Abstract
A history of binge-drinking decreases protein expression of the glutamate-related scaffolding protein Homer2 within the central nucleus of the amygdala (CEA), coinciding with behavioral signs of negative affect. To assess the functional relevance of this protein change for withdrawal-induced hyper-anxiety, adult (PND 56) and adolescent (PND 28) male C57BL/6J mice were administered an intra-CEA infusion of an adeno-associated viral vector (AAV) carrying either cDNA to express Homer2 (H2-cDNA) or GFP as control. Mice underwent 14 days of binge-drinking under multi-bottle, limited-access conditions and were assayed for behavioral signs of negative affect during withdrawal using the light-dark box, marble burying, and forced swim tests (FST). Following behavioral testing, all animals experienced 5 days of drinking to evaluate the effects of prior alcohol experience and Homer2 manipulation on subsequent alcohol consumption. During protracted (4 weeks) withdrawal, adolescent alcohol-experienced GFP controls showed increased signs of negative affect across all 3 assays, compared to water-drinking GFP animals, and also showed elevated alcohol consumption during the subsequent drinking period. Homer2-cDNA infusion in adolescent-onset alcohol-drinking animals was anxiolytic and reduced subsequent alcohol consumption. Conversely, Homer2-cDNA was anxiogenic and increased drinking in water-drinking adolescents. Unfortunately, the data from adult-onset alcohol-drinking animals were confounded by low alcohol consumption and negligible behavioral signs of anxiety. Nevertheless, the present results provide novel cause-effect evidence supporting a role for CEA Homer2 in the regulation of both basal anxiety and the time-dependent intensification of negative affective states in individuals with a history of binge-drinking during adolescence.
Keywords: binge drinking, adolescence, Homer2, amygdala, anxiety, alcoholism
1. Introduction
Binge-drinking is the most prevalent form of alcohol abuse in the United States, with approximately 1 in 6 American engaging in binge drinking an average of 4 times per month (Centers for Disease Control and Prevention, 2013). This pattern of consumption is especially prevalent in adolescents and over 90% of the alcohol consumed by underage individuals is in the form of binge drinks (Centers for Disease Control and Prevention, 2014). Although the majority of binge-drinkers do not meet the criteria for dependence (Esser et al., 2014), frequent binge-drinking is one of the strongest risk factors for alcoholism (Dawson et al., 2005; Hasin & Beseler, 2009; Saha et al., 2007). Chronic binge-drinking often results in the development of tolerance to the pleasurable/hedonic effects of acute alcohol that serve as positive reinforcers of drinking, leading to an escalation of intake (reviewed in Koob & Moal, 1997). Simultaneously, repeated bouts of intoxication and withdrawal can lead to increasing severity of withdrawal symptoms during periods of abstinence, including negative affective consequences such as anxiety, depression, agitation, and general dysphoria. Over time, withdrawal-induced negative affect in chronic binge-drinkers is theorized to facilitate the transition to addiction in non-dependent individuals by shifting the primary motivation for drinking from positive to negative reinforcement (Koob, 2013; Koob & Le Moal, 2001).
The negative affective consequences of alcohol withdrawal are primarily associated with drug-induced adaptations within extended amygdala circuitry- consisting of the bed nucleus of the stria terminalis (BNST), the shell subregion of the nucleus accumbens (AcbSh), and the central nucleus of the amygdala (CEA). Negative affect is classically associated with an increase in amygdalar activation (Beesdo et al., 2009; Davis & Whalen, 2001; Peluso et al., 2009; Shackman & Fox, 2016) and the CEA dysfunction is critically implicated in alcohol withdrawal-induced negative affect (reviewed in detail by Gilpin et al., 2015). Chronic alcohol exposure induces glutamatergic plasticity via both pre- and post-synaptic adaptations (Lack et al., 2007; Lovinger & Roberto, 2013; Siggins et al., 2005; Stuber et al., 2010). These changes contribute to CEA hyperactivation during withdrawal, which is associated with negative affective states.
Prior research has shown that drug-induced restructuring of glutamatergic synapses often involves Homer proteins (reviewed in Szumlinski, Ary, & Lominac, 2008). Homer proteins are intracellular scaffolding proteins located abundantly in the post-synaptic density that exert regulatory control over glutamatergic signaling (Shiraishi-Yamaguchi & Furuichi, 2007). The ‘long-form’ Homer isoforms (Homer1b/c/d, Homer2a/b, and Homer3) are constitutively expressed throughout many addiction-relevant brain regions including the Ach, PFC, and the amygdala (Ary et al., 2013; Soloviev et al., 2000; Verpelli et al., 2012), where they directly influence glutamatergic signaling by regulating the trafficking, distribution, and function of group 1 metabotropic glutamate receptors (mGluRs) and NMDA receptors (Brakeman et al., 1997; Tu et al., 1998).
Alcohol-induced synaptic plasticity within the extended amygdala circuitry is theorized to involve, in part, increases in the expression of Homer proteins - Homer2 isoforms in particular (Cozzoli et al., 2009, 2012, 2014; Haider et al. 2015; Lee et al., 2016; Lum et al., 2014; Obara et al., 2009; see also Cui et al., 2013 and Szumlinski et al., 2008 for review). Homer2 knockout mice are alcohol-averse and –intolerant and these phenotypes are reversed via adeno-associated viral vector (AAV)-mediated Homer2 expression within the nucleus accumbens shell (AcbSh) (Szumlinski et al., 2005). Likewise, AAV-mediated expression of Homer2 within the AcbSh augments alcohol reward, reinforcement and behavioral sensitization (Szumlinski, Ary, Lominac, et al., 2008), while Homer2 knock-down in this region reduces binge-drinking, without influencing water or sweet solution intake (Cozzoli et al., 2009, 2012). Within the CEA, AAV-mediated Homer2 knockdown significantly reduces binge-drinking, particularly of high alcohol concentrations (Cozzoli et al., 2014) and CEA Homer2 maintains voluntary binge-drinking through group 1 mGluR-dependent pathways involving phospholipase C and PKCε (Cozzoli et al., 2016; Cozzoli et al., 2014). Taken together, our prior work argued that a Homer2-dependent hyper-glutamatergic state during alcohol withdrawal promotes the positive reinforcing properties of alcohol to drive excessive intake (Cui et al., 2013; Szumlinski, Ary, & Lominac, 2008).
In contrast to its role in regulating the positive reinforcing properties of alcohol, we know very little regarding Homer2’s potential role in the negative reinforcing properties of this drug. Homer2 knockout mice exhibit wild-type levels of basal emotionality (as well as cognitive, sensorimotor and gross motor function) (Szumlinski et al., 2004, 2005) and do not exhibit stress-alcohol cross-sensitization of locomotor activity (Quadir et al., 2016), but no study to date has examined how Homer2 impacts alcohol-induced changes in affect. Recent western blotting data from our laboratory showed that the manifestation of negative affect during alcohol withdrawal closely aligned with reductions in Homer2 protein expression within the CEA. For example, mice with a 14-day history of binge- drinking during adulthood show increased anxiety and reduced CEA Homer2 expression during acute (24 h) withdrawal and both effects dissipate with the passage of time (Lee et al., 2015, 2017c). In contrast, adolescent binge-drinkers show negligible signs of behavioral dysfunction during acute withdrawal and no change in CeA Homer2 expression (Lee et al., 2016), but a marked negative affective state incubates in these animals during protracted (4 weeks) withdrawal and the mice exhibit hyper-anxiety, as well as reduced CEA H2 expression, when tested as young adults (Lee et al., 2017c). Importantly, irrespective of the age of drinking onset, withdrawal-induced negative affect is positively related to increased subsequent alcohol consumption. Our prior behavioral data are consistent with other clinical and preclinical literature regarding the enduring consequences of adolescent alcohol exposure indicating increased mood disorders, cognitive impairment, and excessive alcohol consumption (McBride et al., 2005; Nixon & McClain, 2010; Spear, 2014; Tapert & Schweinsburg, 2005) and our correlational immunoblotting findings argue a potential role for alcohol withdrawal-induced changes in CEA Homer2 expression in the manifestation of a negative affective state, including the incubation of a negative affective state in those with a history of adolescent-onset binge-drinking.
