Abstract
The long-term performance of different selection strategies was evaluated via simulation using the example of a local cattle breed, German Angler cattle. Different optimum contribution selection (OCS) approaches to maximize genetic gain were compared to a reference scenario without selection and truncation selection. The kinships and migrant contribution (MC) were estimated from genomic data. Truncation selection achieved the highest genetic gain but decreased diversity considerably at native alleles. It also caused the highest increase in MCs. Traditional OCS, which only constrains kinship, achieved almost the same genetic gain but also caused a small increase of MC and remarkably reduced the diversity of native alleles. When MC was required not to increase and the increase of kinship at native alleles was restricted, the MC levels and the diversity at native alleles were well managed, and the genetic gain was only slightly reduced. However, genetic progress was substantially lower in the scenario that aimed to recover the original genetic background. Truncation selection and traditional OCS selection both reduce the genetic originality of breeds with historical introgression. The inclusion of MC and kinship at native alleles as additional constraints in OCS showed great potential for conservation. Recovery of the original genetic background is possible but requires many generations of selection and reduces the genetic progress in performance traits. Hence, constraining MCs at their current values can be recommended to avoid further reduction of genetic originality.
Keywords: optimum contribution selection, conservation, genetic gain, migrant contribution, runs of homozygosity
Crossbreeding can have positive and negative consequences for managed livestock populations. The introgressive hybridization of breeds with high economic value is common to improve performance. Furthermore, the gene flow between populations can counteract the loss of genetic diversity and avoid inbreeding depression. However, it is possible that persistent introgression of genetic material causes breeds to become genetically extinct. For the management of local breeds with historical introgression, three conflicts have to be addressed, i.e., the conflict between increasing genetic gain while managing the inbreeding level, the conflict between maintaining genetic diversity while controlling the loss of genetic uniqueness, and the conflict between increasing genetic gain while recovering the original genetic background. The traditional approach of OCS (traditional OCS) provides a solution to solve the first problem. It aims to maximize genetic gain while controlling the rate of inbreeding by optimizing the genetic contribution of each selection candidate to the next generation (Meuwissen 1997; Grundy et al. 1998; Woolliams et al. 2015). However, traditional OCS cannot solve the other problems. For breeds with historical introgression, although OCS is efficient for controlling the level of kinship and maintaining genetic diversity (Eynard et al. 2016), this diversity may be caused by a large proportion of genetic contributions from other breeds. Additionally, although OCS is efficient in increasing genetic gain, this genetic gain may be achieved by sustained introgression with high-yielding breeds. The reduction of genetic uniqueness due to high MCs and the reduction of diversity at native alleles is a risk for the conservation of the genetic background of the breed. Apart from focusing on high breeding values, reducing MCs to recover genetic originality could also be included as a breeding objective. Advanced OCS approaches could effectively maintain native allele diversity and genetic originality, while ensuring genetic improvement by including MC and kinship at native alleles (Wellmann et al. 2012) as additional constraints, which has been shown for OCS based on pedigree information (Wang et al. 2017).
High-density marker panels of single-nucleotide polymorphisms (SNPs) allow us to obtain more accurate estimates of kinship than pedigrees, as it is common for pedigrees to contain errors (Ron et al. 1996). In addition, genotype-based kinship reflects the actual relatedness between two individuals, whereas pedigree-based estimates are only expectations (Visscher et al. 2006). Furthermore, genotype-based kinship is able to capture the relationships due to distant common ancestors that pedigree-based estimates fail to reflect. Thus, using genotype-based kinship is more efficient than using a pedigree-based approach for the conservation of local breeds (Toro et al. 2014; Mészáros et al. 2015), especially for the removal of undesirable genetic materials (Amador et al. 2013). Currently, there are three approaches to estimate kinships from genome-wide SNPs. The first one is the molecular kinship, i.e., the proportion of SNPs that are identical by state (IBS) (Eding and Meuwissen 2001; Caballero and Toro 2002). The second one is the genomic covariance between individuals computed from gene contents (VanRaden 2008; Yang et al. 2010). The third one is the segment-based kinship computed from shared haplotype segments, which are also known as runs of homozygosity (Gusev et al. 2009; de Cara et al. 2013; Rodríguez-Ramilo et al. 2015; Gómez-Romano et al. 2016). Both molecular kinship and genomic relationship matrices have the disadvantage of being biased due to the preselection of markers included in the SNP panel (Nielsen 2000; McTavish and Hillis 2015). Moreover, increasing genetic diversity by reducing average molecular kinship drives allele frequencies toward 0.5 and increases the frequency of rare deleterious alleles. Thus, it accumulates deleterious variants in the genome and may reduce the fitness of the population (de Cara et al. 2011, 2013). The use of segment-based kinship has been shown to provide a good compromise between maintaining diversity and fitness levels in populations. The estimate based on segments reflects recent identity by descent rather than IBS (Keller et al. 2011). In this study, the segment-based kinship will be used in the optimization process.
Genomic information can also be used to estimate the breed composition of an individual (Frkonja et al. 2012). Software packages for predicting breed composition are usually based on either hidden Markov model clustering algorithms or maximum likelihood procedures (Pritchard et al. 2000; Alexander et al. 2009; Baran et al. 2012). Such analysis can be carried out by Admixture (Alexander et al. 2009) or Structure (Pritchard et al. 2000), where individuals are assumed to be unrelated, and linkage disequilibrium (LD) is not taken into account. Another approach is to assign haplotype segments to the breeds in which they have maximum frequency, which is carried out by optiSel (Wellmann 2017).
The objective of this study was to evaluate the long-term performance of different genomic OCS strategies, using the example of a local cattle breed, by simulating several subsequent generations. The scenarios were compared not only with respect to the genetic gain but also with respect to parameters measuring genetic diversity and genetic uniqueness.
