Abstract
Glycosylation reactions in the Golgi complex and the endoplasmic reticulum utilize nucleotide sugars as donors and produce inorganic phosphate (Pi) and acid (H+) as byproducts. Here we show that homologs of mammalian XPR1 and TMEM165 (termed Erd1 and Gdt1) recycle luminal Pi and exchange luminal H+ for cytoplasmic Ca2+, respectively, thereby promoting growth of yeast cells in low Pi and low Ca2+ environments. As expected for reversible H+/Ca2+ exchangers, Gdt1 also promoted growth in high Ca2+ environments when the Golgi-localized V-ATPase was operational but had the opposite effect when the V-ATPase was eliminated. Gdt1 activities were negatively regulated by calcineurin signaling and by Erd1, which recycled the Pi byproduct of glycosylation reactions and prevented the loss of this nutrient to the environment via exocytosis. Thus, Erd1 transports Pi in the opposite direction from XPR1 and other EXS family proteins and facilitates byproduct removal from the Golgi complex together with Gdt1.
Keywords: Calcium, homeostasis, phosphate, glycosylation, Golgi complex
Secretory proteins, lipids, and carbohydrates can undergo one or more cycles of glycosylation in the lumens of the Golgi complex and the endoplasmic reticulum (ER), often resulting in elaborate glycan chains (Stanley 2011). The glycosyltransferases responsible for these reactions consume nucleotide sugars such as GDP-mannose and UDP-glucose and generate nucleoside diphosphates which are rapidly converted by luminal nucleoside triphosphate diphosphohydrolases (NTPDases, or apyrases) to GMP and UMP plus inorganic phosphate (Pi) and acid (H+) as byproducts (Knowles 2011). Nucleotide sugar transporters embedded in the membranes of these organelles then exchange one luminal nucleoside monophosphate for one cytoplasmic nucleotide sugar to allow additional rounds of glycosylation to occur (Hirschberg et al. 1998). However, the fates of Pi and H+ byproducts of glycosylation reactions are not fully understood, and it is possible that their buildup in secretory organelles could adversely affect glycosylation reactions, sorting and trafficking of secretory proteins, and cell physiology.
Glycosyltransferases usually depend on Ca2+ or Mn2+ ions for maximal activity (Dürr et al. 1998). The SPCA family of P-type ATPases, which is mutated in Hailey–Hailey disease in humans (Hu et al. 2000; Sudbrak et al. 2000), directly transports Ca2+ and Mn2+ ions from the cytoplasm into the lumen of the Golgi complex to satisfy the needs of most glycosyltransferases, as well as the kexin family of proprotein convertases. The first SPCA-family Ca2+/Mn2+ pump, termed Pmr1, was discovered in budding yeast (Rudolph et al. 1989), and the pmr1∆ knockout mutants exhibited strong defects in glycosylation and processing of secretory proteins in the Golgi complex, inability to proliferate in low Ca2+ environments, and hypersensitivity to high Mn2+ in the culture media (Antebi and Fink 1992; Sorin et al. 1997; Dürr et al. 1998). The pmr1∆ mutants also exhibited mild activation of the unfolded protein response, likely because yeast naturally lacks a SERCA-family Ca2+ pump that supplies the ER of most other species with Ca2+ necessary for folding of secretory proteins (Dürr et al. 1998; Strayle et al. 1999). The deficiency of Ca2+ in the Golgi and ER of yeast leads to activation of the cell wall integrity MAP kinase Slt2/Mpk1, which induces expression of the K+ transporter Kch1 that depolarizes the cell membrane and activates a voltage-gated Ca2+ channel (also called HACS, and composed of Cch1, Mid1, and Ecm7) to promote Ca2+ influx, elevation of cytosolic free Ca2+ concentrations, and replenishment of the secretory Ca2+ pools (Locke et al. 2000; Bonilla et al. 2002; Bonilla and Cunningham 2003; Martin et al. 2011; Stefan and Cunningham 2013; Stefan et al. 2013). This mechanism of ER and Golgi Ca2+ homeostasis in yeast is analogous to, but mechanistically distinct from, store-operated Ca2+ entry mechanisms in animals (Smyth et al. 2010). Elevated cytosolic free Ca2+ in yeast also activates calmodulin and the serine/threonine protein phosphatase calcineurin, which induces expression of Pmr1 via activation of the Crz1 transcription factor (Matheos et al. 1997; Stathopoulos and Cyert 1997). Crz1 also induces a PMCA-family Ca2+ pump, termed Pmc1 (Cunningham and Fink 1994), which localizes to the limiting membrane of lysosome-like vacuoles and partially mislocalizes to the Golgi complex and ER in mutants that lack Pmr1(Marchi et al. 1999). Because the vacuole plays a major part in Ca2+ detoxification in yeast, mutants that lack Pmc1 or Crz1 exhibit strong growth defects in medium supplemented with high Ca2+ (Cunningham and Fink 1994; Matheos et al. 1997).
The yeast vacuole, like lysosomes in animals, is strongly acidified by the action of the V-ATPase, which directly transports H+ from the cytoplasm to the lumen (Kane 2016) The CAX-family H+/Ca2+ exchangers in the vacuolar limiting membrane utilize this pH gradient to power transport of Ca2+ from the cytoplasm to the vacuole lumen (Forster and Kane 2000). The first CAX-family H+/Ca2+ exchanger, termed Vcx1, was discovered in yeast based on its ability to confer Ca2+ or Mn2+ resistance when overexpressed (Cunningham and Fink 1996; Pozos et al. 1996). But, surprisingly, vcx1∆ mutants exhibited little hypersensitivity to Ca2+ unless calcineurin was also eliminated by either mutations or inhibitors such as cyclosporine and FK506 (Cunningham and Fink 1996). These studies suggest that activated calcineurin may somehow inhibit Vcx1 function, while independently inducing Pmc1 and Pmr1 expression via Crz1 activation (see Figure 1A). However, the molecular mechanism of Vcx1 inhibition by calcineurin has not been elucidated and the interaction could be indirect.
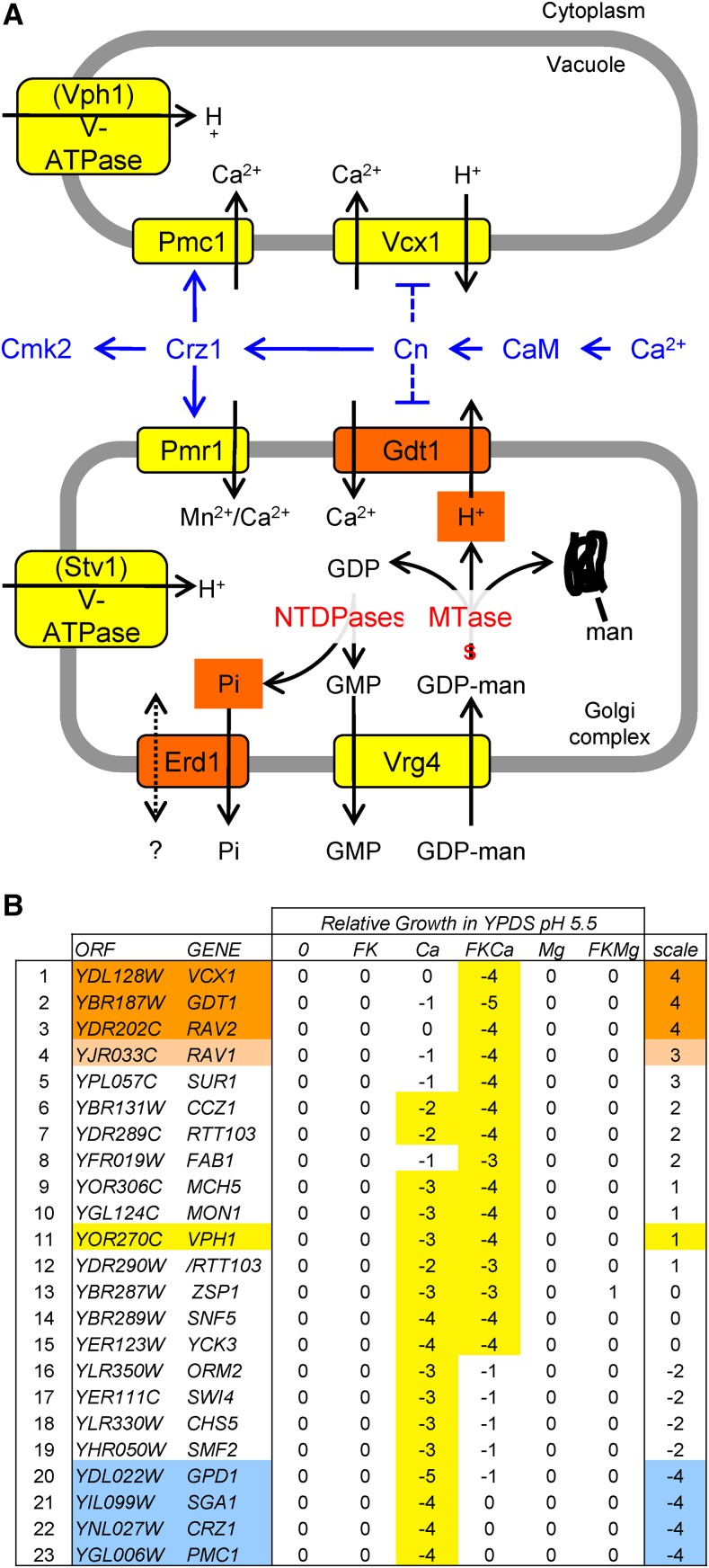
Figure 1.
Model of Ca2+ homeostasis and Golgi glycosylation, with results from a genome-wide screen. (A) Model of known ion or nucleotide sugar transporters (yellow) in the vacuole and Golgi complex that are studied here along with modes of regulation (blue) by the Crz1 transcription factor, calcineurin, calmodulin, and high cytosolic Ca2+. Gdt1 and Erd1 (orange) are putative transporters of the byproducts of glycosylation reactions in the Golgi complex such as H+ [produced by mannosyltransferases (MTases)] and Pi [produced by nucleoside triphosphate diphosphatases (NTDPases)] that were identified in genetic screens. (B) Results of a genetic screen for knockout mutants that specifically exhibit hypersensitivity to elevated Ca2+ and/or Ca2+ plus FK506 in the growth medium. The numbers indicate growth relative to wild-type controls (smaller numbers indicate slower growth) with the strongest effects highlighted (yellow). The 23 filtered mutants were ranked from vcx1∆-like (orange) to pmc1∆-like (blue) based on the difference between the two Ca2+ conditions.