In the present study, we sought to determine if the observed reduction in CEA Homer2 was functionally relevant to the manifestation of anxiety and excessive alcohol consumption during withdrawal using an AAV-mediated gene transfer approach to introduce Homer2 locally within the CEA (H2-cDNA). We presumed that H2-cDNA infusion within the CEA Homer2 should attenuate the binge-drinking induced reduction in protein expression and reduce anxiety in adult and adolescent alcohol-experienced animals, during early and protracted withdrawal, respectively. Moreover, if withdrawal-induced anxiety is a negative reinforcer of subsequent drinking, we hypothesized also that Homer2 ‘rescue’ should block withdrawal-induced drinking.
2. Methods
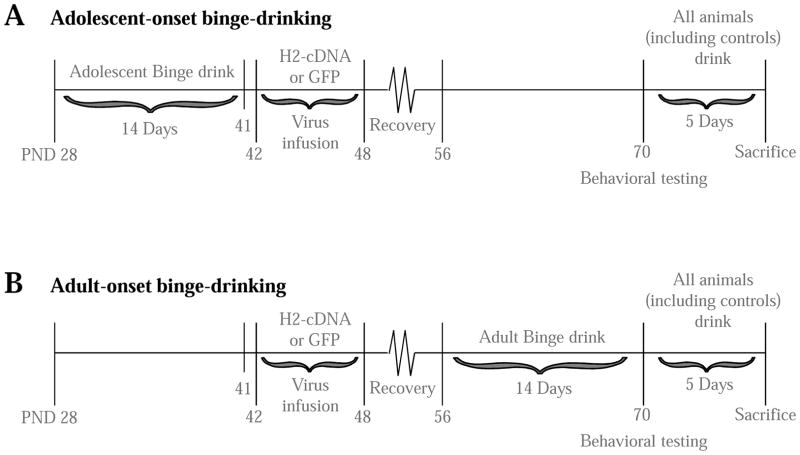
The binge-drinking and behavioral testing procedures in this study were identical to those used previously in our lab (Lee et al., 2015, 2016) and are briefly summarized below. The timeline of experimental procedures is outlined in Figure 1. All procedures were conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 2014) and approved by the IACUC of the University of California, Santa Barbara.
Figure 1.
Experimental timeline for this study of the effects of infusing AAVs into the CEA of mice with a history of binge-drinking during either adulthood or adolescence upon the manifestation of withdrawal-induced anxiety (tested on PND 70) and subsequent drinking.
2.1 Subjects & study design
This study used male C57BL/6J mice (Jackson Laboratory, Sacramento, CA) that were either PND 28 (adolescents) or PND 56 (adults) at the onset of drinking. Animals were housed in age-matched groups of 4 per cage in a climate-controlled vivarium under a reverse light/dark cycle (lights off at 10am), with food and water available ad libitum except during the 2-h alcohol drinking period. The study design consisted of 2 age groups (adults or adolescents), 2 drinking groups (alcohol or water), and 2 AAV groups (H2-cDNA or GFP); n=12/group.
2.2 Viral transfection
2.2.1 Viral vectors
The AAVs were obtained from the laboratory of Dr. M. Klugmann (University of New South Wales, Sydney, Australia), as in our prior transgenic studies (e.g. Cozzoli et al., 2012; Cozzoli et al., 2009; Szumlinski et al., 2006; Szumlinski, Ary, Lominac, et al., 2008) and a description of the procedures for generating the AAVs, as well as the resultant changes in H2 expression, has been described therein. In short, Homer2b was expressed as an N-terminal fusion protein with the hemagglutinin (HA) tag in a recombinant AAV backbone containing the 1.1 kb cytomegalovirus immediate early enhancer/chicken β-actin (CBA) promoter (H2-cDNA). This AAV has been previously validated via western blotting to successfully overexpress H2 (Ary et al., 2013; Haider et al., 2015). The same backbone encoding human Renilla green fluorescent protein (GFP) was used as an AAV control.
2.2.2 Craniotomy & virus infusion
Maximal neuronal transduction by our AAVs is observed approximately 3 weeks post-infusion (Klugmann & Szumlinski, 2008), which complicated the timing of AAV infusion in the younger animals. While it would have been ideal to infuse the AAVs 3 weeks prior to the onset of binge-drinking in both adolescent and adult mice, the obvious difficulties conducting accurate craniotomies on preweanling mice preventing us from adopting this procedural time-line. As such, we opted for both adults and adolescents to undergo surgery at the same age (PND 42-48), approximately 3 weeks prior to behavioral testing to allow for maximal transfection to occur in mice of both drinking-age groups (Klugmann & Szumlinski, 2008). Thus, surgery occurred prior to the 14-day drinking period in adults and afterward in adolescents. Under isoflurane gas anesthesia, 33-gauge injector cannulae (12 mm long; threaded through a 24-gauge adapter for stability) were used to deliver bilateral infusions of H2-cDNA or GFP directly into the CEA [AP:−1.25; ML: ±2.70; DV: −2.70 mm from Bregma, according to Paxinos & Franklin (2004)]. Infusions were delivered at a rate of 0.05 μl/min for 5 minutes (total vol=0.25 μl/side). Injector cannulae were left in place for an additional 5 minutes before removal. The incision site was closed with a small animal wound clip. Mice were left to recover, undisturbed (with the exception of post-operative monitoring and routine cage maintenance) for a minimum of 7 days before further experimentation.
2.3 Multi-Bottle-Choice Drinking-in-the-Dark (DID) Procedures
Half of the animals from each drinking-age group were subjected to 14 consecutive days of binge-drinking under 3-bottle DID procedures. Alcohol access was restricted to 14 days for all animals, corresponding to the approximate duration of early-mid adolescence in mice (Spear, 2000). Each day, animals were separated into individual cages, allowed to habituate to the drinking cage for a minimum of 45 min and then given simultaneous access to bottles containing 10, 20, and 40% (v/v) unsweetened ethanol solutions for 2 h, beginning 3 h into the circadian dark cycle (Lee et al., 2017c; Rhodes et al., 2005). Control animals received water only. Daily alcohol consumption was calculated by weighing the bottles immediately before and after the drinking period and expressed as a function of the animal’s body weight (g/kg). Submandibular blood samples were collected from all alcohol-drinking animals on day 11 of drinking, immediately upon conclusion of the 2-h drinking period. Blood alcohol concentration (BAC) was determined using an Analox alcohol analyzer (model AM1, Analox Instruments USA, Lunenburg, MA).