Methods
Data
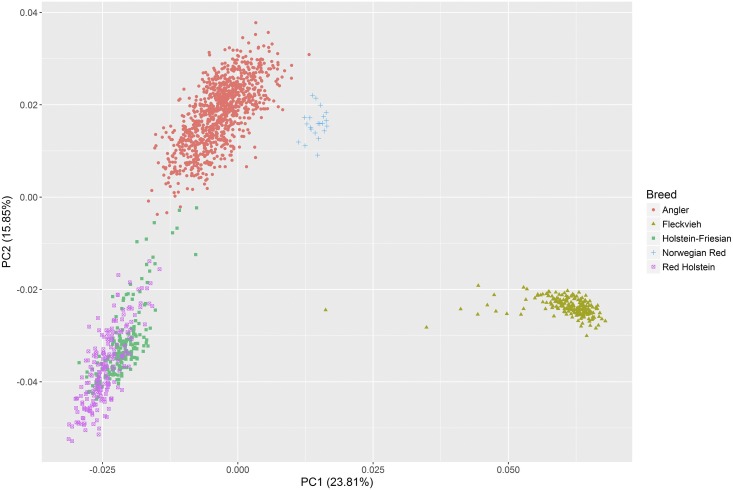
The dataset consisted of genotype information for 889 individuals belonging to five cattle breeds: 268 Angler, 200 Fleckvieh, 200 Holstein-Friesian, 200 Red Holstein, and 21 Norwegian Red. The targeted breed in this study was Angler cattle, which is a dual-purpose cattle breed with an emphasis on milk production. It is mainly located in the northern part of Germany (Bennewitz and Meuwissen 2005). The reference breeds, which include animals from the nontargeted breeds, were only used for the identification of native haplotype segments in Angler cattle. Two hundred Fleckvieh animals were genotyped with the Illumina BovineHD BeadChip (HD), and the remaining animals were genotyped with the Illumina BovineSNP50 BeadChip (50K) with standard quality control parameters. SNPs that were not available for all breeds were discarded. Finally, 23,448 autosomal SNPs were used for the analysis. Haplotypes were phased for all breeds jointly as part of a larger dataset, and missing genotypes were imputed using BEAGLE software (Browning and Browning 2007). To visualize the relationship between Angler cattle and the other four breeds, principal component analysis (PCA) (Price et al. 2006) was performed on the SNP genotypes using PLINK 1.9 software (Chang et al. 2015).
Simulation
The simulations comprised two parts. First, a base population () was generated from the phased genotypes of the Angler cattle. Second, this base population was managed for the following 10 nonoverlapping generations in accordance with the respective scenario.
The base population consisting of 1000 simulated individuals, was generated from genotypes of 131 Angler bulls and 137 Angler cows based on a random sampling of gametes. The animals from other breeds, which were used to identifying native segments, remained the same for each generation. The selection process started from generation For all scenarios, the optimum genetic contribution of each selection candidate () to the next generation was calculated for each generation. The corresponding number of offspring of each parent was generated, and mates were allocated randomly. Offspring received haplotypes from their parents via Mendelian inheritance, allowing recombination to occur according to the length of the chromosomes; i.e., one crossover occurs on average on a chromosome of size 1 M (Weng et al. 2014). For all generations, the population size remained 1000 (500 males and 500 females). For each scenario, five replicates were simulated and the results presented are averages over replicates.
A total of 1500 SNPs were sampled randomly without replacement to become quantitative trait loci (QTL). The QTL effects were sampled from a γ distribution with a shape parameter of 0.4 (Meuwissen et al. 2001) and standardized afterward. The effect of each QTL had a 50% chance of being positive or negative. The highest positive QTL effects were assigned to SNPs that were more frequent in the reference breeds than in Angler cattle. Hence, the mean breeding value in Angler cattle was lower than the mean breeding value in the reference breeds that were used for introgression.
The simulated true breeding value (TBV) of animal was calculated as the sum of all QTL effects:
where is the number of QTL, is the additive effect of QTL and is the QTL genotype of individual j at locus The genotypes were coded as 0, 1, or 2, as the number of copies of the alternative allele. For each individual, an estimated breeding value (EBV) for total merit with the reliability of 0.75 was simulated as:
| , |
where is the mean of the breeding values of the corresponding generation, is a residual term sampled from a normal distribution with mean 0, and variance
MC, kinships, and diversity parameters
For calculating the kinship matrices and MC, the origin of each marker had to be determined for each haplotype from the breed of interest. A haplotype was classified to be native in Angler cattle at a particular marker position if the frequency of the segment containing the marker was sufficiently low in all reference breeds. Only haplotype segments consisting of ≥ 20 consecutive markers and a minimum length of 2.5 Mb were considered. A marker was classified to be native in Angler cattle if the frequency of the segment containing the marker was < 0.01 in all reference breeds. The MC of each individual was calculated as the proportion of its genome that was not classified to be native. The mathematical definitions can be found in the Appendix. For identification of the origin of markers, the R package optiSel (Wellmann 2017) was used.
Two SNP-based kinship parameters were considered, which are denoted as and Kinship between individual and individual (element of the matrix ) is the probability that two alleles taken from a random position from randomly chosen haplotypes of both individuals belong to a shared segment, which is in accordance with de Cara et al. (2013). The mean kinship for the offspring generation is estimated as where is the vector of optimum genetic contributions of all selection candidates. In addition, average kinships among different breeds were calculated from a segment-based kinship matrix that included individuals from all breeds.
For breeds with historical introgression, Wellmann et al. (2012) proposed that kinship at native alleles should be restricted to preserve local breeds. The kinship is the conditional probability that two alleles taken at random from the population belong to a shared segment, given that they are native. For the computation of the segment-based kinship and the kinship at native alleles , we used R package optiSel (Wellmann 2017). The corresponding pedigree-based kinships were referred to as and in Wang et al. (2017). The mathematical definitions can be found in the Appendix.