Here we rule out the V-ATPase as a necessary intermediate in the inhibition of Vcx1 by calcineurin, and we search for possible intermediaries through a genome-wide screen of the yeast gene knockout collection. While the gdt1∆ mutant closely resembled the vcx1∆ mutant in its hypersensitivity to Ca2+ when calcineurin was inhibited or mutated, calcineurin-dependent inhibition of Vcx1 still occurred in gdt1∆ mutants. Gdt1 localizes to the Golgi complex of yeast, promotes glycosylation in high Ca2+ conditions, and transports Ca2+ when expressed in heterologous systems (Demaegd et al. 2013; Colinet et al. 2016; Potelle et al. 2016). The sole human ortholog of Gdt1, termed TMEM165, was previously shown to regulate pH and glycosylation in the Golgi complex and to be deficient in individuals with a congenital disorder of glycosylation (Foulquier et al. 2012; Zeevaert et al. 2013; Potelle et al. 2016). Below we show that Gdt1 and Vcx1 both promote Ca2+ sequestration when their organelles are properly acidified by the V-ATPase, and both promote Ca2+ increases in the cytoplasm when acidification has been disrupted.
To investigate how calcineurin might regulate Gdt1 function, we isolated spontaneous mutations that exhibited elevated Gdt1 function even while calcineurin remained functional. Mutants deficient in Erd1, a polytopic transmembrane protein important for glycosylation and sorting of proteins in the Golgi complex (Hardwick et al. 1990), were recovered. We show evidence that Erd1 recycles Pi byproducts of the glycosylation in the Golgi complex before this important nutrient is lost by exocytosis. Therefore, this study sheds new light on the mechanisms that sustain luminal glycosylation reactions in the Golgi complex and promote Pi, Ca2+, and H+ homeostasis in the cell.
Materials and Methods
Yeast strains and genetic screens
The yeast knockout collection in strain BY4741 background (Giaever et al. 1999) was inoculated into 200 µl yeast extract peptone dextrose (YPD) medium in 96-well dishes and grown to stationary phase (2 d incubation at 30°). Dishes were vortexed briefly to suspend the settled cells, then pinned onto noble agar medium containing YPD medium plus 5 mM succinic acid and supplemented with either 200 mM CaCl2, 200 mM MgCl2, 1 µg/ml FK506, or combinations thereof. After incubation for 3 d at 30°, each strain was scored manually for growth relative to wild-type controls that were included in the arrays, using a scale ranging from 2 (increased growth) through 0 (wild-type growth) to −5 (greatly decreased or no growth). The screen was repeated twice independently. Of the 107 mutant strains that consistently exhibited a significant response to at least one condition, 84 were found to exhibit elevated Ca2+ influx in YPD medium or YPD medium plus FK506 (Martin et al. 2011). The remaining 23 mutants contained vcx1∆ and pmc1∆ and were ranked on a scale from vcx1∆-like to pmc1∆-like based on their distinct behaviors in media containing Ca2+ and Ca2+ plus FK506. A gdt1∆ mutation was generated in a W303-1A strain background and crossed with strains bearing vcx1∆, pmc1∆, crz1∆, and pmr1∆ to produce a panel of isogenic strains bearing many combinations of these mutations in both mating types. Genotypes were confirmed by marker analyses and by polymerase chain reaction (PCR) confirmations.
To identify spontaneous mutants that increase Ca2+ tolerance of crz1∆ pmc1∆ vcx1∆ triple mutants, two strains of opposite mating types and with different selectable markers (DDY19 and K1357) were streaked for single colonies on agar YPD medium, and 36 single colonies were picked, grown further on the same medium to allow spontaneous mutations to accumulate, and then incubated on noble agar YPD medium containing 5 mM succinic acid and 111 mM CaCl2 for several days. A single Ca2+-tolerant colony was picked in each case, purified by restreaking, and then mated with one another in all possible combinations. The resulting diploids were reanalyzed for Ca2+ tolerance. This complementation test indicated that 26 haploid strains contained dominant mutations in unknown genes and 10 haploid strains contained recessive mutations all in the same gene. The 10 recessive mutants were fortuitously found to exhibit hypersensitivity to tunicamycin. One recessive mutant was transformed with a low-copy plasmid library containing fragments of yeast genomic DNA, and nine transformants were replica plated to YPD plus 2 µg/ml tunicamycin to select for strains bearing complementing plasmids. Two different plasmids overlapping the ERD1 gene and several nearby genes were recovered and found to reverse both the tunicamycin hypersensitivity and the Ca2+ tolerance phenotypes upon retransformation into the recessive mutant strains. ERD1 was identified as the defective gene in the recessive mutants by (1) introducing an erd1∆ null mutation into the K1357 strain and obtaining both the tunicamycin hypersensitivity and the Ca2+ resistance phenotypes and (2) demonstrating non-complementation between the erd1∆ null strain and the spontaneous recessive mutant strains. Similar erd1∆ knockout mutations were also introduced by transformation into several other strains; all strains utilized in figures are listed in Supplemental Material, Table S2. Table S1 lists additional strains in the W303 background, their genotypes, and quantitative analyses of their functions in Ca2+ tolerance.
Ion tolerance and Pi dependence assays
Yeast strains were grown to saturation at 30°, typically overnight, in YPD medium for ion tolerance assays or in synthetic complete (SC) medium for Pi dependence assays. They were then diluted 1:1000 into fresh media containing 5 mM succinic acid and various concentrations of CaCl2 in 96-well dishes with and without 1 µg/ml FK506, mixed, and incubated at 30° for 24 hr without shaking. The cells were resuspended by vortex mixing and optical density was measured at 650 nm using a microplate spectrophotometer (Molecular Devices). The concentration of Ca2+ causing a 50% decrease in maximal optical density (the IC50) and the concentration of Pi permitting growth to 50% maximal optical density (the ED50) were calculated for each strain by non-linear regression using the sigmoid equation with four parameters (maximum OD650, minimum OD650, IC50 or ED50, and slope factor). The averages from two independently generated strains of the same genotype (±SD) were calculated and plotted. In Table S1, the coefficient of variation (CV) is listed instead of SD, and the data from two separate experiments (A and B) were normalized to a third reference dataset using linear regression after log-transformation.
PMC1-lacZ expression assays
The PMC1-lacZ reporter gene carried on the high-copy plasmid pKC190 (Cunningham and Fink 1996) was transformed into the indicated strains, and three independent transformants were grown to log phase in SC-ura medium, pelleted briefly, and suspended in YPD medium with 5 mM succinic acid containing 0, 50, or 100 mM CaCl2. After 4 hr incubation at 30°, cells were pelleted, resuspended in Z-buffer, permeabilized with sodium dodecyl sulfate (SDS) and chloroform, and assayed for β-galactosidase activity using o-nitrophenyl-β-galactoside as substrate as described previously (Cunningham and Fink 1996).
Pi export assays
Yeast strains were grown overnight to saturation in SC medium (7.35 mM Pi) and then diluted 1000-fold into fresh SC medium containing tracer quantities (∼30 µCi per ml) of fresh [32]Pi (Perkin Elmer). After 12 hr of incubation with shaking, the log-phase cells were pelleted and washed five times with 5 ml of ice-cold Pi-free SC media to remove all unincorporated [32]Pi from the medium. The cells were then resuspended in Pi-free SC media and incubated on ice or at 30° and sampled at various times. After centrifugation at 15,000 rpm for 1 min, the cell-free supernatant was analyzed by liquid scintillation counting and by thin-layer chromatography (TLC), by spotting 3 μl of sample supernatant onto polyethylenimine (PEI)-cellulose TLC plates, which was then developed in 1 M LiCl with 10 mM HEPES, pH 7.5. The TLC plate was imaged by autoradiography.
Antibodies and western blotting
Cells were lysed via fast alkaline lysis in 0.5 M NaOH with 1.85% beta-mercaptoethanol (BME) in ice water for 10 min. The protein was then isolated through precipitation by addition of trichloroacetic acid (TCA) to a final concentration of 10% (w/w). Equal volumes of protein isolate and 2× SDS sample buffer (0.1 M Tris-HCl, pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol, 2% BME) were then combined, and the samples were incubated at 37° for 30 min. Proteins were processed by separation on a 10% SDS/polyacrylamide gel electrophoresis (PAGE) gel and western blotting, as described previously (Mehta 2009). Blots were probed with anti-TAP polyclonal antibodies from rabbit at 1:10,000 dilution (ThermoFisher, CAB1001) or anti-FLAG polyclonal antibodies from rabbit at 1:10,000 dilution (F7425; Sigma, St. Louis, MO). Protein standards were probed with anti-glucose-6-phosphate dehydrogenase polyclonal antibodies from rabbit at 1:10,000 dilution (A9521; Sigma), or anti-tubulin monoclonal antibodies at 1:10,000 dilution (EMD Millipore, MAB1864).
Statistical tests of significance
Student’s t-tests were implemented on many datasets, as indicated in the figures (* P < 0.05, ** P < 0.01, *** P < 0.001).
Data availability and reagent availability
All yeast strains and plasmids are archived and available upon request. Raw data files used to generate figures and tables are also archived and available upon request. All requests should be made to the corresponding author.
Results
Genetic screen for mutants with altered Ca2+ sensitivity: V-ATPase, Vcx1, and Gdt1
The ability of yeast cells to proliferate in high Ca2+ environments depends mostly on the vacuolar Ca2+ ATPase (Pmc1) when calcineurin is functioning, and mostly on the vacuolar H+/Ca2+ exchanger (Vcx1) when calcineurin has been inhibited or mutated (Cunningham and Fink 1996). To search for additional Ca2+ transporters or regulators of Vcx1, we screened a collection of 4848 non-essential gene knockout mutants of yeast strain BY4741 for their ability to proliferate in media containing 200 mM CaCl2 with and without the calcineurin inhibitor FK506 (see Materials and Methods). A similar genetic screen using cyclosporine instead of FK506 to inhibit calcineurin yielded partially overlapping results (Zhao et al. 2013). After filtering all the mutants that were hypersensitive to FK506 alone or to control osmolyte (200 mM MgCl2), and then filtering out the candidates that had exhibited elevated Ca2+ uptake in response to FK506 in a previous study (Martin et al. 2011), a total of 23 mutants were hypersensitive to Ca2+, Ca2+ plus FK506, or both while passing the stringent filters. These 23 mutants were ranked on a scale ranging from “vcx1∆-like” to “pmc1∆-like” (Figure 1B). As expected, the crz1∆ mutant ranked closest to the pmc1∆ mutant because the calcineurin-dependent transcription activator Crz1 strongly induces expression of Pmc1 (Matheos et al. 1997; Stathopoulos and Cyert 1997). Two other mutants (gpd1∆, sga1∆) exhibited hypersensitivity to Ca2+ but not Ca2+ plus FK506, but were not studied further.
