Abstract
The purpose of this work was to evaluate the intrapatient tumor position reproducibility in a deep inspiration breath‐hold (DIBH) technique based on two infrared optical tracking systems, ExacTrac and ELITETM, in stereotactic treatment of lung and liver lesions. After a feasibility study, the technique was applied to 15 patients. Each patient, provided with a real‐time visual feedback of external optical marker displacements, underwent a full DIBH, a free‐breathing (FB), and three consecutive DIBH CT‐scans centered on the lesion to evaluate the tumor position reproducibility. The mean reproducibility of tumor position during repeated DIBH was in laterolateral (LL), in anteroposterior (AP), and in craniocaudal (CC) direction for lung lesions, and in LL, in AP, and in CC direction for liver lesions. Intra‐and interbreath‐hold reproducibility during treatment, as determined by optical markers displacements, was below 1 mm and 3 mm, respectively, in all directions for all patients. Optically‐guided DIBH technique provides a simple noninvasive method to minimize breathing motion for collaborative patients. For each patient, it is important to ensure that the tumor position is reproducible with respect to the external markers configuration.
PACS numbers: 87.53.Ly, 87.55.km
Keywords: deep inspiration breath‐hold, extracranial stereotactic treatment, optoelectronic system, organ motion
I. INTRODUCTION
Tumors in the thorax and upper abdomen are subject to respiratory‐driven motion. In conventional radiotherapy, large safety margins up to 2.0 cm are added to the clinical target volume (CTV) to account for breathing motion and setup errors. (1) Respiration motion also causes artifacts during image acquisition, such as warping of the target volume and erroneous positional and volumetric information. (2)
There are three techniques, clinically implemented, able to reduce the effects of respiratory motion. The first is based on abdominal pressure obtained with a plastic plate, often applied to extracranial stereotactic treatments using a body frame. (3) The second technique involves the irradiation delivery at a predefined phase of the respiratory cycle, by gating the accelerator while the patient is freely breathing. Methods for gating include monitoring motion of the abdominal wall, spirometer, laser sensor system, and marker tracking. 4 , 5 , 6 , 7 A disadvantage of gating is decreased delivery efficiency, with longer imaging and treatment times. The third technique requires the patient to perform specific maneuvers of respiration, either voluntary 8 , 9 , 10 or with the aid of devices. 11 , 12 , 13 , 14 , 15 Stock et al. (16) developed a noninvasive method based on the opto‐electronic tracking of passive markers. The breath‐hold technique can be performed either at end of expiration, more reproducible but less comfortable for the patient, or at end of inspiration. (17) Deep inspiration breath‐hold has particular advantages in lung tumors, allowing a reduction of lung density with a decrease of normal tissue in the high‐dose region. This has the potential for dose escalation for the same calculated lung morbidity, even without margin reduction. In order to avoid severe pulmonary toxicity, lung volume receiving more than 20 Gy should not exceed 20% of the total volume. (18) Barnes et al. (19) showed a 32.5% reduction of the lung volume receiving more than 20 Gy using a DIBH technique. Furthermore, the reduced target motion during DIBH may justify a smaller safety margin from CTV to planning target volume (PTV). (20)
Nevertheless, in some cases the distance of the PTV to the OARs may increase for other breathing phases and therefore warrant a different phase than DIBH.
In this work, we investigated the use of DIBH technique in stereotactic treatments of lung and liver lesions using two infrared optical tracking systems, ExacTrac (BrainLAB AG, Germany) and ELITE (BTS S.p.a., Milan, Italy), in the attempt to either increase the dose or reduce the safety margin. To guide the DIBH we used the ELITE system, which has already been used in our Institute to evaluate setup errors and their dosimetric consequences in breast treatments, 21 , 22 as well as in frameless body stereotactic treatments. 23 , 24
We first performed a feasibility study on three voluntary subjects to evaluate the reproducibility of the technique, followed by an analysis on eight patients to correlate the patient surface and the tumor position reproducibility. Finally, the results of the first 15 patients treated with the DIBH protocol have been reported.
II. MATERIALS AND METHODS
A. Infra‐red optical tracking
Both optical tracking systems, ExacTrac and ELITETM, use two infrared cameras, which are mounted in the ceiling of the linac vault, to track passive optical markers (radius: 5 mm) placed on selected skin landmarks. A calibration procedure is used in order to determine the position of any passive optical marker relative to the isocenter. Both systems were calibrated with respect to a common isocentric reference frame and they provided submillimetric accuracy in markers 3D localization. (25)
The optical markers were attached to the patient by using an adhesive backing before CT scanning, and their position was referenced with ink marking to aid reapplication for each treatment fraction. CT scan data were sent to the treatment planning system (TPS) (BrainScan, BrainLAB AG), where the isocenter was established relative to the stereotactic coordinate system defined by the optical markers configuration which was used for positioning.
