Abstract
Background
Delirium has been associated with more rapid cognitive decline. However, it is unknown whether increased delirium severity is associated with a higher rate of long-term cognitive decline.
Objective
To evaluate delirium severity and the presence and rate of cognitive decline over 36 months following surgery.
Methods
We examined patients from the Successful Aging after Elective Surgery Study, who were age ≥70 years undergoing major elective surgery (N=560). Delirium severity was determined by the peak Confusion Assessment Method-Severity (CAM-S) score for each patient’s hospitalization and grouped based on the sample distribution: scores of 0–2, 3–7, and 8–19. A neuropsychological composite, General Cognitive Performance (GCP), and proxy-reported Informant Questionnaire for Cognitive Decline (IQCODE) were used to examine cognitive outcomes following surgery at 0, 1, 2 months, and every 6 months for up to 3 years.
Results
No significant cognitive decline was observed for patients with peak CAM-S scores 0–2 (−0.17 GCP units/year, 95% confidence interval [CI] −0.35, 0.01). GCP scores decreased significantly in the group with peak CAM-S scores 3–7 (−0.30 GCP units/year, 95% CI −0.51, −0.09), and decreased almost three times faster in the highest delirium severity group (peak CAM-S scores 8–19; −0.82 GCP units/year, 95% CI −1.28, −0.37). A similar association was found for delirium severity and the proportion of patients who developed IQCODE impairment over time.
Conclusion
Patients with the highest delirium severity experienced the greatest rate of cognitive decline, which exceeds the rate previously observed for patients with dementia, on serial neuropsychological testing administered over 3 years, with a dose-response relationship between delirium severity and long-term cognitive decline.
Keywords: delirium, cognition, dementia, aged
INTRODUCTION
Delirium is a common and serious problem for hospitalized older persons, associated with prolonged hospital stays, higher hospital costs, increased functional decline, higher rates of institutionalization, and greater mortality [1, 2]. There is growing evidence that delirium is associated with a subsequent course of more rapid cognitive decline [3]. Among patients undergoing cardiac surgery, delirium is associated with a significant decline in cognitive ability, with a trajectory characterized by an initial decline and prolonged impairment [4]. Moreover, patients with Alzheimer’s disease (AD) have a 3-fold increase in the rate of cognitive decline following delirium, compared with those without delirium [5, 6]. In patients without dementia at baseline, those who experienced delirium demonstrated a 4.3-fold greater decline in long-term cognitive performance than the effect of a year of cognitive aging [7]. Although this study [7] and others [8–10] demonstrate that incident delirium is associated with long-term cognitive decline, the critical next step to advance understanding of this relationship is to evaluate whether the severity of delirium is associated with the pace of long-term cognitive decline. This would prove useful for monitoring delirium clinically and for providing a quantifiable dose-response measure for intervention trials seeking to prevent or forestall the long-term cognitive decline associated with delirium.
We have previously shown that delirium severity, as measured by the Confusion Assessment Method-Severity (CAM-S) score [11], demonstrated strong predictive validity for important short-term clinical outcomes associated with delirium, including hospital length of stay, healthcare costs, death, institutionalization, and functional decline [11]. Thus, the Aim of this study was to evaluate whether the severity of delirium was associated with the presence and degree of cognitive decline up to 36 months post-surgery in patients who are free of dementia at baseline. We hypothesized that there would be a graded relationship, with increasing severity of delirium associated with increasing degrees of long-term cognitive decline.
MATERIALS AND METHODS
Study Population
The Successful Aging after Elective Surgery (SAGES) Study is an ongoing prospective cohort study of older adults undergoing major elective non-cardiac surgery. The study design and methods have been previously described [12]. Briefly, eligible participants were age ≥70 years, English speaking, scheduled for elective surgery at one of two Harvard-affiliated academic medical centers with an anticipated length of stay ≥3 days. Eligible surgical procedures were: total hip or knee replacement, lumbar, cervical, or sacral laminectomy, lower extremity arterial bypass surgery, open abdominal aortic aneurysm repair, and colectomy. Exclusion criteria included evidence of dementia, delirium, hospitalization within 3 months, terminal condition, legal blindness, severe deafness, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. A total of 566 patients were eligible and enrolled between June 18, 2010 and August 8, 2013. Six patients were subsequently excluded for possible dementia after neuropsychological testing and clinical adjudication (final sample=560; see STROBE diagram and follow-up success rates in the Appendix). This study is in compliance with guidelines on ethical principles for medical research involving human subjects. Written informed consent for study participation was obtained from all participants according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Women’s Hospital, the two study hospitals, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts.
Study Procedures
Trained research assistants conducted a 90-minute baseline interview in participants’ homes about 2 weeks prior to the index surgery [12, 13]. Following surgery, daily interviews were conducted to assess for delirium. After discharge, home-based interviews were conducted by a separate group of trained research assistants (blinded to delirium status) at 1, 2, 6, and every six months up to 36 months. Interviews included assessments of delirium, cognitive and physical function, described below. Medical records were reviewed for the index hospitalization and readmissions.
Main Study Measures
Delirium
The Confusion Assessment Method (CAM) [14] was used to identify delirium at all time points. The CAM provides a standardized method for identification of delirium, with a sensitivity of 94% (95% confidence interval (CI) 91%–97%), specificity of 89% (95% CI 85%–94%), and inter-rater reliability of 0.70–1.00 [15]. All interviewers underwent training and standardization, and inter-rater reliability was determined in 71-paired observations (weighted kappa=0.92) [14]. Delirium was defined as either a positive rating by CAM or by a validated chart review method [16, 17], used to maximize sensitivity.
