Abstract
Background
Alzheimer’s disease (AD) biomarkers are emerging as critically important for disease detection and monitoring. Most biomarkers are obtained through invasive, resource-intense procedures. A cognitive marker, intra-individual cognitive variability (IICV) may provide an alternative or adjunct marker of disease risk for individuals unable or disinclined to undergo lumbar puncture.
Objective
To contrast risk of incident AD and mild cognitive impairment (MCI) associated with IICV to risk associated with well-established biomarkers: cerebrospinal fluid (CSF) phosphorylated tau protein (p-tau181) and amyloid-β 42 (Aβ42) peptide.
Methods
Dispersion in cognitive performance, IICV, was estimated with a published algorithm, and included Trail Making Test A and B, Rey Auditory Verbal Learning Test (RAVLT), and the American National Adult Reading Test (ANART). CSF biomarkers were expressed as a ratio: p-tau181/Aβ42, wherein high values signified pathognomonic profiles. Logistic regression models included longitudinal data from 349 Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants who completed lumbar puncture. All subjects were cognitively healthy (N=105) or diagnosed with MCI (N=244) at baseline. We examined odds of conversion associated with baseline elevations in IICV and/or ratio of CSF p-tau181/Aβ42.
Results
When included in models alone or in combination with CSF p-tau181/Aβ42, one standard IICV unit higher was associated with an estimated odds ratio for incident AD or MCI of 2.81 (95% CI: 1.83–4.33) in the most inclusive sample, and an odds ratio of 3.41 (95% CI: 2.03–5.73) when restricted to participants with MCI. Iterative analyses suggested that IICV independently improved model fit even when individual index components were included in comparative models.
Conclusions
These analyses provide preliminary support for IICV as a marker of incident AD and MCI. This easily-disseminated, non-invasive marker compared favorably to well-established CSF biomarkers.
Keywords (MeSH terms): Cognition, Alzheimer’s Disease, Mild Cognitive Impairment, cognitive dysfunction, biological markers, amyloid beta-Protein, tau Protein, cerebrospinal fluid, incidence studies
Introduction
As the prevalence of Alzheimer’s disease (AD) rises, strategies to address the disease have shifted toward prevention, targeting the disease when it is active but pre-symptomatic [1]. Effectively monitoring AD in preclinical phases requires the use of disease biomarkers. Among the most promising biomarkers are cerebrospinal fluid (CSF) levels of phosphorylated tau protein (p-tau181), amyloid-β 42 (Aβ42) peptide, or the ratio of these analytes (e.g., p-tau181/Aβ42). Although CSF analytes are considered among the most direct measures of the disease’s hallmark neuropathological features [2–4], collection of CSF biomarkers is invasive and aversive to many potential research participants, driving a need to develop non-invasive, convenient markers [5, 6].
We recently demonstrated a relationship between a potential cognitive marker of preclinical disease, intra-individual cognitive variability (IICV), and another robust neuroimaging biomarker, longitudinal hippocampal volume loss (HVL) [7]. The proposed cognitive marker represents the within-person standard deviation across cognitive tests completed during a single session, an approached used by Holtzer et al. [8], and is grounded on the principle put forward by many others [9–16] that a high level of within-person cognitive variability is a marker of brain pathology.
Like Holtzer et al. [8], our approach to estimating IICV emphasizes detection of differences in cognitive performance across domains measured in a single assessment. We selected indices which could be expected to diverge early in dementia due to AD. For example, performing poorly on executive functioning tasks (e.g., Trail Making Test B) in the context of a strong performance on a simple attentional task (e.g., Trail Making Test A) would suggest that an individual is exhibiting executive dysfunction unrelated to simple processing speed and visual scanning [17]. A disparity in performance between the tests would identify someone with very early executive dysfunction yet well-preserved sensory motor abilities, as is typically observed in early AD.
Adding to other investigations of IICV and risk for dementia [8–11, 13, 14], we found that IICV predicted incident AD and mild cognitive impairment (MCI) similar to HVL and Apolipoprotein E (APOE) e4 status [7]. Similarly, using data from the Wisconsin Registry for Alzheimer’s Prevention (WRAP), we noted IICV measured in a younger, i.e., middle-aged cohort, predicted incident cognitive decline occurring approximately a decade later [18].
In the present analysis of Alzheimer’s Disease Neuroimaging Initiative [19–21] (ADNI) data, we contrasted associations of IICV and p-tau181/Aβ42 to incident AD and MCI. Consistent with our overall goal to examine the utility of a non-invasive, easily implemented alternative strategy to traditional biomarkers, we hypothesized that IICV would compare favorably to an established biomarker of underlying AD pathology, i.e., p-tau181/Aβ42. Secondly, using a larger sample of ADNI participants, not limited to those with CSF analyte data, we examined whether individual IICV predicted risk of conversion over and above the individual components of IICV, hypothesizing that IICV would survive a stepwise backward selection analysis, comparing the summary index to its individual components.
Materials and Methods
Study Design
In an ex-post facto designed analysis, cross-sectional measurement of IICV and p-tau181/Aβ42 were used to predict our primary longitudinal outcome: conversion from cognitive healthy to MCI or AD, or from MCI to AD.
Description of ADNI
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI is to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical/neuropsychological assessment can be combined to measure the progression of MCI and early AD. For information, see www.adni-info.org [19–21].
Participants
Participants were enrolled at ADNI sites in the United States and Canada during three ADNI funding cycles (ADNI-1, ADNI-2, and ADNI-GO) [19–21]. All subjects were between age 55 and 91 at baseline; English or Spanish language speakers; non-depressed; and in one of three diagnostic categories: early AD, MCI, or cognitively healthy. Cognitive status was confirmed using cut-off scores from the Clinical Dementia Rating Scale, Mini-Mental State Examination (MMSE), and Wechsler Memory Scale Logical Memory II. Complete ADNI exclusion criteria are found at www.adni-info.org [22]. For the first 24 months, evaluations occurred every 6 months, and every 12 months thereafter, with a mean total follow-up time of 78.2 months (SD=29.7). Results of cognitive assessments, physical examinations, and MRI scans were considered in determining diagnostic status.
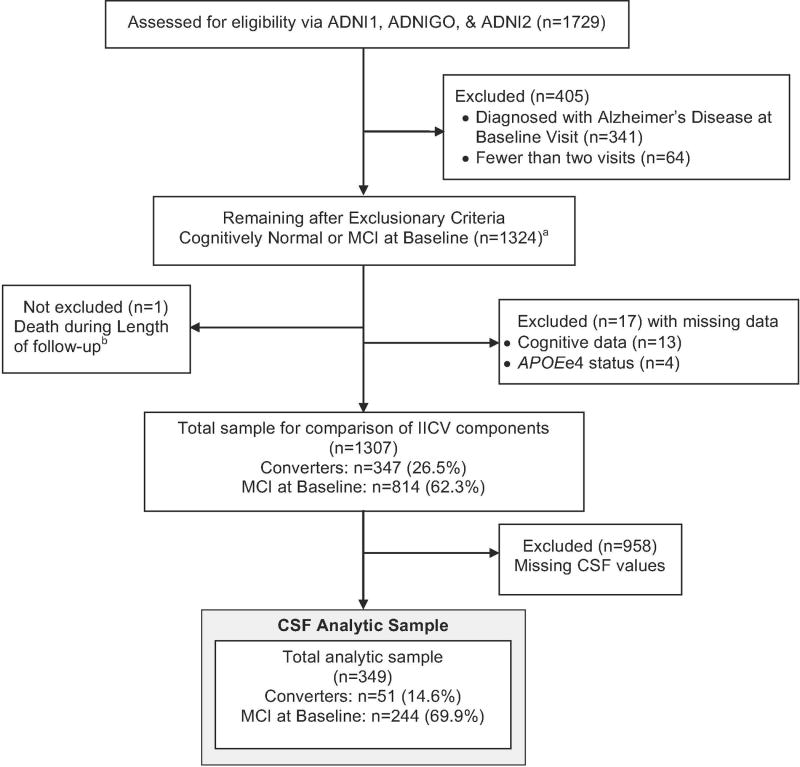
Figure 1 depicts the derivation of the analytic sample. The initial subject pool included 1729 participants with visits up to 10/15/2016. We excluded subjects with fewer than 2 visits, missing demographic data, or baseline diagnosis of AD. After exclusionary criteria were applied, 1307 participants remained in the sample. An analysis comparing the contribution of the components of the IICV to the index itself was performed using this sample. Only a limited number of subjects had CSF data available, resulting in a CSF analytic subsample of 349.
Figure 1.