ExacTrac was the reference system to set up the patient in free breathing, providing the operator with the corrective translations and rotations. ELITE was the reference system for guiding the patient during the DIBH in both CT and treatment rooms. A specific software was implemented to provide the operators with the real‐time 3D displacements of the optical markers configuration with respect to the reference one.
B. Feasibility study of the DIBH technique
B.1. Volunteer study
In order to assess the reproducibility of the technique as a function of the specific feedback to the patient, a feasibility study was performed on three voluntary subjects. Five consecutive acquisitions of 3D marker positions were performed for each subject lying in supine position on the CT couch during a 15 sec DIBH with two feedback conditions: i) verbal instructions by an operator, based on real‐time feedback of marker displacements; ii) direct visual feedback to the subject.
B.2. CT patient study
A CT study was performed on eight patients. Only collaborative patients with a Karnofsky index were enrolled in the feasibility study, as well as in the treatment protocol. The patient characteristics are shown in Table 1 (patients #1‐7, #16). Patients were immobilized supine in an individualized vacuum cushion with an arm holder.
Table 1.
Characteristics of the patients enrolled in the feasibility study and in the treatment protocol
| No. | Age | Sex | Site | Subsite | Comorbidities | Thoracic/Liver Surgery | Treatment/Feasibility Study |
|---|---|---|---|---|---|---|---|
| 1 | 44 | F | Left lung | Upper lobe | Hypertension | No | Feasibility study |
| 2 | 44 | F | Right lung | Lower lobe | Hypertension | No | Feasibility study |
| 3 | 62 | F | Mediastinum | Station R4 | Heart disease | Yes | Feasibility study |
| 4 | 48 | M | Left lung | Lower lobe | ‐ | Yes | Feasibility study |
| 5 | 50 | F | Left lung | Upper lobe | ‐ | No | Feasibility study |
| 6 | 63 | M | Mediastinum | Station 5 | COPD | No | Feasibility study |
| 7 | 59 | M | Left lung | Lower lobe | Heart disease | No | Feasibility study |
| 8 | 61 | M | Right lung | Lower lobe | ‐ | Yes | Treatment |
| 9 | 53 | F | Right lung | Medium lobe | ‐ | No | Treatment |
| 10 | 66 | M | Right lung | Medium lobe | Left Pneumothorax | No | Treatment |
| 11 | 53 | F | Left lung | Lingula | ‐ | No | Treatment |
| 12 | 62 | M | Right lung | Lower lobe | ‐ | No | Treatment |
| 13 | 60 | M | Right lung | Lower lobe | ‐ | Yes | Treatment |
| 14 | 54 | M | Right lung | Upper lobe | COPD | Yes | Treatment |
| 15 | 69 | F | Left lung | Upper lobe | Left Diaphragm Relaxation | No | Treatment |
| 16 | 43 | F | Liver | Hilar node | ‐ | Yes | Feasibility study |
| 17 | 58 | M | Liver | V segment | ‐ | No | Treatment |
| 18 | 59 | M | Liver | VII segment | ‐ | Yes | Treatment |
| 19 | 61 | F | Liver | IV segment | ‐ | No | Treatment |
| 20 | 59 | M | Liver | VII segment | ‐ | Yes | Treatment |
| 21 | 68 | F | Liver | VII segment | ‐ | Yes | Treatment |
| 22 | 56 | F | Liver | VII segment | Hypertension | Yes | Treatment |
| 23 | 54 | F | Liver | IV segment | ‐ | No | Treatment |
Real‐time visual feedback, prompting instantaneous (at video refresh rate) information of the inspiration level, was obtained through an eyewear viewer (SV‐6 PC Viewer; MicroOptical Co., Westwood, MA) (Fig. 1) connected to the ELITE data logger, showing the optical marker displacements in LL, CC, and AP directions, with respect to a reference configuration, as bars. The patient was first instructed to have a reproducible breathing pattern and then was trained to achieve a reproducible DIBH level, keeping the AP bars below a 3 mm threshold. When the bars exceeded the threshold, their color changed from green to red. A configuration of seven optical markers was used, four positioned in stable points to assure a correct setup in FB and three on upper abdomen in points representative of the DIBH maneuver. Acquisition of the reference 3D marker positions was performed at a comfortable level of DIBH and the duration of the breath‐hold was established.