Delirium Severity
The 10-item CAM-S long-form was used to measure delirium severity [11]. Each symptom was rated 0 to 2, except acute onset or fluctuation, which is rated 0 or 1 [11], yielding a summary score from 0 to 19 (19=most severe). Because individual patients had multiple CAM ratings during hospitalization, we utilized the highest CAM-S score (peak CAM-S) across all hospital days for each patient to capture the severity of the delirium episode. Peak CAM-S scores (range 0–19) were divided into three groups. Since a minimum of 3 features is required for CAM delirium, the lowest grouping included peak CAM-S scores of 0–2, representing the group without CAM-defined delirium. While the majority of patients without delirium had a score of 0–2, some patients without delirium received higher scores based on non-specific delirium features (e.g., disorientation, memory impairment, psychomotor agitation), which can be present in conditions unrelated to delirium. Next, the group with delirium (N=134) was divided into two groups based on the median peak CAM-S score. These steps allowed delirium patients to be spread across a range of sub-groups rather than clustering only in the highest group, an approach that is preferred when the sample is imbalanced across the distribution [16]. Thus, a single, median-based cutpoint was applied to our patients with SAGES delirium (N=134) (Table 1), resulting in two delirium groups with CAM-S scores of: 1) 3–7 (N= 67), and 2) 8–19 (N= 66). These cutpoints were then applied across the entire SAGES cohort.
Table 1.
Description of Study Sample
| Characteristic | Full Sample
|
Peak CAM-S score | Rank Correlationc | ||
|---|---|---|---|---|---|
| 0 – 2
|
3 – 7
|
8–19
|
|||
| (N = 560) | (N = 244) | (N = 248) | (N = 68) | ||
| Age - mean (SD) | 76.7 (5.2) | 76.0 (4.7) | 77.2 (5.7) | 77.0 (4.6) | 0.09 |
| Female – n (%) | 326 (58) | 147 (60) | 141 (57) | 38 (56) | −0.04 |
| Nonwhite – n (%) | 42 (8) | 12 (5) | 25 (10) | 5 (7) | 0.07 |
| Education – mean years (SD) | 15.0 (2.9) | 15.6 (2.8) | 14.4 (2.9) | 14.6 (3.0) | −0.17 |
| Married – n (%) | 332 (59) | 142 (58) | 151 (61) | 39 (57) | 0.01 |
| Lives Alone – n (%) | 167 (30) | 79 (32) | 66 (27) | 22 (32) | −0.03 |
| Charlson score - n (%) | 0.12 | ||||
| 0 | 257 (46) | 126 (52) | 102 (41) | 29 (43) | |
| 1 | 139 (25) | 62 (25) | 66 (27) | 11 (16) | |
| 2+ | 164 (29) | 56 (23) | 80 (32) | 28 (41) | |
| GDS15 score - n (%) | 0.18 | ||||
| 0 – 5 | 489 (88) | 225 (93) | 214 (86) | 50 (74) | |
| 6 – 15 | 69 (12) | 17 (7) | 34 (14) | 18 (26) | |
| GCP score - mean (SD) | 57.6 (7.3) | 60.5 (6.7) | 55.8 (7.3) | 53.8 (5.6) | −0.36 |
| 3MS score - n (%) | 0.13 | ||||
| 85–100 | 523 (93) | 237 (97) | 225 (91) | 61 (90) | |
| 71–84 | 37 (7) | 7 (3) | 23 (9) | 7 (10) | |
| Proxy IQCODE (baseline) - n (%) | 0.104 | ||||
| Not Impaired | 430 (78) | 198 (83) | 183 (76) | 49 (72) | |
| Impaired | 118 (22) | 40 (17) | 59 (24) | 19 (28) | |
| ADL impairment – n (%) | 42 (8) | 10 (4) | 24 (10) | 8 (12) | 0.12 |
| IADL impairment – n (%) | 152 (27) | 51 (21) | 77 (31) | 24 (35) | 0.13 |
| Surgery type - n (%) | −0.03 | ||||
| Orthopedic | 454 (81) | 196 (80) | 201 (81) | 57 (84) | |
| Vascular | 35 (6) | 11 (5) | 18 (7) | 6 (9) | |
| General | 71 (13) | 37 (15) | 29 (12) | 5 (7) | |
| Deliriumb - n (%) | |||||
| None | 426 (76) | 243 (100) | 181 (73) | 2 (3) | |
| Delirium | 134 (24) | 1a (0) | 67 (27) | 66 (97) | |
The patient, with a peak CAM-S score of 2, had chart delirium
Delirium status was determined with daily interviews rating the Confusion Assessment Method, augmented by a validated chart review
Spearman rank correlation coefficient indicates the correlation of each variable with the peak CAM-S score
ADL = Activities of Daily Living, impairment indicated by human assistance to complete any activity
CAM-S = Confusion Assessment Method-Severity
GCP = General Cognitive Performance, composite measure of neuropsychological measures reflecting cognitive domains vulnerable to delirium, see text for details
GDS15= Geriatric Depression Scale 15 point version, range (0–15), higher is worse; a score 6 and above is considered impaired
IADL = Instrumental Activities of Daily Living, impairment indicated by human assistance to complete any activity
IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly, score >3.2 indicates cognitive impairment
3MS = Modified Mini-Mental State Exam, range (0–100), lower score indicates impairment; a score ≤84 is considered impaired
SAGES = Successful Aging after Elective Surgery Study
SD= standard deviation.
The Charlson comorbidity score ranged from 0–35, with higher scores indicating more comorbidity.
