Abstract
GM1 has generally been considered as the major receptor that binds to cholera toxin subunit B (CTB) due to its low dissociation constant. However, using a unique nanocube sensor technology, we have shown that CTB can also bind to other glycolipid receptors, fucosyl-GM1 and GD1b. Additionally, we have demonstrated that GM2 can contribute to CTB binding if present in a glycolipid mixture with a strongly binding receptor (GM1/fucosyl-GM1/GD1b). This hetero-multivalent binding result was unintuitive because the interaction between CTB and pure GM2 is negligible. We hypothesized that the reduced dimensionality of CTB-GM2 binding events is a major cause of the observed CTB binding enhancement. Once CTB has attached to a strong receptor, subsequent binding events are confined to a 2D membrane surface. Therefore, even a weak GM2 receptor could now participate in second or higher binding events because its surface reaction rate can be up to 104 times higher than the bulk reaction rate. To test this hypothesis, we altered the surface reaction rate by modulating the fluidity and heterogeneity of the model membrane. Decreasing membrane fluidity reduced the binding cooperativity between GM2 and a strong receptor. Our findings indicated a new protein-receptor binding assay, that can mimic complex cell membrane environment more accurately, is required to explore the inherent hetero-multivalency of the cell membrane. We have thus developed a new membrane perturbation protocol to efficiently screen receptor candidates involved in hetero-multivalent protein binding.
Keywords: Cholera Toxin, multivalent binding, supported lipid bilayer, glycolipid, nanocubes
Graphical abstract
Introduction
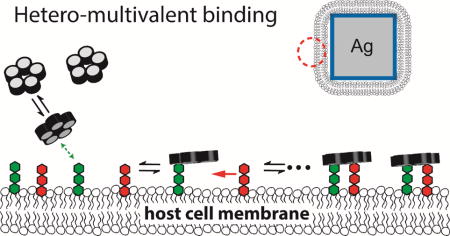
Many proteins recognize glycolipid receptors in cell membranes via multivalent binding mechanisms.[1] Such dynamic binding, driven by a series of binding domains, brings a protein to a membrane surface and initiates biological processes. Interactions between a single glycolipid receptor and a protein binding subunit are often weak, and therefore multivalency enhances the protein binding avidity and specificity to cell surfaces. Cholera toxin (CTx), the virulence factor of Vibrio cholerae, is a type of multivalent glycolipid binding protein. This AB5 toxin consists of a single A subunit associated with five identical B subunits. The B pentamer binds to cell membranes and delivers the catalytic A subunit into the cytoplasm. A potential stepwise reaction of pentavalent cholera toxin subunit B (CTB) binding to the cell membrane [2, 3] is shown in Fig. 1. (1) CTB moves from the solution phase to the membrane surface, followed by one of its binding sites attaching to a glycolipid receptor; (2) Free glycolipids diffuse two dimensionally, encounter the bound CTB, and then enable subsequent binding. The synergistic effort amongst various binding pockets, membrane receptors, and membrane dynamics dramatically influences the overall association.[4]
Fig. 1.
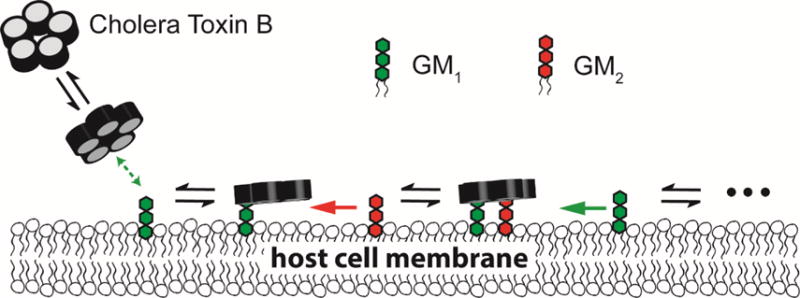
A schematic of the proposed CTB binding mechanism. CTB first diffuses from the solution phase to a membrane surface. One of its binding subunit finds a strongly binding receptor and then forms a relatively stable membrane bound state. Free glycolipid receptors diffuse two dimensionally, encounter the bound CTB, and then enable subsequent binding. The reaction rate on the 2D membrane surface is significantly higher than the rate in 3D bulk solutions. Thus, a weakly binding receptor, such as GM2, can participate in subsequent binding, leading to an enhanced binding capacity.
We recently developed a unique nanocube sensor by integrating supported lipid bilayer and plasmonic sensing technologies.[5] This new tool has enabled label-free detection of protein binding to model membrane surfaces by using a standard laboratory spectrophotometer to observe the extinction spectrum shift of the quadrupolar localized surface plasmon resonance (LSPR) peak.[6] The nanocube sensor was used to investigate the multivalent binding principle of CTB interacting with various glycolipids.[6] We observed that the amount of CTB binding onto the surface containing fucosyl-GM1 was higher than GM1 although the dissociation constant of GM1 was an order of magnitude lower than that of fucosyl-GM1. This unintuitive result might be attributed to a reduced binding cooperativity between fucosyl-GM1 receptors leading to an increased binding capacity.[6] Our previous findings indicated that dissociation constants cannot exclusively represent multivalent CTB bindings and that binding cooperativity also plays an essential role in determining CTB-cell membrane recognition.
Multivalent binding can be either homo-multivalent (i.e. a protein binds to multiple copies of the same type of receptor) or hetero-multivalent (i.e. a protein simultaneously binds to two or more different types of receptors).[7] Due to the complexity of hetero-multivalency, most studies have focused on homo-multivalency. However, homo-multivalent models neglect the inherent heterogeneity of cell membranes. We recently reported that adding a weak glycolipid receptor (GM2) to a model membrane containing fucosyl-GM1 significantly increased the total amount of bound CTB.[6] This was unexpected, as GM2 receptors have negligible binding avidity in bilayers with GM2 as the only glycolipid receptor. A few other studies have also reported that lectin binding to glycan mixtures is stronger than the binding to a single glycan.[8–11] However, the mechanism of such hetero-multivalency is not clear.
