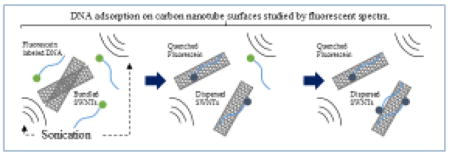
Abstract
Surface modification of single-walled carbon nanotubes (SWNTs) with DNA molecules has attracted much attention in recent years to increase SWNT solubility and make various SWNT-based nanobiodevices. Therefore, there is a critical need to quantify the interaction between DNA molecules and SWNT surfaces, particularly the intermediate structures during DNA adsorption. In this study, we demonstrate the ability to detect the adsorption of DNA oligomers on SWNT surfaces by fluorescence spectroscopy. Fluorescein-labelled, 30 mer, thymine oligonucleotides (F-T30) were employed as a fluorescent probe to study the interaction of DNA with SWNTs. A clear quenching effect was observed when F-T30 was adsorbed onto SWNT surfaces. Using this method, the amount of DNA adsorbed onto the SWNT surfaces was measured under different sonication conditions to correlate absorption efficiency with sonication strength and duration. When a bath-type sonicator was used, mild adsorption of F-T30 on SWNT surfaces was observed. Furthermore, a two-step adsorption was observed in this condition. In contrast, we observed rapid adsorption of F-T30 to SWNT surfaces at the higher sonication amplitude (60% maximal) using a probe-type sonicator, while only slight adsorption of DNA molecules was observed at the lower amplitude (20% maximal). Our data revealed that the quenching effect can be used to evaluate DNA adsorption onto SWNT surfaces. In addition, atomic force microscopy (AFM) and photoacoustic spectroscopy (PAS) were conducted to provide complementary information on the DNA-SWNT nanoconjugates.
Keywords: single-walled carbon nanotube, DNA, fluorescence spectroscopy, atomic force microscopy, photoacoustic spectroscopy, fluorescence quenching
Graphical Abstract
Introduction
The hybridization of DNA to carbon nanotubes (CNTs) has become an established method to prepare monodisperse suspensions of solubilized bundled CNTs. [1–5] This solubilization technique allows for the separation of CNTs based on their chiralities. [6, 7] Furthermore, DNA-CNTs hybrids are attractive candidates for nanobiodevices that can be extensively employed in drug delivery and DNA sensing studies. [8–13]
To allow for the hybridization of DNA with CNTs, suitable amounts of DNA solution and CNT powder are mixed in a sample tube. [14–18] Typically the mixture is sonicated by either probe-type or bath-type sonicators. Sonication of bundled CNTs causes liberation of individual CNTs, which enables DNA molecules to wrap around individual CNT surfaces. Since DNA is a hydrophilic molecule, the DNA-CNT hybrids have hydrophilic surfaces. After completion of the sonication, the separated individual CNTs do not form bundles again.
Although the hybridization of DNA to CNTs is an established technique, the process of DNA adsorption to CNT surfaces has not been well-detected. The majority of research investigations involving DNA-CNT hybridization used extensive sonication periods to prepare the hybrids. [5, 15] However, sonication can also cause damage to DNA molecules and CNTs. In order to evaluate DNA-single-walled carbon nanotube (SWNT) hybrids, several research groups have studied sonication effects during preparation. [19–21] For example, Koh et al. thoroughly investigated the effects of sonication time (between one and twelve hours) on DNA adsorption by near-infrared absorbance spectroscopy/atomic force microscopy (AFM) and Raman spectroscopy. In previous studies, sonication times lasted hours. Therefore, final DNA-SWNT hybrids, rather than intermediate hybrid structures, were characterized. In addition, dispersed SWNTs rather than adsorbed DNA molecules were characterized. Since sonication remains a crucial technique for DNA-CNT hybridization, detecting the intermediate stages of DNA adsorption to CNT surfaces is imperative for determining the optimal conditions to form DNA-CNT hybrids.
Recent studies on the quenching effect of fluorescent molecules in DNA-CNT hybridization have been reported by several groups. [22–24] Fluorescently labelled DNA molecules adsorbed on CNT surfaces completely quenched, even after the DNA-CNT hybrids were irradiated with an excitation beam. Based on this observation, a method for detecting DNA mismatches has been proposed. [25–27]
In this work, we applied the fluorescence quenching method to detect DNA adsorption on CNT surfaces in conjunction with AFM and photoacoustic spectroscopy (PAS). A mixture of fluorescently labelled, 30-mer, single-stranded oligomers of thymine (F-T30) and SWNTs was sonicated under various conditions. By measuring the quenching ratio of F-T30 via fluorescence spectroscopy, we successfully detected DNA adsorption as a function of sonication intensity and duration. However, we did not detect SWNT dispersion in these experiments. These results complement the results mentioned above from previous studies. Furthermore, AFM and PAS were used to determine the dimensions and changes in heat capacity of the DNA-SWNT hybrids, respectively.