Cognitive Outcome Measures: General Cognitive Performance (GCP) and Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)
A neuropsychological test battery, conducted at baseline and each follow-up, included the Visual Search and Attention Test (VSAT) [20], Hopkins Verbal Learning Test-Revised (HVLT-R) [21], Digit Span Forward and Backward [22], Category Fluency (animal naming) [23] Phonemic F-A-S Fluency Tasks [23], Boston Naming Test (BNT) [24], Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Digit Symbol Substitution Test, Trail-Making Tests (Trails) A and B, and intersecting pentagons from the 3MS [25]. We created a weighted composite summary measure, the GCP score following standard procedures (see [26] for a detailed description). We assessed its reliability and validity and calibrated the GCP score to a nationally representative sample of adults age ≥70 years [27] to yield a mean score=50 and standard deviation=10 [25] to improve our ability to make meaningful comparisons to other study populations. The GCP is sensitive to longitudinal change with minimal floor and ceiling effects [26, 28–30].
To account for practice effects, GCP scores were adjusted with a correction factor derived from a control sample of comparable non-surgical patients (N=119) from a primary care clinic, who were administered the identical tests on the same schedule (Appendix). Using an accepted approach [31–33], the mean performance of the control sample at each time point was used to center the observed scores in the surgical sample at matching time points. This control group was used only to correct for retest (learning) effects.
We used IQCODE [34] as a proxy-reported measure of decline in current abilities for daily cognitive tasks (range 1–5). IQCODE ≥3.2 was used to indicate impairment [34].
Death and Nursing Home Placement
We examined death or nursing home placement, obtained from patient/proxy interviews and chart review, as a composite outcome between 6–36 months follow-up. This timeframe was chosen to indicate long-term outcomes, minimizing acute effects of surgery, hospitalization, or rehabilitation.
Other Study Variables
The baseline interview assessed sex, race, ethnicity, education, marital status, living situation, 15-item Geriatric Depression Scale (GDS) [35], Modified Mini-Mental State (3MS) [25], Activities of Daily Living Scale (ADLs) [36], Instrumental Activities of Daily Living Scale (IADLs) [37], and Short Form-12 Health Survey (SF-12) [38]. Age, surgical type, and Charlson comorbidity score [39] were determined from chart review [38].
Statistical Analyses
The overall analytic approaches used general linear mixed effects regression models for the trajectories of GCP score over time. Logistic regression was used for analysis of IQCODE impairment and nursing home placement or death. For GCP, the model included control for delirium severity group, with random effects for baseline GCP level, fixed effects at the 1 and 2 month assessments to capture acute decline and recovery, and random effects for linear change after the 2-month follow-up. The delirium severity group variable and the acute decline, recovery, and linear change were regressed on baseline GCP to capture differential effects by baseline status. Therefore, delirium severity group was treated as both an intermediate outcome (dependent upon baseline GCP and covariates) and as a predictor of model parameters capturing GCP change following baseline. Change over time was modeled using a three-part piecewise linear model to describe the longitudinal pattern, including an immediate decline following pre-operative baseline to month 1 (acute decline), recovery from month 1 to 2 following the acute decline (recovery) and long-term trajectory from month 2 to 36 months (long-term trajectory) (Appendix). All models adjusted for baseline covariates, including age, gender, non-white race, education, Charlson score, GDS score, IADL impairment, surgery type, and IQCODE. Analyses were conducted with Mplus (Version 7.4, Muthén & Muthén, Los Angeles, CA).
For IQCODE, we used a mixed effects generalized linear model with IQCODE impairment as a repeated outcome at all timepoints. A random effect for the linear slope captured variability in the change over time. For death or nursing home placement, logistic regression was used to model the probability of a participant having the composite of either outcome occurring between months 6–36. Delirium severity was entered as a series of categorical indicators. An interaction between time and delirium severity group captured the differences in linear change over time by severity group. For the death or nursing home analyses with IQCODE, the adjusted models controlled for age, gender, non-white race, education, Charlson score, and surgery type. Baseline IADL and IQCODE were not controlled due to collinearity. Analyses were conducted with Stata software (Version 14.1, Stata Corp, College Station, TX). In analyzing this longitudinal data, our approach to handling data missing at random (MAR) aligns with recommendations by the National Research Council [53].
Sensitivity analyses were completed to: (1) assess the extent to which our findings were robust to extreme assumptions regarding cognitive outcomes of persons who left the cohort early due to drop-out, death, or institutionalization (Appendix), and (2) assess the relationship between long-term cognitive decline and sum of all CAM-S scores (an alternate measure of delirium severity that combines both intensity and duration of the delirium episode) [18] (Appendix).
RESULTS
Table 1 reports baseline characteristics overall and stratified by delirium severity group. The mean age was 76.7 years, and 58% were women. Delirium occurred in 24%. Forty-four percent had a peak CAM-S score of 0–2; 44% with peak scores of 3–7; and 12% with peak scores of 8–19. Patients with the most severe delirium (peak CAM-S 8–19) were older, had greater impairment on the Charlson, and lower GCP, 3MS, and GDS (all p<0.05). The Spearman rank correlation coefficients indicating the correlation of each variable with the peak CAM-S score are all trivial to moderate in size.
The median duration of follow-up for this ongoing cohort was 36 months (interquartile range [IQR] 24–37). Deaths occurred in 7% of patients after a median follow-up of 19 months (IQR 12–26). An additional 27 (5%) participants withdrew from follow-up (i.e., drop-outs) after a median of 5 months (IQR 3–12). Rates of death or drop-out differed between the CAM-S groups, and increased with CAM-S severity level (8%, 12%, and 22% respectively, p=0.01) A total of 496 (89%) eligible participants completed all planned study visits, with a range of 1–9 visits per participant. Since this is an ongoing study, the number of visits completed per participant varies according to how long they have been enrolled in the study.
We examined cognitive performance by GCP up to 36 months post-surgery (Table 2) by delirium severity. For all groups, GCP scores declined acutely at one month, returned to baseline or above by two months, then remained stable to 3 years, except for the highest severity group (peak CAM-S =8–19), who experienced progressive decline to 3 years from a mean GCP of 53.8 at baseline to 51.8 at 36 months (2.0 average point decline).