The goal of this study was to gain insight into the mechanism of hetero-multivalent CTB binding. We first investigated the binding cooperativity of CTB to various glycolipid mixtures. Positive cooperativity was observed when GM2 was mixed with any of the other three strongly binding receptors (GM1, fucosyl-GM1, and GD1b). We hypothesized that the increase of CTB binding is caused by a reaction rate enhancement mechanism, “reduction of dimensionality” (Fig. 1). Once CTB has attached to a strong receptor, subsequent binding events are confined on the 2D membrane surface. Therefore, even a weak GM2 receptor could now participate in second or higher binding events because its surface reaction rate is around 104 times higher than the rate in bulk solution. To test this hypothesis, we modulated the fluidity and heterogeneity of the model membrane by adding cholesterol or altering fatty acid composition of phospholipids and observed significant changes in the heterogeneous binding cooperativity. This complies with the surface reaction’s strong dependence on the membrane environment. Our results indicated that the traditional protein binding assay, which detects protein interactions with a specific receptor one by one (e.g. microarray technology), is not appropriate to explore multivalent binding interactions. To discover all possible receptors which could participate in a binding process, we designed a new membrane perturbation protocol that can efficiently screen possible glycolipid receptors involved in multivalent protein binding.
Materials and methods
Materials
Monosialoganglioside GM1 (NH4+salt) (Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glc-Ceramide, GM1), monosialoganglioside GM2 (NH4+salt) (GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glc-Ceramide, GM2), monosialoganglioside GM3 (NH4+4salt) (Neu5Acα2-3Galβ1-4Glc-Ceramide,GM3), fucosylated monosialoganglioside GM1 (NH4+4salt) (Fucα1-2Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glc-Ceramide, fucosyl-GM1) and disialoganglioside GD1b(NH4+salt) (Galβ1-3GalNAcβ1-4(Neu5Acα2-8)(Neu5Acα2-3)Galβ1-4Glc-Ceramide,GD1b) were purchased from Matreya LLC (State College, PA). 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phospho-L-serine - sodium salt (DOPS), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-L-serine – sodium salt (DMPS) were obtained from Avanti Polar Lipids (Alabaster, AL). Cholera Toxin B subunit (CTB, lyophilized powder) from Vibrio cholerae, cholesterol and casein from bovine milk were purchased from Sigma-Aldrich. GM1 oligosaccharide (GM1os) (Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glc) sugar was purchased from Elicityl (Crolles, France). All the CTB binding experiments were performed in Tris-buffered saline-TBS (Sigma Aldrich).
Methods
Synthesis & calibration of the nanocube sensor
Silica coated silver nanocubes were prepared as reported in our previous publication.[6] The silver nanocube synthesis was based on the polyol method. The silica shell synthesis over nanocubes was performed in a scaled-up synthesis batch using 2-propanol as solvent. The quality of the nanocube sensor, including silica shell thickness, nanocube size and uniformity, was confirmed by transmission electron microscopy (FEI Technai G2 F20 FE-TEM). (Fig. S1) The refractive index sensitivity of silica coated silver nanocubes was reported as peak shift (reported in nm) per refractive index unit (RIU). (Fig. S2) Since the change in refractive index is directly proportional to the amount of bound proteins, LSPR peak shift allows an estimation of the amount of protein bound.[5]
Supported lipid bilayer preparation
Lipids stored in organic solvents (chloroform for DOPC, DOPS, DMPC, and DMPS or chloroform/methanol/water mixture for glycolipids) were mixed to obtain the desired final composition. They were then dried using a rotary evaporator (Heidolph Hei-VAP Value®), followed by rehydration with Milli-Q® water. Small unilamellar vesicles (SUVs) were prepared by the standard extrusion protocol described in our prior publication.[6] A previously established modified vesicle fusion technique[6] was used to form supported lipid bilayers. The lipid bilayer coated nanocubes were incubated with 0.5 mg/ml casein in 1X TBS solution for 1 hour to prevent nonspecific binding of CTB.
CTB binding measurement
The lipid bilayer coated nanocubes were incubated with the required CTB concentration for 1.5 hours. Blank solutions were also prepared for each CTB concentration by mixing buffer and CTB corresponding to that composition. The extinction spectra of the solutions were measured in a 384 well plate with a UV/Vis microplate spectrophotometer equipped with a CCD (FLUOstar Omega®, BMG-Labtech). All measurements were carried out at room temperature, except the membrane fluidity experiment involving DMPC. The location of the quadrupolar LSPR peak was calculated by fitting the measured absorption spectra to a seventh order polynomial. Each protein binding measurement was repeated in eleven wells. Each data point is represented as the mean ± standard deviation (S.D.) where n = 11. The experimental conditions for each binding measurement are described below.
Combinatorial glycolipid array
To acquire binding curves for pure glycolipid systems (1 mol% glycolipid along with 89 mol% DOPC and 10 mol% DOPS), the CTB concentration was varied from 0 to 1726 nM. For the binary mixture of glycolipids (1 mol% of each glycolipid along with 88 mol% DOPC and 10 mol% DOPS), the CTB concentrations used were 706 nM and 1726 nM.
GM1os pre-bound CTB binding experiment
345 nM CTB was incubated at various sugar (GM1os) concentrations (0 ~ 38.1 μM) prior to the binding measurement. The resulting GM1os-CTB complex was incubated with the bilayer containing 2 mol% glycolipid along with 88 mol% DOPC and 10 mol% DOPS.
Membrane Perturbation protocol
The reference bilayer comprised of 0.25 mol% of each glycolipid (GM1, GM2, GM3, fucosyl-GM1 and GD1b), 10 mol% DOPS and 88.75 mol% of DOPC. For the perturbed membranes, one of the glycolipids was increased to 2 mol% while other glycolipids were maintained at 0.25 mol% along with 10 mol% DOPS and 87 mol% DOPC. Each experiment was treated with 0.5 mg/ml Casein in 1× TBS buffer to block non-specific binding and then incubated with 1726 nM CTB for 2 hours.
