Abstract
Downregulation of the astroglial glutamate transporter GLT-1 is observed in the nucleus accumbens (NAc) following administration of multiple drugs of abuse. The decrease in GLT-1 protein expression following cocaine self-administration is dependent on both the amount of cocaine self-administered and the length of withdrawal, with longer access to cocaine and longer withdrawal periods leading to greater decreases in GLT-1 protein. However, the mechanism(s) by which cocaine downregulates GLT-1 protein remains unknown. We used qRT-PCR to examine gene expression of GLT-1 splice isoforms (GLT-1A, GLT-1B) in the NAc, prelimbic cortex (PL) and basolateral amygdala (BLA) of rats, following two widely used models of cocaine self-administration: short-access (ShA) self-administration, and the long-access (LgA) self-administration/incubation model. While downregulation of GLT-1 protein is observed following ShA cocaine self-administration and extinction, this model did not lead to a change in GLT-1A or GLT-1B gene expression in any brain region examined. Forced abstinence following ShA cocaine self-administration also was without effect. In contrast, LgA cocaine self-administration and prolonged abstinence significantly decreased GLT-1A gene expression in the NAc and BLA, and significantly decreased GLT-1B gene expression in the PL. No change was observed in NAc GLT-1A gene expression one day after LgA cocaine self-administration, indicating withdrawal-induced decreases in GLT-1A mRNA. In addition, LgA cocaine self-administration and withdrawal induced hypermethylation of the GLT-1 gene in the NAc. These results indicate that a decrease in NAc GLT-1 mRNA is only observed after extended access to cocaine combined with protracted abstinence, and that epigenetic mechanisms likely contribute to this effect.
Keywords: Glutamate, Transporter, GLT-1, Cocaine, Self-Administration, methylation
1. Introduction
Drug-induced adaptations in glutamatergic projections to the nucleus accumbens (NAc) contribute significantly to drug seeking behaviors, including relapse following protracted withdrawal (Cooper et al., 2017; Knackstedt and Kalivas, 2009; Quintero, 2013; Scofield et al., 2016a; van Huijstee and Mansvelder, 2014). In particular, glutamate homeostasis is impaired in the NAc following exposure to different drugs of abuse, including cocaine, heroin, nicotine, and alcohol (reviewed in (Kalivas, 2009; Scofield et al., 2016a)). Glutamate homeostasis refers to the balance between synaptic and extra-synaptic glutamate levels, and a disruption in glutamate homeostasis results in increased drug-seeking following withdrawal (Kalivas, 2009; Scofield and Kalivas, 2014). Elements of impaired glutamate homeostasis include decreased basal levels of glutamate in the NAc (Baker et al., 2003) leading to decreased tone on inhibitory presynaptic metabotropic glutamate receptors (mGluR 2/3), which in turn leads to increased excitatory transmission from the prefrontal cortex (PFC) to the NAc (Kalivas, 2009). This increase in glutamatergic transmission has been observed following self-administration of nicotine, heroin, ethanol and cocaine, indicating that disruptions in glutamate homeostasis may be a common consequence of drug intake (Gipson et al., 2013; Kalivas, 2009; Shen et al., 2014). Moreover, pharmacological treatments that target glutamate homeostasis have shown promise in reducing motivation to seek drug in preclinical animal models, as well as in human addicts (Knackstedt et al., 2010; LaRowe et al., 2013; LaRowe et al., 2006; Reissner et al., 2014; Reissner et al., 2015; Zhou and Kalivas, 2008).
Accordingly, evidence indicates that maintaining glutamate homeostasis is critical in preventing relapse. This maintenance is accomplished largely through the actions of the cystine glutamate exchanger (system xC-) and the glutamate transporter GLT-1. While system xC- is largely responsible for regulation of basal extracellular glutamate levels via the 1:1 release of intracellular glutamate in exchange for uptake of extracellular cystine (Bridges et al., 2012; Kalivas, 2009), GLT-1 is responsible for approximately 90% of synaptic glutamate uptake (Danbolt, 2001). Protein expression of the catalytic subunit of xC- (xCT) and GLT-1 is downregulated in the NAc after cocaine self-administration and withdrawal (Fischer-Smith et al., 2012; Fischer et al., 2013; Knackstedt et al., 2010; Reissner et al., 2014; Reissner et al., 2015; Trantham-Davidson et al., 2012). Furthermore, the magnitude of GLT-1 decrease in the NAc is a function of both duration of access and length of withdrawal from cocaine, with longer access to cocaine and longer withdrawal periods leading to greater decreases in GLT-1 protein expression (Fischer-Smith et al., 2012). This decrease in GLT-1 protein expression is not observed in brain areas such as the prefrontal cortex and striatum (Knackstedt et al., 2010; Parikh et al., 2014; Reissner et al., 2014), highlighting the important role of NAc GLT-1 expression in cocaine-seeking behaviors.
Regulation and function of system xC- also appears to be region- and experience-dependent. For example, stimulation of system xC- with cystine induces glutamate release in cocaine-withdrawn, but not cocaine-naïve animals (Baker et al., 2003). As referenced above, restoration of glutamate homeostasis in the NAc has shown promise in preventing reinstatement to cocaine seeking (Baker et al., 2003; Knackstedt et al., 2010; Reissner et al., 2015; Roberts-Wolfe and Kalivas, 2015; Sondheimer and Knackstedt, 2011); however, neither basal glutamate levels, xCT, or GLT-1 are affected in the PFC in cocaine-withdrawn rats (Knackstedt et al., 2010; Reissner et al., 2014). Moreover, antagonists of system xC- fail to decrease basal glutamate levels in the PFC, as they do in the NAc (Baker et al., 2003; Melendez et al., 2005).
Pharmacological treatments that restore xCT and GLT-1 expression in the NAc have also been shown to decrease reinstatement to cocaine (Knackstedt et al., 2010; Reissner et al., 2014; Reissner et al., 2015). For example, ceftriaxone, a β-lactam antibiotic, and N-acetylcysteine (NAC) both restore xCT and GLT-1 expression in the NAc after cocaine self-administration and extinction training, and importantly attenuate both cue- and cocaine-primed reinstatement (Amen et al., 2011; Knackstedt et al., 2010; LaCrosse et al., 2016; Moussawi et al., 2009; Sondheimer and Knackstedt, 2011). The glial modulator propentofylline (PPF) also impairs reinstatement and restores expression levels of GLT-1 protein (Reissner et al., 2014). Of significance, all three of these interventions require restored expression of both xCT and GLT-1, or GLT-1 specifically (LaCrosse et al., 2017; Reissner et al., 2014; Reissner et al., 2015). These results collectively highlight the important role of GLT-1 regulation within the NAc in cocaine seeking.
