Abstract
S,R(+/−)-3,4-methylenedioxymethamphetamine (SR-MDMA) is an amphetamine derivative with prosocial and putative therapeutic effects. Ongoing clinical trials are investigating it as a treatment for post-traumatic stress disorder (PTSD) and other conditions. However, its potential for adverse effects such as hyperthermia and neurotoxicity may limit its clinical viability. We investigated the hypothesis that one of the two enantiomers of SR-MDMA, R-MDMA, would retain the prosocial and therapeutic effects but with fewer adverse effects. Using male Swiss Webster and C57BL/6 mice, the prosocial effects of R-MDMA were measured using a social interaction test, and the therapeutic-like effects were assessed using a Pavlovian fear conditioning and extinction paradigm relevant to PTSD. Locomotor activity and body temperature were tracked after administration, and neurotoxicity was evaluated postmortem. R-MDMA significantly increased murine social interaction and facilitated extinction of conditioned freezing. Yet, unlike racemic MDMA, it did not increase locomotor activity, produce signs of neurotoxicity, or increase body temperature. A key pharmacological difference between R-MDMA and racemic MDMA is that R-MDMA has much lower potency as a dopamine releaser. Pretreatment with a selective dopamine D1 antagonist prevented SR-MDMA-induced hyperthermia, suggesting that differential dopamine signaling may explain some of the observed differences between the treatments. Together, these results indicate that the prosocial and therapeutic effects of SR-MDMA may be separable from the stimulant, thermogenic, and potential neurotoxic effects. To what extent these findings translate to humans will require further investigation, but these data suggest that R-MDMA could be a more viable therapeutic option for the treatment of PTSD and other disorders for which SR-MDMA is currently being investigated.
Keywords: MDMA, neurotoxicity, PTSD, prosocial, enantiomers
1. Introduction
S,R(+/−)-3,4-methylenedioxymethamphetamine (SR-MDMA) is a substituted phenethylamine with structural and functional similarities to amphetamine-like psychostimulants and mescaline-like hallucinogens. It was first synthesized in the early 20th century but was little studied until the late 1970’s when it became popular as a recreational drug and as an adjunct to psychotherapy (Greer and Tolbert, 1986). Escalating recreational use led to its prohibition, but scientific interest in the drug has persisted due to its unique prosocial effects and potential therapeutic utility. In human volunteers, SR-MDMA increases feelings of closeness towards others, empathy, and sociability (Bedi et al., 2010; Hysek et al., 2013). Recently, double-blind placebo controlled studies have been undertaken to assess the therapeutic efficacy of SR-MDMA (Mithoefer et al., 2011; Oehen et al., 2013). The first of these trials found that just two SR-MDMA-paired psychotherapy sessions significantly reduced the symptoms of post-traumatic stress disorder (PTSD), with a sustained clinical response in 83% of SR-MDMA-treated patients compared to just 25% of those treated with placebo-paired psychotherapy (Mithoefer et al., 2011). These therapeutic gains were mostly maintained upon long-term follow-up an average of 3.8 years later, with 87.5% of SR-MDMA-treated patients no longer meeting the diagnostic criteria for PTSD (Mithoefer et al., 2013). In 2016 the American Food and Drug Administration (FDA) approved a larger Phase III trial to expand testing of SR-MDMA for PTSD, and additional Phase II trials are underway testing SR-MDMA in patients with autism (NCT0200839) and end of life anxiety (NCT02427568).
Although the therapeutic potential of SR-MDMA is promising, debate remains over the possible dangers of using it as a therapeutic (Parrott, 2013). Sold as “Ecstasy” or “Molly”, SR-MDMA is among the most widely used illicit drugs (Center for Behavioral Health Statistics and Quality, 2015), and its recreational use is associated with serious short- and long-term adverse effects. The most dangerous acute effect is hyperthermia, which can occur following even modest doses, and is the most common cause of MDMA-related death (Green et al., 2003; Henry et al., 1992). Hyperthermia and other adverse effects are heavily influenced by the dose and environment, and their risks appear to be mitigated by administration of SR-MDMA in a controlled clinical setting (Liechti, 2014; Vizeli and Liechti, 2017). However, given the large variability in response to SR-MDMA, certain vulnerable individuals may still be at risk. Long-term the greatest concern is neurotoxicity. Although the extent of damage is debated, studies in laboratory animals have reliably demonstrated that SR-MDMA can be neurotoxic, producing long-lasting, potentially permanent brain dysfunction (Biezonski and Meyer, 2011; Capela et al., 2009). While most studies have utilized large repeated doses of SR-MDMA, neurotoxicity has also been observed in non-human primates following single moderate doses (Mueller et al., 2013). Neuroimaging studies of former recreational SR-MDMA users indicate that similar damage can occur in humans (Erritzoe et al., 2011; McCann et al., 2005), with functional consequences including neurocognitive deficits and increased rates of depression (Parrott, 2013; Rogers et al., 2009; Taurah et al., 2014). Signs of neurotoxicity have been correlated with the extent of SR-MDMA use, but it remains unclear whether users with low or moderate lifetime usage are affected or if neurotoxicity is a risk from the clinical use of SR-MDMA (J. H. Halpern et al., 2011; Mueller et al., 2016; Schilt et al., 2007). These potential risks and its questionable margin of safety (P. Halpern et al., 2011) may limit wider clinical use of the drug. There is thus impetus to develop safer medications with similar therapeutic benefits but fewer potential adverse side effects (Nichols et al., 1990).
SR-MDMA is a racemic mixture of two enantiomers: R-MDMA and S-MDMA. Little is known about their relative contributions to the prosocial and therapeutic effects of SR-MDMA, but while S- and SR-MDMA are both neurotoxic and capable of producing severe hyperthermia, there is some evidence that R-MDMA may lack these adverse effects (Frau et al., 2013; Schmidt et al., 1987). If that is the case, and R-MDMA has prosocial and therapeutic effects similar to SR-MDMA, it could potentially be a more viable therapeutic. Both enantiomers function primarily as monoamine releasers (Hiramatsu and Cho, 1990; Johnson et al., 1986; Setola et al., 2003), with S-MDMA being the more potent of the two and generally considered the “active isomer” (Anderson et al., 1978). Yet, the previous studies that evaluated the neurotoxicity of R-MDMA did not account for this lower potency. Since any prosocial or therapeutic effects would likely occur at a significantly higher dose than from SR-or S-MDMA, additional neurotoxic and thermogenic evaluation of R-MDMA is needed that accounts for this higher relative dose.
To test the prosocial effects of SR-MDMA and its enantiomers we utilized a social interaction test that has been previously used to measure the effects of SR-MDMA in rats (Morley and McGregor, 2000). To asses therapeutic-like effects we followed up on a previous study in mice that demonstrated a single treatment of SR- MDMA can facilitate extinction learning and a long-lasting decrease in conditioned fear behavior (Young et al., 2015). This effect is particularly relevant given the clinical interest in using SR-MDMA to treat PTSD, a disorder that is often conceptualized as a deficit of fear extinction learning (VanElzakker et al., 2014). Using this same model, we tested the effects of the enantiomers on facilitation of fear extinction. We next evaluated the neurotoxic and thermogenic effects of R-MDMA relative to SR-MDMA, using a binge dosing regimen that was scaled to their differing behaviorally effective doses. Neurotoxicity was assessed by quantifying reactive astrogliosis, a reliable and universal marker of CNS damage (O’Callaghan and Miller, 1994), as well as dopamine (DA) content and DA transporter (DAT) expression as markers of DA terminal pruning. Although in most species, including primates, SR-MDMA selectively damages serotonin (5-HT) neurons, in mice it produces long-lasting degeneration and dysfunction of DA neurons (Granado et al., 2008).