2.4 Behavioral testing
Based on our previous work showing robust anxiety in adult drinkers during acute (24-h) alcohol withdrawal and adolescent drinkers during protracted (4-weeks), both adult and adolescent drinkers and (water-drinking controls) were behaviorally tested at PND 70 as indicated in Figure 1. Thus, behavioral testing was conducted during adulthood for both age groups. However, in this study, the terms ‘adult’ and ‘adolescent’ refer to the age of alcohol exposure. Behavioral testing was conducted during the animals’ circadian dark phase and consisted of the light-dark box, marble burying test, and Porsolt forced swim test (FST) (exactly as conducted in Lee et al., 2016, 2017a, 2017c). In the light-dark box, the dependent measures were: the number of light-side entries, latency to first light-side entry, total time spent on the light side, and the total distance traveled. Decreased interaction with the light side compared to control animals was interpreted as a sign of increased anxiety. Distance traveled provided an index of general locomotor activity. In the marble burying test, the dependent measures were: the number of marbles buried, latency to first begin burying, and total time spent burying. Increased burying behavior was interpreted as a sign of increased anxiety. In the FST, the dependent measures were: the number of immobile episodes, latency to first immobile episode, and total time spent immobile. In prior work, we have observed an age-dependent effect of alcohol withdrawal in this assay; both adults and adolescents exhibit decreased immobility in early withdrawal (Lee et al., 2016), while adolescents exhibit increased immobility during later withdrawal (Lee et al., 2017a, 2017c). Thus, the FST is a sensitive assay of alcohol-induced affective dysregulation that is capable of detecting age by alcohol interactions in behavior
2.5 Subsequent drinking in adulthood
Approximately 24 h following the conclusion of behavioral testing, all animals, including previously alcohol-naïve animals, were subjected to an additional 5 days of drinking (following the same procedures as the initial 14-day exposure) in order to assess the effects of prior alcohol experience and H2-cDNA on alcohol consumption in adulthood. Additionally, the drinking behavior of previously-naïve H2-cDNA alcohol control animals allowed us to characterize the effects of Homer2 expression on binge-drinking in adulthood, independent of previous alcohol experience.
2.6 Brain tissue collection & verification of AAV transduction
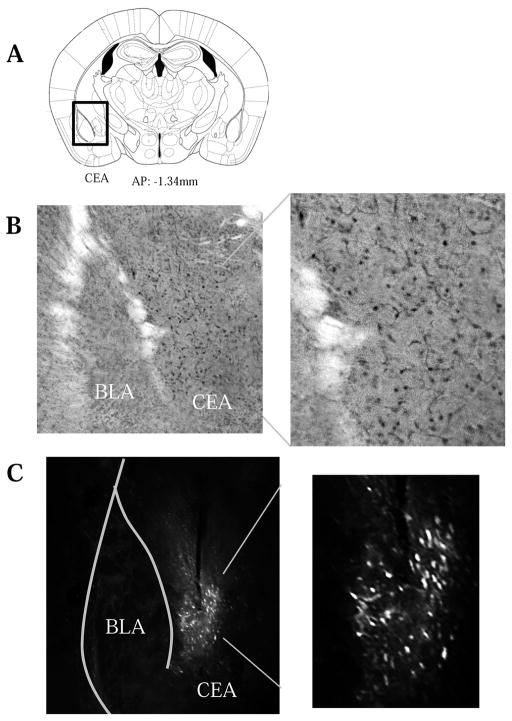
Approximately 24 h following the final alcohol presentation of the 5-day DID, animals were euthanized with an overdose of Euthasol® (Virbac AH, Fort Worth, TX) and transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde, as described previously (e.g. Lee et al., 2015). Brains were removed and cold-stored in cryoprotectant (30% ethylene glycol, 30% glycerol in PBS) until slicing. Tissue was sectioned (40 μm) along the coronal plane on a vibratome at the level of the amygdala (as depicted in Paxinos & Franklin, 2004). Immunohistochemistry was performed using standard procedures employed previously in our laboratory (e.g. Cozzoli et al., 2009; Goulding et al., 2011; Szumlinski, Ary, Lominac, et al., 2008; Szumlinski et al., 2004; Szumlinski et al., 2005) Tissue sections from H2-cDNA infused mice were stained with an antibody against the hemagglutinin (HA) tag using a mouse anti-HA primary antibody (Biolegend, San Diego, CA; 1:1000 dilution), followed by biotinylated anti-mouse secondary IgG (Vector Laboratories, Burlingame, CA; 1:2,000 dilution) and visualized with with 3,3′-diaminobenzidine (DAB). Following staining, sections were mounted on slides, dehydrated, and cover-slipped. GFP transfection of control animals was detected using a GFP tag antibody (Invitrogen, Carlsbad, CA; 1:200 dilution) and fluorescence microscopy. Slides were examined and photographed using a Nikon Eclipse E800 microscope equipped with a Hamamatsu CCD camera (model C4742-95) and MetaMorph® imaging software (Molecular Devices, Sunnyvale, CA). Any animals that failed to show positive AAV transfection within the CEA (Fig. 2) were excluded from the final analysis of the data.
Figure 2.
Verification of viral transduction of neurons selectively within the CEA. (A) Illustration of the sampling region used to verify the subregional selectivity of AAV transduction. Representative 20X micrographs of immunostaining for (B) AAV-transfected HA-tagged Homer2b and (C) AAV-transfected GFP control. The 40X inserts indicate that transduction occurred within both the cell bodies and processes of the neurons.
2.7 Statistical analysis
A repeated-measures ANOVA was used to analyze intake data for all alcohol-drinking animals with age as the between-subject factor in order to determine if there was a difference in alcohol consumption between adults and adolescents across the 14-day drinking period. Adult and adolescent alcohol drinkers were analyzed independently via repeated-measures ANOVA with AAV as the between-subject factor to assess for any intake differences between H2-cDNA and GFP animals. A Pearson’s correlational analysis was conducted for all alcohol-drinking animals to determine the relationship between alcohol intake and resulting BACs sampled on day 11 of drinking. The 5-day drinking data were analyzed separately within each drinking-age group using a repeated-measures ANOVA and Tukey-Kramer multiple comparison tests to compare the effects of H2-cDNA to GFP within alcohol-experienced and -inexperienced animals.
All behavioral data were analyzed separately within each drinking-age group using Tukey-Kramer multiple comparison tests. A significant ANOVA is not a prerequisite for planned pairwise comparison procedures such as Tukey’s HSD, which provides conservative protection against Type I error while maximizing statistical power (Cardinal & Aitken, 2006; Hayter, 1984; Kramer, 1956). This approach is particularly powerful, and often considered preferable to the traditional ANOVA (Games, 1971; Hancock & Klockars, 1996; Rosnow & Rosenthal, 1989; Ruxton & Beauchamp, 2008; Wilkinson & Inference, 1999), in studies such as this that contain multiple a priori comparisons of interest. Specifically, we were interested in between-group comparisons of GFP versus H2-cDNA animals within each drinking group and alcohol versus water within GFP animals (to assess the effect of alcohol, independent of transgenic manipulation). Dependent samples t-tests were conducted separately in alcohol-experienced adult and adolescent drinkers for within-group comparisons of average intake during the initial 14-day drinking period with intake during the subsequent 5-day drinking period.
Statistical outliers were identified using the ±1.5×IQR rule and excluded from analyses. All data depicted in figures represent mean ± SEM of the number of the number of animals indicated in parentheses; α=0.05, though statistical trends (p<0.10) are also reported. Calculations and analyses were performed using add- StatPlus6.0 for Microsoft Excel and SPSS v.21 statistical software (IBM, 2012).
3. Results
3.1Animal exclusion
Animals were omitted entirely from data analysis based unsuccessful or misplaced viral transduction, staining complications rendering it impossible to confirm viral transduction, and attrition due to surgical complications. As presented in Table 1, final sample sizes for analysis were 9–12 per group and the group sizes are indicated in all figures.
Table 1.
