching (Figure 3). It should be noted that oral administration of 20E did not allow Gclc46/Y animals to increase their body size during the third-instar larval stage, to become pupae, or reach later stages (Figure 3, A and B). These results suggest that Gclc loss-of-function impairs ecdysteroid biosynthesis during larval development, with Gclc appearing to have pivotal roles other than ecdysteroid biosynthesis.
Gclc is involved in the regulation of the behavior of cholesterol
Considering that the Halloween gene nobo encodes a GST, we wondered whether a relationship exists between Gclc and nobo for larval development. To address this question, we first performed a feeding experiment with cholesterol, as the nobo loss-of-function phenotype in D. melanogaster is well rescued by oral administration of cholesterol (Chanut-Delalande et al. 2014; Enya et al. 2014). When Gclc46/Y animals were fed standard food supplemented with cholesterol just after hatching, 18% of the animals developed to third-instar larvae, while failing to increase their body size during the third-instar larval stage and grow into pupae or adults (Figure 3, A and B). The rescue ability of cholesterol for Gclc46/Y animals was similar to that of 20E as described above (Figure 3, A and B).
Conversely, we took an approach using LSF (see Materials and Methods). Under our experimental conditions, most control animals fed LSF arrested in the second- or third-instar larval stage, and never grew to pupae or adults (Figure 4A). This phenotype was restored by cholesterol-supplemented LSF, indicating that the larval-arrest phenotype with LSF is due to sterol depletion. We found that the larval-arrest phenotype of Gclc46/Y animals was significantly enhanced in LSF as compared to that in cholesterol-supplemented LSF (Figure 4B). Most Gclc46/Y animals fed LSF arrested in the first-instar larval stage, demonstrating that the larval-arrest phenotype of Gclc mutants is sensitively affected by the amount of cholesterol in food.
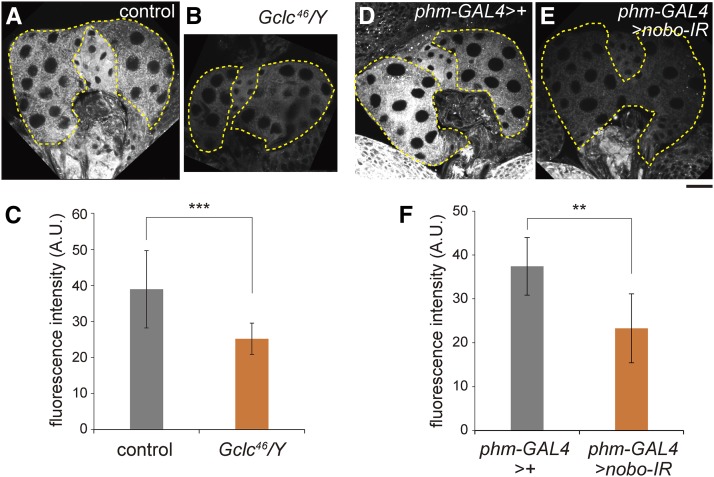
We next examined whether cellular accumulation of neutral lipids, including cholesterol, was affected in PG cells of Gclc46/Y and nobo RNAi second-instar larvae by Nile Red staining (Figure 5). In the control larvae, neutral lipids were highly accumulated in the ring gland, which contains the PG cells, the corpora allata, and the corpora cardiaca. In contrast, the signals were significantly reduced in the ring gland of the Gclc46/Y mutant larvae (Figure 5, B and C). We also observed significant reduction of Nile Red signals in the second-instar larvae of nobo RNAi animals (Figure 5, D–F). Based on the reminiscent phenotype between Gclc46/Y and nobo RNAi animals (Figure 5, B and E), these results suggest that Gclc is involved in cholesterol transport and/or metabolism, as is the case with Nobo.
Figure 5.
Nile Red staining signals are reduced in the prothoracic gland of Gclc46/Y and nobo RNAi animals. (A, B, D, and E) Fluorescence images of the prothoracic gland (PG) from control w1118/Y (A), Gclc46/Y (B), w1118; UAS-dicer2/+; phm-GAL4#22/+ (D), and w1118; UAS-dicer2/+; phm-GAL4#22/UAS-nobo-IR(#40316GD) (E) stained with Nile Red. All animals were in the second-instar larval stage. Areas of the PGs are outlined by dotted lines. Bar, 20 μm. (C and F) Quantification of Nile Red staining signals in the PG area by ImageJ software. (C) N = 10. (F) N = 11. ** P < 0.01 and *** P < 0.001 by Student’s t-test. It should be noted that, even though nobo function was inhibited only in the PG, Nile Red fluorescence was reduced not only in the PG, but also in the corpora allata and the corpora cardiaca for unknown reasons.