CONSORT Diagram – Sample derivation for logistic regression model including subjects with complete longitudinal and APOE e4 and CSF data. Analytic model included baseline age, years of education, APOE e4 status, years of follow-up, and baseline diagnosis.
aThe distributions of cognitive test scores from 1324 participants were used to derive IICV estimates.
bOne subject died during length of follow-up. The subject was included; brain autopsy was used for the final diagnosis instead of clinical examination.
Participants provided written, informed consent per ADNI protocols; study sites obtained approval by the local institutional review boards, ensuring that procedures involving experiments on human subjects were done in accord with the ethical standards.
Cognitive Variability
An IICV index, depicting variability at a single time point was calculated following a previously applied method [8]. Briefly, four test scores were selected to detect across-domain “peaks and valleys” typical in early AD. Specific indices included: Rey Auditory Verbal Learning Test (RAVLT) Total of Learning Trials (memory), American National Adult Reading Test total score (ANART; crystallized reading ability), and Trail Making Test A and B (TMT A and B; speeded attention and executive function, respectively). Prior to calculating baseline IICV index, individual test scores were standardized to mean=0 and SD=1 using score distributions based on the most inclusive sample (n=1324). In addition, standardized TMT scores were multiplied by −1 so that positive z-scores represented better performance for all four test scores. The IICV index corresponded to the standard deviation of four z-transformed baseline scores. Consistency between test scores, regardless of value, resulted in low IICVs, whereas extreme highs and lows between test scores produced high IICVs.
CSF Measurement
CSF data were from the ‘UPENNBIOMK5_10_31_13.csv’ dataset, using CSF aliquots from ADNI-GO and ADNI-2. Details of CSF biomarker analyses are available on the ADNI website.[23] Briefly, CSF Aβ42 and p-tau181 were measured using an xMAP Luminex platform (Luminex Corp) and Innogenetics/Fujirebio AlzBio3 immunoassay kits, following published protocols [24], and standardized with replicate aliquots.
Primary Outcome: Diagnostic Conversion
At each visit, participants were evaluated and diagnosis determined by ADNI clinical investigators. In our analyses, we compared diagnosis at baseline to diagnosis at last available follow-up visit. Conversion was defined as change in diagnosis from cognitively healthy status to MCI or AD, or from MCI to AD.
Statistical Analysis
We previously reported results from survival models including IICV and HVL.[7] In the current project, logistic regression models (R version 3.2.3. [25]) were used to examine whether IICV and ratio of CSF analytes were associated with odds of conversion from a cognitively healthy state to MCI or AD, or MCI to AD, through subjects’ entire follow-up. After cases with missing data were excluded, the IICV analytic sample included 1307 participants. A sub-sample for whom CSF findings were available included n=349. Comparisons of participant demographics by baseline diagnostic groups were performed with Mann-Whitney U tests for continuous variables and Fisher’s exact tests for categorical data.
In logistic regression models, our “base model” included predictors: baseline age, years of education, APOE e4 status, baseline diagnostic status (MCI or cognitively healthy), and total post-baseline follow-up time. Response was conversion status at end of total follow-up time. We tested the following models: 1) base model plus IICV; 2) base model plus ratio of CSF p-tau181/Aβ42, and 3) base model with IICV and CSF p-tau181/Aβ42. Models were tested with subjects whose baseline diagnostic status was either MCI or cognitively healthy, and in a sample restricted to individuals with baseline diagnosis of MCI. Due to low rate of conversion in subjects who were cognitively healthy at baseline, an analysis restricted to this subset was not attempted. Only ten of the 105 individuals in the CSF analytic subsample who were cognitively healthy at baseline (9.5%) converted diagnostically.
To allow for direct comparison of regression coefficients, IICV and CSF ratio values were standardized for analyses (mean of zero and SD = 1). For each item, 1 standard deviation increase in the item’s raw value represented an estimated odds ratio (OR) of conversion of exp(β), where β is the estimated logistic regression coefficient for that item. When not standardized to a z-score scale, the standard deviations of IICV and CSF ratio in these data were 0.405 and 0.201, respectively.
To examine whether individual components of IICV account for associated risk more than variability between scores, we tested a model including base model predictors, IICV, and the individual IICV components (TMT A and B, ANART, and RAVLT raw scores). In other words, does IICV add explanatory power over and above its individual component scores. A stepwise backwards selection was performed on 5 cognitive items (4 individual component scores and IICV), using an Akaike Information Criterion (AIC) [26]. AIC was utilized over other backward selection methods in effort to prioritize relative proximity to the true data generating model, favoring inclusion over exclusion of convergent variables. The AIC method examines the degree to which removal of any component scores or IICV reduced the AIC index and simplified model fit for prediction. An iterative process continued until: (1) removal of a component or IICV score did not reduce AIC or (2) all 5 cognitive predictors were removed.
Results
Participants were average age 72.4 years (SD=7.09) at baseline, and well-educated (mean=16.2 years). Table 1 lists participant characteristics by baseline diagnosis. Most participants were white, n=325 (93.1%), and non-Hispanic n=341 (98.8%). Compared to the larger pool of ADNI Participants included in the CSF analytic subsample were slightly younger than individuals included in the full IICV analytic sample, and less impaired on the Rey AVLT with lower IICV scores (data not included).
Table 1.
Participant characteristics at baseline
| Characteristic | Full Sample with CSF data N=349 | Cognitively Healthy N=105 | Participants wtth MCI N=244 | Comparison of Cognitively healthy and MCI P-value |
|---|---|---|---|---|
| Mean Baseline Age, years (SD) | 72.4 (7.09) | 74.5 (5.84) | 71.5 (7.39) | <0.001 |
| Mean follow-up time, years (SD) | 2.32 (0.682) | 2.01 (0.421) | 2.45 (0.730) | <0.001 |
| Mean Education, years (SD) | 16.2 (2.63) | 16.5 (2.61) | 16.1 (2.64) | 0.221 |
| APOE e4 status, N(%) | - | - | - | - |
| e4 Positive | 132 (37.8) | 24 (23) | 108 (44) | <0.001 |
| e4 Negative | 217 (62.2) | 81 (77) | 136 (56) | |
| Race, N(%) | - | - | - | - |
| African American | 8 (2.3) | 3 (2.9) | 5 (2.0) | 0.914 |
| American Indian/Alaska Native | 2 (0.57) | 1 (0.95) | 1 (0.41) | |
| Asian American | 4 (1.1) | 1 (0.95) | 3 (1.2) | |
| Native Hawaiian/Pacific Islander | 1 (0.29) | 0 (0.00) | 1 (0.41) | |
| White | 325 (93.1) | 99 (94) | 226 (93) | |
| More than one race | 7 (2.0) | 1 (0.95) | 6 (2.5) | |
| Unknown | 2 (0.57) | 0 (0.00) | 2 (0.82) | |
| Ethnicity, N(%) | - | - | - | - |
| Non-Hispanic | 341 (98.8) | 104 (99) | 237 (97) | 0.202 |
| Hispanic | 6 (1.7) | 0 (0.00) | 6 (2.4) | |
| Unknown | 2 (0.57) | 1 (0.95) | 1 (0.41) | |
| Conversion status, N(%) | - | - | - | - |
| No conversion | 298 (85.4) | 95 (90.5) | 203 (83.2) | 0.098 |
| Converted to MCI | 9 (2.6) | 9 (8.6) | - | - |
| Converted to AD | 42 (12.0) | 1 (0.9) | 41 (16.8) | - |
| Mean IICV, value (SD) | 0.620 (0.315) | 0.575 (0.274) | 0.639 (0.329) | 0.198 |
| Mean ANART, score (SD) | 11.0 (8.00) | 9.48 (7.22) | 11.6 (8.24) | 0.015 |
| Mean RAVLT, total of learning trials (SD) | 39.4 (10.9) | 44.4 (10.0) | 37.3 (10.6) | <0.001 |
| Mean time to complete Trails A. seconds (SD) | 36.4 (13.4) | 33.8 (9.78) | 37.5 (14.5) | 0.095 |
| Mean time to complete Trails B. seconds (SD) | 97.7 (52.7) | 83.6 (42.0) | 104 (55.6) | <0.001 |
| Mean CSF Aβ42, ng/l (SD) | 179 (49.9) | 188 (48.4) | 176 (50.2) | 0.029 |
| Mean CSF p-tau181, ng/l (SD) | 38.6 (22.8) | 34.0 (15.3) | 40.5 (25.1) | 0.085 |
| Mean p-tau/Aβ42 ratio (SD) | 0.252 (0.201) | 0.203 (0.127) | 0.273 (0.222) | 0.047 |
Abbreviations:
APOE: Apolipoprotein E
MCI: Mild Cognitive Impairment
AD: Alzheimer’s disease
IICV: Intra-individual cognitive variability
ANART: American National Adult Reading Test
RAVLT: Rey Auditory Verbal Learning Test
Statistical tests:
P-values are from Mann-Whitney U tests for continuous items and Fisher’s Exact test for categorical items
Note:
The CSF analytic sample is a subset of the full IICV dataset, precluding group comparisons.
