Abstract
Understanding how tobacco product flavor additives, such as flavorants in electronic cigarettes, influence smoking behavior and addiction is critical for informing public health policy decisions regarding tobacco product regulation. Here, we developed a combined intraoral (i.o.) and intravenous (i.v.) self-administration paradigm in rats to determine how flavorants influence self-administration behavior. By combining i.o. flavorant delivery with fast scan cyclic voltammetry (FSCV) or i.v. nicotine self-administration in adult, male rats, we examined whether flavors alter phasic dopamine (DA) signaling and nicotine self-administration. Oral administration of 10% sucrose or 0.32% saccharin, but not 0.005% menthol, increased phasic DA release in the nucleus accumbens (NAc). Oral sucrose or saccharin, when combined with i.v. nicotine delivery, also led to increased self-administration behavior. Specifically, combined i.o. sucrose and i.v. nicotine decreased responding compared to sucrose alone, and increased responding compared to nicotine alone. In contrast, i.o. flavorants did not alter motivational breakpoint in a progressive ratio task. Oral menthol, which did not alter i.v. nicotine administration, reversed oral nicotine aversion (50 and 100 mg/L) in a two-bottle choice test. Here, we demonstrate that i.o. appetitive flavorants that increase phasic DA signaling also increase self-administration behavior when combined with i.v. nicotine delivery. Additionally, oral menthol effects were specific to oral nicotine, and were not observed with i.v. nicotine-mediated reinforcement. Together, these preclinical findings have important implications regarding menthol and sweet flavorant additive effects on tobacco product use and can be used to inform policy decisions on tobacco product flavorant regulation.
1. Introduction
The 2009 Family Smoking Prevention and Tobacco Control Act gave the Federal Drug Administration (FDA) power to regulate flavor additives, with the exemption of menthol, in tobacco cigarettes. A primary goal was to reduce smoking in adolescents, primary consumers of flavored cigarettes. However, the advent of flavored electronic cigarettes (e-cigarettes) have reinvigorated these discussions. Recent data suggest that flavored e-cigarettes are the most popular form of smokable tobacco products initiated by high school students (Krishnan-Sarin et al., 2014). Moreover, the availability of appetitive flavors is one of the primary reasons why high school students use e-cigarettes (Kong et al., 2014; Villanti et al., 2013). Currently, there is limited data on oral menthol and appetitive flavorant effects on nicotine self-administration behavior and tobacco product use, although a recent finding in young adult smokers found enhanced reward for flavored versus non-flavored e-cigarettes (Audrain-McGovern et al., 2016).
Individuals who smoke menthol cigarettes have lower quit rates (Delnevo et al., 2011; Gandhi et al., 2009) and a faster time to smoke the day’s first cigarette, compared to non-menthol smokers (Ahijevych and Parsley, 1999). Menthol’s mint-like flavor, cooling, antitussive, and anti-irritant properties are thought to mask the bitter flavor and the irritation of the mouth, lungs, and throat induced by cigarettes (Kreslake et al., 2008; Lee and Glantz, 2011; Strasser et al., 2013; Wickham, 2015; Willis et al., 2011). Menthol also influences the subunit composition, expression, and function of nicotine acetylcholine receptors (nAChRs) in the mesolimbic dopamine (DA) system (Ashoor et al., 2013; Brody et al., 2013; Hans et al., 2012; Henderson et al., 2016). Burst firing of ventral tegmental area (VTA) DA neurons and the subsequent phasic DA release in the nucleus accumbens (NAc) is associated with exposure to rewards and can drive drug-taking and drug-seeking behavior (Day et al., 2007; Phillips et al., 2003; Solecki et al., 2013), while DA release in the NAc core sub-region, in particular, plays a critical role in cue-mediated drug-taking and drug-seeking (Phillips et al., 2003; Solecki et al., 2013). Indeed, based on their recent findings, Wang et al. have proposed that oral menthol may act as a conditioned cue when paired with nicotine (Wang et al., 2014). Due to the complex sensory profiles of menthol and tobacco product flavorants, however, it is unclear whether the tastants and flavorants themselves influence phasic DA signaling and nicotine intake.
In their recent work, Wang et al. specifically sought to examine oral menthol effects on i.v. nicotine taking in rats trained to lick a lickometer that produced simultaneous oral menthol and intravenous (i.v.) nicotine (Wang et al., 2014). However, their model produced low rates of nicotine self-administration and a lack of discrimination between active and inactive operandi. Here, we sought to develop a combined intraoral (i.o.) flavorant and i.v. drug operant paradigm that produced robust rates of nicotine self-administration, a strong dissociation between active and inactive operant responses, and compatibility with in vivo electrochemical techniques. We first combined fast scan cyclic voltammetry (FSCV) with i.o. flavorant delivery to determine whether flavorants alter phasic DA signaling in the NAc. Secondly, we combined i.o. flavorant delivery with i.v. self-administration to demonstrate that oral flavorants which increased phasic DA release also increased self-administration behavior when combined with i.v. nicotine under fixed ratio schedules. In contrast, these i.o. flavorants did not alter the motivation for nicotine taking in a progressive ratio task. These experiments demonstrate the ability of oral flavorants to alter nicotine self-administration and provide a foundation for future investigations of the neurochemical and behavioral effects of other tobacco product flavorants.
2. Materials and Methods
2.1 Animals
Male Sprague Dawley rats (250–350 g, Charles River Laboratories, Wilmington, MA, USA) were placed on ad libitum food and water and housed 2–3 per cage on a 12-h light/dark cycle (lights on at 7 am). One week after combined i.o. and i.v. catheter surgery, rats were maintained at 85–90 % body weight throughout behavioral training. After surgery, each animal was housed individually to prevent cage mates from damaging each other’s catheters. Experiments were conducted according to the Guide for the Care and Use of Laboratory Animals and were approved by the Yale University Institutional Animal Care and Use Committee.
