Summary
Background
Psychological therapies are first-line interventions for depression, but existing provision is not accessible for many adults with intellectual disabilities. We investigated the clinical and cost-effectiveness of a behavioural activation intervention (BeatIt) for people with intellectual disabilities and depression. BeatIt was compared with a guided self-help intervention (StepUp).
Methods
We did a multicentre, single-blind, randomised, controlled trial with follow-up at 4 months and 12 months after randomisation. Participants aged 18 years or older, with mild to moderate intellectual disabilities and clinically significant depression were recruited from health and social care services in the UK. The primary outcome was the Glasgow Depression Scale for people with a Learning Disability (GDS-LD) score at 12 months. Analyses were done on an intention-to-treat basis. This trial is registered with ISCRTN, number ISRCTN09753005.
Findings
Between Aug 8, 2013, and Sept 1, 2015, 161 participants were randomly assigned (84 to BeatIt; 77 to StepUp); 141 (88%) participants completed the trial. No group differences were found in the effects of BeatIt and StepUp based on GDS-LD scores at 12 months (12·03 [SD 7·99] GDS-LD points for BeatIt vs 12·43 [SD 7·64] GDS-LD points for StepUp; mean difference 0·26 GDS-LD points [95% CI −2·18 to 2·70]; p=0·833). Within-group improvements in GDS-LD scores occurred in both groups at 12 months (BeatIt, mean change −4·2 GDS-LD points [95% CI −6·0 to −2·4], p<0·0001; StepUp, mean change −4·5 GDS-LD points [–6·2 to −2·7], p<0·0001), with large effect sizes (BeatIt, 0·590 [95% CI 0·337–0·844]; StepUp, 0·627 [0·380–0·873]). BeatIt was not cost-effective when compared with StepUp, although the economic analyses indicated substantial uncertainty. Treatment costs were only approximately 3·6–6·8% of participants' total support costs. No treatment-related or trial-related adverse events were reported.
Interpretation
This study is, to our knowledge, the first large randomised controlled trial assessing individual psychological interventions for people with intellectual disabilities and mental health problems. These findings show that there is no evidence that BeatIt is more effective than StepUp; both are active and potentially effective interventions.
Funding
National Institute for Health Research.
Introduction
Individuals with intellectual disabilities have higher rates of mental ill health than the general population, with a point prevalence of 40% for adults.1 The term intellectual disabilities refers to people who have substantial impairments of both intellectual and functional ability, with age of onset before adulthood. Approximately 2% of adults and 3·5% of children have an intelligence quotient (IQ) of less than 70, although this figure might be rising because of increasing life expectancy, improving survival outcomes of babies with very low birthweight, and increasing maternal age.2
Depression is as common in adults with intellectual disabilities as in the general population, with a point prevalence of about 5%.1 The disorder is more enduring for these adults than for the general population, suggesting it is either a more severe condition or more poorly managed than in the general population. For example, a study with a British cohort found that adults with intellectual disabilities were four times more likely than the non-intellectually disabled population to meet criteria for chronic depression over a 28-year period.3
Although psychological therapies have become established first-line interventions for depression in the general population, this has not been the case for adults with intellectual disabilities, because of the additional complexities involved in making these interventions accessible to adults with cognitive and verbal communication impairments. Awareness of the inequity in provision of psychological therapies has grown, but substantial limitations remain in the existing evidence base and in its implementation. The available literature was reviewed for the National Institute for Health and Care Excellence (NICE) guideline4 on mental health problems in people with intellectual disabilities. A key recommendation was for modifications to and trials of psychological interventions to ensure they are accessible to adults with intellectual disabilities.
Research in context.
Evidence before this study
In September, 2016, a review for the UK National Institute for Health and Care Excellence (NICE) clinical guideline for mental health problems in people with learning disabilities reported that the only available evidence on psychological interventions for depression in people with intellectual disabilities was for cognitive behavioural therapy (CBT), adapted for people with intellectual disabilities. Of 130 studies identified in that review, three randomised controlled trials and three controlled before-and-after studies investigated CBT for treatment or prevention of depression in adults with intellectual disabilities. NICE concluded that CBT might result in a clinically meaningful reduction in depressive symptoms when compared with treatment as usual at a follow-up of 38 weeks, but graded the evidence as being of very low quality.
Added value of this study
This study is, to our knowledge, the largest randomised controlled trial of individual psychological therapy for adults with intellectual disabilities published to date. Participants were successfully recruited and randomly assigned to receive manualised individual psychological therapies for depression. Participant retention was 88%, and therapies were delivered with excellent fidelity to the manuals. Significant reductions in depression scores were apparent by the end of treatment for both BeatIt and StepUp, and were maintained at 12 months after randomisation. Neither treatment was better than the other. The long-term nature of the follow-up period (12 months after randomisation) and the health economic analysis of the psychological interventions also make important contributions to the existing scientific literature.
Implications of all the available evidence
High-quality randomised controlled trials of individual psychological therapies can be done in adults with intellectual disabilities and mental health problems such as depression. Psychological interventions such as behavioural activation and guided self-help can be manualised and used as first-line interventions for adults with intellectual disabilities and depression. Moreover, health-care workers can be trained and supervised to deliver behavioural activation and guided self-help as part of their regular practice, without requiring specialist therapists.
In behavioural activation, the focus of the intervention is on behaviour change rather than cognition, emphasising engagement with potential environmental reinforcers.5 In the general population, behavioural activation has been shown to be as effective as antidepressant medications, and superior or equivalent to cognitive behavioural therapy (CBT), pill-placebo, and treatment as usual among patients with more severe depression,6, 7 with effects lasting as long as those for CBT following treatment termination.8 Non-specialist health-care workers can be trained to deliver behavioural activation.6 Although there have been promising developments in CBT approaches for people with intellectual disabilities, including a recent pilot randomised controlled trial of a computerised intervention,9 behavioural activation might be more accessible than CBT for people with intellectual disabilities, since it is less cognitively demanding. We therefore aimed to assess the effectiveness of behavioural activation for people with intellectual disabilities and depression. The study reported here was informed by a pilot open trial of behavioural activation alone in people with intellectual disabilities, done at one site.10 Outcomes from the pilot study showed evidence of a reduction in depressive symptoms for those able to self-report on the Glasgow Depression Scale for people with a Learning Disability (GDS-LD)11 before and after the intervention, and at a 3 month follow-up after the end of treatment.
Methods
Study design and participants
We did a multicentre, single-blind, randomised controlled trial comparing adapted behavioural activation (BeatIt) with guided self-help (StepUp). At the request of the funder, the comparator was not treatment as usual. We also did an accompanying health economic evaluation of the interventions. Nested qualitative studies exploring the experiences of participants, supporters, and therapists will be reported elsewhere.
An internal pilot phase was done in Scotland before opening additional study sites in England and Wales. Participants were recruited mostly from specialist intellectual disabilities health services (not specifically mental health services) and social care services, with some recruited from Improving Access to Psychological Therapy (IAPT) services in Lancashire. IAPT services provide first-line psychological interventions for all adults in England with mental health problems. Inclusion criteria were mild to moderate intellectual disabilities, measured by a score of 75 or less on the Weschler Abbreviated Scale of Intelligence;12 the ability to provide informed consent; age 18 years or older; and clinically significant depression as assessed by the Diagnostic Criteria for Psychiatric Disorders for use with Adults with Learning Disabilities.13 Participants also needed to have a supporter (a staff member, family member, or friend) who could provide support in therapy sessions. Participants who were actively suicidal or having difficulties that would prevent them from interacting with the therapist or retaining information from sessions (eg, late-stage dementia), were excluded. Those participating in the trial did not receive another psychological therapy for depression while they were receiving either BeatIt or StepUp.