Mean length of follow-up was 2.32 years (SD=0.682). In total, 42 (12.0%) of the 349 subjects who contributed CSF converted to AD and 9 (2.6%) converted to MCI (Table 1) Converters differed from non-converters in an expected pattern. They were older, performed more poorly on cognitive measures, and demonstrated lower levels of CSF Aβ42 and higher levels of CSF tau181.
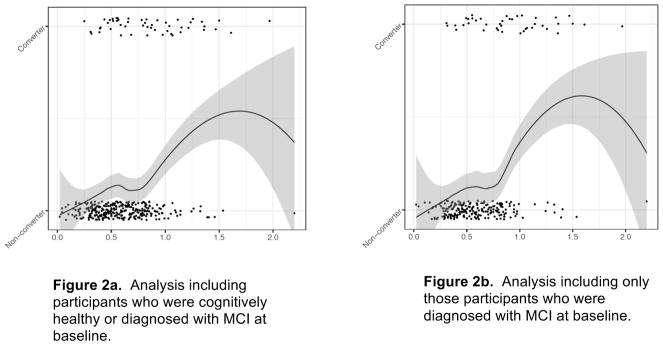
Results from logistic regression models suggest that an increase in IICV elevated an individual’s odds of incident AD and MCI. Figure 2 depicts the positive association of IICV with the proportion of participants who converted diagnostically. The association occurred across the range of IICV values. Regression models in Table 2 indicate an association of CSF with conversion odds in the full sample (n=349) and when the sample is restricted to individuals with a baseline diagnosis of MCI (n=244). Specifically, inclusion of IICV alone resulted in ORs between 2.39 (95% CIs 1.60 to 3.56) and 2.77 (95% CIs 1.72 to 4.46), depending on whether cognitively healthy adults were included in the analytic sample.
Figure 2.
IICV vs. Conversion status with local smoothing trend (LOESS). Data are jittered around conversion status to better illustrate data densities. Figure 2a includes participants with MCI and those who were cognitively healthy at baseline (N=349). Figure 2b includes only those individuals with MCI at baseline (N=244).
Notes:
IICV: Intra-Individual Cognitive Variability. Used raw IICV score for comparison. Conversion is a dichotomous variable: Converters vs. Non-converters. The trend line is smoothed using LOESS in order to illustrate the relationship between conversion and IICV score without linearity assumptions. Additionally, IICV scores are jittered to more clearly see the number of participants obtaining individual IICV scores.
Table 2.
Logistic regression models
| Full Sample, including cognitively healthy and participants with MCI N=349 | Subset of participants with MCI at baseline N=244 | |||||
|---|---|---|---|---|---|---|
| Predictor | OR | 95%CI | P-value | OR | 95%CI | P-value |
| IICV alone | 2.39 | (1.60, 3.56) | <0.0001 | 2.77 | (1.72, 4.46) | <0.0001 |
| Ratio of CSF p-tau181/Aβ42 alone | 2.13 | (1.52, 2.99) | <0.0001 | 2.16 | (1.49, 3.13) | <0.0001 |
| IICV and CSF analytes together | - | - | - | - | - | - |
| IICV | 2.81 | (1.83, 4.33) | <0.0001 | 3.41 | (2.03, 5.73) | <0.0001 |
| Ratio of CSF p-tau181/Aβ42 | 2.48 | (1.72, 3.60) | <0.0001 | 2.63 | (1.73, 4.01) | <0.0001 |
Notes:
Base Model included baseline age, baseline education, APOE e4 status and years of follow-up. For analyses including cognitively healthy participants and those with MCI, base model also includes baseline diagnostic status.
IICV was estimated using baseline data.
Abbreviations:
MCI: Mild Cognitive Impairment
IICV: Intra-individual cognitive variability
CSF: Cerebrospinal fluid
OR: Odd ratio
CI: Confidence Interval
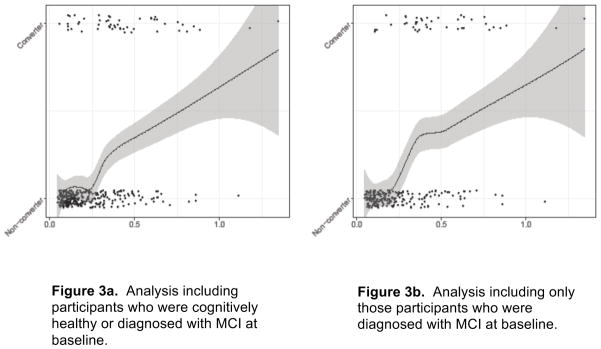
Similarly, estimated OR’s of conversion for 1 standard deviation increase in CSF ratio p-tau181/Aβ42 are 2.13 to 2.16, depending upon the subsample (95% CIs span 1.49 to 3.13). Figure 3 depicts the association of the ratio of standardized CSF p-tau181/Aβ42 and conversion. Like analysis of IICV and conversion, elevated ratio of p-tau181/Aβ42 corresponded to elevations in diagnostic conversion.
Figure 3.
Ratio of CSF p-tau181/Aβ42 vs. Conversion status with local smoothing trend (LOESS). Data are jittered around conversion status to better illustrate data densities. Figure 3a includes participants with MCI and those who were cognitively healthy at baseline (N=349). Figure 3b includes only those individuals with MCI at baseline (N=244).
Notes:
Conversion is a dichotomous variable: Converters vs. Non-converters. The trend line is smoothed using LOESS in order to illustrate the relationship between conversion and CSF biomarkers without linearity assumptions. Additionally, values for the ratio of CSF analytes were jittered to more clearly see the number of participants obtaining individual ratio values.
When IICV and the ratio of CSF analytes were included in the model together, IICV continued associate with conversion odds (See Table 2). When the ratio of CSF analytes was added to models including IICV, the association between IICV and conversion appears to increase, with estimated OR’s between 2.81 (95% CIs 1.83 to 4.33) and 3.41 (95% CIs 2.03 to 5.73), depending on whether cognitively healthy individuals were included in the analysis.
Lastly, we examined whether the individual components of IICV outperformed the overall estimate of variability (i.e., IICV) in predicting conversion to MCI or AD, using a larger analytic sample, not restricted to participants with CSF data (n=1307). Subject characteristics for this group of subjects are provided in Supplementary Table 1. An initial examination comparing conversion status with mean test performance revealed that conversion was not related to overall performance on cognitive tests (see Supplementary Figure 1). Nonetheless, the importance of IICV over and above its individual test components were compared using an AIC backward selection process.
Table 3 displays the backwards selection results comparing IICV with its component tests. Overall, it appears that TMT A does not provide additional explanatory information compared to other items. Importantly, IICV was not excluded in this iterative analysis, and the final model was restricted to ANART, RAVLT, TMT B and IICV. IICV was associated with odds of conversion over and above the tests used to calculate IICV. Thus, IICV appears to provide additional explanatory information beyond its component tests when predicting conversion.
Table 3.
Comparison of IICV components to IICV. Used Akaike Information Criterion (AIC) stepwise backward selection to examine contribution of IICV and individual components of IICV to model fit.
| Predictors and order dropped, or P-Value of retained | ||||||
|---|---|---|---|---|---|---|
| Baseline Diagnostic Status | Selection criteria | IICV | Trails A | Trials B | ANART | RAVLT |
| MCI or Cognitively Healthy N=1307 |
AIC | 0.0153 | 1st | 0.0001 | 0.0028 | <0.0001 |
| MCI N=814 |
AIC | 0.0060 | 1st | <0.0001 | 0.0003 | <0.0001 |
Note: P-values provided by AIC models indicate whether predictors offer predictive value, not the magnitude of added value.
Abbreviations:
MCI: Mild Cognitive Impairment
IICV: Intra-individual cognitive variability
ANART: American National Adult Reading Test
RAVLT: Rey Auditory Verbal Learning Test
AIC: Akaike Information Criterion
Discussion
In these analyses of ADNI data, IICV, an indicator of dispersion between cognitive test scores was associated with elevated odds of conversion from MCI to AD or from a cognitively healthy state to MCI or AD. The IICV index used here characterized dispersion in cognitive abilities including speeded visual motor sequencing with and without an additional divided attention component, crystalized verbal abilities, and verbal memory. Whether used with or without its individual component tests, or the ratio of CSF p-tau181/Aβ42, IICV obtained at a single time point was associated with increased odds of developing incident AD and MCI.