Finally, to begin assessing why R-MDMA may lack certain adverse effects observed with SR-MDMA, we tested the hypothesis that dopaminergic signaling is necessary for the hyperthermic effect of racemic MDMA. Although both enantiomers are monoamine releasers, unlike SR- and S-MDMA, R-MDMA does not increase extracellular DA at behaviorally relevant doses (Hiramatsu and Cho, 1990; Murnane et al., 2010). Differences in dopamine release could explain the distinct thermogenic effects of the drugs and potentially also their differing neurotoxicities. Together, this collection of studies in mice highlights the therapeutic potential of R-MDMA and demonstrates that the prosocial and therapeutic effects of SR-MDMA may be separable from some of its numerous adverse effects.
2. Methods
2.1. Animals
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) aged 10–16 weeks served as subjects in the fear conditioning experiments. Male Swiss Webster mice (Charles River Laboratories, Wilmington, MA) aged 7–10 weeks served as subjects in all other experiments. All mice entered the study drug-naïve with no prior behavioral testing or handing. Mice were housed five per cage in a temperature and humidity controlled colony room at the Yerkes National Primate Research Center with food and water available ad libitum. Lights were set to a 12-hour light/dark cycle. All experiments were performed at an ambient temperature of 22±2°C at 45–50% humidity, during the lights-on phase. All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals, and experimental protocols were approved by the Emory University Institutional Animal Care and Use Committee.
2.2. Drugs
S,R(+/−)-3,4-methylenedioxymethamphetamine (SR-MDMA), S(+)-3,4-methylenedioxymethamphetamine (S-MDMA), and R(−)-3,4-methylenedioxymethamphetamine (R-MDMA) were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC). R(+)-SCH23390 (SCH) was acquired from RBI (now Sigma-Aldrich Research Biochemicals; Natick, MA). Doses were calculated and are expressed as HCl salts. All drugs were dissolved in 0.9% sterile saline and administered via intraperitoneal injection at a volume of 10 ml/kg.
2.3. Behavioral Experiments
2.3.1. Social Interaction Testing
To familiarize subjects with the testing procedure and screen-out aggressive subjects, the social interaction test was performed twice. During the first session, subjects received injections of SR-MDMA, R-MDMA, S-MDMA, or saline, and were isolated in clean cages for 30 minutes. Each subject was then paired with an unfamiliar weight-matched conspecific from the same treatment group in a 30 × 18 cm clear Plexiglas testing chamber for 10 minutes. An experimenter was present during the first test day to separate aggressive subjects. Two saline treated pairs had to be separated and removed from the study because of sustained fighting. These subjects were replaced with new naïve subjects so that each treatment condition had an equal number of non-aggressive subjects (n = 5 pairs/group). No fighting occurred in the other treatment groups. The second test session was performed 48 hours later using the same pairs, treatments, and procedure except that an experimenter was not present in the room during testing. While in the testing arena, subjects were free to move around and interact, allowing a diverse range of observable behaviors. On the second test day, the social pairings were videotaped and the duration of social behavior was quantified using JWatcher or BORIS (Friard and Gamba, 2016) by an observer blind to the experimental conditions. The durations of 3 behaviors were scored: anogenital investigation (sniffing the conspecific’s anogenital area), general investigation (non-anogenital sniffing, grooming, and following the conspecific), and adjacent lying (side-by-side contact or huddling). These behaviors were averaged for each pair and then summed to produce a total social interaction score, upon which statistical analysis was performed.
2.3.2. Locomotor Behavior
Drug effects on locomotor activity were tested in 45 × 39 × 37 cm open field chambers with 16 × 16 photocells positioned 2.5 cm off the chamber floor (San Diego Instruments, San Diego, CA). Mice were treated with SR-MDMA, R-MDMA, S-MDMA, or saline (n = 13/group) immediately before being placed into the chambers for 1 hour. Testing was performed in a dark, enclosed space. Accumulative beam breaks of adjacent photocells were recorded as the measure of locomotor activity.
2.3.3. Fear Conditioning and Extinction
The effects of R-MDMA and S-MDMA on conditioned freezing were evaluated using an established protocol (Young et al., 2015) previously used to test SR-MDMA. For consistency with this prior study, C57BL/6 mice were used in this experiment. Briefly, mice were exposed to cued fear conditioning on day 1, fear extinction training on day 3, and extinction testing on day 4. Cued fear conditioning consisted of a single pairing of a conditioned stimulus (CS) tone (80 dB, 4.5 kHz, 30 s) and an unconditioned stimulus (US) foot shock (1 mA, 2 s). Extinction training was carried out 48 hours later in a new context from conditioning. R-MDMA, S-MDMA, or saline (n = 6–7/group) were administered on day 3, 30 minutes before training. Extinction training consisted of a sub-optimal regimen of 4 CS-tone re-exposures separated by 45 seconds. Extinction testing was performed 24 hours later to determine the lasting effect of treatment on conditioned freezing. Extinction testing was performed in the same context as training and followed the same procedure except that no treatments were administered. Throughout these experiments, percent freezing (the conditioned response) was estimated by scoring the presence or absence of non-respiratory movement every 5 seconds.
2.4. Neurotoxicity
2.4.1. Neurotoxic Dosing and Tissue Collection
Subjects received a total of four injections of either R-MDMA (50 mg/kg), SR-MDMA (20 mg/kg), or saline given twice, two hours apart on two consecutive days. This dosing regimen was slightly modified from one previously shown to produce long lasting evidence of SR-MDMA-induced neurotoxicity in Swiss Webster mice (Itzhak et al., 2003). To account for the lower potency of R-MDMA, the dose administered was scaled relative to SR-MDMA based on the peak effective doses at which each increased social interaction. Subject were isolated during treatment and returned to their home cages 2 hours after the second daily dose. Survival was 75% for SR-MDMA and 100% for R-MDMA. Following treatment, subjects were divided into two groups. 48 hours after the final injection, subjects from group 1 were anesthetized and transcardially perfused with 4% formaldehyde. Their brains were post-fixed overnight and then immersed in 15% sucrose for 48 hours, frozen in chilled methyl butane, sectioned at 35 μm, and stored at −20°C until analysis by immunohistochemistry. Subjects in group 2 were euthanized by cervical dislocation 14 days after the last injection. Their brains were removed and prefrontal cortex, striatum, and hippocampus were rapidly dissected and stored at −80°C for subsequent analysis by high-performance liquid chromatography (HPLC) or Western blot.
2.4.2. Immunohistochemistry
Reactive astrogliosis was assessed by quantification of glial fibrillary acidic protein (GFAP). Tissue sections were washed in PBS and endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 10 minutes. Sections were then blocked with a solution of 2% goat serum and 0.2% Tween 20 in PBS for one hour and then incubated in blocking buffer overnight at 4°C with a primary antibody against GFAP (ab4674, Abcam, Cambridge, UK; 1:8000). They were then rinsed with PBS and incubated with a biotinylated secondary antibody (Vector Labs, Burlingam, CA; 1:200; 1 h). Immunolabeling was visualized with VECTASTAIN ABC and Impact DAB (Vector Labs). Using SPOT Basic imaging software (Sterling Heights, MI), the dorsal striatum was photographed at 10× magnification across 4–6 coronal sections from each subject (n = 7/group). The camera frame was centered within the dorsal striatum, and GFAP immunoreactivity was quantified within the frame using ImageJ by an observer blinded to the treatment condition. Images were converted to 8-bit, and contrast and brightness were adjusted equally for all images using the default settings (imagej.nih.gov/ij/plugins/plane-brightness). Artifacts were removed if needed, and the percent area of immunolabeling was determined by analyzing the particle area with a threshold set to include only immunolabeled cells.