Gclc genetically interacts with nobo for larval development
To gain insights into a genetic interaction between Gclc and nobo, we examined whether the developmental arrest phenotype by nobo RNAi was enhanced by Gclc mutation. For this purpose, we combined Gclc46 mutation with PG-specific nobo RNAi. More than 70% of control larvae (FM7/Y; phm > dicer2, nobo-IR) were in the second-instar larval stage 70–94 hr AEL (Table 1). In contrast, only 13% of the double mutants (Gclc46/Y; phm > dicer2, nobo-IR) became the second-instar larvae, and the majority of them remained in the first-instar larval stage (Table 1). These results suggest that Gclc function is coupled with Nobo for larval development.
Table 1. The larval arrest phenotype of nobo RNAi animals is enhanced by Gclc46 mutation.
| phm-GAL4#22>dicer2, nobo-IR | Control | Gclc46 |
|---|---|---|
| L1 | 16 | 33 |
| L2 | 41 | 5 |
| Total | 57 | 38 |
We combined Gclc46 mutation with prothoracic gland (PG)-specific nobo RNA interference (RNAi), and scored developmental stages of larvae 70–94 hr after egg laying (AEL). Gclc46 mutant genotype: Gclc46/Y; UAS-dicer2/UAS-nobo-IR(#101884KK); phm-GAL4#22/+. Control genotype: FM7/Y; UAS-dicer2/UAS-nobo-IR(#101884KK); phm-GAL4#22/+. After counting the number of the first- and second-instar larvae, genomic DNA from each individual was extracted, and then the genotype of each individual was determined by genomic PCR. The PCR products of GclcWT and Gclc46 alleles are separable as shown in Figure 1C. In this analysis, we could exclude Gclc46 heterozygous females (genotype: Gclc46/FM7; UAS-dicer2/UAS-nobo-IR; phm-GAL4#22/+) by genomic PCR, as Gclc46/FM7 females gave rise to multiple PCR bands (236 and 190 bp).
GST inhibitor-treated larvae phenocopy Gclc mutant animals
To further support our hypothesis that GSH is primarily essential for larval developmental progression, dependent on GST activity, we conducted a feeding experiment of wild-type animals with the general GST inhibitor ethacrynic acid(EA) (Awasthi et al. 1993). We previously demonstrated that EA inhibits Nobo enzymatic activity using an artificial substrate in vitro (Fujikawa et al. 2015). When the wild-type larvae were raised on EA-containing food, they resulted in a developmental-arrest phenotype in a dose-dependent manner (Figure 6, A–D). Moreover, the EA-induced larval-arrest phenotype was rescued by coadministration of 20E or cholesterol (Figure 6, B’–D’). Thus, EA-treated animals phenocopied Gclc mutant animals in terms of the larval-arrest phenotype. These results provide us with another line of evidence showing that Gclc is required for larval development through the function of GST, possibly via Nobo.
Gclc mutant animals exhibit significant reduction in antioxidant capacity
One of the most crucial roles of GSH is the maintenance of redox homeostasis, providing reducing equivalents for the elimination of reactive oxygen species. We therefore investigated whether Gclc mutant animals displayed any defects in the antioxidant system in D. melanogaster larvae.
We first examined whether the sensitivity to oxidative stress was changed between control and Gclc46/Y larvae, as Gclc RNAi adult flies are less resistant to oxidative stress (Luchak et al. 2007). The second-instar larvae of control and Gclc46/Y animals were fed a sucrose–agar medium containing the well-known oxidative stress inducer PQ for 12 hr. The medium did not contain rich nutrients and the survival rate of control animals fed the medium without PQ was 78% (Figure 7A). Under these experimental conditions and on PQ medium, a smaller number (52%) of control animals survived. In contrast, much fewer (23%) Gclc46/Y larvae survived on PQ medium (Figure 7A).
We next examined the antioxidant capacity of Gclc mutant animals. We utilized an established spectrophotometric method based on the Mo blue reaction (Prieto et al. 1999) to quantitatively determine the capacity of larval body fluids. Consistent with the hypersensitivity to oxidative stress, we found that body fluid from Gclc46/Y larvae exhibited a significant reduction in antioxidant capacity compared to control animals (Figure 7B). These results suggest that loss of GSH in Gclc mutants leads to defective antioxidant capacity.
Importantly, neither antioxidant capacity nor the hypersensitivity-to-oxidative stress phenotype of second-instar Gclc46/Y larvae was rescued by oral administration of 20E (Figure 7, A and B). In addition, oral administration of ascorbic acid restored antioxidant capacity (Figure 7B) but did not rescue the second-instar larval-arrest phenotype of Gclc46/Y larvae (Figure 2F). Given that 20E administration rescued the second-instar larval-arrest phenotype of Gclc46/Y animals, allowing them to grow to the third-instar larval stage, these results suggest that the larval-arrest phenotype of Gclc mutant animals does not correlate with the antioxidant-capacity defect. In other words, the primary role of GSH during the early larval stage is to be involved in ecdysteroid biosynthesis, but not maintenance of redox homeostasis.