Figure 1.
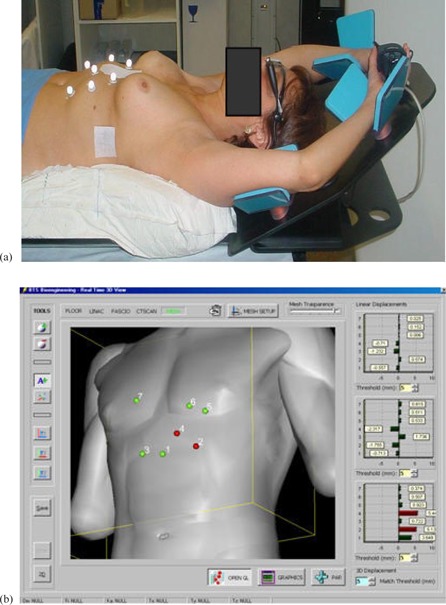
Patient with passive markers and eyewear viewer (a) showing in real‐time marker displacements in LL, CC, and AP directions (with respect to a reference configuration (b)).
Each patient underwent a FB CT scan and three consecutive DIBH CT series spanning the region across the tumor (usually 20 slices), acquired with 3 mm thick slices. Each DIBH CT series was acquired during a single breath‐hold of about 16 sec (one slice acquired in 0.8 sec).
Moreover, shallow inspiration and expiration breath‐hold CT scans centered on the lesion to assess the extent of tumor motion under FB conditions were also acquired.
All CT scans were registered on the FB CT scan using a pair objects technique provided by the TPS; three landmarks on the patient tray and one vertebral body were used. The accuracy of the image fusion, determined by evaluating the contours of fixed anatomical structures, was below 1 mm. The GTV was contoured on each slice by a single radiation oncologist. We measured an intra‐observer variability of 1.5 mm, evaluated by repeating three times the tumor delineation of two patients and considering the shift in the tumor center of mass.
In Fig. 2, three DIBH CT series registered on the FB scan are shown for a lung patient enrolled in the feasibility study. Artifacts in tumor volume and position in the FB CT scan are visible in the sagittal and coronal CT reconstructions.
Figure 2.
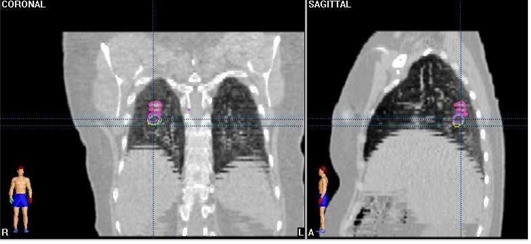
Reproducibility of tumor position during repeated DIBH CT scans fused on the FB CT scan (shown in pink).
We investigated the correlation between the GTV and the optical markers displacement from FB to DIBH, in terms of entity and reproducibility. Tumor displacement was assessed by evaluating the displacement of the GTV center of mass along the LL, CC, and AP directions between FB and DIBH. As a surrogate of surface motion, we considered the mean value of the positions of abdominal optical markers used to guide the DIBH maneuver. The reproducibility of tumor displacement was evaluated considering the SD of the position of the GTV center of mass between consecutive DIBH CT scans, while the reproducibility of marker displacements was evaluated considering the mean SD of the coordinates of abdominal markers digitized on the TPS.
C. clinical implementation of DIBH protocol
C.1. CT simulation
Each patient underwent a full DIBH CT scan, split into segments lasting about 15 sec each, including all involved organs for the DVH analysis and three consecutive DIBH CT scans, centered on the lesion, to evaluate tumor position reproducibility. A full FB scan for setup and shallow inspiration and expiration breath‐hold scans centered on the lesion to assess the extent of tumor motion were also acquired. The same procedure as described in the feasibility study (Materials and Methods Section B above) was followed.
A verification CT in DIBH centered on the lesion was acquired before each treatment section, since a cone beam CT was not available on the linac.
So far, 15 patients have been treated with the DIBH protocol and their characteristics are shown in Table 1 (Patients #8–15, #17–23).