2.4.3. High-Performance Liquid Chromatography
Brain tissue (n = 13–15/group) was homogenized by sonication in 0.1 M perchloric acid and centrifuged for 25 minutes at 14000 × g. The pellet was dissolved in 0.3 M NaOH and total protein content was determined via BCA assay (Pierce Biotechnology, Rockford, IL). HPLC with electrochemical detection was used to determine the quantity of DA and 5-HT in the supernatant. The HPLC system was composed of a small-bore column (3.2 mm × 150 mm × 3 um; 70–0636; Thermo Scientific, Sunnyvale, CA), a Thermo Dionix Ultimate 3000 solvent delivery pump set to a flow rate of 0.6 ml/min, a 5020 ESA guard cell (350 mV; Chelmsford, MA), and an ESA 542 autosampler. Detection was carried out with a dual-channel analytical cell (5014B, Thermo Scientific) and an ESA Coulochem III detector. The analytical cell’s oxidative channel was set to −150 mV and its reductive channel was set to 220 mV. The mobile phase was commercially prepared MDTM (Thermo Scientific). Data were acquired and analyzed using Chromeleon 6.8 software (Thermo Scientific). DA and 5-HT content are presented as a percent of their concentration in saline treated controls.
2.4.4. Western Blot
Striatal tissue (n = 8–9/group) was homogenized by sonication in Tris-HCl buffer and centrifuged for 20 minutes at 18,000 × g. The pellet was suspended in RIPA buffer (Sigma-Aldrich, St. Louis, MO) and shaken on ice for 2 hours. Samples were centrifuged for 20 minutes at 14,000 × g and analysis was performed on the supernatant. Total protein content was determined with a BCA assay (Pierce Biotechnology). Samples were diluted in LDS loading Buffer (Life Technologies, Carlsbad, CA), heated to 70°C for 10 minutes, and then separated on 8% Bis-Tris gels (Life Technologies). Proteins separated by electrophoresis were transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked for 60 minutes at room temperature in TBS containing 5% nonfat dry milk and 0.05% Tween 20. Membranes were incubated overnight in blocking buffer at 4°C with primary antibodies against DAT (AB2231, Millipore, Darmstadt, Germany; 1:20000) and Na+/K+-ATPase (ab76020,1:500000, Abcam). Membranes were washed with TBS containing 0.1% Tween 20 and then incubated for 1 hour at room temperature with an HRP-conjugated secondary antibody (1:200000, Jackson Immuno, West Grove, PA) in blocking buffer. After washing, the antibody complex was visualized by chemiluminescence (Amersham ECL Prime, GE Healthcare, Buckinghamshire, UK). Bands were quantified using ImageJ and the relative expression of DAT was evaluated as a percent of the Na+/K+-ATPase loading control. The effect of treatment is presented as the percent expression relative to saline treated controls.
2.5. Body Temperature Monitoring
The effect of R-MDMA (50 mg/kg) or SR-MDMA (20 mg/kg) on body temperature, given twice at a two-hour interval, was monitored using a rectal thermometer (n = 5/group). Measurements were taken every 30 minutes at an ambient room temperature of 22±2°C. During monitoring, mice were separated to individual holding cages. The ambient temperature within each cage was not measured but may have exceeded the room temperature due to body heat. To determine if dopamine D1 receptor activity was necessary for SR-MDMA induced hyperthermia, a second group of mice received pretreatments of the selective D1 antagonist SCH (0.5 mg/kg) or saline 30 minutes before a single SR-MDMA treatment or saline (saline/saline n = 9, saline/SR-MDMA n = 13, SCH/SR-MDMA n=13, SCH/saline n=9). Statistical analysis was performed on measurements taken post-treatment.
2.6. Statistical analysis
Data were analyzed with Prism 7.01 (Graphpad, La Jolla, CA). Behavioral measures were analyzed using one-factor between-subjects ANOVAs with Dunnett’s post-hoc comparisons of each treatment to saline. GFAP immunoreactivity and DAT expression were analyzed with one-factor between-subjects ANOVAs with Tukey’s post-hoc comparisons, while DA and 5-HT concentrations were analyzed with two-factor ANOVAs with brain region and treatment as factors and a Tukey’s post-hoc comparison. Body temperature measures were analyzed with two-factor mixed-design ANOVAs with time and treatment as factors and Tukey’s post-hoc comparisons. Alpha for all experiments was set at 5%. Error bars represent the standard error of the mean (SEM).
3. Results
3.1. R-MDMA increases murine social interaction without increasing locomotor activity
SR-MDMA dose-dependently increased murine social interaction (Figure 1a; F3,16 = 3.749, p = 0.0326), with a 7.8 mg/kg dose significantly increasing total social interaction compared to saline (95% CI [15.01, 243.7], p = 0.0255). R-MDMA increased total social interaction (Figure 1b; F3,16 = 3.317, p = 0.0468), but was less potent, requiring a higher dose of 17 mg/kg to significantly increase social interaction above that of saline-treated controls (95% CI [15.98, 274], p = 0.0265). S-MDMA did not significantly alter total social interaction (Figure 1c; F4,20 = 1.194, p = 0.344), but there was a trend towards significance at the 7.8 mg/kg dose (p = 0.375).
Figure 1. Effects of SR-MDMA, R-MDMA, and S-MDMA on social interaction and locomotor activity.
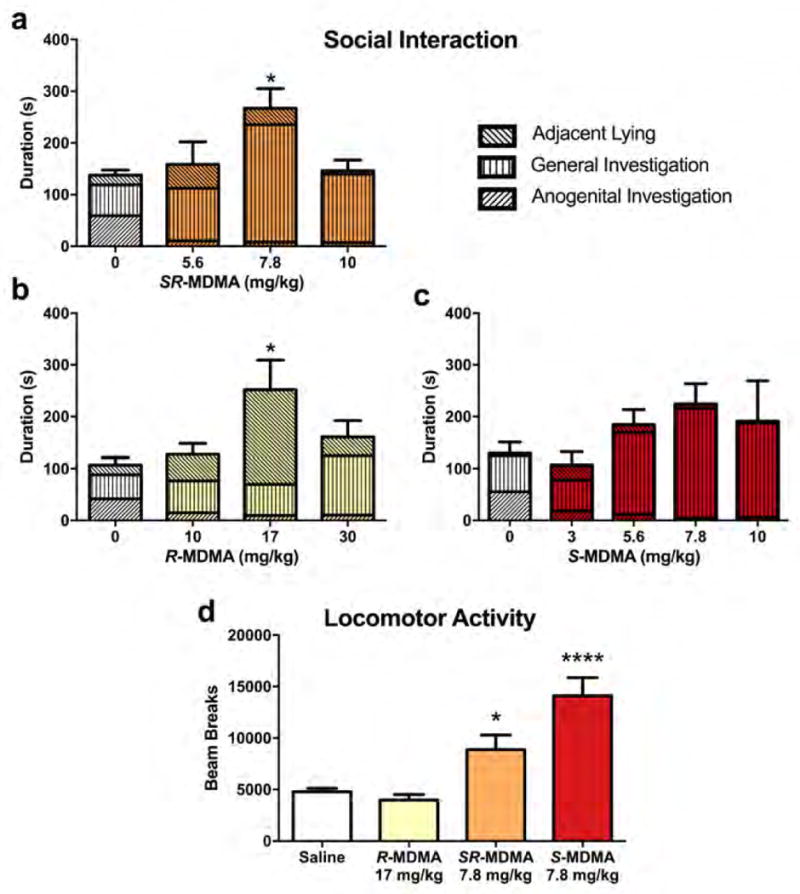
The durations of 3 social behaviors during a 10-minute social interaction test are shown stacked to produce total social interaction. (a) SR-MDMA increased total social interaction with a peak effective dose of 7.8 mg/kg. SR-MDMA preferentially increased general investigation behaviors, mostly nose-to-nose sniffing and non-aggressive following. (b) R-MDMA increased total social interaction with a peak effective dose of 17 mg/kg. R-MDMA preferentially increased adjacent lying behavior. (c) S-MDMA did not significantly alter total social interaction. (d) SR-MDMA and S-MDMA significantly increased locomotor activity compared to saline, while R-MDMA had no effect on locomotor activity. Bars represent mean ± SEM; *p < 0.05, ****p < 0.0001 compared to saline treatment.