Information was provided and presentations were made to health and social care services about the trial. Professionals identified individuals they were working with who might be suitable and passed on details of the study, with advice to talk through the information with a support person of their choosing, since potential participants had few, if any, literacy skills. The researcher then arranged to meet with those who replied, ordinarily alongside a support person, and talked through the information sheet. If the participant was satisfied with the researcher's answers to any questions they had, they were invited to choose whether or not they would like to take part in the study. The West of Scotland Research Ethics Committee 3 gave national approval for the study (NRES: 13/MH97). The full trial protocol has been published.14
Randomisation and masking
Participants who gave written informed consent (they were asked to sign the consent form and their signature was witnessed by a carer or someone independent of the study) were screened and provided baseline data before being randomly assigned. Individuals were allocated in a 1:1 ratio to receive the BeatIt or StepUp intervention, by use of block randomisation. Mixed block sizes of length four and six were used at random. Randomisation was stratified by study centre and use of antidepressants. Changes in the prescription of antidepressants and other mood-stabilising drugs were monitored for the duration of the study.
To conceal the allocation of participants from the research team, each participant was randomly assigned to a treatment group by use of an automated system run by the Robertson Centre for Biostatistics (University of Glasgow, Glasgow, UK). The system did not reveal the random allocation to the researcher but notified the study coordinator, who then contacted clinicians to arrange subsequent treatment visits. Thus, the researchers collecting outcome data remained unaware of the intervention group to which participants had been assigned. Processes were in place to ensure researchers continued to be unaware of participants' designations over the course of their involvement in the trial. On the two occasions that researchers were unmasked, both during the process of arranging data collection, the participants were reassigned to another researcher.
Procedures
The BeatIt intervention was a 12-session manualised approach15 based on a published protocol and carefully adapted and successfully piloted in people with intellectual disabilities. The focus of the intervention was on increasing activity, scheduling activity, and addressing barriers to achieving engagement in activity. The first stage of therapy involved obtaining an insight into the participant's pattern of life and sharing a formulation of their difficulties linked to the underpinning theory of behavioural activation, before working to increase the participant's engagement and overcome emotional and organisational barriers to change. Therapy ended with a plan to maintain or continue progress.
The eight-session guided self-help intervention, StepUp,16 was chosen as the comparator because it was deemed similar to BeatIt in terms of therapist attention, use of a structured, manualised approach, and the presence of a supporter. The self-help materials had been developed before the study by a member of the research team. StepUp was thought to differ from BeatIt in relation to the active components of the intervention, which were perceived to be less person-centred and essentially psychoeducational. After building rapport in the first session, a series of four booklets provided a focus for the next five sessions. The booklets concerned depression and factors linked to low mood, sleep, physical activity, and problem solving. Therapy finished with a review of all booklets and then a final session making a plan for their continued use.
In addition to ensuring that the materials and exercises from both interventions were accessible and engaging, a key adaptation was delivery of the therapy alongside a support person. These were individuals who were able to provide support in sessions and who were therefore available for a minimum of 2 h per week. For BeatIt, people with intellectual disabilities ordinarily relied on others for support to engage in activity and negotiate change. Those taking part in StepUp typically needed help with understanding the booklets and using them in their everyday lives. The involvement of supporters also ensured that participants had help to maintain therapeutic gains once the interventions had finished.
Both interventions were delivered on an outreach basis, by community nurses and allied health professionals with experience of working with people who have intellectual disabilities. Some IAPT workers were also involved. All therapists received 1–2 days of training in the delivery of the intervention and were supervised by clinical psychologists. All therapists were asked, where possible, to record two sessions selected by the trial coordinator, for the purpose of establishing fidelity to the manual and quality of therapy delivery.
Outcomes
The primary outcome measure was the GDS-LD score11 at 12 months after randomisation. Secondary outcomes included carer ratings of depressive symptoms (Intellectual Disabilities Depression Scale; IDDS)17 and aggressive behaviour (Behaviour Problems Inventory short form; BPI-S),18 along with self-reporting of anxiety symptoms (Glasgow Anxiety Scale for people with Intellectual Disabilities; GAS-ID).19 Quality of life was also examined (EuroQol five dimensions questionnaire; EQ-5D-Y),20 along with community involvement (Index of Community Involvement; ICI),21 domestic activity (Index of Participation in Domestic Life; IPDL),22 and perceived social support (Social Support Questionnaire; SSQ3).23 Four subscales of the Adaptive Behavior Scale (ABS)24 were completed. Carer self-efficacy was examined with the Emotional Difficulties Self-Efficacy Scale (EDSE).25 Primary and secondary outcome measures were collected at baseline, 4 months, and 12 months after randomisation. Service use data (reported in the cost analysis) were collected from carers at 4 months and 12 months, and also at 8 months. Participants' life events were recorded (Bangor Life Events Schedule for Intellectual Disabilities; BLESID)26 at baseline and 12 months only, as the BLESID records events that occur during a 12-month period.
Statistical analysis
In the first 18 months of an open trial of BeatIt,10 the mean reduction in GDS-LD scores at a 3 month follow-up after the intervention was 8·50 points (SD 5·24). Our study was powered to detect a mean between-group difference of 0·60 SD units or 3·14 points on the GDS-LD.
If the BeatIt group in the present study could achieve an 8·50-point improvement in GDS-LD scores at 12 months, then this outcome would allow for the StepUp group to show a 5·36-point improvement during the same time period (ie, 63% of the improvement in the BeatIt group). Alternatively, this outcome allowed for detection of a small improvement in the StepUp group in conjunction with a large, short-term improvement in the BeatIt group, followed by some regression. For example, if the BeatIt group showed an improvement from baseline to 12 months of 6·00 points, then the study would be powered to detect a difference if the mean improvement in the StepUp group was 2·86 points.
To have 90% power to detect this difference, this study required 60 participants in each group to provide outcome data at 12 months after randomisation. The primary analysis was an analysis of covariance adjusting for baseline GDS-LD score, which should have the same power to detect smaller intervention effects, depending on the level of correlation in scores over time.
There were no data to inform the effect of clustering of outcomes for participants with intellectual disabilities seen by each therapist. We assumed that each therapist would work with an average of nine participants (ie, several part-time therapists at each site) and we assumed an intraclass correlation of 0·025, resulting in the sample size being increased by 20% to 72 per group, or 144 in total. A recruitment target of 166 participants allowed for up to 13·3% of participants to be lost to follow-up.
The primary analysis compared GDS-LD scores at 12 months after randomisation between intervention groups, adjusting for baseline GDS-LD scores, study centre, and use of antidepressants at baseline as fixed effects within a mixed-effects linear regression model, including random intercepts for therapists. Similar methods were applied to the primary outcome measure at the immediate assessment after the intervention (4 months after randomisation) and to secondary outcome measures at each assessment point. These models were used to estimate between-group differences and within-group changes from baseline. Intraclass correlation coefficients were estimated as the ratio of the between-therapist variance to the sum of between-therapist and residual variance from these models. Additionally, for each outcome measure, data from both follow-up timepoints were analysed simultaneously by use of repeated measures models, adjusting for the baseline value of the outcome, stratification factors, and timepoint as fixed effects, with random intercepts for therapists and participants; intervention-by-time interactions were used to test whether any between-group differences varied over time.
