Abstract
The heat shock response in pancreatitis that is activated via HSP70 protects acinar cells through multiple simultaneous mechanisms. It inhibits trypsinogen activation and modulates NF-κB signaling to limit acinar cell injury. On the other hand, HSP70 is overexpressed in pancreatic cancer and is hijacked by the cellular machinery to inhibit apoptosis. Inhibition of HSP70 in pancreatic cancer by a novel compound, Minnelide, has shown considerable clinical promise.
Keywords: HSP70, pancreatitis, pancreatic cancer, calcium, lysosomes, triptolide, minnelide
Heat Shock Proteins: Chaperones of Cells in Distress
A number of pro-survival pathways have evolved to help cells survive harsh conditions. ‘Heat shock response’ is one such evolutionary conserved pathway. A large number of proteins that play an important role in cellular homeostasis are part of this response. That these ubiquitous polypeptides play an important role in ‘survival’ is clear from that fact that they have been observed in, all the way from, prokaryotes and yeasts to humans [1]. Heat shock proteins have been shown to facilitate protein–protein interactions such as folding and assisting in the establishment of proper protein conformation. When cells undergo acute or chronic stress, multiple signaling pathways are dysregulated resulting in protein misfolding, aggregation and disruption of multiprotein complexes jeopardizing cell survival. In such situations heat shock response is activated and the over expression of heat shock proteins then help restore proper protein confirmation and homeostasis. Traditionally heat shock proteins were believed to help in thermo-tolerance, a process where prior sensitization of thermal stimuli helps cope with subsequent heat exposure [2–4]. However, subsequent research has demonstrated that in addition to heat stress, HSPs respond to a diverse set of cellular stimuli including protection against cytokine release, hypoxia, ischemia, starvation, ultraviolet exposure and acidosis [5–10]. Furthermore, studies suggest that their role may extend beyond their protein chaperone function and that they may have other critical roles including signal transduction. Recently, HSPs have also been associated with antigen presentation and chaperoning immunogenic peptides to Class I and class II major histocompatibility molecules [11–13], thus opening a major area of research where HSPs may be used to modulate immunologic responses in various auto-immune disorders.
Heat Shock Proteins: Family Members Big and Small
The field of heat shock proteins is relatively new. In 1962, Ferrucio Ritossa serendipitously noticed heightened nucleic acid synthesis in salivary gland puffs of Drosophilia which were incubated at higher temperature by mistake. His finding of increased transcriptional activity when cells were exposed to elevated temperature coined the term heat shock response [14]. Initially described heat shock proteins were in the range of 70 kDa but subsequent studies helped discover multiple proteins associated with the heat shock response ranging from 15 KDa to 110 kDa [15–17]. These HSPs are found in multiple subcellular locations and are grouped according to molecular weight. The heavier HSPs are expressed at euthermic or stress temperatures, are ATP dependent and have a diverse set of functions that include protein folding and translocation, cytoprotection, regulation of nuclear hormone receptors as well as regulation of apoptosis [1]. The smaller HSP (sHSPs) are usually tissue specific, are ATP independent and, apart from functioning as chaperones for protein folding, are also strong anti-apoptotic effectors [18].
Heat Shock Protein 70 Superfamily
One of the most conserved and well-studied families of heat shock proteins is HSP70 superfamily. These are ATP dependent chaperones that range in size from 66 to 78 kDa and are encoded by a set of 11 genes in humans. The levels of these proteins are transcriptionally regulated by Heat Shock Factors (HSF) which consists of four members: HSF1, HSF2, HSF3 and HSF4. They possess unique and overlapping functions and have tissue-specific patterns of expression [19]. Of these, HSF-1 is the major transcriptional regulator and is required for transactivation of HSP genes and maintenance of thermotolerance [20]. HSF1, induced during times of stress, binds to the promoter of HSP70 to increase its transcription. The constitutively expressed Hsc70/HSP73 and the highly inducible hsp72/hsp70 are localized to the cytosol and nucleus whereas other members of HSP70 family are found in the mitochondria (mTHSP70) as well as in the endoplasmic reticulum (GRP78/BiP). Increasingly though, the terms ‘constitutive and inducible’ HSPs are becoming obsolete as newer findings suggest that the expression of these proteins may be highly tissue and stimuli specific [21]. Nevertheless, the traditionally described inducible form of HSP70 is sensitive to a wide range of stimuli and its modulation holds promising therapeutic potential. Consequently, our review talks about the emerging role of HSP70 in pathological states starting with a general understanding of HSP70 in human disease and eventually focusing on pancreatitis and pancreatic cancer.
HSP70 in Acute Pancreatitis
Acute Pancreatitis is an inflammatory disease of the pancreas culminating in acinar cell injury and death. Under physiologic conditions, digestive enzymes are synthesized, stored and secreted by the pancreatic acinar cells and their intra-acinar activation is prevented by various protective mechanisms [22]. However, during pancreatitis, the inciting stimuli leads to intra-acinar cell activation of the digestive enzyme zymogens, which in turn are believed to activate the cell death and inflammatory pathways [23]. Research suggest that early on during acute pancreatitis, digestive enzyme zymogens fuse with lysosomes where trypsinogen is activated to trypsin [24, 25]. In parallel but independent to these events, NF-κB dependent pro-inflammatory pathways are activated which lead to release of inflammatory cytokines from acini and thus amplification of the injury at local and systemic levels [26].