Results
CTB binding to glycolipid pairs
Our previous study demonstrated that mixing GM2, a weak binding receptor, with fucosyl-GM1 could enhance the overall CTB binding.[6] In order to understand the mechanism of the hetero-multivalency, we constructed a combinatorial array of glycolipids to evaluate cooperativity of CTB binding. The array was composed of glycolipids like GM1, GM2, GM3, fucosyl-GM1, and GD1b (Fig. 2a). We first examined CTB binding to model membranes containing 1 mol% of a glycolipid (Fig. 2b). The shift in the location of the LSPR peak with respect to the control is directly proportional to the amount of CTB bound. CTB exhibited significant binding to the bilayers containing GM1, fucosyl-GM1, or GD1b. (Fig. 2b) GM2 and GM3 showed negligible binding with CTB even at the highest CTB concentrations (1726 nM); this result is consistent with prior studies.[2, 12, 13] Thus, we categorized GM1/fucosyl-GM1/GD1b as strongly binding receptors and GM2/GM3 as weakly binding receptors.
Fig. 2.
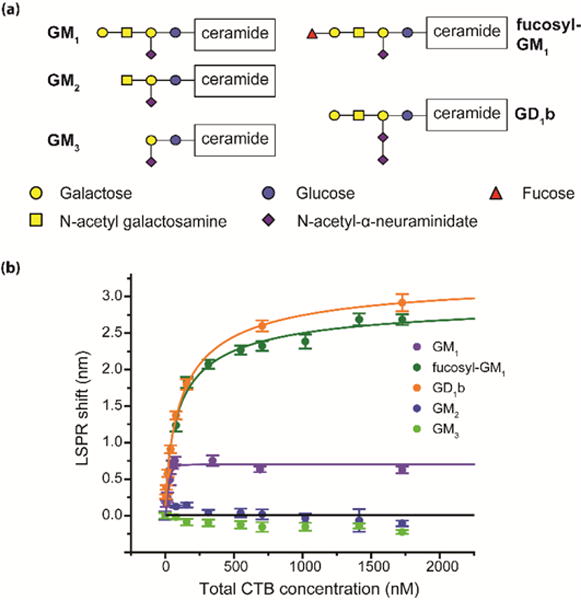
Homo-multivalent CTB binding. (a) Structures of glycolipids used in the study. (b) Equilibrium binding of CTB to pure glycolipids. The glycolipid composition in each case was 1 mol%. Data points are reported as mean ± S.D (n = 11).
The combinatorial array was prepared by mixing two glycolipids in a 1:1 ratio (1 mol% of each glycolipid). The amount of CTB bound to the glycolipid mixtures was measured at two different CTB concentrations (706 nM and 1726 nM). From the CTB-glycolipid binding curves (Fig. 2b), we can see that CTB binding to the model membrane is approximately saturated at 1726 nM. Thus, we used this value to estimate the maximum binding capacity of the model membrane. We also measured the CTB binding at a lower CTB concentration (706 nM) to observe the influence of CTB concentration on binding cooperativity.
To quantify the binding cooperativity of hetero-multivalency, we have defined heterogeneous binding cooperativity (θ) as:
| Equation 1 |
If there is no cooperativity between two glycolipids, θ should equal 1. When θ is larger or smaller than 1, it represents positive or negative cooperativity, respectively. The calculated heterogeneous cooperativity was reported in Table 1. We observed positive cooperativity when GM2 was mixed with any of the strongly binding receptors (GM1, fucosyl-GM1, and GD1b) at both CTB concentrations. Since negligible CTB binding was observed with the model membrane surface containing GM2 as the only glycolipid receptor, the strongly binding receptors seemed to have activated GM2 receptors which led to a higher CTB binding. However, no significant cooperativity was observed when GM3 was mixed with strongly binding receptors. In addition, cooperative action between strong receptors was negligible.
Table 1.
Calculated heterogeneous binding cooperativity between two glycolipids. Column and row headings represent the mixture of two glycolipids. Each cell contains two values that represent the calculated cooperativity at the two CTB concentrations, 706 nM (top)/1726 nM (bottom). Cooperativity values are reported as mean ± S.D (n = 11). The raw data of CTB binding was reported in Fig. S3–S4.
| GM1 | fucosyl-GM1 | GD1b | GM2 | GM3 | |
|---|---|---|---|---|---|
| 1.08 + 0.03 1.12 + 0.03 |
0.92 + 0.02 1.05 + 0.04 |
1.46 + 0.17 1.99 + 0.28 |
1.16 + 0.26 0.92 + 0.20 |
GM1 | |
| 0.94 + 0.02 1.10 + 0.03 |
1.57 + 0.07 1.54 + 0.09 |
1.19 + 0.06 1.11 + 0.04 |
fucosyl-GM1 | ||
| 2.06 + 0.08 1.96 + 0.10 |
1.05 + 0.05 0.98 + 0.05 |
GD1b | |||
| 1.00 + 0.77 1.00 + 0.12 |
GM2 | ||||
| GM3 |
Possible causes of heterogeneous cooperativity
To the best of our knowledge, positive cooperativity between GM2 and other glycolipid receptors has not yet been reported. Several possible reasons may cause this heterogeneous cooperativity, including induced glycolipid cluster formation, allosteric regulation, and reduction of dimensionality. Each hypothesis has been considered and discussed in the following.
Cremer and his coworkers have demonstrated that increasing GM1 density in a model membrane induces the formation of GM1 clusters, leading to weaker CTB binding.[14] If mixing GM2 had induced the disturbance of glycolipid clusters leading to increased CTB binding, the addition of other glycolipids should have altered the clustering of glycolipid receptors and caused some change in binding cooperativity. However, we observed cooperative interactions only between GM2 and other strongly binding glycolipids. Furthermore, the glycolipid concentration was kept relatively low (less than 2 mol%) to minimize any heterogeneous distribution of glycolipids on the membrane surface. Therefore, we believe that it is less likely for induced heterogeneity to be the major cause of positive cooperativity.