The locomotor stimulant effects of SR-MDMA, R-MDMA, and S-MDMA were tested at the doses that produced peak effects during social interaction testing. There was an effect of treatment on the quantity of horizontal beam breaks (Figure 1d; F3,48 = 15.56, p < 0.0001). SR-MDMA and S-MDMA both significantly increased locomotor activity relative to saline (95% CIs [58.37, 8121], [5283, 13345], and p = 0.0461, p < 0.0001, respectively). In contrast, R-MDMA had no effect on locomotor activity relative to saline (p = 0.9299).
3.2. R-MDMA facilitates extinction of conditioned freezing
The timeline of fear conditioning and extinction is shown in Figure 2a. Acute administration of R-MDMA had no effect on conditioned freezing during extinction training (Figure 2b; F3,24 = 1.002, p = 0.4089), but significantly reduced freezing during extinction testing the following day (Figure 2c; F3,24 = 3.64, p = 0.027), suggesting a facilitation of fear extinction learning. As in the social interaction test, the peak effective dose of R-MDMA was 17 mg/kg (95% CI [−32.43, −3.567], p = 0.0124). Unlike R-MDMA, S-MDMA reduced freezing during training (Figure 2d; F3,20 = 5.581, p = 0.006), with 5.6 mg/kg and 7.8 mg/kg S-MDMA significantly reducing freezing compared to saline (95% CIs [−38.21, −5.785], [−33.21, −0.7851], and p = 0.007, p = 0.0387, respectively). However, this reduction did not persist the following day during extinction testing, indicating that extinction learning was not enhanced by S-MDMA (Figure 1e; F3,20 = 1.142, p = 0.3564).
Figure 2. Effects of R-MDMA and S-MDMA on fear extinction.
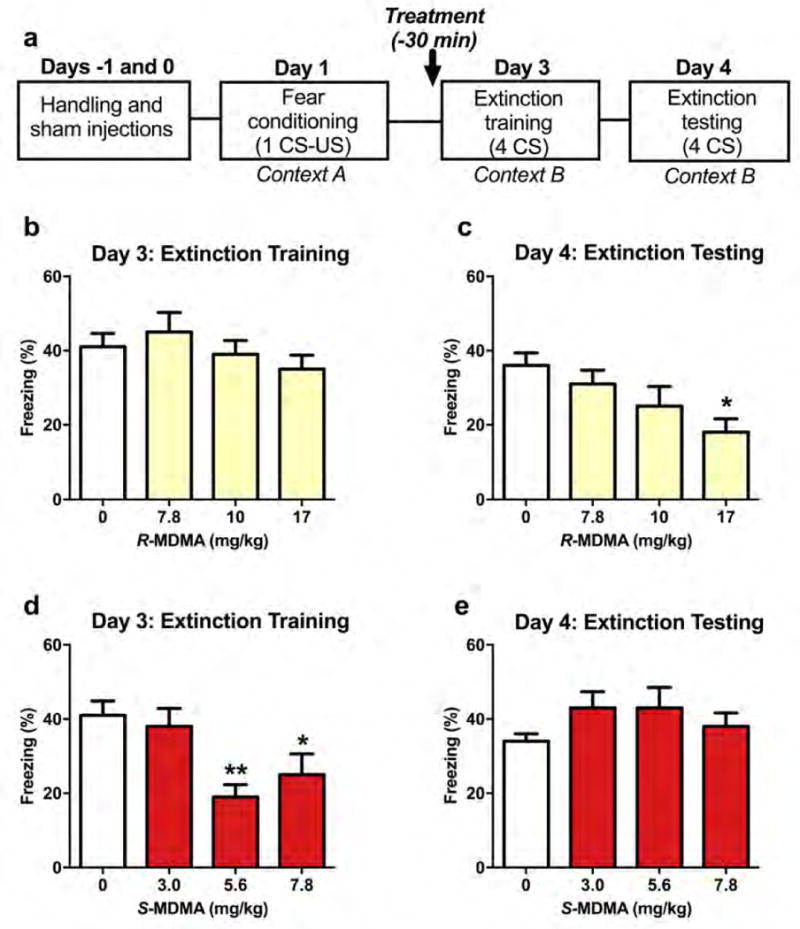
(a) Timeline of fear conditioning and extinction experiment. (b) R-MDMA did not affect freezing during extinction training. (c) But 17 mg/kg R-MDMA given before training significantly reduced freezing during extinction testing performed 24 hours later. (d) S-MDMA decreased freezing during extinction training, with doses of 5.6 mg/kg and 7.8 mg/kg significantly decreasing freezing compared to saline. (e) However, S-MDMA treatment did not facilitate lasting extinction as there was no effect of prior treatment on freezing during extinction testing. Bars represent mean ± SEM of %freezing across 4 CS tones; *p < 0.05, **p < 0.01 compared to saline treatment; CS, conditioned stimulus; US, unconditioned stimulus.
3.3. R-MDMA administration does not produce markers of neurotoxicity associated with SR-MDMA
To assess the potential neurotoxicity of R-MDMA, subjects treated with 4 doses of 50 mg/kg R-MDMA were compared to SR-MDMA (4 × 20 mg/kg; positive control) and saline (negative control) treated subjects. Reactive astrogliosis was assessed in the dorsal striatum 48 hours after treatment (Figure 3a). There was a significant effect of treatment on the percent area of GFAP immunoreactivity (Figure 3b; F2,18 = 6.85, p = 0.0061). Mice treated with SR-MDMA had significantly increased GFAP immunoreactivity relative to saline or R-MDMA treated subjects (95% CIs [0.868, 10.55], [1.552, 11.23], and p = 0.0196, p = 0.0091, respectively). In contrast, R-MDMA had no effect on GFAP immunoreactivity relative to saline treated controls (p = 0.9311).
Figure 3. Astrogliosis 48 hours after treatment with SR-MDMA or R-MDMA.