Multiple studies have evaluated the protective role of heat shock proteins in acute pancreatitis. These studies have used various methods, including thermal stress, to overexpress HSP70. Wagner et. al found that when acini from rat pancreas were exposed to heat, markedly increased expression of the 72 kilodalton HSP-70 molecule was observed [27]. Interestingly, the authors observed that hyperthermia induced HSP70 overexpression protected against organ damage in cerulean induced pancreatitis model. Similar results were observed even in models where HSP70 was induced via non-thermogenic methods. For example, over-expression of HSP70 by beta-adrenergic stimulation protected mice from cerulean induced pancreatitis [28]. Similarly, administration of sodium arsenite induced overexpression of HSP70 provided protection in cerulean as well as in L-arginine-induced model of acute pancreatitis [29] (Figure 1a and 1b). These findings overwhelmingly suggest that HSP70 is protective against acinar cell injury in pancreatitis and that this protection is independent of the mode of induction of HSP70. Although HSP70 was the major protein induced by heat stress as well as pharmacologic manipulation, these interventions induce multiple HSPs and it is possible that the protective effects observed with these interventions are due to events other than overexpression of HSP70. This was investigated by Bhagat et al. who showed that HSP70 overexpression is in fact responsible for the observed protective role of heat stress using in vitro culture of pancreatic lobules [30]. In this elegant study, two different approaches, namely selective inhibition of HSP70 overexpression using an antisense approach and pharmacological inhibition were used to understand the contribution of HSP70 in heat stress induced protection against acute pancreatitis. In the presence of HSP70 anti-sense or the pharmacological inhibitor quercetin, the protection against cerulean induced injury was abrogated. These findings indicated that the protection against cerulean-induced pancreatitis that follows heat stress is mediated by HSP70. The role of HSP70 in protection imparted by heat stress against pancreatic injury was further evaluated in vivo by Bhagat et al.[30] In this study, when antisense oligonucleotide specific to HSP70 was administered prior to heat stress, expression of other stress proteins except HSP70 was observed. The protective effect of pre-induced heat stress on cerulean-induced pancreatitis in these animals was lost indicating that this protective effect is mediated through overexpressed HSP70. But in the group where sense-oligonucleotide for HSP70 was administered prior to heat stress, HSP70 overexpression was not affected and the protective effect of heat stress to cerulean-induced pancreatitis was maintained.
Figure 1.
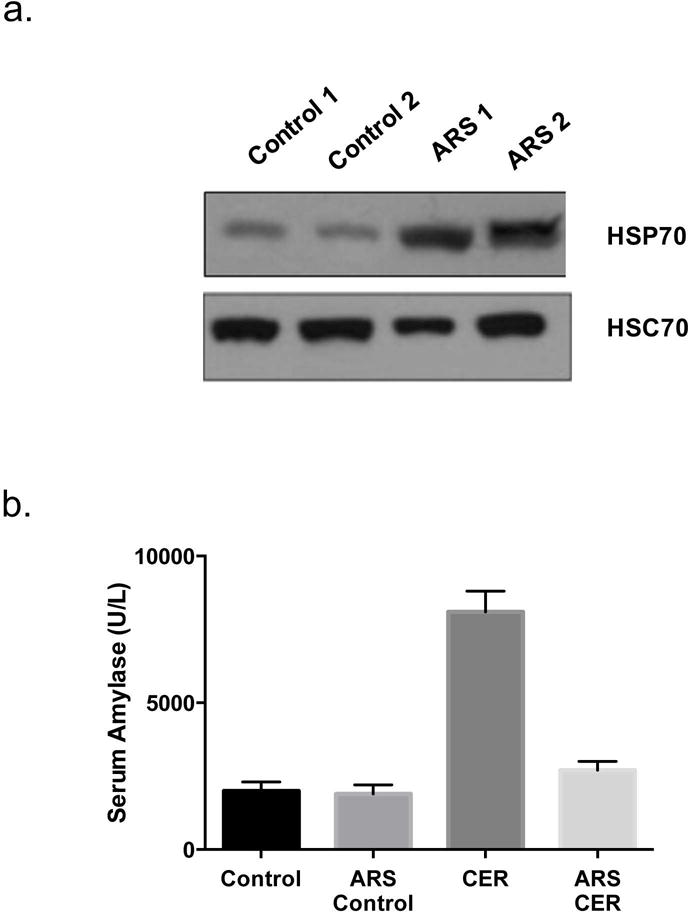
(a) HSP70 Levels are induced by sodium arsenite in rat acini following a single intraperitoneal injection of sodium arsenite (10 mg/kg) and sacrifice at different times after the injection. Pancreas samples from control (CON1 and CON2) and treated rats (ARS) were processed for quantitation of HSP expression by Western blotting. (b) Pre-administration of Sodium Arsenite fourteen hours before induction of acute pancreatitis with Cerulein (ARS + CER, 20 micrograms/kg) reduced serum amylase levels in rats compared to Cerulein treated mice (CER). Adapted from Bhagat et. al [29]
These pre-clinical observations have an intriguing clinical correlate. In an interesting analysis, Balog et. al, found an association between patients with HSP70 polymorphism and severity of acute pancreatitis [31]. A to G polymorphism in the major gene that encodes for HSP70 in humans (i.e., HSP70.2) results in lower levels of inducible HSP70-2 mRNA expression (i.e., individuals with homozygous G allele in HSP70-2 gene). The authors demonstrated that greater number of patients who suffered from severe acute pancreatitis had HSP70.2 G allele polymorphism, when compared to patients with less severe form of pancreatitis or healthy controls. It is possible that in the future HSP70 levels or polymorphism may help in prognostication and that therapeutic induction of HSP70 expression may hold clinical promise in acute pancreatitis.