Materials and Methods
The SWNT powder used in this study was purchased from Cheap Tubes Inc. (sku-0101, Cambridgeport, VT, USA). Non-fluorescently labelled (T30) and fluorescently labelled single-stranded oligomers of thymine 30 mer (F-T30) were obtained from Thermo Fisher Scientific K.K. (Yokohama, JP). DNA solutions were independently prepared by dissolving T30 and F-T30 powders in 200 mM Tris (hydroxymethyl) aminomethane buffered solution (Tris-HCL, pH 7.5) to final concentration of 1.0 mg/mL.
For evaluation of samples by fluorescence spectroscopy and atomic force microscopy (AFM), 0.5 mg/mL SWNT and 0.1 mg/mL F-T30 were prepared in Tris-HCl pH 7.5 in plastic microtubes. Samples were sonicated using either a probe-type sonicator (VCX 130 630-0423: ϕ = 2 mm, 130W; Sonics & Materials Inc., Newtown USA) or a bath-type sonicator (80W; EOL80; Steady Ultrasonic Sdn. Bhd., Selangor Malaysia) [28–30]. The different sonication conditions were as follows: (a) sonication at 60% maximum amplitude of the probe-type sonicator (P60), (b) sonication at 20% maximum amplitude of the probe-type sonicator (P20), (c) bath-type sonication (B), and (d) control (no sonication). For samples sonicated with the bath-type sonicator, the amplitude could not be varied since the sonication power of the instrument could not be modulated. Sample volumes of 30 μL were collected from the mixture at 0, 2, 4, 6, 8, 10, 15, 20, 25, and 30-minute time points during sonication. Due to the heterogeneity of the mixtures, fluorescence intensity measurements were carried out immediately after sample collection. Fluorescence measurements were carried out using the FP-8300 Fluorescence Spectrometer (JASCO Corporation, Tokyo, Japan) on the highest detection sensitivity setting. Fluorescence intensity was measured at 525 nm (excitation wavelength = 495 nm) using 2 μL of sample diluted into 2498 μL of Tris HCl pH 7.5. AFM measurements were carried out using a JSPM-5200 Scanning Probe Microscope (JEOL Ltd., Tokyo, Japan) using the AC imaging mode in air. Appropriate volumes of samples were pipetted onto mica surfaces pretreated with 0.01% ammonium persulfate (APS). [15] After 10 minutes of incubation, the samples were rinsed with 1 mL of water. The diameters and lengths of SWNTs were obtained from the AFM images using scanning frame sizes of 1.5×1.5 μm and 3.0×3.0 μm, respectively.
A photoacoustic scanner (LOIS-2D, TomoWave, Houston, TX) was used in the forward mode to measure the photoacoustic spectrum of the DNA-SWNT samples. A vial with a sample volume of 20 μL was placed in a 200-mL water bath. A tunable, optical parametric oscillator (OPO, Continuum, CA, USA) pumped by a Q-switched Nd:YAG laser (Surelite SLIII-10, Continuum, CA. USA) with a repetition rate of 10 Hz and a 4–6 ns pulse width was used to irradiate the samples at 834, 884, 941, 972, and 1000 nm with energies less than 1.5 mJ/cm2. The energy difference between wavelengths was measured by a photodiode to normalize the photoacoustic signals at different wavelengths. An ultrasound transducer with a central frequency of 5 MHz was used to acquire the photoacoustic signals of the samples. The acquired signal was the average of 10 scans. The peak-to-peak voltages (Vpp) of the central four elements of the transducer were summed and the average of five replicates was used to compare the photoacoustic responses of samples prepared under different sonication conditions.
Results and Discussion
Figure 1 indicates the analytical approaches used in this work. Mixtures of F-T30 or T30 and SWNTs were sonicated under various conditions, and the adsorption of DNA to SWNTs was detected by fluorescence spectroscopy, atomic force spectroscopy (AFM), and photoacoustic spectroscopy (PAS). We first took advantage of the unique fluorescence quenching effect of SWNTs on the adsorbed fluorescently labelled DNA, which provided an effective way to quantify the amount of DNA molecules adsorbed onto the SWNT surfaces. Secondly, as DNA adsorption onto SWNT surfaces causes an increase in SWNT diameter [5, 15, 30], we utilized AFM to measure the dimensions of individual DNA-SWNT hybrids based on their cross-section values in the images. This approach allowed us to directly assess the effects of sonication on the DNA-SWNT hybridization process. Finally, PAS was used to characterize the photoacoustic emission properties of DNA-SWNT hybrids. Although SWNTs have been used as contrast agents for PA imaging in previous studies [31–33], the use of PAS in this study provided a unique method for characterizing DNA-SWNT hybrids by measuring their heat capacity properties.