While a central role for GLT-1 in cellular dynamics that mediate cocaine-seeking has been established, the mechanism(s) responsible for the cocaine-induced downregulation of GLT-1 protein expression in the NAc remains unknown. To investigate whether the cocaine-induced decrease in GLT-1 protein in the NAc is mediated by genetic mechanisms, this study investigated changes in GLT-1 mRNA levels following two widely used models of rodent cocaine self-administration. The short-access (ShA) self-administration and extinction paradigm has been reliably used to test reinstatement to cocaine seeking following extinction training (Venniro et al., 2016). In this model, animals are given limited access (typically 2 hours/day) to cocaine self-administration and, following extinction of cocaine seeking behavior, cocaine seeking is measured upon re-exposure to cocaine or cocaine-related cues in a reinstatement test. In contrast, in the long-access (LgA) and withdrawal model, animals have extended access (often 6 hours/day) to cocaine, followed by protracted withdrawal. This withdrawal period from LgA cocaine self-administration leads to the incubation of cocaine craving, which is characterized by an increase in cocaine seeking across the duration of withdrawal (Grimm et al., 2001; Tran-Nguyen et al., 1998). Although both models have been shown to downregulate GLT-1 protein expression in the NAc (Fischer-Smith et al., 2012; Fischer et al., 2013; Knackstedt et al., 2010), the mechanism(s) responsible for this decrease remain largely unexplored. To examine a possible mechanism for the decrease in GLT-1 protein observed in both models, we examined mRNA levels of GLT-1 and its splice variants (GLT-1A and GLT-1B) in the NAc after both ShA and extinction and withdrawal, as well as after LgA and withdrawal. Furthermore, since the functional significance of GLT-1 protein downregulation has been well characterized in the NAc, and to account for the procedural differences between the two models (extinction vs. withdrawal, and ShA vs. LgA), we examined GLT-1 gene expression specifically within the NAc in two additional groups. NAc GLT-1 gene expression was examined after ShA self-administration and three weeks of withdrawal (to enable comparison of extinction vs. withdrawal after ShA) and following 45 days of withdrawal from ShA self-administration (to compare the effects of prolonged withdrawal after ShA vs. LgA self-administration). In addition to these findings in the NAc, we also investigated GLT-1A and GLT-1B mRNA levels in the prelimbic cortex (PL) and basolateral amygdala (BLA), two regions with important innervations to the NAc, after ShA and extinction, as well as after LgA and withdrawal.
2. Methods
2.1 Surgical Procedures
Male (200–225 g at arrival) Sprague-Dawley rats were individually housed on a 12-hour reverse light cycle (7 AM off, 7 PM on). After a one-week acclimation period to the animal facility, rats were anesthetized with ketamine (100 mg/kg, i.m.) and xylazine (7 mg/kg, i.m.), and a silastic catheter was surgically implanted into the right jugular vein as previously described (Fuchs et al., 2007; Scofield et al., 2016b). Gentamicin (3 mg/kg) and heparinized saline (30 units/kg) were administered i.v. for five days post operatively, as well as throughout all self-administration procedures. All procedures were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
2.2 Self-administration Procedures
All self-administration procedures took place in standard, sound attenuated operant conditioning chambers (Med Associates, St. Albans, VT). Prior to the start of self-administration, to facilitate acquisition to lever-pressing, all animals received at least one session of food training, where responding on the active lever resulted in the delivery of one 45 mg food pellet (Bio Serv, Flemington, NJ). For the ShA self-administration paradigm, rats received two weeks of cocaine self-administration for 2 h/day on an FR1 schedule, followed by three weeks of extinction training (6 extinction sessions/week, 18 total extinction sessions). Responding on the active lever during ShA cocaine self-administration resulted in the delivery of cocaine (0.2 mg/infusion, 0.04 ml total volume over 2.18 seconds), and was also accompanied by a tone and illumination of a stimulus light above the active lever for five seconds. All rats weighed 275–300 g across self-administration, equivalent to 0.67–0.73 mg/kg/infusion during ShA cocaine self-administration. A 20-sec time out period occurred after every infusion of cocaine in which active lever-pressing resulted in no programmed responses. Responding on the inactive lever resulted in no programmed responses. Saline-administering rats received saline (0.9% NaCl), as opposed to cocaine infusions. After 12 days of a minimum of 10 cocaine infusions received per day, rats began extinction training where responding on either lever did not result in the delivery of cocaine, nor the presentation of any audio or visual cues. All rats were trained in extinction for three weeks (6 sessions/week, 18 total extinction sessions), and all cocaine-extinguished rats performed ≤ 20 active lever presses during each of the last 2 sessions. A subset of rats did not undergo extinction training and instead, underwent three weeks or 45 days of withdrawal in the home cage following ShA self-administration. In the LgA self-administration paradigm, rats received 10 days of 6 hours/day of cocaine (0.75 mg/kg/infusion (in 0.045 ml total volume/infusion for a 300 g rat) over 2.18 seconds) or saline self-administration on an FR1 schedule, followed by 24 h or 45 days of withdrawal in the home cage. All other procedures remained identical to the ShA paradigm.
2.3 Tissue collection and sample preparation
For animals that received ShA self-administration, all rats were euthanized via rapid decapitation either twenty-four hours following the last extinction session, or twenty-four hours following the last day of withdrawal, and tissue samples were collected from the NAc and PL. For animals that received LgA self-administration, twenty-four hours following the last day of withdrawal, or twenty-four hours following the last day of self-administration, rats were euthanized via rapid decapitation and tissue samples were collected from the NAc and PL. For all animals, the remaining brain samples were then flash frozen in isopentane and stored at −80 °C. BLA samples were later collected from these samples on a cryostat. All samples were stored in approximately 100 µl RNA later solution (Qiagen, Germantown, MD) until processing for qRT-PCR. For methylated DNA immunoprecipitation (MeDIP) procedures, NAc samples were frozen on dry ice and stored at −80 °C until processing.
2.4 qRT-PCR
Tissue samples from all rats were stored in RNA later and processed either in-house or by the UNC Animal Clinical Chemistry and Gene Expression Laboratories as previously described (Jones et al., 2015; Kim et al., 2002). For tissue samples processed by the UNC Animal Clinical Chemistry and Gene Expression Laboratory, samples first were homogenized in RNA lysis buffer and RNA was isolated using the ABI prism 6700 automated nucleic acid work station (PE Biosystems, Foster City, CA). qRT-PCR amplifications were performed in an ABI prism 7700 sequence detector (PE Biosystems, Foster City, CA), with 10 µl RNA and 20 µl PCR reaction mixture per reaction. Each qRT-PCR amplification was performed in duplicate under the following conditions: 30 min at 48 °C for the RT reaction, and 10 min at 94 °C, followed by 40 temperature cycles (15 s at 94 °C and 1 min at 60 °C). For in-house analysis, RNA was isolated as described above, and each qRT-PCR amplification was performed in duplicate, in a final volume of 20 µl (3 µl cDNA), using the Quantstudio 6 PCR system (ThermoFisher Scientific, Waltham, MA) under the following conditions: hold for 2 min at 50 °C, hold for 2 min at 95 °C, followed by 40 temperature cycles (1 s at 95 °C and 20 sec at 60 °C). For all samples, GAPDH was used as an endogenous control, and the following sequences were used to detect GLT-1 splice variants: GLT-1A Forward: GGAAAGCAACTCTAATCAG/ATG, Reverse: CATTGGCCGCCAGAGTTAC, Probe: FTCT/AATGCCGCACACAACTCTGTCGQ; GLT-1B Forward: GGAAAGCAACTCTAATCAG/ATG, Reverse: TCCAGGAATGGGAAAGGTAC, Probe: FTCT/AATGCCGCACACAACTCTGTCGQ; GAPDH Forward: AGGTCGGTGTGA ACGGATTT, Reverse: GGCAACAATGTCCACTTTGT, Probe: FCGCCTGGTC/TACCAG GGCTGCCQ (F = 5’ Fluorescein (FAM); Q = Quencher (TAMRA)).