Table 2.
Corrected GCP Scores over Time
| Visit month | Full Sample
|
Peak CAM-S score | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 – 2
|
3 – 7
|
8 – 19
|
||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| 0 | 560 | 57.6 (7.3) | 244 | 60.5 (6.7) | 248 | 55.8 (7.3) | 68 | 53.8 (5.6) |
| 1 | 548 | 56.8 (7.9) | 243 | 60.0 (6.9) | 242 | 55.0 (7.9) | 63 | 51.4 (5.8) |
| 2 | 536 | 58.0 (7.9) | 238 | 60.9 (7.1) | 237 | 56.2 (8.1) | 61 | 53.8 (5.3) |
| 6 | 528 | 58.2 (7.5) | 237 | 61.0 (6.5) | 230 | 56.4 (7.9) | 61 | 54.2 (6.1) |
| 12 | 511 | 58.4 (7.6) | 227 | 61.2 (7.0) | 224 | 56.8 (7.6) | 60 | 53.9 (5.4) |
| 18 | 499 | 58.3 (8.0) | 219 | 61.5 (6.9) | 222 | 56.5 (7.9) | 58 | 52.7 (7.2) |
| 24 | 474 | 58.2 (8.0) | 213 | 61.2 (6.8) | 211 | 56.4 (8.1) | 50 | 52.4 (7.2) |
| 30 | 325 | 57.5 (8.2) | 132 | 60.7 (7.5) | 152 | 56.1 (7.9) | 41 | 52.4 (7.3) |
| 36 | 312 | 57.1 (8.4) | 123 | 60.6 (7.4) | 141 | 55.8 (8.2) | 48 | 51.8 (7.7) |
CAM-S = Confusion Assessment Method-Severity
GCP = General Cognitive Performance, composite measure of neuropsychological measures reflecting cognitive domains vulnerable to delirium, see text for details
Notes: All postoperative GCP values corrected for practice effects (see text for details). The number of participants completing each the interview/the number of participants eligible for the interview for each time point follows with amount of attrition from the prior time point in brackets. Baseline: 560/560 [0]; Month 1: 548/552 [8]; Month 2: 536/546 [6]; Month 6: 528/539 [7]; Month 12: 511/527 [8]; Month 18: 499/516 [8]; Month 24: 474/489 [13]; Month 30: 325/342 [6]; Month 36: 312/316 [1]
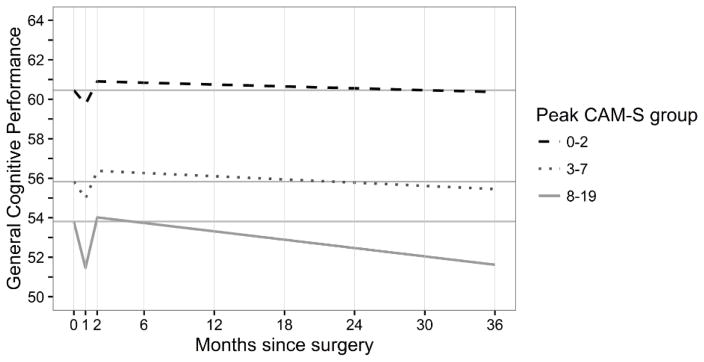
Figure 1 shows the effect of GCP performance over time by delirium severity group. All three groups experienced decline 1 month post-surgery and recovered to baseline or above. The lowest severity group (peak CAM-S=0–2) had no significant decline over months 2–36 (−0.17 GCP units/year, 95% CI −0.35, 0.01). For the group with peak CAM-S=3–7, there was a significant decrease in GCP score (−0.30 GCP units/year, 95% CI −0.51, −0.09). The magnitude of this change was about a third of the change observed in the highest severity grouping, peak CAM-S=8–19 (−0.82 GCP units/year, 95% CI −1.28, −0.37). These results suggest a graded association of delirium severity and the rate of cognitive decline. Compared to patients in the lowest severity group, the most severe delirium group demonstrated a 4.8-fold accelerated decline (−0.82/−0.17). A linear trend test for differences in slope across severity group was significant (p=0.009; Appendix). Moreover, the significant linear relationship between delirium severity and GCP slope remained when peak CAM-S was considered as a continuous measure (see Appendix).
Figure 1.
Trajectory of General Cognitive Performance by Estimated Peak Confusion Assessment Method-Severity (CAM-S) Score
Figure 1 demonstrates the relationship between estimated general cognitive performance (GCP) and time following surgery (months) by delirium severity group. The model is adjusted for baseline GCP, age, gender, non-white race, education, Charlson score, Geriatric Depression Scale score, instrumental Activities of Daily Living (IADL) impairment, surgery type, and proxy Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) impairment. For each group, we plot the model-implied trajectory and a solid gray reference line at the baseline value. The amount of punctuation (acute decline at one month), recovery (up to two months), and long-term decline (two to 36 months) is shown by each CAM-S severity group, 0–2 (dashed black line), 3–7 (dot-dashed black line) and 8–19 (solid gray line). In the acute (punctuation) phase, all groups decline with the most severe group declining the most. This is followed by recovery of cognitive performance, with the less severe groups recovering (at two months) past their baseline (0 months) GCP score, and those in the most severe group showing an incomplete return to baseline. Over long-term follow-up, the less severe groups gradually decline in GCP performance, whereas the most severe group demonstrates a faster pace of decline.