Three additional genetic parameters were calculated to evaluate the level of genetic diversity of Angler cattle, i.e., the average observed heterozygosity the variance of the TBVs (), and the genic variance (). The observed heterozygosity quantifies the amount of genetic variation due to polymorphic loci, which is an important parameter of estimating genetic variation within a population (Gregorius 1978). We calculated the of each generation in each scenario with software PLINK 1.9 (Chang et al. 2015). The genic variance was calculated as
| , |
where is the number of QTL, is the allele frequency at locus , and is the additive effect of QTL (Falconer and Mackay 1996).
Optimization scenarios
Except for the reference scenario, the objective of all the other scenarios was to maximize the genetic gain of the following generation, so the objective function was where is a vector of the EBVs of all selection candidates. Three OCS scenarios were considered and compared to two non-OCS scenarios, i.e., a reference scenario without selection and a truncation selection scenario.
Reference scenario (REF):
In this scenario, all animals were used as parents and each selection candidate had two offspring. Thus, no selection or optimization was done. The effective population size () is 2000, thus, the increase of kinship is negligible.
Truncation selection (TS):
Maintenance of an effective population size of 100 was envisaged, as recommended in Meuwissen (2009). Calculated from (Falconer and Mackay 1996), 26 bulls with the highest EBVs were selected for breeding to create the following generation by truncation selection. In this scenario, all selected bulls had equal contributions to the offspring. Note that the effective size in this scenario is expected to deviate slightly from 100 because the formula does not take into account how the individuals with highest breeding values are related.
Traditional OCS method (OCS-I):
To restrict the rate of inbreeding, the upper bound of kinship was defined as follows. Since the targeted effective population size was =100, the desired rate of inbreeding, which can be calculated from (Falconer and Mackay 1996), was 0.5% per generation. The threshold for of generation was calculated as:
where is the average kinship of the population in generation
OCS with constraint on kinship kinship , and MC (OCS-II):
The constraint of kinship was the same as in the OCS-I scenario. Additionally, constraints on conditional kinship and MC were applied. The upper bound threshold for in generation was calculated as:
| , |
where is the mean kinship at native alleles of the population at generation Additionally, we required that for each generation, the average level for the estimated MC did not exceed the average level in the base generation ().
OCS with constraint on kinship kinship , and reduced level of MC (OCS-III):
The upper bounds of kinship and of kinship were the same as in scenario OCS-II for each generation. Additionally, in this scenario, we required that the MC level estimated from haplotypes decreased by 3% per generation.
Several reasonable conditions were made for all scenarios. The genetic contribution of a selection candidate expressed as the proportion of genetic material originating from this individual in the next generation, was assumed to be nonnegative In diploid species, each sex group contributes half of the genes to the gene pool. Thus, the sum of genetic contribution of all selection candidates of a sex was 0.5; i.e., where and are vectors for indicators of a candidate’s sex. For all OCS scenarios, optimization was done only for males. All females were assumed to have equal numbers of offspring. All 500 males were used as selection candidates, which reflects a breeding program with genomic selection in which a substantial number of the bull calves are genotyped.
The specific values for each constraint are shown in Supplemental Material, Table S1 in File S3. Solver “cccp” (Pfaff 2014), which was called from the R package optiSel version 0.9.1(Wellmann 2017), was used to solve the optimization problems. Five replicates per scenario were simulated and the results presented are averages across replicates.
Data availability
The data used in this study are available as supplemental files. File S1 contains SNP ID numbers and locations. File S2 contains simulated genotypes for each individual of the Angler cattle base generation
Results
Analysis of base generation ()
The simulated base generation reflects the structure of the genotyped animals well. PCA plots of both populations were almost identical (Figure 1 and Figure S1). The first and second principal components (PC1 and PC2) separated animals in the simulated base generation according to their breed (Figure 1). PC1 explained 23.81% of the total variance and distinguished Fleckvieh from the other four breeds. Angler was separated from the Holstein family by PC2, which explained 15.85% of the total variance. Overlap existed between Holstein-Friesian and Red Holstein since Red Holstein is known to be a subpopulation of Holstein-Friesian.
Figure 1.
Plot of the first two principal components (PCs) for the dataset of the simulated base population The analysis was based on 1621 individuals and 23,448 single-nucleotide polymorphisms. Different colors and shapes represent individuals from different breeds.
Relationships within and between breeds are shown in Table 1. The smallest average kinship within a breed was found in Angler (0.048). This shows that Angler has a higher genetic diversity and lower inbreeding than the other breeds. Angler had a close relationship with Red Holstein (0.039) and Holstein-Friesian (0.037), a moderately close relationship with Norwegian Red (0.017), and a distant relationship with Fleckvieh (0.004). This is in agreement with the estimated genetic contributions that the Angler breed has from other breeds, which were 0.448 from Holstein-Friesian and Red Holstein, 0.152 from Norwegian Red, and 0.021 from Fleckvieh (data not shown).
Table 1. Average kinship () among five breeds of the simulated base generation based on shared segments.
| Angler | Red Holstein | Holstein-Friesian | Norwegian Red | Fleckvieh | |
|---|---|---|---|---|---|
| Angler | 0.048 | 0.039 | 0.037 | 0.017 | 0.004 |
| Red Holstein | 0.110 | 0.085 | 0.007 | 0.004 | |
| Holstein-Friesian | 0.095 | 0.007 | 0.004 | ||
| Norwegian Red | 0.086 | 0.002 | |||
| Fleckvieh | 0.061 |
A basic statistical analysis of the simulated TBVs of animals based on each breed group is presented in Table 2. Angler and Fleckvieh had relatively low average TBVs, with a mean of 0.560 and 0.367, respectively. Holstein-Friesian, Norwegian Red, and Red Holstein had relatively high average TBVs, with means of 2.160, 2.390, and 2.431, respectively (as desired). As shown in Figure S2, there was a positive correlation between MC and TBV in the base population of Angler cattle.