As discussed, intra-acinar trypsinogen and NF-κB activation are critical for the pathogenesis of acute pancreatitis [26] and that co-localization of digestive zymogens and lysosomal enzymes is required for intra-acinar trypsin activation. It appears that one of the mechanisms by which HSP70 protects against acinar cell injury is by interfering with the co-localization of trypsinogen containing zymogen granules and lysosomal enzymes [29]. Further dissection of this process suggests that the initial surge of Ca2+ influx followed by a sustained increase in Ca2+ levels is required for co-localization and that chelation of intracellular calcium prevents co-localization [32]. Interestingly, HSP70, at least in non-pancreatic cells, has been shown to attenuate this increase in intra-cellular Ca2+[33]. Besides inhibiting intra-acinar trypsin activation, HSP70 overexpression also decreases NF-κB activation by increasing the levels of its endogenous inhibitor iκBα. In summary, HSP70 protects against pancreatic injury by simultaneously downregulating the two important pathways mediating pancreatic acinar cell injury: Intra acinar activation of digestive enzymes and the NF-kB pathway.
Heat Shock Protein 70 in Chronic Pancreatitis
Contrary to the abundance of literature on acute pancreatitis and heat shock response, relatively little is known about the role of heat shock proteins in chronic pancreatitis. Few studies which have addressed this issue provide conflicting results. Unlike the findings in acute pancreatitis, Lee et al did not find any correlation between HSP70.2 polymorphism and disease severity of alcoholic chronic pancreatitis [34]. Another study however observed that HSP70 levels were increased in patients with chronic pancreatitis compared to healthy controls [35]. Work by Gress et al. has corroborated these results and have demonstrated that HSP70 is over-expressed in pancreatic connective tissues and residual acinar cells from patients with chronic pancreatitis [36]. Results from other chronic fibrotic diseases like pulmonary fibrosis suggest a protective role for HSP70 which is believed to be through inhibition of TGF-β1 [37, 38]. Because our understanding of the fibro-inflammatory infiltrate in chronic pancreatitis is only beginning to evolve, future studies will hopefully decipher the role of HSP70 in regulating disease severity and progression in chronic pancreatitis.
Heat Shock Protein 70 and Pancreatic Cancer
Whether pro-survival role of HSP70 is good or bad, is a matter of perspective. While HSP70 has been shown to help in homeostasis and recovery in multiple inflammatory disorders, it has been found to be overexpressed in multiple cancer types and is believed to, by virtue of its pro-survival role, contribute to their aggressive biology [39]. In pancreatic cancer, the inducible form of HSP70 is highly expressed in multiple pancreatic cancer cell lines when compared with normal ductal cells (the commonly believed cell of origin of pancreatic cancer [40] (Figure 2a). Similarly, HSP70 levels are markedly elevated in human pancreatic cancer tissue when compared with normal pancreatic tissue at the tumor margins (Figure 2b). While overexpression of HSP70 suggests an important role of this protein in cancer cell survival, the definitive evidence regarding the importance of HSP70 in tumorigenesis comes from studies where the impact of manipulation of HSP70 levels on cancer cells is studied. In pancreatic cancer cells, downregulation of HSP70 by siRNA leads to caspase dependent apoptotic cell death [40] (Figure 2c). Similar results were observed when HSP70 is inhibited by quercetin, a naturally occurring flavonoid which inhibits heat shock response [40].
Figure 2.
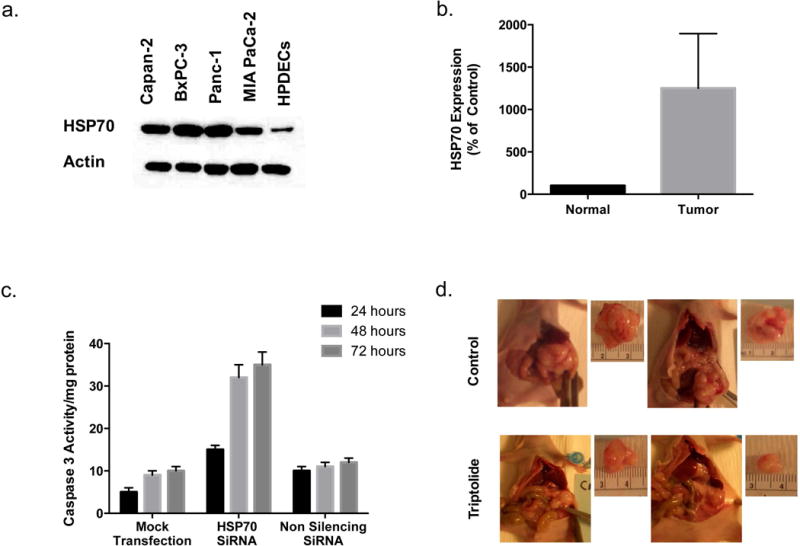
(a) Protein levels of HSP70 were overexpressed in established pancreatic cancer cell lines (Capan-2, BxPC-3, Panc-1, MIA PaCa-2) compared to normal human pancreatic ductal cells (HPDECS). (b) HSP70 Expression was significantly increased in tumors compared to the surrounding normal pancreas. (c) Silencing HSP70 in MIA PaCa-2 cells led to a time dependent apoptosis as seen by an increase in in caspase-3 activity. Adapted from Aghdassi et al.[40] (d) Representative pictures of triptolide treated tumors and controls in nude mice with orthotopic MIA PaCa-2 cancer cells. Mice with orthotopic MiaPaCa-2 tumors who Triptolide were given daily i.p injections (0.2 mg/kg/d) for 60 consecutive days. Adapted from Phillips et al. [54])
The mechanism by which HSP70 protects cancer cells from cell death is still evolving. Dysregulation of the apoptotic machinery is a major hallmark in cancer [41] and a number of studies have implicated HSP70 in preventing programmed cell death [40, 42, 43]. Our studies in pancreatic cancer suggest that HSP70 inhibits apoptosis by two independent but simultaneous mechanisms, namely attenuation of cytosolic calcium level and stabilization of the lysosomes. Intracellular calcium levels are tightly regulated and it is widely believed that increased transport of calcium from the endoplasmic reticulum to the mitochondria results in mitochondrial membrane permeabilization and subsequent activation of apoptosis [44]. We have observed that downregulation of HSP70 by either siRNA or by quercetin leads to time dependent increases in cytosolic calcium levels [45]. Interestingly, prevention of this cytosolic calcium increase by using cell permeable calcium chelators leads to protection from cell death suggesting that this increased cytosolic calcium plays a role in cell death caused by HSP70 downregulation. Besides downregulation of cytosolic calcium, studies from our laboratory and others [45–47] suggest that HSP70 also plays a role in stabilization of lysosomes. The role of lysosomal enzymes in activation of cell death pathways is a newly elucidated concept. We have observed that downregulation of HSP70 in pancreatic cancer cells leads to lysosomal membrane permeabilization and thus release of lysosomal enzymes into the cytosol. Once in the cytosol, lysosomal enzymes activate cell death pathways. Seemingly, of all the lysosomal enzymes, Cathepsin-B plays the most important role as inhibition of Cathepsin-B provides protection against cell death. The fact that chelation of cytosolic calcium or inhibition of lysosomal enzymes only partially protect against cell death and that combination of these strategies provide additive protection against cell death suggests these pathways function simultaneously and independently [45].