2.5 Methylated DNA immunoprecipitation (MeDIP)
DNA from NAc samples were isolated using the DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD), and sonicated using the EpiShear Probe Sonicator (Active Motif, Carlsbad, CA). The quantity and purity of DNA (A260/280 between 1.8–2.0) was verified using a spectrophotometer. Methylated DNA was immunoprecipitated using an antibody against 5-methylcytosine (Active Motif, Carlsbad, CA). For both cocaine and saline groups, 30 µl of input DNA was saved and used to generate qPCR standard curves with known amounts of DNA. Methylated DNA was then amplified using SYBR green under the following PCR conditions: reaction volume of 20 µl (7 µl DNA, 13 µl PCR reaction mixture), 1 cycle at 95 °C for 2 min, 45 temperature cycles (95 °C for 15 sec, 60 °C for 30 sec). The sequence of primers used to amplify the GLT-1 gene was as follows- forward: ACAGCGTCTAAAGATGGGGGG, reverse: GCAGGCGATCGCTCTCTATT.
2.6 Data Analysis
All statistical analysis was conducted on the SigmaPlot 11.0 software. For all behavioral measures, a mixed ANOVA (α = 0.05) was performed with drug (cocaine vs. saline) and time (self-administration session) set as factors. The dependent variable for behavioral measures included active lever presses and the number of infusions received during self-administration. For qRT-PCR, the delta-delta Ct method was used for relative comparisons of GLT-1A and GLT-1B (with GAPDH used as an endogenous control) in cocaine vs. saline groups. For GLT-1 gene methylation, the amount of immunoprecipitated methylated GLT-1 was normalized to saline self-administering animals and the fold change in GLT-1 methylation in cocaine selfadministering animals was assessed relative to the saline group. For both qRT-PCR and GLT-1 methylation a two-tailed, unpaired t-test was conducted to examine statistical differences between saline and cocaine self-administering animals.
3. Results
3.1 Prolonged withdrawal from LgA cocaine self-administration induces brain region and isoform specific decreases in GLT-1 gene expression
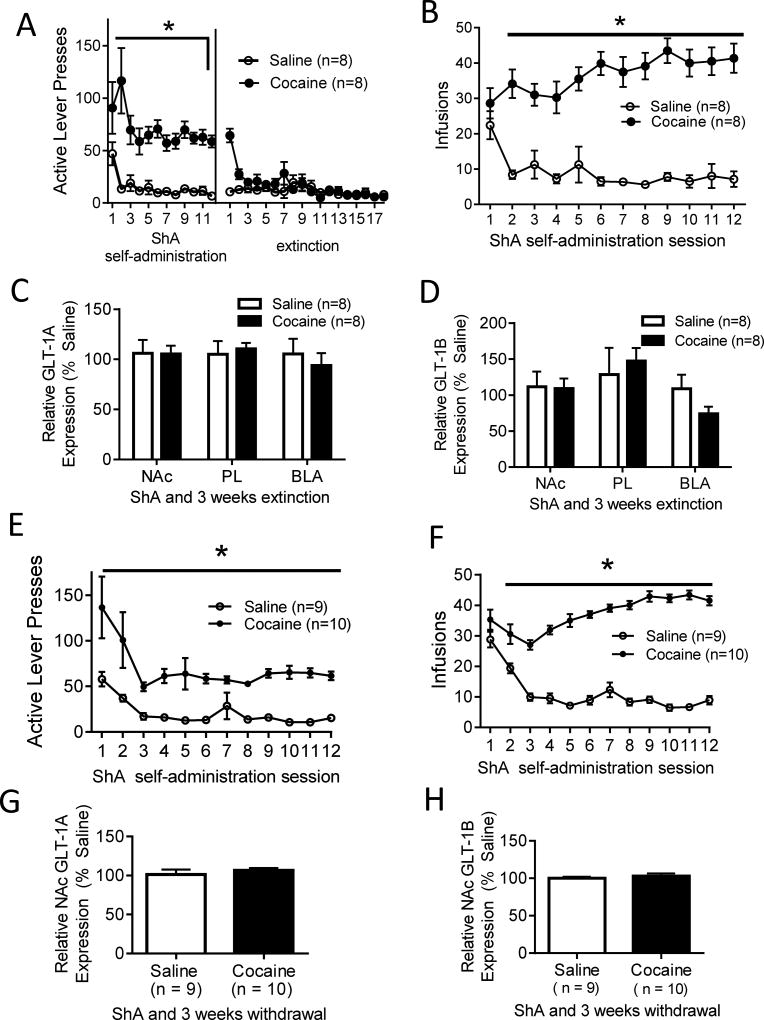
In the ShA and extinction paradigm, cocaine self-administering rats showed a significantly greater number of both active lever presses (F (1, 190) = 44.97, p < .001; Fig 1A) and infusions received (F (1, 190) = 64.82, p < .001; Fig 1B) than saline self-administering animals. For active lever presses, the main effect of time was also significant (F (11, 190) = 3.46, p < .001; Fig 1A), and for infusions, the interaction between drug and time was significant (F (11, 190) = 6.61, p < .001; Fig 1B). No significant difference was found between cocaine and saline groups in active lever presses throughout extinction sessions (F (1, 287) = 3.15, p = .098; Fig 1A). After ShA cocaine self-administration and three weeks of extinction training, no significant differences were observed between cocaine and saline self-administering animals in GLT-1A (t (14) = 0.03, p = 0.96; Fig 1C) or GLT-1B (t (14) = 0.10, p = 0.92; Fig 1D) mRNA levels in the NAc.
Figure 1. Effects of ShA and extinction or forced abstinence on GLT-1A and GLT-1B mRNA levels in NAc, PL, and BLA.
(A, B) Behavioral data for ShA self-administration followed by three weeks of extinction training; (E, F) behavioral data for ShA self-administration followed by three weeks of forced abstinence. (C, D) Relative GLT-1A (C) and GLT-1B (D) mRNA levels in the NAc, PL, and BLA after ShA and three weeks of extinction training. GLT-1A and GLT-1B mRNA levels are unchanged following ShA cocaine self-administration and extinction. (G, H) Relative GLT-1A (G) and GLT-1B (H) mRNA levels in the NAc after ShA self-administration and three weeks of withdrawal. ShA self-administration and three weeks of withdrawal does not change GLT-1A or GLT-1B mRNA levels in the NAc. *Significant difference between cocaine and saline self-administering rats (p < 0.05).