We have previously shown in survival analyses that IICV predicted time to diagnostic conversion. Specifically, our models suggested that an estimate of IICV predicted time to incident AD and MCI even after accounting for a neuroimaging biomarker, HVL and a genetic risk factor, APOE e4 status [7]. Similarly, in a middle-aged cohort enrolled in WRAP study, we noted IICV measured at midlife predicted incident cognitive decline approximately a decade after baseline.[18] In the current analyses we sought to examine how IICV compared to CSF biomarkers.
As anticipated, the ratio of CSF values of phosphorylated tau (p-tau181) and Aβ42 were associated with elevated odds of conversion. These findings are consistent with previously published ADNI analyses, revealing associations between clinical symptoms, incident disease and CSF analytes [27, 28]. A recent summary analysis of multiple studies examining CSF biomarkers for AD-associated neurodegeneration suggested that CSF analytes are robust indicators of underlying disease pathology [2]. In particular, CSF Aβ42, p-tau, total tau (t-tau), and neurofilament light protein (NFL) emerged among several candidate CSF biomarkers as the most useful for differential diagnosis. While unquestionably valuable, these data can only be obtained via lumbar puncture. This invasive procedure must be performed by trained medical personnel in a clinical research setting. Moreover, based on historical research abuses, like the Tuskegee Study of Untreated Syphilis in the Negro Male [29], the LP procedure is associated with one of the most egregious and symbolic cases of research misconduct for African Americans [30]. For participants unwilling to complete an LP, IICV may offer an alternative.
Obtaining a reliable IICV is dependent upon having trained examiners. This would require concerted but not insurmountable effort. Moreover, it is possible that IICV would lend itself to laptop or tablet platforms, reducing the skill level needed to collect data. Altogether, there is justification to explore IICV and other alternatives to CSF biomarkers, especially low-cost, easily disseminated alternatives, not associated with burdensome procedures, especially aversive for African Americans.
When models included both the ratio of CSF p-tau181/Aβ42 and IICV, the odds of incident AD or MCI associated with elevations in IICV remained relevant. Indeed, odds ratios for these predictors remained relatively stable in iterative models, suggesting unique predictive contributions from both IICV and ratio of CSF analytes. Indeed there appears to be little correlation between the two predictors. Supplementary Figure 2 shows a plot of IICV to the ratio of CSF p-tau181/Aβ42. Although studies examining the association of cognitive performance and CSF analytes in pre-clinical or cognitively healthy subjects have reported somewhat mixed findings, Pettigrew et al. [31] and others [4] found associations between cognitive factors and CSF amyloid and CSF tau. Analyses presented here suggest that the processes underlying cognitive variability and those sub-serving CSF amyloid and tau abnormalities may differ, possibly explaining inconsistences in findings, and why others have reported that CSF amyloid and especially tau findings were uncoupled from cognition in the preclinical stages (e.g., Li et al. [32] and Rolstad et al. [33]). Moreover, the finding that neither IICV and CSF biomarkers appeared to suppress the effect of the other when both were included in the model, suggests that the two predictors of risk could be used together in models examining risk for AD.
The concept that dispersion in performance signifies underlying disease is an established and deep-rooted theory in cognitive psychology [9–16]. Many others have examined the relationship between cognitive variability and neurological and psychiatric illnesses, including dementia [7–11, 13, 34–36]. Explanations for the link between inconsistency and brain disease include impaired neural networks, impaired functional connectivity, as well as executive dysfunction [37, 38]. The premise of these theories is that disease results in a disrupted ability to maintain consistent mental efforts.
Like Holtzer et al. [8], our approach to estimating IICV focused on consistency across cognitive domains, rather than consistency between trials of the same task. We intentionally selected indices which could be expected to diverge early in the AD course. For example, Trail Making Test B was contrasted to Trail Making Test A. Trails A requires a person to connect a series of numbered circles as quickly as possible; whereas in Trails B, the individual must alternate between numbers and letters, connecting the circles as rapidly as possible. A disparity in performance between the tests would identify someone with very early executive dysfunction yet well-preserved sensory motor abilities, as is typically observed in AD.
The remaining two cognitive tests contributing to the IICV index used here were included to maximize detection of early AD-related disparities in cognitive performance. A list learning test (RAVLT), selected as a measure of hippocampal-based learning [39], was contrasted with a test of reading ability estimating baseline crystallized intelligence (e.g., semantic knowledge) [40]. In total, the cognitive indices included in our measure of variability were selected if they were likely to either reflect a typically well-preserved ability or a typically diminished ability, representing the peaks and valleys in a cognitive profile.
The older participants included in these analyses were followed for an average of 2.32 years. It is unknown how early cognitive variability appears prior to a diagnosis. Holtzer et al.[8] found that IICV predicted incident dementia on average 3.3 years after initial evaluation. Participants were ~79 y/o and cognitively normal at baseline. In our two previously published papers, we demonstrated IICV’s predictive utility in two different groups, over disparate time periods. Similar to Holtzer et al., we found that older ADNI subjects (~74 y/o) who were cognitive normal or diagnosed with MCI on average evidenced cognitive variability 30.81 months prior to diagnosis [7]. In contrast, Koscik et al. demonstrated the utility of IICV in a much younger cohort (~53 y/o) over a period of 8 to 10 years [18]. In total, variability in cognitive performance appears to indicate elevated risk for later cognitive impairment and/or dementia. The exact time frame for prediction is not yet clear and may depend on how IICV is estimated and/or the characteristics of the cohort being examined.
In our final analysis, we sought to examine the overall contribution of IICV to elevated risk versus the risk for conversion associated with the components of IICV. IICV survived an iterative examination, wherein elements were individually removed from models, suggesting that IICV informed risk for conversion, over and above the contribution of its components alone.
Among the limitations in our analyses, we highlight that incident cognitive impairment in this ADNI sample primarily represented conversion from MCI to AD, i.e., conversion within a clinical population. Given the low rate of conversion in cognitively healthy subjects (N=10), we are unable to make definitive statements regarding detection of preclinical risk. However, in our previous analysis of WRAP data, our sample was middle-aged and cognitively healthy at baseline [18]. IICV predicted incident cognitive decline in the preclinical WRAP cohort. Nonetheless, we emphasize the need for replication, especially in younger cohorts, more remote from conversion. Notably, ADNI participants with MCI were selected for inclusion based on their poor performance on memory measures in the presence of spared global cognition [22]. At the outset, individuals with MCI included in this analysis would demonstrate greater cognitive variability than the general population. Finally, an additional limitation is the relative homogeneity of the sample. Subjects were predominantly non-Hispanic and white, reflecting a selection bias toward those willing to enroll in a longitudinal study involving lumbar puncture. IICV may not be associated with diagnostic conversion for Hispanic, non-white groups or for cases of mixed or non-AD dementias.
Conclusions
If replicated, our IICV findings provide support for a practical alternative to traditional biomarkers. This option would be especially important when patients and subjects are unable or unwilling to travel to research settings, or averse to invasive procedures like lumbar punctures. Moreover, a cognitive marker could be widely disseminated with minimal effort or equipment demands. Altogether, using IICV may permit broader ability to identify at-risk individuals than traditional biomarkers.