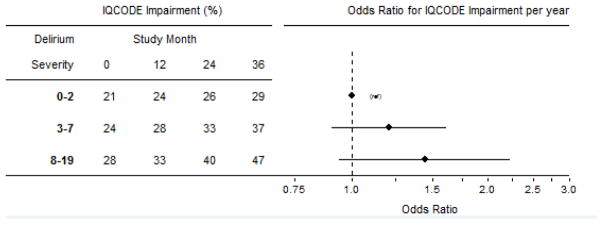
Table 3 shows the prevalence of proxy-rated IQCODE impairment by delirium severity group over time. Sample sizes differ between Table 3 and Table 2 because we could not always locate or interview a suitable proxy informant for every surgical patient. For those in the low severity group (peak CAM-S=0–2), there was no significant change in IQCODE impairment over time. For the other severity groups, the prevalence of IQCODE impairment increased significantly over time, with greater prevalence of IQCODE impairment with increasing delirium severity (odds ratio [OR] 1.2 (95% CI 0.99, 1.5). Similar to the results for GCP, the association with IQCODE impairment suggests a dose response (Figure 2 shows adjusted models), with the strongest effect in the most severe group; however, the linear trend did not achieve statistical significance (p=0.07).
Table 3.
Empirically Observed Prevalence of Proxy IQCODE Impairment over Time
| Visit month | Full Sample
|
Peak CAM-S score | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 – 2
|
3 – 7
|
8 – 19
|
||||||
| N | n (%) | N | n (%) | N | n (%) | N | n (%) | |
| 0 | 548 | 118 (22) | 238 | 40 (17) | 242 | 59 (24) | 68 | 19 (28) |
| 6 | 514 | 135 (26) | 229 | 46 (20) | 226 | 67 (30) | 59 | 22 (37) |
| 12 | 487 | 130 (27) | 217 | 49 (23) | 218 | 60 (28) | 52 | 21 (40) |
| 18 | 480 | 142 (30) | 208 | 49 (24) | 217 | 66 (30) | 55 | 27 (49) |
| 24 | 452 | 125 (28) | 202 | 46 (23) | 205 | 61 (30) | 45 | 18 (40) |
| 30 | 314 | 101 (32) | 127 | 28 (22) | 145 | 54 (37) | 42 | 19 (45) |
| 36 | 287 | 94 (33) | 118 | 25 (21) | 126 | 48 (38) | 43 | 21 (49) |
CAM-S = Confusion Assessment Method-Severity
IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly
Since the IQCODE is proxy-rated, the sample sizes in this table reflect the availability of proxy-informants over time; 12 patients did not have any proxies available at baseline, yielding a total proxy sample of N=548
N=total possible sample, n=number with proxy IQCODE impairment
Figure 2.
Predicted Prevalence of IQCODE impairment by delirium severity group and study month
Figure 2 demonstrates the relationship between the prevalence of proxy Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) impairment (score ≥ 3.2) over time (study month) following surgery, calculated using a mixed effects generalized linear model. The odds ratios (OR) are computed from models that controlled for age, gender, non-white race, education, Charlson score, Geriatric Depression Scale score, and surgery type; and thus differ from the ORs derived from the numbers presented in Table 3. Model-implied (or expected) proportions with IQCODE impairment given mean values on covariates are presented in the table. Odds ratios (and 95% confidence bands) illustrate the size and precision of estimates of the delirium severity group by time (in years following surgery) interaction effects. Over time, all groups have increasing probability of being classified as impaired on the IQCODE (p=.05). The per-year odds of IQCODE ≥3.2 for this group is about two times greater than that observed for the lowest delirium severity group.
In total, 103 participants experienced either death or nursing home placement between 6–36 months. At baseline, these participants were older, fewer were married, had higher Charlson comorbidity scores, more depressive symptoms, more ADL and IADL impairment, lower GCP scores (see Appendix for detailed study sample description). They also had higher peak CAM-S scores during hospitalization relative to the 457 participants who did not die and were not placed in a nursing home. We observed increasing incidence across severity groups (15%, 20%, 28% for peak CAM-S 0–2, 3–7, and 8–19, respectively) and a trend which approached but did not achieve statistical significance (p=0.06) (see Appendix for additional details).
DISCUSSION
In this large prospective cohort of older persons without baseline dementia undergoing elective surgery, patients experiencing higher delirium severity had greater rates of long-term cognitive decline by serial neuropsychological testing (GCP). This finding was supported by analyses examining the proxy IQCODE and risk of death or nursing home placement. These findings suggest a dose-response effect where the risk of poor long-term outcomes increases progressively across severity groups. The risk for greater cognitive decline was substantial and statistically significant in the highest delirium severity grouping.
The findings utilizing the composite GCP measure demonstrated a 4.8-fold more rapid decline between the highest and lowest severity groups. The per-year change in GCP in the long-term (months 2–36) is about −0.17 GCP units/year, or −0.02 (−0.17/7.30) standard deviation (SD) units/year in the lowest delirium severity group (peak CAM-S 0–2). Prior studies report declines with cognitive aging in the absence of dementia to range between −0.01 and −0.04 SD units/year [40–42]. Thus, patients with low delirium severity had a rate of cognitive decline (−0.02 SD per year) comparable to previous studies for cognitively normal persons. By comparison, SAGES patients with moderate severity declined by −0.30 GCP units/year (−0.04 SD units) and those with the most severe delirium declined by −0.82 GCP units/year (−0.11 SD units).
While the substantial short-term adverse outcomes of delirium are well-recognized, our results hold important implications for the longer-term prognosis of delirium. This represents a paradigm shift in the way delirium is currently viewed. Delirium may not be transient and reversible with only acute complications; rather, more severe delirium cases may be associated with long-term and potentially permanent cognitive decline. Furthermore, this work suggests the need to target patients with high delirium severity for strategies to prevent progressive cognitive decline, and potentially increased risk for dementia.