To compare extinction training vs. forced abstinence within the ShA cocaine self-administration paradigm, GLT-1A and GLT-1B mRNA levels were assessed in the NAc of rats following three weeks of withdrawal from ShA self-administration. Comparable to rats in the self-administration and extinction paradigm, cocaine self-administering rats showed a significantly greater number of active lever presses (F (1, 227) = 44.56, p < .001; Fig 1E) and infusions received (F (1, 227) = 365.65, p < .001; Fig 1F) than saline self-administering animals. For active lever presses (F (11, 227) = 5.96, p < .001; Fig 1E), and infusions (F (11, 227) = 59.39 p < .001; Fig 1F), the main effect of time was also significant. For infusions, the interaction between drug and time was also significant (F (11, 227) = 19.53, p < .001; Fig 1F). Similar to rats in the ShA and extinction paradigm, no significant differences in GLT-1A (t (17) = −0.73, p = 0.48; Fig 1G) or GLT-1B (t (17) = −0.69, p = 0.50; Fig 1H) mRNA levels were observed in the NAc of cocaine vs. saline self-administering rats following three weeks of withdrawal from ShA self-administration.
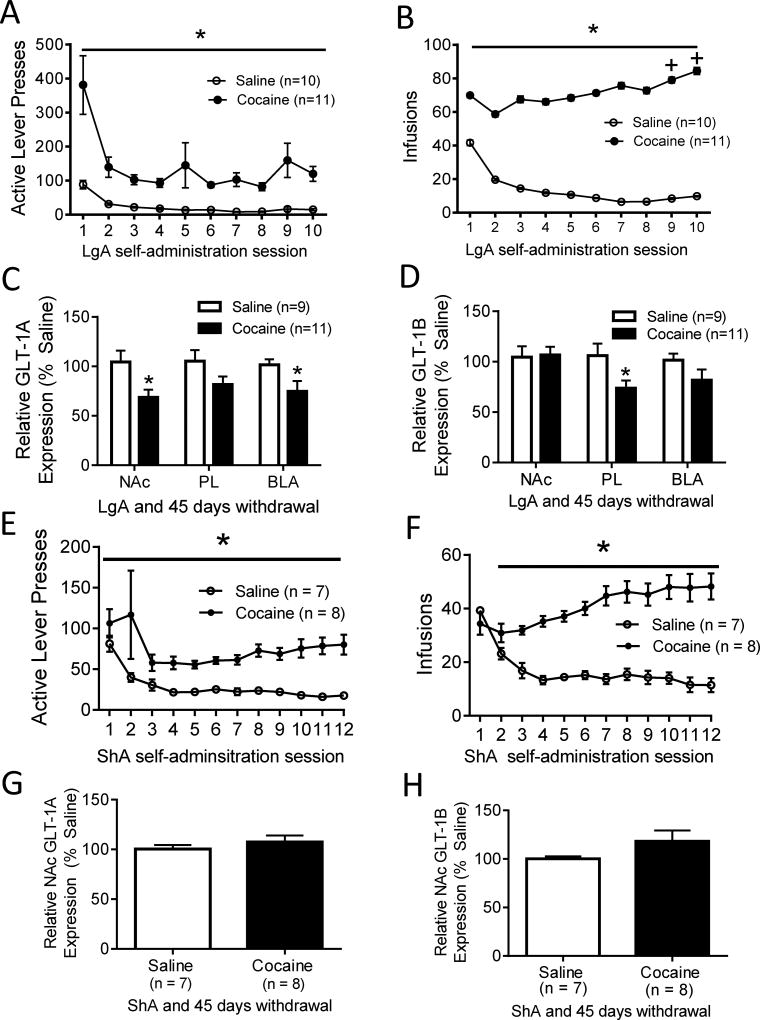
Cocaine self-administering rats in the LgA and 45 days withdrawal group showed a significantly greater number of both active lever presses (F (1, 209) = 10.80, p < .05; Fig 2A) and infusions received (F (1, 209) = 43.61, p < .001; Fig 2B) than saline self-administering animals. For active lever presses (F (9, 209) = 8.12, p < .001; Fig 2A) and infusions received (F (9, 209) = 11.65, p < .001; Fig 2B) the main effect of time was also significant. In addition, the interaction between drug and time was significant for both active lever presses (F (9, 209) = 2.99, p < .05; Fig 2A) and infusions received (F (9, 209) = 13.21, p < .001; Fig 2B). Pairwise comparisons using the Holm-Sidak test showed no significant difference in the number of infusions received across time in saline self-administering animals. However, cocaine self-administering rats received a significantly greater number of infusions received on days 9 (vs. day 2, t = 2.53, p < 0.05) and 10 (vs. day 2, t = 3.05, p < 0.05; vs. day 3, t = 2.23, p < 0.05), indicating an escalation in self-administration. Following 45 days of withdrawal from LgA cocaine self-administration, a significant decrease in GLT-1A (t (18) = 2.68, p < 0.05; Fig 2C) mRNA was found in the NAc. However, no difference was found in NAc GLT-1B mRNA (t (18) = −0.17, p = 0.86; Fig 2D).
Figure 2. Effects of LgA and ShA followed by prolonged abstinence on GLT-1A and GLT1-B levels.
(A, B) Behavioral data for LgA self-administration; (E, F) behavioral data for ShA self-administration, each followed by 45 days of withdrawal. (C, D) Relative GLT-1A and GLT-1B mRNA levels in the NAc, PL, and BLA after following 45 days of withdrawal from LgA self-administration. LgA cocaine self-administration and 45 days of withdrawal significantly decreases GLT-1A gene expression in the NAc and BLA (C), and significantly decreases GLT-1B gene expression in the PL (D). (G, H) Relative GLT-1A (G) and GLT-1B (H) mRNA levels in the NAc following 45 days of withdrawal from ShA self-administration. ShA self-administration and 45 days of withdrawal does not change GLT-1A or GLT-1B mRNA levels in the NAc, in contrast to LgA. *p < 0.05. +Significant difference between day 2 and day 3 of self-administration (p < 0.05).
To compare the effects of withdrawal across ShA and LgA cocaine self-administration, GLT-1A and GLT-1B mRNA levels were assessed in the NAc of rats following 45 days of withdrawal from ShA self-administration. As expected, cocaine self-administering rats showed a significantly greater number of both active lever presses (F (1, 191) = 23.95, p < .001; Fig 2E) and infusions (F (1, 191) = 55.96, p < .001; Fig 2F) than saline self-administering animals. For active lever presses (F (11, 191) = 4.40, p < .001; Fig 2E), and infusions (F (11, 191) = 6.43 p < .001; Fig 2F), the main effect of time was also significant. For infusions, the interaction between drug and time was also significant (F (11, 191) = 20.36, p < .001; Fig 2F). In contrast to the decrease in GLT-1A mRNA in the NAc following 45 days of withdrawal from LgA cocaine self-administration, 45 days of withdrawal from ShA cocaine self-administration did not lead to any changes in NAc GLT-1A mRNA (t (13) = −0.85, p = 0.41; Fig 2G). Moreover, there was no change in NAc GLT-1B mRNA (t (13) = −1.48, p = 0.16; Fig 2H) following 45 days of withdrawal from ShA cocaine self-administration.
In addition to GLT-1A and GLT-1B gene expression in the NAc, we examined GLT-1 gene expression in the PL and BLA after ShA and extinction, as well as after LgA and 45 days of withdrawal. Similarly as observed in the NAc, ShA cocaine self-administration and extinction training did not result in any significant changes in GLT-1A or GLT-1B gene expression in the PL or BLA (Fig 1C, 1D). However, LgA cocaine self-administration and withdrawal led to a significant decrease in GLT-1B mRNA levels (t (19) = 2.32, p < 0.05; Fig 2D), but not GLT-1A mRNA levels in the PL. In the BLA, LgA cocaine self-administration and withdrawal significantly decreased GLT-1A (t (19) = 2.18, p < 0.05; Fig 2C) but not GLT-1B mRNA levels.