The three mutants closest to vcx1 (gdt1∆, rav1∆, rav2∆) may be deficient in positive regulators of Vcx1 or in novel Ca2+ transporters. Rav2 forms a complex with Rav1 and promotes assembly of V1 and V0 sectors of the vacuolar form of the V-ATPase (Smardon et al. 2014). The low residual vacuolar V-ATPase activity present in rav1∆ and rav2∆ mutants may be insufficient to power the H+/Ca2+ exchange activity of Vcx1, thereby producing the vcx1∆-like phenotype. Complete loss of all V-ATPase forms, as observed in vma1∆ and other vma mutants, resulted in hypersensitivity to high Ca2+ and Ca2+ plus FK506, but these mutants were filtered out because of hypersensitivity to high Mg2+ and Mg2+ plus FK506. Surprisingly, a mutant that specifically lacks only the vacuolar form of the V-ATPase (vph1∆) while preserving the Golgi/endosomal forms exhibited no hypersensitivity to high Mg2+ and strong hypersensitivity to high Ca2+ and high Ca2+ plus FK506 conditions (Figure 1, line 11), as if the functions of Vcx1 and Pmc1 were both compromised.
To investigate how the vacuolar V-ATPase affects Pmc1 and Vcx1 function, a pmc1∆ vcx1∆ vph1∆ triple knockout mutant was constructed in the W303-1A strain background along with all possible double and single knockout mutants, and the concentrations of CaCl2 that cause a 50% inhibition of total growth (i.e., the IC50) were compared for all eight strains. Relative to the wild-type parent, the pmc1∆ vcx1∆ vph1∆ triple mutant was ∼19-fold more sensitive to Ca2+ in the medium (Figure 2A, black bars). In the absence of Vph1, restoring Pmc1 increased the IC50 for Ca2+ strongly (4.2-fold), whereas restoring Vcx1 had little effect (1.1-fold) in the presence or absence of Pmc1. Restoring Vph1 function had no significant effect in the cells that lack both Pmc1 and Vcx1 (1.05-fold), but had strong effects on cells expressing only Vcx1 (2.2-fold) or Pmc1 (4.4-fold). Therefore, Pmc1 activity was partially dependent on a functional V-ATPase in the vacuolar membrane, and the relatively low Vcx1 activity detectable in pmc1∆ mutant backgrounds was totally dependent on the vacuolar V-ATPase.
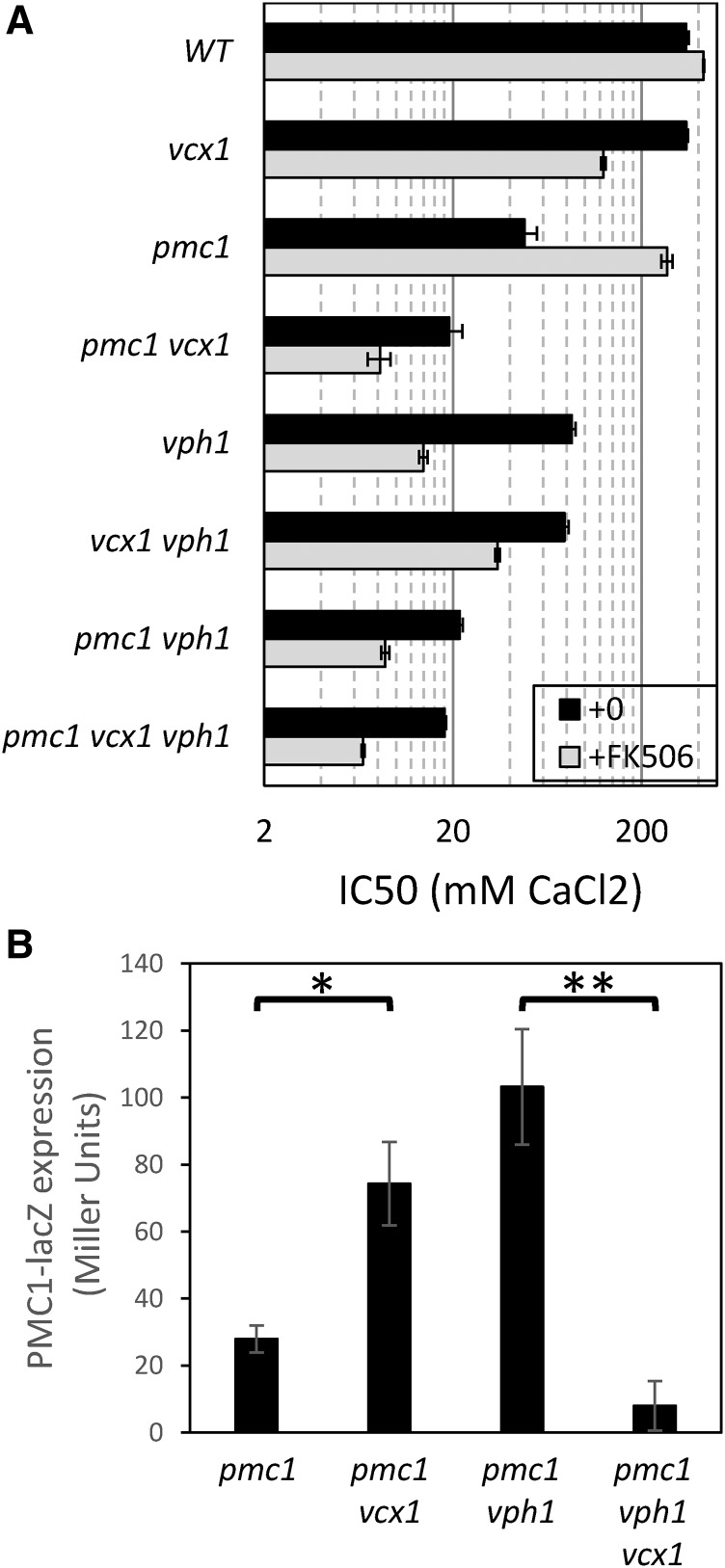
Figure 2.
Vacuolar V-ATPase determines directionality of Vcx1 operation. (A) Ca2+ tolerance assays were performed in duplicate in YPDS medium (black bars) or in the same medium containing 0.2 µg/ml FK506 (gray bars) as described in Materials and Methods, and the derived IC50 values (±SD) for the indicated mutants were plotted on a log scale. (B) The indicated strains were transformed with plasmid pKC190 that bears the PMC1-lacZ reporter gene (Cunningham and Fink 1996) and β-galactosidase activity was measured 4 hr after log-phase cells were shifted to YPDS medium containing 100 mM CaCl2. Results from three independent transformants were averaged (±SD). * P < 0.05, ** P < 0.01.
To test whether the vacuolar V-ATPase mediates the interaction between calcineurin and Vcx1, the same eight mutant strains were reanalyzed in the presence of FK506 (Figure 2A, gray bars). The pmc1∆ vcx1∆ vph1∆ triple mutant became 2.7-fold more sensitive to Ca2+ in the presence of FK506 than in the absence of FK506, presumably owing to diminished function of the Crz1 transcription factor and therefore diminished expression of the Pmr1 Ca2+/Mn2+ ATPase in the Golgi complex when calcineurin is inhibited. Restoring Vcx1 function alone had little effect (1.3-fold increase) in the presence of FK506, whereas restoring Pmc1 function alone had strong effects (5.2-fold increase) despite a lower expression of Pmc1 in the absence of calcineurin (Cunningham and Fink 1994). Importantly, restoring Vcx1 in the vph1∆ mutant actually lowered tolerance to environmental Ca2+ by a significant degree (2.5-fold decrease) in the absence of both calcineurin and only when Pmc1 was functioning. All these findings were reproducible in a replicate experiments and show, for the first time, that Vcx1 can antagonize the function of Pmc1 when the vacuoles lack acidification by the V-ATPase and when the cytoplasm lacks calcineurin signaling. Such antagonism was expected, because H+/Ca2+ exchangers are generally thought to operate in “reverse mode” in conditions where the luminal H+ concentration is low and the Ca2+ concentration is high, potentially causing futile cycles between Vcx1 and Pmc1 in the absence of Vph1. Reverse-mode operation of Na+/Ca2+ exchangers is well established (Harper and Sage 2016). That reverse-mode operation of Vcx1 (observed in vph1∆ mutants with FK506) did not occur in the absence of FK506 suggests that calcineurin regulates both forward and reverse modes of Vcx1 through a process that is independent of the V-ATPase.
Reverse-mode operation of Vcx1 is predicted to increase cytoplasmic free Ca2+ concentrations and enhance calcineurin signaling, whereas forward-mode operation achieves the opposite effects. To test this prediction, we measured expression of a calcineurin-sensitive reporter gene PMC1-lacZ in vcx1∆ vph1∆ double mutants relative to the single mutants and control strain. This experiment was performed in a pmc1∆ mutant background to improve the sensitivity of the reporter and enable detection of forward-mode Vcx1 activity (Cunningham and Fink 1996). As predicted, the pmc1∆ vcx1∆ strain expressed PMC1-lacZ at significantly higher levels than the pmc1∆ strain after exposure to 100 mM CaCl2, whereas the pmc1∆ vph1∆ vcx1∆ triple mutant strain exhibited much lower levels of expression than the pmc1∆ vcx1∆ double mutant strain (Figure 2B). These findings show that Vcx1 can lower calcineurin signaling when the vacuole is properly acidified and increase calcineurin signaling when the vacuolar V-ATPase is inactivated, thus providing independent evidence that Vcx1 transports Ca2+ bidirectionally, similar to Na+/Ca2+ exchangers in animals.
Calcineurin regulates independent functions of Gdt1 and Vcx1
The gdt1∆ mutant, which lacks a probable Golgi-localized H+/Ca2+ exchanger (Demaegd et al. 2013; Colinet et al. 2016), clustered closest to the vcx1∆ mutant in our screening conditions (Figure 1B) and therefore is a potential regulator of Vcx1. To test whether Gdt1 promotes Ca2+ resistance independent of Vcx1, the gdt1∆ knockout mutation was introduced into the pmc1∆ vcx1∆ double mutant and the single mutants in the W303-1A background, and the IC50s of Ca2+ were quantified in the presence and absence of FK506. The gdt1∆ mutation weakly diminished Ca2+ tolerance in every strain background when calcineurin was functional (average decline of 1.35 ± 0.15 fold) but strongly diminished Ca2+ tolerance in WT, pmc1∆, vcx1∆, and pmc1∆ vcx1∆ backgrounds when FK506 was present (by 4.8, 2.9, 5.9, and 2.0 fold, respectively; Figure 3A). Similar results were obtained in four replicate experiments and when calcineurin was inactivated by a cnb1∆ knockout mutation (data not shown). Thus, in the absence of calcineurin signaling, Gdt1 strongly promoted Ca2+ tolerance independent of both Vcx1 and Pmc1, and likely represents a new class of Ca2+ transporter that is partially inhibited by calcineurin.
