Abstract
This aim of this study was to test the feasibility of smartphone-based specular microscopy of the corneal endothelium at a sub-cellular resolution. Quantitative examination of endothelial cells is essential for evaluating corneal disease such as determining a diagnosis, monitoring progression and assessing treatment. Smartphone-based technology promises a new opportunity to develop affordable devices to foster quantitative examination of endothelial cells in rural and underserved areas. In our study, we incorporated an iPhone 6 and a slit lamp to demonstrate the feasibility of smartphone-based microscopy of the corneal endothelium at a sub-cellular resolution. The sub-cellular resolution images allowed quantitative calculation of the endothelial cell density. Comparative measurements revealed a normal endothelial cell density of 2978 cells/mm2 in the healthy cornea, and a significantly reduced cell density of 1466 cells/mm2 in the diseased cornea with Fuchs’ dystrophy. Our ultimate goal is to develop a smartphone-based telemedicine device for low-cost examination of the corneal endothelium, which can benefit patients in rural areas and underdeveloped countries to reduce health care disparities.
Keywords: Cornea, endothelium, endothelial cells, slit lamp microscopy, specular microscopy, smartphone
Quantitative examination of corneal endothelial cells is essential for evaluating the physiological function of the cornea. The main function of the endothelial cell is pumping water out from the cornea to maintain corneal transparency and ensure good visual quality. It is known that healthy corneal endothelium consists of a quasi-regular array of hexagonal cells (1, 2). As endothelial cells die, neighbouring cells enlarge to cover the empty space once occupied by the cell. This, in turn, causes the remaining cells to lose their hexagonal shape. When the endothelium is unable to maintain a fluid balance, the cornea starts to swell and it loses its transparency. Endothelial cells can be destroyed by eye surgeries, corneal diseases, contact lens usage, trauma and ageing (2–4). Quantitative examination of corneal endothelial cells is a routine requirement for patients with corneal endothelial dystrophies like Fuchs’ dystrophy (5), posterior polymorphous dystrophy (6), congenital hereditary endothelial dystrophy (7) and iridocorneal endothelial syndrome (8). The ability to diagnose and treat corneal endothelial dystrophies early can prevent vision loss and allow patients to maintain vision at the initial stage level. Quantitative examination of endothelial cells is also essential for pre-operative evaluation for intraocular surgery. An endothelial cell examination is mandatory for monitoring post-operative changes after a corneal transplant and during follow-up stages (4). Also, endothelial cell density must be continuously assessed in contact lens wearers (3).
Specular and confocal microscopes can be used to quantify endothelial cell density, but the high operation costs limit their clinical deployment (4, 9) in rural areas and underdeveloped countries. The corneal endothelium can also be visualized by slit lamp biomicroscopy (10). A slit lamp biomicroscope equipped with a digital camera is the basic requirement for endothelial cell photography. Unfortunately, commercial slit lamp imaging systems are typically expensive. In addition, traditional slit lamp biomicroscopy does not provide quantitative information on endothelial cells including their density. Smartphone-based technology promises a new opportunity to develop affordable devices. The small pixel size of the camera sensors enables smartphones to capture high resolution images from optical microscopes with moderate system magnification. It was shown that a simple attachment for a smartphone allows 1.5-µm resolution without image processing (11). The high pixel number of the smartphone camera sensor allows easy digital magnification with satisfactory image quality. Here, we demonstrate the feasibility of smartphone-based microscopy of the corneal endothelium at a sub-cellular resolution.