Analyses of the primary outcome were repeated with multiple imputation to assess whether the results were sensitive to missing data. To impute missing values at each timepoint, prediction models were based on age, antidepressant use, and any previous or subsequent measurements of the primary outcome; these prediction models did not include randomised group. Models for the primary outcome were extended to explore the effects of baseline characteristics, including stratification factors, chronicity of depressive symptoms, life events, and history of previous psychological intervention. These models were extended with interaction terms, to assess whether between-group differences varied by subgroup. Selected analyses were repeated in a per-protocol population of participants who attended at least eight BeatIt therapy sessions or at least six StepUp sessions.
For the economic analyses, a UK National Health Service (NHS) and personal social services perspective was taken.27 Hospital and community resource use was collected with the Client Service Receipt Inventory questionnaire (CSRI).28 The CSRI was adapted, on the basis of a similar previous study for a population with learning disabilities, in conjunction with the clinical team.29 Data on therapist and supervisor time incurred and mileage travelled were collected by use of timesheets. Information on medication use was collected by use of a medication inventory. Costs for prescribed medication were taken from the British National Formulary (BNF); other resource costs were taken from the Personal Social Services Research Unit (PSSRU) Unit Costs,30 NHS reference costs,27 and other relevant literature.29 The costs of intervention materials were obtained from the research team. Resources were valued at 2015 costs in pounds sterling, and any costs that needed uplifting (ie, adjusting for inflation) were adjusted by use of the Hospital and Community Health Services (HCHS) index.31 We measured effectiveness in terms of quality-adjusted life-years (QALYs), via the EQ-5D-Y.20 This questionnaire is aimed at young people aged 8 years and older and is adapted directly from the EQ-5D-3L with simplified wording. Since no youth-specific tariff values exist, adult tariffs were used.32 Patient-specific QALYs were estimated from the utility measurements at each follow-up point assuming linear interpolation.
Cost-effectiveness was assessed by use of the cost-utility approach,33 with the difference in mean cost and QALYs between groups compared with regression techniques. Multiple imputaion with chained equations was used to handle missing data34 and uncertainty handled by bootstrapping 1000 non-parametric resamples with results presented on the cost-effectiveness plane and cost-effectiveness acceptability curve. All analyses were done with Stata, version 14.0.
This trial is registered with ISCRTN, number ISRCTN09753005.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. AJ, AM, and RZ had full access to all the data in the study, and AJ had final responsibility for the decision to submit for publication.
Results
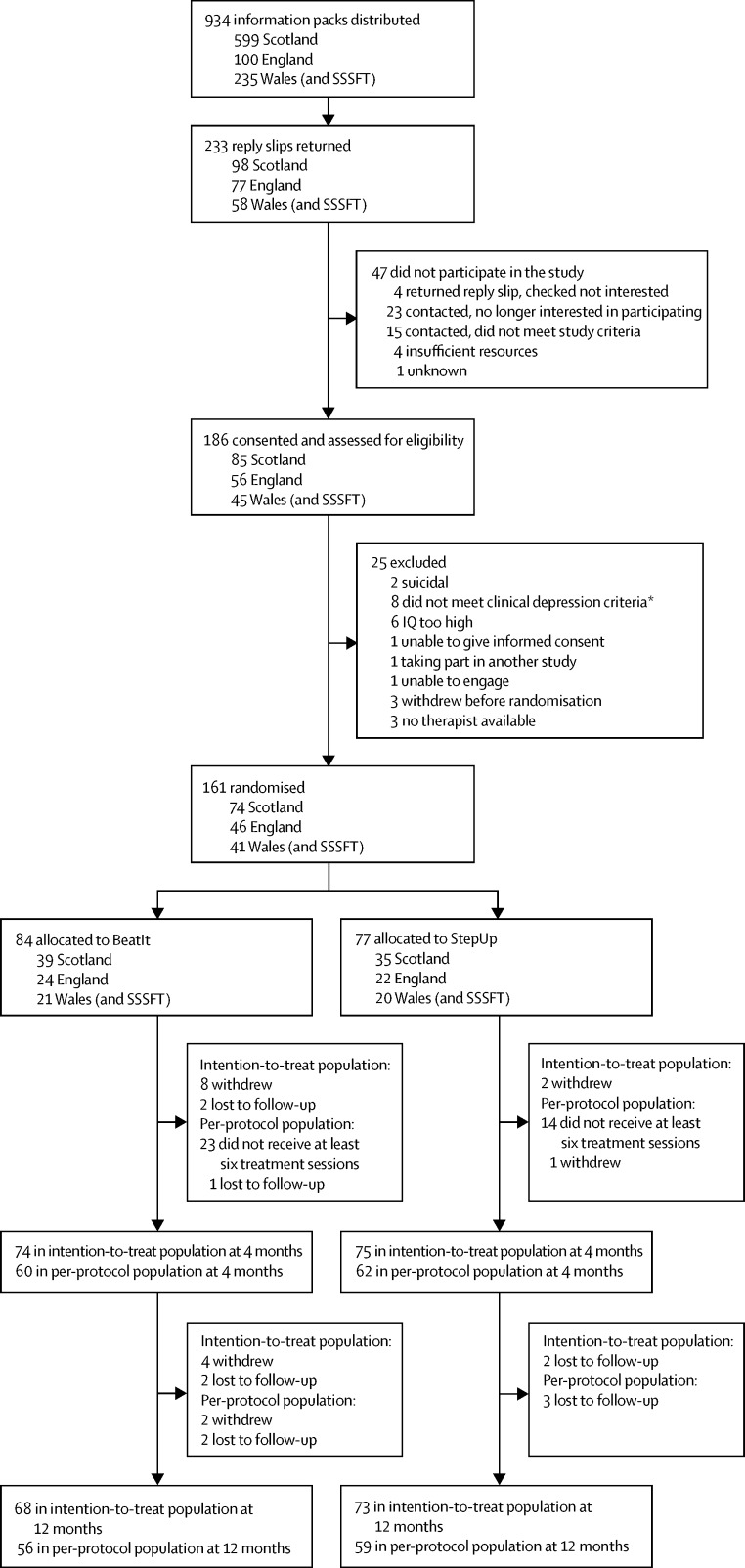
Between Aug 8, 2013, and Sept 1, 2015, 161 participants were recruited and randomly assigned: 84 to BeatIt and 77 to StepUp (figure 1). Of these, 68 (81%) BeatIt and 73 (95%) StepUp participants completed the trial by providing data at the primary endpoint. 56 (67%) BeatIt participants and 59 (77%) StepUp participants completed therapy as defined per protocol. BeatIt participants attended an average of 9·9 sessions (SD 3·2) and StepUp participants an average of 7·1 (2·1). 12 participants withdrew from the BeatIt group compared with two from the StepUp group. However, three of the withdrawals in the BeatIt group happened before participants started therapy and another BeatIt participant withdrew when they were contacted for the 12-month follow-up visit, after the intervention had finished.
Figure 1.
Trial profile
SSSFT=South Staffordshire and Shropshire Healthcare NHS Foundation Trust. IQ=intelligence quotient. Participants recruited within SSSFT were randomly assigned through the Wales study site. *Includes one participant initially allocated to StepUp in error. Data were removed from analysis.
Participants' characteristics were well balanced between the groups at baseline (table 1). The mean full scale IQ scores were 55·44 (SD 8·02) for BeatIt participants and 58·34 (8·38) for StepUp participants. Most participants were receiving support from services at least daily and were being prescribed antidepressant medication (table 1).