Allosteric regulation is another possible cause of positive cooperativity. The bound glycolipids (GM1/fucosyl-GM1/GD1b) could have enhanced the binding energy between GM2 and its adjacent binding sites, enabling GM2 to participate in the CTB binding process and leading to a higher binding capacity (Fig. 3a). To test this hypothesis, we modified the saturation binding assay developed by Leach et al. for detection of allosteric interactions.[15] Klassen and his coworkers have reported that at the equilibrium state CTB forms a binding complex with GM1 oligosaccharide (GM1os), an allosteric modulator that contains the same glycan structure as the GM1 glycolipid without its ceramide tail.[16] We first incubated CTB with various concentrations of GM1os oligosaccharide Then, we measured the binding of GM1os-CTB complex to a model membrane containing 2 mol% glycolipid (GM2 or fucosyl-GM1) at a fixed CTB concentration (345 nM) (Fig. 3b). If the bound GM1os had altered the energetics of the adjacent CTB binding subunit, the allosteric effect should have initiated the attachment of GM1os-CTB complex to the membrane containing GM2. Instead, negligible CTB binding to the lipid bilayer having GM2 was still observed. For the lipid bilayer containing 2 mol% of fucosyl-GM1, the amount of bound GM1os-CTB complex decreased with increased GM1os concentration (Fig. 3b). This is due to competitive binding between GM1os and fucosyl-GM1 receptors. In addition, three different research groups independently evaluated the allosteric effect of GM1os-CTB binding and found that the affinity constants increased by only twofold when the neighboring binding sites were occupied.[13, 16, 17] Turnbull et al. have estimated the dissociation constant for CTB binding with GM2 to be 2 mM.[13] Thus, even twofold enhancement of affinity constant (leading to ~1mM dissociation constant) is not sufficient to promote CTB binding to GM2 at the physiological concentrations. Although we cannot completely exclude the allosteric regulation between GM2 and other strong receptors, it is probably not the major cause for the observed positive cooperativity.
Fig. 3.
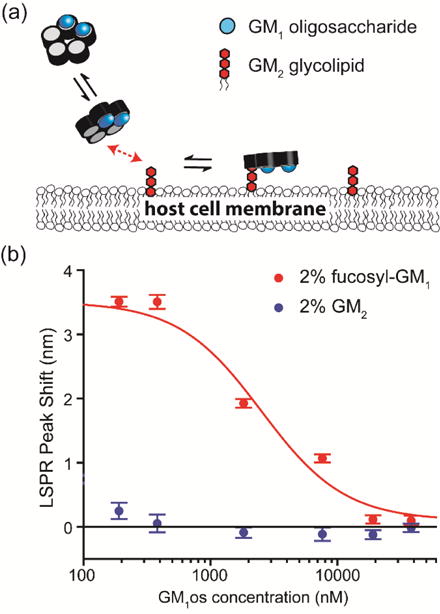
Evaluation of allosteric effect. (a) A schematic of the allosteric regulation hypothesis. CTB was incubated with GM1os to form a GM1os-CTB complex. Then, this GM1os-CTB complex was bound to a model membrane containing GM2. If GM1os modulated the energetics of the adjacent CTB binding pocket, the attachment of GM1os-CTB complex to the membrane containing GM2 should be detectable. (b) Binding of CTB-GM1os complex to membrane surfaces containing 2 mol% fucosyl-GM1 and 2 mol% GM2. Binding of CTB-GM1os complex to the GM2 surface was still negligible; thus, allosteric regulation may not be a major cause of the enhanced CTB binding.
Another possible cause for positive heterogeneous cooperativity is the influence of reduced dimensionality. Searching for reaction partners is much more efficient on a two-dimensional membrane surface than in 3D space. In 1968, Adam and Delbrück first proposed that organisms can shorten the diffusion time of dilute reactants by adsorption to cell membrane surfaces in order to enhance the reaction rates of the biological processes.[18] Many researchers have validated this concept and provided a comprehensive theory to describe this mechanism.[19–24] Recently, Sengers et al. also reported that reduced dimensionality can improve the binding efficiency of a bivalent monoclonal antibody interaction with membrane bound targets by about 104-fold.[25] Thus, it is possible that reduction of dimensionality enhanced the CTB binding to GM2.
The influence of reduced dimensionality
We hypothesized that CTB first moves from the solution phase to the membrane surface and attaches to one of the strongly binding receptors (GM1, fucosyl-GM1, and GD1b). Jobling et al. have shown that a single active binding site on CTB pentamer is sufficient for cell binding and intoxication;[26] therefore, we expected CTB could form a relatively stable membrane-bound state with a single strongly binding receptor (Fig. 1). Once CTB is anchored to the surface, the effective concentration of GM2 on 2-D membrane surface dramatically increases for subsequent bindings. Although the weak binding between GM2 and CTB implies a short lifetime of the CTB-GM2 complex, the enhanced effective concentration allows GM2 to continuously participate in the process to bind to CTB leading to an increase in binding capacity. This hypothesis requires the presentation of a strongly binding receptor in order to anchor CTB to the membrane surface.
In order to verify this hypothesis, we first evaluated the 2D and 3D reaction rates using the established theoretical models.[22–24] The reaction rate, ϕ, can be written as[23]:
| Equation 2 |
Where CA and CB are the number densities of the two reactants, and kobs is the empirical rate constant. In diffusion controlled reactions, kobs is a function of diffusion coefficients (D3D or 2D), the radius of diffusion spaces (b), and the encounter radius of the target receptor (a). Based on our experimental conditions, the bulk concentration of CTB (species A) and glycolipid (species B) were estimated as: CA = 3 × 10−7 mol/L, CB = 3 × 10−7 mol/L. 3D diffusivities of CTB and glycolipid containing liposome were estimated using the Stokes-Einstein equation as DA,3D = 9.77 × 10−11 m2/s and DB,3D = 4.88 × 10−12 m2/s. The measured diffusivity of bound CTB was acquired from literature (DA,2D = 2.5 × 10−13 m2/s).[27, 28] The DOPC lipid diffusivity was DB,2D = 8.25 × 10−12 m2/s.[29] Using different fluorescent labeling approaches, previous researchers have also reported the diffusivity of GM1 in DOPC bilayer to be around 3.6~8 × 10−12 m2/s. [30, 31]
We estimated the 3D reaction rate using Smoluchowski’s relation which gives a steady-state rate constant for fast reactions,[23]
| Equation 3 |
Prior studies derived the approximate solution of kobs for 2D membrane reactions using Smoluchowski theory, mean-passage time theory, and statistical thermodynamic theory (the models are summarized in Supplementary Note).[22–24] Based on our experimental conditions, we found that the 2D reaction rate can be up to 104 higher than 3D reactions. The increased reaction rate implies that effective concentration of reactants on the membrane surface is enhanced by about 104-fold. This calculated enhancement factor has the same order of magnitude of the value in antibody system reported by Sengers et al.[25] In such a case, the reduction of dimensionality could raise the effective GM2 concentration close to or higher than the dissociation constant of CTB-GM2 (2mM). Thus, it is possible that this significant enhancement of reaction rate between bound CTB and GM2 led to higher CTB binding.