Figure 1.
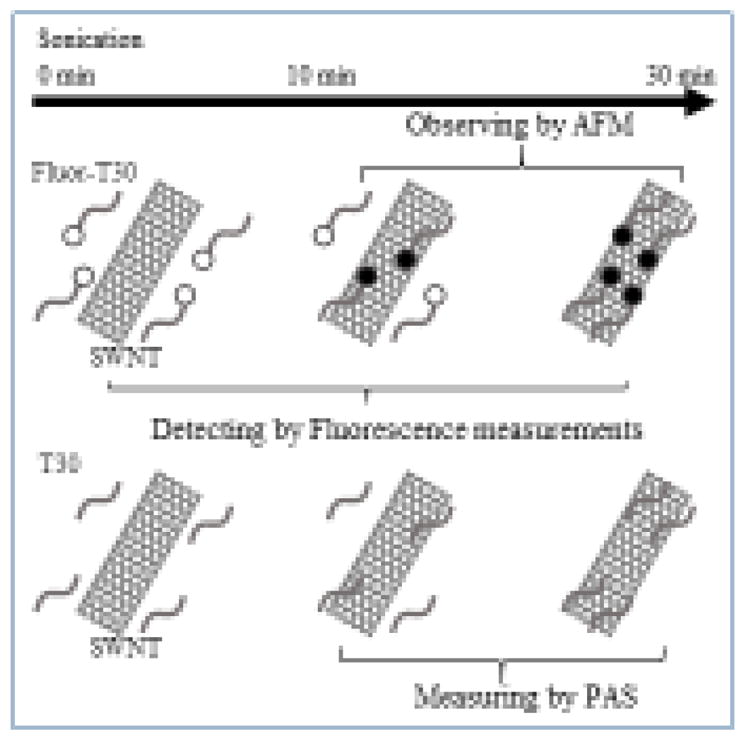
Schematic drawing of our experimental design. The DNA-SWNT hybrid samples are prepared under different sonication conditions and characterized by measuring quenching of fluorescence intensity in conjunction with atomic force microscopy and photoacoustic spectroscopy.
Figure 2 shows the fluorescence intensity of fluorescently labelled DNA -SWNT hybrids synthesized with different sonication methods and durations or in the absence of sonication. When SWNT powder was mixed with F-T30 solution, the fluorescence intensity of F-T30 was slightly decreased even without sonication. This suggests that some of the DNA molecules immediately adsorbed onto SWNT surfaces when the two components were mixed. However, this initial quenching value highly fluctuated, because the SWNT powder was not solubilized at this stage. For this reason, we focused on detecting DNA adsorption during sonication. For each sample, fluorescence intensity measurements were repeated 5 times and were normalized to the maximum fluorescence intensity.
Figure 2.
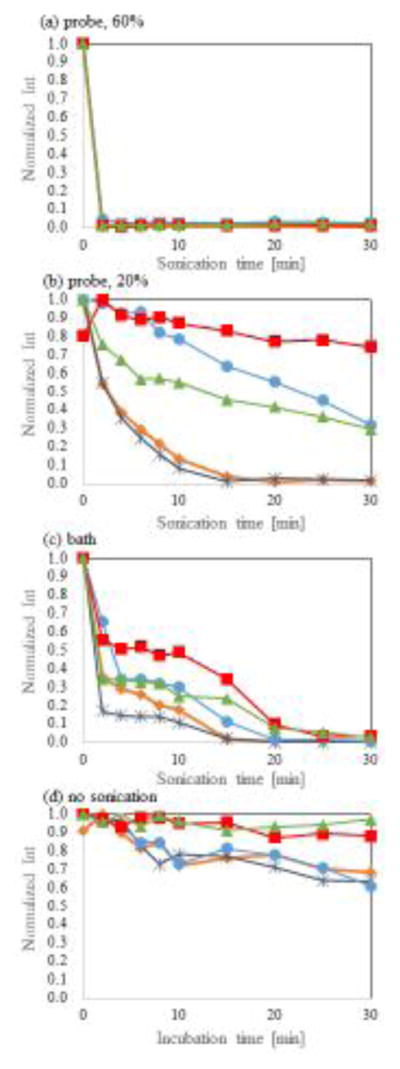
Normalized fluorescence intensity of F-T30 adsorption onto SWNT surfaces as a function of sonication strength and duration. (a) 60% of the maximum amplitude of the probe-type sonicator (P60), (b) 20% of the maximum amplitude of the probe-type sonicator (P20), (c) bath-type sonicator (B), and (d) control (no sonication). Five lines in each figure indicate results of independently repeated five measurements.