While prior work has established the association of incident delirium with long-term cognitive decline,7–10 these findings are novel in demonstrating that delirium severity is directly associated with long-term cognitive decline in an exposure-response fashion. We acknowledge that causal associations cannot be determined from this observational study. However, the observed exposure-response relationship is a critical first step in demonstrating a direct association between delirium severity and long-term cognitive decline, and is an important criterion used in causal inference for epidemiologic studies [42]. The novelty of our study also includes both the use of a comprehensive measure of delirium severity (peak CAM-S scores, reflecting the height of delirium intensity) and in the serial measurement of cognitive function over a 3-year period following surgery. We chose peak CAM-S as our outcome measure to reflect maximal intensity of delirium; however, other measures might have been chosen (e.g., sum CAM-S [18], see Appendix). Future studies should examine other severity measures, including the Memorial Delirium Assessment Scale, Delirium Rating Scale, and Delirium Index have been associated with increased mortality [43, 44], institutionalization [45, 46], and length of stay [47]. Delirium duration has also been associated with increased death, ventilation time, and intensive care unit stay [48–51]. The current study is innovative in enabling examination of exposure-response relationships by examining outcomes across multiple levels of severity. Other strengths include the use of a large cohort with thorough data collection, careful characterization of preoperative cognition, repeated neuropsychological testing over time, standardized delirium assessments, and extended post-surgical follow-up. Additionally, exclusion of mild dementia at baseline facilitated examination of the effects of delirium severity free of this potentially confounding influence. This presented a unique opportunity to study cognitive impairment following delirium occurring largely in non-cognitively impaired older patients. Finally, the careful correction for learning effects over time represents another important advance.
Several caveats about this study deserve mention. Although we controlled for learning effects, patients recovered back to or above baseline levels at 2 months, suggesting that: 1) longer-term follow-up is critical to understanding the trajectory of cognitive recovery post-surgery, and 2) this control for learning effects was either incomplete or that patients had depressed cognitive levels at baseline, which may have been due to preadmission pain medications such as narcotics. We encountered missing data due to deaths and drop-outs, and addressed these in sensitivity analyses to assure the robustness of our conclusions (Appendix). Despite using reasonable and established methods, participants who developed delirium may have been on a downward cognitive trajectory prior to surgery, and we could not completely rule out preclinical (asymptomatic) dementia, or clinically presymptomatic, but AD biomarker positive dementia (as defined by stage 1 of the 2011 NIA criteria for AD), at baseline. Moreover, the observation of a lower GCP in this group was anticipated, given that baseline cognitive impairment has been long recognized as an important risk factor for delirium. Perhaps the more intriguing observation is that participants on average improved back to baseline at 2 months following delirium, and successively declined from 2 to 36 months suggesting a degree of initial resiliency that would not be expected for those with underlying dementia. Similarly, we acknowledge that inclusion of the pending follow-up visits may influence our current findings. In general, we do not anticipate a substantial change in our study conclusions upon incorporating the remaining visits since GCP scores observed for the two lowest delirium severity groups (peak CAM-S 0–2 and 3–7) are relatively stable from around month 24 and onwards, and the GCP scores appear to continue declining in the highest delirium severity group (peak CAM-S 8–19). An additional caveat includes the fact that patients with delirium had lower GCP scores at baseline than those without delirium, although both groups were above the U.S. population mean GCP score=50. It may be that patients who were undergoing cognitive decline prior to surgery may represent individuals at greatest risk for experiencing more severe delirium; however, with only one preoperative cognitive assessment, we were unable to directly test this possibility. We attempted to investigate this possibility by matching patients in the highest severity group (peak CAM-S 8–19) with patients in the other two severity groups on preoperative GCP (see Appendix for Methods and detailed Results), and found the pace of decline was faster in the highest severity group (peak CAM-S 8–19; slope −0.09 SD/year) than in the peak CAM-S 3–7 group (slope −0.04 SD/year), which was in turn faster than the peak CAM-S 0–2 group (slope −0.02 SD/year). We acknowledge that the study population represents a highly educated sample with relatively low racial diversity from a single city; however, the diversity characteristics of our sample (92% white) are representative of the Boston area (2008–2012 census data) [49]. It is important to note that our choice of a dementia-free, relatively robust elective surgical population may have influenced our findings. Patients with dementia might be more vulnerable to decline after milder cases of delirium [5]. Finally, our use of the peak CAM-S does not discern hypoactive from hyperactive delirium, which may have differing prognoses.
While delirium has previously been considered a transient condition of only short-term significance, our results suggest that for patients with moderate to severe delirium, the declines in cognition may be both substantial and long-term, and most notably exceeds the rate of decline observed for patients with dementia. Although it remains critical to prevent and treat all delirium to minimize well-documented short-term adverse outcomes, our results suggest the need for more targeted strategies (e.g., cognitive rehabilitation, as used for patients with brain injuries [54]) in patients with higher delirium severity to prevent long-term cognitive decline. Our findings underscore the need to heighten efforts to better understand the risk factors and pathophysiology of delirium of moderate to high severity, and to better target prevention and management strategies to mitigate the long-term and potentially permanent adverse sequelae associated with this common, morbid, and costly geriatric syndrome.
Supplementary Material
Acknowledgments
Grant Support: The authors thank the patients, family members, nurses, physicians, and research staff who participated in the SAGES Study. This work is dedicated to the memory of Joshua Bryan Inouye Helfand.
Grant Support: Major support was provided by the National Institute on Aging grants T32AG023480 (Dr. Vasunilashorn), R01AG030618, R01AG051658, and K24AG035075 (Dr. Marcantonio), P01AG031720 and K07AG041835 (Dr. Inouye), and R01AG044518 (Drs. Inouye and Jones); and the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustee (Dr. Vasunilashorn). Dr. Inouye is supported by the Milton and Shirley F. Levy Family Chair.
SAGES Study Group
[Presented in alphabetical order; individuals listed may be part of multiple groups, but are listed only once under major activity, listed in parentheses].