Table 2. Basic statistics of the simulated true breeding values of each breed group of base generation .
| Breed | N | Mean | SD |
|---|---|---|---|
| Angler | 1000 | 0.560 | 0.444 |
| Fleckvieh | 200 | 0.367 | 0.275 |
| Holstein-Friesian | 200 | 2.160 | 0.275 |
| Norwegian Red | 200 | 2.390 | 0.270 |
| Red Holstein | 21 | 2.431 | 0.276 |
Values of each parameter obtained in five scenarios
The mean and SD of the parameter values at the starting stage [base generation ()] and final stage [10th generation ()] for all scenarios are shown in Table 3. The mean and SD were estimated from five replicates. The values of the corresponding parameter for all generations can be found in Tables S2–S8 in File S3.
Table 3. Basic statistics of each parameter achieved in base generation and for each selection scenario.
| Parametersa | |||||||
|---|---|---|---|---|---|---|---|
| EBV | MC | ||||||
| Beginning of selection () | |||||||
| 0.561 ± 0.000 | 0.622 ± 0.000 | 0.048 ± 0.000 | 0.061 ± 0.000 | 0.367 ± 0.000 | 0.197 ± 0.000 | 0.075 ± 0.000 | |
| End of selection () | |||||||
| REF | 0.558 ± 0.020 | 0.587 ± 0.002 | 0.044 ± 0.001 | 0.048 ± 0.001 | 0.364 ± 0.001 | 0.091 ± 0.005 | 0.075 ± 0.001 |
| TS | 3.002 ± 0.062 | 0.679 ± 0.005 | 0.115 ± 0.005 | 0.157 ± 0.009 | 0.346 ± 0.002 | 0.044 ± 0.002 | 0.049 ± 0.001 |
| OCS-I | 2.915 ± 0.026 | 0.638 ± 0.008 | 0.094 ± 0.001 | 0.136 ± 0.004 | 0.351 ± 0.001 | 0.049 ± 0.002 | 0.052 ± 0.002 |
| OCS-II | 2.757 ± 0.059 | 0.617 ± 0.001 | 0.085 ± 0.001 | 0.104 ± 0.001 | 0.353 ± 0.001 | 0.056 ± 0.001 | 0.054 ± 0.002 |
| OCS-III | 1.825 ± 0.106 | 0.455 ± 0.001 | 0.073 ± 0.001 | 0.104 ± 0.001 | 0.355 ± 0.001 | 0.065 ± 0.003 | 0.063 ± 0.002 |
EBV, estimated breeding value; MC, migrant contribution; kinship; kinship at native alleles; average heterozygosity; variance of true breeding value; genic variance.
Parameters estimated in each generation of each scenario.
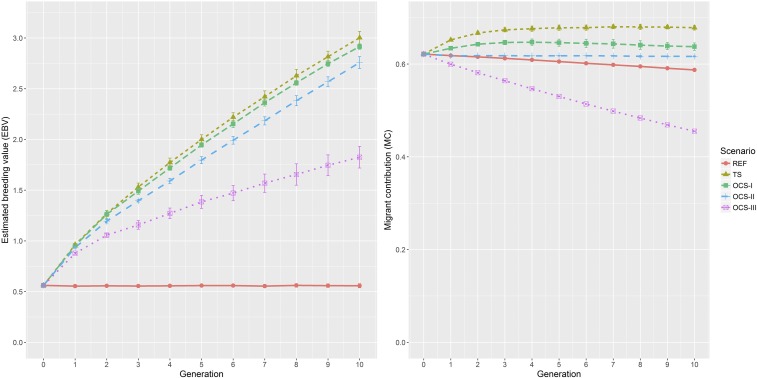
EBV and MC
Except for the reference scenario, the average EBV level of Angler cattle increased in varying degrees from generation to generation in all scenarios, which is shown in Figure 2 (left). The EBV level remained stable at ∼0.558 in REF. The average increase of EBV was ∼0.9 genic SD per generation in TS and OCS-I. The average EBV in was very similar in both scenarios TS and OCS-I. The genetic gain was lower in scenario OCS-II, which achieved an average EBV of 2.757 in and was considerably lower in scenario OCS-III, for which the mean EBV was 1.825 in
Figure 2.
Average estimated breeding values (left) and MC (right) achieved in each generation of each selection scenario. kinship; kinship at native alleles; MC, migrant contribution; OCS, optimum contribution selection; OCS-I, traditional OCS method; OCS-II, OCS with constraint on kinship kinship , and MC; OCS-III, OCS with constraint on kinship kinship , and reduced level of MC; REF, reference scenario; TS, truncation selection.
MC decreased slightly from 0.622 to 0.587 in the reference scenario because old introgressed haplotype segments were split by crossing-over into smaller pieces and could no longer be detected in In contrast, with the increase of EBV in scenarios TS and OCS-I, the level of MC increased to a different extent (Figure 2, right). In scenario TS, MC increased from 0.622 in to 0.676 in and became stable in later generations. Similarly, in scenario OCS-I, MC increased to 0.647 at and became stable afterward. For scenarios OCS-II and OCS-III, MC was set as a constraint. Thus, the average MC values obtained in each generation were approximately equal to the threshold setting in the corresponding generation with a rather small SD; that is, the estimated MC remained 0.618 in OCS-II and decreased by 3% each generation in scenario OCS-III.