Supplementary Material
Acknowledgments
Contributions by first author CEG and co-authors DN, NMD, DTW and RLK are supported by funding from NIH-NIA for African Americans Fighting Alzheimer’s at Midlife (AA-FAIM, R01 AG054059) and the Wisconsin Alzheimer’s Disease Center (P50 AG033514). For coauthors MW, SCJ, CMC, RLK and SA efforts were also supported by funding from the NIH-NIA for the Wisconsin Alzheimer’s Disease Center (WRAP, P50 AG033514). Additional support in the form of statistical and scientific collaboration was provided for co-authors RLK, SCJ and CMC by the Wisconsin Alzheimer’s Institute.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Co-investigator appendix
Michael Weiner, MD (UC San Francisco, PI of ADNI), Paul Aisen, MD (University of Southern California, ADCS PI and Director of Coordinating Center Clinical Core), Michael Weiner, MD (UC San Francisco, Executive Committee), Paul Aisen, MD (University of Southern California, Executive Committee), Ronald Petersen, MD, PhD (Mayo Clinic, Rochester, Executive Committee), Clifford R. Jack, Jr., MD (Mayo Clinic, Rochester, Executive Committee), William Jagust, MD (UC Berkeley, Executive Committee), John Q. Trojanowki, MD, PhD (U Pennsylvania, Executive Committee), Arthur W. Toga, PhD (USC, Executive Committee), Laurel Beckett, PhD (UC Davis, Executive Committee), Robert C. Green, MD, MPH (Brigham and Women’s Hospital/Harvard Medical School, Executive Committee), Andrew J. Saykin, PsyD (Indiana University, Executive Committee), John Morris, MD (Washington University St. Louis, Executive Committee), Leslie M. Shaw, PhD (University of Pennsylvania, Executive Committee), Adam Schwartz, MD (Eli Lilly, ADNI 2 Private Partner Scientific Board (Chair)), Robert C. Green, MD, MPH (Brigham and Women’s Hospital/Harvard Medical School, Data and Publication Committee (Chair)), Tom Montine, MD, PhD (University of Washington, Resource Allocation Review Committee (Chair)), Ronald Petersen, MD, PhD (Mayo Clinic, Rochester, Clinical Core Leaders (Core PI)), Paul Aisen, MD (University of Southern California, Clinical Core Leaders), Ronald G. Thomas, PhD (UC San Diego, Clinical Informatics and Operations), Michael Donohue, PhD (UC San Diego, Clinical Informatics and Operations), Sarah Walter, MSc (UC San Diego, Clinical Informatics and Operations), Devon Gessert (UC San Diego, Clinical Informatics and Operations), Tamie Sather, MA (UC San Diego, Clinical Informatics and Operations), Gus Jiminez, MBS (UC San Diego, Clinical Informatics and Operations), Archana B. Balasubramanian, PhD (UC San Diego, Clinical Informatics and Operations), Jennifer Mason, MPH (UC San Diego, Clinical Informatics and Operations), Iris Sim (UC San Diego, Clinical Informatics and Operations), Laurel Beckett, PhD (UC Davis, Biostatistics Core Leaders and Key Personnel (Core PI)), Danielle Harvey, PhD (UC Davis, Biostatistics Core Leaders and Key Personnel), Michael Donohue, PhD (UC San Diego, Biostatistics Core Leaders and Key Personnel), Clifford R. Jack, Jr., MD (Mayo Clinic, Rochester, MRI Core Leaders and Key Personnel (Core PI)), Matthew Bernstein, PhD (Mayo Clinic, Rochester, MRI Core Leaders and Key Personnel), Joel Felmlee, PhD (Mayo Clinic, Rochester, MRI Core Leaders and Key Personnel), Nick Fox, MD (University of London, MRI Core Leaders and Key Personnel), Paul Thompson, PhD (UCLA School of Medicine, MRI Core Leaders and Key Personnel), Norbert Schuff, PhD (UCSF MRI, MRI Core Leaders and Key Personnel), Charles DeCarli, MD (UC Davis, MRI Core Leaders and Key Personnel), Bret Borowski, RT (Mayo Clinic, MRI Core Leaders and Key Personnel), Jeff Gunter, PhD (Mayo Clinic, MRI Core Leaders and Key Personnel), Matt Senjem, MS (Mayo Clinic, MRI Core Leaders and Key Personnel), Prashanthi Vemuri, PhD (Mayo Clinic, MRI Core Leaders and Key Personnel), David Jones, MD (Mayo Clinic, MRI Core Leaders and Key Personnel), Kejal Kantarci (Mayo Clinic, MRI Core Leaders and Key Personnel), Chad Ward (Mayo Clinic, MRI Core Leaders and Key Personnel), William Jagust, MD (UC Berkeley, PET Core Leaders and Key Personnel (Core PI)), Robert A. Koeppe, PhD (University of Michigan, PET Core Leaders and Key Personnel), Norm Foster, MD (University of Utah, PET Core Leaders and Key Personnel), Eric M. Reiman, MD (Banner Alzheimer’s Institute, PET Core Leaders and Key Personnel), Kewei Chen, PhD (Banner Alzheimer’s Institute, PET Core Leaders and Key Personnel), Chet Mathis, MD (University of Pittsburgh, PET Core Leaders and Key Personnel), Susan Landau, PhD (UC Berkeley, PET Core Leaders and Key Personnel), John C. Morris, MD (Washington University St. Louis, Neuropathology Core Leaders), Nigel J. Cairns, PhD, (FRCPath Washington University St. Louis, Neuropathology Core Leaders), Erin Franklin, MS, CCRP (Washington University St. Louis, Neuropathology Core Leaders), Lisa Taylor-Reinwald, BA, HTL (Washington University St. Louis, Neuropathology Core Leaders), Leslie M. Shaw, PhD (UPenn School of Medicine, Biomarkers Core Leaders and Key Personnel), John Q. Trojanowki, MD, PhD (UPenn School of Medicine, Biomarkers Core Leaders and Key Personnel), Virginia Lee, PhD, MBA (UPenn School of Medicine, Biomarkers Core Leaders and Key Personnel), Magdalena Korecka, PhD (UPenn School of Medicine, Biomarkers Core Leaders and Key Personnel), Michal Figurski, PhD (UPenn School of Medicine, Biomarkers Core Leaders and Key Personnel), Arthur W. Toga, PhD (USC, Informatics Core Leaders and Key Personnel (Core PI)), Karen Crawford (USC, Informatics Core Leaders and Key Personnel), Scott Neu, PhD (USC, Informatics Core Leaders and Key Personnel), Andrew J. Saykin, PsyD (Indiana University, Genetics Core Leaders and Key Personnel), Tatiana M. Foroud, PhD (Indiana University, Genetics Core Leaders and Key Personnel), Steven Potkin, MD, (UC Irvine, Genetics Core Leaders and Key Personnel), Li Shen, PhD (Indiana University, Genetics Core Leaders and Key Personnel), Kelley Faber, MS, CCRC (Indiana University, Genetics Core Leaders and Key Personnel), Sungeun Kim, PhD (Indiana University, Genetics Core Leaders and Key Personnel), Kwangsik Nho, PhD (Indiana University, Genetics Core Leaders and Key Personnel) Michael W. Weiner, MD (UC San Francisco, Initial Concept Planning & Development), Lean Thal, MD (UC San Diego, Initial Concept Planning & Development), Zaven Khachaturian, PhD (Prevent Alzheimer’s Disease 2020, Initial Concept Planning & Development), Leon Thal, MD (UC San Diego, Early Project Proposal Development), Neil Buckholtz (National Institute on Aging, Early Project Proposal Development), Michael W. Weiner, MD (UC San Francisco, Early Project Proposal Development), Peter J. Snyder, PhD (University of Connecticut, Early Project Proposal Development), William Potter, MD (National Institute of Mental Health, Early Project Proposal Development), Steven Paul, MD (Cornell University, Early Project Proposal Development), Marilyn Albert, PhD (John Hopkins University, Early Project Proposal Development), Richard Frank, MD, PhD (Richard Frank Consulting, Early Project Proposal Development), Zaven Khachaturian, PhD (Prevent Alzheimer’s Disease 2020, Early Project Proposal Development),