C.2. Treatment planning
The treatment technique consisted of a single or multiple noncoplanar arcs of 6 MV photon beams conformed to the PTV by means of a dynamic micromultileaf collimator (m3, BrainLAB AG). For metastases, the CTV was equal to GTV, while for primary tumors the CTV was defined as the GTV plus 5 mm margin. The PTV was defined by adding to the CTV drawn on the DIBH CT scan the same margins of the FB plan. Patient‐specific margins of 5–8 mm in LL and 8–18 mm in both AP and CC were used, taking into account organ motion and 3 mm isotropic setup margin. (23) An additional margin of 3 mm was added to the PTV to account for beam penumbra. Moreover, an extra 2 mm margin was used to compensate the reduced accuracy of our dose calculation algorithm, not accounting for lateral electronic disequilibrium in the lung.
We compared the CTV to PTV margins, derived from the FB method, with the uncertainty‐based margins calculated taking into account the following uncertainties, combined in quadrature, involved in the process: patient specific tumor position reproducibility in DIBH (1.5 SD), setup margin, intra‐observer variability in tumor delineation, and uncertainty in image fusion.
Treatment doses, prescribed at the isocenter, ranged between 30 Gy delivered in 2 fractions and 54 Gy delivered in 3 fractions with 48 hours interval.
C.3. Treatment delivery
Patients were treated on a Varian Clinac 600 C/D (Varian Medical Systems, Palo Alto, CA) equipped with an electronic portal imaging device. Patient setup was performed according to ExacTrac. Following initial manual alignment with skin marks and lasers, the position of the couch was automatically adjusted on the basis of optical marker localization in order to achieve a treatment position where the markers matched the reference configuration defined on the treatment planning CT, especially the stable ones. Final patient setup verification was performed by means of a couple of orthogonal electronic portal images acquired in FB and compared with the corresponding DRRs. Bony structures stable during respiration, such as vertebral spine, were used to verify the correct patient position in FB as determined by ExacTrac. Each irradiation was split in 12–15 consecutive breath‐holds lasting about 15–20 sec each, using high‐dose rate (500 MU/min). During each breath‐hold, the beam was switched on manually when the patient reached the correct level given by the ELITE system and switched off before the operator told the patient to freely breath.
We analyzed the intra‐ and inter‐DIBH reproducibility, considering the mean SD and the root mean square error (RMS) of marker displacements, respectively.
III. RESULTS
A. Feasibility study of the reproducibility of DIBH technique
Real‐time visual feedback of marker displacements to the voluntary subjects led to a significantly higher repeatability of surface localization during DIBH, with overall mean 3D displacement of , compared to a mean value of obtained with verbal instructions given by an operator ().
B. CTV to PTV margins
In Table 2 are shown for all patients the SD of the GTV positions in repeated DIBH CT scans and the uncertainty‐based CTV to PTV margins calculated combining in quadrature the patient‐specific tumor position reproducibility in DIBH (1.5 SD), the setup margin (3 mm), the intra‐observer variability in tumor delineation (1.5 mm), and the uncertainty in image fusion (0.5 mm).
Table 2.
Reproducibility of the GTV positions in repeated DIBH CT scans and the uncertainty‐based margins calculated on an individual basis
| SD of GTV Positions in Consecutive DIBHs | CTV‐PTV Margins | |||||
|---|---|---|---|---|---|---|
| Patient N° | LL (mm) | AP (mm) | CC (mm) | LL (mm) | AP (mm) | CC (mm) |