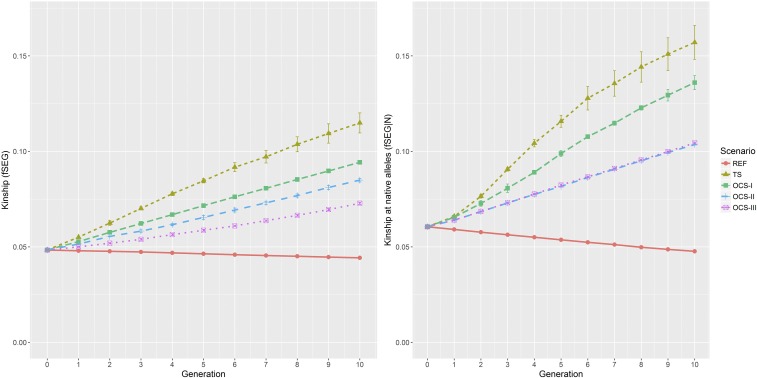
Kinship and kinship
Kinship and kinship increased from generation to generation to varying extents, except for scenario REF, which can be seen in Figure 3 (left: right: ). Kinship had a small reduction in REF from 0.048 to 0.044, which was because old segments were split into smaller pieces, so after some generations, the pieces were no longer involved in shared segments. Kinship increased the most in scenario TS, which moved from 0.048 in to 0.115 in Kinship was set as a constraint in the other three scenarios. For scenario OCS-I, the value of each generation equals the corresponding value of the constraint setting. For scenario OCS-II, in generation the . value increased to 0.085, which is lower than the constraint setting. The smallest mean kinship (0.073) was obtained for scenario OCS-III.
Figure 3.
Average kinship (left) and kinship at native alleles (right) achieved in each generation of each selection scenario. kinship; kinship at native alleles; MC, migrant contribution; OCS, optimum contribution selection; OCS-I, traditional OCS method; OCS-II, OCS with constraint on kinship kinship , and MC; OCS-III, OCS with constraint on kinship kinship , and reduced level of MC; REF, reference scenario; TS, truncation selection.
Estimated kinship decreased from 0.061 in to 0.048 in for the reference scenario because some old introgressed segments were split into small pieces by crossing-over, so the alleles included in the segments were classified as native and contributed to the estimated diversity at native alleles. Kinship increased faster in scenarios TS and OCS-I than kinship The value increased from 0.061 in to 0.157 in TS and to 0.136 in OCS-I. For scenarios OCS-II and OCS-III, was set as a constraint parameter. In all generations of both scenarios, the values were equal to the corresponding constraint setting of with an SD close to zero.
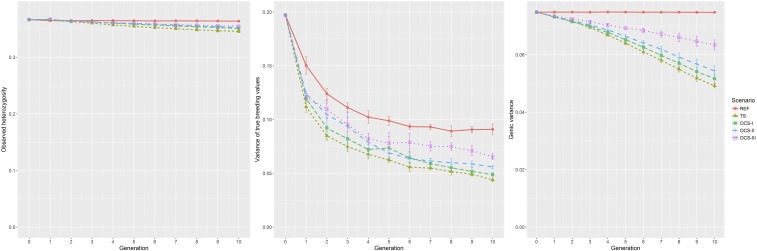
Diversity parameters
The diversity parameters are shown in Figure 4 (left: middle: and right: ). As expected, all diversity values in of REF are higher than the corresponding values of all the other scenarios. The value of and remained nearly unchanged from to The value of decreased considerably from 0.197 to 0.091 from to which is still higher than the level of all the other scenarios (see Table 3). was larger than in which was due to the effects caused by different chromosomes being correlated.
Figure 4.
Average observed heterozygosity (left), variance of true breeding values (middle), and genetic variance (right) achieved in each generation of each selection scenario. kinship; kinship at native alleles; MC, migrant contribution; OCS, optimum contribution selection; OCS-I, traditional OCS method; OCS-II, OCS with constraint on kinship kinship , and MC; OCS-III, OCS with constraint on kinship kinship , and reduced level of MC; REF, reference scenario; TS, truncation selection.
For all scenarios, is relatively stable compared to and The greatest reduction of was found in scenario TS, which moved from 0.367 in to 0.346 in A similar trend but a faster reduction showed the genic variance In scenario TS, the average decreased from 0.075 in to 0.049 in A higher value in was achieved in OCS-I (0.052) and OCS-II (0.054), and the highest genic variance was maintained in OCS-III (0.063).
In all scenarios, the level of decreased considerably from to Thereafter, it decreased at a slower rate and approached the level of the genic variance around For the scenarios with selection, the variance of TBVs in was very similar to the genic variance. It was 0.044 in TS, 0.049 in OCS-I, 0.056 in OCS-II, and the highest level was maintained in scenario OCS-III (0.065). In all scenarios, the average level was much higher than the average genic variance in the first generations. This can be seen in Figure S3 using the example of scenario OCS-II.
Discussion
In this study, we evaluated the long-term performance of five different scenarios for maximizing genetic gain in the context of conserving breeds with historic introgression using the example of Angler cattle. MCs and kinships at native alleles based on shared haplotype segments were restricted in some scenarios. A large proportion of the Angler breed’s genetic background was contributed by other breeds, especially Holstein, which is in accordance with results obtained from pedigree records (Wang et al. 2017). Truncation selection achieved the highest genetic gain among the five scenarios with the highest degree of reduction in genetic diversity. Traditional OCS (OCS-I) achieved a slightly lower genetic gain and slightly higher genetic diversity compared to truncation selection. However, both are inappropriate for the situation of Angler cattle, as both reduced the diversity at native alleles considerably and increased the MC. Constraining MC and kinship at native alleles enabled recovery of the genetic originality but also slowed the genetic progress in performance traits compared to truncation selection and traditional OCS.
Genetic progress vs. genetic conservation
Due to the protocol for simulating QTL effects, a positive correlation between MC and EBV was observed, which is in agreement with results obtained from the pedigree information (Wang et al. 2017). The positive correlation persisted in all generations in all five scenarios (data not shown). There was no genetic progress in REF due to the absence of selection. Truncation selection and traditional OCS achieved similar genetic gain. When MC and kinship at native alleles were constrained, the genetic gain in performance traits was reduced. Hence, to achieve maximum genetic gain, it is essential to allow for the introgression of foreign genetic material.