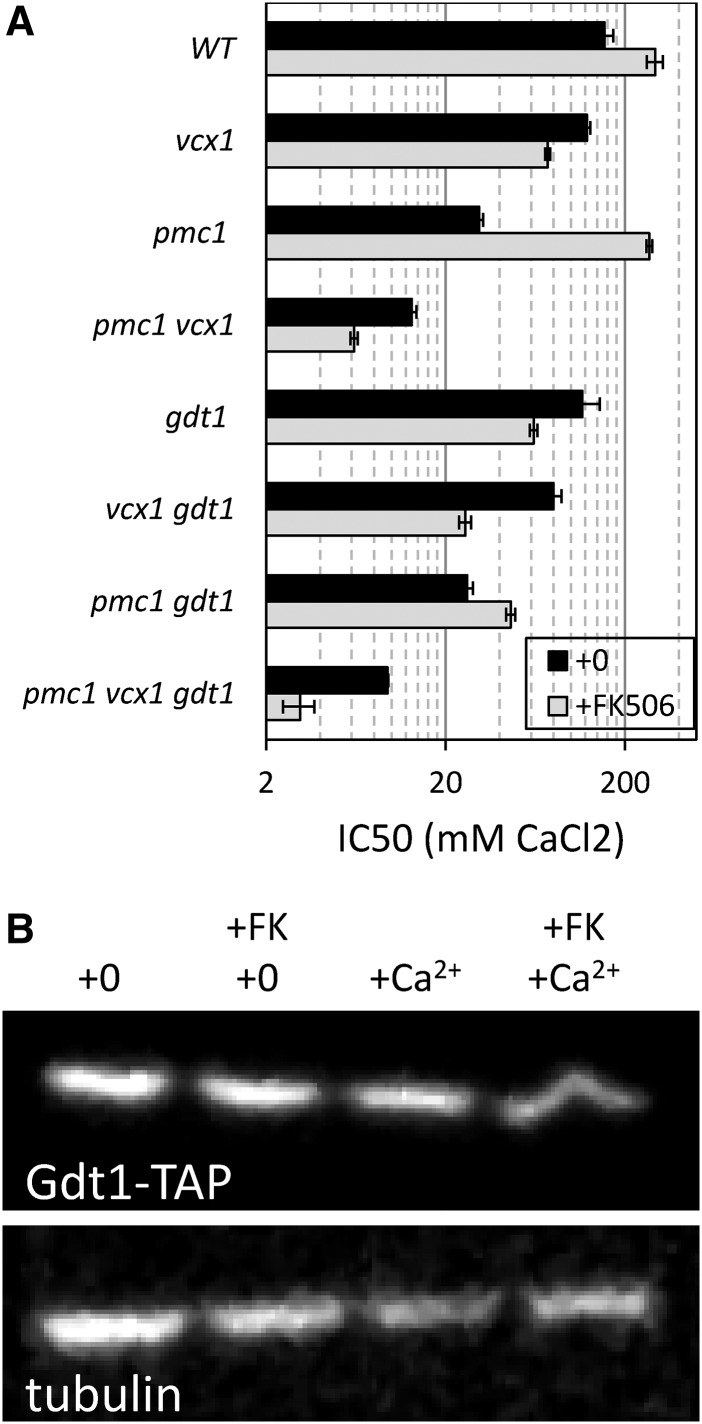
Figure 3.
Gdt1 promotes Ca2+ detoxification independent of Vcx1 and Pmc1. (A) A panel of Saccharomyces cerevisiae strains lacking Gdt1, Vcx1, and Pmc1 in all possible combinations was assayed for Ca2+ tolerance in the presence (gray bars) or absence (black bars) of FK506 as described in Figure 2A. (B) A strain expressing epitope-tagged Gdt1-TAP was grown to log phase in YPDS medium containing 200 mM CaCl2 and/or 1 µg/ml FK506, harvested, lysed, and analyzed by SDS-PAGE and western blotting using anti-TAP polyclonal (top) and anti-tubulin monoclonal (bottom) antibodies.
Calcineurin may directly or indirectly regulate Gdt1 function. To test whether calcineurin inhibits Gdt1 function through its ability to activate Crz1 or to induce expression of Cmk2, we repeated the Ca2+ tolerance experiments described above in crz1∆ cmk2∆ double mutant and single mutant backgrounds. This experiment ruled out Crz1 and Cmk2 as possible intermediaries in the inhibition of Gdt1 by calcineurin (see Table S1). To test whether calcineurin signaling could diminish expression of Gdt1 or alter its mobility on SDS-PAGE, western blots were performed on cells containing a tandem affinity purification (TAP) epitope tag, integrated at the 3′ end of the chromosomal GDT1 gene (Ghaemmaghami et al. 2003), which were exposed to Ca2+ with and without FK506. The TAP tag did not alter the Ca2+ tolerance functions of Gdt1 or its responsiveness to FK506 (not shown). After exposure of the cells to 200 mM CaCl2 with or without 1 µg/ml FK506 for 12 hr, the Gdt1-TAP band maintained the same intensity and migration on the gel (Figure 3B). Therefore, the inhibitory effects of calcineurin on Gdt1 function were not noticeably associated with changes in Gdt1 expression or modification, and are possibly dependent on unknown intermediaries.
Gdt1 promotes H+/Ca2+ exchange in the Golgi complex
Recent studies also showed that Gdt1 localizes to the Golgi complex of yeast (Demaegd et al. 2013), and we have independently confirmed those findings using immunofluorescence microscopy and sucrose gradient fractionation of a functional Gdt1-3HA fusion protein (data not shown). This finding led us to explore the possible interactions between Gdt1 and Pmr1, the secretory pathway Ca2+ ATPase of yeast. To determine whether Gdt1 supplies essential Ca2+ or Mn2+ to the Golgi complex independent of Pmr1, we quantified the tolerance of gdt1∆ pmr1∆ double mutant and single mutant strains to either high Mn2+ (Figure 4A) or a membrane-impermeant chelator of Ca2+, Mn2+, and other divalent cations (BAPTA, Figure 4B) in the culture medium. The Mn2+ tolerance of gdt1∆ mutants and gdt1∆ pmr1∆ double mutants were indistinguishable from control strains (wild type and pmr1∆, respectively), suggesting that Gdt1 cannot remove toxic Mn2+ from the cytoplasm. The gdt1∆ mutant exhibited a strong hypersensitivity to BAPTA relative to the wild-type parent strain, and the gdt1∆ pmr1∆ double mutant exhibited extreme hypersensitivity to BAPTA that was significantly greater than the pmr1∆ mutant (Figure 4B). Thus, Gdt1 appeared to supply essential Ca2+ ions to the Golgi complex independent of Pmr1.
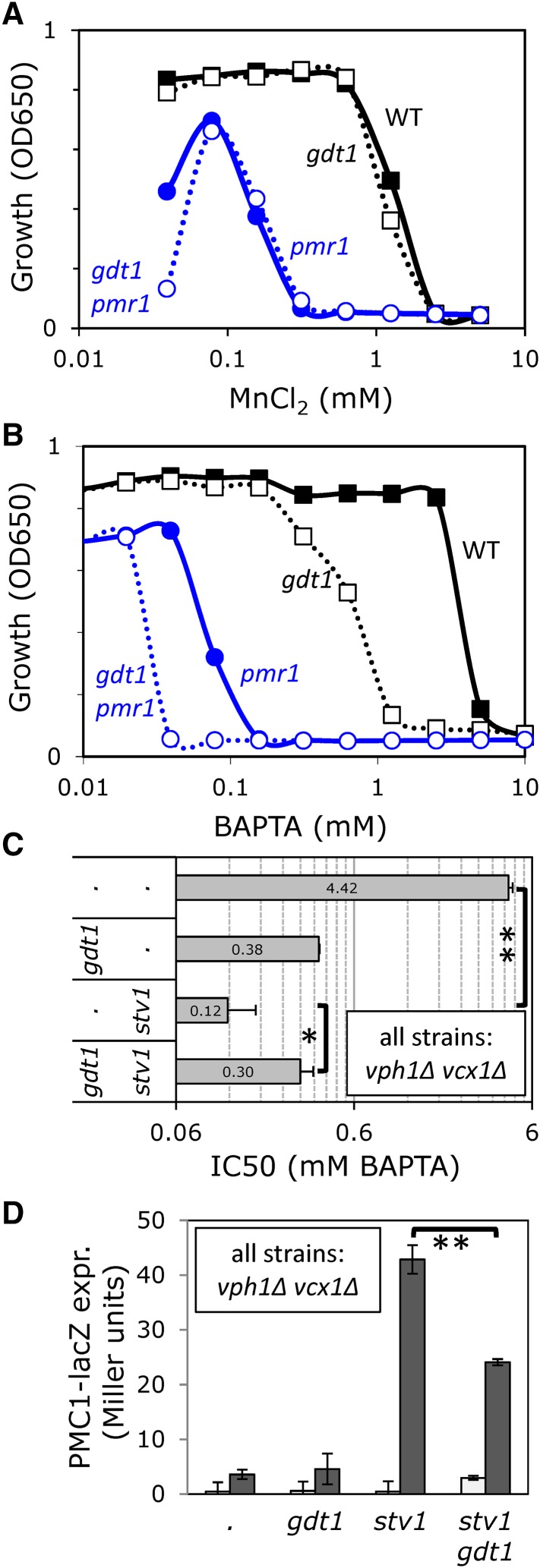
Figure 4.
Gdt1 supplies essential Ca2+ independent of Pmr1, and reverse-mode activity of Gdt1 is blocked by Golgi V-ATPase. Mn2+ (A) and BAPTA (B and C) tolerance assays were performed in YPD medium, and raw data (A and B) or derived IC50 values (C; ±SD) were plotted on log scales. (D) Expression of PMC1-lacZ reporter gene was measured as described in Figure 2B except the medium contained either 0 mM (white bars) or 50 mM (gray bars) supplemental CaCl2. * P < 0.05, ** P < 0.01.
Interestingly, FK506 blocked growth of gdt1∆ pmr1∆ double mutants in all growth media we tested (data not shown). This lethality of FK506 suggests that calcineurin-dependent upregulation of Pmc1 and relocalization to the Golgi complex (Marchi et al. 1999) is crucial to supply essential Ca2+ when Pmr1 and Gdt1 are absent, but further experiments are required to test this hypothesis.
If Gdt1 functions as a H+/Ca2+ exchanger in the Golgi, its contributions to BAPTA tolerance should depend on the Golgi/endosomal forms of the V-ATPase, which employ Stv1 rather than Vph1 in the V0-sector of the enzyme (Manolson et al. 1994). However, stv1∆ mutants alone behaved like wild type in all conditions tested, because the remaining Vph1-containing V-ATPase fully acidifies the Golgi even in the absence of Stv1 (Qi and Forgac 2007). We therefore tested the BAPTA tolerance of gdt1∆ and stv1∆ mutations in a background that lacks Vph1 (and also Vcx1, which could complicate the analyses). In this vph1∆ vcx1∆ double mutant background, the loss of Gdt1 caused a more than 10-fold decrease in BAPTA tolerance and the further loss of Stv1 had little additional effect (Figure 4C). The loss of Stv1 alone caused a more than 25-fold decrease in BAPTA tolerance, and this hypersensitivity was partially reversed (∼2.5-fold) by the further loss of Gdt1. Thus, like Vcx1, Gdt1 appeared to function in reverse mode when the V-ATPase was completely eliminated and in forward mode when the V-ATPase was functional.