Rescue of larval-arrest phenotype of Gclc mutant by Gclc transgene
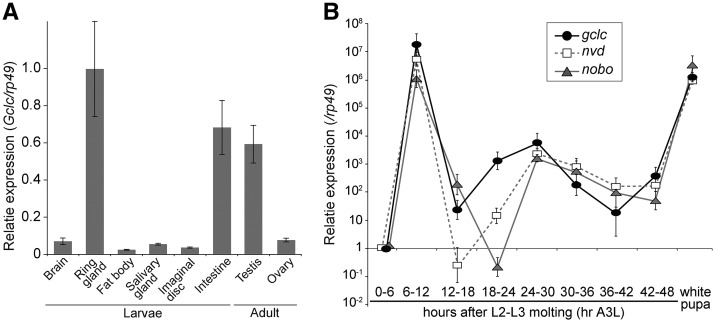
To address the functional specificity of Gclc in the PG, we performed a quantitative RT-PCR experiment to investigate the tissue distribution of the Gclc transcript. In the late third-instar larval stage, Gclc was highly expressed in the ring gland as compared to other tissues (Figure 8A), consistent with a previous transcriptome study (Ou et al. 2016). We also examined a temporal expression pattern of Gclc and nobo in addition to neverland (nvd), encoding the cholesterol 7,8-dehydrogenase for ecdysteroid biosynthesis (Yoshiyama et al. 2006; Yoshiyama-Yanagawa et al. 2011). In the ring glands of the third-instar larvae, the temporal fluctuation of Gclc transcript was well correlated with those of nobo and nvd transcripts (Figure 8B), suggesting that the expression of Gclc is coupled to ecdysteroid biosynthesis in the PG. On the other hand, weaker, yet considerable, expression was observed in many larval tissues other than the PG (Figure 8A). This is in a sharp contrast with nobo and nvd, which are predominantly expressed in the PG during larval development (Yoshiyama et al. 2006; Enya et al. 2014).
Figure 8.
The spatiotemporal expression profile of Gclc in the third-instar larvae. (A) Gclc mRNA amounts were quantified by quantitative RT-PCR. Total RNA samples were prepared from several tissues in wandering third-instar larvae and adult flies. The normalized Gclc mRNA level in the ring gland is set as 1. (B) Total RNA samples were prepared from the ring glands of third-instar larvae. The relative expression levels of Gclc, nvd, and nobo were normalized to the levels of 0–6 hr after L2–L3 molting (hr A3L). Each error bar represents the SEM from at least three independent samples.
To test if Gclc is cell autonomously required in the PG, we induced PG-specific RNAi against Gclc by using phm-GAL4#22 and two independent transgenic UAS-RNAi lines (see Materials and Methods). Unexpectedly, the PG-specific Gclc RNAi larvae exhibited no obvious developmental arrest phenotypes and normally grew up to adulthood (Table S1). This result implies the possibility that Gclc function in tissues other than the PG might affect ecdysteroid biosynthesis.
To address this possibility, we conducted tissue-specific overexpression experiments. The lethality of Gclc46/Y animals was rescued by expression of the UAS-Gclc transgene driven by the ubiquitous tubP-GAL4 driver (Table 2). In addition, the lethality of Gclc46/Y animals was rescued by expression of Gclc in the PG cells by the phm-GAL4 driver, consistent with the idea that GSH plays an essential role in the PG. Unexpectedly, the Gclc46/Y lethal phenotype was also rescued to some degree by several GAL4 drivers that were active in restricted types of tissues or cells other than the PG, including the fat body (ppl-GAL4), the nervous system (elav-GAL4), the hindgut (byn-GAL4), and the wider gut area (NP3084), but not the muscle (Dmef2-Gal4) (Table 2). These results raise the interesting possibility that GSH biosynthesized in peripheral tissues other than the PG might systemically circulate and affect ecdysteroid biosynthesis in the PG.