Maximizing genetic gain is not the only objective of a breeding program. To recover the genetic background of the original endangered population from admixtures, two goals must be set: maintain the genetic diversity at native alleles and remove the introgressed genomic material in the long run. The average MC of the population can be treated as a parameter for measuring genetic uniqueness. Among the five scenarios, truncation selection has the least ability to maintain genetic uniqueness. Although the situation improves in traditional OCS, the estimated MC level in is still higher than at the starting stage and higher than in the reference scenario without selection. This is in accordance with what we obtained from OCS based on pedigrees. The reason why traditional OCS did not cause a larger increase in MC is probably that most MCs were from related Holstein cattle. Thus, increasing genetic gain by increasing MCs would also increase the average kinship in Angler cattle, so restricting average kinship implicitly restricted the MC. Similarly, in Amador et al. (2011), traditional OCS did not eliminate any exogenous representation but kept the value constant, irrespective of the number of generations that had elapsed before management started. Genetic originality could be maintained with the OCS-II method, while genetic gain was only marginally lower, as in traditional OCS and truncation selection.
In OCS-III, the EBV level kept increasing throughout all generations, even though the original genetic background was gradually reconstructed and the highest diversities were maintained with this method. Compared to OCS-II, the reduced genetic progress in OCS-III is directly linked to the strictness of the constraint MC setting. Due to the conflict between achieving genetic gain and maintaining genetic uniqueness, a breeding organization should choose MC constraint settings carefully to achieve both breeding purposes.
In truncation selection, 26 sires with the highest breeding values were selected along with 500 dams to achieve an effective population size of 100 in each generation. However, the formula from which the number of selected sires was obtained did not take into account that the individuals with the highest breeding values were related because they had high genetic contributions from closely related Holstein ancestors. Thus, the rate of inbreeding in truncation selection was higher than in the OCS scenarios. Compared to truncation selection, traditional OCS has good performance in controlling inbreeding via restricting average relatedness in the offspring.
Different kinship estimators
The predictions from and from optiSel were close to the values estimated from offspring haplotypes (results not shown). However, they were slightly larger because some segments were split by crossing-over into small pieces, so they did not contribute to the kinship estimated from offspring haplotypes. This indicates that the estimate obtained from offspring haplotypes is slightly biased. The rate of inbreeding estimated from segments remained 0.5% per generation in traditional OCS, in accordance with the constraint level setting.
Kinship estimates the probability that randomly chosen alleles are IBD. However, it lacks the ability to distinguish whether the alleles originated from native or migrant ancestors. In scenario OCS-I, where was restricted, the increasing rate of was higher than the increasing rate of This suggests that restricting only had the consequence that diversity at introgressed segments was maintained, which tend to have higher breeding values. But a depletion of diversity at native segments could not be avoided. Because kinship and kinship at native alleles are correlated, restricting implicitly restricted so in scenarios OCS-II and OCS-III, the mean kinship was lower than the corresponding constraint setting. This suggests that the constraint for could be skipped if and MC are constrained. Similar results were obtained from pedigree information from Wang et al. (2017).
MCs
In general, it must be distinguished whether MCs predominantly originate from closely related ancestors originating from a single high-yielding breed, or if different unrelated breeds have been used for upgrading. In the Angler breed, they predominantly originated from related Holstein ancestors, so reducing MC in OCS-III was meant to reduce the amount of genetic material contributed by Holstein cattle, which had a positive effect on the genetic diversity. Thus, the mean kinship in OCS-III was smaller than in all other scenarios.
Criteria for detecting shared segments
It has been suggested that the marker density of the SNP chip used, the minimum length of the shared segment, the number of genotyping errors allowed, and the minimum number of SNPs allowed in a single shared segment are likely to remarkably influence kinship estimates based on shared segments (Peripolli et al. 2016). However, to date, there is a lack of consensus in establishing the criteria for determining these parameters, which makes it difficult to compare results from different studies. In this paper, the minimum number of markers in a segment was 20. A shorter minimum length for shared segments allows detection of more ancient inbreeding from common ancestors occurring many generations back (Curik et al. 2014), but it also increases the probability that segments that are identical by chance are considered to be IBD. The minimum length of a segment was 2.50 Mb because, in this case, the correlation between the contribution from the Holstein breed estimated from the pedigree and genotype was high (0.93, data not shown) and the genetic contribution from the Fleckvieh breed was low (∼0.02), in accordance with pedigree records. The average MC of the Angler population was 0.62, which is also similar to the average MC level obtained from the pedigree (Wang et al. 2017). If shorter segments were also to be used, then kinships of individuals would be affected more by very old common ancestors and would consequently be higher.
Reduction of estimates in unselected populations
Estimated parameter values for MC, , and decreased slightly from generation to generation in REF, even though there was no selection in this scenario. This reduction of the above three parameters was caused by recombination, which shortened the length of the haplotype segments (Stam 1980) until they became too short to meet the criteria for being segments, which led to the reduction of Moreover, if recombination occurred near a particular marker position at an introgressed haplotype segment, then the segment containing the marker could no longer be detected in other breeds. Hence, the marker failed to meet the criteria of belonging to a foreign segment. This gave rise to the reduction of estimated MC. Moreover, since the marker was now classified as native, it contributed to the diversity at native alleles, which caused a reduction in Consequently, the estimated MC should be compared with the estimates obtained from the reference scenario rather than with generation In particular, in scenario OCS-II, in which the constraint for MC was set equal to the MC in generation only the estimate of MC was kept constant, whereas the true MC was effectively increased. There are two possibilities to avoid this increase. Either the constraint for MC in generation is set equal to the mean MC in generation (rather than the mean MC in generation ), or another method could be used to estimate the origins of the haplotype segments in generation That is, the origin of a marker could be set equal to the origin of the marker in the parental haplotype from which it originates.