| 1 | 0.9 | 0.5 | 1.6 | 3.6 | 3.5 | 4.2 |
| 2 | 1.0 | 1.4 | 2.9 | 3.7 | 4.0 | 5.5 |
| 3 | 0.3 | 0.8 | 0.4 | 3.4 | 3.6 | 3.4 |
| 4 | 0.2 | 1.7 | 1.8 | 3.4 | 4.2 | 4.3 |
| 5 | 0.4 | 0.1 | 1.5 | 3.4 | 3.4 | 4.1 |
| 6 | 0.4 | 1.7 | 2.1 | 3.4 | 4.2 | 4.6 |
| 7 | 0.9 | 0.9 | 0.8 | 3.7 | 3.7 | 3.6 |
| 8 | 0.7 | 0.5 | 1.4 | 3.6 | 3.5 | 4.0 |
| 9 | 1.2 | 3.6 | 3.3 | 3.8 | 6.4 | 6.0 |
| 10 | 0.5 | 0.6 | 0.5 | 3.5 | 3.5 | 3.5 |
| 11 | 0.1 | 0.1 | 1.8 | 3.4 | 3.4 | 4.3 |
| 12 | 0.2 | 1.1 | 1.5 | 3.4 | 3.8 | 4.1 |
| 13 | 0.4 | 0.2 | 0.5 | 3.4 | 3.4 | 3.5 |
| 14 | 0.2 | 0.5 | 0.9 | 3.4 | 3.5 | 3.7 |
| 15 | 0.4 | 1.0 | 0.4 | 3.4 | 3.7 | 3.4 |
| 16 | 0.6 | 0.5 | 0.4 | 3.5 | 3.5 | 3.4 |
| 17 | 0.9 | 0.9 | 1.2 | 3.6 | 3.7 | 3.8 |
| 18 | 1.1 | 1.6 | 1.3 | 3.8 | 4.1 | 3.9 |
| 19 | 0.4 | 0.8 | 1.3 | 3.4 | 3.6 | 3.9 |
| 20 | 0.0 | 1.0 | 1.1 | 3.4 | 3.7 | 3.8 |
| 21 | 1.4 | 0.5 | 1.6 | 4.0 | 3.5 | 4.2 |
| 22 | 1.9 | 1.9 | 1.7 | 4.4 | 4.4 | 4.2 |
| 23 | 1.6 | 1.5 | 1.0 | 4.2 | 4.1 | 3.7 |
| mean | 0.7 | 1.0 | 1.3 | 3.6 | 3.8 | 4.1 |
| SD | 0.5 | 0.8 | 0.8 | 0.3 | 0.6 | 0.6 |
The mean difference between uncertainty‐based margins in DIBH and the standard ones determined with the FB method (used for the treatment) was in LL, in AP, and in CC directions.
Despite reduced respiratory motion, CTV to PTV margins were not reduced so far, first because we want to acquire more confidence with the technique. The second reason concerns lung treatments and it is related to the dose calculation algorithm implemented in our TPS (see Discussion section below).
All lung patients exhibited a significant decrease in the PTV for the DIBH plan compared to the one obtained in the FB plan, due to target immobilization (). We observed an overall mean variation of with a maximum value of . For patients with liver lesions, although the tumor was visible in all CT scans allowing us to evaluate the center of mass reproducibility during repeated DIBH CT scans, we did not compare the volumes, since we found some differences depending on the time interval from the contrast medium injection and the CT scan acquisition.
C. Displacement of target and passive markers between FB and DIBH
Entity and reproducibility of tumor and markers displacement from FB to DIBH for all patients are shown in Table 3.
Table 3.
Entity and reproducibility of tumor and markers displacement from FB to DIBH CT scans for all patients enrolled in the feasibility study and in the treatment protocol
| LL | AP | CC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Range (mm) |
|
Range (mm) |
|
Range (mm) | ||||||||
| Lung | Tumor | Displacement |
|
|
|
4.5/33.0 |
|
|
|||||
| Reproduc. |
|
0.1/1.2 |
|
0.1/3.6 |
|
0.4/3.3 | |||||||
| Markers | Displacement |
|
|
|
4.4/24.8 |
|
3.0/21.0 | ||||||
| Reproduc. |
|
0.3/1.5 |
|
0.3/3.0 |
|
0.8/2.7 | |||||||
| Liver | Tumor | Displacement |
|
|
|
7.7/36.7 |
|
|
|||||
| Reproduc. |
|
0.0/1.9 |
|
0.5/1.9 |
|
0.4/1.7 | |||||||
| Markers | Displacement |
|
|
|
6.1/25.0 |
|
|
||||||
| Reproduc. |
|
0.3/1.1 |
|
0.6/3.6 |
|
1.0/2.0 | |||||||
Figures 3 and 4 show the tumor and markers displacement from FB to DIBH along the three directions for patients with lung and liver diseases, respectively. For lung tumors situated in the lower lobes, the largest displacement was observed in the caudal direction with a maximum value up to 40 mm, while tumors located in the upper lobes displayed maximal displacement along the AP direction, with a maximum value of 33 mm. Most tumors showed minimal movement along the LL direction, but for three patients it was larger than 5 mm.
Figure 3.
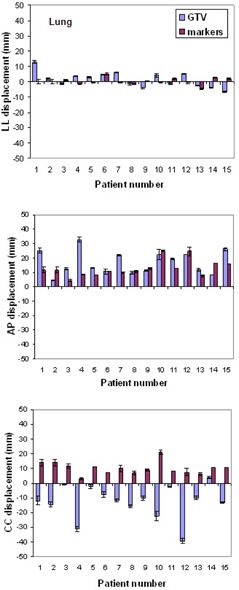
Displacement of GTV and passive markers between FB and DIBH in all directions for lung lesions.