John Hsiao, MD (National Institute on Aging, NIA), Jeffrey Kaye, MD (Oregon Health and Science University, Site Investigator), Joseph Quinn, MD (Oregon Health and Science University, Site Investigator), Lisa Silbert, MD (Oregon Health and Science University, Site Investigator), Betty Lind, BS (Oregon Health and Science University, Site Investigator), Raina Carter, BA (Oregon Health and Science University, Past Site Investigator), Sara Dolen, BS (Oregon Health and Science University, Past Site Investigator), Lon S. Schneider, MD (University of Southern California, Site Investigator), Sonia Pawluczyk, MD (University of Southern California, Site Investigator), Mauricio Becerra, BS (University of Southern California, Site Investigator), Liberty Teodoro, RN (University of Southern California, Site Investigator) Bryan M. Spann, DO, PhD (University of Southern California, Past Site Investigator), James Brewer, MD, PhD (University of California--San Diego, Site Investigator), Helen Vanderswag, RN (University of California--San Diego, Site Investigator), Adam Fleisher, MD (University of California--San Diego, Past Site Investigator), Judith L. Heidebrink, MD, MS, (University of Michigan, Site Investigator), Joanne L. Lord, LPN, BA, CCRC (University of Michigan, Past Site Investigator), Ronald Petersen, MD, PhD (Mayo Clinic, Rochester, Site Investigator), Sara S. Mason, RN (Mayo Clinic, Rochester, Site Investigator), Colleen S. Albers, RN (Mayo Clinic, Rochester, Site Investigator), David Knopman, MD (Mayo Clinic, Rochester, Site Investigator), Kris Johnson, RN (Mayo Clinic, Rochester, Past Site Investigator), Rachelle S. Doody, MD, PhD (Baylor College of Medicine, Site Investigator), Javier Villanueva-Meyer, MD (Baylor College of Medicine, Site Investigator), Valory Pavlik, PhD (Baylor College of Medicine, Site Investigator), Victoria Shibley, MS (Baylor College of Medicine, Site Investigator), Munir Chowdhury, MBBS, MS (Baylor College of Medicine, Past Site Investigator), Susan Rountree, MD (Baylor College of Medicine, Past Site Investigator), Mimi Dang, MD (Baylor College of Medicine, Past Site Investigator), Yaakov Stern, PhD (Columbia University Medical Center, Site Investigator), Lawrence S. Honig, MD, PhD (Columbia University Medical Center, Site Investigator), Karen L. Bell, MD, (Columbia University Medical Center, Site Investigator), Beau Ances, MD (Washington University, St. Louis, Site Investigator), John C. Morris, MD (Washington University, St. Louis, Site Investigator), Maria Carroll, RN, MSN (Washington University, St. Louis, Site Investigator), Mary L. Creech, RN, MSW (Washington University, St. Louis, Site Investigator), Erin Franklin, MS, CCRP (Washington University, St. Louis, Site Investigator) Mark A. Mintun, MD (Washington University, St. Louis, Past Site Investigator), Stacy Schneider, APRN, BC, GNP (Washington University, St. Louis, Past Site Investigator), Angela Oliver, RN, BSN, MSG (Washington University, St. Louis, Past Site Investigator), Daniel Marson, JD, PhD (University of Alabama – Birmingham, Site Investigator), David Geldmacher, MD (University of Alabama – Birmingham, Site Investigator), Marissa Natelson Love, MD (University of Alabama – Birmingham, Site Investigator), Randall Griffith, PhD, ABPP (University of Alabama – Birmingham, Past Site Investigator), David Clark, MD (University of Alabama – Birmingham, Site Investigator), Hillel Grossman, MD (Mount Sinai School of Medicine, Past Site Investigator), Erik Poberson, MD (University of Alabama – Birmingham, Past Site Investigator), Hillel Grossman, PhD (Mount Sinai School of Medicine, Site Investigator), Effie Mitsis, PhD (Mount Sinai School of Medicine, Site Investigator), Raj C. Shah, MD (Rush University Medical Center, Site Investigator), Leyla deToledo-Morrell, PhD (Rush University Medical Center, Past Site Investigator), Ranjan Duara, MD (Wein Center, Site Investigator), Maria T. Greig-Custo, MD (Wein Center, Site Investigator), Warren Barker, MA, MS (Wein Center, Site Investigator), Marilyn Albert, PhD (Johns Hopkins University, Site Investigator), Chiadi Onyike, MD, (Johns Hopkins University, Site Investigator), Daniel D’Agostino II, BS (Johns Hopkins University, Site Investigator), Stephanie Kielb, BS (Johns Hopkins University, Past Site Investigator), Martin Sadowski, MD, PhD (New York University, Site Investigator), Mohammed O. Sheikh, MD (New York University, Site Investigator), Anaztasia Ulysse (New York University, Site Investigator), Mrunalini Gaikwad (New York University, Site Investigator), P. Murali Doraiswamy, MBBS, FRCP (Duke University Medical Center, Site Investigator), Jeffrey R. Petrella, MD (Duke University Medical Center, Site Investigator), Salvador Borges-Neto, MD (Duke University Medical Center, Site Investigator), Terence Z. Wong, MD (Duke University Medical Center, Past Site Investigator), Edward Coleman (Duke University Medical Center, Past Site Investigator), Steven E. Arnold, MD (University of Pennsylvania, Site Investigator), Jason H. Karlawish, MD, (University of Pennsylvania, Site Investigator), David A. Wolk, MD (University of Pennsylvania, Site Investigator), Christopher M. Clark, MD (University of Pennsylvania, Site Investigator), Charles D. Smith, MD (University of Kentucky, Site Investigator), Greg Jicha, MD (University of Kentucky, Site Investigator), Peter Hardy, PhD (University of Kentucky, Site Investigator), Partha Sinah, PhD (University of Kentucky, Site Investigator), Elizabeth Oates, MD (University of Kentucky, Site Investigator), Gary Conrad, MD (University of Kentucky, Site Investigator), Oscar L. Lopez, MD (University of Pittsburgh, Site Investigator), MaryAnn Oakley, MA (University of Pittsburgh, Site Investigator), Donna M. Simpson, CRNP, MPH (University of Pittsburgh, Site Investigator), Anton P. Porsteinsson, MD (University of Rochester Medical Center, Site Investigator), Bonnie S. Goldstein, MS, NP (University of Rochester Medical Center, Site Investigator), Kim Martin, RN (University of Rochester Medical Center, Site Investigator), Kelly M. Makino, BS (University of Rochester Medical Center, Past Site Investigator), M. Saleem Ismail, MD (University of Rochester Medical Center, Past Site Investigator), Connie Brand, RN (University of Rochester Medical Center, Past Site Investigator), Steven G. Potkin, MD (University of California, Irvine, Site Investigator), Adrian Preda, MD (University of California, Irvine, Site Investigator), Dana Nguyen, PhD (University of California, Irvine, Site Investigator), Kyle Womack, MD (University of Texas Southwestern Medical School, Site Investigator), Dana Mathews, MD, PhD (University of Texas Southwestern Medical School, Site Investigator), Mary Quiceno, MD (University of Texas Southwestern Medical School, Site Investigator), Allan I. Levey, MD, PhD (Emory University, Site Investigator), James J. Lah, MD, PhD (Emory University, Site Investigator), Janet S. Cellar, DNP, PMHCNS-BC (Emory University, Site Investigator), Jeffrey M. Burns, MD (University of Kansas, Medical Center, Site Investigator), Russell H. Swerdlow, MD (University of Kansas, Medical Center, Site Investigator), (University of Kansas, Medical Center, Site Investigator), William M. Brooks, PhD (University of Kansas, Medical Center, Site Investigator), Liana Apostolova, MD (University of California, Los Angeles, Site Investigator), Kathleen Tingus, PhD(University of California, Los Angeles, Site Investigator), Ellen Woo, PhD (University of California, Los Angeles, Site Investigator), Daniel H.S. Silverman, MD, PhD (University of California, Los Angeles, Site Investigator), Po H. Lu, PsyD (University of California, Los Angeles, Past Site Investigator), George Bartzokis, MD (University of California, Los Angeles, Past Site Investigator), Neill R Graff-Radford, MBBCH, FRCP (London) (Mayo Clinic, Jacksonville, Site Investigator), Francine Parfitt, MSH, CCRC (Mayo Clinic, Jacksonville, Site Investigator), Kim Poki-Walker, BA (Mayo Clinic, Jacksonville, Site Investigator), Martin R. Farlow, MD (Indiana University, Site Investigator), Ann Marie Hake, MD, Brandy R. Matthews, MD (Indiana University, Past Site Investigator), Jared R. Brosch (Indiana University, Site Investigator), Scott Herring, RN (Indiana University, Site Investigator), Christopher H. van Dyck, MD (Yale University School of Medicine, Site Investigator), Richard E. Carson, PhD (Yale University School of Medicine, Site Investigator), Martha G. MacAvoy, PhD (Yale University School of Medicine, Site Investigator), Pradeep Varma, MD (Yale University School of Medicine, Site Investigator), Howard Chertkow, MD (McGill Univ., Montreal-Jewish General Hospital, Site Investigator), Howard Bergman, MD (McGill Univ., Montreal-Jewish General Hospital, Site Investigator), Chris Hosein, MEd (McGill Univ., Montreal-Jewish General Hospital, Site Investigator), Sandra Black, MD, FRCPC (Sunnybrook Health Sciences, Ontario, Site Investigator), Bojana Stefanovic, PhD (Sunnybrook Health Sciences, Ontario, Site Investigator), Curtis Caldwell, PhD (Sunnybrook Health Sciences, Ontario, Site Investigator), Ging-Yuek Robin Hsiung, MD, MHSc, FRCPC (U.B.C. Clinic for AD & Related Disorders, Site Investigator), Benita Mudge, BS (U.B.C. Clinic for AD & Related Disorders, Site Investigator), Vesna Sossi, PhD (U.B.C. Clinic for AD & Related Disorders, Site Investigator), Howard Feldman, MD, FRCPC (U.B.C. Clinic for AD & Related Disorders, Past Site Investigator), Michele Assaly, MA (U.B.C. Clinic for AD & Related Disorders, Past Site