To further verify this hypothesis, we altered the diffusivity of glycolipids by replacing DOPC with DMPC that has a gel phase transition temperature near room temperature (24 °C). We conducted the measurements of CTB binding to DMPC model membranes with 1 mol% GM1 and GM1:GM2 mixture (1 mol%:1 mol%) at 15 °C and 45 °C. In the DOPC bilayer, which has transition temperature at −20 °C,[32] the cooperativity between GM1 and GM2 at 15 °C was quite similar to what we obtained at room temperature, which implies that such a temperature change does not alter CTB binding much (Fig 4). However, the diffusion of glycolipids in DMPC gel phase is two orders of magnitude lower when compared to the fluidic DMPC membrane.[33, 34] Goins et al. reported GM1 diffusivity to be approximately 1–2 × 10−13 m2/s in DMPC below 20 °C.[35] Under this condition, the 2D reaction rate is only 400–500 times higher than the 3D reaction rate in DMPC gel phase. Thus, we expected that the rate enhancement via reduced dimensionality would be minimized in the DMPC system at 15 °C. Fig. 4 shows that mixing GM2 with GM1 in a DMPC bilayer did not enhance the overall CTB binding at 15 °C; in contrast, binding enhancement was observed in fluidic DMPC bilayer at 45 °C. This result further corroborates our hypotheses that reduction in dimensionality is influencing the binding of CTB with heterogeneous mixtures of glycolipids.
Fig. 4.
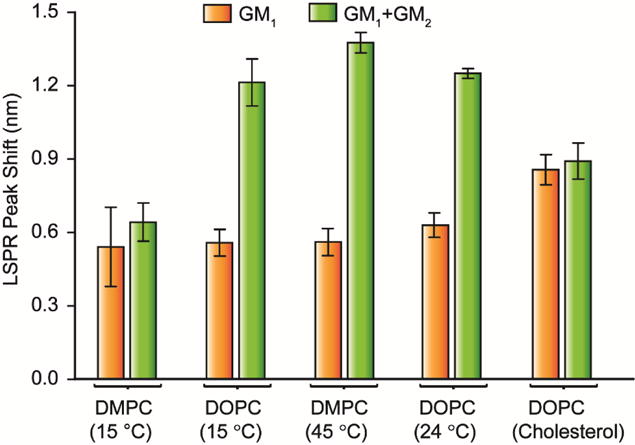
CTB binding to single glycolipid (orange) or paired glycolipids (green) in different membrane environments. (DMPC/DMPS (15 °C), DOPC/DOPS (15 °C), DMPC/DMPS (45 °C), DOPC/DOPS (room temperature) or DOPC/DOPS/cholesterol (room temperature)) The heterogeneous binding cooperativity between GM1 and GM2 depends on the fluidity and heterogeneity of membranes. Data points are reported as mean ± S.D (n = 11).
In addition, 10 mol% of cholesterol was added to DOPC bilayer in order to alter the fluidity and the heterogeneity of model membranes. Similar to the DMPC system, changing the membrane environment altered the heterogeneous binding cooperativity (Fig. 4). This result is not surprising because many studies have shown the compositions of fatty acids and cholesterol in host cells can influence the toxin potency.[36, 37] Previous studies have also reported that surface diffusion and heterogeneity can influence the homo-multivalent CTB-GM1 binding.[38] Our result indicated that the membrane environment is also essential in hetero-multivalent binding process.
The other question is why mixing GM3 with the other receptor did not enhance CTB binding. The only difference in the structure of GM2 and GM3 is that GM2 contains an additional N-acetyl galactosamine (GalNAc) in its glycan portion. The crystal structure of CTB-GM1 complex indicates that the sugar groups of galactose (Gal), GalNAc, and sialic acid (Neu5Ac) in GM1 were buried in the CTB binding subunit and contribute to 39%, 17%, and 43% of the contact surface area respectively.[39] CTB binding to GM3 that has only one Neu5Ac epitope should be weaker than GM2 receptor. In fact, Turnbull et al. estimated the dissociation constant for α-methyl sialoside, which contains only Neu5Ac epitope, to be 210 mM.[13] Even though the mechanism of reduced dimensionality could increase the reaction rate around 104-fold, the effective concentration of GM3 on membrane surfaces is still far below the dissociation constant between CTB and sialic acid residual. Therefore, it wasn’t surprising that no cooperativity was found between GM3 and the other binding receptors.
A new perturbation protocol for screening glycolipid receptors in multivalent interactions
One of the difficulties in observing hetero-multivalency is that some receptors, such as GM2, only exhibit significant binding when they form a partnership with other receptors. Traditional ligand-receptor binding assays (e.g. microarray technology) cannot reflect such hetero-multivalency because they screen only one specific receptor at a time. Thus, the contribution of GM2 was often ignored since CTB binding to pure GM2 was only detected at the CTB concentration far beyond physiologically relevant conditions. To address this issue, previous studies have developed combinatorial arrays that mix two different receptors in 1:1 ratio.[9] However, this labor-intensive method cannot observe hetero-multivalent binding involving more than two receptors.
In order to efficiently discover receptor candidates for multivalent binding proteins, we designed a new membrane perturbation protocol. This protocol first involves constructing a membrane that contains all receptor candidates with known compositions as a reference. The reference membrane is then perturbed by increasing the density of a desired glycolipid receptor. If a specific receptor can either directly bind to the target protein or indirectly form a binding complex with the assistance of other glycolipids; the perturbation will alter the overall protein binding irrespective of the mechanism.
As a proof-of-concept, we constructed a reference membrane consisting of GM1, GM2, GM3, fucosyl-GM1, and GD1b (0.25 mol% of each glycolipid). We then perturbed the reference membrane by increasing one of the glycolipid receptor to 2 mol%. The CTB binding to the reference membrane and each perturbed membrane is shown in Fig. 5. As expected, CTB binding was significantly enhanced when the densities of GM1, fucosyl-GM1, and GD1b were increased. The positive binding cooperativity between GM2 and the other glycolipids present in the reference membrane also enhanced the overall CTB binding. In addition, increasing GM3 density did not enhance CTB binding. Thus, we could exclude GM3 as a CTB receptor candidate without conducting the entire combinatorial array measurement. In order to identify receptors of multivalent protein from a large library of molecules, this perturbation method can be more efficient than combinatorial glycolipid arrays.