The ability of Gdt1 to decrease and increase cytosolic free Ca2+ was also examined by measuring expression of the calcineurin-dependent PMC1-lacZ reporter gene described earlier. In the vph1∆ vcx1∆ double mutant background that acidifies the Golgi complex, the additional gdt1∆ mutation did not significantly alter expression of the reporter gene after addition of 50 mM CaCl2 (Figure 4D), and so forward-mode activity of Gdt1 was not detectable above the high influences of Pmc1 and Pmr1 in these conditions. However, reverse-mode activity of Gdt1 was detected in the vph1∆ vcx1∆ stv1∆ triple mutant background, because the additional gdt1∆ mutation significantly lowered expression of the reporter gene (Figure 4D). Together with the earlier findings on Gdt1 and Vcx1 bidirectionality, these findings provide strong support for the hypothesis that Gdt1 functions as a reversible H+/Ca2+ exchanger of the Golgi complex in vivo.
Erd1 recycles inorganic phosphate from the ER and Golgi complex and limits the functions of Pmr1 and Gdt1
If activated calcineurin inhibits Gdt1 function, it may be possible to isolate variants of Gdt1 that are hyperactive when calcineurin is fully functional, similar to the hyperactive Vcx1-D mutants that have been isolated previously (Cunningham and Fink 1996). To focus on mutants that disrupt the interaction between calcineurin and Gdt1, we generated strains simultaneously lacking Pmc1, Vcx1, and Crz1, and selected for rare spontaneous mutants that enabled growth in medium supplemented with high concentrations of CaCl2 (see Materials and Methods). Of 36 independent spontaneous mutations, 26 were found to be dominant in heterozygous diploids. The GDT1 coding sequence from all 26 strains was amplified by PCR and sequenced. No mutations were identified in the GDT1 coding sequences. The mechanism of Gdt1 regulation by calcineurin remains unknown and may involve several unknown intermediary steps (see Discussion).
In addition to the 26 dominant mutants, 10 independent recessive mutants were recovered, and these mutants defined a single complementation group. All of the recessive Ca2+-resistant mutants were found to be hypersensitive to tunicamycin, an inhibitor of N-glycosylation reactions in the ER (Lehle and Tanner 1976), and we isolated two low-copy plasmids from a library of random genomic DNA fragments that complemented this phenotype. The ERD1 gene was found to be necessary and sufficient for complementation of both phenotypes, and the erd1∆ knockout mutation was found to recapitulate both phenotypes in the starting strain background. Though the precise function of Erd1 has not yet been determined, earlier studies show that Erd1 is a polytopic membrane protein important for two Golgi-localized processes: glycosylation of secretory proteins, and retrieval of escaped HDEL-containing proteins back to the ER (Hardwick et al. 1990). To determine its role in Ca2+ homeostasis, the erd1∆ mutation was introduced into backgrounds that also lacked Pmc1, Vcx1, Gdt1, and Crz1, and the IC50s for CaCl2 were measured as before. These experiments showed that Erd1 significantly decreased the Ca2+ tolerance of the pmc1∆ vcx1∆ crz1∆ triple mutant (1.9-fold decrease), and that this effect was abolished if Gdt1 were eliminated (Table S1). Erd1 actually increased Ca2+ tolerance or was neutral in most other conditions. These results are consistent with models where Erd1 selectively diminishes the forward function of Gdt1 in the Golgi complex.
Erd1 contains an EXS domain similar to that of human XPR1 and plant PHO1 proteins, which recently have been shown to export Pi from the cell (Hamburger et al. 2002; Giovannini et al. 2013). Because Pi is produced in the lumen of the Golgi complex and ER as a byproduct of glycosylation reactions (Figure 1A), it is possible that erd1∆ mutants fail to recycle Pi to the cytoplasm, and the backlog of Pi in the lumen increases the buffer capacity for Ca2+ while also interfering with normal glycosylation and sorting reactions. To test the hypothesis that Erd1 recycles the Pi byproduct of glycosylation from the lumen of secretory organelles, several experiments were performed.
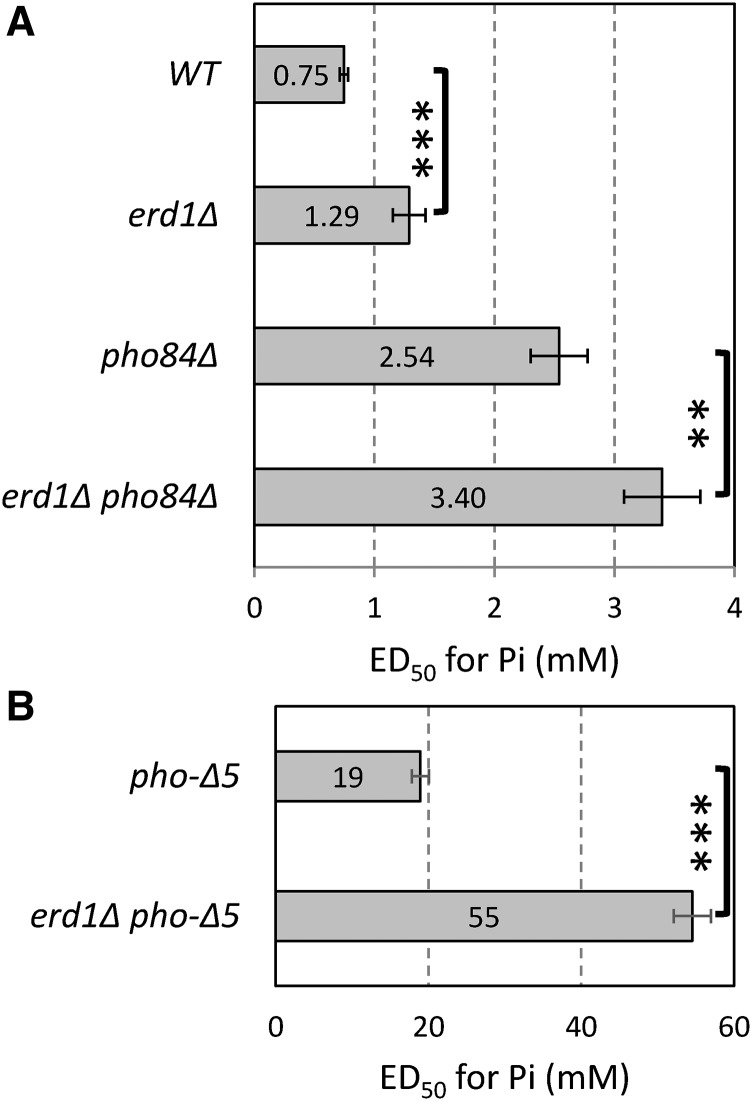
First, because Pi is an important nutrient to yeast, we explored the possibility that Erd1 is important for growth in low-Pi environments. Interestingly, the concentration of Pi in the medium required for 50% maximal growth (i.e., the ED50) was about 1.5-fold higher for erd1∆ mutants compared with the wild-type control strain (Figure 5A). The erd1∆ mutation caused a similar increase in the requirement for Pi when introduced into a pho84∆ mutant background, which lacks a high-affinity Pi transporter in the plasma membrane (Bun-Ya et al. 1991) and requires approximately threefold higher concentrations of Pi than wild type for growth (Figure 5A). The erd1∆ mutation strongly increased the ED50 of Pi nearly threefold when introduced into a pho84∆ pho87∆ pho89∆ pho90∆ pho91∆ quintuple mutant background (Figure 5B) that lacked all five of the known Pi transporters in yeast (Wykoff and O’Shea 2001; Samyn and Persson 2016). Therefore, Erd1 supplies essential Pi to the cytoplasm of yeast cells through a process that does not rely on any of the known Pi transporters.
Figure 5.
Erd1 supplies essential Pi. The indicated mutant strains were washed and inoculated into an SC medium containing various concentrations of Pi. After 24 hr incubation, the concentration of Pi that enabled 50% maximal growth (i.e., ED50) was derived and the averages (±SD) of triplicate measurements were plotted. The erd1∆ mutation increased the ED50 by 1.5-fold, 1.4-fold, and 2.9-fold, respectively, in the wild-type (WT), pho84∆, and pho-∆5 (pho84∆ pho87∆ pho89∆ pho90∆ pho91∆) backgrounds. ** P < 0.01, *** P < 0.001. Note the 15-fold change of scale between pho-∆5 strains (B) and other strains (A).