Discussion
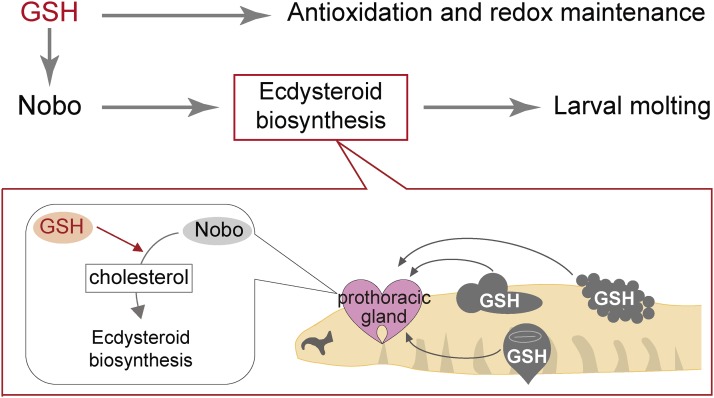
In this study, we report the effect of GSH deficiency employing genetic, developmental, and physiological approaches, using complete Gclc loss-of-function mutant animals of D. melanogaster. It is widely accepted that GSH serves as not only the substrate of GSTs but also the most abundant intracellular nonprotein thiol in most organisms. Indeed, a number of pioneering studies using adult D. melanogaster have supported the antioxidant role of GSH in maintaining redox homeostasis (Orr et al. 2005; Luchak et al. 2007; Beaver et al. 2012; Radyuk et al. 2012; Klichko et al. 2015; Mercer et al. 2016; Moskalev et al. 2016). In this sense, the unexpected finding in this study is that the second-instar larval-arrest phenotype of Gclc mutant animals is not due to defective antioxidant function, but rather due to an impairment of ecdysteroid biosynthesis. Given that the ecdysteroid biosynthesis pathway requires a specialized GST, namely Nobo, we propose our hypothesis that GSH is primarily important for ecdysteroid biosynthesis through the regulation of the enzymatic activity of Nobo during early larval development (Figure 9).
Figure 9.
Model of the systemic action of glutathione (GSH) in controlling larval development.
However, it must be noted that GSH loss in vivo may also result in pleiotropic effects in the PG, as GSH is involved in many biological processes such as antioxidant function and xenobiotics. We cannot completely rule out the possibility that ecdysteroid biosynthesis is indirectly affected, independently from Nobo function. In future studies, it would be worth examining whether a mutated Nobo protein that selectively abolishes GSH binding is dysfunctional for ecdysteroid biosynthesis or not.
We must also take into consideration that GSH plays crucial roles in physiological processes other than ecdysteroid biosynthesis after the third-instar larval stage. This consideration is based on our observation that Gclc46/Y animals on a 20E-supplemented diet never grow into later third-instar larvae or pupae. One possibility is that GSH serves as a substrate for not only Nobo, but also other nonecdysteroidogenic GSTs in developing third-instar larvae and pupae. The D. melanogaster genome possesses at least 36 independent cytosolic GSTs (Wai et al. 2007). Previous studies have demonstrated that a number of cytosolic GST genes are expressed in cells other than PG cells, such as neurons and midgut cells, of third-instar larvae (Li et al. 2008; Deng and Kerppola 2013, 2014; Harrop et al. 2014). Some of these genes are responsible for the xenobiotic response. Related to this point, a recent important finding is that the CncC‐dKeap1 pathway, a major upstream regulator of xenobiotic detoxification, is essential for the larva-to-pupa transition and has significant effects on transcription of the GSTd1 and GSTe1 xenobiotic response genes (Deng and Kerppola 2013, 2014). Thus, GCLc-dependent GSH biosynthesis might play more versatile roles in later development as compared to earlier larval development.
Our study shows that Gclc46/Y animals can complete embryogenesis and first-instar larval development. In contrast, complete nobo loss-of-function animals exhibit embryonic lethality with typical Halloween mutant phenotypes (Chanut-Delalande et al. 2014; Enya et al. 2014), implying that GSH is necessary for embryonic development. To address if GSH is maternally loaded into eggs, we generated a Gclc germline clone, but observed no obvious phenotype of their offspring (data not shown). This result implies that maternal GSH is not supplied from germline, but instead from somatic follicle cells. Alternatively, GSH could be loaded maternally from food; in the yeast Saccharomyces cerevisiae, which is contained in the standard food for rearing D. melanogaster, GSH is present in high concentrations of up to 10 mM (Penninckx 2002).
Another important finding in this study is that GSH might systemically circulate throughout the larval body to be eventually taken up by PG cells (Figure 9). To incorporate extracellular GSH into PG cells, GSH transporters must be present in the hemolymph. In S. cerevisiae and the plant Arabidopsis thaliana, Hgt1p and AtOPT4 are identified as high-affinity GSH transporters (Bourbouloux et al. 2000; Zhang et al. 2016), while their orthologs have not been found in animal genomes. In mammals, two organic-anion transporters, OAT1 and OAT3, are potential candidates that may be responsible for extracellular GSH uptake, whereas these transporters seem not to be specific for GSH and their in vivo function has not been evaluated (Bachhawat et al. 2013). Further studies are required for the identification and characterization of GSH transporters underlying the systemic action of GSH in animals.
It has been reported that GSH amounts and redox status fluctuate in a temporal and developmental stage-specific manner during zebrafish early embryogenesis (Timme-Laragy et al. 2013). Moreover, Gclc homozygous knockout mice are embryonic lethal and die before gestational day 13 (Dalton et al. 2000). However, a functional role of GCLc and GSH during embryogenesis is still largely unclear, as embryonic phenotypes of Gclc mutants in either zebrafish or mice have not been extensively investigated. It would be intriguing to examine whether GSH-dependent steroid hormone biosynthesis is also involved in early developmental processes in vertebrates.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300391/-/DC1.