The mixtures of F-T30 and SWNTs sonicated under the P60 condition (probe-type sonicator with amplitude set to 60% of its maximum value) had almost complete quenching of fluorescence intensity within 2 minutes (Figure 2a). This indicates that most of the F-T30 molecules were adsorbed within 2 minutes, although this does not suggest complete monodispersity of F-T30-SWNT hybrids within this timeframe.
When mixtures were sonicated under the P20 condition (probe-type sonicator with amplitude set to 20% of its maximum value), the resulting data revealed high fluctuations among the five independent repetitions. Fluorescence intensity gradually decreased in two samples and was significantly quenched after 30 minutes of sonication (Figure 2b). However, the same degree of quenching was not obtained in the other three samples. The large difference between replicates indicates that the samples may be highly affected by conditions not considered in these experiments, such as the position of the sonicating probe in the sample. At high sonication power, such as in the P60 condition, the position of the probe would have less effect since enough sonication power can reach throughout the sample. However, the P20 condition, which uses a lower sonication amplitude, may not be sufficient to fully homogenize samples. Thus, probe location would have a greater effect on hybrid formation. The difference between replicates suggests 20% maximal amplitude is close to a lower limit threshold value for causing adsorption of F-T30 to SWNT surfaces.
When samples were sonicated under condition B (bath-type sonication), two-step quenching was observed. Fluorescence intensity dramatically decreased within the first 4 minutes of sonication. The decrease in fluorescence intensity was highly restrained up to the 10-minute time point, followed by a resurgence in successive quenching until the samples were fully quenched at 30 minutes (Figure 2c). Although further experiments are necessary to understand the molecular basis for the two-step quenching effect, one possible explanation is that some of the F-T30 molecules are adsorbed onto the bundled SWNTs prior to dissociation of individual SWNTs.
Under the control condition without sonication, the F-T30 solution and SWNT powder were mixed into a solution. Fluorescence intensities of some hybrids were slightly lower than that of the control F-T30 solution without SWNTs (Figure 2d). This finding suggests that some F-T30 molecules adsorb to SWNT surfaces almost immediately after introducing the two components in solution. During the remaining 30 minutes of incubation without sonication, fluorescence intensity only slightly decreased in four out of the five samples.
The average quenching efficiencies of the samples are included in Table 1 and in the supplementary information section (Table S1). After 2 minutes of sonication, the quenching efficiencies were 98%±1.5%, 19%±27%, 58%±17%, and 0.62%±5.2% for P60, P20, B, and the control condition, respectively. After 30 minutes of sonication, the efficiencies were 98%±0.83%, 69%±33%, 99%±1.4%, and 23%±14%, respectively.
The fluorescence quenching data suggest that the lower sonicator strength setting at 20% (P20) is inefficient for producing DNA-SWNT hybrids. However, increasing the sonication amplitude to 60% (P60) resulted in much higher quenching efficiencies. Therefore, the higher sonication strength significantly improved the production efficiency of DNA-SWNT hybrids by allowing DNA molecules to more effectively attach to SWNT surfaces. Although probe-type sonicators were more commonly used in previous studies than bath-type sonicators for preparing DNA-SWNT hybrids, our results indicate the two methods had similar adsorption efficiencies after 30 minutes. Despite this, there are other considerations in choosing which type of sonication instrument to use. For probe-type sonicators, sample tubes remain open in order to insert the probe, whereas the sample vials can remain sealed in bath-type sonication, thus preventing samples from evaporating during sonication. Additionally, sonicating probes must be washed in volatile solvents to remove residual contaminants, while bath-type sonication does not require this step. Therefore, although using a sonicating probe at 60% maximal amplitude (P60) is an effective approach to promote rapid attachment of DNA to SWNTs, bath-type sonicators are also effective and have their own advantages.