Table 1.
Participants' characteristics at baseline
| BeatIt (n=84) | StepUp (n=77) | ||
|---|---|---|---|
| Age, years | 40·3 (11·7) | 40·1 (12·0) | |
| Sex | |||
| Male | 38 (45·2%) | 38 (49·4%) | |
| Female | 46 (54·8%) | 39 (50·6%) | |
| Ethnicity* | |||
| White | 81 (97·6%) | 75 (98·7%) | |
| Other | 2 (2·4%) | 1 (1·3%) | |
| Marital status† | |||
| Married or cohabiting | 5 (6·0%) | 7 (9·3%) | |
| Separated, divorced, or widowed | 6 (7·2%) | 1 (1·3%) | |
| Single | 73 (86·9%) | 67 (89·3%) | |
| Deprivation decile | 4·5 (2·6) | 3·8 (2·1) | |
| Previous psychological therapies for depression | |||
| Yes | 17 (20·2%) | 14 (18·2%) | |
| No | 67 (79·8%) | 63 (81·8%) | |
| Intelligence quotient (IQ) | |||
| Verbal | 58·87 (8·67) | 63·14 (10·15) | |
| Performance | 57·84 (9·18) | 58·45 (8·11) | |
| Full scale | 55·44 (8·02) | 58·34 (8·38) | |
| Support with living | |||
| Less than daily support | 25 (29·8%) | 24 (31·2%) | |
| Daily support (contact at some point 7 days per week) | 59 (70·2%) | 53 (68·8%) | |
| Vision | |||
| Visual impairment | 55 (65·5%) | 45 (58·4%) | |
| No visual impairment | 29 (34·5%) | 32 (41·6%) | |
| Hearing | |||
| Hearing impairment | 20 (23·8%) | 8 (10·4%) | |
| No hearing impairment | 64 (76·2%) | 69 (89·6%) | |
| Mobility | |||
| Mobility problems | 19 (22·6%) | 20 (26·0%) | |
| No mobility problems | 65 (77·4%) | 57 (74·0%) | |
| Anti-epileptic medication | |||
| Yes | 4 (4·8%) | 9 (11·7%) | |
| No | 80 (95·2%) | 68 (88·3%) | |
| Antidepressants | |||
| Yes | 53 (63·1%) | 51 (66·2%) | |
| No | 31 (36·9%) | 26 (33·8%) | |
| Mood stabilisers | |||
| Yes | 11 (13·1%) | 15 (19·5%) | |
| No | 73 (86·9%) | 62 (80·5%) | |
Data are mean (SD) or n (%).
Two participants declined to respond (n=83 for BeatIt, n=76 for StepUp).
Two participants declined to respond (n=84 for BeatIt, n=75 for StepUp).
42 therapists delivered BeatIt and 34 therapists delivered StepUp. Most therapists were community intellectual disabilities nurses (51 [67%] of 76), were experienced in working with people who have intellectual disabilities (48 [63%] for 5 years or more), and had received some previous therapy training (46 [61%]). The mean ratio of sessions to supervisions delivered was 1·90 (SD 1·36). The expected ratio was 2·00, and a mean of less than 2·00 indicates a slightly higher than expected level of supervision.
Fidelity recordings were obtained from 44 (52%) of 84 BeatIt and 49 (63%) of 77 StepUp participants. No significant differences were observed in baseline scores for GDS-LD, IDDS, BPI-S (aggressive behaviour scale), and the age or sex of the 93 participants with fidelity recordings and of the 68 participants without recordings. The scale had a Cronbach's alpha of 0·76 and a mean adjusted item-total correlation of 0·42 (SD 0·10; range 0·31–0·58). The stability of the fidelity score was explored with the first and second fidelity ratings for the 51 participants with two recordings. The first fidelity rating for these participants had a mean score of 33·6 (SD 3·6) and the second rating had a mean score of 33·7 (3·7); this difference was not significant (p=0·89). The scores for the first and second ratings were correlated (Pearson's r 0·64, p<0·0001).
Fidelity scores did not differ significantly between the two groups (32·3 [SD 4·1] for BeatIt, 33·9 [3·9] for StepUp; p=0·13). In terms of fidelity to the manual, 33 (75%) of 44 BeatIt therapists and 42 (86%) of 49 StepUp therapists whose data were included in the fidelity assessments obtained maximum scores. Measures of therapy quality showed high scores for empathy (maximum score 4; BeatIt score 3·55 [SD 0·5]; StepUp score 3·61 [0·57]) and warmth (BeatIt score 3·68 [SD 0·47]; StepUp score 3·61 [0·64]) by both sets of therapists.
Primary and secondary study outcome data at baseline, 4 months, and 12 months after randomisation and estimated between-group differences at follow-up are presented in table 2. We found no evidence of a difference in treatment effect between BeatIt and StepUp based on the GDS-LD scores at 12 months (12·03 GDS-LD points [SD 7·99] with BeatIt; 12·43 GDS-LD points [7·64] with StepUp; mean difference 0·26 GDS-LD points [95% CI −2·18 to 2·70]; p=0·833). Secondary outcomes (table 2) were consistent with the findings for the primary outcome, with no statistically significant between-group differences at 4 months and 12 months. One exception was the ABS-socialisation score at 4 months, which was lower in the Step-Up group than in the BeatIt group at 4 months (p=0·017) but similar at 12 months (p=0·750). Analyses of GDS-LD scores were repeated with multiple imputation for missing outcome data, and analyses of primary and secondary outcomes were repeated for the per-protocol population. Although the estimates for effects changed slightly, the overall pattern of findings was similar (appendix).
Table 2.