2.2 Drugs
Nicotine hydrogen tartrate salt (Sigma, St. Louis MO) was dissolved in 0.9 % saline solution (pH = 7.0) and filtered using a 0.22 μm filter. Nicotine was delivered at 30 μg/kg/infusion (free base) and infused for 6 s at 17.7 μL/s (106 μL total). For i.o. delivery, 10% sucrose, 0.32% saccharin, 0.005% menthol (all w/v) were prepared in deionized (DI) water and infused at a rate of 33 μL/s for 6 s (362 μL). Flavorants and doses were selected to allow for examination of caloric and non-caloric appetitive flavorants (sucrose and saccharin, respectively) and menthol, encompassing multiple types of tobacco product flavorants. The reinforcing 10% sucrose served as a positive control for our newly established behavioral methodology. The appetitive saccharin dose was selected from two-bottle choice tests showing that 0.32% saccharin was most preferred compared to water. For menthol, 0.001%, 0.005% and 0.01% all showed equal preference to water (two bottle choice data not shown). Here, we chose to use the 0.005% concentration to maintain consistency with recently published work in mice showing the ability of 0.005% menthol to decrease oral nicotine aversion, even with demonstrated equal preference between water and 0.005% menthol (Fan et al., 2016). For two-bottle choice experiments, nicotine hydrogen tartrate salt (Sigma, St. Louis MO) was dissolved in DI water and 0.1 M NaOH was used to set the pH to ~7.4.
2.3 Surgical procedures
Rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p. Sigma Aldrich, USA). First, we implanted a silastic catheter into the external jugular vein, as described by previously (Solecki et al., 2013). Second, we implanted an ethylene oxide sterilized polyethylene i.o. catheter that was anchored to the first molar and protruded dorsally through the skin between the ears. For additional, detailed i.o. surgical methodology, see (Wickham et al., 2015). Carprofen (5 mg/kg, s.c.) was administered prior to any surgical incision and was administered for three days post-surgery. In preparation for voltammetry experiments, a guide cannula (Bioanalytical Systems, West Lafayette, IL) was positioned above the NAc (AP +1.2 mm, ML −1.4 mm) and an Ag/AgCl reference electrode (previously baked at 120°C for 1 h) was implanted in the contralateral hemisphere (Wickham et al., 2015). Subsequently, a bipolar stimulating electrode was implanted in the VTA/substantia nigra (SN) (AP −5.2 mm, ML −0.5 to −1.5 mm, DV −8.0 to −9.0 mm). Dental cement (Dentsply, Milford, DE) and screws (Gexpro, High Point, NC) were used to secure the cannula and reference electrode to the skull.
2.4 Measurement of phasic DA combined with intraoral infusion
A micromanipulator containing a carbon fiber microelectrode was lowered to the NAc core and a low-pass filtered (2 kHz), a triangular potential waveform (−0.4 V to +1.3 V and back to −0.4 V, at a rate of 400 V/s) was applied at 60 Hz for 15 min, and then applied at 10 Hz for recordings. An initial training set of DA and pH was collected by stimulating the VTA at varying frequencies and pulses (10–20 Hz, 10–40 pulses, all at 150 μA). Then, a single flavor was administered (Fig. 1A) at pseudorandom intervals of 60, 120, and 180 s, for a total of 25 infusions, similar to previously published methodology (Roitman et al., 2008; Wickham et al., 2015).
Figure 1.
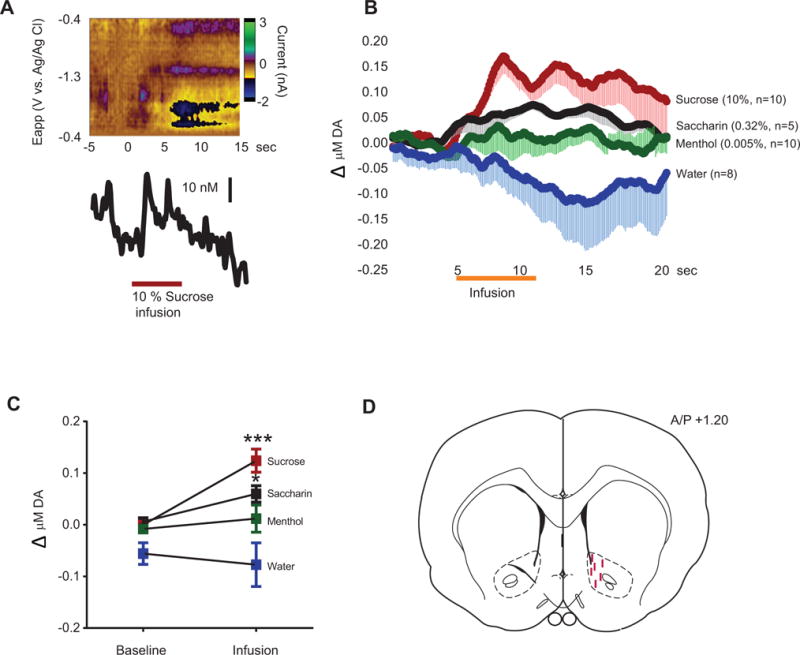
(A) Representative color plot of DA response to 10% sucrose. (B) Average DA response to 10% sucrose, 0.32% saccharin, 0.005% menthol, and water. Comparison of DA concentration before versus during (orange bar) flavor infusion. (C) Average change in DA concentration during the 6 s infusion versus the 5 s pre-infusion baseline. (D) Representative carbon fiber microelectrode placements. Post-hoc with Bonferroni correction for multiple comparisons: * p < 0.05, *** p < 0.001, baseline versus infusion. Error bars indicate standard error of the mean (SEM).
After the experiment, the carbon fiber microelectrode was removed and calibrated in vitro to determine DA concentrations. Using principal component regression and the DA training set, we identified and quantified phasic DA responses to flavorants, consistent with published methodology (Keithley et al., 2010; Keithley et al., 2009). In a subset of rats, the carbon fiber was used to lesion the recording site for histological verification (Fig. 1D) as previously described (Addy et al., 2010). Since the lesioned electrode could not be used for calibration, in rats used for histology, we used our obtained average calibration factor (7 nA/μM DA), consistent with our published methodology (Addy et al., 2010).
2.5 Combined intraoral and intravenous self-administration training and behavior
Sucrose or food pre-training is often necessary for obtaining robust nicotine self-administration in rats (Corrigall and Coen, 1989), although some have obtained nicotine self-administration without food pre-training (Levin et al., 2012; Smith et al., 2013). Here, sucrose training (10 days) was performed using i.o. delivery, where rats pressed a lever for 10% sucrose, with the reinforcement schedule gradually increasing from FR1 to FR5 (20 infusions per session, one session a day). On the last two days of training, rats were habituated to performing behavior with both their i.o. and i.v. catheters attached (no i.v. drugs delivered).