Figure 4.
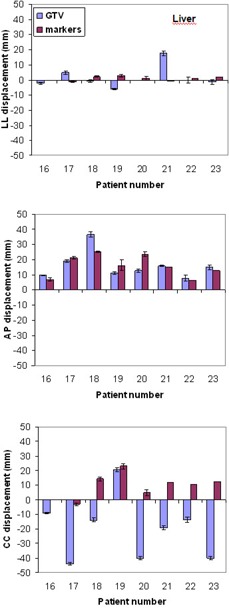
Displacement of GTV and passive markers between FB and DIBH in all directions for liver lesions.
We did not find any correlation (Pearson correlation coefficient) between the marker and the tumor displacement in any directions among the patients.
All patients with liver lesions, except one, exhibited a caudal displacement of the target, caused by the increased lung volume pushing down the liver. The only patient showing target displacement in the opposite direction had a lesion very close to the diaphragm pushed up during DIBH. Relevant displacements were also observed in AP direction. One lesion, located on the diaphragm, exhibited a displacement of 17.7 mm in LL direction.
A correlation between tumor and marker displacement in CC direction (regression line ) has been found for liver lesions.
We observed a high variability of tumor displacement between FB and DIBH among the patients, which may be caused by different respiration modality, thoracic or abdominal, as well as position of the lesion.
Data from the verification CT acquired before each treatment section confirmed the reproducibility of tumor position during DIBH assessed during the simulation CT for all patients.
D. Patient performance during the DIBH treatment
The mean number of breath‐holds per fraction was (range: 6.0 to 16.0). The mean number of frames acquired during a single breath‐hold was , with mean breath‐hold duration of about 15 sec and a sample rate of 20 Hz. Intra‐ and interbreath‐hold reproducibility, as determined by marker displacements, for all treated patients is shown in Table 4.
Table 4.
Intra‐ and interbreath‐hold reproducibility of marker displacements for all treated patients
| LL | AP | CC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reproducibility |
|
Max (mm) |
|
Max (mm) |
|
Max (mm) | |||
| Intra ‐ DIBH |
|
1.6 |
|
1.7 |
|
2.0 | |||
| Inter ‐ DIBH |
|
3.9 |
|
2.3 |
|
3.7 | |||
We found larger RMS errors in LL and CC directions due to the fact that, although the patients could have real‐time feedback of markers displacements along the three directions, they were told to reproduce as better as possible their reference positions along the AP direction.
IV. DISCUSSION
A. Feasibility study on the reproducibility of DIBH technique
Preliminary study showed that self‐visual feedback increased the reproducibility of the patient surface. Our results are in agreement with other studies reported in the literature. (26) Some authors found a reproducibility of tumor position under patient self breath‐hold without any respiratory monitoring device, with a difference of tumor position less than 3 mm in all directions. (10)
B. CTV to PTV margins
The PTV was smaller in DIBH compared to FB, even using the same margins derived from the FB plan, due to the fact that artifacts in shape and location of the lesion caused by the CT acquisition were minimized. Since the tumor is immobilized during DIBH, it might be possible to reduce the internal margin, taking into account only the residual tumor motion during breath‐hold. Nevertheless, a margin reduction should be considered carefully if a pencil beam algorithm is used for dose calculation, as in our TPS. Since DIBH reduces lung density, the effect of a broadened penumbra at field edges due to electronic disequilibrium will be increased and may cause under dosage of targets, as pointed out by Fogliata et al. (27) Hanley et al. (20) showed that for 6 MV photon beams the penumbra broadening should have little clinical significance for DIBH treatments. Yorke et al. (28) found, using Monte Carlo, that lateral disequilibrium caused more decreased target coverage for DIBH than for FB. However, if DIBH enables higher prescription doses exceeding 10%, despite lateral disequilibrium, higher doses would be delivered to target volume.
We have planned to change our dose calculation algorithm with a more accurate one. In the meantime, the implementation of the DIBH technique allowed us to escalate the dose for selected patients. Two patients with a primary lung tumor received a treatment dose of 54 Gy in 3 fractions, while our standard protocol was 45 Gy in 3 fractions.