Summary of the final sample sizes employed in the statistical analyses of the data.
| Water | Alcohol | |||
|---|---|---|---|---|
| GFP | cDNA | GFP | cDNA | |
| Adults | 11 | 11 | 12 | 11 |
| Adolescents | 12 | 9 | 11 | 10 |
3.2 Alcohol consumption
Across the entire 14-day drinking period, adult animals consumed an average of 2.96 ± 0.12 g/kg and adolescents an average of 6.12 ± 0.19 g/kg. The repeated-measures ANOVA showed a significant between-subjects effect of age [F(1,42)=48.42, p<0.001], but no within-subject effect of Day or Age by Day interaction (p’s>0.05). Thus, adolescents drank significantly more alcohol than adults across the entire 14-day drinking period and adults maintained low levels of alcohol intake throughout. Within each age group, the repeated-measures ANOVA showed no significant difference in alcohol consumption between adult animals previously infused with GFP or H2-cDNA [GFP: 3.01 ± 0.20 g/kg vs. H2-cDNA: 2.93 ± 0.22 g/kg; p=0.793] and there was also no differences in the alcohol intake between adolescent mice subsequently infused with GFP or H2-cDNA [GFP: 5.96 ± 0.35 g/kg vs. H2-cDNA: 6.26 ± 0.32 g/kg; F(1,21)=0.426, p=0.521]. On day 11 of drinking when blood was sampled, adolescent alcohol drinkers had an average alcohol consumption of 6.25 ± 0.29 g/kg with a resulting BAC of 105.49 ± 4.84 mg/dl, which is above the 80 mg/dl NIAAA criterion for binge-drinking. Unfortunately, adult animals had an average alcohol consumption of 2.78 ± 0.17 g/kg with a resulting BAC of 57.27 ± 3.54 mg/dl and thus, were not engaged in binge-drinking. Alcohol intake was significantly correlated with BAC across all animals (r=0.892, p<0.001). While we were concerned that the low levels of alcohol intake by adult mice would be insufficient to elicit changes in affect, little is known regarding the behavioral or biological consequences of repeated low-dose alcohol consumption and thus, we retained the adult animals in the study.
3.3 Effects of alcohol and CEA Homer2 in adolescent animals during protracted withdrawal
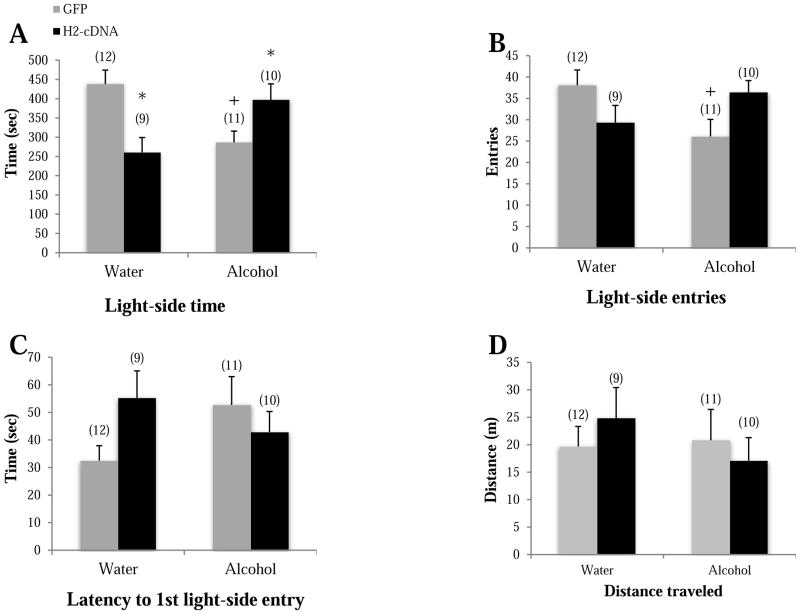
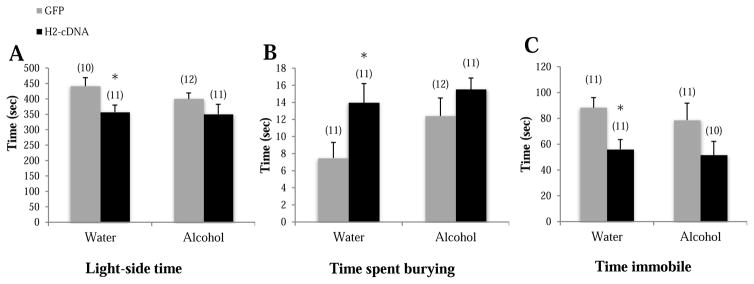
When tested as adults on PND70 in the light-dark box, GFP alcohol-drinking mice spent less time on the light side (p=0.004; Fig. 3A), made fewer light-side entries (p=0.019; Fig. 3B), and trended toward a longer latency to first light-side entry (p=0.079; Fig. 3C), compared to GFP water-drinking mice, supporting greater anxiety-like behavior during protracted withdrawal in adolescent-onset alcohol-drinking mice. In alcohol-drinking mice specifically, H2-cDNA significantly increased light-side time (p=0.039) and a trend toward more light-side entries (p=0.052), compared to GFP-infused alcohol-drinking controls. No H2-cDNA effect was observed on the latency to first light-side entry p>0.10). Thus, CEA H2-cDNA blunts the negative affective state produced by a history of adolescent-onset alcohol-drinking. In water-drinking mice, the effects of H2-cDNA were mixed in the light-dark box test; relative to GFP-infused mice, H2-cDNA significantly decreased light-side time (p=0.002) and tended to increase the latency to first light-side entry (p=0.062), but had no effect on light-side entries (p>0.10). There were no significant group differences in total distance traveled (p’s>0.10; Fig. 3D), indicating that neither a history of adolescent binge-drinking or CEA Homer2 expression influences gross motor activity.
Figure 3.
The effects of adolescent alcohol experience and intra-CEA infusion of Homer2-cDNA (H2-cDNA) on (A) the time on the light side (sec), (B) the number of light-side entries, (C) the latency to the first light entry (sec) and (D) the total distance traveled in the light-side in a light-dark box test (m). In GFP-infused animals (grey bars), alcohol-experienced mice (Alcohol) exhibited signs of hyper-anxiety, but no change in locomotor activity. H2-cDNA infusion (black bars) reversed the hyper-anxiety exhibited by alcohol-experienced mice, while this same treatment produced mixed effects in water-drinking mice. + p<0.05 vs. respective water control (adolescent drinking effect). * p<0.05 vs. respective GFP control (H2-cDNA effect).
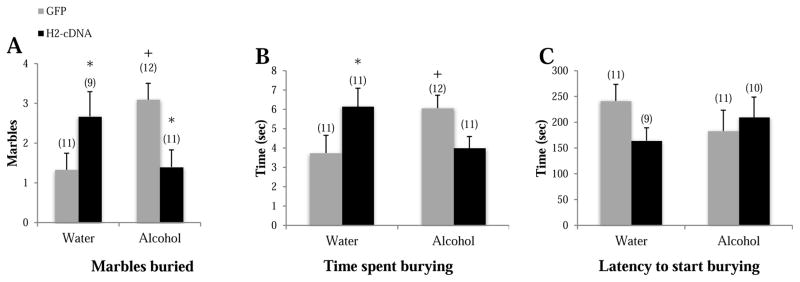
In the marble burying test, GFP alcohol-drinking animals buried more marbles (p=0.008; Fig. 4A) and spent more time burying (p=0.039; Fig. 4B), compared to GFP water-drinking animals, confirming the presence of an anxiogenic state in control mice. In alcohol-drinking mice, H2-cDNA significantly decreased marbles buried (p=0.015) and tended towards less time spent burying (p=0.077) in alcohol-drinking mice, compared to GFP alcohol-drinking animals, but there was no effect on the latency to start burying (p>0.10). In water-drinking animals, H2-cDNA increased marbles buried (p=0.025) and time spent burying (p=0.044), compared to GFP water-drinking animals. There were no significant group differences in the latency to begin burying (p’s>0.10; Fig. 4C). Thus, as observed in the light-dark back, the effects of an intra-CEA H2-cDNA infusion upon an animal’s affective state varied as a function of adolescent-onset drinking experience.
Figure 4.
The effects of adolescent alcohol experience and intra-CEA infusion of Homer2-cDNA (H2-cDNA) on (A) the number of marbles buried, (B) the time spent burying (sec), (C) the latency to begin burying (sec) in a marble-burying test. In GFP-infused animals (grey bars), alcohol-experienced mice (Alcohol) exhibited some signs of hyper-anxiety. H2-cDNA infusion (black bars) reversed the hyper-anxiety exhibited by alcohol-experienced mice, and produced an anxiogenic effect in water-drinking mice. + p<0.05 vs. respective water control (adolescent drinking effect). * p<0.05 vs. respective GFP control (H2-cDNA effect).