After pre-training, rats were trained on combined self-administration, where active lever depression led to simultaneous delivery of i.o. flavorant and i.v. drug for 6 s with simultaneous cue light presentation (20 s) and the house light being turned off (20 s). The 20 s timeout period (no drug available), co-terminated with the cue light turning off and the house light turning on. Each session lasted 1 hour. Active and inactive lever presses were recorded both when nicotine was available and during the timeouts. A short training regimen, performed over five FR1 sessions, was utilized for rats that were subsequently tested on conditioned reinforcement (Table 1). A long training regimen (FR1 sessions 1 to 5, FR2 sessions 6 to 8, FR5 sessions 9 to 14) was used for rats subsequently tested on a progressive ratio task (Table 1) – permitting us to analyze the ability of flavors to influence nicotine intake over a range of reinforcement schedules and to build extinction resistance often needed for progressive ratio tests. After i.v. self-administration experiments, catheter patency was verified using i.v. methohexital (0.5 mg/kg) administration, to induce a temporary (5 sec) loss of sternal posture in subjects with patent catheters. Any subjects with non-patent catheters, verified by a lack of response to methohexital administration, were not included in experimental analyses.
Table 1.
Summary of experiments and results
| Figures and Experimental Description | Summary of Results |
|---|---|
|
Figure 1 Measurement of phasic DA combined with intraoral infusion (Section 2.4). |
I.o. infusion of 10% sucrose or 0.32% saccharin, but not 0.005% menthol nor water, increases phasic DA release in the NAc core. |
|
Figures 2–4 Combined i.o. and i.v. self-administration training and behavior using long training regimen (Section 2.5). |
Combined i.o. infusion of sucrose or saccharin with i.v. nicotine elevates self-administration compared to i.o. water or menthol combined with i.v. nicotine. Combined i.o. sucrose plus i.v. nicotine leads to decreased self-administration compared to i.v. nicotine alone. |
|
Figure 5 Progressive ratio test (Section 2.6) one day after long-training regimen. Rats used were the same as in Figures 2–4. |
No differences in sucrose, menthol, nor saccharin in breakpoint. |
|
Figure 6 Conditioned reinforcement test (Section 2.7) after receiving short training regimen (Section 2.5) |
Conditioned reinforcement for saccharin nor menthol were altered by nicotine pairings compared to saline pairings. |
|
Figure 7 Two-bottle choice (Section 2.8) |
Menthol or saccharin increase oral consumption of nicotine. |
2.6 Progressive Ratio
Rats underwent progressive ratio testing in which the number of lever presses required to receive co-infusion of i.v. drug and i.o. flavor increased based on the formula [5-e 0.2n)-5], where n was the position in the following series of values: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95 (Arnold and Roberts, 1997). The experimental session terminated when a rat stopped responding on the active lever for 5 minutes.
2.7 Conditioned Reinforcement
Rats were placed in the same operant chamber as training, except that the levers were replaced with illuminated noseports. Each nosepoke produced a 362 μL infusion of the flavor previously paired with nicotine or saline.
2.8 Two-bottle choice
Rats were first acclimated to housing and husbandry for 5 to7 days after arrival. Prior to the experiment, home cage water bottles were first placed on one side of the cage (24 hours) and then placed on the opposite (24 hours) to prevent side biases. On test day, rats were first water deprived for 4 hours. Immediately after the deprivation period, 2 previously weighed bottles were placed on opposite sides of the cage for a one hour two-bottle choice test session. Bottle side assignments were made using a counterbalanced design. After the test session, the amount of each solution consumed was determined by comparing pre-session and post-session bottle weights.
2.9 Voltammetry statistical analysis
All voltammetry data were analyzed via a two-way repeated measures ANOVA (with flavor and time as factors). Post-hoc tests were performed using a Bonferroni correction.
2.10 Behavioral statistical analysis
All behavioral data were analyzed via a two-way repeated measures ANOVA (with either flavor and time or lever and time as factors). Post hoc tests were performed using a Bonferroni correction or a Holm-Sidok correction (CR experiment) for multiple comparisons. Statistical analyses were performed using Graph Pad Prism 7 (Graph Pad Software, La Jolla, CA). Effect size measurements were calculated using Cohen’s d.
3. Results
3.1 Intraoral delivery of saccharin and sucrose, but not menthol, increases phasic DA release
Rats received i.o. 10% sucrose, 0.32% saccharin, 0.005% menthol, or water while phasic DA release was monitored. Analyses revealed a significant interaction between time and flavor (F57,627 = 2.169, p < 0.0001; Fig. 1B) with main effects of time (F19,627 = 1.612, p < 0.05) and flavor (F3,33 = 10.78, p < 0.0001). Moreover, comparing average phasic DA release during the 6 s infusion to the 5 s pre-infusion baseline revealed a significant interaction between infusion period and flavor (F3,33 = 4.416, p < 0.05, Fig. 1C), with main effects of period (F1,33 = 7.104, p < 0.05) and flavor (F3,33 = 10.43, p < 0.0001). Post-hoc comparisons revealed that sucrose (p < 0.001) and saccharin (p < 0.05), but not menthol (p = 0.49) nor water (p = 0.48), increased phasic DA concentrations compared to baseline.