Acknowledgments
We thank Reiko Kise for technical support. We are also grateful to Tobias P. Dick, Ryutaro Murakami, Michael B. O’Connor, Eric N. Olson, William C. Orr, David Ron, Hiroko Sano, Tadashi Uemura, Takeo Usui, Alisa Zyryanova, the Bloomington Drosophila Stock Center, Kyoto Stock Center, the Vienna Drosophila RNAi Center, and the National Institute of Genetics for stocks and reagents. We thank Akira Ogawa for helpful discussion. S.E. was a recipient of research fellowships for young scientists from the Japan Society for the Promotion of Science (JSPS). This work was supported by JSPS Grants-in-Aid for Scientific Research (KAKENHI) grant numbers 12J01444 to S.E. and 15K14719 to R.N.; Japan Science and Technology Agency (JST) Precursory Research for Embryonic Science and Technology grant number JPMJPR12M1 to R.N.; the Naito Foundation and the Inoue Foundation to Y.S.-N.; and the JST and Japan Agency for Medical Research and Development project for the Development of Systems and Technology for Advanced Measurement and Analysis to T.M.
Footnotes
This article is dedicated to Tsutomu Masujima, who died during the course of the study.
Communicating editor: B. Sullivan
Literature Cited
- Abbott J. J., Ford J. L., Phillips M. A., 2002. Substrate binding determinants of Trypanosoma brucei γ-glutamylcysteine synthetase. Biochemistry 41: 2741–2750. [DOI] [PubMed] [Google Scholar]
- Aquilano K., Baldelli S., Ciriolo M. R., 2014. Glutathione: new roles in redox signalling for an old antioxidant. Front. Pharmacol. 5: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S., Srivastava S. K., Ahmad F., Ahmad H., Ansari G. A., 1993. Interactions of glutathione S-transferase-π with ethacrynic acid and its glutathione conjugate. Biochim. Biophys. Acta 1164: 173–178. [DOI] [PubMed] [Google Scholar]
- Bachhawat A. K., Thakur A., Kaur J., Zulkifli M., 2013. Glutathione transporters. Biochim. Biophys. Acta, Gen. Subj. 1830: 3154–3164. [DOI] [PubMed] [Google Scholar]
- Beaver L. M., Klichko V. I., Chow E. S., Kotwica-Rolinska J., Williamson M., et al. , 2012. Circadian regulation of glutathione levels and biosynthesis in Drosophila melanogaster. PLoS One 7: e50454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbouloux A., Shahi P., Chakladar A., Delrot S., Bachhawat A. K., 2000. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275: 13259–13265. [DOI] [PubMed] [Google Scholar]
- Bradford M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Carvalho M., Schwudke D., Sampaio J. L., Palm W., Riezman I., et al. , 2010. Survival strategies of a sterol auxotroph. Development 137: 3675–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut-Delalande H., Hashimoto Y., Pelissier-Monier A., Spokony R., Dib A., et al. , 2014. Pri peptides are mediators of ecdysone for the temporal control of development. Nat. Cell Biol. 16: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Chávez V. M., Marqués G., Delbecque J. P., Kobayashi K., Hollingsworth M., et al. , 2000. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127: 4115–4126. [DOI] [PubMed] [Google Scholar]
- Chou T. B., Perrimon N., 1992. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics 131: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J., Raisin S., Pantalacci S., Radimerski T., Montagne J., et al. , 2003. A nutrient sensor mechanism controls Drosophila growth. Cell 114: 739–749. [DOI] [PubMed] [Google Scholar]
- Dalton T. P., Dieter M. Z., Yang Y., Shertzer H. G., Nebert D. W., 2000. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem. Biophys. Res. Commun. 279: 324–329. [DOI] [PubMed] [Google Scholar]
- Deng H., Kerppola T. K., 2013. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet. 9: e1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Kerppola T. K., 2014. Visualization of the Drosophila dKeap1-CncC interaction on chromatin illumines cooperative, xenobiotic-specific gene activation. Development 141: 3277–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya S., Ameku T., Igarashi F., Iga M., Kataoka H., et al. , 2014. A Halloween gene noppera-bo encodes a glutathione S-transferase essential for ecdysteroid biosynthesis via regulating the behaviour of cholesterol in Drosophila. Sci. Rep. 4: 6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya S., Daimon T., Igarashi F., Kataoka H., Uchibori M., et al. , 2015. The silkworm glutathione S-transferase gene noppera-bo is required for ecdysteroid biosynthesis and larval development. Insect Biochem. Mol. Biol. 61: 1–7. [DOI] [PubMed] [Google Scholar]
- Fedulova N., Raffalli-Mathieu F., Mannervik B., 2010. Porcine glutathione transferase Alpha 2-2 is a human GST A3-3 analogue that catalyses steroid double-bond isomerization. Biochem. J. 431: 159–167. [DOI] [PubMed] [Google Scholar]
- Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J., et al. , 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44: D279–D285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K. P., Leonard M. W., Engel J. D., 1993. Quantitation of RNA using the polymerase chain reaction. Trends Genet. 9: 380–385. [DOI] [PubMed] [Google Scholar]
- Fraser J. A., Saunders R. D. C., McLellan L. I., 2002. Drosophila melanogaster glutamate-cysteine ligase activity is regulated by a modifier subunit with a mechanism of action similar to that of the mammalian form. J. Biol. Chem. 277: 1158–1165. [DOI] [PubMed] [Google Scholar]