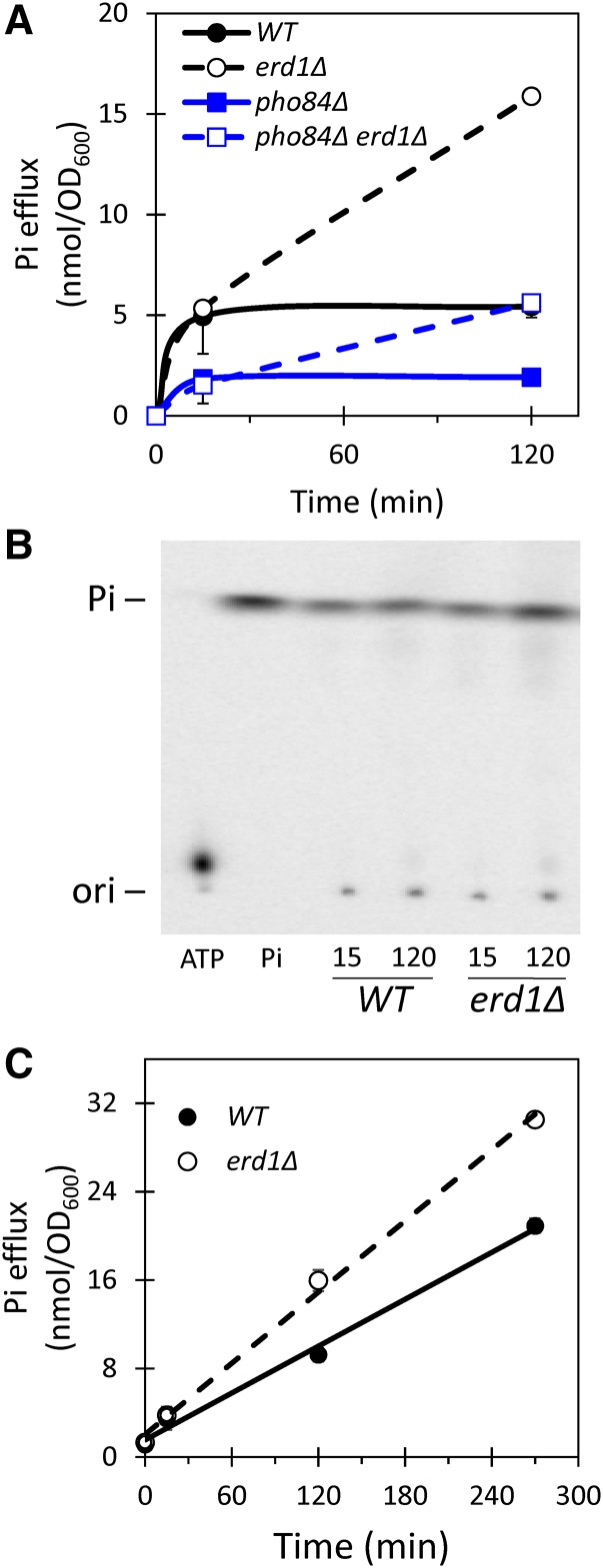
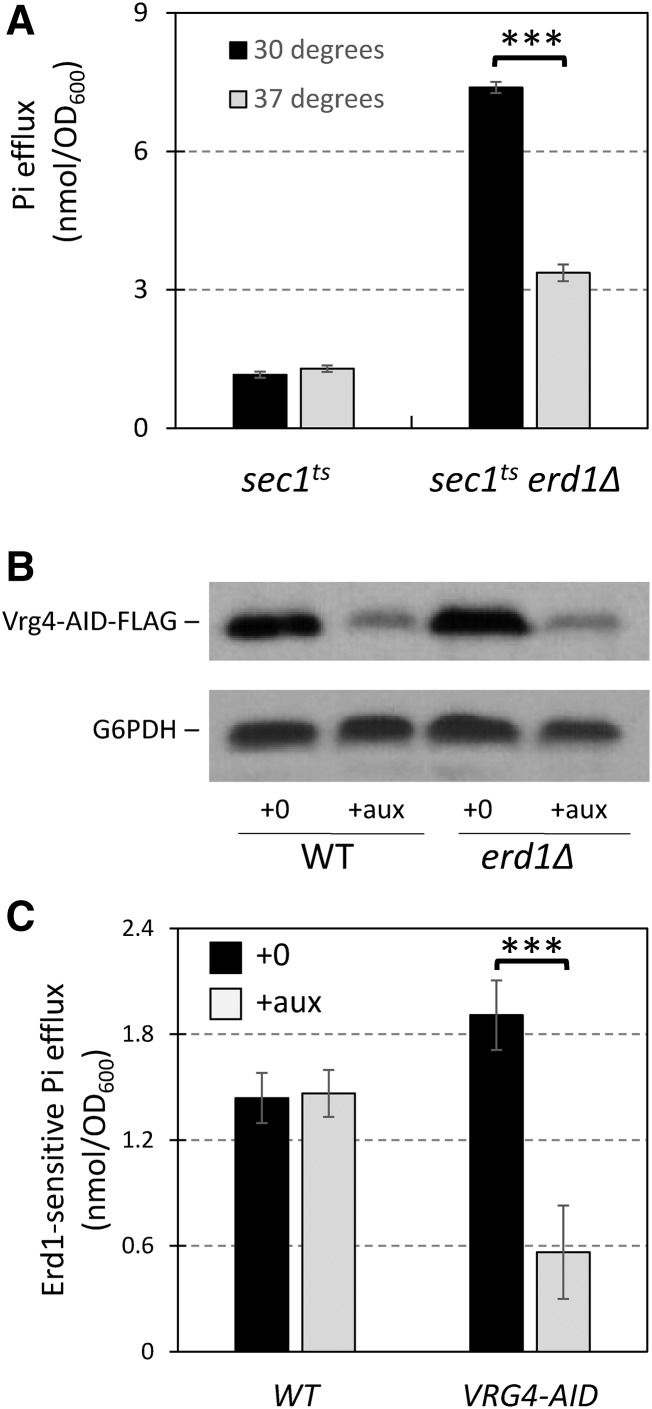
Second, we tested whether erd1∆ mutants exhibited higher rates of Pi export into the culture medium via exocytosis, which would be predicted if Erd1 normally recycles this nutrient from the Golgi complex. For this experiment, the erd1∆ and wild-type strains were cultivated for >10 generations in standard synthetic medium (7.35 mM Pi) supplemented with tracer amounts of [32]Pi, chilled, and the cells were washed extensively in fresh medium lacking Pi. The temperature was then raised to 30° and aliquots were removed at different times, centrifuged to pellet the cells, and the cell-free supernatants were collected and analyzed by liquid scintillation counting and by TLC. In the first 0.25 hr of incubation at 30°, both erd1∆ and wild-type strains exported similar amounts of radioactivity. However, the erd1∆ mutant exported strikingly more Pi than wild type over the next 1.75 hr of incubation (Figure 6A, black curves). Similar trends were obtained in the pho84∆ background (Figure 6A, red curves), though these strains started with <30% of the wild-type levels of Pi owing to their defect in Pi accumulation prior to the start of the experiment. The large majority of radioactivity exported to the culture medium comigrated with Pi standards on TLC (Figure 6B). When the chase experiment was repeated in SC medium containing 7.35 mM Pi, the influx of non-radioactive Pi permits sustained efflux of [32]Pi from wild-type cells for several hours, and still the erd1∆ mutant exported more [32]Pi (Figure 6C). If this Erd1-sensitive Pi efflux depends on exocytosis, such efflux may be diminished in a sec1-1ts mutant background, which quickly accumulates post-Golgi secretory vesicles when shifted to the non-permissive temperature of 37° owing to defects in the exocytotic machinery (Novick et al. 1981). Remarkably, the Erd1-sensitive efflux of Pi was markedly diminished in the sec1-1ts strain at 37° but not in the wild-type control strain (Figure 7A). These data suggest that Erd1 normally limits the export of Pi from cells via exocytosis.
Figure 6.
Erd1 prevents Pi loss to the environment. The indicated mutant strains were grown to log phase in SC medium containing tracer levels of [32]Pi radioisotope, washed extensively in Pi-free SC medium, and then in the same medium (A and B) or in SC medium (C). At the indicated times of incubation at 30°, aliquots were removed and cell-free supernatants were analyzed by liquid scintillation counting (A and C) or by TLC (B). Charts illustrate the averages (±SD) of three biological replicates.
Figure 7.
Erd1-sensitive losses of Pi depend on exocytosis and on transport of GDP-mannose into the Golgi complex. (A) Pi losses were measured after 2 hr incubation as in Figure 6A except that sec1-1ts and sec1-1ts erd1∆ mutant strains were used at a permissive temperature (30°; black bars) or a non-permissive temperature (37°; gray bars) during the incubation in Pi-free medium. (B) Western blot of Vrg4-AID-FLAG strains after 1 hr exposure to 100 µM auxin in SC medium. (C) Erd1-sensitive losses of Pi (i.e., the difference between erd1∆ and ERD1 pairs of strains) were measured for wild-type and Vrg4-AID-FLAG strain backgrounds that were exposed to 100 µM auxin (gray bars) or not (black bars) starting 1 hr before the shifts to Pi-free medium. *** P < 0.001.
If glycosylation reactions in the Golgi complex produce the Pi that is recycled by Erd1, the Erd1-sensitive Pi efflux may be diminished by inactivation of Vrg4, the main nucleotide sugar transporter of the Golgi complex that primarily supplies GDP-mannose in exchange for luminal GMP (Dean et al. 1997). Vrg4 is essential for yeast growth and is not inhibited by any known molecules. To render Vrg4 druggable, we introduced an auxin-inducible degron tag at its C-terminus along with a FLAG tag and an expression cassette for osTIR, an auxin-sensitive E3 ubiquitin ligase (Nishimura et al. 2009; Morawska and Ulrich 2013). Strains bearing a Vrg4-AID-FLAG fusion protein grew as well as wild type in the absence of auxin, but grew much more slowly than wild type in the presence of 100 µM auxin (data not shown), suggesting that Vrg4 expression became growth-limiting in these conditions. Indeed, just 1 hr exposure to 100 µM auxin resulted in a 70% decline in Vrg4-AID-FLAG expression (Figure 7B). The erd1∆ mutation was introduced into the Vrg4-AID-FLAG and wild-type control strains, and Erd1-sensitive Pi efflux was measured as above. After a 2 hr chase period in Pi-free culture medium, the Erd1-sensitive Pi efflux from wild-type cells was unaffected by exposure to auxin (Figure 7C). The Erd1-sensitive Pi efflux from the Vrg4-AID-FLAG strain was similar to wild type in the absence of auxin, and was greatly reduced when the cells were exposed to auxin beginning 1 hr before the washes and continuously during the chase period (Figure 7C). These findings show that the GDP-mannose transporter Vrg4 is a major source of the Pi that is lost to the environment in erd1∆ mutants. Altogether, the results indicate that Erd1 facilitates recycling of the Pi byproduct of glycosylation in the Golgi complex, which alters buffering of Ca2+ and performance of Gdt1.
Discussion
The Golgi complex of all eukaryotes is a hub for glycosylation, sorting, and processing of secreted proteins, transmembrane proteins, ceramides, and other lipids. In yeast, the glycosylation system of the Golgi complex is essential for viability, as mutations that eliminate the major nucleotide-sugar transporter (Vrg4) or the luminal nucleoside triphosphate diphosphatases (Gda1 and Ynd1) are lethal (Dean et al. 1997; Gao et al. 1999). While the nucleoside monophosphate byproduct of glycosylation is exchanged from the Golgi complex for another nucleotide sugar molecule, the fate of the other byproducts, Pi and H+, were elusive until now. Our findings above suggest that Erd1 facilitates transport of the luminal Pi to the cytoplasm where it can be reutilized and that Gdt1 transports luminal H+ to the cytoplasm in exchange for cytoplasmic Ca2+. Because neither protein has been purified to homogeneity and analyzed in reconstituted liposomes, it remains possible that Erd1 and Gdt1 do not directly transport these byproducts of glycosylation and instead regulate unknown transporters that have these properties. Nevertheless, the simplest model of inorganic ion homeostasis in the Golgi complex (summarized in Figure 1A) is consistent with numerous genetic observations in both yeast and humans, and it also raises several new questions as well as new opportunities for treating a congenital disorder of glycosylation in humans (Dulary et al. 2017).
Pi homeostasis in the Golgi complex
Our findings that Erd1 decreases the loss of Pi from the Golgi complex to the environment and promotes growth of yeast cells in low-Pi environments independent of all known Pi transporters suggest that yeast normally recycles this byproduct of glycosylation for reuse in the cytoplasm. This function is somewhat surprising because XPR1, a homolog of Erd1 in the basolateral plasma membrane of humans, has been shown to promote export of cytoplasmic Pi from the cytoplasm rather than import (Giovannini et al. 2013). XPR1 orthologs in zebrafish may have similar roles in Pi export, as knockout mutations resulted in failure to produce osteoclasts (Meireles et al. 2014), which are thought to experience massive Pi uptake and efflux during resorption of bone. PHO1, an ortholog of XPR1 in the plant Arabidopsis thaliana, has also been shown to promote Pi export from root stelar cells to the xylem, resulting in Pi deficiencies in the shoots (Hamburger et al. 2002; Wege and Poirier 2014). PHO1 localizes primarily to the Golgi complex rather than the plasma membrane and thus may export Pi through exocytosis pathways (Arpat et al. 2012). The transmembrane topologies of Erd1 (Kim et al. 2006) and PHO1 (Wege et al. 2016) are similar, so the directionality of net Pi transport by members of the ESX family may depend on other factors such as unknown ions or molecules that could be co- or countertransported with Pi.