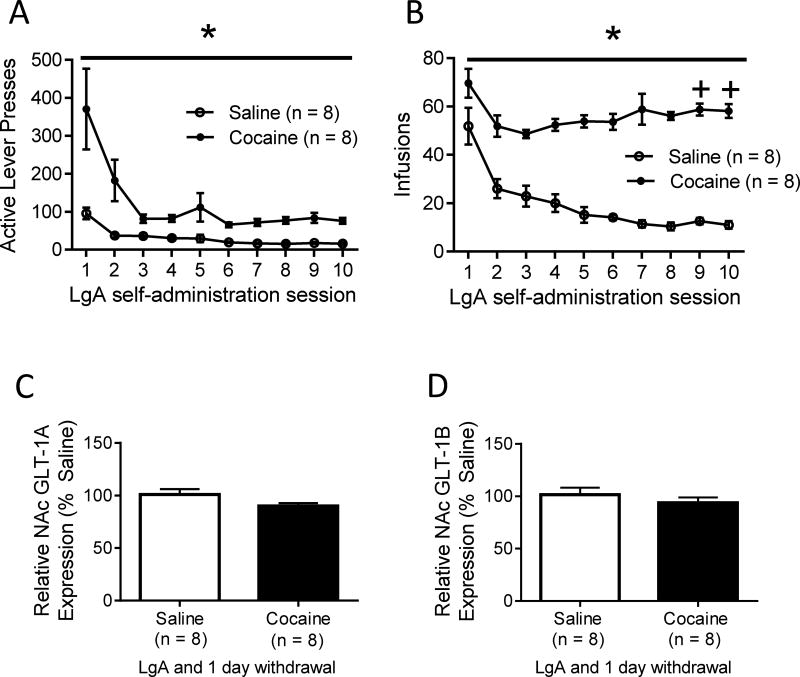
3.2 The decrease in GLT-1A in the NAc after LgA cocaine self-administration requires withdrawal
Behavioral data for all animals in the LgA self-administration and 1 day of withdrawal group is shown in figure 3 (A: active lever presses, B: infusions). Cocaine self-administering rats showed a significantly greater number of active lever presses (F (1, 157) = 25.34, p < .001; Fig 3A) and infusions (F (1, 157) = 114.19, p < .001; Fig 3B) than saline self-administering animals. For active lever presses (F (9, 157) = 8.97, p < .001; Fig 3A) and infusions received (F (9, 157) = 16.21, p < .001; Fig 3B) the main effect of time was also significant. In addition, the interaction between drug and time was significant for both active lever presses (F (9, 157) = 3.31, p < .05; Fig 3A) and infusions received (F (9, 157) = 7.03, p < .001; Fig 3B). Pairwise comparisons using the Holm-Sidak test showed no significant difference in the number of infusions received on the last two days (vs. days 2 and 3) of self-administration in saline self-administering animals. However, cocaine self-administering rats received a significantly greater number of infusions received on day 9 (vs. day 3 t = 10.13, p < 0.05) and day 10 (vs. day 3 t = 2.32, p < 0.05). To further assess the decrease in GLT-1A mRNA levels after LgA cocaine self-administration and withdrawal, we examined GLT-1A and GLT-1B gene expression in the NAc of animals 24 hours after the conclusion of LgA self-administration. In this case, measurements were taken 24 hours after the last LgA session, as opposed to 45 days of withdrawal. Although LgA cocaine self-administration led to a trend toward a marginal decrease in GLT-1A levels in the NAc, this decrease was not statistically significant (p = 0.072; Fig 3C). Likewise, there was no significant difference between cocaine and saline self-administering animals in GLT-1B mRNA levels in the NAc directly after LgA self-administration (p = 0.38; Fig 3D).
Figure 3. Effect of LgA and one day of abstinence on NAc GLT-1 mRNA levels.
(A, B) Behavioral data for LgA self-administration followed by one day of withdrawal. (C, D) Relative GLT-1A (C) and relative GLT-1B (D) gene expression in the NAc after one day of withdrawal from LgA self-administration. No significant changes in GLT-1A or GLT-1B were observed. *(p < 0.05) +Significant difference between day 3 of self-administration (p < 0.05).
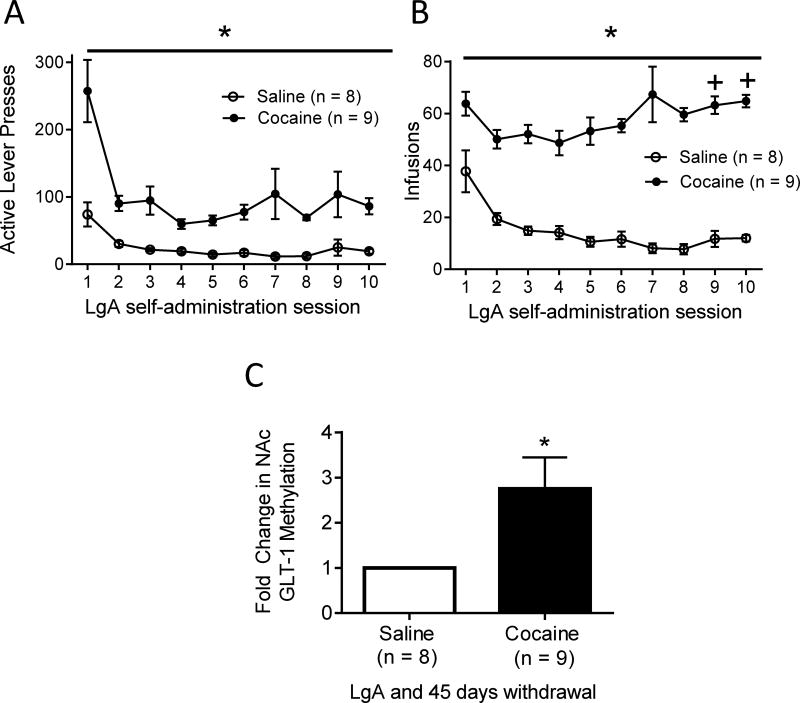
3.3 The GLT-1 gene is hypermethylated in the NAc after LgA cocaine self-administration and withdrawal
Behavioral data for all animals used in MeDIP experiments is shown in figure 4 (A: active lever presses, B: infusions). As expected, cocaine self-administering rats showed a significantly greater number of both active lever presses (F (1, 169) = 59.94, p < .001; Fig 4A) and infusions received (F (1, 169) = 134.19, p < .001; Fig 4B) than saline self-administering animals. For active lever presses (F (9, 169) = 8.46, p < .001; Fig 4A) and infusions received (F (9, 169) = 4.92, p < .001; Fig 4B) the main effect of time was also significant. In addition, the interaction between drug and time was significant for both active lever presses (F (9, 169) = 2.47, p < .05; Fig 4A) and infusions received (F (9, 169) = 4.53, p < .001; Fig 4B). Pairwise comparisons using the Holm-Sidak test showed no significant difference in the number of infusions received on the last two days (vs. days 2 and 3) of self-administration in saline self-administering animals. However, cocaine self-administering rats received a significantly greater number of infusions received on days 9 (vs. day 2, t = 2.66, p < 0.05; vs. day 3, t = 2.26, p < 0.05) and 10 (vs. day 2, t = 2.98, p < 0.05; vs. day 3, t = 2.57, p < 0.05). To examine a possible epigenetic mechanism for the observed decrease in GLT-1A mRNA levels in the NAc after LgA cocaine and withdrawal, we next examined the methylation state of the GLT-1 gene after LgA cocaine self-administration and withdrawal by using MeDIP. In comparison to saline self-administering animals, LgA cocaine self-administration and withdrawal induced a significant 2.75-fold increase in GLT-1 gene methylation (t (15) = −2.379, p < 0.05; Fig 4C).