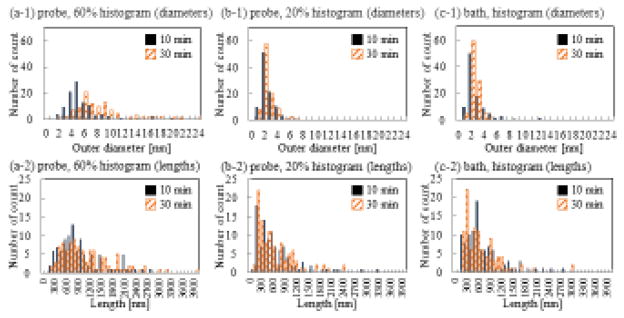
Changes in fluorescence intensity can be used to detect the adsorption of F-T30 to SWNT surfaces based on the quenching effect of SWNTs, but it does not assess the monodispersity of SWNTs. Since size analyses of nanoconjugates can be used to evaluate the dispersion and solubility of SWNTs, we employed AFM to measure the diameter and length of the SWNT samples or DNA-SWNT hybrids. The supplementary Figures S1 and S2 are AFM images showing that F-T30-SWNT hybrids were well-observed in the samples prepared using the P60, P20, and B sonicating conditions. Figure 3 is a histogram showing the diameters and lengths of each sample after 10 and 30 minutes of sonication. The diameter data was obtained from 100 cross-sections for 20 F-T30-SWNTs.
Figure 3.
Histograms of diameters and lengths of F-T30-SWNT hybrids measured by atomic force microscopy. (a) diameters. (b) lengths. (1) P60. (2) P20. (3) B.
Interestingly, the diameter distribution of DNA-SWNT hybrids prepared under P60 sonication was clearly broader than those prepared under P20 and B conditions. The average diameters of the DNA-SWNT hybrids prepared under the P60 condition at 10 min and 30 min were 5.2±2.8 nm and 7.9 ± 4.1 nm, respectively. The diameters of the hybrids were markedly larger than that of pure SWNTs (1–4 nm) under the same corresponding sonication conditions, indicating attachment of DNA onto the SWNT surfaces. The average diameters of the DNA-SWNT hybrids in the P20 and B conditions were 2.0 ± 1.1 nm and 2.5 ± 2.0 nm at 10 min, respectively, and 2.0 ± 1.1 nm and 1.8 ± 0.74 nm at 30 min, respectively. A similar average value (2.0 ± 0.79 nm) was obtained when a mixture of T30 and SWNTs were sonicated under the standard conditions for SWNT solubilization that was employed by many other research groups (DNA: SWNTs = 1:1 in weight ratio; 90 min sonication under P60 condition). [5, 15] The data suggest that DNA-SWNTs had high monodispersity under the P20 and B conditions. On the other hand, there was no significant difference in lengths of DNA-SWNTs for all of the conditions tested. The length data was obtained from 100 cross-sections for 100 DNA-SWNT hybrids.
Figure 4 shows the photoacoustic (PA) signals of each sample excited at five different laser wavelengths. The signals are normalized by corresponding sample concentration and represented as Vpp (voltage of peak to peak) response of the transducer. The PA signal of the P20 samples collected after 10 minutes of sonication was significantly higher than those of other samples, while the PA signal of P60 samples was the lowest among all the samples. Since the same SWNT powder was employed in all of the samples to ensure consistency, the difference in PA signals may be attributed to different amounts of T30 adsorbed onto the SWNTs. In addition, there was a correlation between PA signal of the T30-SWNT hybrids and the fluorescence signal of the F-T30-SWNT hybrids. As shown in Table 1, higher PA signal strength correlated with lower fluorescence quenching efficiency of the corresponding sample, indicating less adsorbed F-T30. This result can be explained based on the fact that PA signals are inversely proportional to the heat capacity of the sample. For example, the low PA signals observed from samples prepared under P60 sonication conditions indicated an increase in heat capacity of the T30-SWNTs, as almost all the DNA molecules are adsorbed onto the SWNTs. On the other hand, the high PA signals observed for samples prepared under the P20 sonication condition for 10 minutes indicated low heat capacity of the hybrids, as few DNA molecules were adsorbed on the SWNTs. Thus, this study demonstrates that PAS provides a unique method to detect hybridization of DNA with SWNT by measuring the change in heat capacity of DNA-SWNT hybrids.
Figure 4.