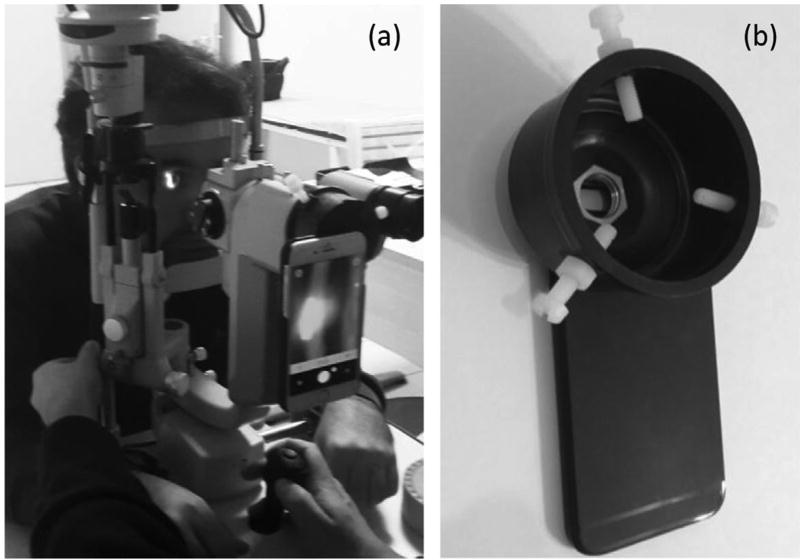
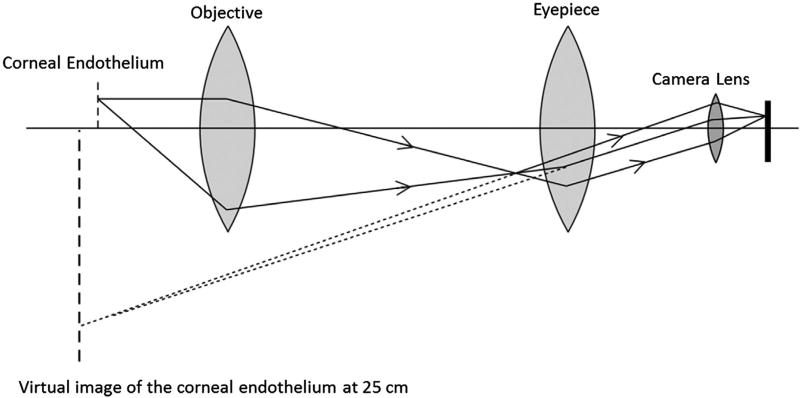
This study was approved by the Institutional Review Board of the University of Illinois at Chicago and was in compliance with the Declaration of Helsinki. Figure 1(a) shows the slit lamp (Topcon SL-D7, Oakland, NJ, USA) used for this study. One iPhone 6 was attached to the left eyepiece (Figure 1(a)) of the biomicroscope through a custom-made adapter (Figure 1(b)). Figure 2 illustrates a schematic diagram of the optical layout of the imaging system shown in Figure 1(a). As shown in Figure 2, the objective was used to produce a real image before the eyepiece. This corresponded to a virtual image at 250 mm from the camera (or the observer’s eye). The employed magnification 40× of the biomicroscope indicated that the height of the virtual image was 40 times that of the observed object, i.e. the cornea in this study.
Figure 1.
(a) Photographs of the slit lamp microscope (b) and custom-made smartphone adapter.
Figure 2.
Optical diagram of the imaging system. For simplification, the image inverting prism system is ignored and optical system is assumed as one objective and one eye piece.
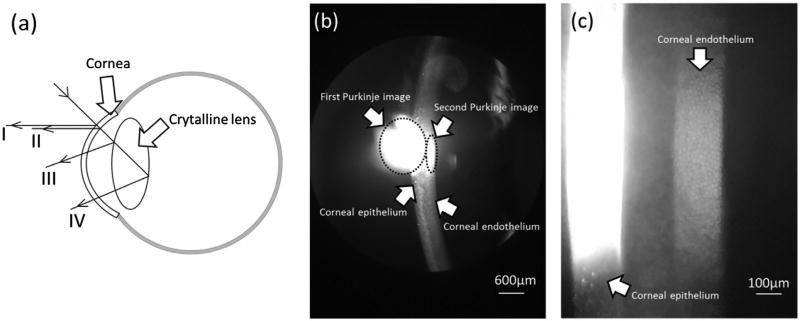
Camera + (an app for Apple’s iOS mobile operating system http://campl.us/), which enables manually focusing the iPhone 6 camera, was used for imaging the corneal endothelium. During the recording, a subject was seated with their chin placed on the chinrest and forehead against the headrest. The length and width of the slit were adjusted to 10 and 2 mm, respectively. The light source was swung 60° left relative to the optical axis of the biomicroscope. The slit lamp magnification was set to 16 × for preliminary adjustment, and Camera + software was started. Purkinje images were used to guide the focusing adjustment (Figure 3(a)). As shown in Figure 3(a), specular reflection from the epithelial layer (anterior surface) of the cornea forms the first Purkinje image. Second Purkinje image is formed by the specular reflection from the endothelial layer (posterior surface) of the cornea and it can be used for imaging the endothelium cells. The third and fourth Purkinje images are related to the anterior and posterior surfaces of the crystalline lens (Figure 3(a)) (12, 13). During the experiment, the corneal epithelial bright reflex of the slit light (first Purkinje image, Figure 3(b)) was found on the smartphone screen. After accessing the first Purkinje image, the magnification of the microscope was switched to 40 ×. By gently moving the biomicroscope and decreasing the width of the slit light, first and second Purkinje images were clearly identified. The digital zoom level of the smartphone was set to the maximum (6×). The cell mosaic of the endothelium was manually focused using the Camera +. Digital photographs were taken when the endothelium was optimally focused (Figure 3(c)).