Outcomes after intervention (4 months) and at follow-up (12 months)
| BeatIt | StepUp | Between-group difference* | p value | ICC | |
|---|---|---|---|---|---|
| GDS-LD | |||||
| Baseline | 16·60 (7·91) | 16·90 (6·73) | .. | .. | .. |
| After intervention (4 months) | 11·91 (7·43) | 12·94 (7·77) | −0·75 (−2·80 to 1·31) | 0·471 | 0·022 |
| Follow-up (12 months) | 12·03 (7·99) | 12·43 (7·64) | 0·26 (−2·18 to 2·70) | 0·833 | 0·085 |
| IDDS | |||||
| Baseline | 83·87 (32·70) | 73·57 (31·37) | .. | .. | .. |
| After intervention (4 months) | 60·37 (29·01) | 59·66 (34·13) | −4·49 (−13·72 to 4·75) | 0·336 | <0·001 |
| Follow-up (12 months) | 61·02 (31·82) | 55·02 (30·06) | 1·36 (−9·19 to 11·92) | 0·797 | 0·079 |
| GAS-ID | |||||
| Baseline | 25·05 (11·15) | 24·71 (11·00) | .. | .. | .. |
| After intervention (4 months) | 20·96 (11·18) | 21·39 (11·70) | −0·77 (−3·38 to 1·84) | 0·559 | <0·001 |
| Follow-up (12 months) | 20·77 (11·36) | 20·07 (11·15) | 0·43 (−2·59 to 3·45) | 0·776 | 0·143 |
| BPI-S | |||||
| Baseline | 1·96 (2·74) | 2·10 (3·61) | .. | .. | .. |
| After intervention (4 months) | 1·24 (2·19) | 2·09 (3·84) | −0·65 (−1·55 to 0·25) | 0·154 | 0·066 |
| Follow-up (12 months) | 1·09 (1·85) | 1·82 (3·42) | −0·67 (−1·46 to 0·12) | 0·093 | <0·001 |
| EQ-5D-Y | |||||
| Baseline | 0·46 (0·44) | 0·62 (0·38) | .. | .. | .. |
| After intervention (4 months) | 0·64 (0·41) | 0·69 (0·36) | 0·01 (−0·12 to 0·14) | 0·878 | 0·084 |
| Follow-up (12 months) | 0·68 (0·41) | 0·70 (0·35) | 0·04 (−0·08 to 0·15) | 0·546 | <0·001 |
| EQ-5D-VAS | |||||
| Baseline | 46·56 (28·63) | 56·96 (29·53) | .. | .. | .. |
| After intervention (4 months) | 48·72 (28·21) | 56·64 (29·61) | 8·15 (−1·16 to 17·45) | 0·085 | <0·001 |
| Follow-up (12 months) | 49·23 (28·76) | 57·36 (28·92) | 6·75 (−3·07 to 16·56) | 0·174 | 0·002 |
| ICI (total) | |||||
| Baseline | 49·81 (15·94) | 51·26 (17·35) | .. | .. | .. |
| After intervention (4 months) | 53·71 (16·80) | 51·47 (17·44) | 3·23 (−0·33 to 6·78) | 0·074 | <0·001 |
| Follow-up (12 months) | 51·45 (15·34) | 48·52 (16·95) | 3·11 (−0·68 to 6·91) | 0·106 | <0·001 |
| IPDL | |||||
| Baseline | 19·07 (8·46) | 18·19 (9·03) | .. | .. | .. |
| After intervention (4 months) | 18·34 (9·24) | 18·46 (8·45) | −0·51 (−2·16 to 1·14) | 0·539 | <0·001 |
| Follow-up (12 months) | 16·47 (8·01) | 15·86 (7·97) | 0·75 (−1·11 to 2·61) | 0·424 | 0·003 |
| SSQ3, size of support network | |||||
| Baseline | 4·89 (3·2) | 4·62 (2·6) | .. | .. | .. |
| After intervention (4 months) | 5·31 (2·95) | 4·92 (2·99) | 0·09 (−0·68 to 0·86) | 0·823 | 0·039 |
| Follow-up (12 months) | 4·92 (2·91) | 4·71 (2·74) | 0·04 (−0·79 to 0·86) | 0·932 | 0·001 |
| SSQ3, satisfaction | |||||
| Baseline | 2·48 (0·53) | 2·62 (0·55) | .. | .. | .. |
| After intervention (4 months) | 2·59 (0·49) | 2·65 (0·53) | −0·02 (−0·17 to 0·12) | 0·741 | <0·001 |
| Follow-up (12 months) | 2·62 (0·50) | 2·66 (0·44) | 0·001 (−0·15 to 0·15) | 0·987 | <0·001 |
| ABS, socialisation | |||||
| Baseline | 20·92 (3·55) | 20·75 (3·12) | .. | .. | .. |
| After intervention (4 months) | 21·37 (3·19) | 20·20 (3·57) | 1·11 (0·20 to 2·02) | 0·017 | <0·001 |
| Follow-up (12 months) | 21·59 (3·17) | 21·47 (2·52) | 0·14 (−0·72 to 0·10) | 0·750 | <0·001 |
| ABS, self-direction | |||||
| Baseline | 15·38 (5·41) | 14·60 (5·49) | .. | .. | .. |
| After intervention (4 months) | 16·87 (4·68) | 15·54 (4·83) | 0·98 (−0·15 to 2·12) | 0·089 | <0·001 |
| Follow-up (12 months) | 16·94 (4·50) | 16·59 (4·28) | 0·13 (−1·13 to 1·39) | 0·838 | <0·001 |
| ABS, responsibility | |||||
| Baseline | 8·27 (1·77) | 7·62 (2·10) | .. | .. | .. |
| After intervention (4 months) | 8·28 (1·84) | 7·72 (2·02) | −0·16 (−0·59 to 0·27) | 0·452 | <0·001 |
| Follow-up (12 months) | 8·20 (1·76) | 8·40 (1·51) | −0·44 (−0·92 to 0·05) | 0·076 | 0·035 |
| EDSE | |||||
| Baseline | 20·73 (4·98) | 21·04 (4·68) | .. | .. | .. |
| After intervention (4 months) | 22·45 (4·06) | 22·01 (4·80) | 0·86 (−0·44 to 2·16) | 0·192 | 0·025 |
| Follow-up (12 months) | 21·98 (4·34) | 22·67 (3·83) | −0·36 (−1·60 to 0·83) | 0·527 | <0·001 |
| BLESID | |||||
| Baseline | 2·04 (2·35) | 1·79 (2·03) | .. | .. | .. |
| Follow-up (12 months) | 1·56 (1·86) | 1·29 (1·72) | 0·25 (−0·34 to 0·83) | 0·406 | <0·001 |
Data are mean (SD) or mean (95% CI). ICC=Intraclass Correlation Coefficient for clustering within therapists. GDS-LD=Glasgow Depression Scale for people with Learning Disabilities. IDDS=Intellectual Disabilities Depression Scale. GAS-ID=Glasgow Anxiety Scale for People with Intellectual Disabilities. BPI-S=Behaviour Problems Inventory for Individuals with Intellectual Disabilities short form. EQ-5D-Y=EuroQol five-dimensional questionnaire, Youth version. EQ-5D-VAS=EuroQol five-dimensional questionnaire Visual Analogue Scale. ICI=Index of Community Involvement. IPDL=Index of Participation in Domestic Life. SSQ3=Social Support Questionnaire. ABS=Adaptive Behaviour Scale, Residential and Community: second edition. EDSE=Emotional Difficulties Self-Efficacy Scale. BLESID=Bangor Life Events Schedule for Intellectual Disabilities.
Models adjusted for baseline scores, antidepressant use, and site, with therapist as random effect.