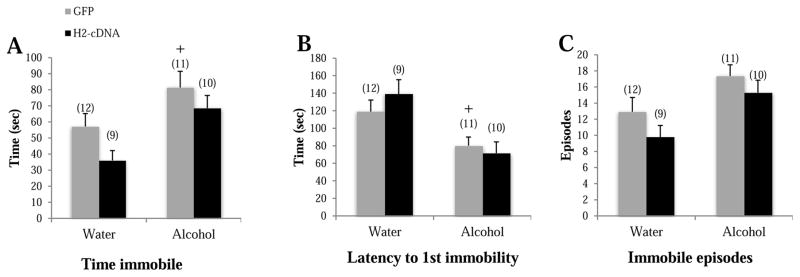
In GFP-infused animals. alcohol-drinking mice spent more time immobile compared to their water-drinking animals (p=0.039; Fig. 5A), had a shorter latency to first immobility (p=0.024; Fig. 5B), and tended to show more immobile episodes (p=0.059; Fig. 5C) in the FST. In water-drinking animals, there was a trend toward less time spent immobile in H2-cDNA animals compared to their GFP controls (p=0.088). However, there was no other effect of H2-cDNA in either the water or alcohol-drinking groups (p’s>0.10). Thus, CEA Homer2 expression does not appear to play a major role in regulating depressive-like behaviors in the FST.
Figure 5.
The effects of adolescent alcohol experience and intra-CEA infusion of Homer2-cDNA (H2-cDNA) on (A) the time spent immobile (sec), (B) the latency to first immobile episode (sec), (C) the number of immobile episodes in a Porsolt swim test. In GFP-infused animals (grey bars), alcohol-experienced mice (Alcohol) exhibited signs of depressive-like behavior. H2-cDNA infusion (black bars) did not significantly influence behavior of either alcohol- or water-drinking (Water) animals. + p<0.05 vs. respective water control (adolescent drinking effect).
3.4 Effects of alcohol and CEA Homer2 in adult animals during early withdrawal
Not surprisingly given their low levels of alcohol intake (see Sect. 3.1.), adult mice infused with GFP did not exhibit signs of anxiety-like behavior on any of our assays (see Table 2 and Fig. 6 grey bars; p’s>0.065). Likewise, there were also no significant effects of H2-cDNA infusion on the behavior of the alcohol-experienced animals (p’s>0.10; Table 2 and Fig. 6, black vs. grey bars).
Table 2.
Summary of the negative results regarding the effects of adult-onset binge-drinking in GFP-infused controls, as well as the effects of intra-CEA H2-cDNA infusion upon our behavioral measures observed in early withdrawal. The data represent the means ± SEMs of the number of mice indicated in parentheses.
| Test | Dependent measure | Water | Alcohol | ||
|---|---|---|---|---|---|
| GFP (11) | H2-cDNA (11) | GFP (12) | H2-cDNA (11) | ||
| Light-dark box | Light-side entries | 36.70 ± 2.84 | 29.36 ± 3.22# | 32.42 ± 2.30 | 28.90 ± 2.83 |
| Time spent on light side | Fig. 6A | ||||
| Latency to first light-side entry | 37.07 ± 5.85 | 44.75 ± 8.63 | 34.34 ± 8.73 | 38.81 ± 4.86 | |
| Marble burying | Marbles buried | 1.18 ± 0.30 | 1.82 ± 0.72 | 1.55 ± 0.39 | 1.09 ± 0.34 |
| Time spent burying | Fig. 6B | ||||
| Latency to begin burying | 106.57 ± 21.44 | 70.28 ± 14.95 | 79.30 ± 16.15 | 94.48 ± 27.11 | |
| Forced swim test | Immobile episodes | 18.91 ± 2.52 | 12.18 ± 2.02# | 15.17 ± 2.08 | 14.00 ± 2.73 |
| Time spent immobile | Fig. 6C | ||||
| Latency to first immobility | 72.85 ± 8.42 | 94.28 ± 9.88 | 67.33 ± 8.99 | 87.86 ± 8.24 | |
p<0.10 vs. water-GFP.
Figure 6.
The effects of adult alcohol experience and intra-CEA infusion of Homer2-cDNA (H2-cDNA) on (A) the time spent in the light-side of the light-dark box (sec), (B) the time spent marble-burying (sec), (C) the total time spent immobile in the FST (sec). The remainder of the data is presented in Table 2. In GFP-infused animals (grey bars), alcohol-experienced mice (Alcohol) failed to exhibit signs of anxiety- or depressive-like behavior. H2-cDNA infusion (black bars) produced an anxiogenic effect in water-drinking controls (Water), but did not significantly influence behavior in alcohol-experienced animals. * p<0.05 vs. GFP control (cDNA effect).
However, we did observe some effects of intra-CEA H2-cDNA infusion on the behavior of water-drinking animals and the significant findings are presented in Fig. 6 (left columns),. Specifically, H2-cDNA animals spent less time on the light-side compared to GFP (p=0.028; Fig. 6A), with a similar trend observed for light-side entries (p=0.075; data not shown), but no effects on the latency to first light-side entry (p>0.10) or distance traveled (p>0.10). In the marble-burying test, H2-cDNA animals spent more time burying compared to GFP (p=0.023; Fig. 6B), but no group differences were observed for the number of marbles buried or the latency to start burying (p’s>0.10). In the FST, H2-cDNA-infused animals spent less time immobile, compared to GFP controls (p=0.042; Fig. 6C), and there was also a trend toward fewer immobile episodes (p=0.062), but no effect on the latency to first immobility in either drinking group (p’s>0.10).
3.5 Effect of H2-cDNA on alcohol consumption in alcohol-experienced and alcohol-inexperienced animals
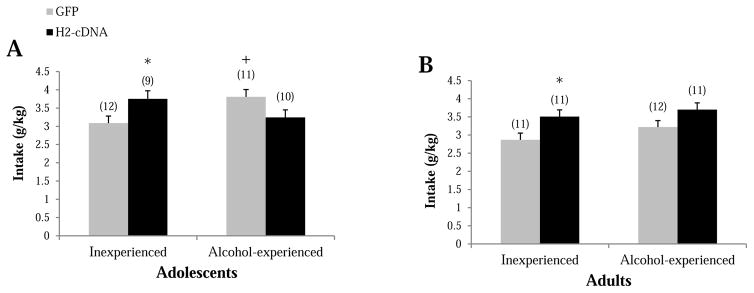
During the subsequent 5-day drinking period, adolescent alcohol-experienced GFP-infused animals drank more alcohol compared to their alcohol-inexperienced GFP counterparts (p=0.013, Fig. 7A), suggesting the maintenance of a more “adolescent-like” drinking phenotype in the alcohol-experienced adolescent controls. H2-cDNA significantly increased intake in alcohol-inexperienced mice, compared to their GFP controls (p=0.029). In contrast, H2-cDNA tended to reduce alcohol consumption in alcohol-experienced adolescent mice (p=0.057; Fig. 7A). There was no difference in intake between adult alcohol-experienced and -inexperienced animals infused with GFP (p>0.10; Fig. 7B). However, as observed for alcohol-inexperienced adolescent mice, H2-cDNA significantly increased alcohol intake in alcohol-inexperienced adult mice (p=0.019; Fig. 7B), with a similar trend observed in alcohol-experienced animals (p=0.066; Fig. 7B). Dependent-samples t-tests conducted within each drinking group showed that both H2-cDNA [t(9)=8.86, p’s<0.001] and GFP [t(10)=6.31, p<0.001] animals with adolescent alcohol experience drank significantly less alcohol, on average, during the subsequent drinking period compared to the initial 14-day drinking period. This likely reflects the well-characterized ontogeny of alcohol-intake (e.g., Lee et al., 2016; Moore et al., 2010; Spear & Varlinskaya, 2005; Strong et al., 2010;), given that the 2nd round of drinking occurred during adulthood (starting on PND 71). In contrast, there was a significant escalation of alcohol intake in H2-cDNA adult alcohol-drinking mice [t(10)=3.68, p=0.004], but not in their GFP-infused counterparts [t(11)=1.12, p=0.288].