Figure 4. Effect of LgA and prolonged abstinence on GLT-1 gene methylation in the NAc.
(A, B) Behavioral data for animals in MeDIP experiments after LgA self-administration and 45 days of withdrawal. (C) Methylation state of the GLT-1 gene in the NAc after LgA cocaine or saline self-administration and withdrawal. LgA cocaine self-administration and withdrawal induces a significant 2.75-fold increase in GLT-1 gene methylation in the NAc. *p < 0.05. +Significant difference between day 2 and day 3 of self-administration (p < 0.05).
4. Discussion
4.1 Genetic regulation of GLT-1 by cocaine depends on behavioral paradigm, isoform, and brain region
Numerous studies have indicated that GLT-1 protein expression is downregulated in the NAc following exposure to multiple drugs of abuse (Gipson et al., 2013; Knackstedt et al., 2009; Knackstedt et al., 2010; Sari et al., 2013; Shen et al., 2014), and that restored expression of GLT-1 is central to the mechanism for several candidate pharmacotherapies for relapse (Knackstedt et al., 2010; LaCrosse et al., 2017; Reissner et al., 2014; Reissner et al., 2015). However, the mechanism(s) responsible for drug effects on GLT-1 protein levels is unknown. The results from this study show that GLT-1 gene expression after cocaine self-administration and withdrawal is dependent on self-administration paradigm, as well as GLT-1 splice isoform and brain region. Neither three weeks of extinction training nor three weeks of withdrawal following ShA cocaine self-administration affected mRNA levels of either GLT-1 splice isoform in the NAc, despite known effects on GLT-1 protein. Furthermore, ShA cocaine self-administration and extinction training did not affect GLT-1 mRNA levels in the PL or BLA. In contrast, prolonged (45 days) but not short (1 day) withdrawal from LgA cocaine self-administration significantly decreased GLT-1A gene expression in the NAc. LgA cocaine self-administration and prolonged withdrawal also significantly decreased GLT-1A mRNA in the BLA, and significantly decreased GLT-1B mRNA in the PL. These results indicate that extended access to cocaine combined with a prolonged withdrawal period reduces GLT-1 gene expression, and that this decrease is dependent on splice isoform and brain region examined. A summary of the effects of cocaine self-administration on GLT-1 mRNA levels in the NAc is shown in Table 1.
Table 1.
Changes in NAc GLT-1 Gene Expression after Cocaine Self-Administration
| ShA Cocaine Self-Administration | LgA Cocaine Self-Administration | ||||
|---|---|---|---|---|---|
| 3 weeks extinction |
3 weeks of withdrawal |
45 days of withdrawal |
1 day of withdrawal |
45 days of withdrawal |
|
| GLT-1A | No change (Figure 1C) | No change (Figure 1G) | No change (Figure 2G) | No change (Figure 3C) | ⬇ 41 ± 7% *p < .05 (Figure 2C) |
| GLT-1B | No change (Figure 1D) | No change (Figure 1H) | No change (Figure 2H) | No change (Figure 3D) | No change (Figure 2D) |
The three main splice isoforms of GLT-1, which all vary in the C-terminus, are GLT-1A, GLT-1B, and GLT-1C (Holmseth et al., 2009). GLT-1A is considered to be the dominant GLT-1 isoform, accounting for over 90% of all GLT-1 expression in the brain (Holmseth et al., 2009; Meabon et al., 2012). GLT-1B accounts for approximately 6% of GLT-1 expression, while GLT-1C accounts for roughly 1% (Holmseth et al., 2009). Although GLT-1A is more abundantly expressed overall in the rat brain, studies have shown brain region-specific differences in the expression of GLT-1 splice isoforms (Lehre et al., 1995; Reye et al., 2002). For example, GLT-1A expression is more evident in parts of the pituitary gland and external capsule, while GLT-1B is more highly expressed in parts of the cerebellar nuclei (Reye et al., 2002). The results from the present study suggest that LgA cocaine self-administration and prolonged withdrawal may regulate GLT-1 gene expression differently based on isoform and brain region. It is possible that region-specific differences in mRNA expression of GLT-1 splice isoforms contributed to the differential effects of LgA cocaine self-administration and prolonged withdrawal on GLT-1A and GLT-1B gene expression in the NAc, PL, and BLA.
A recent report by Shin et al (Shin et al., 2016) showed an increase in cue-elicited glutamate release in the ventromedial prefrontal cortex following 30 but not 3 days of withdrawal from LgA cocaine self-administration. These results suggest that GLT-1 may be regulated in the prefrontal cortex following prolonged withdrawal from LgA cocaine self-administration, in accordance with our observation that GLT-1B mRNA is significantly reduced in the PL after prolonged abstinence following LgA (Fig 2D). Thus, while previous reports have not indicated effects of ShA on GLT-1 in the PFC (Knackstedt et al., 2010; Reissner et al., 2014), engagement of modifications to glutamate homeostasis and GLT-1 expression may become recruited following prolonged access to cocaine.
In the NAc, ShA cocaine self-administration and extinction did not result in any changes in GLT-1A or 1B mRNA levels, while LgA cocaine self-administration and prolonged withdrawal selectively decreased GLT-1A mRNA levels. This result was somewhat surprising since both models of cocaine self-administration downregulate GLT-1 protein levels in the NAc (Fischer-Smith et al., 2012; Knackstedt et al., 2010; Reissner et al., 2015; Trantham-Davidson et al., 2012), suggesting that post-transcriptional or post-translational mechanisms may be responsible for protein downregulation observed following the ShA paradigm, while genetic mechanisms may be responsible for the protein downregulation after LgA and withdrawal. Accordingly, we propose a dual model of GLT-1 regulation dependent on cocaine self-administration paradigm. The downregulation of GLT-1 protein in the NAc after ShA cocaine self-administration and extinction likely reflects changes in GLT-1 protein trafficking or degradation (discussed in more detail below), whereas the decrease in GLT-1 protein in the NAc after LgA and withdrawal engage changes in genetic regulation of GLT-1.