3.2 Co-administration of i.v. nicotine with sweet flavors, but not menthol, increases self-administration
In animals co-administering i.o. water and i.v. nicotine, analyses revealed an interaction between lever and time (F13,130 = 4.75, p < 0.0001), with main effects of both time (F13,130 = 5.378, p < 0.0001, Fig. 2A) and lever (F1,10 = 25.25, p < 0.001, Fig. 2A). Post-hoc tests revealed significant differences between active and inactive levers for FR5 Sessions 9 through 14. In i.o. water and i.v. saline rats, analyses revealed an interaction between time and lever (F13,130 = 5.79, p < 0.0001, Fig. 2B) along with significant main effects of time (F13,130 = 7.9, p < 0.0001, Fig. 2B), and lever (F1,10 = 17.92, p < 0.001, Fig. 2B). Post-hoc differences were only observed during the first three days of FR1 training, thus i.o. water plus i.v. saline rats did not discriminate between active and inactive levers by the end of training. In i.o. water animals, direct comparison of i.v. infusions in nicotine versus saline subjects (Fig. 2C) revealed an interaction between time and drug (F13,260 = 5.192, p < 0.0001) and main effects of time (F13,260 = 10.97, p < 0.0001) and drug (F1,20 = 15.66, p < 0.001), with significant post-hoc differences between nicotine and saline in FR5 sessions 10 through 14. Similar self-administration results were observed when i.v. nicotine was combined with i.o. menthol (Supplementary Fig. 1) or saccharin (Supplementary Fig. 2). Animals receiving i.o. sucrose plus i.v. saline obtained more infusions than i.o. sucrose plus i.v. nicotine rats throughout training (main effect of drug (F1,14 = 22.5, p<0.001, Fig. 3C), without an interaction (F13,182 = 1.429, p = 0.15) nor an effect of time (F13,182 = 1.61, p = 0.09). Analysis of self-administration behavior across all four i.o. groups revealed a significant interaction between time and flavor (F39,455 = 1.63, p < 0.05, Fig. 4A), with main effects of time (F13,455 = 4.34, p < 0.0001) and flavor (F3,455 = 10.03, p < 0.0001). Specifically, sucrose + nicotine rats, but not menthol + nicotine nor saccharin + nicotine rats, showed greater self-administration infusions compared to water + nicotine rats (p < 0.001, post-hoc, Fig. 4A). To more specifically analyze self-administration behavior during the initial stage of training, we examined the average number of infusions during the initial FR1 phase of nicotine self-administration (Fig. 4B). Analysis revealed a main effect of flavor (F3,50 = 13.77, p < 0.0001), with greater infusions in sucrose (p < 0.0001, Cohen’s d =1.76) and saccharin (p < 0.05, Cohen’s d = 1.10), but not menthol rats (p = 0.82, Cohen’s d = 0.23), compared to i.o. water rats (Fig. 4B). Additionally, for combined i.o. flavorant and i.v. nicotine self-administration, both sucrose (p < 0.05) and saccharin (p < 0.05) rats showed greater infusions compared to menthol rats (Fig. 4B).
Figure 2.
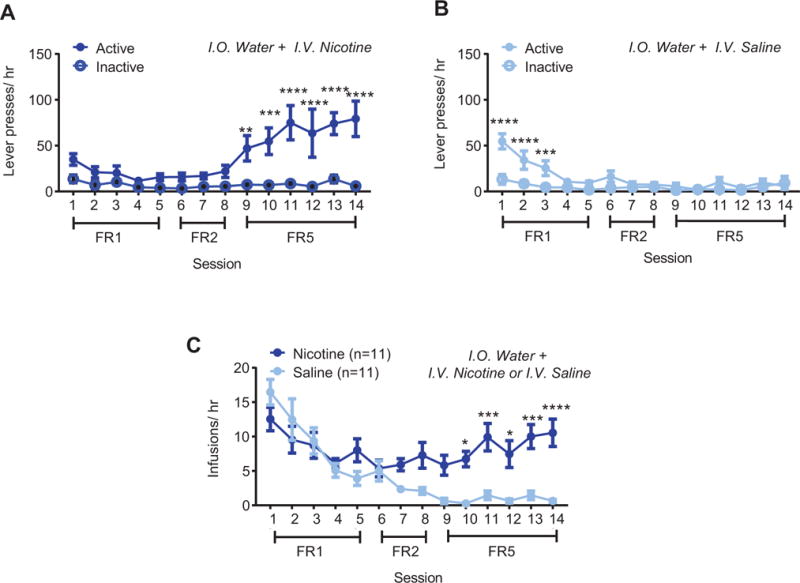
Combined intraoral (i.o.) and intravenous (i.v.) delivery supports self-administration (A) Active and inactive lever presses for i.o. water and i.v. nicotine or (B) i.v. saline across FR1, FR2, and FR5 reinforcement schedules. (C) Total i.v. infusions earned per session in nicotine vs. saline animals. Post-hoc with Bonferroni correction: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 between active and inactive presses in (A) and (B) and between nicotine and saline in (C). Error bars indicate SEM.
Figure 3.
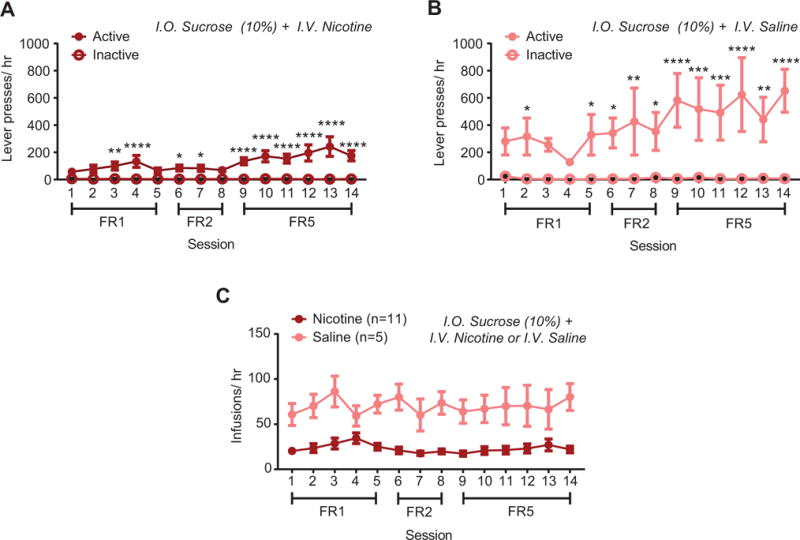
Combined i.o. sucrose and i.v. drug delivery. (A) Active and inactive lever presses for i.o. sucrose and i.v. nicotine self-administration or (B) i.o. sucrose and i.v. saline self-administration. (C) Total i.v. sessions earned per session in i.o. sucrose animals with combined i.v. nicotine or i.v. saline. Post-hoc with Bonferroni correction: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 between active and inactive presses in (A) and (B) and between nicotine and saline in (C). Error bars indicate SEM.
Figure 4.