C. Displacement of target and passive markers between FB and DIBH
The tumor displacement between FB and DIBH varied significantly among patients. We could not find any correlation between the movement of the markers used to guide the DIBH and tumor displacements for patients with lung lesions, while a weak linear regression was found in CC direction for patients with liver metastases. Nevertheless, this is not an important issue once the tumor position is reproducible with regard of the external markers position.
For each patient it is important to ensure that the tumor position is reproducible with respect to the external markers configuration by acquiring repeated DIBH CT scans during simulation. The availably of a cone‐beam CT (CBCT) on the linac would allow one to acquire a DIBH‐gated CBCT in order to verify the tumor reproducibly just before the treatment.
Our results are in agreement with those obtained by other investigators. 20 , 29
D. Patient performance during the DIBH treatment
Only patients who could achieve sufficient reproducibility of DIBH after the training were included in the treatment protocol. All patients were able to complete the stereotactic treatment, some of them exhibiting fatigue at the end of the fraction.
Berson et al. (30) found a reduction of treatment time by a factor of 2 using breath‐hold compared to gating. This is particularly important for hypofractionated stereotactic treatments, where high doses per fraction are delivered.
V. CONCLUSIONS
The DIBH technique, supported by an opto‐electronic system, provides a simple and noninvasive method to minimize breathing motion for collaborative patients. A visual feedback provided to the patient led to a higher reproducibility of the technique. We strongly recommend an accurate CT study for each patient to evaluate the reproducibility of tumor position during the DIBH maneuver with respect to the external markers configuration.
REFERENCES
- 1. van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14(1):52–64. [DOI] [PubMed] [Google Scholar]
- 2. Chen GTY, Kung JH, Beaudette KP. Artifacts in computed tomography scanning of moving objects. Semin Radiat Oncol. 2004;14(1):19–26. [DOI] [PubMed] [Google Scholar]
- 3. Murray B, Forster K, Timmerman R. Frame‐based immobilization and targeting for stereotactic body irradiation therapy. Med Dosim. 2007;32(2):86–91. [DOI] [PubMed] [Google Scholar]
- 4. Kubo HD and Hill BC. Respiration gated radiotherapy treatment: a technical study. Phys Med Biol. 1996;41(1):83–91. [DOI] [PubMed] [Google Scholar]
- 5. Zhang T, Keller H, O'Brien MJ, Mackie TR, Paliwal B. Application of the spirometer in respiratory gated radiotherapy. Med Phys. 2003;30(12):3165–71. [DOI] [PubMed] [Google Scholar]
- 6. Tsunashima Y, Sakae T, Shioyama Y et al. Correlation between the respiratory waveform measured using a respiratory sensor and 3D tumor motion in gated radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(3):951–58. [DOI] [PubMed] [Google Scholar]
- 7. Murphy MJ. Tracking moving organs in real time. Sem Radiat Oncol. 2004;14(1):91–100. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura K, Shioyama Y, Nomoto S et al. Reproducibility of the abdominal and chest wall position by voluntary breath‐hold technique using a laser‐based monitoring and visual feedback system. Int J Radiat Oncol Biol Phys. 2007;68(1):267–72. [DOI] [PubMed] [Google Scholar]
- 9. Kim D, Murray B R, Halperin R, Roa WH. Held‐breath self‐gating technique for radiotherapy of non‐small‐cell lung cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2001;49(1):43–49. [DOI] [PubMed] [Google Scholar]
- 10. Onoshi H, Kuriyama K, Komiyama T et al. CT evaluation of patient deep inspiration self‐breath‐holding: how precisely can patients reproduce the tumor position in the absence of respiratory monitoring devices? Med Phys. 2003;30(6):1183–87. [DOI] [PubMed] [Google Scholar]
- 11. Kimura T, Murakami Y, Kenjo M et al. Interbreath‐hold reproducibility of lung tumour position and reduction of the internal target volume using a voluntary breath‐hold methods with spirometer during stereotactic radiotherapy for lung tumours. Br J Radiol. 2007;80(953):355–61. [DOI] [PubMed] [Google Scholar]