Overall Principal Investigator: Sharon K. Inouye, MD, MPH (Overall PI, Administrative Core, Project 1; HSL, BIDMC, HMS).
Project and Core Leaders: David Alsop, PhD (Project 3; BIDMC, HMS); Richard Jones, ScD (Data Core, Project 4; Brown University); Thomas Travison, PhD (Data Core, HSL, HMS); Edward R. Marcantonio, MD, SM (Overall Co-PI, Epidemiology Core, Project 2; BIDMC, HMS).
Executive Committee: Steven Arnold, MD, (MGH); Zara Cooper, MD, MSc (HMS, BWH); Bradford Dickerson, MD (MGH, HMS); Tamara Fong, MD, PhD (HMS, HSL, BIDMC); Towia Libermann, PhD (HMS, BIDMC); Eran Metzger, MD, (HMS, HSL, BIDMC); Alvaro Pascual-Leone, MD (HMS, BIDMC); Eva M. Schmitt, PhD (Overall Project Director, HSL); Mouhsin Shafi, MD (HMS, BIDMC).
Other Co-investigators: Michele Cavallari, MD (BWH); Weiying Dai, PhD (BIDMC); Simon T. Dillon, PhD (HMS, BIDMC); Janet McElhaney, MD (UConn); Charles Guttmann, MD (BWH, HMS); Tammy Hshieh, MD (BWH); George Kuchel, MD, FRCP, (UCONN); Long Ngo, PhD (HMS, BIDMC); Daniel Press, MD (HMS, BIDMC); Jane Saczynski, PhD, (UMASS); Sarinnapha Vasunilashorn, PhD (HMS, BIDMC).
Clinical Consensus Panel: Margaret O’Connor, PhD (HMS, BIDMC); Eyal Kimchi, MD, PhD (MGH), Jason Strauss, MD (Cambridge Health Alliance); Bonnie Wong, PhD (BIDMC).
Surgical Leaders: Michael Belkin, MD (HMS, BWH); Douglas Ayres, MD (HMS, BIDMC); Mark Callery, MD (HMS, BIDMC); Frank Pomposelli, MD (HMS, BIDMC); John Wright, MD (HMS, BWH); Marc Schermerhorn, MD (HMS, BIDMC).
Epidemiology Core: Asha Albuquerque (HSL); Amanda Brown M.Ed. (HSL); Amy Callahan (BIDMC), Sarah Dowal, MSW, LCSW, MPH (HSL); Meaghan Fox (BIDMC); Jacqueline Gallagher, MS (BIDMC); Rebecca Anna Gersten; Ariel Hodara (BIDMC); Ben Helfand, MPH (BIDMC); Jennifer Inloes (HSL); Jennifer Kettell (HSL); Aleksandra Kuczmarska (BIDMC); Jacqueline Nee (HSL); Emese Nemeth (HSL); Lisa Ochsner (BWH); Kerry Palihnich (BIDMC); Katelyn Parisi (HSL); Margaret Puelle (HSL); Sarah Rastegar, MA (HSL); Margaret Vella (HSL), Guoquan Xu, MD, PhD (HSL).
Data Management and Statistical Analysis Core: Margaret Bryan (HSL); Jamey Guess (BIDMC); Dee Enghorn (HSL); Alden Gross, PhD, MHS (John Hopkins School of Medicine); Yun Gou, MA (HSL); Daniel Habtemariam (HSL); Ilean Isaza, PhD (HSL); Cyrus Kosar, MA (HSL); Christopher Rockett, PhD (HSL); Douglas Tommet, MPH (Brown University).
Fiscal Management Committee: Ted Gruen (HSL); Meg Ross (HSL); Katherine Tasker (Chair, HSL).
Scientific Advisory Board: James Gee, PhD (University of Pennsylvania); Ann Kolanowski, PhD, RN, FAAN (Pennsylvania State University); Margaret Pisani, MD, MPH (Yale University); Sophia de Rooij, MD, PhD (Academic Medical Center, Amsterdam); Selwyn Rogers, MD, MPH (Temple University), Stephanie Studenski, MD (Chair, NIA); Yaakov Stern, PhD (Columbia University); Anthony Whittemore, MD (BWH, HMS).
Internal Advisory Board: Gary Gottlieb, MD, MBA (BWH, MGH, HMS); John Orav, PhD (BWH, HMS); Reisa Sperling, MD, MMSc (BWH, HMS).
Abbreviations: BIDMC, Beth Israel Deaconess Medical Center; BWH, Brigham and Women’s Hospital; HMS, Harvard Medical School; HSL, Hebrew SeniorLife; MGH, Massachusetts General Hospital; PI, principal investigator; UCONN, University of Connecticut Health Center.
Footnotes
Conflict of Interest Disclosures
The authors state no conflicts of interest to report.