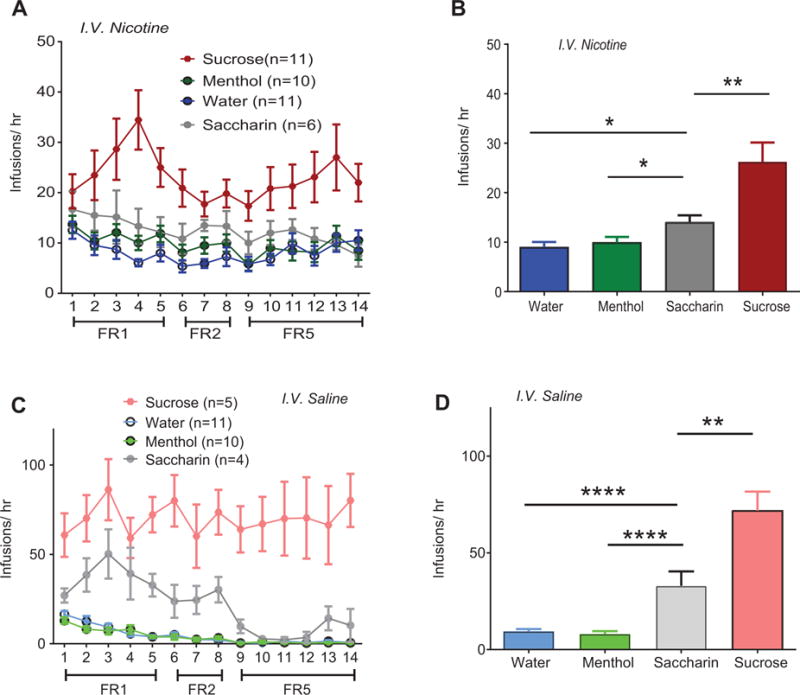
(A) Total infusions in combined i.o. flavorant and i.v. nicotine subjects across all reinforcement schedules. (B) Average nicotine infusions during FR1 sessions. (C) Total infusions earned for combined i.o. flavorant and i.v. saline subjects across all reinforcement schedules. (D) Average saline infusions during FR1 sessions. Post-hoc with Bonferroni correction: * p < 0.05, ** p < 0.01, **** p < 0.0001 between flavors in (B) and (D). Error bars indicate SEM. Rats in the i.o. water and nicotine/saline groups (Figure 2) and from the i.o. sucrose and nicotine/saline groups (Figure 3) are replotted here for comparison.
To examine how rats responded to the flavors in the absence of nicotine, we trained an additional group of animals for i.v. saline and i.o. flavorant self-administration. Analyses revealed a significant interaction between time and flavor (F39,338 = 3.39, p < 0.0001, Fig. 4C) with main effects of time (F13,338 = 9.54, p < 0.0001) and flavor (F3,338 = 40.2, p < 0.0001). Analysis of average infusion during FR1 training also revealed a main effect of flavor (F3,28 = 41.14, p < 0.0001, Fig. 4D), with sucrose (p < 0.0001) and saccharin (p < 0.0001), but not menthol (p = 0.99, post hoc) rats receiving more infusions compared to i.o. water rats. Sucrose self-administration rats also obtained more infusions, compared to saccharin rats (p < 0.01). Together, these data indicate that i.o. sucrose and saccharin, but not menthol, support operant behavior alone. Secondly, the data reveal that combined i.o. sucrose plus i.v. nicotine decreases responding compared to nicotine alone (Fig. 3C). Finally, the data demonstrate that i.o sucrose and saccharin, but not menthol, when combined with nicotine leads to a greater nicotine taking compared to i.o. vehicle (Fig. 4B).
3.3 Motivation to take nicotine is not altered by i.o. flavorant co-administration
To assess whether flavors influence motivation to take nicotine, we tested rats in a progressive ratio (PR) task. We examined PR in the 3 flavorant groups (water, menthol, saccharin) that showed similar rates of nicotine self-administration at the end of FR5 training (Fig. 4C). Nicotine subjects had a higher PR breakpoint than saline subjects, as indicated by a main effect of drug (F1,33 = 19.0, p < 0.001), but there was no effect of flavor (F2,33 = 0.14, p = 0.86) and no interaction (F2,33 = 0.18, p = 0.83, Fig. 5). Thus, the motivation for nicotine taking was unchanged by co-administration with i.o. flavorants.
Figure 5.
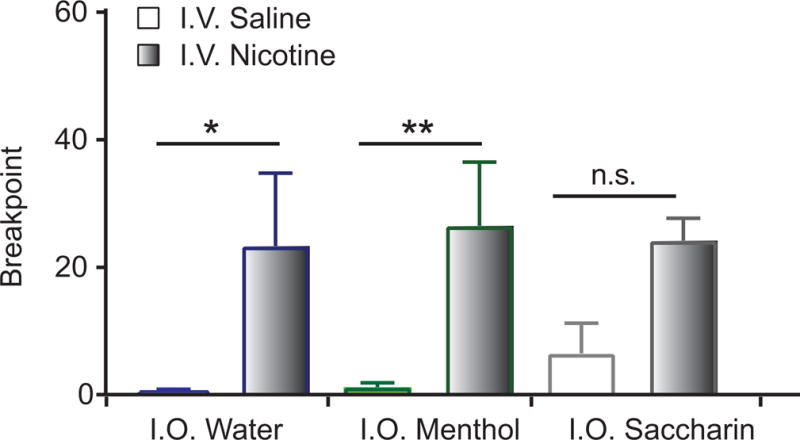
Breakpoint to self-administer i.o. flavorant and i.v. saline or nicotine in the progressive ratio task.
3.4 Nicotine does not confer additional conditioned reinforcing properties to flavors
Nicotine has been shown to enhance the reinforcing value of cues and to permit cues to influence subsequent nicotine-taking (Caggiula et al., 2009; Caggiula et al., 2001, 2002b). To determine whether flavors acquired additional reinforcing value by association with nicotine, we performed a conditioned reinforcement test after nicotine self-administration (Table 1), where rats nosepoked on the conditioned reinforced (CR) noseport to receive only the flavor. Nosepoking in the non-conditioned reinforced (NCR) noseport had no programmed consequence. Since repeated flavorant exposure prior to pairing with nicotine could alter subsequent conditioned reinforcement, this experiment did not include i.o. sucrose and water groups (due to previous sucrose exposure during pre-training and water exposure in the home cage). For saccharin, analysis revealed a significant main effect of noseport (F1,9 = 16.94, p < 0.01, Fig. 6A), but no significant interaction between i.v. drug and nosepoke (F1,9 = 0.03, p = 0.85) nor a main effect of i.v. drug (F1,9 = 1.89, p = 0.20). Thus, nicotine did not alter the conditioned reinforcing value of saccharin. For i.o. menthol animals, analysis revealed no interaction (F1,9 = 0.001, p = 0.99, Fig. 6B) and no main effects of drug treatment (F1,9 = 0.17, p = 0.69) nor nosepoke (F1,9 = 0.26, p = 0.61). Thus, animals did not show conditioned reinforcement for menthol, and nicotine did not alter this lack of conditioned reinforcement.