Fig. 5.
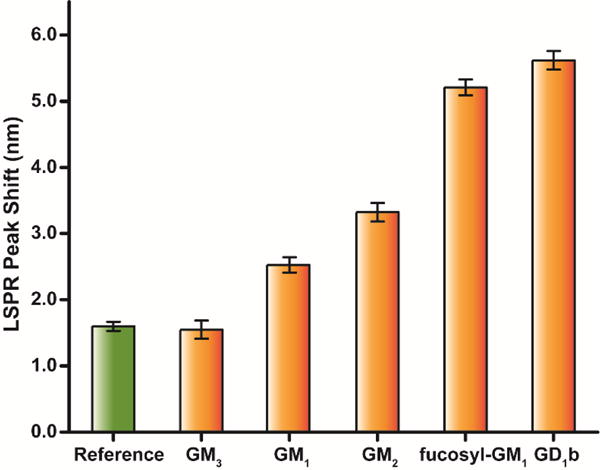
The demonstration of membrane perturbation protocol. 1726 nM CTB was bound to the reference and perturbed membranes that preserved all receptor candidates. The reference membrane contained 88.75 mol% DOPC, 10 mol% DOPS, 0.25 mol% of each GM1, GM2, GM3, GD1b and fucosyl-GM1. The reference membrane was perturbed by increasing the density of a specific glycolipid to 2 mol%. Data points are reported as mean ± S.D (n = 11).
Discussion
In this study, significant enhancement of CTB binding was observed when a strongly binding receptor was mixed with a weakly binding receptor (GM2). When investigated further, the reduction of dimensionality looks like the most likely cause. If this mechanism is valid, a fraction of bound CTB should simultaneously bind to GM2 and other strong binding receptors. Most recently, Klassen and his coworkers demonstrated the same heterogeneous binding cooperativity using catch-and-release electrospray ionization-mass spectrometry (CaR-ESI-MS) assay.[40, 41] Mass spectrometry allows identifying the types of receptors binding to CTB. Using CaR-ESI-MS assay, Klassen and his coworkers observed that CTB could bind to very weak binding receptors GM2 and GM3 when 7 different glycolipids (GM1, GM2, GM3, GD1a, GD1b, GD2, and GT1b) were mixed in either picodiscs or micelles systems, but no binding was observed when GM2 or GM3 was the only receptor. Their results provide evidence that CTB can directly bind to weakly binding receptors when they are mixed with strongly binding receptors. It is worth noting that we did not observe binding cooperativity between GM1 and GM3, but Klassen and his coworkers observed CTB binding to GM3. This is probably due to the difference of lipid bilayer conditions. In our experiment, surface density of glycolipid receptor was maintained at 1mol%. CaR-ESI-MS assay mixed 7 glycolipid receptors equally resulting in 14mol% of each glycolipid. The reaction enhancement via reduced dimensionality was higher in CaR-ESI-MS assay; thus, it is not surprising that Klassen and his coworkers observed CTB binding to GM3.
Reduction of dimensionality provided a potential mechanism to answer a long-standing question, why CTB binding does not correlate with GM1 level on cell surfaces.[42] Yanagisawa et al. observed strong reactivity between CTB and embryonic neuroepithelial cells in the absence of GM1.[43] Kirkeby stained GM1 with CTB and anti-GM1 antibody, and found that both labeling reagents were not co-localized.[44] In addition, GM1 is of very low abundance (0.0015–0.003 mol% of glycosphingolipids) in human small intestinal epithelial cells[45]; thus, a recent publication raised a question, whether GM1 is sufficient to induce cholera toxin attachment.[46] In the reduction of dimensionality model, high-affinity receptors can serve as initiators, and then activate weak receptors, leading to higher retention of CTB on the cell surface. Thus, the overall CTB binding is not simply controlled by a single GM1 receptor; the weakly binding receptors can contribute to CTB binding via reduction of dimensionality. Surface diffusion and local density of membrane receptors can influence the 2D reaction rate, membrane fluidity and heterogeneity (i.e. lipid raft) which can also play essential roles in CTB binding process.
The mechanism of reduced dimensionality has also been used to explain unexpected phenomena in various multivalent binding studies.[3, 8, 25] For example, Mazor et al. observed that the binding avidity of a bispecific antibody to receptors confined in cell membrane surfaces were significantly higher than the binding avidity to free receptors in solution.[47] Sengers et al. established a mathematical model based on the reduced dimensionality hypothesis to describe the mechanism of bivalent antibody binding to heterogeneous membrane targets, and estimated that the effective affinity of bivalently bound antibody can be enhanced by approximately 4 orders of magnitude.[25] These studies, combined with our own CTB binding measurements suggest the importance of the role of reduced dimensionality in multivalent protein-cell membrane recognition. Further kinetic studies are necessary in order to verify the hypothesis and establish a comprehensive model of hetero-multivalent recognition.
Since the complex interplay between multiple membrane receptors is critical, we also developed a new membrane perturbation protocol to efficiently screen receptor candidates. This protocol measured CTB binding to perturbed membranes that preserve all receptor candidates; therefore, the interplay between different receptors can be monitored. This new protocol is more efficient in screening the potential receptors than the combinatorial array, which detects proteins binding to the binary mixture of glycolipids. For example, if we plan to screen 20 receptor candidates, the membrane perturbation protocol required only 21 measurements instead of 190 measurements in a combinatorial array.
Conclusion
In summary, we elucidated the essence of hetero-multivalency in CTB-cell membrane recognition using a high-throughput and easy-to-use nanocube sensors. We believe that the detection protocols presented here can provide a systematic and efficient strategy to investigate multivalent protein-cell membrane recognition.
Supplementary Material
Highlights.
A weak receptor can bind to CTB when present with a high-affinity receptor.
Reduced dimensionality of receptor diffusion causes the hetero-multivalent binding.