In the present study, twenty-four hours after the last LgA cocaine self-administration session, we observed a non-significant trend toward a minimal decrease in NAc GLT-1 mRNA levels, but observed a robust decrease in NAc GLT-1A mRNA following prolonged withdrawal from LgA cocaine self-administration. This is also consistent with the greater magnitude of GLT-1 protein downregulation observed following prolonged withdrawal from LgA cocaine self-administration (Fischer-Smith et al., 2012). Furthermore, we found no effect of ShA cocaine self-administration and prolonged withdrawal on GLT-1 mRNA expression. This is in agreement with a recent report finding no change in NAc GLT-1 mRNA in cocaine-extinguished rats, despite a decrease in protein expression (LaCrosse et al., 2017). Since GLT-1 transcription is decreased only following extended access to cocaine followed by prolonged withdrawal, this decrease in GLT-1 mRNA in the NAc may be considered to reflect a neurochemical correlate of the incubation of cocaine craving, akin to other previously reported (Wolf, 2016).
4.2 Genetic and post-transcriptional regulation of GLT-1
In vitro and in vivo studies have shown that post-transcriptional and post-translational mechanisms can lead to decreased cell surface expression of GLT-1 protein. For example, numerous studies using cell cultures have shown that SUMOylation (Foran et al., 2014) and ubiquitination (García-Tardón et al., 2012; Ibáñez et al., 2016; Martinez-Villarreal et al., 2012; Sheldon et al., 2008) can lead to internalization and decreased cell surface expression of GLT-1. Further, in response to activation of protein kinase C, increased ubiquitin levels are observed leading to the internalization and degradation of GLT-1 (García-Tardón et al., 2012; Sheldon et al., 2008; Susarla and Robinson, 2008). In addition, heat shock protein (Hsp90β) which aids in the degradation of GLT-1 protein, is increased in reactive astrocytes of patients with temporal lobe epilepsy, as well as in a mouse model of epilepsy (Sha et al., 2017). In the same study, an inhibitor of HSP90 was then shown to increase GLT-1 levels (Sha et al., 2017), further suggesting regulation of GLT-1 protein by HSP90. Since GLT-1 gene expression was not downregulated after extinction training or prolonged withdrawal following ShA cocaine self-administration, it will be interesting to determine whether these or other post-translational mechanisms contribute to the widely observed downregulation of GLT-1 protein expression after ShA cocaine self-administration.
The results from this study also show an increase in GLT-1 gene methylation in the NAc following LgA cocaine self-administration and prolonged withdrawal, providing one epigenetic mechanism for the observed decrease in GLT-1A mRNA in the NAc. Increased DNA methylation leads to a decrease in transcription via recruitment of methyl-CpG binding proteins which prevent the binding of transcription factors, or by directly preventing the binding of transcription factors to DNA sequences (Nestler, 2014). Studies using cell culture have shown region specific increases in DNA methylation at shore regions of GLT-1 CpG islands, with higher methylation patterns observed in the cerebellum vs. the cortex (Perisic et al., 2012). Other studies using cultured astrocytes from the cerebellum have shown that inhibitors of DNA methyltransferase can increase GLT-1 gene transcription (Zschocke et al., 2005). Furthermore, numerous studies have shown cocaine-induced changes in DNA methylation patterns, including changes in gene expression of DNA methyltransferase 3A and 3B in the NAc (Anier et al., 2010; LaPlant et al., 2010), as well as hypermethylation at the protein phosphatase-1 (PP1c) promoter (Anier et al., 2010). Microarray analysis used to examine the methylation state of various genes in the NAc after LgA cocaine self-administration and withdrawal indicated a hypermethylation of genes related to glutamatergic signaling (Massart et al., 2015). Similarly, in the medial prefrontal cortex of mice, prolonged abstinence from cocaine self-administration altered DNA methylation patterns in 28 different genomic locations (Baker-Andresen et al., 2015). The results from this present study add to these findings and show that LgA cocaine self-administration and withdrawal results in the hypermethylation of the GLT-1 gene in the NAc. Although there was only a decrease in GLT-1A and not GLT-1B mRNA in the NAc following LgA cocaine self-administration and withdrawal, hypermethylation of the GLT-1 gene in the NAc was still evident. This provides further evidence that in the NAc, GLT-1A is the dominant isoform.
4.4 Conclusions
The results from this study show that LgA cocaine self-administration and withdrawal, but not ShA cocaine self-administration and extinction, decreases GLT-1 gene expression, indicating both transcriptional and post-transcriptional mechanisms that contribute to the downregulation of GLT-1 protein are observed in these paradigms. This decrease is specific to GLT-1 splice isoform and brain region, and requires extended access to cocaine followed by a prolonged withdrawal period. Moreover, the decrease in GLT-1 mRNA in the NAc is associated with a significant increase in methylation of the GLT-1 gene, indicating epigenetic regulation of GLT-1 expression in the NAc following LgA and prolonged withdrawal. Future studies will further elucidate what mechanism(s) may be responsible for the observed decrease in GLT-1 protein in the NAc after ShA and extinction. Possibilities include post-transcriptional mechanisms and/or post-translational mechanisms. It remains to be studied if these mechanisms play a role in the downregulation of GLT-1 protein after ShA cocaine self-administration and extinction.
Moreover, while our results show that hypermethylation of the GLT-1 gene is one contributing mechanism responsible for the observed decreases in GLT-1 mRNA, other epigenetic mechanisms, such as histone modifications, likely also play a role in the regulation of GLT-1 expression. Indeed, numerous studies have reported cocaine-induced changes in histone modifications (Kennedy et al., 2013; Kumar et al., 2005; LaPlant and Nestler, 2011), and future studies can examine if histone modifications on the GLT-1 gene influences GLT-1 gene expression after LgA cocaine self-administration and withdrawal. Furthermore, although the main focus of this study was to characterize the changes in GLT-1 mRNA after cocaine self-administration in the NAc, where protein downregulation is most readily observed, we also observed significant decreases in GLT-1A mRNA in the BLA, and GLT-1B mRNA in the PL following LgA and prolonged withdrawal. Current experiments are in progress to determine if GLT-1 gene methylation is also a possible mechanism for decreases in GLT-1 mRNA observed in these brain regions, and if the changes in GLT-1 mRNA in these brain regions are evident 24 hours following the last LgA self-administration session and/or following 45 days of withdrawal from ShA self-administration. Finally, future studies can examine the epigenetic mechanism(s) leading to the changes in GLT-1 gene expression beyond the NAc following more extensive cocaine use, in order to determine whether protracted use and withdrawal recruits epigenetic mechanisms to suppress GLT-1 expression in regions beyond the NAc.
Highlights.