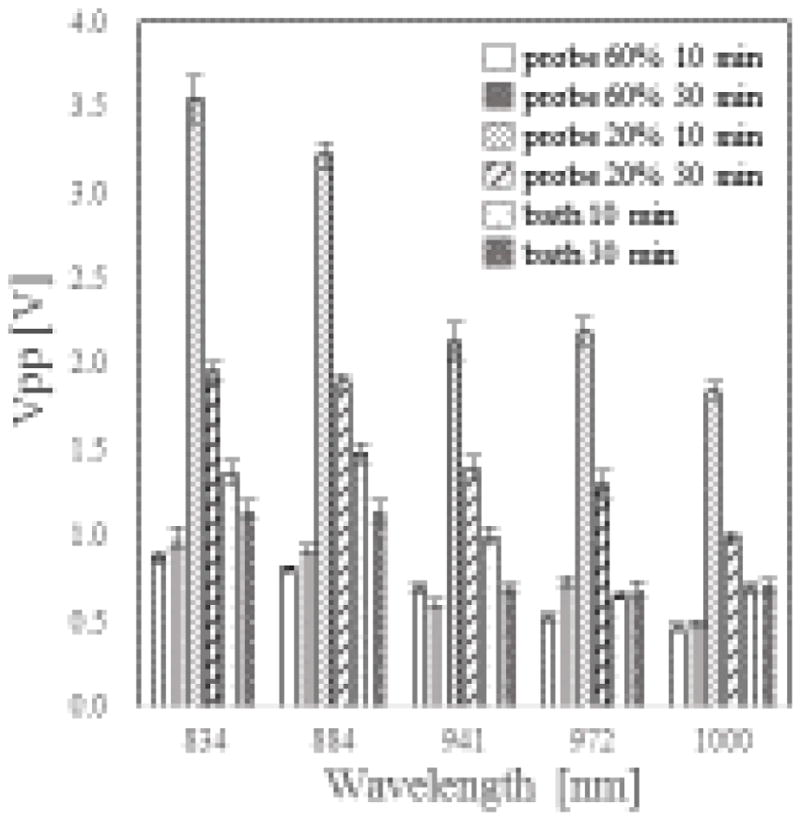
PA intensities from T30-SWNT hybrids prepared under different sonication conditions. Excitation wavelengths were 834, 884, 941, 972, and 1000 nm, respectively.
Table 1.
Summary of the results from fluorescence spectroscopy, atomic force microscopy, and photoacoustic spectroscopy experiments. Values are represented as mean ± standard deviation (n = 5 for fluorescent spectroscopy, n = 100 for atomic force microscopy (AFM), and n = 5 for photoacoustic spectroscopy (PAS)).
| 10 min | 30 min | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Fluor-T30-SWNT | T30-SWNT | Fluor-T30-SWNT | T30-SWNT | |||||
|
| ||||||||
| Quenching efficiency | Outer diameters [nm] | Length [nm] | PA signal [V] wavelength 941 nm | Quenching efficiency | Outer diameters [nm] | Length [nm] | PA signal [V] wavelength 941 nm | |
| Probe 60% | 98% ±0.72% | 5.2 ±2.8 | 937 ±580 | 0.69 ±0.028 | 98% ±0.83% | 7.9 ±4.1 | 1093 ±714 | 0.60 ±0.024 |
| Probe 20% | 47% ±38% | 2.0 ±1.1 | 648 ±568 | 2.13 ±0.11 | 69% ±33% | 2.0 ±1.1 | 588 ±504 | 1.39 ±0.068 |
| Bath | 74% ±13% | 2.5 ±2.0 | 707 ±524 | 0.99 ±0.045 | 99% ±1.4% | 1.8 ±0.74 | 617 ±504 | 0.69 ±0.028 |
| No sonication | 16% ±9.5% | - | - | - | 23.% ±14% | - | - | - |
Our data indicated that adsorption of T30-SWNT hybrids were strongly affected by the sonication conditions. Although our original research target was to propose a new method for detecting DNA adsorption onto SWNT surfaces rather than determining the most effective sonication conditions, our data provide useful insights on this topic. As being employed in many previous papers have employed, P60 is useful as a general approach for general preparation of T30-SWNT hybrids. Under the P60 condition, DNA rapidly adsorb onto SWNT surfaces, and long hybrids can be obtained. The P20 and B conditions led to mild adsorption. However, the reproducibility of the data under condition B was better than that of the P20 condition. Shorter SWNTs were obtained under these two conditions. It is likely that longer SWNTs were not solubilized. Although we did not focus on obtaining detailed structural information in this work, precise structural characterization of DNA-SWNT hybrids would be useful in future studies for determining the best sonication conditions.
Conclusions
Fluorescence spectroscopy, atomic force microscopy (AFM), and photoacoustic spectroscopy were effectively used to evaluate the adsorption profiles of DNA onto SWNT surfaces under differing sonication conditions. Adsorption of DNA onto SWNT surfaces using fluorescence spectroscopy was based on the fluorescence quenching effect of fluorescently labeled, 30-mer, single-stranded thymine oligonucleotides (F-T30) by SWNTs. Our experimental results indicated that the fluorescence intensity of F-T30 were quenched in two steps, i.e., naturally by SWNTs without sonication and enhanced by sonication. AFM measurements provided dimensions of the F-T30-SWNT hybrids. This study also showed that the PAS results were consistent with the results obtained from the fluorescence spectroscopy measurements, indicating the effectiveness of using PAS for characterizing T30-SWNT hybrids. Our results revealed useful information for understanding the mechanism of DNA adsorption onto SWNT surfaces.