Fluidity of membrane plays an essential role in multivalent binding.
A new ligand-receptor binding assay is required to explore the hetero-multivalency.
A new protocol is developed to efficiently screen receptor candidates.
Acknowledgments
This work was supported by funds from the National Institutes of Health under award number R03AI113585.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. Cold Spring Harbor; NY: 2009. [PubMed] [Google Scholar]
- 2.Lauer S, Goldstein B, Nolan RL, Nolan JP. Analysis of cholera toxin−ganglioside interactions by flow cytometry. Biochemistry. 2002;41:1742–1751. doi: 10.1021/bi0112816. [DOI] [PubMed] [Google Scholar]
- 3.Fishman PH, Atikkan EE. Mechanism of action of cholera toxin: effect of receptor density and multivalent binding on activation of adenylate cyclase. The Journal of membrane biology. 1980;54:51–60. doi: 10.1007/BF01875376. [DOI] [PubMed] [Google Scholar]
- 4.Fasting C, Schalley CA, Weber M, Seitz O, Hecht S, Koksch B, Dernedde J, Graf C, Knapp EW, Haag R. Multivalency as a chemical organization and action principle. Angewandte Chemie. 2012;51:10472–10498. doi: 10.1002/anie.201201114. [DOI] [PubMed] [Google Scholar]
- 5.Wu HJ, Henzie J, Lin WC, Rhodes C, Li Z, Sartorel E, Thorner J, Yang PD, Groves JT. Membrane-protein binding measured with solution-phase plasmonic nanocube sensors. Nat Methods. 2012;9:1189–U1181. doi: 10.1038/nmeth.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worstell NC, Krishnan P, Weatherston JD, Wu HJ. Binding cooperativity matters: A GM1-like ganglioside-cholera toxin B subunit binding study using a nanocube-based lipid bilayer array. PLoS ONE. 2016;11:e0153265. doi: 10.1371/journal.pone.0153265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller C, Despras G, Lindhorst TK. Organizing multivalency in carbohydrate recognition. Chemical Society Reviews. 2016;45:3275–3302. doi: 10.1039/c6cs00165c. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Wang Y, Lin C-I, Liu H-W, Guo A, Zhu XY. Membrane environment can enhance the interaction of glycan binding protein to cell surface glycan receptors. ACS Chemical Biology. 2014;9:1877–1884. doi: 10.1021/cb5004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinaldi S, Brennan KM, Goodyear CS, O’Leary C, Schiavo G, Crocker PR, Willison HJ. Analysis of lectin binding to glycolipid complexes using combinatorial glycoarrays. Glycobiology. 2009;19:789–796. doi: 10.1093/glycob/cwp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallegos KM, Conrady DG, Karve SS, Gunasekera TS, Herr AB, Weiss AA. Shiga toxin binding to glycolipids and glycans. Plos One. 2012;7:e30368. doi: 10.1371/journal.pone.0030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega-Muñoz M, Perez-Balderas F, Morales-Sanfrutos J, Hernandez-Mateo F, Isac-García J, Santoyo-Gonzalez F. Click multivalent heterogeneous neoglycoconjugates – modular synthesis and evaluation of their binding affinities. European Journal of Organic Chemistry. 2009;2009:2454–2473. [Google Scholar]
- 12.Kuziemko GM, Stroh M, Stevens RC. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 13.Turnbull WB, Precious BL, Homans SW. Dissecting the cholera toxin-ganglioside GM1 interaction by isothermal titration calorimetry. J Am Chem Soc. 2004;126:1047–1054. doi: 10.1021/ja0378207. [DOI] [PubMed] [Google Scholar]
- 14.Shi JJ, Yang TL, Kataoka S, Zhang YJ, Diaz AJ, Cremer PS. GM(1) clustering inhibits cholera toxin binding in supported phospholipid membranes. J Am Chem Soc. 2007;129:5954–5961. doi: 10.1021/ja069375w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leach K, Sexton PM, Christopoulos A. Current Protocols in Pharmacology. John Wiley & Sons, Inc; 2001. Quantification of allosteric interactions at G protein– coupled receptors using radioligand binding assays. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Kitova EN, Klassen JS. Measuring positive cooperativity using the direct ESI-MS assay. Cholera toxin B subunit homopentamer binding to GM1 pentasaccharide. Journal of the American Society for Mass Spectrometry. 2014;25:104–110. doi: 10.1007/s13361-013-0751-5. [DOI] [PubMed] [Google Scholar]
- 17.Schafer DE, Thakur AK. Quantitative description of the binding of GM1 oligosaccharide by cholera enterotoxin. Cell Biophysics. 1982;4:25–40. doi: 10.1007/BF02788553. [DOI] [PubMed] [Google Scholar]
- 18.Adam G, Delbruck M. Reduction of dimensionality in biological diffusion processes. In: Rich A, Davidson N, editors. Structural chemistry and molecular biology. W. H. Freeman and Co.; San Francisco: 1968. pp. 198–215. [Google Scholar]
- 19.Axelrod D, Wang M. Reduction-of-dimensionality kinetics at reaction-limited cell surface receptors. Biophysical journal. 1994;66:588. doi: 10.1016/s0006-3495(94)80834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCloskey MA, Poo M. Rates of membrane-associated reactions: reduction of dimensionality revisited. The Journal of cell biology. 1986;102:88–96. doi: 10.1083/jcb.102.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg HC, Purcell EM. Physics of chemoreception. Biophys J. 1977;20:193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardt SL. Rates of diffusion controlled reactions in one, two and three dimensions. Biophysical Chemistry. 1979;10:239–243. doi: 10.1016/0301-4622(79)85012-7. [DOI] [PubMed] [Google Scholar]
- 23.Keizer J. Diffusion effects on rapid bimolecular chemical reactions. Chemical Reviews. 1987;87:167–180. [Google Scholar]
- 24.Szabo A, Schulten K, Schulten Z. First passage time approach to diffusion controlled reactions. The Journal of Chemical Physics. 1980;72:4350–4357. [Google Scholar]