Genetic diversity parameters
Different parameters can be used to measure genetic diversity, such as the percentage of polymorphic loci, the number of alleles per locus, expected heterozygosity, etc. (Harper and Hawksworth 1994). The genetic variation within a breed is of major importance for the conservation of local breeds. In addition to and three further parameters were considered for evaluation of the level of genetic diversity, i.e., the average observed heterozygosity the variance of TBV (), and the genic variance (). Restricting kinship at native alleles and MCs not only had an impact on recovering the original genetic background, but also showed the most potential in conserving genetic diversity among all scenarios. In this study, a similar decreasing pattern of and was observed, with a smaller extent of reduction for parameter This is because is predominantly influenced by neutral alleles (Gregorius 1978).
The additive genetic variance was substantially larger than the genic variance in the first generations and decreased to a large amount from generation to generation This was predominantly because the genetic effects of different chromosomes were correlated in the Angler breed. The contribution of the covariance between different chromosomes to the variance of TBV was 0.089, so in the absence of the covariance, the variance of TBV should be 0.108. The Angler cattle in generation had different contributions from the high-yielding Holstein cattle. For an individual with a high contribution from Holstein cattle, the breeding values of all chromosomes tended to be high, whereas for an individual with a low contribution from Holstein, the breeding values of all chromosomes tended to be low. Consequently, in the first generations, there was covariance between effects of different chromosomes, which contributed to the variance of the breeding values. Additionally, the Bulmer Effect (Bulmer 1971) and the changes in LD due to selection (Bijma 2012; Gorjanc et al. 2015) contributed to the difference between and
Conclusions
Advanced OCS strategies enable the achievement of a balance between the different breeding goals of populations with historic introgression, which are to improve the genetic progress, recover the original genetic background, and conserve genetic diversity. Here, truncation selection and traditional OCS achieved the highest genetic gain, but both reduced the genetic originality of the breed by depleting diversity at native alleles and increasing MCs. However, maintaining genetic originality is crucial for conserving breeds with historical introgression. The inclusion of MC and kinship at native alleles as additional constraints in OCS showed great potential for conservation. Recovering the original genetic background is possible but requires many generations of selection and reduces the genetic progress. Thus, it is essential to set an appropriate constraint for MC in order to balance both breeding goals, which are to achieve genetic progress and to recover the original genetic background of local breeds.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300272/-/DC1.
Acknowledgments
We thank the editor and two anonymous reviewers for their comments and advice. This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft). The authors declare that they have no competing interests.
Appendix
Native Segments
Each individual has a maternal haplotype and a paternal haplotype For haplotype of Angler the frequency of the segment at marker in the set of animals from reference breed was calculated as:
where is the number of individuals from breed set contains all markers belonging to segments, which are identical in haplotypes and and if marker belongs to such a segment.
A marker from haplotype was classified as native () if the frequency of the segment containing marker is smaller than in all reference breeds. That is,
where the maximum was taken over all reference breeds, which were Fleckvieh, Holstein-Friesian, Red Holstein, and Norwegian Red in our study.
The native contribution of individual is the proportion of the genome included in native segments. That is,
Here, is the length of the genome region in megabases represented by marker and is the length of the genome in megabases. The MC of individual is
Kinship and
The kinship between individuals and (element of matrix ) is the probability that two alleles taken at random from individuals and belong to identical segments:
where all parameters involved are the same as previously explained.
The kinship in the offspring is the conditional probability that two alleles taken at random from the offspring belong to identical segments, given that they are native. The mean kinship of the following generation is estimated as:
where is a matrix containing the probabilities that two alleles taken at random from both individuals belong to identical segments and are native, and is a matrix containing the probabilities that two alleles taken at random from both individuals are native:
| . |
Footnotes
Communicating editor: G. de los Campos
Literature Cited
- Alexander D. H., Novembre J., Lange K., 2009. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador C., Toro M. Á., Fernández J., 2011. Removing exogenous information using pedigree data. Conserv. Genet. 12: 1565–1573. [Google Scholar]
- Amador C., Fernández J., Meuwissen T. H. E., 2013. Advantages of using molecular coancestry in the removal of introgressed genetic material. Genet. Sel. Evol. 45: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y., Pasaniuc B., Sankararaman S., Torgerson D. G., Gignoux C., et al. , 2012. Fast and accurate inference of local ancestry in Latino populations. Bioinformatics 28: 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennewitz J., Meuwissen T. H. E., 2005. Estimation of extinction probabilities of five German cattle breeds by population viability analysis. J. Dairy Sci. 88: 2949–2961. [DOI] [PubMed] [Google Scholar]
- Bijma P., 2012. Accuracies of estimated breeding values from ordinary genetic evaluations do not reflect the correlation between true and estimated breeding values in selected populations. J. Anim. Breed. Genet. 129: 345–358. [DOI] [PubMed] [Google Scholar]
- Browning S. R., Browning B. L., 2007. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81: 1084–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer M. G., 1971. The effect of selection on genetic variability. Am. Nat. 105: 201–211. [Google Scholar]
- Caballero A., Toro M. Á., 2002. Analysis of genetic diversity for the management of conserved subdivided populations. Conserv. Genet. 3: 289–299. [Google Scholar]
- Chang C. C., Chow C. C., Tellier L. C., Vattikuti S., Purcell S. M., et al. , 2015. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curik I., Ferenčaković M., Sölkner J., 2014. Inbreeding and runs of homozygosity: a possible solution to an old problem. Livest. Sci. 166: 26–34. [Google Scholar]