Other studies have elucidated additional mechanisms by which HSP70 protects cancer cells as well as cells in general from cell death. Investigations in various cellular systems have suggested that HSP70 can protect against cell death by interfering with apoptotic cell death pathways both upstream and downstream of mitochondria. HSP70 has been shown to inhibit apoptosome formation, an important event which is downstream of mitochondrial release of cytochrome c [48, 49]. Studies have also suggested that HSP70 downregulation leads to the release of cytochrome c, suggesting that HSP70 inhibits the apoptosis pathway upstream of mitochondria as well [50]. In fact, HSP70 has been shown to inhibit translocation of the pro-apoptotic molecule Bax to the mitochondrial membrane and thus prevent mitochondrial membrane permeabilization and cytochrome c release [51]. HSP70 has also been shown to inhibit c Jun N-terminal kinase and thus prevent Bid dependent cytochrome c release from the mitochondria [52]. These findings show the often complementary and redundant pathways through which HSP70 serves to prevent apoptosis in pancreatic cancer cells.
Inhibition of HSP70 in Pancreatic Cancer
While the heat shock response is involved at multiple levels in evading apoptosis in cancer cells, it also underscores a vulnerability that could be therapeutically targeted. A small molecule screen conducted to discover HSP70 inhibitors identified triptolide, a natural compound derived from the Chinese herb Tryptergium wilfordii, as an effective inhibitor of Heat shock response [53]. In our initial studies we observed that triptolide at nano-molar doses inhibited HSP70 levels in multiple pancreatic cell lines and at these doses it led to the activation of apoptotic cell death [54]. Interestingly while it effectively decreased both transcription as well as protein levels of HSP70 in pancreatic cancer cells, triptolide did not affect HSP70 levels in normal human pancreatic ductal cells, pointing to its selectivity towards a transformed system that had an abnormal overexpression of HSP70.
Furthermore, administration of triptolide in mouse models of aggressive orthotopic pancreatic cancer cells produced dramatic decrease in tumor growth (Figure 2d). Although these findings were promising, triptolide was water insoluble and thus had limited clinical application moving forward. To overcome this, we generated a water soluble pro-drug of triptolide called, Minnelide. On addition of a phosphate group to triptolide, the drug was rendered water soluble and in the presence of phosphatases, that are universally present in all tissue compartments, Minnelide rapidly converted to its active form triptolide [55]. Minnelide in vitro (in the presence of alkaline phosphatase) caused a dose and time dependent cytotoxicity in cell lines derived from primary as well as metastatic pancreatic cancer cells. We evaluated Minnelide in multiple animal models of pancreatic cancer simulating multiple clinical scenarios. Minnelide markedly reduced tumor growth in multiple orthotopic models of aggressive pancreatic cancer. Minnelide treated mice did not reach median survival even after treatment for one year in an animal model derived from pancreatic cancer cell line AsPC-1, whereas saline treated mice had a median survival of only 36 days (Figure 3a) In addition to local tumor control, Minnelide treatment abrogated the ability of local tumors to form metastases in a model involving S2-013 cells. In tumors derived from patients, Minnelide treated xenograft tumors regressed to the point of disappearance and the effect was sustained even when Minnelide treatment was stopped. This effect was recapitulated in a genetic model of pancreatic cancer (KPC Model) which like its human counterpart is notorious for being therapy resistant [56] Minnelide treatment in KPC mice resulted in an impressive increase in median survival (148 vs 89.5 days) when compared to saline treated mice, as well as significantly decreased tumor size when compared to conventional therapy with Gemcitabine[55, 57]. In summary Minnelide is effective across multiple independent and complementary models in decreasing tumor burden, metastasis and increasing overall survival in pancreatic cancer and these studies set the stage for its phase I trial.
Figure 3.