Figure 7.
The effects of alcohol experience and intra-CEA infusion of H2-cDNA on the alcohol intake exhibited by mice with a prior history of drinking either water (Inexperienced) or alcohol (Alcohol-experienced) during (A) adolescence or (B) adulthood. In GFP-infused animals (grey bars), adolescent-onset alcohol-experienced mice exhibited greater alcohol intake relative to first-time drinkers. This alcohol history effect was less pronounced in adult mice. H2-cDNA infusion (black bars) augmented the alcohol intake exhibited by first-time drinkers but did not significant alter alcohol intake by alcohol-experienced animals. + p<0.05 vs. respective Inexperienced control (drinking history effect). * p<0.05 vs. GFP control (cDNA effect).
4. Discussion
Replicating our prior work (Lee et al., 2016, 2017a, 2017b, 2017c) and other reports in the literature (Doremus et al., 2005; Moore et al., 2010; Spear & Varlinskaya, 2005; Strong et al., 2010; Vetter et al., 2007), adolescent animals in the present study consumed significantly more alcohol across the 14-day drinking period than adults and were, by definition, engaged in binge-drinking. However, the average alcohol intake and resultant BACs in adult animals were below the levels typically observed under in our multi-bottle, limited alcohol-access, binge-drinking models (e.g. Cozzoli et al., 2014; Lee et al., 2016, 2017a, 2017b, 2017c). Indeed, the adult mice in this study were infused intra-CeA with H2-cDNA/GFP prior to the onset of their initial binge-drinking session, raising the possibility that our AAV infusion procedures or post-infusion effects of these procedures negatively impacted the initial alcohol drinking of the adult mice in the present study. It is noteworthy that we observed no overt signs of AAV-induced neurotoxicity or tissue damage upon histological examination of the adult tissue - the appearance of these sites were comparable to those exhibited by the adolescent-onset animals infused post-drinking. Further, we have never observed detrimental effects of our AAV-GFP vector (or our H2-cDNA vector, for that matter) upon any measure of alcohol reward or reinforcement in adult laboratory rodents when infused 2–3 weeks prior to testing (e.g., Cozzoli et al., 2012, 2014; Haider et al., 2015; Szumlinski et al., 2005, 2008).
While infrequent, it is not unprecedented for an occasional cohort of animals to exhibit low alcohol intakes. To date, we have been unable to pin-point a specific cause for this low-dose alcohol intake, but it does not appear to vary systemically with the season, the colony room in which the mice are housed, the experimenter or the animal care staff. Nevertheless, the adult mice in this study provided an opportunity to extend our earlier indication that the severity of alcohol withdrawal-induced negative affect is proportional to the amount of alcohol consumed during adulthood (Lee et al., 2015, 2017a, 2017c) by demonstrating that a repeated history of low-dose alcohol consumption (~ 3 g/kg/day) produces negligible signs of withdrawal-induced negative affect nor does it increase the subsequent propensity to binge-drink. Thus, while precluding our ability to assay the effects of CEA H2-cDNA infusion upon withdrawal-induced negative affect in adult-onset drinkers, the present results nonetheless argue that repeated low-dose alcohol consumption in adulthood is insufficient to elicit the neurobiological adaptations that drive alcohol withdrawal-induced changes in affect.
Also replicating our prior work (Lee et al., 2015, 2016, 2017a, 2017b, 2017c), a history of binge alcohol-drinking elicited a hyper-anxious state during withdrawal, which is theorized to contribute to the negative reinforcing properties of alcohol abuse (Koob & Le Moal, 1997; Koob & Moal, 1997). This negative affective state is associated with reduced Homer2 expression within the CEA (Lee et al., 2017c) and the present data for adolescent-onset binge-drinking mice provides novel evidence that this neuroadaptation actively regulates alcohol withdrawal-induced anxiety.
4.1 Homer2 cDNA infusion reverses the hyper-anxious phenotype of adolescent-onset drinking mice during protracted withdrawal
Replicating the results from our previous reports (Lee et al., 2016, 2017a, 2017c), animals with adolescent alcohol-experience showed robust signs of negative affect during protracted withdrawal, as indicated by increased indices of anxiety-like behavior in the light-dark box and marble-burying test and increased signs of depression-like ‘behavioral despair’ (i.e. increased immobility) in the FST. The fact that adolescent-onset drinkers do not exhibit overt signs of negative affective during early withdrawal (Lee et al., 2016) argue that this negative affective state, and its neurobiological underpinnings, incubate during protracted withdrawal (Lee et al., 2017c). Indeed, mice with a history of adolescent-onset binge-drinking exhibit a number of protein changes within the AcbSh and CeA in protracted withdrawal that are not manifest in early withdrawal (Lee et al., 2016, 2017c). Further, many of the protein changes observed during protracted withdrawal in adolescent-onset drinkers resemble those exhibited by adult-onset drinkers during early withdrawal when they manifest a hyper-anxious phenotype (Lee et al., 2016, 2017c). One such change that we pursued in the present study is reduced Homer2 expression within the CEA (Lee et al., 2017c). This neuroadaptation is opposite that reported in our prior immunoblotting studies of mice with a 30-day history of binge-drinking (Cozzoli et al., 2014), but its functional relevance has not been pursued in our prior work.
Herein, an intra-CEA infusion of H2-cDNA, administered following a 2-week history of binge-drinking during adolescence, exerted an anxiolytic effect, as indicated by a reversal of some of the signs of anxiety-like behavior in the light-dark box and marble-burying tests. Notably, however, the cDNA-mediated reversal of hyper-anxiety in these assays was not complete; some measures appeared to be more sensitive to the effects of CEA H2-cDNA infusion than others. These data argue, not unexpectedly, that the withdrawal-induced decrease in CEA Homer2 expression is not the sole mediator of withdrawal-induced negative affect. Consistent with this notion, H2-cDNA infusion did not alter any dependent measure in the FST, despite the manifestation of depressive-like signs in GFP-infused controls. The disparate findings between our assays of anxiety- versus depression-like behaviors may relate to the nature of the stressor (physical versus psychological) or the possibility that H2-cDNA selectively gates output from the CEA to mesocorticolimbic structures driving withdrawal-induced anxiety, but not depressive-like states. Nevertheless, it is important to note that not only did intra-CEA H2-cDNA infusion reverse the hyper-anxious phenotype of adolescent-onset drinkers, it also attenuated their subsequent alcohol consumption. These data provide novel evidence to support an important role for CEA Homer2 expression in gating the negative reinforcing properties of alcohol that drive excessive intake. Given the limitations of our study of adult alcohol-experienced animals, an obvious future direction is to replicate this aspect of the experiment in an adult cohort that exhibits binge-levels of alcohol consumption and determine whether or not reversing the reduction in CEA Homer2 expression observed during early withdrawal in adult-onset drinkers can attenuate the manifestation their hyper-anxious phenotype. Further, future studies should probe how Homer2 influences CEA output, as well as characterize the Homer2-dependent molecular interactions within the CEA that drive that output, in animals with histories of adolescent- versus adult-onset drinking to better understand their unique temporal patterning of withdrawal-induced negative affect (Lee et al., 2017c).