GLT-1 mRNA was examined after different paradigms of cocaine self-administration
ShA self-administration and extinction did not affect GLT-1 mRNA levels
ShA self-administration and abstinence did not affect GLT-1 mRNA levels
LgA self-administration/withdrawal decreased GLT-1A/1B in a region-specific manner
LgA self-administration and withdrawal led to hypermethylation of the GLT-1 gene
Acknowledgments
This work was supported by NIDA R01-DA041455 (KJR) and NIDA T32-DA07244 (RK). The authors thank members of the Reissner lab for constructive criticism of a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Abbreviations: GLT-1, NAc, PL, BLA, ShA, LgA, MeDIP, qRT-PCR
References
- Amen SL, Piacentine LB, Ahmad ME, Li S-J, Mantsch JR, Risinger RC, Baker DA. Repeated N-Acetyl Cysteine Reduces Cocaine Seeking in Rodents and Craving in Cocaine-Dependent Humans. Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2010;35:2450–2461. doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Andresen D, Zhao Q, Li X, Jupp B, Chesworth R, Lawrence AJ, Bredy T. Persistent variations in neuronal DNA methylation following cocaine self-administration and protracted abstinence in mice. Neuroepigenetics. 2015;4:1–11. doi: 10.1016/j.nepig.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA. Thinking Outside the Cleft to Understand Synaptic Activity: Contribution of the Cystine-Glutamate Antiporter (System x(c)(−)) to Normal and Pathological Glutamatergic Signaling. Pharmacological Reviews. 2012;64:780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Robison AJ, Mazei-Robison MS. Reward Circuitry in Addiction. Neurotherapeutics. 2017:1–11. doi: 10.1007/s13311-017-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston ACW, Rebec GV. Differential effects of cocaine access and withdrawal on GLT1 expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–339. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston ACW, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core vs. shell in cue-induced cocaine seeking behavior. J Neurosci. 2013;33:9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran E, Rosenblum L, Bogush A, Pasinelli P, Trotti D. Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization. Glia. 2014;62:1241–1253. doi: 10.1002/glia.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- García-Tardón N, González-González IM, Martínez-Villarreal J, Fernández-Sánchez E, Giménez C, Zafra F. Protein Kinase C (PKC)-promoted Endocytosis of Glutamate Transporter GLT-1 Requires Ubiquitin Ligase Nedd4-2-dependent Ubiquitination but Not Phosphorylation. The Journal of Biological Chemistry. 2012;287:19177–19187. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–1071. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Ibáñez I, Díez-Guerra FJ, Giménez C, Zafra F. Activity dependent internalization of the glutamate transporter GLT-1 mediated by β-arrestin 1 and ubiquitination. Neuropharmacology. 2016;107:376–386. doi: 10.1016/j.neuropharm.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Barrus D, Lysle DT. The Role of Brain Interleukin-1 in Stress-Enhanced Fear Learning. Neuropsychopharmacology. 2015;40:1289–1296. doi: 10.1038/npp.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, Bassel-Duby R, Olson EN, Nestler EJ. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16:434–440. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-S, Lee G, John SWM, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci U S A. 2002;99:4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and Reinstatement. Current opinion in pharmacology. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The Role of Cystine-Glutamate Exchange in Nicotine Dependence in Rats and Humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine-seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- LaCrosse AL, Hill K, Knackstedt LA. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. European Neuropsychopharmacology. 2016;26:186–194. doi: 10.1016/j.euroneuro.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCrosse AL, O'Donovan SM, Sepulveda-Orengo MT, McCullumsmith RE, Reissner KJ, Schwendt M, Knackstedt LA. Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression. The Journal of Neuroscience. 2017;37:5809–5821. doi: 10.1523/JNEUROSCI.3717-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Nestler EJ. CRACKing the histone code: cocaine's effects on chromatin structure and function. Horm Behav. 2011;59:321–330. doi: 10.1016/j.yhbeh.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A Double-Blind Placebo-Controlled Trial of N-Acetylcysteine in the Treatment of Cocaine Dependence. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2013;22:443–452. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and Tolerability of N-Acetylcysteine in Cocaine-Dependent Individuals. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Villarreal J, Garcia Tardon N, Ibanez I, Gimenez C, Zafra F. Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia. 2012;60:1356–1365. doi: 10.1002/glia.22354. [DOI] [PubMed] [Google Scholar]
- Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, Kennedy P, Nestler EJ, Szyf M, Yadid G. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 2015;35:8042–8058. doi: 10.1523/JNEUROSCI.3053-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meabon JS, Lee A, Meeker KD, Bekris LM, Fujimura RK, Yu C-E, Watson G, Pow DV, Sweet IR, Cook DG. Differential Expression of the Glutamate Transporter GLT-1 in Pancreas. Journal of Histochemistry and Cytochemistry. 2012;60:139–151. doi: 10.1369/0022155411430095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of Extracellular Glutamate in the Prefrontal Cortex: Focus on the Cystine Glutamate Exchanger and Group I Metabotropic Glutamate Receptors. Journal of Pharmacology and Experimental Therapeutics. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine Reverses Cocaine Induced Metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Naughton SX, Shi X, Kelley LK, Yegla B, Tallarida CS, Rawls SM, Unterwald EM. Cocaine-induced neuroadaptations in the dorsal striatum: Glutamate dynamics and behavioral sensitization. Neurochemistry international. 2014;75:54–65. doi: 10.1016/j.neuint.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Perisic T, Holsboer F, Rein T, Zschocke J. The CpG island shore of the GLT-1 gene acts as a methylation-sensitive enhancer. Glia. 2012;60:1345–1355. doi: 10.1002/glia.22353. [DOI] [PubMed] [Google Scholar]
- Quintero GC. Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatric Disease and Treatment. 2013;9:1499–1512. doi: 10.2147/NDT.S45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW. Chronic Administration of the Methylxanthine Propentofylline Impairs Reinstatement to Cocaine by a GLT-1- Dependent Mechanism. Neuropsychopharmacology. 2014;39:499–506. doi: 10.1038/npp.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction biology. 2015;20:316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Scott H, Pow DV. Distribution of two splice variants of the glutamate transporter GLT-1 in rat brain and pituitary. Glia. 2002;38:246–255. doi: 10.1002/glia.10059. [DOI] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS & neurological disorders drug targets. 2015;14:745–756. doi: 10.2174/1871527314666150529144655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee R, Choi D-S. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of molecular neuroscience : MN. 2013;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith ACW, Roberts-Wolfe D, Kalivas PW. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological Reviews. 2016a;68:816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist. 2014;20:610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, Boger HA, Kalivas PW, Reissner KJ. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry. 2016b;80:207–215. doi: 10.1016/j.biopsych.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L, Wang X, Li J, Shi X, Wu L, Shen Y, Xu Q. Pharmacologic inhibition of Hsp90 to prevent GLT-1 degradation as an effective therapy for epilepsy. The Journal of Experimental Medicine. 2017;214:547–563. doi: 10.1084/jem.20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon AL, Gonzalez MI, Krizman-Genda EN, Susarla BT, Robinson MB. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochem Int. 2008;53:296–308. doi: 10.1016/j.neuint.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-w, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic Glutamate Spillover Due to Impaired Glutamate Uptake Mediates Heroin Relapse. The Journal of Neuroscience. 2014;34:5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK. Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology. 2016;102:103–110. doi: 10.1016/j.neuropharm.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susarla BT, Robinson MB. Internalization and degradation of the glutamate transporter GLT-1 in response to phorbol ester. Neurochem Int. 2008;52:709–722. doi: 10.1016/j.neuint.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LTL, Fuchs RA, Coffey GP, Baker DA, Odell LE, Neisewander JL. Time-Dependent Changes in Cocaine-Seeking Behavior and Extracellular Dopamine Levels in the Amygdala during Cocaine Withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huijstee AN, Mansvelder HD. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Frontiers in Cellular Neuroscience. 2014;8:466. doi: 10.3389/fncel.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y. Chapter 2 - Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. In: Hamed E, Martin PP, editors. Progress in Brain Research. Elsevier; 2016. pp. 25–52. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem. 2005;280:34924–34932. doi: 10.1074/jbc.M502581200. [DOI] [PubMed] [Google Scholar]