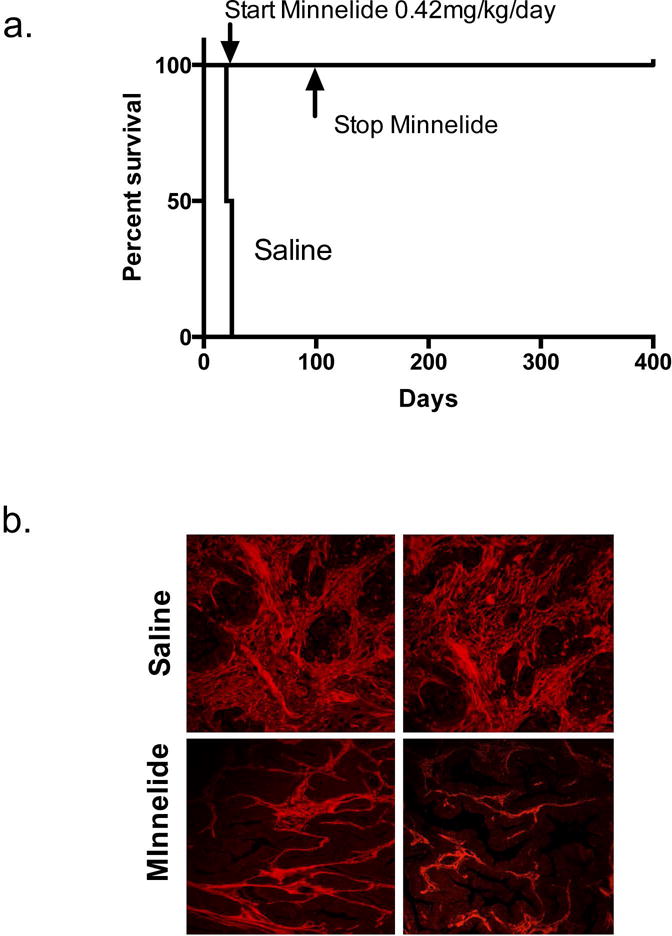
(a) Overall survival in athymic nude mice in an aggressive orthotopic model of AsPC-1 shows increased survival with Minnelide treated mice even after stopping therapy at 100 days and follow up till 100 days whereas the median survival in saline treated mice was only 36 days. Adapted from Chugh et al. [55] (b) Tumors from Minnelide treated mice (0.4mg/kg/day) had considerably less collagen content in an aggressive syngeneic mouse model of pancreatic cancer (KPC). Adapted from Banerjee et. al ([57]
Is Killing Cancer Cells Enough? Impact of Minnelide on Tumor Stroma
Pancreatic Ductal Adenocarcinoma is characterized by a dense desmoplasia [58]. Apart from the cancer associated fibroblasts that make up the bulk of the tumor mass, it also contains a variety of immune, endothelial and neural cells enmeshed in an extra-cellular matrix composed of collagen, fibronectin, proteoglycans and hyaluronic acid. This results in a tumor that is hypoxic, disorganized and poorly perfused posing significant challenge to drug delivery and anti-tumor immune response [59]. Not surprisingly, the concentration of active intracellular metabolite of gemcitabine, 2′, 2′-difluoro-2′-deoxycytidine triphosphate (dFdCTP), was high in stroma-poor xenografts but almost absent in genetically engineered mouse models secondary to vascular compromise. Therefore, a number of studies have proposed that modulating the pro-tumorigenic stroma may aid in therapy against this difficult disease [60, 61]. Nevertheless, owing to yet an incomplete understanding of the complex tumor microenvironment, a blunt approach in targeting stroma may have un-intended consequences at times. For example, inhibition of hedgehog signaling in patients with PDAC was prematurely terminated due to worsened survival (NCT01130142) and targeted deletion of cancer associated fibroblasts in a genetic model eventually gave way to a more aggressive form of disease [62]. Therefore, an approach that modulates the stroma, rather than eliminates it completely, may be a better alternative moving forward. For example, depletion of hyaluronan, a glycosaminoglycan that increases interstitial pressure in the tumor ECM, improves vascular patency, drug delivery and ultimately improves survival [63, 64]. In that vein, Minnelide has been shown to decrease HA synthesis in stroma rich models of PDAC and human xenografts by decreasing the expression of hyaluron synthase enzymes [57]. Additionally, tumor collagen content was reduced secondary to decreased activity of PLOD1-4 (procollagen lysine 5 dioxygenase), an enzyme that is involved in collagen stabilization (Figure 3b). The cellular compartment of the stroma also showed a reduction in the number of activated stellate cells with Minnelide treatment. The resulting tumor microenvironment had greater functional vessels (four times compared to untreated animals) and consequently better drug delivery inside these poorly perfused tumors. Importantly though, these changes translated to a significantly improved survival in the aggressive KPC mouse model compared to saline treated mice. These preliminary results hint towards an exciting possibility that Minnelide, while inhibiting HSP70 globally, may also result in a tumor microenvironment that is not conducive for tumor growth. Early evidence towards this fact was shown by Gabai et. al where HSP70 inhibitors that were not effective in vitro on resistant cancer cells were surprisingly able to reduce tumor growth in vivo, a finding, they attribute to the inhibition of HSP70 in the stroma [65]. Together, Minnelide appears to combine stromal modulation with cancer cell death that may hold the key to designing stroma directed therapy in the future.
Cancer Stem Cells, Their Role in Recurrence and Minnelide
A subpopulation of cancer cells has been consistently identified in a number of cancers that share stem-like features [66]. They are analogous to normal stem cells in terms of self-renewal and tumorigenicity [67]. The terminology used can be often contentious with some preferring to call them ‘tumor initiating cells’ due to their ability to from tumors in mice at very low numbers [68]. In pancreatic cancer, a number of markers have been used to identify cells that have heightened tumorigenic capability, are resistant to conventional chemotherapy and are able to lie dormant by evading routine immune surveillance. Though there is still considerable debate over which cancer stem cell marker(s) (if any) truly represents this phenotype, a number of candidate markers have been described [69]. These include CD44, CD24, ESA, CXCR4, C-met and CD-133. One of the most well studied markers is the glycoprotein receptor CD-133. Banerjee et al., isolated CD133+ cells from a spontaneous genetic mouse model of pancreatic cancer and found that the CD133+ fraction also included subpopulations of cells with markers such as CD44, CD24 and ESA+ cells [70]. On animal studies, CD133+ cells were able to form tumors with an inoculum load as low as 10 cells in immunocompetent mice, a finding that was not described before. In vitro, expression of CD133 co-related with invasiveness in cancer cell lines. Subsequent studies also showed that CD133+ cells were able to influence cancer cell invasion and metastasis via the NF-κB pathway [71]. Another therapeutically significant characteristic shared by these cancer stem cells is their resistance to conventional chemo-therapy. CD133+ cells in pancreatic cancer have been shown to be highly resistant to Gemcitabine, Paclitaxel and 5-FU, three of the most commonly used components of various chemotherapy regimens against pancreatic cancer [70, 72]. This resistance to treatment from a quiescent sub-population may partially explain tumor recurrence in pancreatic cancer where most patients will ultimately recur at local or distant sites. This has been shown to be true in breast cancer after systemic chemotherapy where a bulk of cell death due to cytotoxic therapy enriches for cancer cells with stem like features [73].