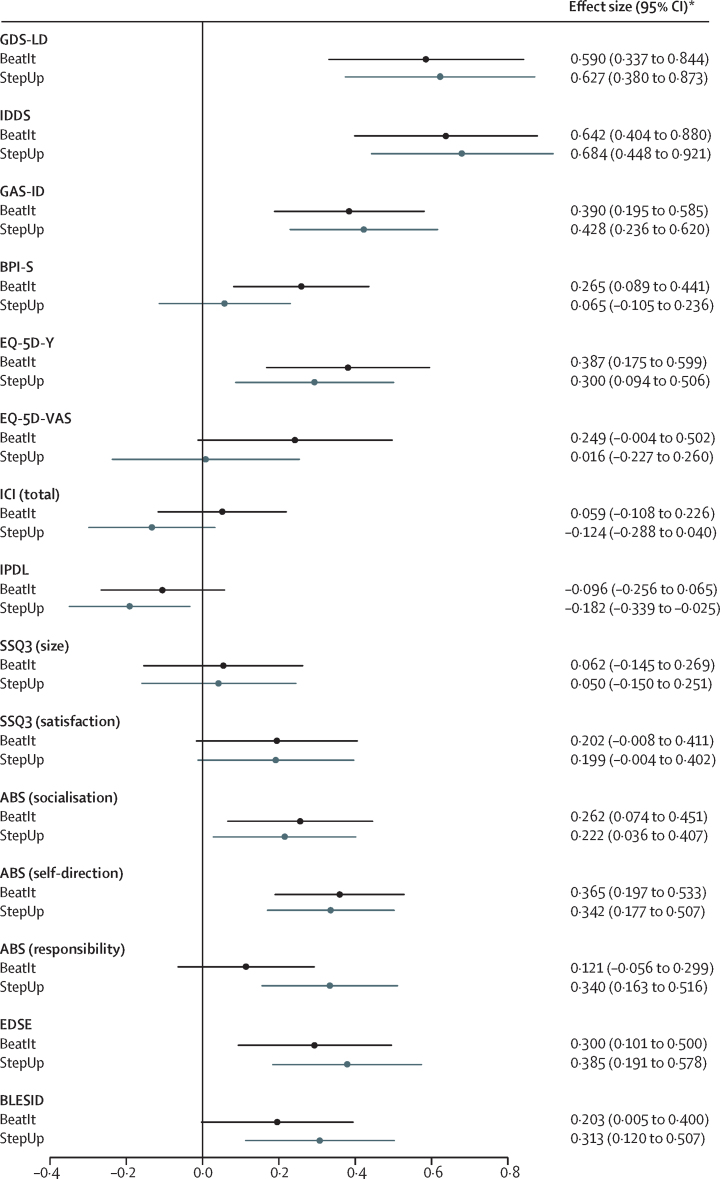
Compared with baseline, significant improvements in the primary outcome were observed for both study groups at 4 months (BeatIt, mean change −5·1 GDS-LD points [95% CI −6·7 to −3·6], p<0·0001; StepUp, mean change −4·4 GDS-LD points [–5·9 to −2·9], p<0·0001) and at 12 months (BeatIt, mean change −4·2 GDS-LD points [–6·0 to −2·4], p<0·0001; StepUp, mean change −4·5 GDS-LD points [–6·2 to −2·7], p<0·0001), with large effect sizes, as shown in figure 2. Repeated measures analysis found no evidence of a change in mean GDS-LD scores between 4 months and 12 months (p=0·66), and no evidence of a change in the between-group difference over time (treatment × time interaction, p=0·68). The intraclass correlation observed in the primary outcome at 12 months (0·085; table 2) was higher than assumed in the sample size calculation. However, given the greater number of therapists involved in the trial, and the smaller number of participants per therapist, the design effect due to clustering was 1·10 rather than 1·20 according to our initial assumptions, so this outcome did not adversely affect the power of the study.
Figure 2.
Effect estimates and 95% CIs at 12 months
The forest plot shows effect sizes and 95% CIs for the primary and secondary outcomes for both groups of the study. Effect sizes were calculated as the estimated between-group differences for each outcome at each timepoint, divided by the SD of the outcome measure at baseline across all participants. Measurements were taken at baseline and 12 months. GDS-LD=Glasgow Depression Scale for people with Learning Disabilities. IDDS=Intellectual Disabilities Depression Scale. GAS-ID=Glasgow Anxiety Scale for people with Intellectual Disabilities. BPI-S=Behaviour Problems Inventory for Individuals with Intellectual Disabilities, short form. EQ-5D-Y=EuroQol five-dimensional questionnaire, Youth version. EQ-5D-VAS=EuroQol five-dimensional questionnaire Visual Analogue Scale. ICI=Index of Community Involvement. IPDL=Index of Participation in Domestic Life. SSQ3=Social Support Questionnaire. ABS=Adaptive Behavior Scale (Residential and Community: second edition). EDSE=Emotional Difficulties Self-Efficacy Scale. BLESID=Bangor Life Events Schedule for Intellectual Disabilities. *Where a decrease in score indicated a positive change, effect sizes were reported as positives (GDS-LD, IDDS, GAS-ID, BPI-S, and BLESID had negative effect sizes, indicating a positive change).
This pattern of within-group change was mirrored by improvements in secondary measures of emotional difficulties and quality of life (IDDS, GAS-ID, and EQ-5D-Y) for both interventions. Once again, these changes were observed at 4 months after the intervention and maintained until the 12 month follow-up (table 2).
Carers' confidence in supporting participants with their emotional difficulties (EDSE scores) significantly improved for both groups at the 4 month follow-up, after the intervention, and was maintained at the 12 month follow-up (table 2). Finally, the negative effect of life events (BLESID) for both groups of participants was also reduced at the 12 month follow-up.
No deaths were reported during the course of the study. 19 adverse events were reported by 15 participants allocated to StepUp and 21 adverse events reported by 18 allocated to BeatIt. None of the 24 serious adverse events were treatment or study related and they were all hospital admissions. Four BeatIt and five StepUp participants were admitted to a mental health ward, of whom two BeatIt and three StepUp participants presented with suicidal ideation. One attempted overdose was reported during the trial. The participant had been randomised but had not started therapy.
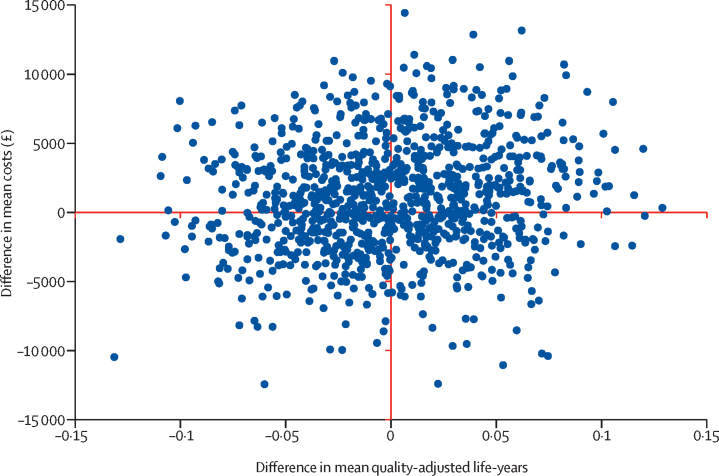
For the health economic analysis, missing data were less than 5% in both resource use and EQ-5D-Y questionnaires. A significant difference in baseline utilities between groups favoured StepUp. However, at the 4 month and 12 month follow-ups no significant difference in utilities was observed. After adjustment for missing data and baseline imbalance, the initial QALY gain favouring StepUp was reversed to favour BeatIt, although neither result was significant. Mean intervention costs differed significantly between groups, with BeatIt being around £769 (95% CI £622–917) more expensive than StepUp. However, we found no significant difference in non-intervention costs or total costs (table 3; appendix). The incremental costs for StepUp versus BeatIt were £1593 (standard error £1827); the incremental adjusted QALYs were −0·002 (standard error 0·043); and the incremental cost-effectiveness ratio showed that BeatIt dominates (table 3). Although the adjusted multiple imputation results suggested that BeatIt dominates, the associated 95% CI ranges from BeatIt dominating to being dominated by StepUp, indicating substantial uncertainty. A dominated intervention is more costly and less effective than the comparator. The only certainty in the results is that the BeatIt intervention is more costly than StepUp. Exclusion of non-intervention costs and calculation of an incremental cost-effectiveness ratio (ICER) solely with intervention costs for BeatIt compared with StepUp results in an ICER of £385 000 per QALY gained (95% CI £8000 to dominated), which would not be considered cost-effective. The cost-effectiveness plane in figure 3 shows that incremental costs and QALYs are spread in all quadrants, indicating that there is uncertainty in the results (see the appendix for the cost-effectiveness acceptability curve).
Table 3.