Figure 3.
Purkinje images I to IV (a). Images of corneal layers taken at 40× magnification (b) and 40× magnification of the biomicroscope with 6× digital magnification of the smartphone (c).
Overall magnification, Mo, of the system, as in Figure 3, can be estimated as follows:
where Mb is the magnification (40×) of the biomicroscope; Dv is the distance (250 mm) between the virtual image and camera lens; fc is the focal length (4.2 mm) of the iPhone 6 camera; and Mb is the digital magnification (6×) of the iPhone camera. Therefore,
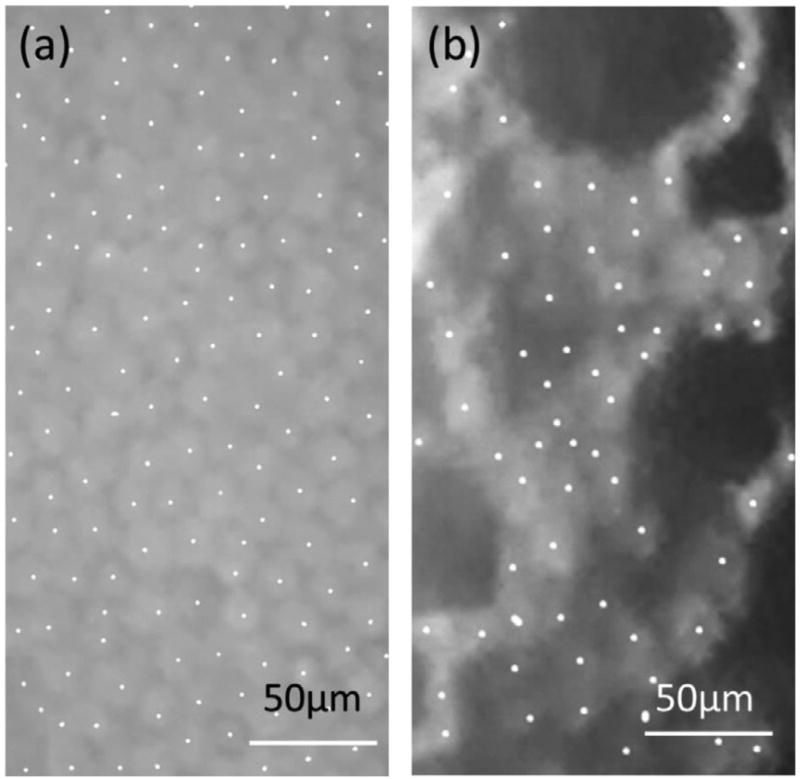
The size of the iPhone 6 front camera was 3672 × 4896 µm, which corresponds to 911 × 1214 µm at the cornea. For the 1.5-µm pixel size of the iPhone 6 camera, it corresponds to a pixel resolution of 0.37 µm, i.e. subcellular-level resolution. As shown in Figure 4(b), individual endothelial cells were unambiguously observed. The sub-cellular-level resolution enabled quantitative analysis of the endothelial cell density (Figure 4). Frame method was used to count corneal endothelial cells (14). The best visible field of the image was captured and marked in a rectangle frame using Paint software inside the Windows 7 operating system (Microsoft Company; Figure 4). The centre of the cells was marked by dots. Cells extending from the left and upper borders of the frame to the outside were counted. Extending outside cells from the right and lower borders of the frame were excluded. Image J was used for cell counting. The vertical and horizontal sizes of the image were defined using the ‘Set scale’ function of Image J. Further area measurements were done based on that defined size.
Figure 4.
Centrally marked cells of a healthy cornea (a) and cornea with Fuchs’ dystrophy (b).
In Figure 4(a), the total area and cell number were 45,000 µm2 and 134, respectively, which corresponded to a cell density of 2978 cells/mm2, which is consistent with reported endothelial cell densities of a healthy cornea (9). In contrast, for the same total area in a cornea with Fuchs’ dystrophy, as shown in Figure 4(b), the observed cell number was 66 in 45,000 µm2, which corresponded to a cell density of 1466 cells/mm2, which was related to the endothelium affected by the disease (15).