Minnelide, on the other hand, is highly effective in reducing proliferation and inducing apoptosis in CD133+ cancer cells in vitro. Furthermore, tumors derived from CD133+ cells from KPC tumors as well as human PDACs showed significantly slower tumor growth. In another study, Minnelide treated mice did not have evidence of tumor recurrence at 400 days in a highly aggressive orthotopic model of pancreatic cancer even after stopping therapy whereas untreated mice had a median survival of 36 days [55]. When tumors were analyzed for CD133 expression, the fraction of CD133+ cells was greatly reduced (8 vs 4%) in Minnelide treated tumors compared to saline controls. Taken together these findings suggest that Minnelide is highly effective in targeting cancer stem cells and consequently reducing tumor recurrence.
Minnelide: Bench to Bedside…. And Back to Bench
Encouraged by the results of the pre-clinical studies, Minnelide is being evaluated in clinical trials. A Phase I Dose escalation trial for Minnelide showed promising evidence of clinical activity and a favorable safety profile [74]. In these patients with advanced solid organ malignancies (17 pancreatic, 7 colorectal, 3 other GI), Minnelide, given at doses ranging from 0.16 mg to 0.8 mg/m2, was well tolerated with the most common toxicity being hematologic and rarely cerebellar (1/24). Pharmacokinetic studies showed rapid conversion of Minnelide to triptolide in the blood stream with corresponding decrease in serum HSP70 levels. Peak triptolide concentrations were reached within five minutes of drug infusion. On evaluation with the RECIST criteria after 2 cycles, 10% (1/10) patients showed partial remission and 60% (6/10) patients showed stable disease for up to six months. That Minnelide demonstrated clinical activity in a phase I clinical trial itself is very promising. Chemotherapy in pancreatic cancer is fraught with one significant problem, the acquisition of resistance to an initially susceptible drug. Drug resistance leads to recurrence and advancing disease in almost all pancreatic cancer patients treated with systemic chemotherapy [75]. In this scenario, Minnelide may provide an important advantage in overcoming this challenge [76]. Platinum compounds form a major component of the armamentarium against pancreatic cancer and are part of regimens such as FOLFIRINOX and GEM-OX (Gemcitabine Oxaliplatin)[77, 78]. A very common platinum compound used in PDAC chemotherapy regimens is Oxaliplatin which causes cell death by incorporating itself into the DNA and causing inter and intra-strand links and ultimately precluding transcription. Understandably, one of the major mechanism of platinum resistance in cancer cells then involves overexpression of the components of nucleotide excision repair (NER) pathway which excises and repairs these crosslinks [79]. We found that Minnelide treatment of pancreatic cancer cells suppressed Nucleotide Exchange Repair (NER) pathway via a decrease in AP-1 promoter activity [76]. This decrease in the NER pathway led to a synergistic effect when combined with Oxaliplatin. Combination of Oxaliplatin and triptolide/Minnelide led to greater cell death and apoptosis in vitro and significant decrease in tumor growth in vivo compared to either drug alone. These promising results form the basis for more combination studies evaluating the role of Minnelide together with the current standard of care chemotherapy in future studies.
How Does Minnelide Reduce HSP70 Levels?
The mechanism of how exactly Minnelide inhibits HSP70 is still unclear. Triptolide, the biologically active form of Minnelide, is known to decrease global transcription by inhibiting RNA polymerase II at very high doses [80]. These concentrations, however, are never reached in vitro (Triptolide 0–200 nM) and in vivo (Minnelide 0.4 mg/kg/day) before observing cell death or unacceptable toxicity. Multiple reports from ours and other laboratories have shown that triptolide inhibits NF-κB [81, 82] and induces endoplasmic reticulum (ER) stress [83], ultimately leading to cancer cell death. These multiple effects could perhaps be explained by triptolide’s effect on SP-1, a major transcription regulator [81]. We have shown that inhibition of O-Glycolysation of SP-1 by triptolide prevents its nuclear localization and its subsequent DNA binding. SP-1 besides modulating ER is also an upstream regulator of NF-κB and HSF-1. HSF-1 in turn is the master heat shock transcription factor which controls the expression of HSP70. Thereby Minnelide in downregulating HSP70 and its downstream anti-apoptotic drives cancer cells towards cell death.
Conclusion
Through our journey of studying HSP70 we have found that it plays a dual role in pancreatic pathology. In the acute inflammatory condition of the pancreas, HSP70 serves to protect acinar cells by modulating intracellular calcium signaling, lysosomal membrane permeabilization and counteracting inflammatory cascade secondary to NF-κB and trypsin activation. It eventually leads the pancreas away from a fate that would result in acinar cell death, necrosis and its painful aftermath of cytokine storm and multi-organ failure. In that essence, HSP70 is a reliable friend. However, cancer cells have found a way to hijack this mechanism that turns its cyto-protective role to its own advantage. HSP70 opposes apoptosis in transformed cells at multiple levels. Fortunately, we have seen promising results obtained by inhibiting HSP70 in pancreatic cancer where this dependence of neoplastic cells on HSP70 may ultimately help us defeat it. With that perspective HSP70 definitely seems like a friend on our side.