Supplementary Material
Highlights.
DNA adsorption to SWNT surfaces were monitored by fluorescence spectroscopy.
The DNA adsorption was affected by sonication conditions.
AFM and PAS provided helpful information.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (26400436) from the Japan Society for the Promotion of Science (JSPS) and NIH RISE R25GM060655. It was also supported in part by the CONNECT grant, the CPRIT grant RP120558, and NIH R21EB008765.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakashima N, Okuzono S, Murakami H, Nakai T, Yoshikawa K. DNA dissolves single-walled carbon nanotubes in water. Chem Lett. 2003;32:456–457. [Google Scholar]
- 2.Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 3.Pividori MI, Alegret S. DNA adsorption on Carbonaceous materials. In: Wittmann C, editor. Immobilisation of DNA on Chips I. Springer-Verlag; Berlin, Berlin: 2005. pp. 1–36. [Google Scholar]
- 4.Nakashima N. Solubilization of single-walled carbon nanotubes with condensed aromatic compounds. Sci Technol Adv Mater. 2006;7:609–616. [Google Scholar]
- 5.Umemura K. Hybrids of Nucleic Acids and Carbon Nanotubes for Nanobiotechnology. Nanomaterials. 2015;5:321–350. doi: 10.3390/nano5010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu XM, Zheng M. A DNA-Based Approach to the Carbon Nanotube Sorting Problem. Nano Res. 2008;1:185–194. [Google Scholar]
- 7.Zhao YL, Stoddart JF. Noncovalent Functionalization of Single-Walled Carbon Nanotubes. Accounts Chem Res. 2009;42:1161–1171. doi: 10.1021/ar900056z. [DOI] [PubMed] [Google Scholar]
- 8.Karachevtsev VA, Glamazda AY, Leontiev VS, Lytvyn OS, Dettlaff-Weglikowska U. Glucose sensing based on NIR fluorescence of DNA-wrapped single-walled carbon nanotubes. Chem Phys Lett. 2007;435:104–108. [Google Scholar]
- 9.Xu Y, Pehrsson PE, Chen LW, Zhang R, Zhao W. Double-stranded DNA single-walled carbon nanotube hybrids for optical hydrogen peroxide and glucose sensing. J Phys Chem C. 2007;111:8638–8643. [Google Scholar]
- 10.Sanchez-Pomales G, Santiago-Rodriguez L, Cabrera CR. DNA-Functionalized Carbon Nanotubes for Biosensing Applications. J Nanosci Nanotechnol. 2009;9:2175–2188. doi: 10.1166/jnn.2009.se47. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JQ, Boghossian AA, Barone PW, Rwei A, Kim JH, Lin DH, Heller DA, Hilmer AJ, Nair N, Reuel NF, Strano MS. Single Molecule Detection of Nitric Oxide Enabled by d(AT)(15) DNA Adsorbed to Near Infrared Fluorescent Single-Walled Carbon Nanotubes. J Am Chem Soc. 2011;133:567–581. doi: 10.1021/ja1084942. [DOI] [PubMed] [Google Scholar]
- 12.Zhang WX, Zhang ZZ, Zhang YG. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett. 2011;6:22. doi: 10.1186/1556-276X-6-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensafi AA, Amini M, Rezaei B. Biosensor based on ds-DNA decorated chitosan modified multiwall carbon nanotubes for voltammetric biodetection of herbicide amitrole. Colloid Surf B-Biointerfaces. 2013;109:45–51. doi: 10.1016/j.colsurfb.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Hayashida T, Kawashima T, Nii D, Ozasa K, Umemura K. Kelvin Probe Force Microscopy of Single-walled Carbon Nanotubes Modified with DNA or Poly(ethylene glycol) Chem Lett. 2013;42:666–668. [Google Scholar]
- 15.Hayashida T, Umemura K. Surface morphology of hybrids of double-stranded DNA and single-walled carbon nanotubes studied by atomic force microscopy. Colloid Surf B-Biointerfaces. 2013;101:49–54. doi: 10.1016/j.colsurfb.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Nii D, Hayashida T, Umemura K. Controlling the adsorption and desorption of double-stranded DNA on functionalized carbon nanotube surface. Colloid Surf B-Biointerfaces. 2013;106:234–239. doi: 10.1016/j.colsurfb.