- de Cara M. Á. R., Fernández J., Toro M. Á., Villanueva B., 2011. Using genome-wide information to minimize the loss of diversity in conservation programmes. J. Anim. Breed. Genet. 128: 456–464. [DOI] [PubMed] [Google Scholar]
- de Cara M. Á. R., Villanueva B., Toro M. Á., Fernández J., 2013. Using genomic tools to maintain diversity and fitness in conservation programmes. Mol. Ecol. 22: 6091–6099. [DOI] [PubMed] [Google Scholar]
- Eding H., Meuwissen T. H. E., 2001. Marker-based estimates of between and within population kinships for the conservation of genetic diversity. J. Anim. Breed. Genet. 118: 141–159. [Google Scholar]
- Eynard S. E., Windig J. J., Hiemstra S. J., Calus M. P. L., 2016. Whole-genome sequence data uncover loss of genetic diversity due to selection. Genet. Sel. Evol. 48: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F., 1996. Introduction to Quantitative Genetics. Pearson Education Limited, Harlow, UK. [Google Scholar]
- Frkonja A., Gredler B., Schnyder U., Curik I., Sölkner J., 2012. Prediction of breed composition in an admixed cattle population. Anim. Genet. 43: 696–703. [DOI] [PubMed] [Google Scholar]
- Gómez-Romano F., Villanueva B., Sölkner J., de Cara M. Á. R., Mészáros G., et al. , 2016. The use of coancestry based on shared segments for maintaining genetic diversity. J. Anim. Breed. Genet. 133: 357–365. [DOI] [PubMed] [Google Scholar]
- Gorjanc G., Bijma P., Hickey J. M., 2015. Reliability of pedigree-based and genomic evaluations in selected populations. Genet. Sel. Evol. 47: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorius H.-R., 1978. The concept of genetic diversity and its formal relationship to heterozygosity and genetic distance. Math. Biosci. 41: 253–271. [Google Scholar]
- Grundy B., Villanueva B., Woolliams J. A., 1998. Dynamic selection procedures for constrained inbreeding and their consequences for pedigree development. Genet. Res. 72: 159–168. [Google Scholar]
- Gusev A., Lowe J. K., Stoffel M., Daly M. J., Altshuler D., et al. , 2009. Whole population, genome-wide mapping of hidden relatedness. Genome Res. 19: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. L., Hawksworth D. L., 1994. Biodiversity: measurement and estimation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 345: 5–12. [DOI] [PubMed] [Google Scholar]
- Keller M. C., Visscher P. M., Goddard M. E., 2011. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics 189: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish E. J., Hillis D. M., 2015. How do SNP ascertainment schemes and population demographics affect inferences about population history? BMC Genomics 16: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros G., Boison S. A., Pérez O’Brien A. M., Ferenčaković M., Curik I., et al. , 2015. Genomic analysis for managing small and endangered populations: a case study in Tyrol Grey cattle. Front. Genet. 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T., 2009. Genetic management of small populations: a review. Acta Agric. Scand. A Anim. Sci. 59: 71–79. [Google Scholar]
- Meuwissen T. H. E., 1997. Maximizing the response of selection with a predefined rate of inbreeding. J. Anim. Sci. 75: 2575–2583. [DOI] [PubMed] [Google Scholar]
- Meuwissen T. H. E., Hayes B. J., Goddard M. E., 2001. Prediction of total genetic value using genome-wide dense marker maps. Genetics 157: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., 2000. Estimation of population parameters and recombination rates from single nucleotide polymorphisms. Genetics 154: 931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peripolli E., Munari D. P., Silva M. V. B., Lima A. L. F., Irgang R., et al. , 2016. Runs of homozygosity: current knowledge and applications in livestock. Anim. Genet. 48: 255–271. [DOI] [PubMed] [Google Scholar]
- Pfaff B., 2014. The R package cccp: design for solving cone constrained convex programs. R Finance. Available at: http://www.pfaffikus.de/files/conf/rif/rif2014.pdf. Accessed: May 16, 2014.
- Price A. L., Patterson N. J., Plenge R. M., Weinblatt M. E., Shadick N. A., et al. , 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38: 904–909. [DOI] [PubMed] [Google Scholar]
- Pritchard J. K., Stephens M., Donnelly P., 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Ramilo S. T., García-Cortés L. A., de Cara M. Á. R., 2015. Artificial selection with traditional or genomic relationships: consequences in coancestry and genetic diversity. Front. Genet. 6: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M., Blanc Y., Band M., Ezra E., Weller J. I., 1996. Misidentification rate in the Israeli dairy cattle population and its implications for genetic improvement. J. Dairy Sci. 79: 676–681. [DOI] [PubMed] [Google Scholar]
- Stam P., 1980. The distribution of the fraction of the genome identical by descent in finite random mating populations. Genet. Res. 35: 131. [Google Scholar]
- Toro M. Á., Villanueva B., Fernández J., 2014. Genomics applied to management strategies in conservation programmes. Livest. Sci. 166: 48–53. [Google Scholar]
- VanRaden P. M., 2008. Efficient methods to compute genomic predictions. J. Dairy Sci. 91: 4414–4423. [DOI] [PubMed] [Google Scholar]
- Visscher P. M., Medland S. E., Ferreira M. A. R., Morley K. I., Zhu G., et al. , 2006. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet. 2: e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Bennewitz J., Wellmann R., 2017. Novel optimum contribution selection methods accounting for conflicting objectives in breeding programs for livestock breeds with historical migration. Genet. Sel. Evol. 49: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann R., 2017. optiSel: optimum contribution selection and population genetics. R Package. version 0.9.1 https://CRAN.R-project.org/package=optiSel.
- Wellmann R., Hartwig S., Bennewitz J., 2012. Optimum contribution selection for conserved populations with historic migration. Genet. Sel. Evol. 44: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Z.-Q., Saatchi M., Schnabel R. D., Taylor J. F., Garrick D. J., 2014. Recombination locations and rates in beef cattle assessed from parent-offspring pairs. Genet. Sel. Evol. 46: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolliams J. A., Berg P., Dagnachew B. S., Meuwissen T. H. E., 2015. Genetic contributions and their optimization. J. Anim. Breed. Genet. 132: 89–99. [DOI] [PubMed] [Google Scholar]
- Yang J., Benyamin B., McEvoy B. P., Gordon S., Henders A. K., et al. , 2010. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available as supplemental files. File S1 contains SNP ID numbers and locations. File S2 contains simulated genotypes for each individual of the Angler cattle base generation