This dual, chimeric role of HSP70 in pancreatic pathology, if understood and harnessed well, has broad clinical applicability not only in inflammatory diseases but also in cancer.
Synopsis.
HSP70 modulates inflammation in pancreatitis mainly by upregulating cell survival mechanisms. In pancreatic cancer, inhibition of HSP70 has shown great promise in various pre-clinical models.
Acknowledgments
Funding: This study was funded by NIH grants R01-CA170946 and R01-CA124723 (to AKS); Authors also want to acknowledge the intra-mural support from Sylvester Comprehensive Cancer Center.
Footnotes
Disclosures: The University of Minnesota has a patent for Minnelide™ (which has been licensed to Minneamrita Therapeutics LLC, Moline, IL). AS has ownership interests (including patents) and is a consultant/advisory board member for Minneamrita Therapeutics LLC. Dr. Sulagna Banerjee is a consultant for Minneamrita Therapeutics, licensee of the intellectual property (Minnelide) being described in the study. The other authors have nothing to disclose.
References
- 1.Kregel KC. Invited Review: Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. Journal of Applied Physiology. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 2.Li GC, Meyer JL, Mak JY, Hahn GM. Heat-induced protection of mice against thermal death. Cancer Res. 1983;43:5758–5760. [PubMed] [Google Scholar]
- 3.Landry J, Bernier D, Chretien P, et al. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42:2457–2461. [PubMed] [Google Scholar]
- 4.Landry J, Chretien P. Relationship between hyperthermia-induced heat-shock proteins and thermotolerance in Morris hepatoma cells. Can J Biochem Cell Biol. 1983;61:428–437. doi: 10.1139/o83-058. [DOI] [PubMed] [Google Scholar]
- 5.Guttman SD, Glover CV, Allis CD, Gorovsky MA. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980;22:299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- 6.Hahn GM, Li GC. Thermotolerance and heat shock proteins in mammalian cells. Radiat Res. 1982;92:452–457. [PubMed] [Google Scholar]
- 7.Marber MS, Mestril R, Chi SH, et al. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzel G, Pilatus U, Rensing L. Similar dose response of heat shock protein synthesis and intracellular pH change in yeast. Exp Cell Res. 1985;159:252–256. doi: 10.1016/s0014-4827(85)80054-9. [DOI] [PubMed] [Google Scholar]
- 9.Jaattela M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993;177:231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988;241:1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–177. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 12.Todryk S, Melcher AA, Hardwick N, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 13.Moseley PL. Exercise, stress, and the immune conversation. Exerc Sport Sci Rev. 2000;28:128–132. [PubMed] [Google Scholar]
- 14.Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 15.Stricker EM, Hainsworth FR. Evaporative cooling in the rat: Effects of dehydration. Canadian Journal of Physiology and Pharmacology. 1970;48:18–27. doi: 10.1139/y70-003. [DOI] [PubMed] [Google Scholar]
- 16.Tissiéres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. Journal of Molecular Biology. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 17.Welch WJ. How cells respond to stress. Sci Am. 1993;268:56–64. doi: 10.1038/scientificamerican0593-56. [DOI] [PubMed] [Google Scholar]
- 18.Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: Role in cellular functions and pathology. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Åkerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMillan DR, Xiao X, Shao L, et al. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 22.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 23.Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 24.Saluja AK, Donovan EA, Yamanaka K, et al. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304–310. doi: 10.1016/s0016-5085(97)70108-2. [DOI] [PubMed] [Google Scholar]
- 25.Van Acker GJ, Saluja AK, Bhagat L, et al. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G794–800. doi: 10.1152/ajpgi.00363.2001. [DOI] [PubMed] [Google Scholar]
- 26.Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation is required for early pancreatic injury but not for inflammation during acute pancreatitis. Gastroenterology. 2011;141:2210–2217. e2212. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner AC, Weber H, Jonas L, et al. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996;111:1333–1342. doi: 10.1053/gast.1996.v111.pm8898648. [DOI] [PubMed] [Google Scholar]
- 28.Frossard JL, Bhagat L, Lee HS, et al. Both thermal and non-thermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50:78–83. doi: 10.1136/gut.50.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhagat L, Singh VP, Dawra RK, Saluja AK. Sodium arsenite induces heat shock protein 70 expression and protects against secretagogue-induced trypsinogen and NF-kappaB activation. J Cell Physiol. 2008;215:37–46. doi: 10.1002/jcp.21286. [DOI] [PubMed] [Google Scholar]
- 30.Bhagat L, Singh VP, Song AM, et al. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156–165. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 31.Balog A, Gyulai Z, Boros LG, et al. Polymorphism of the TNF-alpha, HSP70-2, and CD14 genes increases susceptibility to severe acute pancreatitis. Pancreas. 2005;30:e46–50. doi: 10.1097/01.mpa.0000153329.92686.ac. [DOI] [PubMed] [Google Scholar]
- 32.Bhagat L, Saluja AK, Akella U, et al. Role of calcium in lysosomal enzyme redistrmution and intra-acinar cell activation of trypsinogen in an in vitro model of pancreatitis. Gastroenterology. 2000;118:A165. [Google Scholar]
- 33.Kiang JG, Ding XZ, McClain DE. Overexpression of HSP-70 attenuates increases in [Ca2+]i and protects human epidermoid A-431 cells after chemical hypoxia. Toxicol Appl Pharmacol. 1998;149:185–194. doi: 10.1006/taap.1997.8364. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Ryu JK, Jeong JB, et al. Polymorphisms of the MCP-1 and HSP70-2 Genes in Korean Patients with Alcoholic Chronic Pancreatitis. Digestive Diseases and Sciences. 2008;53:1721–1727. doi: 10.1007/s10620-007-0049-1. [DOI] [PubMed] [Google Scholar]