- 12. Remouschamps VM, Huyskens DP, Mertens I et al. The use of magnetic sensors to monitor moderate deep inspiration breath hold during breast irradiation with dynamic MLC compensator. Radiother Oncol. 2007;82(3):341–48. [DOI] [PubMed] [Google Scholar]
- 13. Murphy MJ, Martin D, Whyte R, Hai J, Ozhasoglu C, Le QT. The effectiveness of breath‐holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys. 2002;53(2):475–82. [DOI] [PubMed] [Google Scholar]
- 14. Mah D, Hanley J, Rosenzweig KE et al. Technical aspects of the deep inspiration breath‐hold technique in the treatment of thoracic cancer. Int J Radiat Oncol Biol Phys. 2000;48(4):1175–85. [DOI] [PubMed] [Google Scholar]
- 15. Wong JW, Sharpe MB, Jaffray DA et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44(4):911–19. [DOI] [PubMed] [Google Scholar]
- 16. Stock M, Kontrisova K, Dieckmann K, Bogner J, Poetter R, Georg D. Development and application of a real‐time monitoring and feedback system for deep inspiration breath hold based on external marker tracking. Med Phys. 2006;33(8):2868–77. [DOI] [PubMed] [Google Scholar]
- 17. Kontrisova K, Stock M, Dieckmann K, Bogner J, Pötter R, Georg D. Dosimetric comparison of stereotactic body radiotherapy in different respiration conditions: a modeling study. Radiother Oncol. 2006;81(1):97–104. [DOI] [PubMed] [Google Scholar]
- 18. Martel MK, Ten Haken RK, Hauza MB, Turrisi AT, Fraass BA, Lichter AS. Dose‐volume histograms and 3‐D treatment planning evaluation of patients with pneumonities. Int J Radiat Oncol Biol Phys. 1994;28(3):575–81. [DOI] [PubMed] [Google Scholar]
- 19. Barnes EA, Murray BR, Robinson D, Underwood LJ, Hanson J, Roa WH. Dosimetric evaluation of lung tumor immobilization using breath hold at deep inspiration. Int J Radiat Oncol Biol Phys. 2001;50(4):1091–98. [DOI] [PubMed] [Google Scholar]
- 20. Hanley J, Debois M, Mah D et al. Deep inspiration breath‐hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation, Int J Radiat Oncol Biol Phys. 1999;45(3):603–11. [DOI] [PubMed] [Google Scholar]
- 21. Spadea MF, Baroni G, Riboldi M et al. Patient set‐up verification by infrared optical localization and body surface sensing in breast radiation therapy. Radiother Oncol. 2006;79(2):170–78. [DOI] [PubMed] [Google Scholar]
- 22. Baroni G, Garibaldi C, Scabini M et al. Dosimetric effects within target and organs at risk of interfractional patient mispositioning in left breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(3):861–71. [DOI] [PubMed] [Google Scholar]
- 23. Baroni G, Garibaldi C, Riboldi M et al. 3D optoelectronic analysis of interfractional patient setup variability in frameless extracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(2):635–42. [DOI] [PubMed] [Google Scholar]
- 24. Riboldi M, Baroni G, Spadea MF et al. Robust frameless stereotactic localization in extra‐cranial radiotherapy. Med Phys. 2006;33(4):1141–52. [DOI] [PubMed] [Google Scholar]
- 25. Baroni G, Ferrigno G, Pedotti A. Implementation and application of real‐time motion analysis based on passive markers. Med Biol Eng Comput. 1998;36(6):693–703. [DOI] [PubMed] [Google Scholar]
- 26. George R, Chung T, Vedam SS et al. Audio‐visual biofeedback for respiratory‐gated radiotherapy: impact of audio instruction and audio‐visual biofeedback on respiratory‐gated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65(3):924–33. [DOI] [PubMed] [Google Scholar]
- 27. Fogliata A, Nicolini G, Vanetti E, Clivio A, Winkler P, Cozzi L. The impact of photon dose calculation algorithms on expected dose distributions in lungs under different respiration phases. Phys Med Biol. 2008;53(9):2375–90. [DOI] [PubMed] [Google Scholar]
- 28. Yorke ED, Wang L, Rosenzweig KE, Mah D, Paoli JB, Chni CS. Evaluation of deep inspiration breath‐hold lung treatment plans with Monte Carlo dose calculation. Int J Radiat Oncol Biol Phys. 2002;53(4):1058–70. [DOI] [PubMed] [Google Scholar]
- 29. Seppenwoolde Y, Shirato H, Kitamura K et al. Precise and real‐time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53(4):822–34. [DOI] [PubMed] [Google Scholar]
- 30. Berson AM, Emery R, Rodriguez L et al. Clinical experience using respiratory gated radiation therapy: comparison of free‐breathing and breath‐hold techniques. Int J Radiat Oncol Biol Phys. 2004;60(2):419–26. [DOI] [PubMed] [Google Scholar]