In summary, we incorporated an iPhone 6 and a slit lamp to demonstrate the feasibility of smartphone-based microscopy of the corneal endothelium at a sub-cellular resolution. Given the 1.5-µm pixel size of the iPhone 6 camera, 0.37 µm was readily achieved in coordination with 40× magnification of the slit biomicroscope and 6× digital magnification of the smartphone. The sub-cellular resolution images allowed quantitative calculation of the endothelial cell density. Comparative measurements revealed a normal endothelial cell density of 2978 cells/mm2 in the healthy cornea, while there was a reduced cell density of 1466 cells/mm2 in the diseased cornea with Fuchs’ dystrophy. For clinical deployments, the most challenging part of our current system is accurate alignment of the focal plane of specular reflection and the imaging system using manual focusing control. Our next step is to develop a smartphone-based portable device with automatic focusing adjustment and automatic quantification of cell density to enable low-cost telemedicine examination of the corneal endothelium, which can benefit patients in rural areas and underdeveloped countries to reduce health disparities.
Acknowledgments
We thank Professor Sait Egrilmez, Ege University School of Medicine, Izmir, Turkey for inspiring us and providing valuable suggestions during this project. Toslak is supported by a visiting scholarship from the Department of Public Hospitals Authority, Turkey. Erol is supported by Antalya Training and Research Hospital. Thapa and Yao are supported in part by NIH R01 EY023522, NIH R01 EY024628, NIH P30 EY001792 and NSF CBET-1055889.
Funding
This work was supported by Division of Chemical, Bioengineering, Environmental, and Transport Systems [grant number CBET-1055889] and National Eye Institute [grant number NIH R01 EY023522, NIH R01 EY024628, NIH P30 EY0017].
Footnotes
Disclosure statement
This manuscript has not been published or submitted elsewhere. We have no financial conflict to report.
References
- 1.Tuft SJ, Coster DJ. The Corneal Endothelium. Eye. 1990;4(3):389–424. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- 2.Krachmer JH, Mannis JM, Holland EJ. Cornea. Philidelphia: Elsevier Mosby; 2005. [Google Scholar]
- 3.Efron N. Contact Lens Complications. Edinburgh, UK: Elsevier Saunders; 2012. [Google Scholar]
- 4.Jonuscheit S, Doughty MJ, Ramaesh K. In Vivo Confocal Microscopy of the Corneal Endothelium: Comparison of Three Morphometry Methods after Corneal Transplantation. Eye. 2011;25(9):1130–1137. doi: 10.1038/eye.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamis AP, et al. Fuchs’ Endothelial Dystrophy of the Cornea. Surv Ophthalmol. 1993;38(2):149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 6.Krachmer JH. Posterior Polymorphous Corneal Dystrophy: A Disease Characterized by Epithelial-like Endothelial Cells Which Influence Management and Prognosis. Trans Am Ophthalmol Soc. 1985;83:413–475. [PMC free article] [PubMed] [Google Scholar]
- 7.Vithana EN, et al. Mutations in Sodium-Borate Cotransporter SLC4A11 Cause Recessive Congenital Hereditary Endothelial Dystrophy (CHED2) Nature Genet. 2006;38(7):755–757. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- 8.Price MO, Price FW., Jr Descemet Stripping with Endothelial Keratoplasty for Treatment of Iridocorneal Endothelial Syndrome. Cornea. 2007;26(4):493–497. doi: 10.1097/ICO.0b013e318030d274. [DOI] [PubMed] [Google Scholar]
- 9.Bourne WM, Kaufman HE. Specular Microscopy of Human Corneal Endothelium in VIVO. Am J Ophthalmol. 1976;81(3):319–323. doi: 10.1016/0002-9394(76)90247-6. [DOI] [PubMed] [Google Scholar]
- 10.Rose GE. Clinical Assessment of Corneal Endothelial Cell Density: An Original System of Grading Using a Slit-Lamp Biomicroscope. Br J Ophthalmol. 1986;70(7):510–515. doi: 10.1136/bjo.70.7.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith ZJ, Chu K, Espenson AR, Rahimzadeh M, Gryshuk A, Molinaro M. Cell-Phone-Based Platform for Biomedical Device Development and Education Applications. PLoS ONE. 2011;6(3):e17150. doi: 10.1371/journal.pone.0017150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji Woo L, Chul Woo C, Kwang Yong S, Eui Chul L, Kang Ryoung P. 3D Gaze Tracking Method Using Purkinje Images on Eye Optical Model and Pupil. 2012;50(5):736–751. [Google Scholar]
- 13.Bruce James LB. Ophthalmology: Investigation and Examination Techniques. New York: Elsevier; 2007. [Google Scholar]
- 14.McCarey BE, Edelhauser HF, Lynn MJ. Review of Corneal Endothelial Specular Microscopy for FDA Clinical Trials of Refractive Procedures, Surgical Devices and New Intraocular Drugs and Solutions. Cornea. 2008;27(1):1–16. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eghrari AO, Gottsch JD. Fuchs’ Corneal Dystrophy. Expert Rev Ophthalmol. 2010;5(2):147–159. doi: 10.1586/eop.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]