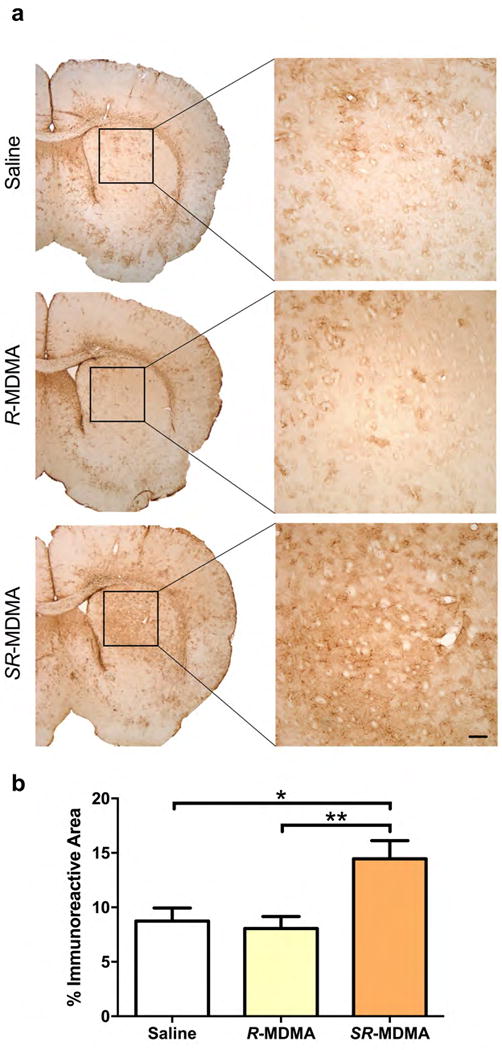
(a) Reactive astrogliosis, a marker of CNS damage, was assessed by quantification of GFAP immunoreactivity in the dorsal striatum 48 hours after a neurotoxic dosing regimen of SR-MDMA (20 mg/kg × 4) or an equivalent regimen of R-MDMA (50 mg/kg × 4). (b) SR-MDMA significantly increased GFAP immunoreactivity in the striatum. R-MDMA did not affect GFAP immunoreactivity. Bars represent mean ± SEM; Scale bar, 100 μm; *p < 0.05, **p < 0.01; GFAP, glial fibrillary acidic protein.
Long-lasting changes to DA and 5-HT content and DAT expression were assessed two weeks after treatment. There was a significant effect of treatment on DA tissue content (Figure 4a, F2,110 = 4.023, p = 0.0206). Mice treated with SR-MDMA had lower DA content across all brain regions compared to saline (95% CI [−43.84, −2.337], p = 0.0253) or R-MDMA treated mice (95% CI [−41.13, −0.0784], p = 0.0489), whereas R-MDMA treated mice were not significantly different from saline-treated controls (p = 0.9517). 5-HT concentrations across the same brain regions were not significantly affected by either treatment (Figure 4b; F2,110 = 1.112, p = 0.3326), indicating that neurotoxicity was likely selective to DA neurons. DAT expression in the striatum was quantified as an additional marker of DA neuronal pruning. DAT expression relative to Na+/K+-ATPase loading controls was normalized to expression as a percentage of saline treated controls. There was a significant effect of treatment on striatal DAT expression (Figure 4c, F2,22 = 4.807, p = 0.0185). DAT was significantly decreased in SR-MDMA treated mice compared to R-MDMA treated subjects (95% CI [−119.3, −8.672], p = 0.0215). R-MDMA had no effect on DAT expression relative to saline (p = 0.9106), whereas SR-MDMA appeared to decrease DAT relative to saline with a trend towards significance (p = 0.0601).
Figure 4. Markers of neuronal terminal pruning.
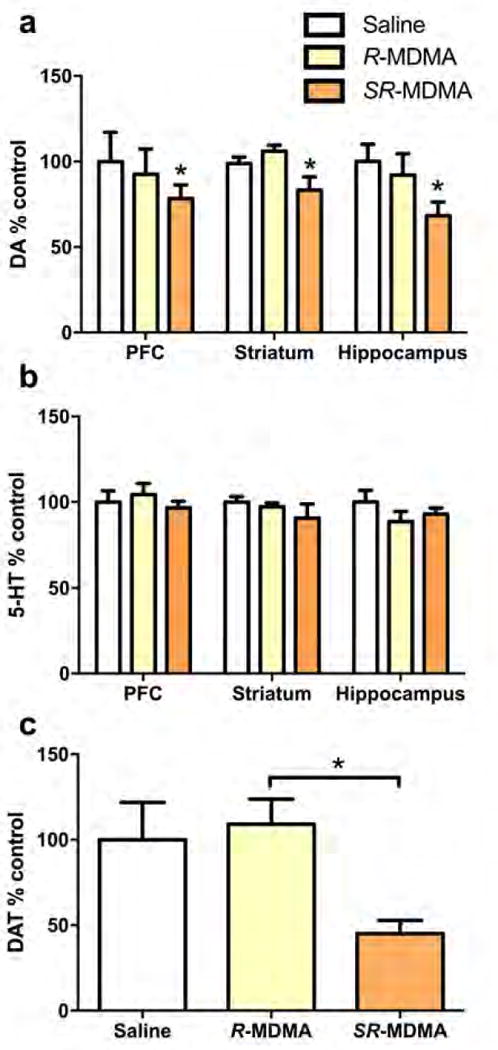
2 weeks after binge dose treatments of SR-MDMA (20 mg/kg × 4) or R-MDMA (50 mg/kg × 4), markers of neuronal terminal pruning were assayed. (a) DA content was assessed in 3 brain regions as a marker of DA terminal pruning. SR-MDMA treatment significantly decreased DA content across the regions. R-MDMA had no effect on DA concentrations relative to saline treated controls. (b) There was no effect of either treatment on 5-HT content in the same brain regions, suggesting that pruning was isolated to DA neurons. (c) DAT expression in the striatum was quantified as an additional marker of DA neuronal pruning. SR-MDMA treated mice had significantly lower striatal DAT expression compared to R-MDMA treated subjects, which were not significantly different from saline treated controls. Bars represent % of saline control ± SEM; *p < 0.05; DA, dopamine; DAT, dopamine transporter.
3.4. R-MDMA administration does not increase body temperature
Murine body temperature was monitored after treatment with SR-MDMA and R-MDMA (Figure 5a). There was a significant effect of treatment (F2,12 = 26.85, p < 0.0001), time (F7,84 = 4.646, p = 0.0002), and an interaction (F14,84 = 6.586, p < 0.0001). SR-MDMA significantly increased body temperature relative to saline (95% CI [3.073, 0.8174], p = 0.0016) or R-MDMA (95% CI [4.188, 1.932], p < 0.0001). Conversely, R-MDMA appeared to decrease body temperature compared to saline with a trend towards significance (p = 0.0527).
Figure 5. Effects of SR-MDMA and R-MDMA on body temperature.