Though absent from Erd1, SPX domains are found at the N-termini of XPR1, PHO1, and many other proteins that regulate Pi homeostasis (Secco et al. 2012). Deletion of the SPX domain did not diminish Pi transport activity or alter directionality (Wege et al. 2016). The SPX domain can also be found at the N-termini of numerous other proteins involved in Pi homeostasis in yeast, such as Pi transporters of the SL13 family (Pho87, Pho90, Pho91), subunits of the vacuolar poly-Pi synthase (Vtc2, Vtc3, Vtc4, Vtc5), a cyclin-dependent kinase (Pho85) and its inhibitor (Pho81) that govern the response to Pi starvation, glycerophosphocholine phosphodiesterase 1 (Gde1), and another ESX domain protein of unknown function (Syg1) (Secco et al. 2012). Several SPX domains have now been shown to bind inositol polyphosphates such as IP6 and IP7 (Lee et al. 2007; Wild et al. 2016), which have important regulatory effects on Pi homeostasis. The lack of an SPX domain in Erd1, and the inability of Pho4 to stimulate transcription of the ERD1 gene in Pi-limiting conditions, suggest that Erd1 may escape the conventional regulation imposed on other Pi homeostasis regulators.
The evolutionary origins of Erd1 also may be instructive about its functions in cell physiology. Our searches of protein databanks and multiple sequence alignments (not shown) reveal orthologs of Erd1 in virtually all species of fungi, in a unicellular relative of fungi (the nucleariid Fonticula alba), and in a unicellular relative of metazoans (the choanoflagellate Monosiga brevicollis), but not in metazoans. This suggests that the Erd1 subfamily of ESX-domain proteins originated prior to the divergence of fungi and animals but were uniquely lost from the metazoan lineage. This loss in metazoans could have been enabled by the gain of another Pi transporter in the Golgi complex. Alternatively, the benefits of Pi recycling from the Golgi complex may have diminished in metazoans, where the loss of Pi to the extracellular fluids is not harmful or is potentially even beneficial for Pi storage, for pH buffering, or for creating extracellular structures.
In addition to defects in Pi recycling, erd1∆ mutants of yeast exhibit striking deficiencies in secretory protein glycosylation and in sorting by the H/KDEL receptor (Erd2), which ordinarily retrieves many ER-resident molecular chaperones and enzymes that have escaped the ER (Hardwick et al. 1990). How elevated Pi in the lumen of Golgi complex can cause such disparate effects is not clear. Conceivably, high luminal Pi may competitively inhibit the activities of the NTPDases that produce it, or the glycosyltransferases, nucleotide sugar transporters, and other factors involved directly in promoting glycosylation. The binding of H/KDEL peptides to their receptor is highly sensitive to the luminal pH (Wilson et al. 1993), which might be altered in erd1∆ mutants through accumulation of H2PO4− and dissociation to H+ and HPO42−. An inability of erd1∆ mutants to transport Pi out of the Golgi complex may increase the buffering of Ca2+, Mn2+, and Mg2+ in the lumen as well, perhaps altering the structure of the organelle or decreasing the performance of glycosylation and ER retrieval systems. Lastly, the concentration of some other molecules in the Golgi complex could be directly disrupted in erd1∆ mutants if Erd1 (or possibly its associated catalytic partner) also co- or countertransports some other molecule together with Pi. Direct transport studies using liposomes with purified and reconstituted Erd1 protein and other biochemical experiments will be needed to test these possibilities.
Ca2+ homeostasis in the Golgi complex and vacuole
Here we show that Gdt1 promotes growth of yeast cells in both low and high Ca2+ environments independent of the known Ca2+ transporters (Pmr1, Pmc1, Vcx1) and only when the Golgi complex receives sufficient acidification from the V-ATPase. In the absence of all V-ATPase activity, Gdt1 seemed to partially undo the work of the other transporters and to have the opposite effects on cell growth and on calcineurin activation in the cytoplasm. By showing that Gdt1 can operate in reverse mode when the V-ATPase is eliminated, we add strong experimental support to the hypothesis that Gdt1 catalyzes H+/Ca2+ exchange in the Golgi complex (Demaegd et al. 2013; Colinet et al. 2016). But unlike Pmr1, which transports both Ca2+ and Mn2+ to the Golgi complex, we did not detect any hypersensitivity of gdt1∆ mutants to elevated Mn2+ in the medium even in the absence of Pmr1 (data not shown), suggesting that Gdt1 may not transport significant levels of Mn2+. Consistent with these findings, the partial rescue of glycosylation defects in pmr1∆ mutants by supplemental Ca2+ depended on Gdt1, but the partial rescue by supplemental Mn2+ occurred independent of Gdt1 (Colinet et al. 2016). While these findings suggest that Gdt1 does not play a direct part in Mn2+ transport into the Golgi complex, direct transport of Mn2+ by Gdt1 was proposed to explain the observation that gdt1∆ mutants exhibit glycosylation defects in high Ca2+ conditions that can be rescued by supplemental Mn2+ and Pmr1 function (Potelle et al. 2016). Our demonstration of reverse-mode operation of Gdt1 strengthens an alternative hypothesis to explain this observation: in high Ca2+ conditions, Gdt1 may normally promote net Ca2+ efflux from the Golgi complex and thereby allow Pmr1 to cycle more rapidly and effectively transport more Mn2+ for stimulation of glycosyltransferases.
Findings from mammalian cells indicate broad conservation of Gdt1 and TMEM165 function in the Golgi complex. Simultaneous knockouts of TMEM165 and SPCA1 genes (a homolog of Pmr1 encoded by ATP2C1) result in synthetic lethality in the human HAP1 cell line (Blomen et al. 2015), suggesting that these Golgi proteins share important functions. Deficiency of TMEM165 in human cells disrupts pH homeostasis of lysosomes and late endosomes (Demaegd et al. 2013), as expected if TMEM165 normally catalyzes H+/Ca2+ exchange in the forward mode in these cells. Interestingly, knockdown of TMEM165 in human cell lines resulted in glycosylation defects that could be rescued by low concentrations of Mn2+ (Potelle et al. 2016). Such rescue provides clues for therapies to treat rare deficiencies of TMEM165 in humans, which cause a type-II congenital disorder of glycosylation that manifests with glycosylation defects, bone dysplasias, and other abnormalities (Dulary et al. 2017). TMEM165 appears to be expressed in virtually all tissues, so understanding how such specific developmental abnormalities arise from defects in a housekeeping gene function will require much more work. The recent identification of TMEM165 splice variants localized to the ER, rather than the Golgi, raises the possibility of additional functions of TMEM165 that have yet to be identified (Krzewinski-Recchi et al. 2017).
We also provide evidence that activated calcineurin can inhibit the forward-mode activity of Gdt1 in vivo, similar to that of Vcx1: in the presence of the calcineurin inhibitor FK506, gdt1∆ and vcx1∆ mutants were far more hypersensitive to Ca2+ than in the absence of the calcineurin inhibitor, even when Pmc1 and Crz1 were eliminated. Gdt1 did not mediate the inhibition of Vcx1 by calcineurin, and Vcx1 did not mediate the inhibition of Gdt1 by calcineurin, as calcineurin still retains its inhibitory effects when one or the other transporter has been eliminated. The molecular mechanism(s) by which calcineurin regulates the function of Gdt1 and Vcx1 remain unknown, as neither protein undergoes changes in expression or mobility on SDS-PAGE upon activation/inhibition of calcineurin. Our finding that calcineurin still inhibited reverse-mode activity of Vcx1 in the absence of Vph1 suggests that neither the vacuolar V-ATPase nor luminal H+ are key mediators of this regulation. We also ruled out Erd1 as an intermediary of Gdt1 inhibition by calcineurin, as this regulation persisted in erd1∆ vcx1∆ strains (Table S1). Because Gdt1 and Vcx1 both seem to promote H+/Ca2+ exchange, it is tempting to speculate that calcineurin regulates the pH of the cytoplasm or organelles in high Ca2+ conditions, which would alter the ability of transporters to bind Ca2+. Indeed, new evidence suggests that calcineurin may downregulate the plasma membrane H+ pump Pma1 (P. Kane, personal communication), potentially causing acidification of the cytoplasm and diminishing forward Ca2+ transport by H+/Ca2+ exchangers.
Because Gdt1 and Vcx1 may also inhibit calcineurin activation by removing Ca2+ from the cytoplasm, both proteins have the potential to form double-negative feedback loops with calcineurin (Figure 1A). Double-negative feedback loops generate positive feedback in signaling networks, and tend to promote switch-like transitions between two stable states (Ferrell 2002). In such a scenario, calcineurin is less likely to become activated when Gdt1 and Vcx1 are fully functional, but as cytosolic Ca2+ concentrations rise and calcineurin becomes activated, both Gdt1 and Vcx1 may become progressively inhibited, thus accelerating the activation of calcineurin and the further inhibition of H+/Ca2+ exchangers. Double-negative feedback loops can contribute to heterogeneity in clonal cell populations and a form of cellular memory (hysteresis), as observed previously during the switching between high- and low-affinity Pi transporters in yeast (Wykoff et al. 2007). In addition to these advantages, yeast cells may also inhibit the H+/Ca2+ exchangers in high Ca2+ conditions to avoid reverse-mode operation of Vcx1 and Gdt1 and possible futile cycling with the Ca2+ ATPases (Pmc1 and Pmr1, which are upregulated by calcineurin signaling). It will be interesting to determine precisely how and why calcineurin inhibits Gdt1 and Vcx1 functions in vivo, and whether such regulation contributes to the unexplained “bursts” of free Ca2+ elevation and calcineurin signaling that have been observed through real-time imaging in single yeast cells (Cai et al. 2008; Carbo et al. 2017).
A better understanding of H+, Ca2+, and Pi homeostasis in the Golgi complex may help explain how this organelle can operate so differently in different tissues of humans. In addition to a general housekeeping function in nonsecretory cells, specialized secretory cells may require huge increases in glycosylation and corresponding increases in byproduct production. In the case of alveolar epithelial cells of the mammary gland, which can produce massive quantities of lactose (a product of glycosylation) and casein micelles (rich in Ca2+ and Pi) in the Golgi complex during lactation (Neville 2005), retrieval of the Pi byproduct may not be beneficial and removal of the H+ byproduct in exchange for Ca2+ may be exceptionally important. Interestingly, to potentially meet this demand, expression of TMEM165 mRNA and protein becomes massively increased in alveolar epithelial cells just as milk production begins (Reinhardt et al. 2014). The full repertoire of functions carried out by the Gdt1 and the Erd1 families of proteins will be fascinating to unravel in the many different species and cellular situations.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300339/-/DC1.