Outcomes of health economics analyses (adjusted multiple imputation)
| StepUp | BeatIt | Difference | p values | 95% CI | |
|---|---|---|---|---|---|
| Intervention costs | £1019 (£36·40) | £1789 (£65·90) | −£769 (£75·10) | 0·000 | −£917 to –£622 |
| Non-intervention costs | £27 181 (£2491) | £24 630 (£2503) | £2552 (£1807) | 0·160 | −£1021 to £6215 |
| Total costs* | £27 962 (£2347) | £26 369 (£2382) | £1593 (£1827) | 0·384 | −£2008 to £5194 |
| Total QALYs† | 0·655 (0·029) | 0·657 (0·031) | −0·002 (0·043) | 0·965 | −0·085 to 0·082 |
Data are mean (standard error). QALY=quality-adjusted life-year. Cost data adjusted by use of multivariable generalised linear model; gamma family and identity link. QALY data adjusted by use of multivariable generalised linear model; Gauss family and identity link.
Original sample sizes: StepUp, n=68 (93·2%); BeatIt, n=58 (85·3%).
Original sample sizes: StepUp, n=68 (93·2%); BeatIt, n=61 (89·7%).
Figure 3.
Cost-effectiveness plane (multiple imputation, adjusted results)
Discussion
We found no evidence of the effectiveness of BeatIt compared with StepUp, on primary or secondary outcomes. Both interventions were associated with significant reductions in depressive symptoms. Within-group improvements were observed at 4 months after randomisation (at the end of treatment) and were maintained until the 12-month follow-up. The same pattern was found for secondary measures of mental health and wellbeing, with moderate to large effect sizes.
Given the similar outcomes for both interventions, it was unsurprising that we found no evidence that BeatIt is cost-effective when compared with StepUp. No differences in resource use were found at 12 months, although BeatIt did cost more to deliver than StepUp. However, overall, most of the support costs for participants in both treatment groups were not related to the treatments themselves: intervention costs were approximately 3·6–6·8% of total support costs for participants.
A key question when interpreting these findings is whether the associated improvements in depressive symptoms simply reflect spontaneous recovery. The background characteristics of study participants indicate that many had unsuccessful treatment histories. Approximately two thirds of participants in both groups were taking antidepressants, and 20% allocated to BeatIt and 18% allocated to StepUp had received psychological therapy before enrolling in the trial, which suggests that these participants might have been at the severe and enduring end of the depression spectrum. It is known from UK birth cohorts that adults with intellectual disabilities have a more severe and enduring pattern of depression than the general population.3 Moreover, the results of six small pilot trials35, 36, 37, 38, 39, 40 of psychological interventions for depression in people with mild to moderate intellectual disabilities reviewed by NICE27 reported that for participants receiving treatment as usual, in all six studies, there was virtually no change in depression scores from baseline to follow-up, including up to 11 months after baseline. Consequently, it seems unlikely (although it cannot be ruled out) that the marked improvements in self-reported depressive symptoms in both BeatIt and StepUp groups were due to spontaneous recovery of symptoms.
No related serious adverse events were reported for either of the intervention groups. Although there were few withdrawals overall, there were more from the BeatIt group than from StepUp. This difference might, in part, reflect the greater number of BeatIt sessions and the higher expectations made of BeatIt participants, with the aim of supporting participants and supporters to engage in scheduled activities between sessions.
This study is, to our knowledge, the first randomised controlled trial of behavioural activation for people with intellectual disabilities and depression, and provides the first data collected about the use of guided self-help in this population. This study is also the first large-scale randomised controlled trial of any individually delivered psychological therapy for a mental health problem in people with intellectual disabilities. Overall, the successful delivery of the trial refutes a widely held contention that it is not possible to recruit people with intellectual disabilities into large-scale randomised controlled trials of individual psychological therapies.41
The main limitation of the trial was the absence of a treatment as usual condition. It might have been more difficult to recruit participants and deliver the intervention if participants faced the prospect of being randomly assigned to treatment as usual, especially since they were recruited through intermediaries who acted as gatekeepers, seeking help for those they support or those with whom they work. One of the main reasons a guided self-help intervention was selected as the comparison group was because it was seen as a less person-centred and more rigid psychoeducational approach than BeatIt, with a different set of active components from behavioural activation. What was not anticipated was the ability of therapists to personalise the materials, as highlighted in the strong fidelity scores for non-specific therapy factors, and the agency shown by the participants and their supporters to follow through on ideas and plans made in therapy sessions, as suggested in the nested qualitative studies to be reported elsewhere.
A potential limitation of the trial was the differential follow-up rate in the two randomised groups (81% for BeatIt vs 95% for StepUp). Some baseline characteristics were associated with dropout (eg, female sex, higher IPDL scores, higher ABS [responsibility] scores), but none of these characteristics translated into significant baseline differences between randomised groups for those with follow-up data (table 2). With the exception of EQ-5D-Y, we did not observe any between-group differences for study outcome measures at baseline, for those included in either the intention-to-treat or per-protocol analyses, and all analyses were adjusted for baseline values of the outcome being analysed, so differential follow-up is unlikely to have biased our results to a substantial degree.
In conclusion, the lessons learned from this study have important implications for the conduct of future trials of psychological therapies involving people with intellectual disabilities. The successful delivery of this trial, carried out in existing services, shows the possibility of training professional groups, who are already working with people who have intellectual disabilities and depression, to deliver focused psychological interventions such as behavioural activation and guided self-help. This implementation work is a priority and might help to address the inequities faced by people with intellectual disabilities, who often do not have access to psychological therapies for commonly occurring mental health problems.
For the online version of the British National Formulary see https://www.medicinescomplete.com
Acknowledgments
Acknowledgments
This independent research study was funded by the UK National Institute for Health Research Health Technology Assessment Programme. The views expressed in this publication are those of the authors and not necessarily of the National Institute for Health Research or the Departments of Health in Scotland, England, or Wales. We thank all NHS and social care services in Scotland, England, and Wales who contributed to the study—the therapists and study researchers. Most of all, we express our gratitude to the participants who took part in the study along with their supporters.
Contributors
AJ, CM, RH, CH, S-AC, DD, AM, RJ, CW, and AB designed and managed the study. KA, KS, LF, RK, and DK were responsible for data collection and trial management. AM, RZ, NM, and DD did the data analysis. IS, KM, HL, and GT provided expert advice on service, clinical, and patient-related matters. All authors contributed to writing and editing the paper.
Declaration of interests
AJ, CM, RH, CH, S-AC, DD, AM, RJ, CW, and AB all report receiving grant funding from the National Institute for Health Research during the course of the study. All other authors declare no competing interests.