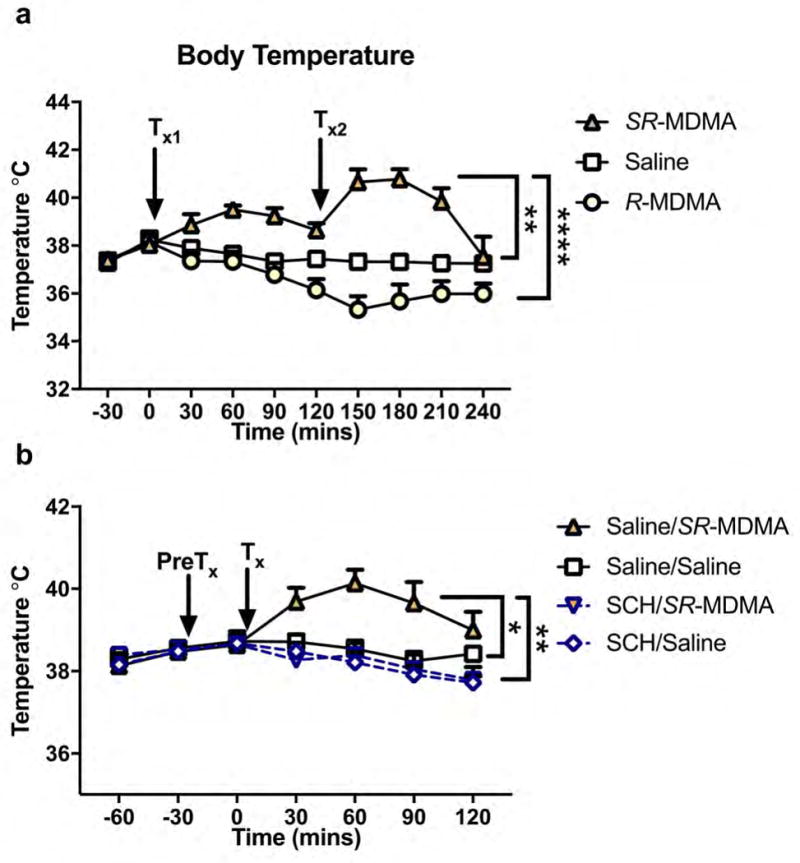
(a) Subjects were treated with SR-MDMA (20 mg/kg), R-MDMA (50 mg/kg), or saline at times Tx1 and Tx2. Relative to saline or R-MDMA treated subjects, SR-MDMA significantly increased body temperature. (b) To investigate the role of DA in the hyperthermic effect of SR-MDMA, subjects were pretreated (PreTx) with the D1 receptor antagonist R(+)-SCH23390 (SCH; 0.5 mg/kg) or saline 30 minutes before treatment (Tx) with SR-MDMA (20 mg/kg) or saline. SR-MDMA increased body temperature compared to saline treated controls. Pretreatment with SCH attenuated this effect, but did not significantly reduce baseline body temperature. Symbols represent mean ± SEM; *p < 0.05, **p < 0.01, ****p < 0.0001; DA, dopamine; SCH, R(+)-SCH23390.
3.5. SR-MDMA-induced hyperthermia is dopamine D1 receptor dependent
To investigate the role of DA in the hyperthermic effect of SR-MDMA, subjects were pretreated with the D1 receptor antagonist SCH (Figure 5b). There was a significant effect of treatment (F3,40 = 6.923, p = 0.0007) and time (F3,120 = 10.02, p < 0.0001). Post-hoc analysis revealed that SR-MDMA increased body temperature relative to saline (Saline/SR-MDMA × Saline/Saline, 95% CI [0.026, 2.247], p = 0.0431). Pretreatment with SCH attenuated this effect (SCH/SR-MDMA × Saline/SR-MDMA, 95% CI [−0.4918, −2.501], p = 0.0015), but did not significantly affect baseline body temperature (SCH/Saline × Saline/Saline, p = 0.811).
4. Discussion
The prosocial effects of SR-MDMA have been extensively studied in humans (Kamilar-Britt and Bedi, 2015), and similar effects have been observed in rodents, with SR-MDMA increasing social interaction (Morley and McGregor, 2000) and preference for social contexts (Kuteykin-teplyakov and Maldonado, 2014). However, the prosocial effects of MDMA’s enantiomers have not been previously studied in rodents. We found that although R-MDMA was less potent than SR-MDMA, both increased total murine social interaction with similar magnitude. S-MDMA treatment did not meet the predefined level of significance to reject the null hypothesis, but subjectively it appears likely that this enantiomer also increased social interaction but possibly to a lesser extent or with greater variability than SR- or R-MDMA. Additional study and a larger sample size will be necessary to clarify what effect this enantiomer has on murine social interaction. These behavioral results were recently replicated in a study of non-human primates that similarly found that SR- and R-MDMA both robustly increase affiliative social behavior, while S-MDMA is less effective (Pitts et al., 2017). In the present study, R-MDMA preferentially increased adjacent lying, which is a commonly reported behavioral effect of SR-MDMA in rats (Kamilar-Britt and Bedi, 2015) and possibly analogous to huddling observed in MDMA treated squirrel monkeys (Pitts et al., 2017). This behavior, however, did not appear to increase in SR-MDMA or S-MDMA treated mice. One potential explanation for this difference is that locomotor activity was significantly increased in SR- and S-MDMA treated mice but not R-MDMA treated ones. Hyperactivity might mask certain social behaviors, particularly in S-MDMA treated mice, which had especially high locomotor activity.
Before its prohibition, SR-MDMA was used as an adjunct to facilitate psychotherapy and was claimed to have wide ranging therapeutic effectiveness, earning the nickname “penicillin for the soul” (Shulgin and Shulgin, 1991). Recently, clinical trials have begun evaluating whether SR-MDMA is in fact effective. Much of the focus thus far has been on PTSD, a disorder often conceptualized in terms of Pavlovian fear-conditioning, whereby neutral stimuli associated with a trauma continue to trigger fear responses even though they no longer signal a threat. Normally, such associations would fade as they no longer predict danger, but in PTSD this process of extinction appears deficient (VanElzakker et al., 2014). SR-MDMA may help to treat PTSD by facilitating extinction learning (Mithoefer et al., 2016; Young et al., 2015). A previous study with mice found that 7.8 mg/kg SR-MDMA, given prior to extinction training, facilitates a long-lasting extinction of conditioned freezing, a species-typical fear behavior (Young et al., 2015). In the present study, we found that R-MDMA also facilitates extinction learning in mice, while S-MDMA, at least at the doses tested, does not. Although S-MDMA had no lasting effect on extinction, it did acutely decrease freezing during training. This may have been due to the locomotor stimulant effect of the drug, or could indicate an effect similar to that of benzodiazepines or alcohol which can acutely decrease fear responses but do not facilitate lasting extinction (Bouton et al., 1990). Regardless, previous studies have demonstrated that fear behavior during training is irrelevant to the lasting extinction improvements by SR-MDMA (Young et al., 2017, 2015).
In both the social interaction and fear extinction tests, SR-MDMA was more potent than would be expected from the effects of the individual enantiomers. This phenomenon has been observed numerous times with MDMA (Anderson et al., 1978; Pitts et al., 2017; Young and Glennon, 2008), and suggests that the enantiomers act synergistically to produce these effects. S-MDMA is a potent monoamine releaser that increases levels of 5-HT, norepinephrine (NE), and DA (Setola et al., 2003). In contrast, R-MDMA is a less potent but more selective 5-HT and NE releaser, and a partial agonist at 5-HT2A receptors (Huot et al., 2011). Due to functional selectivity at 5-HT2A receptors, the effects of R-MDMA on downstream signaling likely differ from endogenous 5-HT (Urban et al., 2007). Clinical studies have found that the majority of SR-MDMA’s subjective effects can be attributed to release of 5-HT (Liechti et al., 2000a), NE (Hysek et al., 2012, 2011), and activation of 5-HT2A receptors (Liechti et al., 2000b). Each of these factors is also necessary for the facilitative effect of SR-MDMA on fear extinction in mice (Young et al., 2017), and 5-HT2A activation is necessary for the increased affiliative behaviors in SR-MDMA treated squirrel monkeys (Pitts et al., 2017). Thus, as a racemic mixture, S-MDMA may provide the necessary 5-HT and NE release, while R-MDMA provides the necessary 5-HT2A activation. Our findings indicate that by increasing the dosage of R-MDMA it can produce prosocial and therapeutic-like effects on its own, possibly because at higher doses it releases sufficient 5-HT and NE. Although it is possible that higher doses of S-MDMA might also produce these behavioral effects, its side effects observed here or previously documented in the literature such as hyperactivity, hyperthermia (Fantegrossi et al., 2003) and neurotoxicity (Frau et al., 2013; Schmidt et al., 1987), make its clinical use at higher doses unviable.