4.2 CEA Homer2 cDNA infusion drives hyper-anxiety and alcohol consumption in alcohol-naïve mice
Fascinatingly, the capacity of CEA H2-cDNA infusion to reverse the hyper-anxious phenotype of adolescent-onset binge-drinkers did not reflect some general anxiolytic effect of AAV infusion. In fact, intra-CEA H2-cDNA infusion was anxiogenic in both water-drinking adolescent and adult controls on measures obtained from the marble-burying and light-dark shuttle tests. Furthermore, in adult water-drinking controls, H2-cDNA infusion reduced the time spent immobile in the forced swim test. While reduced immobility in this assay is classically interpreted as an “anti-depressive” phenotype (e.g., Porsolt et al., 1977), we have shown consistently that adult mice in early alcohol withdrawal exhibit reduced immobility on the forced swim test (Lee et al., 2015, 2016, 2017a,b,c) – an effect that can be reversed by pretreatment with anxiolytic drugs (Lee et al., 2017b). Based on this prior work and the fact that H2-cDNA control adults exhibited hyper-anxiety on certain measures in the light-dark shuttle and the marble-burying tests, we interpret the he reduced immobility expressed by these animals in the forced swim test as reflecting a panicked state (see also Ferre et al., 1994).
Further, consistent with the prior evidence that AchSh H2-cDNA infusion augments alcohol reward and reinforcement (Szumlinski et al., 2005, 2008) and our previous indication that CEA Homer2 drives binge-drinking behavior in adult mice (Cozzoli et al., 2014), CEA H2-cDNA infusion also augmented the alcohol intake exhibited by adult mice drinking alcohol for the first time. It is interesting to note that when behavioral effects of H2-cDNA were observed in adult water controls (e.g., Fig. 6; 7b), the direction of the effects was comparable to that observed in their alcohol-experienced counterparts, but with a lesser magnitude (i.e., the GFP-cDNA differences were statistically significant in water controls, but not in alcohol-experienced animals). The present data are the first to demonstrate that CEA H2-cDNA infusion during late adolescence/early adulthood is sufficient to both induce some signs of hyper-anxiety and augment subsequent alcohol consumption, raising the possibility that elevated CEA Homer2 expression might drive directly the negative reinforcing properties of alcohol.
4.3. Reconciling disparities in the role for CEA Homer2 in regulating anxiety
Homer2 is glutamate receptor scaffolding protein that regulates various aspects of glutamate transmission within extended amygdala structures, ranging from the maintenance of basal extracellular glutamate content to the activational state of specific intracellular signaling pathways and its expression is generally considered necessary for normal excitatory neurotransmission (for reviews, Szumlinski et al., 2008). Consistent with this reasoning, the anxiogenic effects of H2-cDNA infusion within the CEA of alcohol-naïve mice are in line with an abundant literature implicating amygdalar hyper-activation in clinical anxiety, depression and withdrawal-induced negative affect (Davidson, 2002; Davis, 1992; Gilpin et al., 2015; Ketter et al., 1996; Servan-Schreiber et al., 1998; Stein et al., 2007). However, our 2-week binge-drinking paradigm reduces CEA Homer2 expression (Lee et al., 2017c) and herein, H2-cDNA infusion exerted an anxiolytic effect on some measures in mice with a prior history of adolescent-onset binge-drinking. While the precise relation between the chronicity/amount of binge-drinking and the direction of the effect of alcohol withdrawal upon CEA Homer2 expression requires a considerable parametric study, the disparities in results across our studies argue that either increased or decreased CEA Homer2 expression can result in the manifestation of a negative affective state that augments subsequent alcohol-taking, likely via shifts in group 1 mGlu receptor function.
Indeed, several lines of evidence support the notion that an imbalance in the relative expression of different Homer isoforms results in behavioral and neurochemical anomalies of relevance to a number of different neuropsychiatric disorders, including depression and addiction (Ary et al., 2013; Gould et al., 2015; Szumlinski et al., 2006). As inducible Homer1 gene products (e.g., Homer1a) act as dominant negatives to disrupt constitutive Homer isoform binding to interacting partners and alter mGlu and NMDA receptor function (e.g., Kammermeier et al., 2000; Park et al., 2013; Xiao et al., 1998), the majority of this work has focused on the interplay between constitutively expressed versus inducible Homer1 gene products (e.g., Klugmann et al., 2005; Lominac et al., 2005; Szumlinski et al., 2006; Tappe and Kuner, 2009). Even within this line of Homer1-related work, both over- or under-expression of Homer1a results in comparable cognitive dysfunction in fear-conditioning models (Banerjee et al., 2016; Mahan et al., 2012), which is a finding in-line with our disparate results for CEA Homer2 expression and anxiety.
To date, we have failed to detect any alcohol-induced changes in the expression of constitutively expressed Homer1 isoforms in the brain across a variety of alcohol delivery paradigms (Cozzoli et al., 2012; Cozzoli et al., 2014; Cozzoli et al., 2009; Goulding et al., 2011; Lee et al., 2015, 2017c; Obara et al., 2009; Szumlinski, Ary, Lominac, et al., 2008). Thus, either an increase or a decrease in CEA Homer2 levels will be predicted to imbalance the relative expression of Homer1 versus Homer2 isoforms in this region. Less well-studied than the effects of imbalancing different Homer1 gene products, we have reported previously that cocaine-induced changes in the relative levels of constitutively expressed Homer1 and Homer2 gene products, at least within the prefrontal cortex, are critical for gating the rewarding and reinforcing properties of this drug (Ary et al., 2013; Gould et al., 2013). Thus, it is entirely possible that any alcohol-induced disruption in the Homer1-Homer2 balance within the CEA is sufficient to produce a negative affective state that drives excessive drinking. Indeed, Homer2 is more occlusive than Homer1 with respect to the efficiency through which Group1 mGlu receptors signal to voltage-gated ion channels in vitro (Kammermeier & Worley, 2007) notably, to inhibit Cav2.2 and Cav2.3 (Beqollari & Kammermeier, 2013; Kammermeier, 2008; Kammermeier et al., 2000; Won et al., 2009), but it remains to be determined how this property relates to Homer2 regulation of group1 mGlu function in vivo, let alone behavior. Given that capacity of an intra-CEA infusion of mGlu1 and mGlu5 inhibitors to regulate binge-drinking require Homer2 (Cozzoli et al., 2014), and systemic treatment with mGlu5 inhibitors blunt alcohol withdrawal-induced negative affect (Lee et al., 2017a), future work will focus on better understanding the biochemical consequences of manipulating CEA Homer2 expression in vivo in relation to the negative reinforcing properties of alcohol.
4.5 Conclusions
In conclusion, the present study provides novel insight into the role of CEA Homer2 in basal and alcohol-induced increases in anxiety, as well as alcohol consumption. Together these data provide evidence of an anxiogenic and pro-drinking effect of H2-cDNA infusion within the CEA. Conversely, restoring CEA Homer2 levels in animals with adolescent alcohol experience exerts a protective effect against withdrawal-induced anxiety and escalated alcohol consumption in adulthood. If relevant to humans, these data argue idiopathic or alcohol-induced changes in CEA Homer2 expression as important for the etiology of negative affect, the negative reinforcing properties of alcohol and the ontogeny of alcohol abuse.
Highlights.
Age-related differences were observed with respect to alcohol intake and the subsequent manifestation of a negative affective state during withdrawal.
The negative affective state elicited by adolescent-onset binge-drinking incubated with the passage of time in withdrawal.
Over-expression of Homer2 within the central nucleus of the amygdala (CeA) exerted an anxiolytic effect and reduced subsequent alcohol consumption in adolescent-onset drinking mice.