- Fraser J. A., Kansagra P., Kotecki C., Saunders R. D. C., McLellan L. I., 2003. The modifier subunit of Drosophila glutamate-cysteine ligase regulates catalytic activity by covalent and noncovalent interactions and influences glutathione homeostasis in vivo. J. Biol. Chem. 278: 46369–46377. [DOI] [PubMed] [Google Scholar]
- Fujii T., Matsuda S., Tejedor M. L., Esaki T., Sakane I., et al. , 2015. Direct metabolomics for plant cells by live single-cell mass spectrometry. Nat. Protoc. 10: 1445–1456. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y., Morisaki F., Ogura A., Morohashi K., Enya S., et al. , 2015. A practical fluorogenic substrate for high-throughput screening of glutathione S-transferase inhibitors. Chem. Commun. (Camb.) 14: 1–4. [DOI] [PubMed] [Google Scholar]
- Gilbert L. I., 2004. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 215: 1–10. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Mulcahy R. T., 1999. The enzymes of glutathione synthesis: γ-glutamylcysteine synthetase. Adv. Enzymol. Relat. Areas Mol. Biol. 73: 209–267. [DOI] [PubMed] [Google Scholar]
- Hamilton D., Wu J. H., Alaoui-Jamali M., Batist G., 2003. A novel missense mutation in the γ-glutamylcysteine synthetase catalytic subunit gene causes both decreased enzymatic activity and glutathione production. Blood 102: 725–730. [DOI] [PubMed] [Google Scholar]
- Harrop T. W. R., Pearce S. L., Daborn P. J., Batterham P., 2014. Whole-genome expression analysis in the third instar larval midgut of Drosophila melanogaster. G3 (Bethesda) 4: 2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikiba J., Ogihara M. H., Iga M., Saito K., Fujimoto Y., et al. , 2013. Simultaneous quantification of individual intermediate steroids in silkworm ecdysone biosynthesis by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 915–916: 52–56. [DOI] [PubMed] [Google Scholar]
- Igarashi F., Hikiba J., Ogihara M. H., Nakaoka T., Suzuki M., et al. , 2011. A highly specific and sensitive quantification analysis of the sterols in silkworm larvae by high performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal. Biochem. 419: 123–132. [DOI] [PubMed] [Google Scholar]
- Imura E., Yoshinari Y., Shimada-Niwa Y., Niwa R., 2017. Protocols for visualizing steroidogenic organs and their interactive organs with immunostaining in the fruit fly Drosophila melanogaster. J. Vis. Exp. 122: e55519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki D. D., Lengyel J. A., 2002. A delta-notch signaling border regulated by engrailed/invected repression specifies boundary cells in the Drosophila hindgut. Mech. Dev. 114: 71–84. [DOI] [PubMed] [Google Scholar]
- Jumbo-Lucioni P. P., Hopson M. L., Hang D., Liang Y., Jones D. P., et al. , 2013. Oxidative stress contributes to outcome severity in a Drosophila melanogaster model of classic galactosemia. Dis. Model. Mech. 6: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jünger M. A., Rintelen F., Stocker H., Wasserman J. D., Végh M., et al. , 2003. The Drosophila Forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klichko V. I., Chow E. S., Kotwica-Rolinska J., Orr W. C., Giebultowicz J. M., et al. , 2015. Aging alters circadian regulation of redox in Drosophila. Front. Genet. 6: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., 2014. New horizons in genome engineering of Drosophila melanogaster. Genes Genet. Syst. 89: 3–8. [DOI] [PubMed] [Google Scholar]
- Kondo S., Ueda R., 2013. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics 195: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L., 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461. [DOI] [PubMed] [Google Scholar]
- Li H. M., Buczkowski G., Mittapalli O., Xie J., Wu J., et al. , 2008. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol. Biol. 17: 325–339. [DOI] [PubMed] [Google Scholar]
- Loncle N., Boube M., Joulia L., Boschiero C., Werner M., et al. , 2007. Distinct roles for mediator Cdk8 module subunits in Drosophila development. EMBO J. 26: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. C., 2014. Glutathione synthesis. Biochim. Biophys. Acta 1830: 3143–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchak J. M., Prabhudesai L., Sohal R. S., Radyuk S. N., Orr W. C., 2007. Modulating longevity in Drosophila by over- and underexpression of glutamate-cysteine ligase. Ann. N. Y. Acad. Sci. 1119: 260–273. [DOI] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N., 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8: 1787–1802. [DOI] [PubMed] [Google Scholar]
- McBrayer Z., Ono H., Shimell M., Parvy J. P., Beckstead R. B., et al. , 2007. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev. Cell 13: 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer S. W., La Fontaine S., Warr C. G., Burke R., 2016. Reduced glutathione biosynthesis in Drosophila melanogaster causes neuronal defects linked to copper deficiency. J. Neurochem. 137: 360–370. [DOI] [PubMed] [Google Scholar]
- Moskalev A., Shaposhnikov M., Proshkina E., Belyi A., Fedintsev A., et al. , 2016. The influence of pro-longevity gene Gclc overexpression on the age-dependent changes in Drosophila transcriptome and biological functions. BMC Genomics 17: 1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki T., Niwa R., Sakudoh T., Shirai K. I., Takeuchi H., et al. , 2005. Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 337: 367–374. [DOI] [PubMed] [Google Scholar]