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 17.Nii D, Hayashida T, Yamaguchi Y, Ikawa S, Shibata T, Umemura K. Selective binding of single-stranded DNA-binding proteins onto DNA molecules adsorbed on single-walled carbon nanotubes. Colloid Surf B-Biointerfaces. 2014;121:325–330. doi: 10.1016/j.colsurfb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Oura S, Ito M, Nii D, Homma Y, Umemura K. Biomolecular recognition ability of RecA proteins for DNA on single-walled carbon nanotubes. Colloid Surf B-Biointerfaces. 2015;126:496–501. doi: 10.1016/j.colsurfb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Koh B, Cheng W. The Impact of Sonication on the Surface Quality of Single-Walled Carbon Nanotubes. J Pharm Sci. 2015;104:2594–2599. doi: 10.1002/jps.24483. [DOI] [PubMed] [Google Scholar]
- 20.Koh B, Park JB, Hou XM, Cheng W. Comparative Dispersion Studies of Single-Walled Carbon Nanotubes in Aqueous Solution. J Phys Chem B. 2011;115:2627–2633. doi: 10.1021/jp110376h. [DOI] [PubMed] [Google Scholar]
- 21.Yang QH, Wang Q, Gale N, Oton CJ, Cui L, Nandhakumar IS, Zhu ZP, Tang ZY, Brown T, Loh WH. Loosening the DNA wrapping around single-walled carbon nanotubes by increasing the strand length. Nanotechnology. 2009;20:5. doi: 10.1088/0957-4484/20/19/195603. [DOI] [PubMed] [Google Scholar]
- 22.Hedderman TG, Keogh SM, Chambers G, Byrne HJ. Solubilization of SWNTs with organic dye molecules. J Phys Chem B. 2004;108:18860–18865. [Google Scholar]
- 23.Satishkumar BC, Brown LO, Gao Y, Wang CC, Wang HL, Doorn SK. Reversible fluorescence quenching in carbon nanotubes for biomolecular sensing. Nat Nanotechnol. 2007;2:560–564. doi: 10.1038/nnano.2007.261. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CF, Dementev N, Borguet E. Fluorescence Quenching of Dyes Covalently Attached to Single-Walled Carbon Nanotubes. J Phys Chem A. 2011;115:9579–9584. doi: 10.1021/jp200152z. [DOI] [PubMed] [Google Scholar]
- 25.Li HL, Tian JQ, Wang L, Zhang YW, Sun XP. Multi-walled carbon nanotubes as an effective fluorescent sensing platform for nucleic acid detection. J Mater Chem. 2011;21:824–828. [Google Scholar]
- 26.Wang L, Li HL, Luo YL, Zhang YW, Tian JQ, Sun XP. Detection of single-stranded nucleic acids by hybridization of probe oligonucleotides on polystyrene nanospheres and subsequent release and recovery of fluorescence. RSC Adv. 2011;1:1318–1323. [Google Scholar]
- 27.Chen J, Huang Y, Shi M, Zhao SL, Zhao YC. Highly sensitive multiplexed DNA detection using multi-walled carbon nanotube-based multicolor nanobeacon. Talanta. 2013;109:160–166. doi: 10.1016/j.talanta.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Hayashida T, Umemura K. Atomic Force Microscopy of DNA-wrapped Single-walled Carbon Nanotubes in Aqueous Solution. Colloid Surf B-Biointerfaces. 2016;143:526–531. doi: 10.1016/j.colsurfb.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 29.Umemura K, Ishibashi Y, Oura S. Physisorption of DNA molecules on chemically modified single-walled carbon nanotubes with and without sonication. Eur Biophys J Biophys Lett. 2016;45:483–489. doi: 10.1007/s00249-016-1116-3. [DOI] [PubMed] [Google Scholar]
- 30.Umemura K, Izumi K, Oura S. Probe Microscopic Studies of DNA Molecules on Carbon Nanotubes. Nanomaterials. 2016;6:14. doi: 10.3390/nano6100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pramanik M, Song KH, Swierczewska M, Green D, Sitharaman B, Wang LHV. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys Med Biol. 2009;54:3291–3301. doi: 10.1088/0031-9155/54/11/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu ZA, Yang K, Lee ST. Single-walled carbon nanotubes in biomedical imaging. J Mater Chem. 2011;21:586–598. [Google Scholar]
- 33.Judkins J, Lee HH, Tung S, Kim JW. Diffusion of Single-Walled Carbon Nanotube Under Physiological Conditions. J Biomed Nanotechnol. 2013;9:1065–1070. doi: 10.1166/jbn.2013.1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.