- 35.Dutta SK, Girotra M, Singla M, et al. Serum HSP70: a novel biomarker for early detection of pancreatic cancer. Pancreas. 2012;41:530–534. doi: 10.1097/MPA.0b013e3182374ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gress TM, Muller-Pillasch F, Weber C, et al. Differential expression of heat shock proteins in pancreatic carcinoma. Cancer Res. 1994;54:547–551. [PubMed] [Google Scholar]
- 37.Hagiwara S, Iwasaka H, Matsumoto S, et al. Association Between Heat Stress Protein 70 Induction and Decreased Pulmonary Fibrosis in an Animal Model of Acute Lung Injury. Lung. 2007;185:287–293. doi: 10.1007/s00408-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Tanaka Y, Namba T, et al. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol. 2010;80:920–931. doi: 10.1016/j.bcp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Garrido C, Brunet M, Didelot C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 43.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis. 2004;25:179–187. doi: 10.1093/carcin/bgh001. [DOI] [PubMed] [Google Scholar]
- 44.Rizzuto R, Pinton P, Ferrari D, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 0000(22):8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 45.Dudeja V, Mujumdar N, Phillips P, et al. Heat Shock Protein 70 Inhibits Apoptosis in Cancer Cells Through Simultaneous and Independent Mechanisms. Gastroenterology. 2009;136:1772–1782. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nylandsted J, Gyrd-Hansen M, Danielewicz A, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bivik C, Rosdahl I, Öllinger K. Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis. 2006;28:537–544. doi: 10.1093/carcin/bgl152. [DOI] [PubMed] [Google Scholar]
- 48.Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 49.Li CY, Lee JS, Ko YG, et al. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 50.Steel R, Doherty JP, Buzzard K, et al. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279:51490–51499. doi: 10.1074/jbc.M401314200. [DOI] [PubMed] [Google Scholar]
- 51.Stankiewicz AR, Lachapelle G, Foo CP, et al. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 52.Park HS, Lee JS, Huh SH, et al. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. Embo j. 2001;20:446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 54.Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide Induces Pancreatic Cancer Cell Death via Inhibition of Heat Shock Protein 70. Cancer Research. 2007;67:9407. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 55.Chugh R, Sangwan V, Patil SP, et al. A Preclinical Evaluation of Minnelide as a Therapeutic Agent Against Pancreatic Cancer. Sci Transl Med. 2012;4:156–ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee S, Modi S, McGinn O, et al. Impaired Synthesis of Stromal Components in Response to Minnelide Improves Vascular Function, Drug Delivery, and Survival in Pancreatic Cancer. Clinical Cancer Research. 2016;22:415. doi: 10.1158/1078-0432.CCR-15-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 59.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 61.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 62.Özdemir Berna C, Pentcheva-Hoang T, Carstens Julienne L, et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell. 25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabai VL, Yaglom JA, Wang Y, et al. Anticancer Effects of Targeting Hsp70 in Tumor Stromal Cells. Cancer Res. 2016;76:5926–5932. doi: 10.1158/0008-5472.CAN-16-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 67.Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 68.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 69.Abel EV, Simeone DM. Biology and Clinical Applications of Pancreatic Cancer Stem Cells. Gastroenterology. 144:1241–1248. doi: 10.1053/j.gastro.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 70.Banerjee S, Nomura A, Sangwan V, et al. CD133+ Tumor Initiating Cells in a Syngenic Murine Model of Pancreatic Cancer Respond to Minnelide. Clinical Cancer Research. 2014;20:2388–2399. doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nomura A, Banerjee S, Chugh R, et al. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 2015;6:8313–8322. doi: 10.18632/oncotarget.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hermann PC, Huber SL, Herrler T, et al. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greeno E, Borazanci E, Gockerman J, et al. Abstract CT207: Phase I dose escalation and pharmokinetic study of 14-O-phosphonooxymethyltriptolide. AACR; 2015. [Google Scholar]
- 75.Chand S, O’Hayer K, Blanco FF, et al. The Landscape of Pancreatic Cancer Therapeutic Resistance Mechanisms. Int J Biol Sci. 2016;12:273–282. doi: 10.7150/ijbs.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Modi S, Kir D, Giri B, et al. Minnelide Overcomes Oxaliplatin Resistance by Downregulating DNA Repair Pathway in Pancreatic Cancer. J Gastrointest Surg. 2016;20:13–24. doi: 10.1007/s11605-015-3000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alberts SR, Townley P, Goldberg R, et al. Gemcitabine and oxaliplatin for metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group phase II study. Annals of oncology. 2003;14:580–585. doi: 10.1093/annonc/mdg170. [DOI] [PubMed] [Google Scholar]
- 78.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 79.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 80.Titov DV, Gilman B, He Q-L, et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7:182–188. doi: 10.1038/nchembio.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banerjee S, Sangwan V, McGinn O, et al. Triptolide-induced cell death in pancreatic cancer is mediated by O-GlcNAc modification of transcription factor Sp1. J Biol Chem. 2013;288:33927–33938. doi: 10.1074/jbc.M113.500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yinjun L, Jie J, Yungui W. Triptolide inhibits transcription factor NF-kappaB and induces apoptosis of multiple myeloma cells. Leuk Res. 2005;29:99–105. doi: 10.1016/j.leukres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Mujumdar N, Banerjee S, Chen Z, et al. Triptolide activates unfolded protein response leading to chronic ER stress in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1011–1020. doi: 10.1152/ajpgi.00466.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