Unlike SR- and S-MDMA, which have both previously been shown to be neurotoxic across species, clear evidence of neurotoxicity has not been observed for R-MDMA. Herein we demonstrate that even at very high doses that account for its lower potency relative to SR-MDMA, R-MDMA produced no signs of neurotoxicity, including reactive astrogliosis and markers of neuronal terminal pruning. Also, unlike SR-MDMA it did not produce hyperthermia or any visual signs of acute toxicity such as excessive salivation and hind-limb rigidity. A key pharmacological difference that may explain these differential effects is that unlike SR- and S-MDMA, R-MDMA does not increase extracellular DA at behaviorally relevant doses (Hiramatsu and Cho, 1990; Murnane et al., 2010). Unlike 5-HT and NE, DA appears to play very little role in the subjective effects of SR-MDMA in humans (Liechti and Vollenweider, 2001; Schmid et al., 2015), and no role in the facilitation of fear extinction in mice (Young et al., 2017). However, it may be a critical factor for many of SR-MDMA’s adverse effects. DA signaling has previously been shown to be necessary for the locomotor-stimulant effects of SR-MDMA (Benturquia et al., 2008), and in the present study, pretreatment with a selective D1 receptor antagonist also prevented hyperthermia. This finding supports previous evidence that DA release may be necessary for MDMA-induced hyperthermia (Mechan et al., 2002; Shioda et al., 2008), likely in combination with NE and 5-HT which have also been implicated in thermogenesis (Liechti, 2014). Neurotoxic damage by SR-MDMA is typically correlated with the degree of hyperthermia and in many cases can be attenuated or eliminated by preventing hyperthermia (Green et al., 2003). Furthermore, DA catabolism has been implicated as a potential mechanism of SR-MDMA neurotoxicity across species (Sprague et al., 1998). DA released by SR-MDMA can be taken up by monoamine transporters into DA and 5-HT terminals where its deamination by monoamine oxidase (MAO) produces reactive oxygen species (ROS) that can lead to cellular degeneration (Cadet and Brannock, 1997). Thus, DA release may be necessary for some of SR-MDMA’s adverse effects but unnecessary for the prosocial and therapeutic effects.
The findings presented herein raise the possibility that R-MDMA may produce prosocial and therapeutic effects similar to SR-MDMA, but with a lower risk for certain adverse effects including neurotoxicity and hyperthermia. However, the extent to which these findings will translate to humans is unknown and will require further study. In particular, given the differences in SR-MDMA neurotoxicity between mice and primates, our findings regarding the neurotoxicity of R-MDMA should be confirmed in a species with closer homology to humans. It is also possible that R-MDMA may have adverse effects that were not evaluated in this study, or that adverse effects may occur at higher doses than were tested here. Also, because only males were evaluated in this study, these is the possibility that sex-differences in these effects may exist. Despite these limitations, further investigation of R-MDMA appears warranted. Very little is known about the psychoactive effects of R-MDMA in humans; to our knowledge, only a single study has evaluated these effects. At 200 mg, which was the highest dose tested, it reportedly produced none of the physical side effects (jaw clenching and mydriasis) and lacked most of the psychotropic effects that occurred with SR- or S-MDMA at lower doses (Anderson et al., 1978). By using the interspecies scaling equation Dose1 = Dose2(Weight1/Weight2)b, approximate human doses can be calculated from the doses used in mice (West, 2005). Assuming a 30 g mouse, 75 kg human, and an exponent of 0.8, the peak-effective dose of SR-MDMA in this study is estimated to be 122 mg in a human, approximately the same dose used in recent clinical trials of SR-MDMA (125 mg) (Mithoefer et al., 2011; Oehen et al., 2013). Based on this same equation, an effective dose of R-MDMA in humans may be around 267 mg or 3.55 mg/kg.
5. Conclusions
The primary findings of the present study are that R-MDMA increases social interaction and facilitates extinction of a conditioned fear response in a manner similar to racemic MDMA, yet even when administered at high repeated doses, it does not produce hyperthermia or evidence of neurotoxicity in mice. Although some safety concerns remain and additional studies are needed to investigate its toxicity in other species, these findings suggest that R-MDMA may be a safer therapeutic than SR-MDMA. Clinical study of R-MDMA seems warranted to determine if it has similar prosocial effects in humans and the same therapeutic efficacy that has been demonstrated with racemic MDMA. But perhaps more significantly, this study suggests that the prosocial and therapeutic effects of SR-MDMA can be isolated from its stimulant, neurotoxic, and hyperthermic effects. Development of safer and more targeted therapeutics might therefore be possible that would make clinical use of MDMA, in any form, unnecessary.
Highlights.
R-MDMA increases prosocial behavior and facilitates fear-extinction learning in mice.
High doses of R-MDMA do not produce hyperthermia or signs of neurotoxicity in mice.
Lower dopamine release may explain why R-MDMA lacks these adverse effects of SR-MDMA.
R-MDMA may be a safer and more viable therapeutic than racemic MDMA.
Acknowledgments
We thank the animal care and veterinary staff at the Yerkes National Primate Research Center (YNPRC) for maintaining the health and well-being of our research subjects. We also thank our research subjects for their contribution. Juliet Brown, Brian Zhao, and Maylen Perez Diaz contributed to the collection of these data.