References
- 1.Cole MG, Primeau FJ. Prognosis of delirium in elderly hospital patients. CMAJ. 1993;149:41–46. [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross AL, Jones RN, Habtemariam DA, Fong TG, Tommet D, Quach L, Schmitt E, Yap L, Inouye SK. Delirium and long-term cognitive trajectory among persons with dementia. Arch Int Med. 2012;172:1–8. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, ME, Kosar CM, Tommet D, Schmitt EM, Travison TG, Saczynski JS, Ngo LH, Alsop DC, Jones RN. The short- and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, Cunningham C, Polvikoski T, Sulkava R, MacLullich AM, Brayne C. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard TD, Yende S. Cognitive impairment and critical illness: A chicken and an egg. Crit Care Med. 2016;44:2115–2116. doi: 10.1097/CCM.0000000000001934. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Jones RN. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160:526–33. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO, Jr, Fong TG, Metzger E, Inouye SK SAGES Study Group. Novel risk markers and long-term outcomes of delirium: The Successful Aging after Elective Surgery (SAGES) Study design and methods. J Am Med Dir Assoc. 2012;13:818. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt EM, Saczynski JS, Kosar CM, Jones RN, Alsop DC, Fong TG, Metzger E, Cooper Z, Marcantonio ER, Travison T, Inouye SK Successful Aging after Elective Surgery Study Group. The Successful Aging After Elective Surgery Study: Cohort Description and Data Quality Procedures. J Am Geriatr Soc. 2015;63:2463–2471. doi: 10.1111/jgs.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 15.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 17.Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, Marcantonio ER, Wong B, Isaza I, Inouye SK. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62:518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasunilashorn SM, Marcantonion ER, Gou Y, Pisani MA, Travison TG, Schmitt EM, Jones RN, Inouye SK. Quantifying the severity of a delirium episode throughout hospitalization: The combined importance of intensity and duration. J Gen Intern Med. 2016;31:1164–1171. doi: 10.1007/s11606-016-3671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Trenerry MR. Psychological Assessment Resources. 1990. Visual Search and Attention Test: VSAT. [Google Scholar]
- 21.Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin Neuropsychologist. 1991;5:125–142. [Google Scholar]
- 22.Wechsler D. WAIS-R manual: Wechsler adult intelligence scale-revised. Psychological Corporation; 1981. [Google Scholar]
- 23.Spreen OB. Neurosensory Center Comprehensive Examination for Aphasia: Manual of instructions. NCCEA; 1977. [Google Scholar]
- 24.Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. J Gerontol. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 26.Jones RN, Rudolph JL, Inouye SK, Yang FM, Fong TG, Milberg WP, Tommet D, Metzger E, Cupples LA, Marcantonio ER. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol. 2010;32:1041–1049. doi: 10.1080/13803391003662728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, Burke JR, Fisher GG, Fultz NH, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Weir DR, Willis RJ. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 28.Gross AL, Jones RN, Fong TG, Tommet D, Inouye SK. Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology. 2014;42:144–153. doi: 10.1159/000357647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavallari M, Hshieh TT, Guttmann CR, Ngo LH, Meier DS, Schmitt EM, Marcantonio ER, Jones RN, Kosar CM, Fong TG, Press D, Inouye SK, Alsop DC SAGES Study Group. Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging. 2015;36:2122–2129. doi: 10.1016/j.neurobiolaging.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saczynski JS, Inouye SK, Kosar CM, Tommet D, Marcantonio ER, Fong T, Hshieh T, Vasunilashorn S, Metzger ED, Schmitt E, Alsop DC, Jones RN SAGES Study Group. Cognitive and brain reserve and the risk of postoperative delirium in older patients: analysis of data from a prospective observational study. Lancet Psychiatry. 2014;1:437–443. doi: 10.1016/S2215-0366(14)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 32.Lewis M, Maruff P, Silbert B. Statistical and conceptual issues in defining post-operative cognitive dysfunction. Neurosci Biobehav Rev. 2004;28:433–440. doi: 10.1016/j.neubiorev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Soinne L, Helenius J, Tikkala I, Saimanene E, Salonen O, Hietanen M, Lindsberg PJ, Kaste M, Tatlisumak T. The effect of severe carotid occlusive disease and its surgical treatment on cognitive functions of the brain. Brain Cogn. 2009;69:353–359. doi: 10.1016/j.bandc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage J, Sheikh JI. Geriatric Depression Scale (GDS) Recent Evidence and Development of a Shorter Version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 36.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–991. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 37.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 38.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Hayden KM, Reed BR, Manly JJ, Tommet D, Pietrzak RH, Chelune GJ, Yang FM, Revell AJ, Bennett DA, Jones RN. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. 2011;40:684–689. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson JK, Gross AL, Pa J, McLaren DG, Park LQ, Manly JJ. Longitudinal change in neuropsychological performance using latent growth models: a study of mild cognitive impairment. Brain Imaging Behav. 2012;6:540–550. doi: 10.1007/s11682-012-9161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill AB. The environment and disease: Association or causation? Proc Royal Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly KG, Zisselman M, Cutillo-Schmitter T, Reichard R, Payne D, Denman SJ. Severity and course of delirium in medically hospitalized nursing facility residents. Am J Geriatr Psychiatry. 2001;9:72–77. [PubMed] [Google Scholar]
- 44.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162:457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 45.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 46.Dasgupta M, Brymer C. Prognosis of delirium in hospitalized elderly: worse than we thought. Int J Geriatr Psychiatry. 2014;29:497–505. doi: 10.1002/gps.4032. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Hu W, Shen M, Ye X, Huang Y, Sun Y. Profiles of delirium and the clinical outcomes of patients who underwent coronary artery bypass grafting: a prospective study from China. J Clin Nurs. 2016;25:631–641. doi: 10.1111/jocn.13089. [DOI] [PubMed] [Google Scholar]
- 48.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 50.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 51.Bellelli G, Mazzola P, Morandi A, Bruni A, Carnevali L, Corsi M, Zatti G, Zambon A, Corrao G, Olofsson B, Gustafson Y, Annoni G. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62:1335–1340. doi: 10.1111/jgs.12885. [DOI] [PubMed] [Google Scholar]
- 52.U.S. Census, Bureau. [Accessed on August 30, 2016];2008–2012 American Community Survey. http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- 53.National Research Council Panel on Handling Missing Data in Clinical Trials. The Prevention and Treatment of Missing Data in Clinical Trials. National Academies Press; Washington DC: 2010. [PubMed] [Google Scholar]
- 54.Park HY, Maitra K, Martinez KM. The effect of occupation-based cognitive rehabilitation for traumatic brain injury: A meta-analysis of randomized controlled trials. Occup Ther Int. 2015;22:104–116. doi: 10.1002/oti.1389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.