- 25.Sengers BG, McGinty S, Nouri FZ, Argungu M, Hawkins E, Hadji A, Weber A, Taylor A, Sepp A. Modeling bispecific monoclonal antibody interaction with two cell membrane targets indicates the importance of surface diffusion. MAbs. 2016;8:905–915. doi: 10.1080/19420862.2016.1178437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jobling MG, Yang Z, Kam WR, Lencer WI, Holmes RK. A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway. mBio. 2012;3:e00401–00412. doi: 10.1128/mBio.00401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh CL, Spindler S, Ehrig J, Sandoghdar V. Tracking single particles on supported lipid membranes: multimobility diffusion and nanoscopic confinement. The Journal of Physical Chemistry B. 2014;118:1545–1554. doi: 10.1021/jp412203t. [DOI] [PubMed] [Google Scholar]
- 28.Day CA, Kenworthy AK. Mechanisms underlying the confined diffusion of cholera toxin B-subunit in intact cell membranes. PLoS ONE. 2012;7:e34923. doi: 10.1371/journal.pone.0034923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindblom G, Orädd G. Lipid lateral diffusion and membrane heterogeneity. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2009;1788:234–244. doi: 10.1016/j.bbamem.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Burns AR, Frankel DJ, Buranda T. Local mobility in lipid domains of supported bilayers characterized by atomic force microscopy and fluorescence correlation spectroscopy. Biophys J. 2005;89:1081–1093. doi: 10.1529/biophysj.105.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachl R, Amaro M, Aydogan G, Koukalova A, Mikhalyov II, Boldyrev IA, Humpolickova J, Hof M. On multivalent receptor activity of GM1 in cholesterol containing membranes. Biochimica et biophysica acta. 2015;1853:850–857. doi: 10.1016/j.bbamcr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Curry DE, Liu J. Driving adsorbed gold nanoparticle assembly by merging lipid gel/fluid interfaces. Langmuir : the ACS journal of surfaces and colloids. 2015;31:13271–13274. doi: 10.1021/acs.langmuir.5b03606. [DOI] [PubMed] [Google Scholar]
- 33.Forstner MB, Yee CK, Parikh AN, Groves JT. Lipid lateral mobility and membrane phase structure modulation by protein binding. J Am Chem Soc. 2006;128:15221–15227. doi: 10.1021/ja064093h. [DOI] [PubMed] [Google Scholar]
- 34.Scomparin C, Lecuyer S, Ferreira M, Charitat T, Tinland B. Diffusion in supported lipid bilayers: influence of substrate and preparation technique on the internal dynamics, The European physical journal. E, Soft matter. 2009;28:211–220. doi: 10.1140/epje/i2008-10407-3. [DOI] [PubMed] [Google Scholar]
- 35.Goins B, M M, Barisas B, Freire E. Lateral diffusion of ganglioside GM1 in phospholipid bilayer membranes. Biophys J. 1986;49:849–856. doi: 10.1016/S0006-3495(86)83714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alouf JE, Popoff MR. The comprehensive sourcebook of bacterial protein toxins. Third. Academic Press; London: 2006. [Google Scholar]
- 37.Goluszko P, Nowicki B. Membrane cholesterol: a crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infection and immunity. 2005;73:7791–7796. doi: 10.1128/IAI.73.12.7791-7796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bricarello DA, Mills EJ, Petrlova J, Voss JC, Parikh AN. Ganglioside embedded in reconstituted lipoprotein binds cholera toxin with elevated affinity. Journal of lipid research. 2010;51:2731–2738. doi: 10.1194/jlr.M007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merritt EA, Sarfaty S, Akker FVD, L’Hoir C, Martial JA, Hol WGJ. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Science. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Fan X, Kitova EN, Zou C, Cairo CW, Eugenio L, Ng KK, Xiong ZJ, Prive GG, Klassen JS. Screening glycolipids against proteins in vitro using picodiscs and catch-and-release electrospray ionization-mass spectrometry. Analytical chemistry. 2016;88:4742–4750. doi: 10.1021/acs.analchem.6b00043. [DOI] [PubMed] [Google Scholar]
- 41.Han L, Kitova EN, Klassen JS. Detecting protein-glycolipid interactions using glycomicelles and CaR-ESI-MS. Journal of the American Society for Mass Spectrometry. 2016;27:1878–1886. doi: 10.1007/s13361-016-1461-6. [DOI] [PubMed] [Google Scholar]
- 42.Frances GR, Platt M, Dwek Raymond A, Butters Terry D. Extensive glycosphingolipid depletion in the liver and lymphoid organs of mice treated with N-Butyldeoxynojirimycin. The Journal of Biological Chemistry. 1997;272:19365–19372. doi: 10.1074/jbc.272.31.19365. [DOI] [PubMed] [Google Scholar]
- 43.Yanagisawa M, Ariga T, Yu RK. Cholera toxin B subunit binding does not correlate with GM1 expression: a study using mouse embryonic neural precursor cells. Glycobiology. 2006;16:19G–22G. doi: 10.1093/glycob/cwl003. [DOI] [PubMed] [Google Scholar]
- 44.Kirkeby S. Cholera toxin B subunit-binding and ganglioside GM1 immunoexpression are not necessarily correlated in human salivary glands. Acta Odontologica Scandinavica. 2014;72:694–700. doi: 10.3109/00016357.2014.898090. [DOI] [PubMed] [Google Scholar]
- 45.Breimer ME, Hansson GC, Karlsson KA, Larson G, Leffler H. Glycosphingolipid composition of epithelial cells isolated along the villus axis of small intestine of a single human individual. Glycobiology. 2012;22:1721–1730. doi: 10.1093/glycob/cws115. [DOI] [PubMed] [Google Scholar]
- 46.Wands AM, Fujita A, McCombs JE, Cervin J, Dedic B, Rodriguez AC, Nischan N, Bond MR, Mettlen M, Trudgian DC, Lemoff A, Quiding-Järbrink M, Gustavsson B, Steentoft C, Clausen H, Mirzaei H, Teneberg S, Yrlid U, Kohler JJ. Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife. 2015;4:e09545. doi: 10.7554/eLife.09545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazor Y, Hansen A, Yang C, Chowdhury PS, Wang J, Stephens G, Wu H, Dall’Acqua WF. Insights into the molecular basis of a bispecific antibody’s target selectivity. mAbs. 2015;7:461–469. doi: 10.1080/19420862.2015.1022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