Supplementary Material
References
- 1.Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. Mental ill-health in adults with intellectual disabilities: prevalence and associated factors. Br J Psychiatry. 2007;190:27–35. doi: 10.1192/bjp.bp.106.022483. [DOI] [PubMed] [Google Scholar]
- 2.Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32:419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Collishaw S, Maughan B, Pickles A. Affective problems in adults with mild learning disability: the roles of social disadvantage and ill health. Br J Psychiatry. 2004;185:350–351. doi: 10.1192/bjp.185.4.350. [DOI] [PubMed] [Google Scholar]
- 4.NICE . National Institute for Health and Care Excellence; London: 2016. Mental health problems in people with learning disabilities: prevention, assessment and management. NICE guideline NG54. [PubMed] [Google Scholar]
- 5.Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif. 2011;35:111–161. doi: 10.1177/0145445510390929. [DOI] [PubMed] [Google Scholar]
- 6.Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. Br J Psychiatry. 2011;198:66–72. doi: 10.1192/bjp.bp.110.079111. [DOI] [PubMed] [Google Scholar]
- 7.Richards DA, Ekers D, McMillan D. Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet. 2016;388:871–880. doi: 10.1016/S0140-6736(16)31140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobson KS, Dimidjian S, Kohlenberg RJ. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney P, Jackman C, Coyle D, O'Reilly G. Computerised cognitive-behavioural therapy for adults with intellectual disability: randomised controlled trial. Br J Psychiatry. 2017;211:95–102. doi: 10.1192/bjp.bp.117.198630. [DOI] [PubMed] [Google Scholar]
- 10.Jahoda A, Melville CA, Pert C. A feasibility study of behavioural activation for depressive symptoms in adults with intellectual disabilities. J Intellect Disabil Res. 2015;59:1010–1021. doi: 10.1111/jir.12175. [DOI] [PubMed] [Google Scholar]
- 11.Cuthill FM, Espie CA, Cooper SA. Development and psychometric properties of the Glasgow Depression Scale for people with a learning disability: individual and carer supplement versions. Br J Psychiatry. 2003;182:347–353. doi: 10.1192/bjp.182.4.347. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D. NCS Pearson; San Antonio, TX: 2011. Wechsler abbreviated scale of intelligence, second edition (WASI-II) [Google Scholar]
- 13.Cooper S-A, Melville CA, Einfeld SL. Psychiatric diagnosis, intellectual disabilities and diagnostic criteria for psychiatric disorders for use with adults with learning disabilities/mental retardation (DC-LD) J Intellect Disabil Res. 2003;47(suppl 1):3–15. doi: 10.1046/j.1365-2788.47.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 14.Jahoda A, Melville C, Cooper S-A. BEAT-IT: comparing a behavioural activation treatment for depression in adults with intellectual disabilities with an attention control: study protocol for a randomised controlled trial. Trials. 2015;16:595. doi: 10.1186/s13063-015-1103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melville C, Cooper S-A, Jahoda A, Pert C. University of Glasgow; Glasgow: 2009. Guided self help package for people with learning disabilities and low mood. [Google Scholar]
- 16.Jahoda A, Melville C, Pert C. University of Glasgow; Glasgow: 2012. A behavioural activation treatment manual for people with learning disabilities and depression. [Google Scholar]
- 17.Evans KM, Cotton MM, Einfeld SL, Florio T. Assessment of depression in adults with severe or profound intellectual disability. J Intellect Dev Disabil. 1999;24:147–160. [Google Scholar]
- 18.Rojahn J, Rowe EW, Sharber AC. The Behavior Problems Inventory-Short Form for individuals with intellectual disabilities: part II: reliability and validity. J Intellect Disabil Res. 2012;56:546–565. doi: 10.1111/j.1365-2788.2011.01506.x. [DOI] [PubMed] [Google Scholar]
- 19.Mindham J, Espie CA. Glasgow Anxiety Scale for people with an Intellectual Disability (GAS-ID): development and psychometric properties of a new measure for use with people with mild intellectual disability. J Intellect Disabil Res. 2003;47:22–30. doi: 10.1046/j.1365-2788.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 20.Wille N, Badia X, Bonsel G. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res. 2010;19:875–886. doi: 10.1007/s11136-010-9648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raynes N, Sumpton R, Pettipher C. Manchester University, Department of Social Policy and Social Work; Manchester: 1989. The index of community involvement. [Google Scholar]
- 22.Raynes N, Sumpton R, Pettipher C. Manchester University, Department of Social Policy and Social Work; Manchester: 1989. Index of participation in domestic life. [Google Scholar]
- 23.Hastings RP, Brown T. Behavior problems of children with autism, parental self-efficacy, and mental health. Am J Ment Retard. 2002;107:222. doi: 10.1352/0895-8017(2002)107<0222:BPOCWA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Nihira K, Lambert NM, Leland H. Pro-Ed; Austin, TX: 1993. AAMR adaptive behavior scale: residential and community. Examiner's manual. [Google Scholar]
- 25.Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: practical and theoretical implications. J Soc Pers Relat. 1987;4:497–510. [Google Scholar]
- 26.Hulbert-Williams L, Hastings RP, Crowe R, Pemberton J. Self-reported life events, social support and psychological problems in adults with intellectual disabilities. J Appl Res Intellect Disabil. 2011;24:427–436. [Google Scholar]
- 27.NICE Guide to the methods of technology appraisal. April, 2013. https://www.nice.org.uk/article/pmg9/chapter/foreword (accessed Oct 29, 2015).
- 28.Chisholm D, Knapp MRJ, Knudsen HC. Client socio-demographic and service receipt inventory—European version: development of an instrument for international research—EPSILON Study 5. Br J Psychiatry. 2000;177:S28–S33. doi: 10.1192/bjp.177.39.s28. [DOI] [PubMed] [Google Scholar]
- 29.Willner P, Rose J, Jahoda A. A cluster randomised controlled trial of a manualised cognitive-behavioural anger management intervention delivered by supervised lay therapists to people with intellectual disabilities. Health Technol Assess. 2013;17:1–173. doi: 10.3310/hta17210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis L, Burns A. Unit costs of health and social care 2015, Personal Social Services Research Unit, University of Kent, Canterbury. http://www.pssru.ac.uk/project-pages/unit-costs/2015/ (accessed Aug 21, 2017).
- 31.Department of Health NHS reference costs 2014 to 2015. Nov 18, 2015. https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed Aug 21, 2017).
- 32.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Ramsey SD, Willke RJ, Glick H. Cost-effectiveness analysis alongside clinical trials II—an ISPOR good research practices task force report. Value in Health. 2015;18:161–172. doi: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Briggs A, Clark T, Wolstenholme J, Clarke P. Missing presumed at random: cost-analysis of incomplete data. Health Economics. 2003;12:377–392. doi: 10.1002/hec.766. [DOI] [PubMed] [Google Scholar]
- 35.McCabe MP, McGillivray JA, Newton DC. Effectiveness of treatment programmes for depression among adults with mild/moderate intellectual disability. J Intellect Disabil Res. 2006;50:239–247. doi: 10.1111/j.1365-2788.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 36.McGillivray JA, McCabe MP, Kershaw MM. Depression in people with intellectual disability: an evaluation of a staff-administered treatment program. Res Dev Disabil. 2008;29:524–536. doi: 10.1016/j.ridd.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Hassiotis A, Serfaty M, Azam K. Manualised individual cognitive behavioural therapy for mood disorders in people with mild to moderate intellectual disability: a feasibility randomised controlled trial. J Affect Disord. 2013;151:186–195. doi: 10.1016/j.jad.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 38.Hartley SL, Esbensen AJ, Shalev R, Vincent LB, Mihaila I, Bussanich P. Cognitive behavioral therapy for depressed adults with mild intellectual disability: a pilot study. J Ment Health Res Intellect Disabil. 2015;8:72–97. doi: 10.1080/19315864.2015.1033573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsay WR, Tinsley S, Beail N. A preliminary controlled trial of a trans-diagnostic programme for cognitive behaviour therapy with adults with intellectual disability. J Intellect Disabil Res. 2015;59:360–369. doi: 10.1111/jir.12145. [DOI] [PubMed] [Google Scholar]
- 40.McGillivray JA, Kershaw MM. The impact of staff initiated referral and intervention protocols on symptoms of depression in people with mild intellectual disability. Res Dev Disabil. 2013;34:730–738. doi: 10.1016/j.ridd.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Iacono T. Ethical challenges and complexities of including people with intellectual disability as participants in research. J Intellect Dev Disabil. 2006;31:173–179. doi: 10.1080/13668250600876392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.