Figure 6.
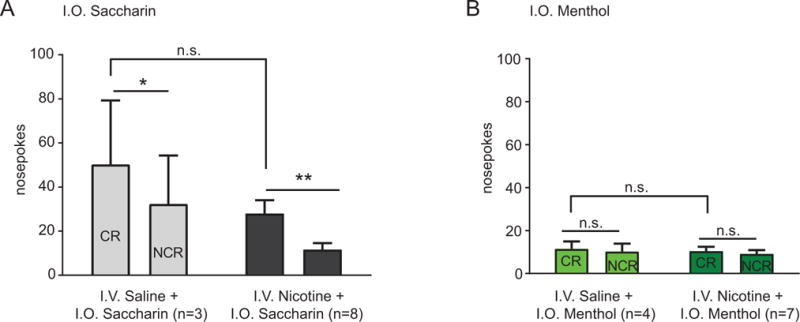
Active (CR) and inactive (NCR) nosepokes for i.o. saccharin (A) or i.o. menthol (B) after five days of FR1 training. Post-hoc test using a Holm-Sidak correction for multiple corrections: * p < 0.05, ** p < 0.01 between CR and NCR.
3.5 Menthol increases oral nicotine consumption
Given the lack of a menthol effect, we sought to determine whether a dissociation exists between oral menthol effects on oral nicotine aversion and i.v. nicotine-mediated reinforcement. We first performed a two-bottle choice experiment to identify nicotine doses that produced oral nicotine aversion (Fig. 7A). Here, nicotine (50 mg/L and 100 mg/L) produced oral aversion compared to water, as revealed by a main effect of drug (F1,59 = 72.361, p < 0.05, 2 × 2 repeated measure ANOVA, Fig. 7A). In a subsequent experiment in a new cohort, rats showed greater oral intake of 0.005% menthol plus nicotine compared to nicotine alone (F1,31 = 171.125, p < 0.001, main effect of drug, with nicotine and menthol factors; planned comparisons: low dose nicotine plus menthol versus low dose nicotine, p<0.0001, Cohen’s d = 2.80; high dose nicotine plus menthol versus high dose nicotine, p<0.0001, Cohen’s d = 3.53, Fig. 7B). To determine whether a sweet flavorant could also alter oral nicotine aversion, outside of potential caloric effects, we also performed a two-bottle choice experiment with saccharin (Fig. 7C). Here, combining 0.32% saccharin with 100 mg/L nicotine also led to increased oral intake compared to nicotine alone (p < 0.05, Cohen’s d= 1.02, Fig. 7C). Thus, oral menthol effects were specific to oral nicotine, while oral saccharin increased both oral and i.v. nicotine intake.
Figure 7.
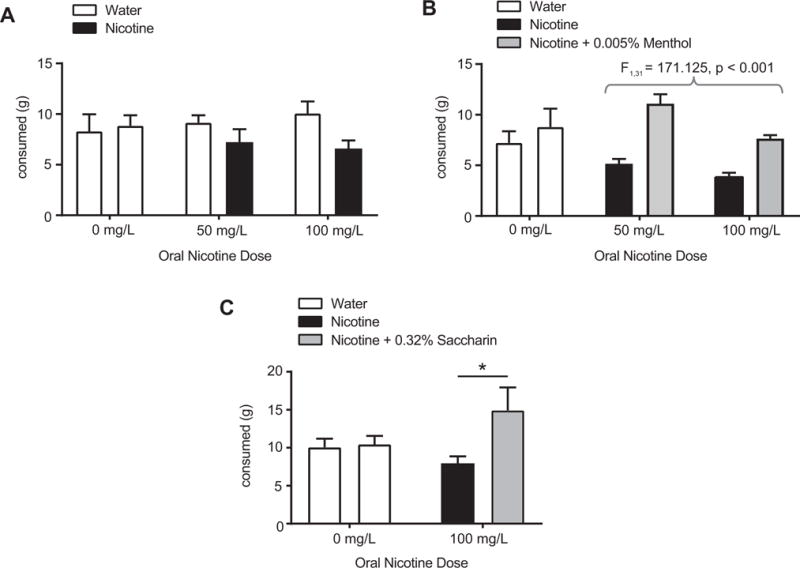
Two-bottle choice experiments. (A) Dose response demonstration of oral nicotine aversion in rats (F1,59 = 72.361, p < 0.05, 2 × 2 repeated measures ANOVA). (B) 0.005% menthol + nicotine increases oral intake compared to nicotine alone (main effect of menthol, F1,31 = 171.125, p < 0.001, 2 × 2 repeated measures ANOVA). (C) 0.32% saccharin + nicotine increases oral intake compared to nicotine alone: * p < 0.05.
4. Discussion
Here, we developed a preclinical model for examining oral flavorant effects on DA signaling and nicotine taking and seeking. We found that reinforcing oral flavorants enhanced phasic DA signaling and, when combined with i.v. nicotine, led to greater self-administration behavior compared to i.o. vehicle, but did not alter motivation for nicotine taking. Given that many tobacco products contain appetitive candy and fruit flavor additives, our findings suggest that appetitive flavorants combined with nicotine delivery could lead to greater self-administration behavior, and thus greater nicotine intake, compared to the absence of these appetitive flavorants. Such findings can inform policy decisions on flavorant regulation and the effects of such regulation on tobacco product use.