Investigator), Elizabeth Finger, MD (Cognitive Neurology - St. Joseph’s, Ontario, Site Investigator), Stehphen Pasternack, MD, PhD (Cognitive Neurology - St. Joseph’s, Ontario, Site Investigator), Irina Rachisky, MD (Cognitive Neurology - St. Joseph’s, Ontario, Site Investigator), Andrew Kertesz, MD (Cognitive Neurology - St. Joseph’s, Ontario, Past Site Investigator), Dick Trost, PhD (Cognitive Neurology - St. Joseph’s, Ontario, Past Site Investigator), Charles Bernick, MD (Cleveland Clinic Lou Ruvo Center for Brain Health, Site Investigator), Donna Munic, PhD (Cleveland Clinic Lou Ruvo Center for Brain Health, Site Investigator), Marek-Marsel Mesulam, MD (Northwestern University, Site Investigator), Emily Rogalski, PhD (Northwestern University, Site Investigator), Kristina Lipowski, MA (Northwestern University, Site Investigator), Sandra Weintraub, PhD, (Northwestern University, Site Investigator), Borna Bonakdarpour, MD (Northwestern University, Site Investigator), Diana Kerwin, MD (Northwestern University, Past Site Investigator), Chuang-Kuo Wu, MD, PhD (Northwestern University, Past Site Investigator), Nancy Johnson, PhD (Northwestern University, Past Site Investigator), Carl Sadowsky, MD (Premiere Research Inst (Palm Beach Neurology), Site Investigator), Teresa Villena, MD (Premiere Research Inst (Palm Beach Neurology), Site Investigator), Raymond Scott Turner, MD, PhD (Georgetown University Medical Center, Site Investigator), Kathleen Johnson, NP (Georgetown University Medical Center, Site Investigator), Brigid Reynolds, NP (Georgetown University Medical Center, Site Investigator), Reisa A. Sperling, MD (Brigham and Women’s Hospital, Site Investigator), Keith A. Johnson, MD (Brigham and Women’s Hospital, Site Investigator), Gad Marshall, MD (Brigham and Women’s Hospital, Past Site Investigator), Jerome Yesavage, MD (Stanford University, Site Investigator), Joy L. Taylor, PhD (Stanford University, Site Investigator), Barton Lane, MD (Stanford University, Site Investigator), Allyson Rosen, PhD (Stanford University, Past Site Investigator), Jared Tinklenberg, MD (Stanford University, Past Site Investigator), Marwan Sabbagh, MD, FAAN, CCRI (Banner Sun Health Research Institute, Site Investigator), Christine Belden, PsyD (Banner Sun Health Research Institute, Site Investigator), Sandra Jacobson, MD (Banner Sun Health Research Institute, Site Investigator), Sherye A. Sirrel, CCRC (Banner Sun Health Research Institute, Site Investigator), Neil Kowall, MD (Boston University, Site Investigator), Ronald Killiany, PhD (Boston University, Site Investigator), Andrew E. Budson, MD (Boston University, Site Investigator), Alexander Norbash, MD (Boston University, Past Site Investigator), Patricia Lynn Johnson, BA (Boston University, Past Site Investigator), Thomas O. Obisesan, MD, MPH (Howard University, Site Investigator), Saba Wolday, MSc (Howard University, Site Investigator), Joanne Allard, PhD (Howard University, Site Investigator), Alan Lerner, MD (Case Western Reserve University, Site Investigator), Paula Ogrocki, PhD (Case Western Reserve University, Site Investigator), Curtis Tatsuoka, PhD (Case Western Reserve University, Site Investigator), Parianne Fatica, BA, CCRC (Case Western Reserve University, Site Investigator), Evan Fletcher, PhD (University of California Davis – Sacramento, Site Investigator), Pauline Maillard, PhD (University of California Davis – Sacramento, Site Investigator), John Olichney, MD (University of California, Davis – Sacramento, Site Investigator), Charles DeCarli, MD (University of California, Davis – Sacramento, Past Site Investigator), Owen Carmichael, PhD (University of California, Davis – Sacramento, Past Site Investigator), Smita Kittur, MD (Neurological Care of CNY, Past Site Investigator), Michael Borrie, MB ChB (Parkwood Hospital, Site Investigator), T-Y Lee, PhD (Parkwood Hospital, Site Investigator), Rob Bartha, PhD (Parkwood Hospital, Site Investigator), Sterling Johnson, PhD (University of Wisconsin, Site Investigator), Sanjay Asthana, MD (University of Wisconsin, Site Investigator), Cynthia M. Carlsson, MD, MS (University of Wisconsin, Site Investigator), Steven G. Potkin, MD (University of California, Irvine – BIC, Site Investigator), Adrian Preda, MD (University of California, Irvine – BIC, Site Investigator), Dana Nguyen, PhD (University of California, Irvine – BIC, Site Investigator), Pierre Tariot, MD (Banner Alzheimer’s Institute, Site Investigator), Anna Burke, MD (Banner Alzheimer’s Institute, Site Investigator), Ann Marie Milliken, NMD (Banner Alzheimer’s Institute, Site Investigator), Nadira Trncic, MD, PhD, CCRC (Banner Alzheimer’s Institute, Past Site Investigator), Adam Fleisher, MD (Banner Alzheimer’s Institute, Past Site Investigator), Stephanie Reeder, BA (Banner Alzheimer’s Institute, Past Site Investigator), Vernice Bates, MD (Dent Neurologic Institute, Site Investigator), Horacio Capote, MD (Dent Neurologic Institute, Site Investigator), Michelle Rainka, PharmD, CCRP (Dent Neurologic Institute, Site Investigator), Douglas W. Scharre, MD (Ohio State University, Site Investigator), Maria Kataki, MD, PhD (Ohio State University, Site Investigator), Brendan Kelley, MD (Ohio State University, Site Investigator), Earl A. Zimmerman, MD (Albany Medical College, Site Investigator), Dzintra Celmins, MD (Albany Medical College, Site Investigator), Alice D. Brown, FNP (Albany Medical College, Past Site Investigator), Godfrey D. Pearlson, MD (Hartford Hosp, Olin Neuropsychiatry Research Center, Site Investigator), Karen Blank, MD (Hartford Hosp, Olin Neuropsychiatry Research Center, Site Investigator), Karen Anderson, RN (Hartford Hosp, Olin Neuropsychiatry Research Center, Site Investigator), Laura A. Flashman, PhD (Dartmouth-Hitchcock Medical Center, Site Investigator), Marc Seltzer, MD (Dartmouth- Hitchcock Medical Center, Site Investigator) Mary L. Hynes, RN, MPH (Dartmouth-Hitchcock Medical Center, Site Investigator), Robert B. Santulli, MD (Dartmouth- Hitchcock Medical Center, Past Site Investigator), Kaycee M. Sink, MD, MAS (Wake Forest University Health Sciences, Site Investigator), Leslie Gordineer (Wake Forest University Health Sciences, Site Investigator), Jeff D. Williamson, MD, MHS (Wake Forest University Health Sciences, Past Site Investigator), Pradeep Garg, PhD (Wake Forest University Health Sciences, Past Site Investigator), Franklin Watkins, MD (Wake Forest University Health Sciences, Past Site Investigator), Brian R. Ott, MD (Rhode Island Hospital, Site Investigator), Geoffrey Tremont, PhD (Rhode Island Hospital, Site Investigator), Lori A. Daiello, PharmD, ScM (Rhode Island Hospital, Site Investigator), Stephen Salloway, MD, MS (Butler Hospital, Site Investigator), Paul Malloy, PhD (Butler Hospital, Site Investigator), Stephen Correia, PhD (Butler Hospital, Site Investigator), Howard J. Rosen, MD (UC San Francisco, Site Investigator), Bruce L. Miller, MD (UC San Francisco, Site Investigator), David Perry, MD (UC San Francisco, Site Investigator), Jacobo Mintzer, MD, MBA (Medical University South Carolina, Site Investigator), Kenneth Spicer, MD, PhD (Medical University South Carolina, Site Investigator), David Bachman, MD (Medical University South Carolina, Site Investigator), Elizabether Finger, MD (St. Joseph’s Health Care, Site Investigator), Stephen Pasternak, MD (St. Joseph’s Health Care, Site Investigator), Irina Rachinsky, MD (St. Joseph’s Health Care, Site Investigator), John Rogers, MD (St. Joseph’s Health Care, Site Investigator), Andrew Kertesz, MD (St. Joseph’s Health Care, Past Site Investigator), Dick Drost, MD (St. Joseph’s Health Care, Past Site Investigator), Nunzio Pomara, MD (Nathan Kline Institute, Site Investigator), Raymundo Hernando, MD (Nathan Kline Institute, Site Investigator), Antero Sarrael, MD (Nathan Kline Institute, Site Investigator), Susan K. Schultz, MD (University of Iowa, Site Investigator), Karen Ekstam Smith, RN (University of Iowa, Site Investigator), Hristina Koleva, MD (University of Iowa, Site Investigator), Ki Won Nam, MD (University of Iowa, Site Investigator), Hyungsub Shim, MD (University of Iowa, Past Site Investigator), Norman Relkin, MD, PhD (Cornell University, Site Investigator), Gloria Chaing, MD (Cornell University, Site Investigator), Michael Lin, MD (Cornell University, Site Investigator), Lisa Ravdin, PhD (Cornell University), Amanda Smith, MD (University of South Florida, Site Investigator), Balebail Ashok Raj, MD (University of South Florida, Site Investigator), Kristin Fargher, MD (University of South Florida, Past Site Investigator)