- Nehme N. T., Liégeois S., Kele B., Giammarinaro P., Pradel E., et al. , 2007. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3: 1694–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R., Niwa Y. S., 2014. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci. Biotechnol. Biochem. 78: 1283–1292. [DOI] [PubMed] [Google Scholar]
- Niwa R., Matsuda T., Yoshiyama T., Namiki T., Mita K., et al. , 2004. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 279: 35942–35949. [DOI] [PubMed] [Google Scholar]
- Niwa R., Namiki T., Ito K., Shimada-Niwa Y., Kiuchi M., et al. , 2010. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the “Black Box” of the ecdysteroid biosynthesis pathway. Development 137: 1991–1999. [DOI] [PubMed] [Google Scholar]
- Niwa Y. S., Niwa R., 2014. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster. Genes Genet. Syst. 89: 27–34. [DOI] [PubMed] [Google Scholar]
- Niwa Y. S., Niwa R., 2016. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Dev. Growth Differ. 58: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E., Kluding H., 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Rouxs Arch. Dev. Biol. 193: 267–282. [DOI] [PubMed] [Google Scholar]
- Ono H., Rewitz K. F., Shinoda T., Itoyama K., Petryk A., et al. , 2006. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 298: 555–570. [DOI] [PubMed] [Google Scholar]
- Orr W. C., Radyuk S. N., Prabhudesai L., Toroser D., Benes J. J., et al. , 2005. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J. Biol. Chem. 280: 37331–37338. [DOI] [PubMed] [Google Scholar]
- Ou Q., Zeng J., Yamanaka N., Brakken-Thal C., O’Connor M. B., et al. , 2016. The insect prothoracic gland as a model for steroid hormone biosynthesis and regulation. Cell Rep. 16: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx M. J., 2002. An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2: 295–305. [DOI] [PubMed] [Google Scholar]
- Petryk A., Warren J. T., Marqués G., Jarcho M. P., Gilbert L. I., et al. , 2003. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 100: 13773–13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P., Pineda M., Aguilar M., 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269: 337–341. [DOI] [PubMed] [Google Scholar]
- Radyuk S. N., Gambini J., Borras C., Serna E., Klichko V. I., et al. , 2012. Age-dependent changes in the transcription profile of long-lived Drosophila over-expressing glutamate cysteine ligase. Mech. Ageing Dev. 133: 401–413. [DOI] [PubMed] [Google Scholar]
- Raffalli-Mathieu F., Mannervik B., 2005. Human glutathione transferase A3–3 active as steroid double-bond isomerase. Methods Enzymol. 401: 265–278. [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G., Schulz R. A., Olson E. N., 1996. Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev. Biol. 176: 143–148. [DOI] [PubMed] [Google Scholar]
- Rewitz K. F., Rybczynski R., Warren J. T., Gilbert L. I., 2006. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem. Soc. Trans. 34: 1256–1260. [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W., 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., 1999. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 27: 916–921. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy A. R., Goldstone J. V., Imhoff B. R., Stegeman J. J., Hahn M. E., et al. , 2013. Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo. Free Radic. Biol. Med. 65: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu O., Ameku T., Niwa R., 2015. Recent progress in understanding the role of ecdysteroids in adult insects: germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool. Lett. 1: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai Y. L., Hooi L. N., Morton C. J., Parker M. W., Batterham P., et al. , 2007. Molecular evolution of glutathione S-transferases in the genus Drosophila. Genetics 177: 1363–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. T., Petryk A., Marques G., Jarcho M., Parvy J.-P., et al. , 2002. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 99: 11043–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. T., Petryk A., Marqués G., Parvy J. P., Shinoda T., et al. , 2004. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 34: 991–1010. [DOI] [PubMed] [Google Scholar]
- Yamanaka N., Rewitz K. F., O’Connor M. B., 2013. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58: 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama T., Namiki T., Mita K., Kataoka H., Niwa R., 2006. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development 133: 2565–2574. [DOI] [PubMed] [Google Scholar]
- Yoshiyama-Yanagawa T., Enya S., Shimada-Niwa Y., Yaguchi S., Haramoto Y., et al. , 2011. The conserved rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J. Biol. Chem. 286: 25756–25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Xie Q., Jobe T. O., Kau A. R., Wang C., et al. , 2016. Identification of AtOPT4 as a plant glutathione transporter. Mol. Plant 9: 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains and materials are available upon request. Supplemental Material, File S1 contains detailed descriptions of animal count data represented in Figure 2, Figure 3, Figure 4, Figure 6, and Figure 7.