Previous flavorant models have used drinking assays, where nicotine and a flavor were dissolved in solution and the amount consumed was used as a metric of nicotine taking (Smith and Roberts, 1995). These models are helpful for examining how flavors mask the aversive effects of oral nicotine, potentially modeling the oral bitterness associated with nicotine taking in humans. As demonstrated in a recent study in mice (Fan et al., 2016), and as replicated here in rats, oral menthol (0.005%) is sufficient to decrease the aversive effects of oral nicotine in a two-bottle choice test (Fan et al., 2016). While this recent study provides important insight regarding nicotine and menthol interactions in the oral cavity, our utilization of i.v. nicotine self-administration provides a model where nicotine has faster rates of entry into the brain that is more comparable to human tobacco product use than orally administered nicotine (reviewed in (Matta et al., 2007)). Although i.v. nicotine delivery does not mimic the route of administration experienced by human tobacco product users, it does provide a useful model for investigating operant self-administration behavior for nicotine delivery with rapid access to the central nervous system (CNS). One potential weakness of our model, as indicated above, is that it does not directly model the combined oral flavorant and oral nicotine effects experienced by human tobacco product users. However, we note recent evidence revealing the ability of systemically, non-orally administered menthol to modulate central nAChR desensitization (Ton et al., 2015), nAChR upregulation (Henderson et al., 2017; Henderson et al., 2016) and nicotine reward (Henderson et al., 2017). Here, we specifically sought to examine the ability oral menthol or oral sweet flavorants to alter CNS-mediated nicotine reinforcement, outside of nicotine’s oral aversive effects. Previously, Wang et al. combined i.v. nicotine delivery with licking for oral flavorants cues. However, their model produced very low levels of nicotine self-administration (approximately 1–2 infusions/hr in the i.o. vehicle group, compared to 10 infusions/hr in our model) and a lack of operant discrimination between active and inactive spouts (Wang et al., 2014). Here, our combined i.o. and i.v. self-administration model provides several advantages including: (a) robust i.v. nicotine self-administration and operant discrimination (10 infusions/hr, 16:1 active/inactive ratio for i.o. water/i.v. nicotine groups), (b) the ability to isolate oral flavorant effects on nicotine taking (outside of the oral nicotine masking effects of oral flavorants), (c) and compatibility with in vivo electrochemical methods.
The ability of saccharin or sucrose co-administration with nicotine to enhance early self-administration behavior raises the possibility that appetitive flavorants in tobacco products may also enhance self-administration behavior in new tobacco product users – resulting in greater nicotine exposure in these individuals. We note that nicotine self-administering rats in this study had prior sucrose pre-training, a translationally relevant design, given that human tobacco product users likely have numerous sweet caloric experiences prior to tobacco product use. Indeed, the observed enhanced self-administration behavior may be due to the reinforcing properties of the appetitive flavorants themselves. We note that in our combined model, rats did not self-administer i.v. nicotine without prior sucrose training (data not shown) – a result which guided our decision to use i.o. sucrose pre-training. However, one caveat of our pre-training experimental design is the potential, initial contribution of negative contrast effects after the transition from i.o. sucrose pre-training to less appetitive i.o. flavorants (saccharin, menthol or water) for the combined i.v. self-administration training. Additionally, it is possible that extinction from sucrose pre-training during the early phase of combined i.o. plus i.v. training could have facilitated the initial responding to i.o. saccharin, water, and menthol. Despite these caveats, i.o. saccharin plus i.v. nicotine was self-administered at a higher rate than i.o. menthol or water, demonstrating the ability of a non-caloric, appetitive flavorant to facilitate self-administration behavior. However, given the potential contributions of extinction and negative contrast effects during the initial day of combined i.o. and i.v. self-administration behavior, future experimental designs could include the use of more traditional operant food pre-training or the use of i.o. water pre-training instead of i.o. sucrose.
In contrast to i.o. saccharin and sucrose, co-administered i.o. menthol had no effect on self-administration behavior, but did reverse oral nicotine aversion in rats, similar to previous findings in mice (Fan et al., 2016). Together, these data suggest that the effects of oral menthol were specific to oral nicotine, and not due to modulation of the nicotine reinforcement mediated by i.v. nicotine. However, our observed lack of i.o. menthol effects on i.v. nicotine self-administration differs from recent studies where oral or i.p. menthol altered i.v. nicotine self-administration in rats (Biswas et al., 2016; Wang et al., 2014). However, Wang et al. used licking for 0.01% menthol drinking in female rats, whereas we used lever pressing for direct, i.o. menthol at 0.005% in male rats. Further, rats in the Wang et al. paradigm showed poor discrimination between active and inactive spouts, and had low rates of self-administration (~5 infusions of 30 μg/kg/nicotine over 3 hours) compared to the higher rates in our study and others’ (~10 to 15 infusions of 30 μg/kg/nicotine over 45 min to 1 h) (Caggiula et al., 2002a; Johnson et al., 2012). Thus, the Wang paradigm produced mild nicotine reinforcement that was enhanced by menthol (although menthol plus nicotine rats still showed poor operant discrimination) (Wang et al., 2014). Interestingly, this menthol-induced enhancement of low-rate nicotine self-administration is similar to another study, where systemic menthol enhanced self-administration only at low nicotine doses (7.55 and 15 μg/kg/infusion) (Biswas et al., 2016). In future work, one could apply our combined i.o. and i.v. method to determine whether i.o. menthol also enhances self-administration of low dose i.v. nicotine. Given our DA findings, one could also determine whether i.p. menthol effects on self-administration are mediated by phasic DA signaling. Indeed, recent studies have revealed the ability of menthol to alter VTA DA firing and excitability and to upregulate VTA α4 and α6-containing nAChRs (Henderson et al., 2016) that are known to play a crucial role in nicotine self-administration (Pons et al., 2008; Tapper et al., 2004).
We also found that flavors do not influence PR, despite flavorant-induced increases in FR1 self-administration behavior. However, we note that the non-caloric flavorant, saccharin, only altered self-administration behavior at lower, and not higher, reinforcement schedules. In previous work, others have also observed no change in motivation despite changes during FR training (Neugebauer et al., 2014) or with no change during FR training (Garcia et al., 2014). It is possible that motivation differences may have emerged if PR was tested immediately after FR1 training, or if we had chosen a longer break point (i.e. 10 or 20 minutes (Brunzell et al., 2010)). With respect to nicotine-associated cues, nicotine has been shown to enhance the reinforcing properties of environmental cues (Caggiula et al., 2009; Caggiula et al., 2001; Chaudhri et al., 2006). However, our conditioned reinforcement results showed that the reinforcing value of the flavor did not change with nicotine pairings, suggesting that appetitive properties of the flavor, and not its cue properties, contributed to the enhanced self-administration behavior. While nicotine is typically thought to confer conditioned reinforcement to smoking-associated stimuli, it is also likely that intrinsically reinforcing smoking stimuli (i.e. appetitive flavors) could increase nicotine’s reinforcing value. Although beyond the scope of our study, which addresses whether flavors can augment self-administration behavior, the aforementioned hypothesis could be examined in the future by pairing an appetitive flavorant with nicotine followed by testing for nicotine reinforcement without the flavor.