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 3.Seppala TT, Nerg O, Koivisto AM, Rummukainen J, Puli L, Zetterberg H, Pyykko OT, Helisalmi S, Alafuzoff I, Hiltunen M, Jaaskelainen JE, Rinne J, Soininen H, Leinonen V, Herukka SK. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology. 2012;78:1568–1575. doi: 10.1212/WNL.0b013e3182563bd0. [DOI] [PubMed] [Google Scholar]
- 4.Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher LC, Burke KE, Caine PL, Rinne NL, Braniff CA, Davis HR, Miles KA, Packer C. Diagnosing Alzheimer’s disease: are we any nearer to useful biomarker-based, non-invasive tests? GMS Health Technol Assess. 2013;9:Doc01. doi: 10.3205/hta000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma SL, Lam LC. Panel of Genetic Variations as a Potential Non-invasive Biomarker for Early Diagnosis of Alzheimer’s Disease. Clin Psychopharmacol Neurosci. 2011;9:54–66. doi: 10.9758/cpn.2011.9.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ED, Wahoske M, Huber M, Norton D, Li Z, Koscik RL, Umucu E, Johnson SC, Jones J, Asthana S, Gleason CE Alzheimer’s Disease Neuroimaging I. Cognitive variability-A marker for incident MCI and AD: An analysis for the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement (Amst) 2016;4:47–55. doi: 10.1016/j.dadm.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300:823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao JL, Cheung RT, Chan YS, Chu LW, Lee TM. Increased prospective memory interference in normal and pathological aging: different roles of motor and verbal processing speed. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20:80–100. doi: 10.1080/13825585.2012.672948. [DOI] [PubMed] [Google Scholar]
- 10.Hilborn JV, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. J Clin Exp Neuropsychol. 2009;31:412–424. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- 11.Ownby RL, Loewenstein DA, Schram L, Acevedo A. Assessing the cognitive abilities that differentiate patients with Alzheimer’s disease from normals: single and multiple factor models. Int J Geriatr Psychiatry. 2004;19:232–242. doi: 10.1002/gps.1056. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald SWS, Hultsch DF, Dixon RA. Performance variability is related to change in cognition: Evidence from the Victoria Longitudinal Study. Psychology and Aging. 2003;18:510–523. doi: 10.1037/0882-7974.18.3.510. [DOI] [PubMed] [Google Scholar]
- 13.Matarazzo JD, Daniel MH, Prifitera A, Herman DO. Inter-Subtest Scatter in the WAIS-R Standardization Sample. Journal of Clinical Psychology. 1988;44:940–950. [Google Scholar]
- 14.Salthouse TA, Soubelet A. Heterogeneous ability profiles may be a unique indicator of impending cognitive decline. Neuropsychology. 2014;28:812–818. doi: 10.1037/neu0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon RA, Lentz TL, Garrett DD, MacDonald SWS, Strauss E, Hultsch DF. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- 16.Bunce D, Bielak AAM, Cherbuin N, Batterham PJ, Wen W, Sachdev P, Anstey KJ. Utility of Intraindividual Reaction Time Variability to Predict White Matter Hyperintensities: A Potential Assessment Tool for Clinical Contexts? Journal of the International Neuropsychological Society. 2013;19:971–976. doi: 10.1017/S1355617713000830. [DOI] [PubMed] [Google Scholar]
- 17.Reitan RM, Wolfson D. Category Test and Trail Making Test as Measures of Frontal-Lobe Functions. Clinical Neuropsychologist. 1995;9:50–56. [Google Scholar]
- 18.Koscik RL, Berman SE, Clark LR, Mueller KD, Okonkwo OC, Gleason CE, Hermann BP, Sager MA, Johnson SC. Intraindividual Cognitive Variability in Middle Age Predicts Cognitive Impairment 8–10 Years Later: Results from the Wisconsin Registry for Alzheimer’s Prevention. J Int Neuropsychol Soc. 2016;22:1016–1025. doi: 10.1017/S135561771600093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner MW, Veitch DP. Introduction to special issue: Overview of Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2015;11:730–733. doi: 10.1016/j.jalz.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aisen PS, Petersen RC, Donohue M, Weiner MW ADNI Investiagators. Alzheimer’s Disease Neuroimaging Initiative 2 Clinical Core: Progress and plans. Alzheimers & Dementia. 2015;11:734–739. doi: 10.1016/j.jalz.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW ADNI Investiagators. Clinical core of the Alzheimer’s disease neuroimaging initiative: Progress and plans. Alzheimers & Dementia. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed December 16, 2016];Alzheimer’s Disease Neuroimaging Initiative Procedures Manual [online] https://adni.ucsd.edu/. Last updated 2006.
- 23. [Accessed on December 16, 2016];Alzheimer’s Disease Neuroimaging Initiative Biomarkers Analysis Manual [online] http://adni.loni.usc.edu/methods/biomarker-analysis/
- 24.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. Vienna Austria: [Accessed December 2016]. [online], https://www.r-project.org/. Last updated 2015. [Google Scholar]
- 26.Seber GAF, Lee AJ. Linear regression analysis. Wiley-Interscience; Hoboken, N.J: 2003. [Google Scholar]
- 27.Andriuta D, Moullart V, Schraen S, Devendeville A, Meyer ME, Godefroy O ADNI Investigators. What are the Most Frequently Impaired Markers of Neurodegeneration in ADNI Subjects? Journal of Alzheimers Disease. 2016;51:793–800. doi: 10.3233/JAD-150829. [DOI] [PubMed] [Google Scholar]
- 28.Dowling NM, Johnson SC, Gleason CE, Jagust WJ ADNI Investigators. The mediational effects of FDG hypometabolism on the association between cerebrospinal fluid biomarkers and neurocognitive function. Neuroimage. 2015;105:357–368. doi: 10.1016/j.neuroimage.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White RM. Effects of untreated syphilis in the negro male, 1932 to 1972: a closure comes to the Tuskegee study, 2004. Urology. 2006;67:654. doi: 10.1016/j.urology.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Shavers VL, Lynch CF, Burmeister LF. Knowledge of the Tuskegee study and its impact on the willingness to participate in medical research studies. J Natl Med Assoc. 2000;92:563–572. [PMC free article] [PubMed] [Google Scholar]
- 31.Pettigrew C, Soldan A, Moghekar A, Wang MC, Gross AL, O’Brien R, Albert M. Relationship between cerebrospinal fluid biomarkers of Alzheimer’s disease and cognition in cognitively normal older adults. Neuropsychologia. 2015;78:63–72. doi: 10.1016/j.neuropsychologia.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, Mayer C, Shofer JS, Raskind MA, Quinn JF, Galasko DR, Montine TJ. Cross-Sectional and Longitudinal Relationships Between Cerebrospinal Fluid Biomarkers and Cognitive Function in People Without Cognitive Impairment From Across the Adult Life Span. JAMA Neurology. 2014;71:742–751. doi: 10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, Wallin A. Amyloid-beta(4)(2) is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J Alzheimers Dis. 2011;26:135–142. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- 34.Reckess GZ, Varvaris M, Gordon B, Schretlen DJ. Within-person distributions of neuropsychological test scores as a function of dementia severity. Neuropsychology. 2014;28:254–260. doi: 10.1037/neu0000017. [DOI] [PubMed] [Google Scholar]
- 35.Shin YS, Kim SN, Shin NY, Jung WH, Hur JW, Byun MS, Jang JH, An SK, Kwon JS. Increased Intra-Individual Variability of Cognitive Processing in Subjects at Risk Mental State and Schizophrenia Patients. Plos One. 2013:8. doi: 10.1371/journal.pone.0078354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinowitz AR, Arnett PA. Intraindividual cognitive variability before and after sports-related concussion. Neuropsychology. 2013;27:481–490. doi: 10.1037/a0033023. [DOI] [PubMed] [Google Scholar]
- 37.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.West R, Murphy KJ, Armilio ML, Craik FIM, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- 39.Creasey H, Rapoport SI. The aging human brain. Ann Neurol. 1985;17:2–10. doi: 10.1002/ana.410170103. [DOI] [PubMed] [Google Scholar]
- 40.Horn JL. Psychometric studies of aging and intelligence. Psychopharmacol Bull. 1975;11:44–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.