Figure 2.
Larval lethality and developmental-arrest phenotype of Gclc46/Y larvae. (A and B) The survival rate and developmental progression of control w1118/Y (A) and Gclc46/Y (B) animals. L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60. (C–E) Comparison of body size and developmental stage between control w1118/Y (left) and Gclc46/Y (right) animals. Typically, control animals were second-instar larvae, third-instar larvae, and pupae at 48 hr (C), 96 hr (D), and 168 hr (E) after egg laying (AEL), respectively. In contrast, Gclc46/Y animals in these photos are all second-instar larvae, collected at the same time points. Bar, 0.5 mm. (F) The larval-arrest phenotype of Gclc46/Y animals was partly rescued by oral administration of glutathione (GSH) from food. The survival rate and developmental progression of w1118/Y and Gclc46/Y animals at 96 hr AEL, fed standard food supplemented with vehicle ethanol (−) with or without GSH and ascorbic acid (Asc.) just after hatching. GSH concentration in food ranged 0–20 mg/ml. Asc. concentration was 1 mg/ml. N = 60.
Figure 3.
Molting defect of Gclc46/Y animals is rescued by feeding 20-hydroxyecdysone (20E) and cholesterol. (A and B) The survival rate and developmental progression of control w1118/Y and Gclc46/Y animals that were fed standard food supplemented with vehicle ethanol (−), 20E, and cholesterol (CLR). The number of larvae was counted at 96 hr (A) and 168 hr (B) after egg laying (AEL). L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60. (C–H) Whole bodies (C and F), anterior tracheal pits (D and G), and dissected mouth hooks (E and H) of Gclc46/Y larvae at 96 hr AEL. The animals were reared on standard food supplemented with control ethanol (C–E) and 20E (F–H). Gclc46/Y larvae raised on a control diet died in the second-instar larval stage, exhibiting singular insertions of anterior tracheal pits (D) and 2–5 teeth on mouth hooks ((E); red arrowheads). In contrast, 20E-fed Gclc46/Y larvae molted in the third-instar larval stage as judged by the branched morphology of the anterior tracheal pits (G) and numerous small teeth on the mouth hook ((H); red arrowheads), typical features of third-instar larvae. Bars, 1 mm for (C and F), 148 μm for (D and G), and 125 μm for (E and H).
Figure 6.
Larval-arrest phenotype induced by ethacrynic acid (EA) is rescued by feeding 20-hydroxyecdysone (20E) and cholesterol (CLR). (A–D) The survival rate and developmental progression of wild-type (w1118) animals that were fed standard food supplemented with vehicle ethanol 0%, (A), 0.1% (B), 0.2% (C), and 0.5% (D) EA. (B’–D’) The survival rate at 192 hr after egg laying (AEL) was examined when vehicle ethanol (−), 20E, and CLR were added with 0.1% (B’), 0.2% (C’), and 0.5% (D’) EA to standard food. L1, L2, and L3 indicate first-, second-, and third-instar larvae, respectively. N = 60.
Figure 7.
Oxidative stress response and antioxidant-capacity phenotypes of Gclc46/Y are not rescued by 20-hydroxyecdysone (20E) administration. (A) The survival rate and developmental progression of control w1118/Y and Gclc46/Y animals that were fed sucrose‐agar medium containing paraquat (PQ) and/or 20E. Second-instar larvae at 48 hr after egg laying (AEL) were transferred to the medium, then fed media for 12 hr. *** P < 0.001 chi-square test with Bonferroni correction. n.s., not significant. (B) Antioxidant capacity of body fluids isolated from control w1118/Y and Gclc46/Y second-instar larvae (60 hr AEL) that were fed standard food supplemented with vehicle ethanol (−), 20E, ascorbic acid (asc.), and glutathione (GSH). * P < 0.05 and ** P < 0.01 by Dunnett’s test.