5. Conclusion
In conclusion, our findings demonstrate that appetitive oral flavorants when combined with i.v. nicotine delivery lead to greater self-administration behavior, compared to i.v. nicotine self-administration behavior in the absence of an appetitive flavorant. Although i.o. menthol did not increase i.v. nicotine self-administration, we provide evidence that menthol can “mask” the bitter flavor of nicotine and increase nicotine drinking, consistent with previous reports in mice (Fan et al., 2016). Given the recent findings of flavorant impacts on adolescent e-cigarette perception and initiation (Kong et al., 2014; Krishnan-Sarin et al., 2014; Villanti et al., 2013), our findings can inform decisions regarding tobacco product flavorant regulation. With our established preclinical model, future investigations can also determine whether appetitive flavorant modulation of self-administration behavior differs in adolescence versus adulthood and in males versus females, and can also investigate flavorant effects on relapse to nicotine taking.
Supplementary Material
Intraoral appetitive flavorant delivery enhances phasic dopamine signaling in rats
Combined oral flavorant and i.v. drug delivery supports operant self-administration
Appetitive flavorants, when combined with i.v. nicotine, enhance self-administration
Oral menthol did not alter dopamine signaling nor nicotine self-administration
Oral menthol reversed oral nicotine aversion, in a two-bottle choice test
Acknowledgments
We thank Dr. Stephanie O’Malley for helpful feedback and comments on the manuscript.
Funding
This research was supported by Yale TCORS grant P50 DA036151 from the National Institute on Drug Abuse (NIDA) and the Federal Drug Administration (FDA) Center for Tobacco Products (NAA, RJW, EJN, SW and JP), by the National Institute of General Medical Sciences (NIGMS) grant R25 GM104553 (SH), and by NSF Graduate Research Fellowships (RJW and PRS). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH or FDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors do not have any competing financial interests to declare.
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol. 2010;104:922–931. doi: 10.1152/jn.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24:115–120. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ashoor A, Nordman JC, Veltri D, Yang KH, Al Kury L, Shuba Y, Mahgoub M, Howarth FC, Sadek B, Shehu A, Kabbani N, Oz M. Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PLoS One. 2013;8:e67674. doi: 10.1371/journal.pone.0067674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 2016;166:263–267. doi: 10.1016/j.drugalcdep.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas L, Harrison E, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T, Lage J, Liu X. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl) 2016;233:3417–3427. doi: 10.1007/s00213-016-4391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern MA. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol. 2013;16:957–966. doi: 10.1017/S1461145712001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002a;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002b;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB. Smoking-cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med. 2011;41:357–365. doi: 10.1016/j.amepre.2011.06.039. [DOI] [PubMed] [Google Scholar]
- Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR, Picciotto MR, Jordt SE. Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tob Control. 2016;25:ii50–ii54. doi: 10.1136/tobaccocontrol-2016-053209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63:360–367. doi: 10.1111/j.1742-1241.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- Garcia KL, Le AD, Tyndale RF. Effect of food training and training dose on nicotine self-administration in rats. Behav Brain Res. 2014;274:10–18. doi: 10.1016/j.bbr.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses. 2012;37:463–469. doi: 10.1093/chemse/bjr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA. Menthol Enhances Nicotine Reward-Related Behavior by Potentiating Nicotine-Induced Changes in nAChR Function, nAChR Upregulation, and DA Neuron Excitability. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, Nichols WA, Moaddel R, Xiao C, Lester HA. Menthol Alone Upregulates Midbrain nAChRs, Alters nAChR Subtype Stoichiometry, Alters Dopamine Neuron Firing Frequency, and Prevents Nicotine Reward. J Neurosci. 2016;36:2957–2974. doi: 10.1523/JNEUROSCI.4194-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology (Berl) 2012;222:269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Carelli RM, Wightman RM. Rank estimation and the multivariate analysis of in vivo fast-scan cyclic voltammetric data. Anal Chem. 2010;82:5541–5551. doi: 10.1021/ac100413t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. Multivariate concentration determination using principal component regression with residual analysis. Trends Analyt Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for Electronic Cigarette Experimentation and Discontinuation Among Adolescents and Young Adults. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslake JM, Wayne GF, Connolly GN. The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res. 2008;10:705–715. doi: 10.1080/14622200801979134. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette Use Among High School and Middle School Adolescents in Connecticut. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YO, Glantz SA. Menthol: putting the pieces together. Tob Control. 2011;20(Suppl 2):ii1–7. doi: 10.1136/tc.2011.043604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res. 2012;14:299–305. doi: 10.1093/ntr/ntr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Cortright JJ, Sampedro GR, Vezina P. Exposure to nicotine enhances its subsequent self-administration: contribution of nicotine-associated contextual stimuli. Behav Brain Res. 2014;260:155–161. doi: 10.1016/j.bbr.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nature Neuroscience. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Roberts DC. Oral self-administration of sweetened nicotine solutions by rats. Psychopharmacology (Berl) 1995;120:341–346. doi: 10.1007/BF02311182. [DOI] [PubMed] [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15:1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki W, Wickham RJ, Behrens S, Wang J, Zwerling B, Mason GF, Addy NA. Differential role of ventral tegmental area acetylcholine and N-methyl-d-aspartate receptors in cocaine-seeking. Neuropharmacology. 2013;75C:9–18. doi: 10.1016/j.neuropharm.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiol Biomarkers Prev. 2013;22:382–389. doi: 10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Ton HT, Smart AE, Aguilar BL, Olson TT, Kellar KJ, Ahern GP. Menthol Enhances the Desensitization of Human alpha3beta4 Nicotinic Acetylcholine Receptors. Mol Pharmacol. 2015;88:256–264. doi: 10.1124/mol.115.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Richardson A, Vallone DM, Rath JM. Flavored tobacco product use among U.S. young adults. Am J Prev Med. 2013;44:388–391. doi: 10.1016/j.amepre.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham RJ. How Menthol Alters Tobacco-Smoking Behavior: A Biological Perspective. Yale J Biol Med. 2015;88:279–287. [PMC free article] [PubMed] [Google Scholar]
- Wickham RJ, Park J, Nunes EJ, Addy NA. Examination of Rapid Dopamine Dynamics with Fast Scan Cyclic Voltammetry During Intra-oral Tastant Administration in Awake Rats. J Vis Exp. 2015 doi: 10.3791/52468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. Faseb Journal. 2011;25:4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