Acknowledgments
The authors thank Lauren Meyer for excellent technical assistance and Young-Sam Lee for thoughtful advice. This research was generously supported by grants from the National Institute of Child Health and Human Development (R21HD080102), the National Institute of Allergy and Infectious Disease (R21AI115016), the National Institute of General Medical Sciences (T32GM007231), and Johns Hopkins University. The authors express no conflicts of interest.
Footnotes
Communicating editor: C. Hoffman
Literature Cited
- Antebi A., Fink G. R., 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3: 633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpat A. B., Magliano P., Wege S., Rouached H., Stefanovic A., et al. , 2012. Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J. 71: 479–491. [DOI] [PubMed] [Google Scholar]
- Blomen V. A., Majek P., Jae L. T., Bigenzahn J. W., Nieuwenhuis J., et al. , 2015. Gene essentiality and synthetic lethality in haploid human cells. Science 350: 1092–1096. [DOI] [PubMed] [Google Scholar]
- Bonilla M., Cunningham K. W., 2003. Mitogen-activated protein kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell 14: 4296–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla M., Nastase K. K., Cunningham K. W., 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21: 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y., 1991. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 11: 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Dalal C. K., Elowitz M. B., 2008. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbo N., Tarkowski N., Ipina E. P., Dawson S. P., Aguilar P. S., 2017. Sexual pheromone modulates the frequency of cytosolic Ca2+ bursts in Saccharomyces cerevisiae. Mol. Biol. Cell 28: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet A. S., Sengottaiyan P., Deschamps A., Colsoul M. L., Thines L., et al. , 2016. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 6: 24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R., 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R., 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N., Zhang Y. B., Poster J. B., 1997. The VRG4 gene is required for GDP-mannose transport into the lumen of the Golgi in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 272: 31908–31914. [DOI] [PubMed] [Google Scholar]
- Demaegd D., Foulquier F., Colinet A. S., Gremillon L., Legrand D., et al. , 2013. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc. Natl. Acad. Sci. USA 110: 6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulary E., Potelle S., Legrand D., Foulquier F., 2017. TMEM165 deficiencies in congenital disorders of glycosylation type II (CDG-II): clues and evidences for roles of the protein in Golgi functions and ion homeostasis. Tissue Cell 49: 150–156. [DOI] [PubMed] [Google Scholar]
- Dürr G., Strayle J., Plemper R., Elbs S., Klee S. K., et al. , 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9: 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14: 140–148. [DOI] [PubMed] [Google Scholar]
- Forster C., Kane P. M., 2000. Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J. Biol. Chem. 275: 38245–38253. [DOI] [PubMed] [Google Scholar]
- Foulquier F., Amyere M., Jaeken J., Zeevaert R., Schollen E., et al. , 2012. TMEM165 deficiency causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 91: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. D., Kaigorodov V., Jigami Y., 1999. YND1, a homologue of GDA1, encodes membrane-bound apyrase required for Golgi N- and O-glycosylation in Saccharomyces cerevisiae. J. Biol. Chem. 274: 21450–21456. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., et al. , 2003. Global Analysis of Protein Expression in Yeast. Nature 425: 737–741. [DOI] [PubMed] [Google Scholar]
- Giaever G., Shoemaker D. D., Jones T. W., Liang H., Winzeler E. A., et al. , 1999. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat. Genet. 21: 278–283. [DOI] [PubMed] [Google Scholar]
- Giovannini D., Touhami J., Charnet P., Sitbon M., Battini J. L., 2013. Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 3: 1866–1873. [DOI] [PubMed] [Google Scholar]
- Hamburger D., Rezzonico E., MacDonald-Comber Petetot J., Somerville C., Poirier Y., 2002. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K. G., Lewis M. J., Semenza J., Dean N., Pelham H. R., 1990. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 9: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. G., Sage S. O., 2016. TRP-Na+/Ca2+ exchanger coupling. Adv. Exp. Med. Biol. 898: 67–85. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Robbins P. W., Abeijon C., 1998. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 67: 49–69. [DOI] [PubMed] [Google Scholar]
- Hu Z., Bonifas J. M., Beech J., Bench G., Shigihara T., et al. , 2000. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 24: 61–65. [DOI] [PubMed] [Google Scholar]
- Kane P. M., 2016. Proton transport and pH control in fungi. Adv. Exp. Med. Biol. 892: 33–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Melen K., Osterberg M., von Heijne G., 2006. A global topology map of the Saccharomyces cerevisiae membrane proteome. Proc. Natl. Acad. Sci. USA 103: 11142–11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles A. F., 2011. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 7: 21–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewinski-Recchi M. A., Potelle S., Mir A. M., Vicogne D., Dulary E., et al. , 2017. Evidence for splice transcript variants of TMEM165, a gene involved in CDG. Biochim. Biophys. Acta 1861: 737–748. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Mulugu S., York J. D., O’Shea E. K., 2007. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L., Tanner W., 1976. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 72: 167–170. [DOI] [PubMed] [Google Scholar]
- Locke E. G., Bonilla M., Liang L., Takita Y., Cunningham K. W., 2000. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 20: 6686–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Wu B., Proteau D., Taillon B. E., Roberts B. T., et al. , 1994. STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H(+)-ATPase subunit Vph1p. J. Biol. Chem. 269: 14064–14074. [PubMed] [Google Scholar]
- Marchi V., Sorin A., Wei Y., Rao R., 1999. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett. 454: 181–186. [DOI] [PubMed] [Google Scholar]
- Martin D. C., Kim H., Mackin N. A., Maldonado-Baez L., Evangelista C. C., et al. , 2011. New regulators of a high affinity Ca2+ influx system (HACS) revealed through a genome-wide screen in yeast. J. Biol. Chem. 286: 10744–10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos D. P., Kingsbury T. J., Ahsan U. S., Cunningham K. W., 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11: 3445–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Li H., Hogan P. G., Cunningham K. W., 2009. Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol. Cell. Biol. 10: 2777–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles A. M., Shiau C. E., Guenther C. A., Sidik H., Kingsley D. M., et al. , 2014. The phosphate exporter xpr1b is required for differentiation of tissue-resident macrophages. Cell Rep. 8: 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska M., Ulrich H. D., 2013. An expanded tool kit for the auxin-inducible degron system in budding yeast. Yeast 30: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. C., 2005. Calcium secretion into milk. J. Mammary Gland Biol. Neoplasia 10: 119–128. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M., 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6: 917–922. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R., 1981. Order of events in the yeast secretory pathway. Cell 25: 461–469. [DOI] [PubMed] [Google Scholar]
- Potelle S., Morelle W., Dulary E., Duvet S., Vicogne D., et al. , 2016. Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum. Mol. Genet. 25: 1489–1500. [DOI] [PubMed] [Google Scholar]
- Pozos T. C., Sekler I., Cyert M. S., 1996. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16: 3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Forgac M., 2007. Cellular environment is important in controlling V-ATPase dissociation and its dependence on activity. J. Biol. Chem. 282: 24743–24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt T. A., Lippolis J. D., Sacco R. E., 2014. The Ca/H antiporter TMEM165 expression, localization in the developing, lactating and involuting mammary gland parallels the secretory pathway Ca2+ATPase (SPCA1). Biochem. Biophys. Res. Commun. 445: 417–421. [DOI] [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., et al. , 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58: 133–145. [DOI] [PubMed] [Google Scholar]
- Samyn D. R., Persson B. L., 2016. Inorganic phosphate and sulfate transport in S. cerevisiae. Adv. Exp. Med. Biol. 892: 253–269. [DOI] [PubMed] [Google Scholar]
- Secco D., Wang C., Shou H., Whelan J., 2012. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 586: 289–295. [DOI] [PubMed] [Google Scholar]
- Smardon A. M., Diab H. I., Tarsio M., Diakov T. T., Nasab N. D., et al. , 2014. The RAVE complex is an isoform-specific V-ATPase assembly factor in yeast. Mol. Biol. Cell 25: 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J. T., Hwang S. Y., Tomita T., DeHaven W. I., Mercer J. C., et al. , 2010. Activation and regulation of store-operated calcium entry. J. Cell. Mol. Med. 14: 2337–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorin A., Rosas G., Rao R., 1997. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 272: 9895–9901. [DOI] [PubMed] [Google Scholar]
- Stanley P., 2011. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 3: a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A. M., Cyert M. S., 1997. Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11: 3432–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan C. P., Cunningham K. W., 2013. Kch1 family proteins mediate essential responses to endoplasmic reticulum stresses in the yeasts Saccharomyces cerevisiae and Candida albicans. J. Biol. Chem. 288: 34861–34870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan C. P., Zhang N., Sokabe T., Rivetta A., Slayman C. L., et al. , 2013. Activation of an essential calcium signaling pathway in Saccharomyces cerevisiae by Kch1 and Kch2, putative low-affinity potassium transporters. Eukaryot. Cell 12: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayle J., Pozzan T., Rudolph H. K., 1999. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 µM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 18: 4733–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbrak R., Brown J., Dobson-Stone C., Carter S., Ramser J., et al. , 2000. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum. Mol. Genet. 9: 1131–1140. [DOI] [PubMed] [Google Scholar]
- Wege S., Poirier Y., 2014. Expression of the mammalian xenotropic polytropic virus receptor 1 (XPR1) in tobacco leaves leads to phosphate export. FEBS Lett. 588: 482–489. [DOI] [PubMed] [Google Scholar]
- Wege S., Khan G. A., Jung J. Y., Vogiatzaki E., Pradervand S., et al. , 2016. The EXS domain of PHO1 participates in the response of shoots to phosphate deficiency via a root-to-shoot signal. Plant Physiol. 170: 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild R., Gerasimaite R., Jung J. Y., Truffault V., Pavlovic I., et al. , 2016. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352: 986–990. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Lewis M. J., Pelham H. R., 1993. pH-dependent binding of KDEL to its receptor in vitro. J. Biol. Chem. 268: 7465–7468. [PubMed] [Google Scholar]
- Wykoff D. D., O’Shea E. K., 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159: 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff D. D., Rizvi A. H., Raser J. M., Margolin B., O’Shea E. K., 2007. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol. Cell 27: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaert R., de Zegher F., Sturiale L., Garozzo D., Smet M., et al. , 2013. Bone dysplasia as a key feature in three patients with a novel congenital disorder of glycosylation (CDG) Type II due to a deep intronic splice mutation in TMEM165. JIMD Rep. 8: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Du J., Zhao G., Jiang L., 2013. Activation of calcineurin is mainly responsible for the calcium sensitivity of gene deletion mutations in the genome of budding yeast. Genomics 101: 49–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All yeast strains and plasmids are archived and available upon request. Raw data files used to generate figures and tables are also archived and available upon request. All requests should be made to the corresponding author.