Funding
This work was supported by NIH grants: T32 DA015040 (for DWC), MK12 GM000680 (for MBY), P51 OD011132 (to the YNPRC), and K05 DA031246 (to LLH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have financial or other conflicts of interest to report
References
- Anderson GM, Braun G, Braun U, Nichols DE, Shulgin AT. Absolute configuration and psychotomimetic activity. NIDA Res Monogr. 1978:8–15. [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–40. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benturquia N, Courtin C, Noble F, Marie-Claire C. Involvement of D1 dopamine receptor in MDMA-induced locomotor activity and striatal gene expression in mice. Brain Res. 2008;1211:1–5. doi: 10.1016/j.brainres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Biezonski DK, Meyer JS. The Nature of 3, 4-Methylenedioxymethamphetamine (MDMA)-Induced Serotonergic Dysfunction: Evidence for and Against the Neurodegeneration Hypothesis. Curr Neuropharmacol. 2011;9:84–90. doi: 10.2174/157015911795017146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037/0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1997;32:117–131. doi: 10.1016/S0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remião F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–71. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. HHS Pulication No. SMA 15-4927, NSDUH Ser. 2015;H-50:64. [Google Scholar]
- Erritzoe D, Frokjaer VG, Holst KK, Christoffersen M, Johansen SS, Svarer C, Madsen J, Rasmussen PM, Ramsøy T, Jernigan TL, Knudsen GM. In vivo imaging of cerebral serotonin transporter and serotonin(2A) receptor binding in 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) and hallucinogen users. Arch Gen Psychiatry. 2011;68:562–76. doi: 10.1001/archgenpsychiatry.2011.56. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–11. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Frau L, Simola N, Plumitallo A, Morelli M. Microglial and astroglial activation by 3,4-methylenedioxymethamphetamine (MDMA) in mice depends on S(+) enantiomer and is associated with an increase in body temperature and motility. J Neurochem. 2013;124:69–78. doi: 10.1111/jnc.12060. [DOI] [PubMed] [Google Scholar]
- Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol. 2016 doi: 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- Granado N, Escobedo I, O’Shea E, Colado I, Moratalla R. Early loss of dopaminergic terminals in striosomes after MDMA administration to mice. Synapse. 2008;62:80–4. doi: 10.1002/syn.20466. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs. 1986;18:319–327. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Sherwood AR, Hudson JI, Gruber S, Kozin D, Pope HG, Pope HG., Jr Residual neurocognitive features of long-term ecstasy users with minimal exposure to other drugs. Addiction. 2011;106:777–86. doi: 10.1111/j.1360-0443.2010.03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern P, Moskovich J, Avrahami B, Bentur Y, Soffer D, Peleg K. Morbidity associated with MDMA (ecstasy) abuse: a survey of emergency department admissions. Hum Exp Toxicol. 2011;30:259–66. doi: 10.1177/0960327110370984. [DOI] [PubMed] [Google Scholar]
- Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“ecstasy”) Lancet. 1992;340:384–387. doi: 10.1016/0140-6736(92)91469-O. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Cho AK. Enantiomeric differences in the effects of 3,4-methylenedioxymethamphetamine on extracellular monoamines and metabolites in the striatum of freely-moving rats: an in vivo microdialysis study. Neuropharmacology. 1990;29:269–75. doi: 10.1016/0028-3908(90)90012-g. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM. Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of ON-time. J Neurosci. 2011;31:7190–8. doi: 10.1523/JNEUROSCI.1171-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. 2013;222:293–302. doi: 10.1093/scan/nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, Huwyler J, Liechti ME. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (“ecstasy”) in humans. Clin Pharmacol Ther. 2011;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola VG, Vischer N, Donzelli M, Krähenbühl S, Grouzmann E, Huwyler J, Hoener MC, Liechti ME, Krahenbuhl S, Grouzmann E, Huwyler J, Hoener MC, Liechti ME. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF, Achat CN, Anderson KL. Relevance of MDMA (“ecstasy”)-induced neurotoxicity to long-lasting psychomotor stimulation in mice. Psychopharmacol. 2003;166:241–248. doi: 10.1007/s00213-002-1320-y. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Kamilar-Britt P, Bedi G. The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neurosci Biobehav Rev. 2015;57:433–446. doi: 10.1016/j.neubiorev.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuteykin-teplyakov K, Maldonado R. Looking for prosocial genes : ITRAQ analysis of proteins involved in MDMA-induced sociability in mice. Eur Neuropsychopharmacol. 2014;24:1773–1783. doi: 10.1016/j.euroneuro.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Liechti ME. Effects of MDMA on body temperature in humans. Temp (Austin, Tex) 2014;1:192–200. doi: 10.4161/23328940.2014.955433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000a;22:513–21. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000b;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol. 2001;16:589–598. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET Studies of the Serotonin Transporter in MDMA Users and Controls Using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) to rats. Br J Pharmacol. 2002;135:170–80. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Grob CS, Brewerton TD. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. The Lancet Psychiatry. 2016;3:481–488. doi: 10.1016/S2215-0366(15)00576-3. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25:439–52. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, Michel Y, Brewerton TD, Doblin R. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol. 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KC, McGregor IS. (+/−)-3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) increases social interaction in rats. Eur J Pharmacol. 2000;408:41–9. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- Mueller F, Lenz C, Steiner M, Dolder PC, Walter M, Lang UE, Liechti ME, Borgwardt S. Neuroimaging in moderate MDMA use: A systematic review. Neurosci Biobehav Rev. 2016;62:21–34. doi: 10.1016/j.neubiorev.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Mueller M, Yuan J, McCann UD, Hatzidimitriou G, Ricaurte GA. Single oral doses of (±) 3,4-methylenedioxymethamphetamine (’Ecstasy’) produce lasting serotonergic deficits in non-human primates: relationship to plasma drug and metabolite concentrations. Int J Neuropsychopharmacol. 2013;16:791–801. doi: 10.1017/S1461145712000582. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther. 2010;334:642–50. doi: 10.1124/jpet.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Brewster WK, Johnson MP, Oberlender R, Riggs RM. Nonneurotoxic tetralin and indan analogs of 3,4-(methylenedioxy)amphetamine (MDA) J Med Chem. 1990;33:703–710. doi: 10.1021/jm00164a037. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–51. [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (± 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) J Psychopharmacol. 2013;27:40–52. doi: 10.1177/0269881112464827. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychobiology of MDMA or “Ecstasy”: an overview of 25 years of empirical research. Hum Psychopharmacol. 2013;28:289–307. doi: 10.1002/hup.2318. [DOI] [PubMed] [Google Scholar]
- Pitts EG, Minerva AR, Oliver EB, Kohn JN, Logun MT, Sulima A, Rice KC, Howell LL. 3, 4-Methylenedioxymethamphetamine Increases Affiliative Behaviors in Squirrel Monkeys in a Serotonin 2A Receptor-Dependent Manner. Neuropsychopharmacology. 2017:1–10. doi: 10.1038/npp.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, Zawada A, Somerville M. The harmful health effects of recreational ecstasy: a systematic review of observational evidence. Health Technol Assess (Rockv) 2009;13:1–315. doi: 10.3310/hta13050. [DOI] [PubMed] [Google Scholar]
- Schilt T, de Win MML, Koeter M, Jager G, Korf DJ, van den Brink W, Schmand B. Cognition in novice ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch Gen Psychiatry. 2007;64:728–36. doi: 10.1001/archpsyc.64.6.728. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Rickli A, Schaffner A, Duthaler U, Grouzmann E, Hysek CM, Liechti ME. Interactions between Bupropion and 3,4-Methylenedioxymethamphetamine in Healthy Subjects. J Pharmacol Exp Ther. 2015;353:102–111. doi: 10.1124/jpet.114.222356. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Levin JA, Lovenberg W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem Pharmacol. 1987;36:747–55. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [pii] [DOI] [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yoshino T, Kuboshima K, Iwamura T, Yui K, Kato S. Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology. 2008;29:1030–6. doi: 10.1016/j.neuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PIHKAL: A Chemical Love Story. Transform Press; Berkeley: 1991. [Google Scholar]
- Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19:427–41. [PubMed] [Google Scholar]
- Taurah L, Chandler C, Sanders G. Depression, impulsiveness, sleep, and memory in past and present polydrug users of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) Psychopharmacology (Berl) 2014;231:737–51. doi: 10.1007/s00213-013-3288-1. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional Selectivity and Classical Concepts of Quantitative Pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Kathryn Dahlgren M, Caroline Davis F, Dubois S, Shin LM. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeli P, Liechti ME. Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol. 2017 doi: 10.1177/0269881117691569. 26988111769156. [DOI] [PubMed] [Google Scholar]
- West GB. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- Young MB, Andero R, Ressler KJ, Howell LL. 3,4-Methylenedioxymethamphetamine facilitates fear extinction learning. Transl Psychiatry. 2015;5:1–8. doi: 10.1038/tp.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MB, Norrholm SD, Khoury LM, Jovanovic T, Rauch SAM, Reiff CM, Dunlop BW, Rothbaum BO, Howell LL. Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3,4-methylenedioxymethamphetamine (MDMA) Psychopharmacology (Berl) 2017:1–13. doi: 10.1007/s00213-017-4684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. MDMA (N-methyl-3,4-methylenedioxyamphetamine) and its stereoisomers: Similarities and differences in behavioral effects in an automated activity apparatus in mice. Pharmacol Biochem Behav. 2008;88:318–31. doi: 10.1016/j.pbb.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