Conversely, Homer2 over-expression was anxiogenic and increased drinking in alcohol-naïve controls.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, et al. Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. Journal of Neuroscience. 2013;33(19):8101–8113. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Luong JA, Ho A, Saib AO, Ploski JE. Overexpression of Homer1a in the basal and lateral amygdala impairs fear conditioning and induces an autism-like social impairment. Molecular Autism. 2016;7:16. doi: 10.1186/s13229-016-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beqollari D, Kammermeier PJ. The interaction between mGluR1 and the calcium channel Cav(2).(1) preserves coupling in the presence of long Homer proteins. Neuropharmacology. 2013;66:302–310. doi: 10.1016/j.neuropharm.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386(6622):284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MRF. ANOVA for the behavioural sciences researcher. Mahwah, N.J: L. Erlbaum; 2006. [Google Scholar]
- Centers for Disease Control and Prevention. Binge drinking: Nationwide problem, local solutions. CDC Vital Signs. 2013 Retrieved from https://www.cdc.gov/vitalsigns/bingedrinking/
- Centers for Disease Control and Prevention. Fact Sheets - Underage Drinking. 2014 from http://www.cdc.gov/alcohol/fact-sheets/underage-drinking.htm.
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, et al. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcoholism, Clinical and Experimental Research. 2012;36(9):1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Rostock C, Campbell RR, Wroten MG, McGregor H, et al. Protein Kinase C Epsilon Activity in the Nucleus Accumbens and Central Nucleus of the Amygdala Mediates Binge Alcohol Consumption. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, et al. Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdale. Neuropsychopharmacology. 2014;39(2):435–444. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. Journal of Neuroscience. 2009;29(27):8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, et al. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67:223–232. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M. The Role of the Amygdala in Fear and Anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.neuro.15.1.353. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcoholism, Clinical and Experimental Research. 2005;29(5):902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, Clinical and Experimental Research. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of Alcohol Dependence Among US Adult Drinkers, 2009–2011. Preventing Chronic Disease. 2014;11 doi: 10.5888/pcd11.140329. ARTN E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games PA. Multiple Comparisons of Means. American Educational Research Journal. 1971;8(3):531–565. doi: 10.3102/00028312008003531. [DOI] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biological Psychiatry. 2015;77(10):859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould AT, Sacramento AD, Wroten MG, Miller BW, von Jonquieres G, Klugmann M, et al. Cocaine-elicited imbalances in ventromedial prefrontal cortex Homer1 versus Homer2 expression: implications for relapse. Addiction Biology. 2015;20(1):148–157. doi: 10.1111/adb.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, et al. Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2011;10(1):111–126. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Woodward NC, Lominac KD, Sacramento AD, Klugmann M, Bell RL, et al. Homer2 within the nucleus accumbens core bidirectionally regulates alcohol intake by both P and Wistar rats. Alcohol. 2015;49(6):533–542. doi: 10.1016/j.alcohol.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock GR, Klockars AJ. The quest for alpha: Developments in multiple comparison procedures in the quarter century since Games (1971) Review of Educational Research. 1996;66(3):269–306. [Google Scholar]
- Hasin DS, Beseler CL. Dimensionality of lifetime alcohol abuse, dependence and binge drinking. Drug and Alcohol Dependence. 2009;101(1–2):53–61. doi: 10.1016/j.drugalcdep.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter AJ. A Proof of the Conjecture That the Tukey-Kramer Multiple Comparisons Procedure Is Conservative. Annals of Statistics. 1984;12(1):61–75. doi: 10.1214/aos/1176346392. [DOI] [Google Scholar]
- Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. Journal of Neuroscience. 2008;28(34):8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(14):6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. Journal of Neuroscience. 2000;20(19):7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI, et al. Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Archives of General Psychiatry. 1996;53(1):59–69. doi: 10.1001/archpsyc.1996.01830010061009. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Szumlinski KK. Targeting Homer genes using adeno-associated viral vector: lessons learned from behavioural and neurochemical studies. Behavioural Pharmacology. 2008;19(5–6):485–500. doi: 10.1097/FBP.0b013e32830c369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Frontiers in psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Moal ML. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kramer CY. Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications. Biometrics. 1956;12(3):307–310. doi: 10.2307/3001469. [DOI] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. Journal of Neurophysiology. 2007;98(6):3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK. Binge alcohol drinking elicits persistent negative affect in mice. Behavioural Brain Research. 2015;291:385–398. doi: 10.1016/j.bbr.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, Szumlinski KK. mGlu5-dependent modulation of anxiety during withdrawal from binge-drinking in adult and adolescent male mice. Drug and Alcohol Dependence. 2017a doi: 10.1016/j.drugalcdep.2017.10.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK. Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Frontiers in Cellular Neuroscience. 2016;10:265. doi: 10.3389/fncel.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Bocz MD, Szumlinski KK. Anxiolytic effects of buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress. 2017b doi: 10.1177/2470547017712985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Solton NR, Szumlinski KK. Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, but not adult, mice. Frontiers in Psychology. 2017c;8:1128. doi: 10.3389/fpsyg.2017.01128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Current Topics in Behavioral Neurosciences. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Mou L, Shah N, Hu JH, Worley PF, Ressler KJ. Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. Journal of Neuroscience. 2012;32(13):4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Developments in Alcoholism. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol: Clinical and Experimental Research. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., & Institute for Laboratory Animal Research (U.S.) Guide for the care and use of laboratory animals. 2011 (pp. 1 online resource (xxv, 220 pages)). Retrieved from Ebrary. Restricted to UCSD IP addresses http://site.ebrary.com/lib/ucsd/docDetail.action?docID=10443276.
- National Academies Press. http://uclibs.org/PID/227484.
- Nixon K, McClain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry. 2010;23(3):227–232. doi: 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcoholism, Clinical and Experimental Research. 2009;33(11):1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- Peluso MA, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, et al. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Research. 2009;173(2):158–161. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R. Statistical Procedures and the Justification of Knowledge in Psychological Science. American Psychologist. 1989;44(10):1276–1284. doi: 10.1037//0003-066x.44.10.1276. [DOI] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behavioral Ecology. 2008;19(3):690–693. doi: 10.1093/beheco/arn020. [DOI] [Google Scholar]
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug and Alcohol Dependence. 2007;89(1):82–92. doi: 10.1016/j.drugalcdep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Perlstein WM, Cohen JD, Mintun M. Selective pharmacological activation of limbic structures in human volunteers: a positron emission tomography study. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(2):148–159. doi: 10.1176/jnp.10.2.148. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. Contributions of the Central Extended Amygdala to Fear and Anxiety. Journal of Neuroscience. 2016;36(31):8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biology. 2007;8(2):206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacology & Therapeutics. 2005;107(1):80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. European Journal of Biochemistry. 2000;267(3):634–639. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology. 2014;41:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism. 2005;17:143–159. [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164(2):318–327. doi: 10.1176/appi.ajp.164.2.318. [DOI] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones and Behavior. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Current Topics in Behavioral Neurosciences. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31(4):768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochemical Pharmacology. 2008;75(1):112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33(6):1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43(3):401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, et al. Homer2 is necessary for EtOH-induced neuroplasticity. Journal of Neuroscience. 2005;25(30):7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD. The human adolescent brain and alcohol use disorders. Recent Developments in Alcoholism. 2005;17:177–197. doi: 10.1007/0-306-48626-1_9. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21(4):717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Schmeisser MJ, Sala C, Boeckers TM. Scaffold proteins at the postsynaptic density. Advances in Experimental Medicine and Biology. 2012;970:29–61. doi: 10.1007/978-3-7091-0932-8_2. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism, Clinical and Experimental Research. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L, Inference TFS. Statistical methods in psychology journals - Guidelines and explanations. American Psychologist. 1999;54(8):594–604. doi: 10.1037//0003-066x.54.8.594. [DOI] [Google Scholar]
- Won YJ, Puhl HL, 3rd, Ikeda SR. Molecular reconstruction of mGluR5a-mediated endocannabinoid signaling cascade in single rat sympathetic neurons. Journal of Neuroscience. 2009;29(43):13603–13612. doi: 10.1523/JNEUROSCI.2244-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]